SUMMARY
Introduction
Leptospirosis is a zoonotic bacterial infection with significant mortality and morbidity, especially in resource-limited settings. This systematic review aimed to study the clinical profile and outcome of patients with leptospirosis in India.
Methodology
All articles up to 02.08.2022 were searched using the two databases, PubMed and Scopus. A total of 542 articles were found using the search terms related to ‘leptospirosis’ and ‘India’. After two rounds of screening, 55 articles were included. The data were collected on epidemiology, clinical features, laboratory features and treatment of patients with leptospirosis.
Results
Most cases of leptospirosis were reported from the coastal belt. A large percentage of patients were identified as farmers, and exposure to rainfall was identified as an important risk factor. Fever was present in 97%, and conjunctival suffusion was present in 35% of cases. Haemoptysis, gastrointestinal bleeding, and haematuria were present in 5%, 5% and 12% of patients, respectively. Liver and kidney were involved in 34% and 35% of the patients, respectively. The average haemoglobin, leucocyte count and platelet count across various studies ranged from 9.6–12.5 grams/dl, 8.8–11.3 thousand/μl and 20–130 thousand/μl, respectively. Treatment details were sparsely available in some studies, with penicillin, ceftriaxone, and doxycycline used commonly. The pooled mortality across various studies was calculated as 11% [95% CI-8–15%, I2=93%, P<0.001].
Conclusions
Leptospirosis is associated with significant mortality in Indian settings. There is a need for studies focussing on treatment modalities.
Keywords: Leptospira, Weil’s disease, acute febrile illness
INTRODUCTION
Leptospirosis is a zoonotic bacterial infection associated with significant morbidity and mortality. It is one of the most widespread yet neglected zoonoses, with most reports from South America, the Caribbean, and South Asia [1]. An estimate suggests that a total of 58,900 deaths are reported every year [2]. More than three-fourths of cases occur between the tropics of Cancer and the tropic of Capricorn [3]. In a systematic review (1970–2012), 13% of all the outbreaks were reported from South Asia [4]. An evidence gap map on leptospirosis in India suggested a paucity of evidence on management and control [5]. This systematic review aimed to study the epidemiological features, clinical profile, laboratory parameters, diagnostics, treatment and outcome of patients with leptospirosis in India.
METHODOLOGY
A systematic review of the literature was performed in two databases (PubMed and Scopus) to identify leptospirosis studies reported up to 02.08.2022. Studies (randomized controlled trials, cohort studies, case-control studies, cross-sectional studies, and case series) with clinical, laboratory and treatment details of diagnosed leptospirosis cases from India were included. In-vitro studies, case reports or studies with non-human subjects were excluded. In addition, those studies that did not have sufficient clinical data were also excluded. The following search string was used:
leptospirosis OR leptospira OR Weil’s AND disease) AND (clinical OR epidemiology OR symptoms OR sign OR haemorrhagic OR neurological OR laboratory OR treatment OR penicillin OR doxycycline OR azithromycin) AND (India).
The title-abstract and full-text screening were done by two authors (PR and WW). Wherever there was a lack of consensus, the third author (NG) was consulted to arrive at a consensus. The included studies’ data (epidemiology, clinical features, laboratory parameters, treatment and outcome) were entered into a pre-defined workbook. NG did the data analysis. The pooled proportion was calculated for all the included variables. A pooled mortality was calculated using the Revman software (version 5.3, Cochrane Nordic, Copenhagen, Denmark). A random effects model was used to generate the forest plot.
RESULTS AND DISCUSSION
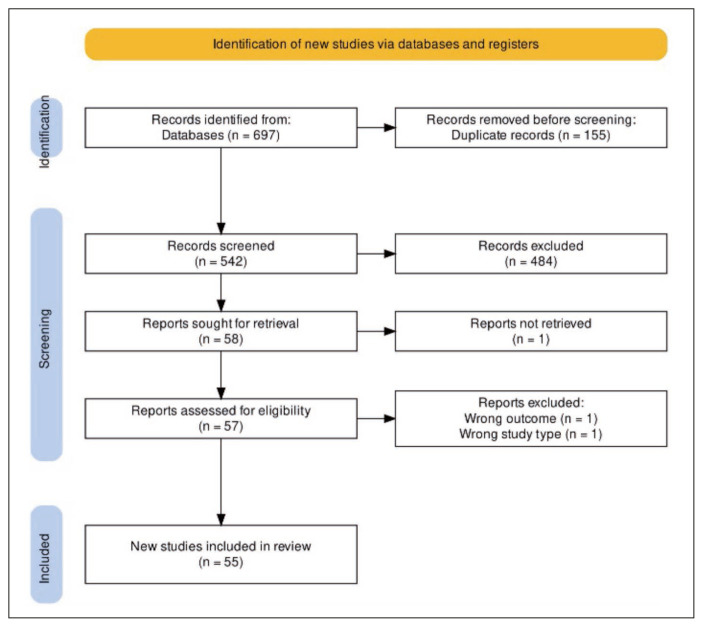
A total of 318 articles from PubMed and 375 articles from Scopus were found using the search term. Four articles were added from other sources (references of screened articles). After deleting 155 duplicates, 542 articles were included in the title-abstract screening. The full text could not be retrieved for one article. A total of 55 articles with 11,303 cases were included in the final analysis (Figure 1) [6–61]. Two articles were excluded because they did not meet the inclusion criteria.
Figure 1.
PRISMA chart showing the screening and final inclusion of studies used in this Systematic review.
Considering the vastness and the heterogeneity of the collected data, the results and discussion section have been organized into sub-headings.
Biology
Leptospira spp is a spirochaete traditionally divided into the pathogenic L. interrogans and the saprophytic L. biflexa [1]. In the present day, Leptospira spp. is divided into multiple serovars. The most common serovar identified in the various Indian sites were icterohaemorrhagiae, australis, autumnalis, grippotyphosa, canicola, pomona and pyrogenes (Table 1) [9, 11, 24, 30, 33, 40, 51, 55]. Their distribution varies across geographical regions [1, 40]. For example, icterohaemorrhagiae is significantly more frequent in rural regions [40]. The association between serovars and clinical manifestations has not been clear-cut, but icterohaemorrhagiae has been associated with severe manifestations in some reports [1].
Table 1.
Infective serovars/serogroups identified across different studies in India.
| Sn | Author | Icterohaemorrhagiae | Australis | Autumnalis | Grippotyphosa | Louisiana | Canicola | Pomona |
|---|---|---|---|---|---|---|---|---|
| 1 | Jeyakumar 2008 [51] | 3% | 6% | 8% | 3% | 11% | ||
| 2 | Kuriakose 1996 [30] | 12% | 20% | 27% | 7% | 10% | 5% | 1% |
| 3 | Manocha 2004 [24] | 50% | 50% | |||||
| 4 | Murhekar 1998 [55] | 38% | 73% | 65% | 38% | |||
| 5 | Muthusethupathi 1995 [33] | 4% | 2% | 74% | 2% | |||
| 6 | Narayanan 2016 [11] | 37% | 27% | 5% | 16% | 1% | 5% | 8% |
| 7 | Prabhakaran 2014 [40] | 10% | 6% | 9% | 8% | 37% | 18% | |
| 8 | Sehgal 2002 [9] | 11% | 89% |
Epidemiology
Leptospirosis is a zoonotic disease with the infection maintained in the kidney of reservoir animals like rodents, cattle, goats, pigs etc. [6]. Humans acquire the infection when they come in contact with water contaminated with the urine of an affected animal. In a study by Padmakumar et al., more than half of the leptospirosis cases had a history of positive contact with animals [10]. In our review, exposure to rats was found in 67% of the leptospirosis cases. In a study by Narayanan et al., stagnant water and rat infestation in the house were independently associated with leptospirosis infection [11]. In a study by Vimala et al., 42% of trapped rats (Rattus rattus and Rattus norvegicus) were positive for leptospirosis [25]. Exposure to stagnant water was found in 45% of the patients. Chakurkar et al. divided the pattern of leptospirosis in India into rural and urban patterns [12]. Cases in rural regions were primarily associated with farming, while urban cases were primarily related to poor sewage disposal and rodent infestation. Farming is associated with higher exposure to contaminated water, especially in the case of farming crops (paddy, sugarcane, banana, etc.) requiring substantial water irrigation [6]. The percentage of patients identified as farmers in our review was 41%. Other occupations at risk of leptospirosis include sewage workers and veterinarians [7]. Water exposure can also occur during recreational sports such as rafting, kayaking or canoeing [7]. In a study by Balasundaram et al., 55% of the cases had a history of recreational water sports [8].
