Abstract
Purpose of the review:
Congenital CMV infection (cCMV) is the most frequent congenital infection and a leading non-genetic cause of sensorineural hearing loss (SNHL) and brain disease. The purpose of this review is to highlight recent developments in the diagnosis and management of children with cCMV.
Recent Findings:
Progress is being made in the efforts to identify more infants with cCMV, especially those with asymptomatic infection. Largely due to efforts by various advocacy/parent groups, a number of states in the US and many hospital systems have implemented hearing targeted CMV screening and mandated education of pregnant women about CMV.
Summary:
cCMV is an important cause of SNHL and neurologic morbidity worldwide. Early identification of infected children is critical to improve outcomes by providing timely interventions and guidance for long-term follow up. The fact that most infants with cCMV have no abnormal clinical findings, and the need to obtain samples for diagnosis within the first 2–3 weeks of life, makes it challenging to identify a majority of infants with cCMV without universal newborn CMV screening.
Keywords: Congenital cytomegalovirus, newborn screening, sensorineural hearing loss, treatment
Introduction:
Congenital CMV infection (cCMV) is the most common congenital viral infection and a leading non-genetic cause of sensorineural hearing loss (SNHL) and brain disease. Diagnostic methodologies to enable early detection of children with cCMV through targeted screening and the urgent need for universal screening and maternal education are discussed. Finally, treatment options during pregnancy and in the newborn period with long-term follow up recommendations are outlined in this review.
Prenatal diagnosis:
It has been demonstrated that majority of infants with cCMV are born to women with preexisting seroimmunity to CMV (non-primary maternal infection) and therefore, routine serologic screening of all pregnant women for CMV antibodies is not recommended (1–4). In addition, screening of pregnant women can result in unnecessarily invasive testing and pregnancy termination (5).
Pregnant women are usually tested for CMV IgG as part of a diagnostic evaluation in the setting of a mononucleosis-like illness or upon detection of fetal abnormalities on prenatal ultrasound examination that raise suspicion of cCMV. When cCMV is suspected, the analysis of the amniotic fluid for the presence of CMV DNA is the appropriate approach to confirm an infected fetus. It is critical that amniocentesis should be performed after 21 weeks of gestation because of the insufficient sensitivity of the PCR to detect CMV DNA before then (6, 7). Therefore, performing amniocentesis in early gestation or soon after identifying primary maternal infection is only reliable when positive, and in cases of a negative amniotic fluid PCR, the testing should be repeated later in gestation. Higher levels of amniotic fluid viral loads have been associated with symptomatic cCMV (8). In women with primary CMV infection during pregnancy, maternal viremia at the time of amniocentesis was associated with increased risk of fetal infection (9). A recent prospective French study of 37 pregnant women with primary maternal CMV infection showed that fetal CMV infection can be diagnosed during the first trimester by PCR of chorionic villi sampling (10). However, the findings of the study have to be validated in studies with larger sample size because of the invasive nature of chorionic villi sampling that can be associated with rare adverse pregnancy outcomes.
Identification of infants with congenital CMV infection:
Infants with cCMV shed large amounts of virus in saliva and urine and therefore, either specimen can be used to identify congenitally infected infants. Saliva samples are easier to collect than urine from newborns and in addition, saliva specimens are less susceptible to contamination. It is important to note that regardless of the specimen tested, these samples should be collected from the infant within the first 2–3 weeks of life so that congenital infection can be distinguished from CMV infection acquired in the immediate postnatal period.
Viral isolation by culture from urine or saliva has long been the gold standard for identifying infants with cCMV (11, 12). However, culture-based methods are labor intensive, requires cell culture facilities, and could take several weeks to finalize the results. To overcome these challenges, a rapid culture method was developed based on centrifugation-enhancement and detection of viral antigens using monoclonal antibodies against immediate early antigens of CMV (11, 12). In a study from Brazil, PCR-based testing of urine samples for the detection of CMV DNA to diagnose cCMV has demonstrated high sensitivities (ranging between 93% and 100%) (13). Reports from more recent studies have documented high sensitivity and specificity of real-time PCR testing of newborn saliva and urine for the diagnosis of cCMV and for screening newborns to identify infected infants (14–16). PCR-based assays are less expensive with rapid turnaround times, and without the need for tissue culture facilities, these methods are now used widely for identifying infants with cCMV. An additional advantage of testing saliva specimens is that real-time PCR testing can be carried out without the need for extracting DNA (14, 16). Furthermore, PCR-based methods are less likely to be impacted by conditions of specimen storage and transport. Finally, the molecular methods can be adapted for high throughput technology and suitable for universal newborn CMV screening.
