SUMMARY
Staphylococci are the most frequent cause of vertebral osteomyelitis, but infections due to unusual pathogens are also reported. We describe a rare case of spondylodiscitis due to Lactobacillus paracasei. A 74-year-old diabetic male was evaluated for fever and back pain. Blood cultures and vertebral biopsy were positive for Lactobacillus paracasei. He often took laxatives and probiotics for chronic constipation. After target treatment the patient improved but he died for a heart attack two months after the end of the treatment. Although Lactobacillus paracasei is usually not pathogenic, sepsis is described in immunocompromised patients while vertebral osteomyelitis is rare.
Keywords: Lactobacillus, vertebral osteomyelitis, sepsis, probiotics
INTRODUCTION
Vertebral osteomyelitis, also called spondylodiscitis, is a challenge for physicians [1]. An insidious onset with progressive worsening is usually described. A late diagnosis can have serious consequences in particular neurological damage [2,3]. Vertebral osteomyelitis are grouped into brucellar, tuberculous or pyogenic. The most frequent etiology is Staphylococcus aureus but infections due to Gram-negatives and other Gram positive microorganisms are also reported [4]. Infections due to unusual pathogen are rare [5].
We describe a case of vertebral osteomyelitis due to Lactobacillus paracasei in a diabetic patient.
CASE REPORT
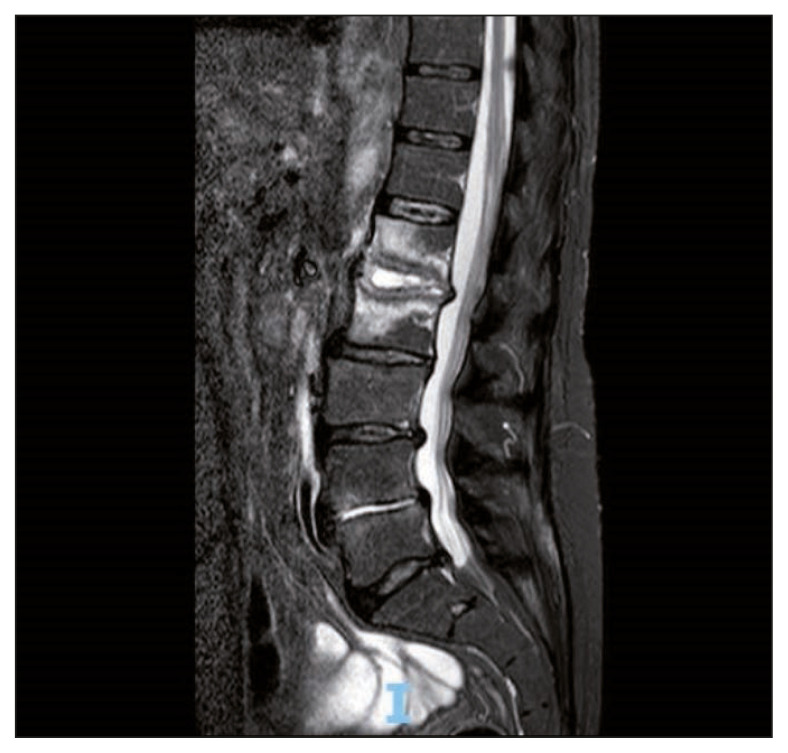
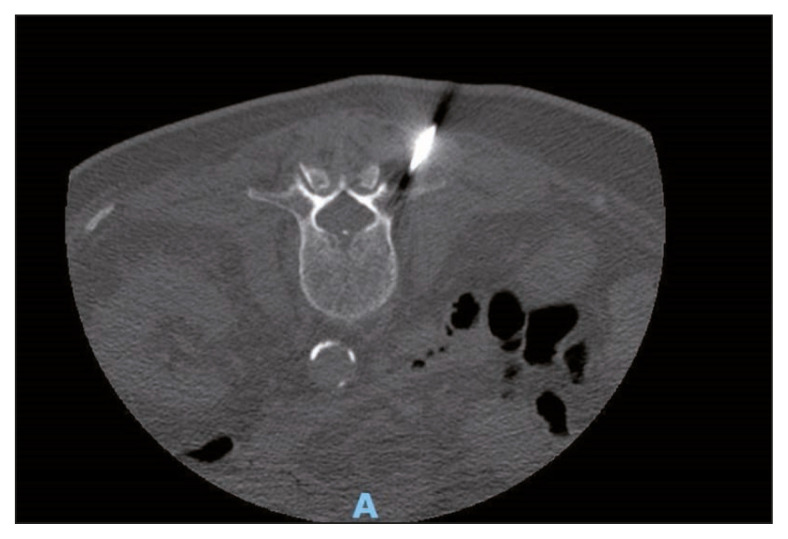
A 74-year-old man was admitted in our center because of fever and back pain. He had type II diabetes and ischemic heart disease. He was not a smoker. He often took laxatives and probiotics for chronic constipation. Other chronic treatments were low-dose salicylate and metformin. One month before, he was admitted in the emergency department for intestinal sub-occlusion. At admission in our center, he was febrile, with severe backache. Blood investigations showed mild anemia, while white cell count and platelets were in the normal range. Erythrocyte sedimentation rate was 91 mm/1st h, C-reactive protein was 33 mg/dl (normal range <5 mg/dl). He had mild renal insufficiency (creatinine 1,43 mg/dl clearance 46 ml/min), glycemia 71 mg/dl, glycosylated hemoglobin 7.1%. Neoplastic markers and HIV test were negatives. Magnetic resonance imaging (MRI) showed an alteration of the signal affecting the intervertebral disc and the somatic cancellous bone of L1 and L2. Extensive erosions of the opposing somatic plates, more evident on L1, were associated. After administration of contrast medium, peripheral somatic bone and disc impregnation was observed around a central component with a necrotic-colliquative appearance. There were signs of inflammation of the anterolateral paravertebral tissues, with possible initial involvement of the left psoas muscle, and the neural foramina (Figure 1). No sign of endocarditis was observed on trans-thoracic echocardiography. Abdominal computed tomography scan (CT) and colonoscopy were negative. Six blood