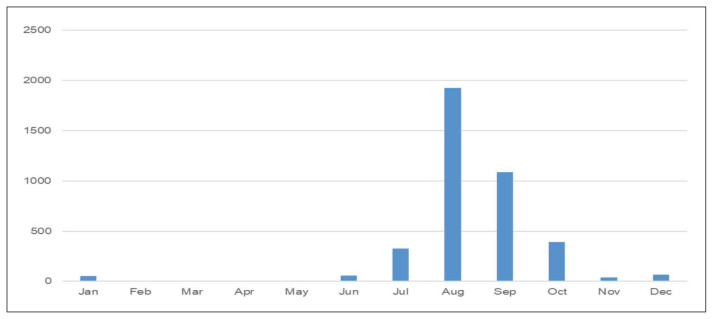
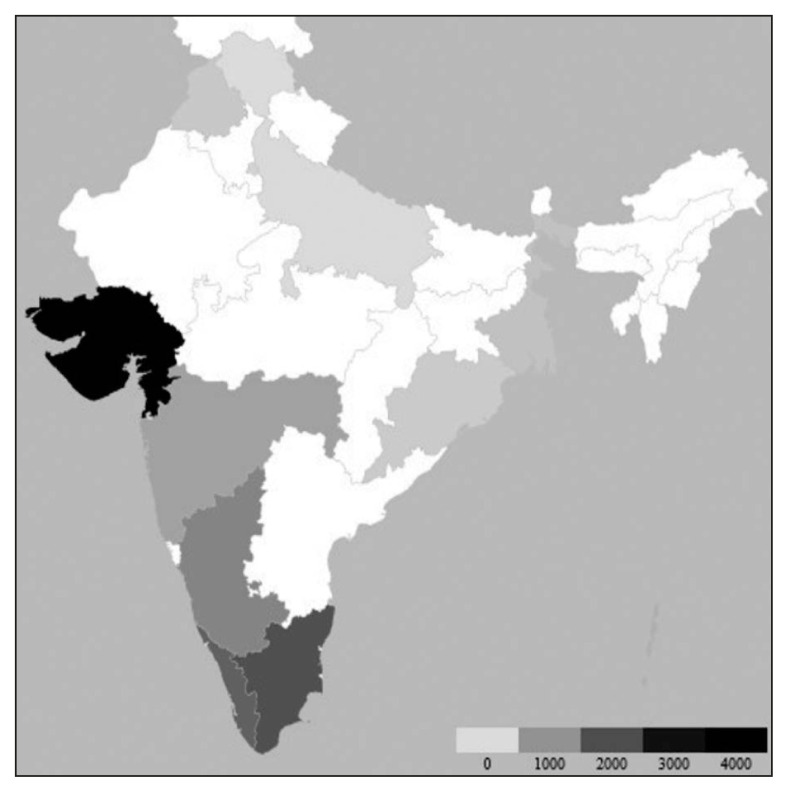
A significant increase in the number of leptospirosis cases has been observed after rainfall-related flooding. Contaminated animal urine on soil mix easily with surface water during floods, making a large population, especially those living in low-lying areas, susceptible to leptospirosis [14]. This explains the higher frequency of the included cases in the rainy season (July to October) (Figure 2). Of the included studies, 18 were directly linked to rainfall. This also included 12 studies where flood-related events were described. In the study by Karande et al., all children diagnosed with leptospirosis had either played in or waded in the water-logged areas [14]. In another study by Sehgal et al., the patient’s residence near a river was a risk factor for infection [9]. Most cases were reported from the coastal belt (Gujarat, Tamil Nadu, Maharashtra, Kerala, Karnataka, Andaman and Nicobar Islands) (Figure 3). Since most of these regions have deltas of large rivers, there are prone to frequent flooding. The population density next to the rivers might have contributed to the outbreaks as well. Also, the coastal areas have a higher amount of rainfall. This situation increases the probability of human exposure to contaminated waters. Another reason for the increased incidence in the southern coastal areas could be the increased survival of leptospires in warm and humid environments [1].
Figure 2.
Seasonal distribution of leptospirosis cases across studies from India.
Abbreviations: Jan: January, Feb: February, Mar: March, Apr: April, Jun: June, Jul: July, Aug- August, Sep: September, Oct- October, Nov- November, Dec- December.
Figure 3.
State-wise distribution of cases of leptospirosis across India*.
*The colour scale at the bottom represents the number of leptospirosis cases. As the number of cases increases, the shade becomes darker.
Clinical features
Acute febrile illness
The most common manifestation of leptospirosis is an undifferentiated acute febrile illness. Fever at presentation has been reported in 97% of the included cases and lasted usually for 4–5 days (Table 2). Headache (43%), myalgia (51%) and arthralgia (18%) were common constitutional features. Some studies suggested that muscle tenderness (localized or general) was significantly more common in cases of leptospirosis when compared to other causes of febrile illnesses (Table 2) [8, 10, 14]. Lymphadenopathy (5%) and rash (6%) were relatively uncommon. Gastrointestinal manifestations such as diarrhoea (18%) and abdominal pain (24%) were also seen (Table 2). Hepatomegaly (31%) and splenomegaly (29%) were not uncommon.
Table 2.
Clinical features of adult patients with leptospirosis presenting as Acute Febrile illness.