Testing of dried blood spots (DBS) collected for routing newborn screening for genetic and metabolic disease has been found to be helpful to retrospectively diagnose cCMV when children present with hearing loss or neurodevelopmental delays beyond the first few weeks of life (17, 18). However, the sensitivity of DBS PCR for detecting CMV DNA is low (19). Therefore, a positive DBS PCR confirms cCMV but a negative results does not rule out cCMV.
Newborn CMV screening:
As about 90% of infants with cCMV do not exhibit clinical abnormalities at birth (asymptomatic infection), most infected infants are not identified at birth. Between 10 to 15% of children with asymptomatic cCMV develop sequelae with SNHL being the most frequent long-term complication (20). Therefore, universal newborn CMV screening is proposed so that babies with asymptomatic cCMV who are at risk for developing SNHL can be identified early in life (1). The value of providing early interventions after the diagnosis of hearing loss in improving speech and language function outcomes has been clearly documented (21, 22). Thus, identification of children with CMV-related SNHL as early as possible is crucial to improve language/literacy outcomes.
Both urine and saliva from newborns have been shown to be reliable specimens for CMV screening (13). Saliva real-time PCR was shown to have high sensitivity and specificity (>97%) for identifying infants with cCMV in a large prospective multicentre study (14). Testing of DBS collected at birth for CMV screening has been proposed because these specimens are collected from all infants routinely and therefore, it will be easier to integrate CMV screening with the routine newborn screening program. However, DBS PCR was shown to have low sensitivity for using in large scale newborn CMV screening (19). Using modified DNA extraction protocol and PCR conditions, a recent newborn screening study reported improved DBS PCR sensitivity of 85.7% when the results from two study sites were combined. However, the sensitivity of the assay performed at the individual sites ranged between 73.2% and 76.8% (23). Even though the sensitivity of DBS PCR has been improved, this sample is not yet appropriate for newborn CMV screening because 1 in 4 infected infants will be missed.
There has been a substantial interest in screening all newborns for cCMV to identify all CMV infected newborns. In June 2021, Minnesota became the first state to enact universal newborn CMV screening and in February 2022, the state Commissioner of Health approved the recommendation to begin implementing the addition of CMV to its newborn screening program (24). Currently, seven states require both education of pregnant women and hearing targeted newborn CMV screening (25). An additional eleven states require the state to educate the public and professionals about congenital CMV. Also, many hospitals across US are currently implementing targeted CMV screening for infants who fail newborn hearing screening for the identification of babies with CMV-related SNHL at birth (26). Although this hearing targeted screening strategy identifies some of the infants with CMV-related SNHL, a large multicentre study showed that only 57% of CMV-infected infants with confirmed SNHL at birth would be detected by this approach (27). In addition, SNHL appears after early infancy in almost half of congenitally infected children with hearing loss and the hearing loss will often be progressive during early childhood. Therefore, universal CMV screening of all newborns has been advocated for the detection of children with cCMV early in life so that interventions can be provided on a timely basis to improve outcome. A recent study has shown that both targeted and universal CMV screening are cost-effective approaches (28).
Management:
Long-term Sequelae and Predictors of Outcome:
Overall, SNHL is the most common sequela of cCMV. Of those with symptomatic cCMV, 40–60% will develop permanent sequelae - SNHL is the most common, followed by developmental disabilities, chorioretinitis, and cerebral palsy (29–31). Compared to infants with symptomatic cCMV, fewer children with asymptomatic cCMV have permanent sequelae - 10–15% with SNHL and much lower rates of ophthalmological and neurodevelopmental abnormalities compared to symptomatic cCMV (29, 32–34). SNHL in cCMV is variable with respect to time of onset, laterality, progression and fluctuance over time (31, 35–37). Approximately 33–50% of SNHL due to cCMV develops beyond the neonatal period (late-onset) with a later onset in asymptomatic compared to symptomatic cCMV. Nearly 50% with SNHL will continue to have further deterioration (progressive loss) with greater degree of severity and earlier progression in children with symptomatic cCMV. A characteristic feature of CMV-related hearing loss is fluctuating hearing loss that may occur unilaterally or bilaterally.