cultures and a CT-guided needle biopsy on L2 were performed (Figure 2). Histological examination revealed inflammation without neoplastic cells. Blood cultures and biopsy specimen yielded a strain of Lactobacillus paracasei (identified using Matrix Assisted Laser Desorption Ionization – Time of Fligh -MALDI-TOF- M, Becton Dikinson, USA). The patient was initially treated with ampicillin (3 gr i.v. q 6 h) and levofloxacin (750 mg po q 24 h). Antimicrobial susceptibility of the isolates was determined by Kirby Bauer method and showed sensitivity to clindamycin, levofloxacin, tetracyclines, and resistance to ampicillin. Treatment was switched to clindamycin (600 mg IV q 8 h). Meanwhile ESBL positive E. coli was isolated from urine culture and a 10 days course of imipenem/cilastatin (500 mg IV q6 h) was associated. Clinical symptoms evolved favorably with defervescence and progressive reduction of backache. C-reactive protein progressively decreased to normal range.
Figure 1.
Magnetic resonance image of the spine showing high signal intensity on L1 and L2 vertebrae and paravertebral tissue.
Figure 2.
CT-guided needle vertebral biopsy.
During hospitalization, the patient had a heart attack and cardiac arrest, which he survived.
Antibiotic therapy was discontinued after 6 weeks. One month after the end of treatment, MRI showed reduced but still present the uptake in the L1–L2 vertebral bodies and minimum signal in left psoas muscle. A PET/CT showed minimal F-FDG uptake in L1–L2 level. C-reactive protein was in the normal range.
We decided to monitor clinical and laboratory test without other antibiotic treatment. Two months after stopping treatment, patient died for another fatal heart attack.
DISCUSSION
The Lactobacillus spp. are Gram-positive, nonspore forming rods or cocco-bacilli widely distributed in the environment and humans. They colonize the oral cavity, the gastrointestinal and genital tract and are present in many foods and para pharmaceutical products, including over-the-counter probiotics. Even if their real utility is questionable, these compounds are often prescribed in patients with type 2 diabetes because of reported positive impact on the metabolic control [6, 7]. Lactobacillus spp. is considered not pathogenic but sepsis, with or without organ involvement, have been recently and widely reviewed [8–10]. The most severe cases are described in patients with severe underlying conditions and in preterm infant [8–13]. Spondylodiscitis is a rare localization and a challenging diagnosis [4, 5]. Symptoms and imaging allow for clinical suspicion, but microbiological diagnosis is essential for an effective therapy and successful case management. In our case we had 6 positive blood cultures, but in presence of unusual pathogens the Infectious Diseases Society of America Guideline recommends vertebral biopsy [1]. A CT-scan guided vertebral biopsy was performed and resulted positive for the same pathogen confirming Lactobacillus-bacteremia as responsible for bone vertebral infection.