| Sn | Author/Year | N | Fever | Head | Msl | LN | Rash | Drrh | Abd pain | Liver | Spleen |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Narayanan 2016 [11] | 118 | 98.3% | 36% | 32% | 15% | |||||
| 2 | Mathur 2019 [15] | 237 | 100% | 39% | 8% | 4% | |||||
| 3 | Padmakumar 2016 [10] | 45 | – | – | – | 2% | 22% | 27% | 33% | – | |
| 4 | Sethi 2003 [16] | 20 | 100% | 95% | 90% | 30% | – | 25% | 25% | – | |
| 5 | Sehgal 2002 [9] | 569 | 100% | 26% | 83% | – | – | 27% | – | ||
| 6 | Mathew Thomas et al. 2006 [17] | 31 | 97% | 71% | – | – | 23% | 13% | |||
| 7 | Chaudhry 2017 [18] | 107 | 90% | 31% | 39% | – | 43% | 27% | |||
| 8 | Salkade 2005 [19] | 62 | 79% | – | 13% | – | – | 29% | 29% | – | |
| 9 | Holla 2018 [21] | 202 | 92% | 18% | 36% | 19% | |||||
| 10 | Shah Kinjal 2009 [13] | 24 | 100% | – | – | – | – | ||||
| 11 | Chakurkar 2008 [12] | 44 | 100% | 4% | 36% | 7% | 30% | 16% | |||
| 12 | Somasundaram Aravindh 2014 [22] | 122 | 97% | 87% | 54% | – | – | – | |||
| 13 | Patil 2017 [7] | 193 | 81% | 52% | 51% | – | – | – | |||
| 14 | Chawla 2004 [23] | 60 | 97% | – | – | – | – | ||||
| 15 | Manocha 2004 [24] | 25 | 100% | 24% | 16% | – | – | 8% | 40% | – | |
| 16 | Vimala 2014 [25] | 10 | 100% | 70% | 60% | – | – | – | |||
| 17 | Madhusudhana 2015 [26] | 42 | 100% | – | – | – | – | ||||
| 18 | Trivedi 2010 [27] | 144 | 100% | 76% | 97% | – | – | – | |||
| 19 | George Thomas 2012 [28] | 467 | 89% | 19% | 8% | – | – | 15% | 26% | – | |
| 20 | Adiga Deepa 2017 [29] | 130 | 89% | 41% | 29% | – | – | 26% | 9% | – | |
| 21 | Balasundaram Padmakumar 2020 [8] | 110 | – | – | 83% | – | 2% | 37% | 39% | – | |
| 22 | Kuriakose 1997 [30] | 978 | – | – | – | – | 11% | – | |||
| 23 | DebMandal 2011 [31] | 214 | 100% | 100% | 0.5% | – | 87% | 87% | |||
| 24 | Saravanan 2014 [32] | 894 | 100% | – | – | – | – | ||||
| 25 | Muthusethupathi 1995 [33] | 57 | 100% | 25% | 82% | – | – | 26% | 18% | – | |
| 26 | Majumdar 2013 [34] | 77 | 100% | – | – | 12% | 9% | ||||
| 27 | Patel 2011 [35] | 44 | 100% | 89% | 95% | 20% | 4% | 25% | 23% | ||
| 28 | Bhardwaj Pankaj 2008 [36] | 62 | – | – | 50% | – | – | – | |||
| 29 | Sethi 2010 [37] | 232 | 97% | 14% | 11% | 2% | 2% | 13% | 20% | 10% | |
| 30 | Chauhan 2010 [38] | 13 | 100% | 92% | 77% | – | – | – | |||
| 31 | Pappachan 2002 [39] | 282 | 100% | 78% | 93% | 7% | 6% | 28% | 58% | 2% | |
| 32 | Prabhakaran 2014 [40] | 410 | 100% | 45% | 52% | – | – | 6% | 26% | – | |
| 33 | Zala/2018 [41] | 154 | 100% | 38% | 29% | – | 14% | 38% | – | ||
| 34 | Gupta 2021 [42] | 63 | 100% | 22% | 54% | – | 5% | 21% | 40% | 29% | 17% |
| 35 | Varma/2013 [43] | 100 | 100% | 49% | 69% | – | – | 29% | 43% | 50% | 17% |
| 36 | Jagadishchandra 2003 [44] | 84 | 100% | 64% | 30% | – | – | 24% | 30% | – | |
| 37 | Datta 2011 [45] | 51 | 100% | 41% | 78% | – | – | 73% | 72% |
Abbreviation: S.n- Serial number, N- Sample size, Msl- Muscle involvement, LN- Lymphadenopathy, Drrh- Diarrhoea, Abd pain- Abdominal pain, Liver- Hepatomegaly, Spleen- Splenomegalya.
An important feature that helps differentiate leptospirosis from other viral causes of Acute febrile illness is the presence of leucocytosis (Table 3) [42, 43]. In our review, leukocytosis was seen in 54% of the patients. Leucopenia was uncommonly reported in 8% of the patients. Raised Erythrocyte Sedimentation Rate (ESR), C-Reactive Protein (CRP), and procalcitonin have also been shown to help differentiate leptospirosis from other viral causes [42, 43].
Table 3.
Laboratory parameters of adult patients with leptospirosis.
| Sn | Author/Year | N | ↑TLC | ↓TLC | ↓Plt | ↑Bil | ↑AST | ↑ALT | ↑ALP | ↑Cr |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mathur 2019 [15] | 237 | 14% | |||||||
| 2 | Padmakumar 2016 [10] | 45 | 78% | 2% | 56% | 89% | 87% | 87% | 53% | |
| 3 | Mathew Thomas 2006 [17] | 31 | 55% | 74% | ||||||
| 4 | Chaudhry 2017 [18] | 107 | 42% | 27% | 32% | |||||
| 5 | Mathew Anoop 2018 [20] | 113 | ||||||||
| 6 | Holla 2018 [21] | 202 | 81% | 78% | 80% | 78% | 79% | 73% | 53% | |
| 7 | Shah 2009 [13] | 24 | 54% | 58% | 100% | 80% | 80% | |||
| 8 | Chakurkar 2008 [12] | 44 | 20% | 9% | 57% | 25% | 14% | 14% | ||
| 9 | Trivedi 2010 [27] | 144 | 10% | 77% | ||||||
| 10 | George Thomas 2020 [28] | 467 | ||||||||
| 11 | Adiga Deepa 2017 [29] | 130 | ||||||||
| 12 | Balasundaram 2020 [8] | 110 | 83% | 63% | 53% | 93% | 91% | 84% | 49% | |
| 13 | DebManda 2011 [31] | 214 | 92% | 81% | 64% | |||||
| 14 | Muthusethupathi 1995 [33] | 57 | 23% | 44% | 47% | |||||
| 15 | Patel 2011 [35] | 44 | 50% | 75% | 98% | 73% | ||||
| 16 | Sethi 2010 [37] | 232 | 23% | 7% | 30% | 60% | ||||
| 17 | Chauhan 2010 [38] | 13 | 100% | 62% | 77% | |||||
| 18 | Ittyachen 2007 [49] | 53 | 74% | 74% | ||||||
| 19 | Pappachan 2002 [39] | 282 | 51% | 18% | 46% | 16% | 47% | |||
| 20 | Unnikrishnan 2005 [48] | 92 | 50% | |||||||
| 21 | Zala 2018 [41] | 154 | 52% | 88% | 79% | 88% | 86% | 41% | ||
| 22 | Gupta 2021 [42] | 63 | 63% | 8% | 78% | 76% | ||||
| 23 | Varma 2013 [43] | 100 | 47% | 71% | 80% | |||||
| 24 | Datta 2011 [45] | 51 | 55% | 73% | 78% | 78% | 78% | 71% | 41% |
Abbreviation: S.n- Serial number, N- Sample size, ↑TLC- Leucocytosis, ↓TLC- Leucopenia, ↓Plt- Thrombocytopenia, ↑Bil- Increased bilirubin, ↑AST- Increased Aspartate transaminase, ↑ALT- Increased Alanine Transaminase, ↑ALP- Increased Alkaline phosphatase, ↑Cr- Increased Creatinine.
Haemorrhagic fever
Conjunctival suffusion has been reported to be more common in leptospirosis cases when compared to other causes of acute febrile illnesses [8]. According to an estimate, conjunctival suffusion has been seen in less than 10% of the cases of febrile illnesses due to causes other than leptospirosis [8]. Our review showed it in 35% of leptospirosis cases (Table 4). Conjunctival congestion, especially in combination with icterus, is considered virtually pathognomonic for leptospirosis [1, 8]. Haemoptysis, gastrointestinal bleeding, and haematuria were present in 5%, 5% and 12% of patients, respectively (Table 4). Bleeding manifestations in leptospirosis could be attributed to thrombocytopenia, coagulation abnormalities and uraemia [62]. Thrombocytopenia is commonly seen in many causes of acute febrile illnesses, including leptospirosis. The average platelet count across various studies ranged from 20 to 130 thousand/μl. Thrombocytopenia was seen in 50% of the patients in this review (Table 3). It is hypothesized to be a result of the direct effect of the organism, bone marrow suppression and increased destruction of the platelets [29]. In a study by Adiga et al., the severity of the disease was found to be proportional to the severity of thrombocytopenia [29].
Table 4.