Overall, compared to children with symptomatic cCMV, neurodevelopmental and other sequelae are uncommon in children with asymptomatic cCMV, particularly in children with no evidence of SNHL prior to 2 years of age (38). Increasingly, vestibular dysfunction with visual and balance disorders are being reported in children with symptomatic and asymptomatic cCMV with and without hearing loss (39–41).
Multiple studies have explored utilizing prenatal and neonatal findings as biomarkers for early identification cCMV sequelae with limited success. Among children with symptomatic cCMV, infants with CNS involvement have been shown to have poor cognitive outcomes compared to those with only non-neurologic symptoms (42). Recently, in a study of a small subset of the Houston Congenital CMV Longitudinal Study cohort, adults born with asymptomatic cCMV with or without SNHL were shown to have similar quality of life ratings compared with uninfected controls suggesting that SNHL in children with asymptomatic cCMV may not have an adverse impact on quality of life measures (43). However, the small cohort size limits the significance of the findings. In two different cohorts of cCMV from Taiwan, identified by a combination of newborn screening and screening of symptomatic infants, symptomatic infection, high CMV viral load at birth and failed newborn hearing screening were identified as prognostic indicators for SNHL in the newborn period (44) while low birth weight, gestational age and prolonged viral shedding correlated with late-onset SNHL (45). None of these risk-factors predicted vestibular dysfunction. However, most of these studies are limited by sample sizes and inclusion of predominantly symptomatic children, suggesting the need for further validation of the findings. Additionally, studies that evaluated fetal and neonatal cranial imaging (ultrasound and MRI) for abnormalities as prognostic indicators have revealed variable findings with a possible association between presence of imaging abnormalities, symptomatic disease and hearing loss (46, 47).
Treatment of Intrauterine Infection:
While CMV testing during pregnancy is not routinely performed, treatment with valganciclovir (VGCV) and CMV hyperimmune globulin have the potential to decrease viral load and have been used as potential therapeutic options to decrease vertical transmission during pregnancy and sequelae in newborns. In a randomized controlled trial from Israel, of 100 pregnant women with primary CMV infection diagnosed periconceptionally or in the first trimester, treatment with VGCV significantly reduced the odds of acquiring fetal CMV infection, with 11% and 48% of treated cases and those from the placebo group found to have fetal CMV infection, respectively (OR - 0.29; P = .027) (48). In a different study by Leruez-Ville et al, the impact of high-dose VGCV on clinical outcomes in 43 French women was examined with an endpoint of confirmed fetal CMV infection by amniocentesis with features suggestive of mild symptomatic disease and demonstrated a significant decrease in the proportion of symptomatic infants compared to a historical cohort (43% vs 82% respectively) in the treated cohort (49). In a randomized controlled trial by Hughes et al, the utility of treatment with monthly HIG was compared to placebo in 394 women with singleton pregnancies and primary CMV infection at <24 weeks’ gestation. The study documented no significant difference in fetal transmission between the treatment arms (relative risk - 1.17; p = 0 .42) (50). Overall, the role of VGCV and CMV HIG in CMV infections in pregnancy is still being defined with no high-quality evidence for wide spread use at this time.