Lactobacillus spp and Bifidobacterium spp are the bacteria most frequently present in probiotics. We performed a MEDLINE search using as keywords Lactobacillus, Bifidobacterium, probiotics, bacteremia (last search July 7th 2023). Table 1 summarizes cases of probiotic-associated bacteremias included in the reviews and in the MEDLINE search [8–23].
Table 1.
Case reports and literature reviews of bacteremias associated with probiotics (some cases were reported simultaneously in different reviews).
| Reference | Pathogen | Number of cases | Localization | Risk factors/copathologies | Outcome |
|---|---|---|---|---|---|
| Lactobacillus | |||||
| Kullar et al. 2023 (*)(^) | Lactobacillus spp | 25 | Bacteremia | Short gut syndrome, Ulcerative colitis, Preterm Low birth weight, C. difficile associated disease, Acute leukemia, Cancer, Hematopoietic stem cell transplantation, central venous catheter | Recovered: 25 |
| Rahman et al. 2023 | Lactobacillus casei | 1 | Bacteremia + endocarditis | Chronic steroid intake | Recovered |
| Hefter et al. 2023 (@) | Lactobacillus spp | 3 | Bacteremia | Impaired intestinal function, Central venous catheter | Recovered: 3 |
| Mikucka et al. 2022 | Lactobacillus rhamnosus | 2 | Bacteremia | Intensive care unit admission | Died |
| Karime et al. 2022 | Lactobacillus rhamnosus | 1 | Bacteremia + endocarditis | Ulcerative colitis | Recovered |
| Rossi et al. 2022 (§)(^) | Lactobacillus spp | 3 | Bacteremia, Bacteremia + meningo encephalitis, Interstitial pneumonia | Extremely low birth weight neonates, Promyelocytic leukemia Cancer, Diabetes |
NR |
| Rubin et al. 2020 | Lactobacillus rhamnosus | 1 | Bacteremia | Parenteral feeding | Recovered |
| Matkowska et al. 2021 (^) | Lactobacillus spp | 28 | Bacteremia ± endocarditis, Empyema, Liver abscess, Pneumonia, Cholecystitis | No risk factors (3 cases), Cardiac valvular disease, Liver cirrhosis, Short bowel syndrome, Severe neurological impairment, Lung transplant, Diabetes, Immunocompromission | Recovered 27 Died 1 |
| Falci et al. 2015 | Lactobacillus rhamnosus | 1 | Bacteremia | Kidney transplant recipient | Recovered |
| Antoun et al. 2020 | Lactobacillus rhamnosus | 1 | Bacteremia + endocarditis | Uncontrolled diabetes | Recovered |
| Bifidobacterium | |||||
| Matkowska et al. 2021 (^) | Bifidobacterium spp | 3 | Bacteremia | Preterm low birth weight neonates, acute lymphoblastic leukemia | Recovered: 3 |
| Weber et al. 2015 (#) (^) | Bifidobacterium spp | 6 | Bacteremia | Preterm low birth weight neonates, | Recovered: 6 |
| Acuna-Gonzales et al. 2023 (^) | Bifidobacterium longum subsp. infantis | 7 | Bacteremia | extremely low birth weight neonates | Recovered |
| Pruccoli et al. 2019 (^) | Bifidobacterium sp. | 16 | Bacteremia | Preterm neonates some with comorbidities; Acute lymphoblastic leukemia; Intrauterine growth restriction, congenital heart disease | Recovered 15 Dead: 1 |
| Sakurai et al. 2022 | Bifidobacterium breve | 6 | Bacteremia | Preterm neonates | Recovered |
NR=not reported
review with possible presence of duplicate cases with other similar papers
3 further cases not matched for same strain found using genomic analysis in the blood sample and probiotic; other Lactobacillus spp. bacteremias with no evidence of oral probiotic administration or cases where the blood isolate did not match the probiotic strain
other 20 further isolated bacteremias and 12 with deep organ localizations without report of probiotic use
other 15 cases not related or with no data on probiotics assumption
5 further cases not associated with probiotics assumption
Noteworthy, 5 cases of Lactobacillus rhamnosus bacteremia, with associated endocarditis in 2, were reported in patients with diabetes and taking probiotics [8, 14]. Three further cases of Lactobacillus spp bacteremia, apparently not associated with probiotics, were described in diabetic patients (in one case diabetes was diagnosed at time of Lactobacillus infection) [8, 22, 23]. Two cases of discitis/osteomyelitis, one due to Lactobacillus sp and the other to Lactobacillus casei/paracasei have been described in an intravenous drug abuser and in a patient affected with stroke, diabetes, hypertension, hip prothesis, cardiac pacemaker and umbilical hernia [5, 23]. Both cases, apparently not related with probiotics assumption, were diagnosed in absence of positive blood cultures.