Bleeding manifestations in adult patients with leptospirosis.
| Sn | Author/Year | N | Bleeding [NOS] | Hematuria | GI bleeding | Hemoptysis | Conjunctival suffusion |
|---|---|---|---|---|---|---|---|
| 1 | Narayanan 2016 [11] | 118 | 5% | ||||
| 2 | Mathur 2019 [15] | 237 | 15% | 24% | |||
| 3 | Padmakumar 2016 [10] | 45 | – | – | 53% | ||
| 4 | Sethi 2003 [16] | 20 | – | 5% | 50% | ||
| 5 | Sehgal/2002 [9] | 569 | – | 20% | 2% | 25% | |
| 6 | Mathew Thomas/2006 [17] | 31 | – | – | – | 23% | |
| 7 | Chaudhry 2017 [18] | 107 | 6% | 4% | 1% | 4% | 30% |
| 8 | Salkade 2005 [19] | 62 | 29% | – | – | 29% | 15% |
| 9 | Holla 2018 [21] | 202 | 4% | ||||
| 10 | Shah 2009 [13] | 24 | – | – | – | 13% | |
| 11 | Chakurkar 2008 [12] | 44 | 7% | 4% | 4% | 20% | 14% |
| 12 | Somasundaram 2014 [22] | 122 | – | – | – | 16% | |
| 13 | Clerke 2002 [46] | 38 | – | – | – | 26% | |
| 14 | Chawla 2004 [23] | 60 | 7% | – | – | 40% | |
| 15 | Manocha 2004 [24] | 25 | – | 4% | 4% | ||
| 16 | Trivedi 2010 [27] | 144 | – | – | – | 25% | |
| 17 | Balasundaram 2020 [8] | 110 | – | – | – | 58% | |
| 18 | Kuriakose 1997 [30] | 978 | – | – | – | 49% | |
| 19 | Debmandal 2011 [31] | 214 | – | 5% | 5% | ||
| 20 | Muthusethupathi 1995 [33] | 57 | – | – | 26% | 9% | 58% |
| 21 | Panicker/2001 [47] | 40 | 5% | – | – | ||
| 22 | Patel 2011 [35] | 44 | 23% | – | – | 23% | |
| 23 | Bhardwaj 2008 [36] | 62 | – | – | – | 3% | |
| 24 | Sethi 2010 [37] | 232 | – | 2% | 2% | 7% | |
| 25 | Chauhan 2010 [38] | 13 | – | – | – | 54% | |
| 26 | Pappachan 2002 [39] | 282 | – | – | – | 5% | 81% |
| 27 | Prabhakaran 2014 [40] | 410 | – | – | – | 41% | |
| 28 | Zala 2018 [41] | 154 | – | – | – | 9% | 22% |
| 29 | Gupta 2021 [42] | 63 | 3% | – | – | 29% | |
| 30 | Varma/2013 [43] | 100 | 25% | – | – | 35% | |
| 31 | Jagadishchandra 2003 [44] | 84 | – | 27% | 2% | 30% | |
| 32 | Datt 2011 [45] | 51 | 35% | – | – |
Abbreviation: S.n- Serial number, N- Sample size, NOS- Not otherwise specified, GI- Gastrointestinal.
Weil’s disease
Concurrent icterus and Acute Kidney Injury has been classically described under the heading of Weil’s disease. Icterus results from intracellular cholestasis and was seen in 34% of the patients (Table 5). Intracellular cholestasis as the primary mechanism of liver involvement can also be extrapolated from the increased bilirubin, increased alkaline phosphate and relatively normal transaminase levels (Table 3) [22]. The average bilirubin, AST and ALT were 2.7–14.6 grams/dl, 58–524 IU/l and 58–503 IU/l, respectively. In contrast, yellow fever has a marked increase in transaminase levels owing to hepatocellular injury. Acute kidney injury resulting from tubulointerstitial nephritis was seen in 35% of the patients (Table 5). The average creatinine at presentation ranged from 1.8–5.4 mg/dl (Table 3).
Table 5.
Organ Involvement in adult patients with Leptospirosis.
| Sn | Author/Year | N | Liver | Kidney | Lungs | Heart | CNS | |
|---|---|---|---|---|---|---|---|---|
| Icterus | Oliguria | Dyspnoea | Myocarditis | Neck stiffness | Altered sensorium | |||
| 1 | Narayanan 2016 [11] | 118 | 6% | 3% | ||||
| 2 | Mathur 2019 [15] | 237 | 81% | 37% | 2% | |||
| 3 | Padmakumar 2016 [10] | 45 | 20% | 38% | ||||
| 4 | Sethi 2003 [16] | 20 | 80% | 45% | 5% | 20% | 20% | |
| 5 | Sehgal 2002 [9] | 569 | 25% | 39% | 21% | 2% | ||
| 6 | Mathew Thomas 2006 [17] | 31 | 45% | 65% | 81% | |||
| 7 | Chaudhry 2017 [18] | 107 | 19% | 14% | ||||
| 8 | Salkade 2005 [19] | 62 | 40% | 29% | 44% | 39% | ||
| 9 | Mathew Anoop 2018 [20] | 113 | 50% | 27% | ||||
| 10 | Holla 2018 [21] | 202 | 22% | 26% | ||||
| 11 | Shah 2009 [13] | 24 | 71% | 13% | 46% | 96% | ||
| 12 | Chakurkar 2008 [12] | 44 | 27% | 32% | 100% | 93% | 11% | |
| 13 | Somasundram 2014 [22] | 122 | 34% | 25% | 10% | |||
| 14 | Clerke/2002 [46] | 38 | 71% | |||||
| 15 | Patil 2017 [7] | 193 | 18% | 22% | ||||
| 16 | Chawla 2004 [23] | 60 | 67% | 55% | ||||
| 17 | Manocha 2004 [24] | 25 | 68% | 24% | 12% | |||
| 18 | Trivedi 2010 [27] | 144 | ||||||
| 19 | George Thomas 2020 [28] | 467 | 21% | 13% | 6% | 4% | ||
| 20 | Adiga Deepa 2017 [29] | 130 | 14% | 18% | ||||
| 21 | Balasundaram 2020 [8] | 110 | 36% | 11% | 71% | |||
| 22 | Kuriakose 1997 [30] | 978 | 14% | 17% | ||||
| 23 | Debmandal 2011 [31] | 214 | 94% | |||||
| 24 | Sarvanan 2014 [32] | 894 | 15% | |||||
| 25 | Muthusethupathi 1995 [33] | 57 | 84% | 72% | 7% | 42% | ||
| 26 | Majumdar 2013 [34] | 77 | 40% | |||||
| 27 | Panicker 2001 [47] | 40 | 33% | |||||
| 28 | Patel 2011 [35] | 44 | 86% | 39% | 5% | |||
| 29 | Bhardwaj 2008 [36] | 62 | 15% | |||||
| 30 | Sethi 2010 [37] | 232 | 27% | 11% | 12% | 3% | 14% | |
| 31 | Chauhan 2010 [38] | 13 | 77% | 54% | ||||
| 32 | Ittyachen 2007 [49] | 53 | ||||||
| 33 | Pappachan 2002 [39] | 282 | 70% | 24% | 9% | 4% | 10% | |
| 34 | Prabhakaran 2014 [40] | 410 | 37% | 25% | ||||
| 35 | Unnikrishnan 2005 [48] | 92 | 67% | 10% | ||||
| 36 | Zala 2018 [41] | 154 | 55% | 18% | ||||
| 37 | Gupta/2021 [42] | 63 | 59% | 63% | 8% | 32% | ||
| 38 | Varma 2013 [43] | 100 | 63% | 56% | 33% | 12% | ||
| 39 | Jagadishchandra 2003 [44] | 84 | 35% | 27% | 13% | 2% | 14% | |
| 40 | Datta 2011 [45] | 51 | 75% | 29% | 25% | 6% | 22% | |
Abbreviation: S.n- Serial number, N- Sample size.
Pulmonary leptospirosis
Pulmonary involvement in the form of dyspnoea was noticed in 17% of the patients (Table 5). It can result from pneumonitis, pulmonary haemorrhage or acute respiratory distress syndrome (ARDS) [28]. Pneumonitis presents as a consolidation on imaging. Pulmonary haemorrhage has been suggested to be one of the most important causes of mortality in Indian leptospirosis patients [27]. Pulmonary haemorrhage in leptospirosis is considered an immune-mediated phenomenon and presents most commonly as haemoptysis with diffuse small round opacities on imaging [27].
Cardiac leptospirosis
Heart involvement in the form of myocarditis is reported in 30% of the patients (Table 5). This myocarditis in leptospirosis has been described to be similar to septic cardiomyopathy [20]. Sinus bradycardia and first-degree atrioventricular block were found to be common in those with cardiac involvement [46]. In an autopsy study, inflammation and haemorrhages were noticed in all three layers of the heart [13]. It must be noted that cardiac involvement may be masked by pulmonary or hepatorenal involvement [13].