Treatment of Neonatal Infection:
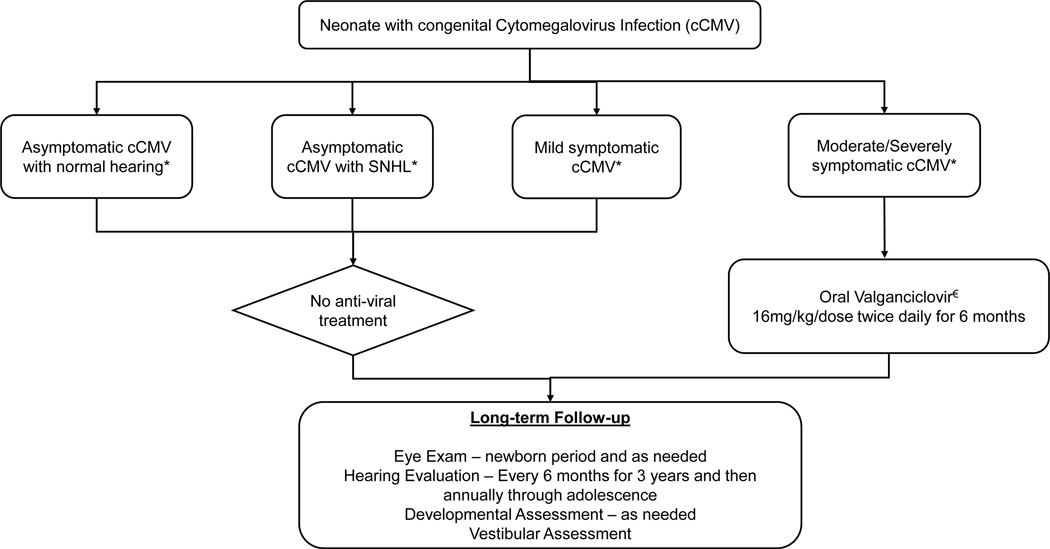
Antiviral treatment with oral VGCV for 6 months compared to 6 weeks has been shown to be beneficial for hearing and neurodevelopment outcomes in newborns with moderate-severely symptomatic cCMV if initiated within the first month of life and is considered standard of care (51, 52). Myelosuppression and liver damage are the anticipated side-effects from VGCV, and close follow-up is recommended for the entire duration of treatment. Due to lack of established efficacy, antiviral treatment is currently not recommended for children with asymptomatic cCMV or those with isolated SNHL pending data from ongoing studies (NCT03107871, NCT03301415) (1). Few studies have explored long-term effects of antiviral treatment on cCMV sequelae. In a longitudinal study of 76 children with symptomatic cCMV by Lanzieri et al, compared hearing outcomes between children who did not receive antiviral treatment vs those treated with intravenous ganciclovir and reported no significant differences in frequency of severe hearing loss in these groups (53). A retrospective review of long-term hearing outcomes in 16 children with symptomatic cCMV who received VGCV showed a measurable worsening of hearing function over time suggesting that VGCV therapy may only provide short-term benefit (54).
While no alternate treatment regimens are recommended for cCMV, a recent study of a small cohort of children with symptomatic cCMV, neurological outcomes were compared at 18 months between children who received a combination of fetal immunoglobulin therapy and antiviral treatment in the neonatal period vs neonatal antiviral treatment alone (55). The proportion of infants with severe impairments in the group that received the combination therapy was significantly lower than that in the group receiving neonatal antiviral treatment alone (18.2 % vs 64.3 %, p < 0.05). However, given the small sample size of this cohort and lack of longer follow-up, further validation of these findings is needed.
While ganciclovir/valganciclovir resistance has only occasionally been reported in children with cCMV undergoing treatment, it is necessary to reassess the risk-benefit ratio for antiviral treatment, including the possibility of resistance onset, alongside improvement in SNHL and neurodevelopmental outcomes (56, 57).
Recommended Long-term follow up:
Although no standard guidelines exist at this time for long-term follow up of children with cCMV, consensus recommendations published in 2017 provides guidance and recommends close follow up to monitor for audiological, ophthalmological and neurodevelopmental outcomes and are outlined in Figure 1 (1, 58). It is recommended that children with cCMV should be followed with serial audiological assessments initiated in the newborn period, repeated at 6-month intervals for the first 3 years of life, and annually thereafter through adolescence. With emerging evidence that vestibular dysfunction occurs frequently in children with symptomatic and asymptomatic cCMV and the association between SNHL and vestibular dysfunction, it is likely that infected children will benefit from routine periodic vestibular assessments, incorporated in to the newborn hearing screening and audiological protocols (59, 60).
Figure 1: Schema for Treatment and Follow-up of Neonates with cCMV.
cCMV – congenital Cytomegalovirus
SNHL – sensorineural hearing loss
* Severity of cCMV as described by Rawlinson et al (1)
€ Absolute neutrophil counts should be followed weekly for 6 weeks, then at 8 weeks and then monthly for the duration of treatment. Transaminase levels should be followed monthly while on treatment.
Conclusion:
In conclusion, cCMV remains an important cause of SNHL and developmental disabilities with early identification enabled by CMV DNA detection by real-time PCR in saliva or urine samples obtained from newborns. Efforts by advocacy groups are leading to implementation of CMV education and newborn universal/targeted screening programs. Anti-viral treatment in select cases and long-term follow up for hearing, ophthalmological, developmental and vestibular outcomes are recommended for all children with cCMV.