Lactobacillus antimicrobial susceptibilities are poorly defined [25–27]. Different methods are recommended by Clinical Laboratory Standards Institute (CLSI) while the European Committee on Antimicrobial Susceptibility Testing (EUCAST) did not indicate any antibiotic susceptibility threshold for this pathogen [28]. According to the disk diffusion method, our isolate resulted in vitro resistant to ampicillin and susceptible to clindamycin, levofloxacin and tetracyclines. The patient improved with targeted antibiotic therapy. Unfortunately, the follow up was short due to unexpected death not related to the infection. Our case focuses on the difficulties in diagnosing spondylodiscitis by unusual pathogens that require an aggressive, multidisciplinary approach. A possible role of Lactobacillus in the development of invasive infections should be considered in patients who regularly take probiotics [29]. The benefits of using probiotics should be weighed against the potential risks, especially in the most fragile patients.
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest regarding the publication of this paper.
Funding
None to declare
REFERENCES
- 1. Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin Infect Dis. 2015;61(6):e26–e46. doi: 10.1093/cid/civ482. [DOI] [PubMed] [Google Scholar]
- 2. Gentile L, Benazzo F, De Rosa F, et al. A systematic review: characteristics, complications and treatment of spondylodiscitis. Eur Rev Med Pharmacol Sci. 2019;23(2 Suppl):117–128. doi: 10.26355/eurrev_201904_17481. [DOI] [PubMed] [Google Scholar]
- 3. Rutges JP, Kempen DH, van Dijk M, Oner FC. Outcome of conservative and surgical treatment of pyogenic spondylodiscitis: a systematic literature review. Eur Spine J. 2016;25(4):983–999. doi: 10.1007/s00586-015-4318-y. [DOI] [PubMed] [Google Scholar]
- 4. Herren C, Young N, Pishnamaz M, et al. Spondylodiscitis: diagnosis and treatment options. A systematic review. Dtsch Arztebl Int. 2017;114(51–52):875–882. doi: 10.3238/arztebl.2017.0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Obeidat Y, Suliman MS, Mullins M, Saunders E. Lactobacillus Discitis/Osteomyelitis in an intravenous drug abuser. Cureus. 2020;12(7):e9219. doi: 10.7759/cureus.9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Food and Agriculture Organization of the United Nations (FAO); World Health Organization (WHO) Report of a joint FAO/WHO expert consultation on the evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. 2001. [Last accessed Jul 05, 2022]. http://www.fao.org/3/a-a0512e.pdf .
- 7. Kocsis T, Molnár B, Németh D, et al. Probiotics have beneficial metabolic effects in patients with type 2 diabetes mellitus: a meta-analysis of randomized clinical trials. Scient Rep. 2020;10:11787. doi: 10.1038/s41598-020-68440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matkowska M, Garbacz K, Kusiak A. Probiotics: should all patients take them? Microorganisms. 2021;9:2620. doi: 10.3390/microorganisms9122620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kullar R, Goldstein EJC, Johnson S, McFarland LV. Lactobacillus bacteremia and probiotics: a review. Microorganisms. 2023;11(4):896. doi: 10.3390/microorganisms11040896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rossi F, Amadoro C, Gasperi M, et al. Lactobacillii infection case reports in the last three years and safety implications. Nutrients . 2022;14:1178. doi: 10.3390/nu14061178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pruccoli G, Silvestro E, Pace Napoleone C, et al. Are probiotics safe? Bifidobacterium bacteremia in a child with severe heart failure. Infez Med. 2019;27(2):175–178. [PubMed] [Google Scholar]
- 12. Weber E, Reynaud Q, Suy F, et al. Bifidobacterium species bacteremia: risk factors in adults and infants. Clin Infect Dis. 2015;61(3):482–484. doi: 10.1093/cid/civ347. 1048.1093/cid/civ347. [DOI] [PubMed] [Google Scholar]
- 13. Acuna-Gonzalez A, Kujawska M, Youssif M, et al. Bifidobacterium bacteraemia is rare with routine probiotics use in preterm infants: A further case report with literature review. Anaerobe. 2023;80:102713. doi: 10.1016/j.anaerobe.2023.102713. [DOI] [PubMed] [Google Scholar]