Neuroleptospirosis
The neurological manifestation of leptospirosis has been linked to both direct invasions by leptospires and immune complexes formed in the second week of illness [47]. The direct invasion leads to altered sensorium but aseptic meningitis is related to vasculitis resulting from the immune-mediated reaction [47]. Altered sensorium could also be explained partly by metabolic encephalopathy secondary to hepatorenal dysfunction [17]. Neck stiffness and altered sensorium were seen in 10% and 18% of leptospirosis patients, respectively in this review (Table 5).
Paediatric patients
A total of 346 children diagnosed with leptospirosis were included in the review. The presence of fever was seen in 95% of the children (Table 6). Headache, myalgia and conjunctival suffusion were present in 54%, 47% and 33% of the patients, respectively (Table 6). In a study by Karande et al., tenderness of the abdominal muscles was significantly associated with the diagnosis of leptospirosis in children [53]. In another study, a straight leg raising test to demonstrate myalgia helped differentiate leptospirosis from other causes [58]. Bleeding manifestations were present in 6% of the patients (Table 6). Jaundice and acute kidney injury was seen in 13% and 6% of the patients, respectively (Table 6). In a study by Narayanan et al., no significant difference in pulmonary, cardiac or neurological involvement was seen between adult and paediatric cases of leptospirosis [11].
Table 6.
Clinical features in paediatric patients diagnosed with leptospirosis.
| Sn | Author/Year | N | Fever | Headache | Myalgia | Bleeding | Conjunctival suffusion | Jaundice | Renal involvement |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Narayanan 2016 [11] | 35 | 100% | 83% | 63% | 26% | 31% | ||
| 2 | Mathur 2019 [15] | 86 | 100% | – | 76% | 12% | 55% | 2% | 5% |
| 3 | Karande 2005 [54] | 15 | 60% | 47% | 40% | 13% | 13% | ||
| 4 | Karande 2002 [14] | 26 | 77% | 54% | – | 15% | 15% | 8% | 8% |
| 5 | Rajajee 2002 [58] | 139 | 96% | – | 24% | – | 19% | 18% | 1% |
| 6 | Karande 2003 [53] | 18 | 100% | 50% | 61% | – | 28% | 0% | – |
| 7 | Zaki 2010 [61] | 27 | 81% | 18% | 44% | 4% | 63% | 15% | 26% |
Abbreviation: S.n- Serial number, N- Sample size.
Diagnostics
Leptospirosis needs to be differentiated from other Acute Febrile illnesses that are frequent in Indian settings during the monsoons. The most common differentials are dengue, scrub typhus, enteric fever and chikungunya. The diffuse erythematous rash seen in dengue, pathognomonic eschar of scrub typhus, gastrointestinal involvement in enteric fever and the small joint arthralgia of chikungunya is rarely seen in leptospirosis. Icterus and conjunctival suffusion seen in leptospirosis are very uncommon in any of the other differentials. Despite these differences, it is difficult to differentiate leptospirosis from these febrile illnesses without the use of microbiological methods.
The diagnosis of leptospirosis can be achieved by many methods. In the first week, the diagnostic modality of choice is polymerization chain reaction assay (PCR) or culture of the blood [1]. The sensitivity and specificity of PCR for the diagnosis of scrub typhus in a study were 97 and 96%, respectively [11]. After the first week, the sensitivity of these tests on blood decreases substantially. PCR or culture in the urine samples is useful in the second week. These tests are, however, limited by availability, cost and resource intensiveness [1]. In the included studies, PCR was done in two studies, whereas culture was done in three studies (Table 7) [11, 30, 50].
Table 7.
Details of methods used for diagnosis of leptospirosis in the included studies.
| Sn | Author/Year | MFC | DGM | IgM ELISA | Rapid | MAT | PCR | Culture |
|---|---|---|---|---|---|---|---|---|
| 1 | Narayanan 2016 [9] | Yes | Yes | Yes | Yes | Yes | ||
| 2 | Mathur 2019 [16] | Yes | Yes | Yes | ||||
| 3 | Padmakumar 2016 [10] | Yes | ||||||
| 4 | Sethi 2003 [16] | Yes | Yes | |||||
| 5 | Sehgal 2002[13] | Yes | Yes | Yes | ||||
| 7 | Chaudhry 2017 [19] | Yes | Yes | |||||
| 8 | Salkade 2005 [20] | Yes | ||||||
| 9 | Mathew Anoop 2018 [21] | Yes | Yes | |||||
| 10 | Holla 2018 [22] | Yes | ||||||
| 11 | Shah Kinjal 2009 [14] | Yes | ||||||
| 12 | Somasundaram Aravindh 2014 [23] | Yes | Yes | Yes | ||||
| 13 | Chawla 2004 [24] | Yes | ||||||
| 14 | Manocha 2004 [25] | Yes | Yes | Yes | ||||
| 15 | Vimala 2014 [26] | Yes | ||||||
| 16 | Madhusudhana 2015 [27] | Yes | Yes | |||||
| 17 | Trivedi 2010 [28] | Yes | Yes | |||||
| 18 | George Thomas 2012 [29] | Yes | ||||||
| 19 | Adiga Deepa 2017 [30] | Yes | ||||||
| 20 | Balasundaram Padmakumar 2020 [12] | Yes | Yes | |||||
| 21 | Kuriakose 1997 [31] | Yes | Yes | Yes | ||||
| 22 | DebMandal 2011 [32] | Yes | ||||||
| 23 | Saravanan 2014 [33] | Yes | ||||||
| 24 | Muthusethupathi 1995 [34] | Yes | Yes | |||||
| 25 | Majumdar 2013 [35] | Yes | ||||||
| 26 | Patel 2011 [36] | Yes | Yes | |||||
| 27 | Bhardwaj Pankaj 2008 [37] | Yes | ||||||
| 28 | Sethi 2010 [38] | Yes | Yes | Yes | ||||
| 29 | Chauhan 2010 [39] | Yes | ||||||
| 30 | Pappachan 2002 [40] | Yes | ||||||
| 31 | Prabhakaran/2014 [41] | Yes | Yes | |||||
| 32 | Zala 2018 [42] | Yes | ||||||
| 33 | Gupta2021 [43] | Yes | Yes | |||||
| 34 | Varma 2013 [44] | Yes | ||||||
| 35 | Jagadishchandra 2003 [44] | Yes | ||||||
| 36 | Datta 2011 [46] | Yes | Yes | |||||
| 37 | Ittyachen 2007 [47] | Yes | ||||||
| 38 | Jena 2004 [50] | Yes | Yes | Yes | ||||
| 39 | Jeyakumar 2008 [51] | Yes | ||||||
| 40 | Kamath 2014 [52] | Yes | ||||||
| 41 | Karande 2002 [14] | Yes | ||||||
| 42 | Karande 2003 [53] | Yes | ||||||
| 43 | Karande 2005 [54] | Yes | Yes | Yes | ||||
| 44 | Murhekar 1998 [55] | Yes | ||||||
| 45 | Panicker 2001 [47] | Yes | ||||||
| 46 | Pappachan 2007 [56] | Yes | Yes | |||||
| 47 | Patel 2006 [57] | Yes | Yes | |||||
| 48 | Rajajee 2002 [58] | Yes | Yes | Yes | ||||
| 49 | Sehgal 2003 [59] | Yes | Yes | |||||
| 50 | Sugunan 2009 [60] | Yes | ||||||
| 51 | Unnikrishnan 2005 [48] | Yes | ||||||
| 52 | Zaki 2010 [61] | Yes | Yes |
Abbreviation: S.n- Serial number, N- Sample size, MFC- Modified Faine’s criteria, DGM- Dark ground microscopy, ELISA-Enzyme linked immunosorbent assay, Rapid- Rapid diagnostic test, MAT- Microagglutination test, PCR- Polymerase chain reaction assay.