Key Points:
Saliva or urine remain the most appropriate samples from newborns for detecting the presence of CMV by real-time PCR
Early identification of infants with cCMV including those with asymptomatic infection is critical to implement intervention measures to improve clinical outcomes in affected children
Efforts by advocacy groups are resulting in more states in the US implementing CMV education and newborn screening measures. Minnesota became the first state in the US to mandate screening of all newborns for cCMV.
CMV hyperimmune globulin failed to prevent transplacental transmission of CMV in women with primary CMV infection during pregnancy.
Financial Support and sponsorship:
Kaul Pediatric Research Institute at Children’s of Alabama, and NIH/NIAID 1R01AI109001 - 04A1.
Footnotes
Conflicts of interest: SB is a member of CMV Vaccine Advisory Committees of Merck, Moderna and GSK.
Contributor Information
Swetha Pinninti, Heersink School of Medicine I University of Alabama at Birmingham.
Suresh Boppana, Heersink School of Medicine I University of Alabama at Birmingham.
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
¥ of special interest
¥¥ of outstanding interest
- 1.Rawlinson WD, Boppana SB, Fowler KB, Kimberlin DW, Lazzarotto T, Alain S, et al. Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis. 2017;17(6):e177–e88. [DOI] [PubMed] [Google Scholar]
- 2.Luck SE, Wieringa JW, Blázquez-Gamero D, Henneke P, Schuster K, Butler K, et al. Congenital Cytomegalovirus: A European Expert Consensus Statement on Diagnosis and Management. Pediatr Infect Dis J. 2017;36(12):1205–13. [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Li F, Freed DC, Finnefrock AC, Tang A, Grimes SN, et al. Quantitative analysis of neutralizing antibody response to human cytomegalovirus in natural infection. Vaccine. 2011;29(48):9075–80. [DOI] [PubMed] [Google Scholar]
- 4.Townsend CL, Forsgren M, Ahlfors K, Ivarsson SA, Tookey PA, Peckham CS. Long-term outcomes of congenital cytomegalovirus infection in Sweden and the United Kingdom. Clin Infect Dis. 2013;56(9):1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerra B, Simonazzi G, Banfi A, Lazzarotto T, Farina A, Lanari M, et al. Impact of diagnostic and confirmatory tests and prenatal counseling on the rate of pregnancy termination among women with positive cytomegalovirus immunoglobulin M antibody titers. Am J Obstet Gynecol. 2007;196(3):221.e1–6. [DOI] [PubMed] [Google Scholar]
- 6.Azam AZ, Vial Y, Fawer CL, Zufferey J, Hohlfeld P. Prenatal diagnosis of congenital cytomegalovirus infection. Obstet Gynecol. 2001;97(3):443–8. [DOI] [PubMed] [Google Scholar]
- 7.Liesnard C, Donner C, Brancart F, Gosselin F, Delforge ML, Rodesch F. Prenatal diagnosis of congenital cytomegalovirus infection: prospective study of 237 pregnancies at risk. Obstet Gynecol. 2000;95(6 Pt 1):881–8. [DOI] [PubMed] [Google Scholar]
- 8.Lazzarotto T, Varini S, Guerra B, Nicolosi A, Lanari M, Landini MP. Prenatal indicators of congenital cytomegalovirus infection. J Pediatr. 2000;137(1):90–5. [DOI] [PubMed] [Google Scholar]
- 9.Simonazzi G, Cervi F, Zavatta A, Pellizzoni L, Guerra B, Mastroroberto M, et al. Congenital cytomegalovirus infection: prognostic value of maternal DNAemia at amniocentesis. Clin Infect Dis. 2017;64(2):207–10. [DOI] [PubMed] [Google Scholar]
- 10. Faure-Bardon V, Fourgeaud J, Guilleminot T, Magny JF, Salomon LJ, Bernard JP, et al. First-trimester diagnosis of congenital cytomegalovirus infection after maternal primary infection in early pregnancy: feasibility study of viral genome amplification by PCR on chorionic villi obtained by CVS. Ultrasound Obstet Gynecol. 2021;57(4):568–72. ¥ Recent study detailing pre-natal diagnosis of cCMV