- 14. Antoun M, Hattab Y, Akhrass F, Hamilton LD. Uncommon pathogen, Lactobacillus, causing infective endocarditis: case report and review. Case Rep Infect Dis . 2020;2020:8833948. doi: 10.1155/2020/8833948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Falci DR, Rigatto MH, Cantarelli VV, et al. Lactobacillus rhamnosus bacteremia in a kidney transplant recipient. Transpl Infect Dis. 2015;17(4):610–612. doi: 10.1111/tid.12410. [DOI] [PubMed] [Google Scholar]
- 16. Rubin IMC, Stevnsborg L, Mollerup S, et al. Bacteraemia caused by Lactobacillus rhamnosus given as a probiotic in a patient with a central venous catheter: a WGS case report. Infect Prev Pract. 2022;4(1):100200. doi: 10.1016/j.infpip.2022.100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karime C, Barrios MS, Wiest NE, et al. Lactobacillus rhamnosus sepsis, endocarditis and septic emboli in a patient with ulcerative colitis taking probiotics. BMJ Case Rep. 2022;15(6):e249020. doi: 10.1136/bcr-2022-249020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mikucka A, Deptuła A, Bogiel T, et al. Bacteraemia caused by probiotic strains of Lacticaseibacillus rhamnosus-case studies highlighting the need for careful thought before using microbes for health benefits. Pathogens. 2022;11(9):977. doi: 10.3390/pathogens11090977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hefter Y, Powell L, Tabulov CE, et al. An 11-year review of lactobacillus bacteremia at a pediatric tertiary care center. Hosp Pediatr. 2023:e2022006892. doi: 10.1542/hpeds.2022-006892. [DOI] [PubMed] [Google Scholar]
- 20. Rahman A, Alqaisi S, Nath J. A Case of Lactobacillus casei endocarditis associated with probiotic intake in an immunocompromised patient. Cureus. 2023;15(4):e38049. doi: 10.7759/cureus.38049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sakurai Y, Watanabe T, Miura Y, Uchida T, Suda N, Yoshida M, Nawa T. Clinical and bacteriologic characteristics of six cases of Bifidobacterium breve bacteremia due to probiotic administration in the neonatal intensive care unit. Pediatr Infect Dis J. 2022;41(1):62–65. doi: 10.1097/INF.0000000000003232. [DOI] [PubMed] [Google Scholar]
- 22. Lnu K, Abdesalam A, Bambach W. Lactobacillus bacteremia: a tell-tale sign for diabetes? J Community Hosp Med Perspect. 2022;12:86–88. doi: 10.55729/2000-9666.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramos-Coria D, Canto-Losa J, Carrillo-Vazquez, et al. Lactobacillus gasseri liver abscesses and bacteremia: a case report. BMC Infect Dis. 2021;21:518. doi: 10.1186/s12879-021-06181-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pailhoriès H, Sanderink D, Abgueguen P, Lemarié C. A neglected pathogen responsible for deep infections: a case report of spondylodiscitis due to Lactobacillus sp. C. Médecine Mal Infect. 2017;47:302–303. doi: 10.1016/j.medmal.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 25. Goldstein EJ, Tyrrell KL, Citron DM. Lactobacillus species: taxonomic complexity and controversial susceptibilities. Clin Infect Dis. 2015;60(Suppl 2):S98–S107. doi: 10.1093/cid/civ072. [DOI] [PubMed] [Google Scholar]
- 26. Lee MR, Tsai CJ, Liang SK, et al. Clinical characteristics of bacteraemia caused by Lactobacillus spp. and antimicrobial susceptibilities of the isolates at a medical centre in Taiwan, 2000.2014. International journal of antimicrobial agents. 2015;46:439–445. doi: 10.1016/j.ijantimicag.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 27. Anisimova EA, Yarullina DR. Antibiotic resistance of lactobacillus strains. Curr Microbiol. 2019;76(12):1407–1416. doi: 10.1007/s00284-019-01769-7. [DOI] [PubMed] [Google Scholar]
- 28.European Committee on Antimicrobial Susceptibility Testing. [last access July 7, 2023]. http://www.eucast.org .
- 29. Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. 2015;60(Suppl 2):S129–S134. doi: 10.1093/cid/civ085. [DOI] [PMC free article] [PubMed] [Google Scholar]