After the first week, serology is the preferred method. Microagglutination test [MAT] is the gold standard serological method of choice, but it requires maintenance of live cultures of leptospires and is usually available at reference centres [1]. A total of 24 studies used MAT to diagnose leptospirosis [Table 7]. In the absence of MAT, IgM Enzyme-linked immunosorbent assay (IgM ELISA) is the most commonly used serological method for diagnosing leptospirosis. A total of 38 studies used IgM ELISA in this review (Table 7). In a study by Narayanan et al., the sensitivity and specificity of IgM ELISA were found to be 100 and 93%, respectively [11]. Conventional serological methods like ELISA and MAT are limited by laboratory requirements, expertise and considerable turn-around time. For this reason, there has been increasing use of rapid tests for making a diagnosis of leptospirosis. Sensitivity issues limit darkfield microscopy for direct observation of leptospires in blood or urine sample. Nevertheless, it was used in two studies (Table 7) [54,61]. Rapid tests of different formats were used across 13 of the included studies (Table 7). Most commonly used rapid tests were based on lateral flow assay-based immunochromatography (ICT). In a diagnostic randomized controlled trial, rapid ICT-based tests had high agreement with the conventional tests. They had a significantly less turn-around time but did not have an impact on days of hospitalisation or antimicrobial consumption [63].
It should be noted here that serological tests are associated with their inherent fallacies. In endemic areas, false positives are not uncommon. Therefore, they must be interpreted carefully, especially in those patients presenting with atypical symptoms. Some studies used paired sampling two weeks apart to increase the specificity of serological tests [46]. A validated scoring system called Modified Faine’s criteria uses a combination of epidemiological features, clinical findings and diagnostic tests to diagnose leptospirosis. This criterion was used for diagnosis in nine studies (Table 7).
Treatment details and outcome
Treatment details were sparsely available in some studies. Nine studies used penicillin, while ceftriaxone was used in three studies (Table 8). In one recent study by Gupta et al., piperacillin-tazobactam and meropenem were used empirically as the patient presented with severe manifestations, and other Gram-negative bacterial infections were in the differentials [42]. Doxycycline was used in three studies, while azithromycin was used in one study (Table 8) [31, 37, 42]. The most common duration of intravenous penicillin or doxycycline in the included studies was seven days. Two studies used corticosteroids, especially in patients with pulmonary involvement [27, 37]. Dialysis is often required in patients with severe renal involvement.
Table 8.
Drugs used in different studies used in patients with leptospirosis.
| Authors | Penicillin | Ampicillin | Doxycycline | Azithromycin | Ceftriaxone | Piperacillintazobactam | Meropenem | Ciprofloxacin |
|---|---|---|---|---|---|---|---|---|
| Chakurkar 2017 [12] | Yes | Yes | ||||||
| Gupta 2021 [42] | Yes | Yes | Yes | Yes | Yes | Yes | ||
| Karande 2003 [53] | Yes | |||||||
| Karande 2005 [54] | Yes | |||||||
| Patel 2006 [57] | Yes | |||||||
| Sethi 2010 [37] | Yes | Yes | ||||||
| Somasundaram 2014 [22] | Yes | |||||||
| Trivedi 2010 [27] | Yes | |||||||
| Zaki 2010 [61] | Yes | Yes | ||||||
| Debmandal 2011 [31] | Yes | Yes | Yes |
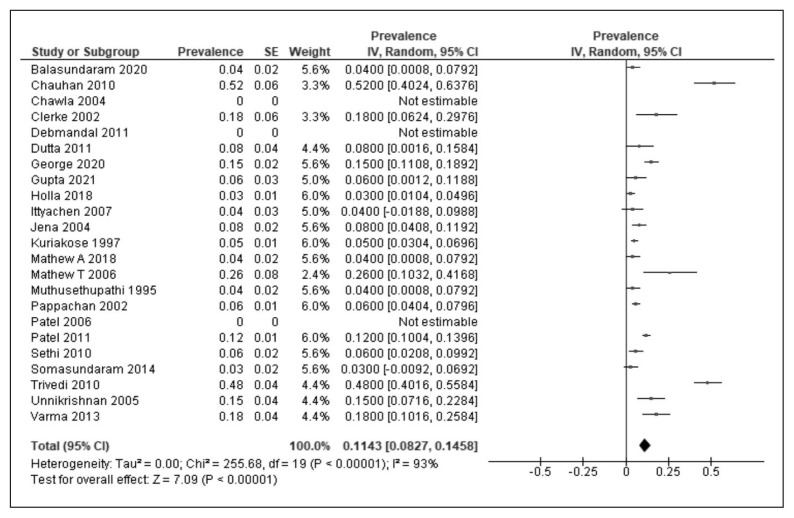
Mortality outcomes were reported in 23 studies. In a study by Chawla et al., male gender, alcohol dependence, higher age, multi-organ dysfunction, acidosis and ARDS were identified as poor prognostic factors [23]. In a study by Somasundaram et al., mortality and organ dysfunction outcomes were poorer in leptospirosis patients with pre-existing decompensated liver disease [23]. In a study by Pappachan et al., pulmonary and neurological involvement were independently associated with mortality [39]. In another study by Unnikrishnan et al., cardiac involvement and bleeding manifestations were also associated with mortality [48]. In another study by Varma et al., liver/kidney involvement, thrombocytopenia, and creatine kinase elevation were predictors of death. The mortality rates ranged from 0–52% in various studies. The mortality outcomes in children were reported in two studies, ranging from 6–13% [54,58]. In a study that compared paediatric and adult cases of leptospirosis, worse outcomes were more commonly seen in adult cases of leptospirosis [11]. Using the random effect model, the pooled mortality across various studies was calculated as 11% [Figure 4] [95% CI-8–15%, I2=93%, P<0.001].
Figure 4.
Meta-analysis of mortality using random-effects model in leptospirosis cases reported in studies from India. Abbreviations: SE: Standard error, CI-Confidence interval.
Chemoprophylaxis
Chemoprophylaxis for individuals at high risk of exposure (sewage workers, paddy farmers) during the peak transmission season (monsoon) has shown to be effective. Mass chemoprophylaxis in regions with heavy floods has also been advocated. Doxycycline has been used in most studies with a dose of 200 mg weekly for a maximum period of 6 months [6]. In a randomized controlled trial, weekly doxycycline did not decrease the infection rates but reduced the incidence of clinical disease significantly [64]. In a case-control study by Desai et al., leptospirosis cases received doxycycline chemoprophylaxis for significantly less duration than similarly exposed controls [6].
LIMITATIONS OF THE STUDY
This review had several limitations. Most of the studies were retrospective observational studies with their inherent biases. The mapping of the disease burden was limited to published cases with available clinical details. The presence of leptospirosis in regions without published cases cannot be ruled out. Treatment details were missing in most of the cases. The studies included for the calculation of pooled mortality had considerable heterogeneity.
CONCLUSIONS
Leptospirosis is reported across India, with most reports coming from the coastal belt. It is primarily reported in farmers, with a definite seasonal distribution. Most outbreaks follow heavy rainfalls, especially when they are associated with floods. Liver and Kidney involvement are amongst the most common complications. The disease is associated with a high mortality rate in hospital settings.