- 11.Boppana SB, Smith R, Stagno S, Britt WJ. Evaluation of a microtiter plate fluorescent antibody assay for rapid detection of human cytomegalovirus infections. J Clin Microbiol. 1992;30:721–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balcarek KB, Warren W, Smith RJ, Lyon MD, Pass RF. Neonatal screening for congenital cytomegalovirus infection by detection of virus in saliva. J Infect Dis. 1993;167(6):1433–6. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto AY, Mussi-Pinhata MM, Marin LJ, Brito RM, Oliviera PF, Coelho TB. Is saliva as reliable as urine for detection of cytomegalovirus DNA for neonatal screening of congenital CMV infection? J Clin Virol. 2006;36:228–30. [DOI] [PubMed] [Google Scholar]
- 14.Boppana SB, Ross SA, Shimamura M, Palmer AL, Ahmed A, Michaels MG, et al. Saliva polymerase-chain-reaction assay for congenital cytomegalovirus screening in newborns. N Engl J Med. 2011;364:2111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross SA, Ahmed A, Palmer AL, Michaels MG, Sánchez PJ, Bernstein DI, et al. Detection of Congenital Cytomegalovirus Infection by Real-Time Polymerase Chain Reaction Analysis of Saliva or Urine Specimens. J Infect Dis. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinninti SG, Ross SA, Shimamura M, Novak Z, Palmer AL, Ahmed A, et al. Comparison of saliva PCR assay versus rapid culture for detection of congenital cytomegalovirus infection. Pediatr Infect Dis J. 2015;34(5):536–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Binda S, Caroppo S, Didò P, Primache V, Veronesi L, Calvario A, et al. Modification of CMV DNA detection from dried blood spots for diagnosing congenital CMV infection. J Clin Virol. 2004;30(3):276–9. [DOI] [PubMed] [Google Scholar]
- 18.Atkinson C, Walter S, Sharland M, Tookey P, Luck S, Peckham C, et al. Use of stored dried blood spots for retrospective diagnosis of congenital CMV. J Med Virol. 2009;81(8):1394–8. [DOI] [PubMed] [Google Scholar]
- 19.Boppana SB, Ross SA, Novak Z, Shimamura M, Tolan RW, Palmer AL, et al. Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA. 2010;303(14):1375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fowler KB, Boppana SB. Congenital cytomegalovirus infection. Semin Perinatol. 2018;42(3):149–54. [DOI] [PubMed] [Google Scholar]
- 21.Downs MP, Yoshinaga-Itano C. The efficacy of early identification and intervention for children with hearing impairment. Pediatr Clin North Am. 1999;46(1):79–87. [DOI] [PubMed] [Google Scholar]
- 22. Yoshinaga-Itano C, Mason CA, Wiggin M, Grosse SD, Gaffney M, Gilley PM. Reading Proficiency Trends Following Newborn Hearing Screening Implementation. Pediatrics. 2021;148(4). ¥ Study highlighting importance of early detection and intervention for hearing loss.
- 23.Dollard SC, Dreon M, Hernandez-Alvarado N, Amin MM, Wong P, Lanzieri TM, et al. Sensitivity of Dried Blood Spot Testing for Detection of Congenital Cytomegalovirus Infection. JAMA Pediatr. 2021;175(3):e205441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DOH M, News Release: Congenital cytomegalovirus approved for addition to newborn screening panel. State would be first to screen all newborns for the infection-caused condition. . 2022, MDH Communications. https://content.govdelivery.com/bulletins/gd/MNMDH-308c2bf. [Google Scholar]
- 25.Foundation NC Congenital CMV Legislation in the United States. 2022; Available from: https://www.nationalcmv.org/about-us/advocacy
- 26. Screening N. Hospitals providing hearing targeted early CMV screening. 2022. [cited 2022 July 15 2022]; Available from: https://www.nationalcmv.org/overview/newborn-screening. ¥¥ Foundation highlighting advocacy groups working to increase CMV awareness and newborn screening implementation
- 27.Fowler KB, McCollister FP, Sabo DL, Shoup AG, Owen KE, Woodruff JL, et al. A Targeted Approach for Congenital Cytomegalovirus Screening Within Newborn Hearing Screening. Pediatrics. 2017;139(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gantt S, Dionne F, Kozak FK, Goshen O, Goldfarb DM, Park AH, et al. Cost-effectiveness of universal and targeted newborn screening for congenital cytomeaglovirus infection. JAMA Pediatr. 2016;170(12):1173–80. [DOI] [PubMed] [Google Scholar]