Footnotes
Conflict of interest
None to declare
Funding
None to declare
REFERENCES
- 1. Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Torgerson PR, Hagan JE, Costa F, et al. Global Burden of Leptospirosis: Estimated in Terms of Disability Adjusted Life Years. PLoS Negl Trop Dis. 2015;9(10):e0004122. doi: 10.1371/journal.pntd.0004122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Costa F, Hagan JE, Calcagno J, et al. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl Trop Dis. 2015;9(9):e0003898. doi: 10.1371/journal.pntd.0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Munoz-Zanzi C, Groene E, Morawski BM, et al. A systematic literature review of leptospirosis outbreaks worldwide, 1970–2012. Rev Panam Salud Pública. 2020;44:e78. doi: 10.26633/RPSP.2020.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beri D, Moola S, Jagnoor J, Salam A, Bhaumik S. Prevention, control and management of leptospirosis in India: an evidence gap map. Trans R Soc Trop Med Hyg. 2021;115(12):1353–1361. doi: 10.1093/trstmh/trab036. [DOI] [PubMed] [Google Scholar]
- 6. Desai KT, Patel F, Patel PB, Nayak S, Patel NB, Bansal RK. A case-control study of epidemiological factors associated with leptospirosis in the South Gujarat region. J Postgrad Med. 2016;62(4):223–227. doi: 10.4103/0022-3859.188551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patil DY, Dahake RV, Chowdhary AS, Deshmukh RA. Clinico-epidemiological observations of human leptospirosis from Mumbai, India. J Infect Public Health. 2017;10(2):247–248. doi: 10.1016/j.jiph.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 8. Balasundaram PK, Kanakamma LG, Jayageetha K, Selvarajan B. Epidemiological, clinical and laboratory features of leptospirosis compared to other acute febrile illnesses. J R Coll Physicians Edinb. 2020;50(2):118–213. doi: 10.4997/JRCPE.2020.208. [DOI] [PubMed] [Google Scholar]
- 9. Sehgal SC, Sugunan AP, Vijayachari P. Outbreak of leptospirosis after the cyclone in Orissa. Natl Med J India. 2002;15(1):22–23. [PubMed] [Google Scholar]
- 10. Padmakumar B, Kumari Jayageetha PB, Libu GK. Dani Dius Fernandes. A Study on Epidemiological, Clinical and Laboratory Profile of Patients with Leptospirosis Admitted in a Tertiary Care Centre in Central Kerala, India. JK Science . 2016;18(4) [Google Scholar]
- 11. Narayanan R, Sumathi G, Prabhakaran SG, Shanmughapriya S, Natarajaseenivasan K. Paediatric leptospirosis: A population-based case-control study from Chennai, India. Indian J Med Microbiol. 2016;34(2):228–232. doi: 10.4103/0255-0857.180353. [DOI] [PubMed] [Google Scholar]
- 12. Chakurkar G, Vaideeswar P, Pandit SP, Divate SA. Cardiovascular lesions in leptospirosis: an autopsy study. J Infect. 2008;56(3):197–203. doi: 10.1016/j.jinf.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 13. Shah K, Amonkar GP, Kamat RN, Deshpande JR. Cardiac findings in leptospirosis. J Clin Pathol. 2010;63(2):119–123. doi: 10.1136/jcp.2009.069575. [DOI] [PubMed] [Google Scholar]
- 14. Karande S, Kulkarni H, Kulkarni M, De A, Varaiya A. Leptospirosis in children in Mumbai slums. Indian J Pediatr. 2002;69(10):855–858. doi: 10.1007/BF02723705. [DOI] [PubMed] [Google Scholar]
- 15. Mathur M, De A, Turbadkar D. Leptospirosis outbreak in 2005: LTMG hospital experience. Indian J Med Microbiol. 2009;27(2):153–155. doi: 10.4103/0255-0857.49431. [DOI] [PubMed] [Google Scholar]
- 16. Sethi S, Sood A, Pooja N, Sharma S, Sengupta C, Sharma M. Leptospirosis in northern India: a clinical and serological study. Southeast Asian J Trop Med Public Health. 2003;34(4):822–825. [PubMed] [Google Scholar]
- 17. Mathew T, Satishchandra P, Mahadevan A, et al. Neuroleptospirosis - revisited: experience from a tertiary care neurological centre from south India. Indian J Med Res. 2006;124(2):155–162. [PubMed] [Google Scholar]
- 18. Chaudhry R, Saigal K, Bahadur T, Kant K, Chourasia B, Gupta N. Varied presentations of leptospirosis: experience from a tertiary care hospital in north India. Trop Doct. 2017;47(2):128–132. doi: 10.1177/0049475516687431. [DOI] [PubMed] [Google Scholar]
- 19. Salkade HP, Divate S, Deshpande JR, et al. A study of autopsy findings in 62 cases of leptospirosis in a metropolitan city in India. J Postgrad Med. 2005;51(3):169–173. [PubMed] [Google Scholar]
- 20. Mathew A, Shanks M, Punnoose E, et al. Cardiac involvement in critically ill patients with leptospirosis: A prospective study using myocardial deformation imaging. Eur Heart J Acute Cardiovasc Care. 2020;9(8):975–983. doi: 10.1177/2048872618809319. [DOI] [PubMed] [Google Scholar]
- 21. Holla R, Darshan B, Pandey L, Unnikrishnan B, Kumar N, Thapar R, et al. Leptospirosis in Coastal South India: a facility based study. BioMed Res Int. 2018;2018:e1759125. doi: 10.1155/2018/1759125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Somasundaram A, Loganathan N, Varghese J, Shivakumar S, Jayanthi V. Does leptospirosis behave adversely in cirrhosis of the liver? Indian J Gastroenterol. 2014;33(6):512–516. doi: 10.1007/s12664-014-0500-0. [DOI] [PubMed] [Google Scholar]
- 23. Chawla V, Trivedi TH, Yeolekar ME. Epidemic of leptospirosis: an ICU experience. J Assoc Physicians India. 2004;52:619–622. [PubMed] [Google Scholar]
- 24. Manocha H, Ghoshal U, Singh SK, Kishore J, Ayyagari A. Frequency of leptospirosis in patients of acute febrile illness in Uttar Pradesh. J Assoc Physicians India. 2004;52:623–625. [PubMed] [Google Scholar]
- 25. Vimala G, Rani AMJ, Gopal VR. Leptospirosis in Vellore: a clinical and serological study. Int J Microbiol. 2014;2014:643940. doi: 10.1155/2014/643940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Madhusudhana M, Chandrashekar S, Revathy R. An Epidemiological investigation of Leptospirosis outbreak in Shimoga District of Karnataka, India. Indian J Public Health Res Dev. 2015;6:106. [Google Scholar]
- 27. Trivedi SV, Vasava AH, Bhatia LC, Patel TC, Patel NK, Patel NT. Plasma exchange with immunosuppression in pulmonary alveolar haemorrhage due to leptospirosis. Indian J Med Res. 2010;131:429–433. [PubMed] [Google Scholar]
- 28. George T, Pais ML, Adnan M, Pereira R, Jakribettu RP, Baliga MS. Clinicolaboratory profile of leptospirosis: observations from a tertiary care hospital. J Appl Hematol. 2020;11(3):102. [Google Scholar]
- 29. Adiga DSA, Mittal S, Venugopal H, Mittal S. Serial Changes in Complete Blood Counts in Patients with Leptospirosis: Our Experience. J Clin Diagn Res . 2017;11(5):EC21–EC24. doi: 10.7860/JCDR/2017/25706.9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuriakose M, Eapen CK, Paul R. Leptospirosis in Kolenchery, Kerala, India: epidemiology, prevalent local serogroups and serovars and a new serovar. Eur J Epidemiol. 1997;13(6):691–697. doi: 10.1023/a:1007300729615. [DOI] [PubMed] [Google Scholar]
- 31. DebMandal M, Mandal S, Pal NK. Serologic evidence of human leptospirosis in and around Kolkata, India: a clinico-epidemiological study. Asian Pac J Trop Med. 2011;4(12):1001–1006. doi: 10.1016/S1995-7645(11)60234-4. [DOI] [PubMed] [Google Scholar]
- 32. Saravanan S, Palanivel KM, Geetha M, Rishikesavan R. Epidemiology of Leptospirosis in humans with pyrexia of unknown origin. J Pure Appl Microbiol. 2014;8(3):2501–2503. [Google Scholar]