- 29.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17(5):355–63. [DOI] [PubMed] [Google Scholar]
- 30.Fowler KB, Stagno S, Pass RF, Britt WJ, Boll TJ, Alford CA. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med. 1992;326(10):663–7. [DOI] [PubMed] [Google Scholar]
- 31.Dahle AJ, Fowler KB, Wright JD, Boppana SB, Britt WJ, Pass RF. Longitudinal investigation of hearing disorders in children with congenital cytomegalovirus. J Am Acad Audiol. 2000;11(5):283–90. [PubMed] [Google Scholar]
- 32.Fowler KB, McCollister FP, Dahle AJ, Boppana S, Britt WJ, Pass RF. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J Pediatr. 1997;130(4):624–30. [DOI] [PubMed] [Google Scholar]
- 33.Fowler KB, Boppana SB. Congenital cytomegalovirus (CMV) infection and hearing deficit. J Clin Virol. 2006;35(2):226–31. [DOI] [PubMed] [Google Scholar]
- 34. Puhakka L, Lappalainen M, Lönnqvist T, Nieminen T, Boppana S, Saxen H, et al. Hearing outcome in congenitally CMV infected children in Finland - Results from follow-up after three years age. Int J Pediatr Otorhinolaryngol. 2022;156:111099. ¥ Recent study detailing hearing outcomes in children identified through newborn screening
- 35.Fowler KB, McCollister FP, Dahle AJ, Boppana SB, Britt WJ, Pass RF. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J Pediatr. 1997;130(4):624–30. [DOI] [PubMed] [Google Scholar]
- 36.Fowler KB, Dahle AJ, Boppana SB, Pass RF. Newborn hearing screening: will children with hearing loss caused by congenital cytomegalovirus infection be missed? J Pediatr. 1999;135(1):60–4. [DOI] [PubMed] [Google Scholar]
- 37. Cushing SL, Purcell PL, Papaiaonnou V, Neghandi J, Daien M, Blaser SI, et al. Hearing Instability in Children with Congenital Cytomegalovirus: Evidence and Neural Consequences. Laryngoscope. 2022. ¥ Study highlighting hearing instability in cCMV
- 38.Lopez AS, Lanzieri TM, Claussen AH, Vinson SS, Turcich MR, Iovino IR, et al. Intelligence and Academic Achievement With Asymptomatic Congenital Cytomegalovirus Infection. Pediatrics. 2017;140(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chebib E, Maudoux A, Benoit C, Bernard S, Van Den Abbeele T, Teissier N, et al. Audiovestibular Consequences of Congenital Cytomegalovirus Infection: Greater Vulnerability of the Vestibular Part of the Inner Ear. Ear Hear. 2022. [DOI] [PubMed] [Google Scholar]
- 40.Pinninti S, Christy J, Almutairi A, Cochrane G, Fowler KB, Boppana S. Vestibular, Gaze, and Balance Disorders in Asymptomatic Congenital Cytomegalovirus Infection. Pediatrics. 2021;147(2). [DOI] [PubMed] [Google Scholar]
- 41. Dhondt C, Maes L, Rombaut L, Martens S, Vanaudenaerde S, Van Hoecke H, et al. Vestibular Function in Children With a Congenital Cytomegalovirus Infection: 3 Years of Follow-Up. Ear Hear. 2021;42(1):76–86. ¥ studies highlighting vestibular involvement in cCMV
- 42.Dreher AM, Arora N, Fowler KB, Novak Z, Britt WJ, Boppana SB, et al. Spectrum of disease and outcome in children with symptomatic congenital cytomegalovirus infection. J Pediatr. 2014;164(4):855–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katusic MZ, Mensah-Bonsu NE, Miller JA, Turcich MR, Iovino I, Vinson-Sellers S, et al. The Impact of Asymptomatic Congenital Cytomegalovirus on Adult Quality of Life. J Dev Behav Pediatr. 2021;42(1):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lo TH, Lin PH, Hsu WC, Tsao PN, Liu TC, Yang TH, et al. Prognostic determinants of hearing outcomes in children with congenital cytomegalovirus infection. Sci Rep. 2022;12(1):5219. ¥ study detailing predictors of hearing outcome
- 45.Wu PH, Lee CY, Huang JY, Yang SF, Shih CP. The correlation between neonatal parameters and late-onset inner ear disorders in congenital cytomegalovirus infection: a 10-year population-based cohort study. Clin Otolaryngol. 2022;47(1):107–14. [DOI] [PubMed] [Google Scholar]
- 46.Craeghs L, Goderis J, Acke F, Keymeulen A, Smets K, Van Hoecke H, et al. Congenital CMV-Associated Hearing Loss: Can Brain Imaging Predict Hearing Outcome? Ear Hear. 2021;42(2):373–80. [DOI] [PubMed] [Google Scholar]
- 47.Lucignani G, Rossi Espagnet MC, Napolitano A, Figà Talamanca L, Calò Carducci FI, Auriti C, et al. A new MRI severity score to predict long-term adverse neurologic outcomes in children with congenital Cytomegalovirus infection. J Matern Fetal Neonatal Med. 2021;34(6):859–66. [DOI] [PubMed] [Google Scholar]
- 48.Shahar-Nissan K, Pardo J, Peled O, Krause I, Bilavsky E, Wiznitzer A, et al. Valaciclovir to prevent vertical transmission of cytomegalovirus after maternal primary infection during pregnancy: a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396(10253):779–85. [DOI] [PubMed] [Google Scholar]
- 49.Leruez-Ville M, Ghout I, Bussières L, Stirnemann J, Magny JF, Couderc S, et al. In utero treatment of congenital cytomegalovirus infection with valacyclovir in a multicenter, open-label, phase II study. Am J Obstet Gynecol. 2016;215(4):462.e1-.e10. [DOI] [PubMed] [Google Scholar]
- 50. Hughes BL, Clifton RG, Rouse DJ, Saade GR, Dinsmoor MJ, Reddy UM, et al. A Trial of Hyperimmune Globulin to Prevent Congenital Cytomegalovirus Infection. N Engl J Med. 2021;385(5):436–44. ¥¥ Study detailing the futility of hyperimmune globulin in preventing CMV transmisison in pregnancy
- 51.Kimberlin DW, Jester PM, Sánchez PJ, Ahmed A, Arav-Boger R, Michaels MG, et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med. 2015;372(10):933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ross SA, Kimberlin D. Clinical outcome and the role of antivirals in congenital cytomegalovirus infection. Antiviral Res. 2021;191:105083. [DOI] [PubMed] [Google Scholar]
- 53.Lanzieri TM, Caviness AC, Blum P, Demmler-Harrison G. Progressive, Long-Term Hearing Loss in Congenital CMV Disease After Ganciclovir Therapy. J Pediatric Infect Dis Soc. 2022;11(1):16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCrary H, Sheng X, Greene T, Park A. Long-term hearing outcomes of children with symptomatic congenital CMV treated with valganciclovir. Int J Pediatr Otorhinolaryngol. 2019;118:124–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanimura K, Shi Y, Uchida A, Uenaka M, Imafuku H, Ikuta T, et al. Immunoglobulin fetal therapy and neonatal therapy with antiviral drugs improve neurological outcome of infants with symptomatic congenital cytomegalovirus infection. J Reprod Immunol. 2021;143:103263. [DOI] [PubMed] [Google Scholar]
- 56.Torii Y, Horiba K, Kawada JI, Haruta K, Yamaguchi M, Suzuki T, et al. Detection of antiviral drug resistance in patients with congenital cytomegalovirus infection using long-read sequencing: a retrospective observational study. BMC Infect Dis. 2022;22(1):568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garofoli F, Lombardi G, Angelini M, Campanini G, Zavattoni M, Baldanti F. Onset of valganciclovir resistance in two infants with congenital cytomegalovirus infection. Int J Infect Dis. 2020;98:150–2. [DOI] [PubMed] [Google Scholar]
- 58.Park A, Doutre S, Schleiss MR, Shoup A. All Cytomegalovirus-infected Children Need Hearing and Neurologic Follow-up. Clin Infect Dis. 2020;70(1):173. [DOI] [PubMed] [Google Scholar]
- 59.Martens S, Dhooge I, Dhondt C, Vanaudenaerde S, Sucaet M, Van Hoecke H, et al. Three Years of Vestibular Infant Screening in Infants With Sensorineural Hearing Loss. Pediatrics. 2022;150(1). [DOI] [PubMed] [Google Scholar]
- 60.Janky KL, Yoshinaga-Itano C. The Feasibility of Performing Vestibular Newborn Screening. Pediatrics. 2022;150(1). [DOI] [PubMed] [Google Scholar]