- 33. Muthusethupathi MA, Shivakumar S, Suguna R, et al. Leptospirosis in Madras-a clinical and serological study. J Assoc Physicians India. 1995;43(7):456–458. [PubMed] [Google Scholar]
- 34. Majumdar M, Bhaduri A, Mandal M, Haldar K. A study on serological correlation of clinically suspected leptospirosis cases in West Bengal, India. Ann Trop Med-Public Health. 2013;6(1) [Google Scholar]
- 35. Patel DK, Purohit P, Jena SK, Patel S, Padhi PK. Clinical profile of leptospirosis in a tertiary medical center of Western Orissa, India. Orr Physic J . 2011;7 [Google Scholar]
- 36. Bhardwaj P, Kosambiya JK, Desai VK. A case-control study to explore the risk factors for acquisition of leptospirosis in Surat city, after flood. Indian J Med Sci. 2008;62(11):431–438. [PubMed] [Google Scholar]
- 37. Sethi S, Sharma N, Kakkar N, Taneja J, Chatterjee SS, Banga SS, et al. Increasing trends of Leptospirosis in Northern India: a clinico-epidemiological study. PLoS Negl Trop Dis. 2010;4(1):e579. doi: 10.1371/journal.pntd.0000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chauhan V, Mahesh D, Panda P, Mokta J, Thakur S. Profile of patients of leptospirosis in sub-Himalayan region of North India. J Assoc Physicians India. 2010;58:354–356. [PubMed] [Google Scholar]
- 39. Pappachan MJ, Mathew S, Aravindan KP, et al. Risk factors for mortality in patients with leptospirosis during an epidemic in northern Kerala. Natl Med J India. 2004;17(5):240–242. [PubMed] [Google Scholar]
- 40. Prabhakaran SG, Shanmughapriya S, Dhanapaul S, James A, Natarajaseenivasan K. Risk factors associated with rural and urban epidemics of leptospirosis in Tiruchirappalli District of Tamilnadu, India. J Public Health. 2014;22(4):323–333. [Google Scholar]
- 41. Zala D, Khan D, Das V. Leptospirosis in the Union Territory of Dadra & Nagar Haveli, India. Indian J Public Health Res Dev. 2018;9:84–89. [Google Scholar]
- 42. Gupta N, Wilson W, Ravindra P, Joylin S, Bhat R, Saravu K. Clinical profile, management and outcome of patients with leptospirosis during the times of COVID-19 pandemic: A prospective study from a tertiary care centre in South India. Infez Med. 2021;29(3):393–401. doi: 10.53854/liim-2903-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Varma MD, Vengalil S, Vallabhajosyula S, Krishnakumar PC, Vidyasagar S. Leptospirosis and dengue fever: a predictive model for early differentiation based on clinical and biochemical parameters. Trop Doct. 2014;44(2):100–102. doi: 10.1177/0049475513515212. [DOI] [PubMed] [Google Scholar]
- 44. Jagadishchandra K, Prathb AG, Rao SP. Clinical and epidemiological correlation of leptospirosis among patients attending KMCH, Manipal. Indian J Med Sci. 2003;57(3):101–104. [PubMed] [Google Scholar]
- 45. Datta S, Sarkar RN, Biswas A, Mitra S. Leptospirosis: an institutional experience. J Indian Med Assoc. 2011;109(10):737–738. [PubMed] [Google Scholar]
- 46. Clerke AM, Leuva AC, Joshi C, Trivedi SV. Clinical profile of leptospirosis in South Gujarat. J Postgrad Med. 2002;48(2):117–118. [PubMed] [Google Scholar]
- 47. Panicker JN, Mammachan R, Jayakumar RV. Primary neuro leptospirosis. Postgrad Med J. 2001;77(911):589–590. doi: 10.1136/pmj.77.911.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Unnikrishnan D, Pisharody R, Vijayalakshmy N. Prognostic Factors in Leptospirosis: A Study From Kerala, India. Infect Dis Clin Pract. 2005;13(3):104–107. [Google Scholar]
- 49. Ittyachen AM, Krishnapillai TV, Nair MC, Rajan AR. Retrospective study of severe cases of leptospirosis admitted in the intensive care unit. J Postgrad Med. 2007;53(4):232–235. doi: 10.4103/0022-3859.37510. [DOI] [PubMed] [Google Scholar]
- 50. Jena AB, Mohanty KC, Devadasan N. An outbreak of leptospirosis in Orissa, India: the importance of surveillance. Trop Med Int Health. 2004;9(9):1016–1021. doi: 10.1111/j.1365-3156.2004.01293.x. [DOI] [PubMed] [Google Scholar]
- 51. Jeyakumar N, Ranjithsingh AJA, Shankar SK, Hepzibah Beula. Epidemiological study on the seroprevalence of leptospirosis in Salem and Namakkal districts, the major centre for livestock in Tamilnadu. Asian J Microbiol . 2008;10(2):239–244. [Google Scholar]
- 52. Kamath R, Swain S, Pattanshetty S, Nair NS. Studying risk factors associated with human leptospirosis. J Glob Infect Dis. 2014;6(1):3–9. doi: 10.4103/0974-777X.127941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Karande S, Bhatt M, Kelkar A, Kulkarni M, De A, Varaiya A. An observational study to detect leptospirosis in Mumbai, India, 2000. Arch Dis Child. 2003;88(12):1070–1075. doi: 10.1136/adc.88.12.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Karande S, Gandhi D, Kulkarni M, Bharadwaj R, Pol S, Thakare J, De A. Concurrent outbreak of leptospirosis and dengue in Mumbai, India, 2002. J Trop Pediatr. 2005;51(3):174–181. doi: 10.1093/tropej/fmh100. [DOI] [PubMed] [Google Scholar]
- 55. Murhekar MV, Sugunan AP, Vijayachari P, Sharma S, Sehgal SC. Risk factors in the transmission of leptospiral infection. Indian J Med Res. 1998;107:218–223. [PubMed] [Google Scholar]
- 56. Pappachan JM, Mathew S, Thomas B, Renjini K, Scaria CK, Shukla J. The incidence and clinical characteristics of the immune phase eye disease in treated cases of human leptospirosis. Indian J Med Sci. 2007;61(8):441–447. [PubMed] [Google Scholar]
- 57. Patel BK, Gandhi SJ, Desai DC. Clinico-epidemiological aspect of leptospirosis in South Gujarat. Indian J Med Microbiol. 2006;24(4):322–325. doi: 10.4103/0255-0857.29409. [DOI] [PubMed] [Google Scholar]
- 58. Rajajee S, Shankar J, Dhattatri L. Pediatric presentations of leptospirosis. Indian J Pediatr. 2002;69(10):851–853. doi: 10.1007/BF02723704. [DOI] [PubMed] [Google Scholar]
- 59. Sehgal SC, Sugunan AP, Vijayachari P. Leptospirosis disease burden estimation and surveillance networking in India. Southeast Asian J Trop Med Public Health. 2003;34(Suppl 2):170–177. [PubMed] [Google Scholar]
- 60. Sugunan AP, Vijayachari P, Sharma S, Roy S, Manickam P, Natarajaseenivasan K, Gupte MD, Sehgal SC. Risk factors associated with leptospirosis during an outbreak in Middle Andaman, India. Indian J Med Res. 2009;130(1):67–73. [PubMed] [Google Scholar]
- 61. Zaki SA, Shanbag P. Clinical manifestations of dengue and leptospirosis in children in Mumbai: an observational study. Infection. 2010;38(4):285–291. doi: 10.1007/s15010-010-0030-3. [DOI] [PubMed] [Google Scholar]
- 62. Petakh P, Nykyforuk A. Predictors of lethality in severe leptospirosis in Transcarpathian region of Ukraine. Infez Med. 2022;30(2):272–276. doi: 10.53854/liim-3002-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gupta N, Joylin S, Ravindra P, et al. A diagnostic randomised controlled trial to study the impact of rapid diagnostic tests in patients with Acute febrile illness when compared to conventional diagnostics (DRACO study) J Infect. 2021;82(6):e6–e8. doi: 10.1016/j.jinf.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 64. Sehgal SC, Sugunan AP, Murhekar MV, Sharma S, Vijayachari P. Randomized controlled trial of doxycycline prophylaxis against leptospirosis in an endemic area. Int J Antimicrob Agents. 2000;13(4):249–255. doi: 10.1016/s0924-8579(99)00134-x. [DOI] [PubMed] [Google Scholar]