Abstract
Background:
Group A Streptococcus (GAS) skin infections can lead to invasive sepsis, post-streptococcal glomerulonephritis, and potentially rheumatic heart disease (RHD). Within a study to identify predisposing factors of RHD in Ugandan schoolchildren, we determined the prevalence of skin infections and assessed the clinical features and antibiotic susceptibility of GAS skin infection.
Methods:
Cross-sectional study conducted at 3 urban primary schools in Western and Northern Uganda in March 2017. A dermatologist rendered clinical diagnoses and obtained a skin swab specimen from lesions with signs of bacterial infection. Beta-hemolytic colonies underwent Lancefield grouping, species identification by polymerase chain reaction, and antimicrobial susceptibility testing.
Results:
From 3265 schoolchildren, we observed 32% with ≥ 1 fungal, 1.8% with ≥ 1 bacterial, 0.9% with ≥ 1 viral, and 0.2% with ≥ 1 ectoparasitic infection. 32% (25 of 79) of specimens were GAS-positive, of which one-third demonstrated tetracycline resistance. Of 17 impetigo cases, 13 (76%) were located on the leg/foot and 3 (18%) on the head/neck. Prevalence of GAS skin infection was 0.8% (25 of 3265). In Northern Uganda, where subclinical definite RHD prevalence is 1.1%, GAS skin infection prevalence was 1.2% (4 of 343) and 0.9% (3 of 352).
Conclusion:
This study identifies tetracycline-resistant GAS in Ugandan communities, suggests modified skin examination of exposed anatomic locations may be appropriate for population-based GAS skin infection studies, and underscores need for clear case definitions of GAS skin infection. Future studies are needed to evaluate the role of GAS skin infection in development of RHD in Ugandan communities.
Keywords: Group A Streptococcus, Streptococcus pyogenes, skin infection, children, Africa, Uganda
Background
Impetigo is the most common skin infection in children worldwide.(1) Globally, the population of children with impetigo at any one time is estimated to be at least 162 million, predominantly in tropical, resource-limited settings.(2) In low- and middle-income countries, the Lancefield group A β-hemolytic streptococcus (GAS, Streptococcus pyogenes) is the primary etiologic agent for impetigo(3), though Staphylococcus aureus can also cause the condition. Impetigo from GAS and S. aureus can causes complicated local skin infections, such as cellulitis and abscesses, as well as invasive infections that can be fatal. Additionally, it has been hypothesized that GAS skin infection could be linked to the pathophysiology of acute rheumatic fever and rheumatic heart disease (RHD) in impetigo-endemic settings.(4, 5) While RHD is endemic in Africa, there is little high-quality disease surveillance data on GAS.
Within the structure of a larger GAS study aimed at understanding drivers of RHD in Ugandan schoolchildren, we recognized an opportunity to address gaps in knowledge related to skin infections, including those of GAS etiology. In this cross-sectional study, we aimed to determine the prevalence of skin infections in urban Ugandan schoolchildren and assess the clinical features and antibiotic susceptibility of GAS skin infection in this population.
Methods
Study Setting
This cross-sectional study was conducted during the first week of March 2017 at 3 public primary schools in urban locations: 1 in Western Uganda (Mbarara) and 2 in Northern Uganda (Gulu). These schools were chosen because they were existing sites for enrollment in the National RHD Registry.(6) Additionally, the pediatric RHD prevalence was well characterized in Gulu (7), and small-scale epidemiological surveillance (unpublished) had also been conducted in Mbarara. Primary school enrollment, as a percentage of primary school-age children, is estimated to be 91% in Uganda.(8) Mbarara and Gulu have a tropical climate with year-round average high temperatures of 25.8C and 28.3C, respectively. Mbarara has four seasons that alternate between rainy and dry. In March, Mbarara is in the middle of its first rainy season. Gulu has a long rainy season that runs from March to November and a shorter dry season from December to February.
Skin Examination & Clinical Diagnosis
By school class, participants were separated by gender, brought to a waiting area by their teacher, and examined in a private setting. Skin examination occurred in two-phases: 1) Phase 1: Full body skin screening examination by a local healthcare professional (nurse, clinical officer, or pediatrician) who had received training on identification of abnormal skin findings, including clinical signs of skin infection (bacterial, fungal, viral) and ectoparasitic infestation. Examination of the groin/buttocks and female breasts was deferred. 2) Phase 2: Positive phase one participants were evaluated by a board-certified dermatologist (A.Y.C), who rendered a clinical diagnosis. Clinical signs of bacterial infection were defined as pus, pustule, crust, or erythema. A distinction was made in clinical diagnosis between folliculitis of the scalp versus non-scalp as asymptomatic scalp carriage of GAS has been reported.(9)
Case Definition
GAS skin infection was defined as identification of GAS by polymerase chain reaction (PCR) assay from a skin specimen collected from a lesion with clinical signs of bacterial infection, as defined above.
Laboratory Methods
Skin Swab Collection
If crust, pus, or pustules were present, a skin surface swab was obtained from a single representative lesion—regardless of the clinical diagnosis. Of note, tinea capitis commonly presents with pustules and crust. Skin swabs were obtained in accordance with the study protocol. Fungal culture and potassium hydroxide staining were not performed. Similarly, folliculitis lesions were swabbed, though folliculitis can be caused by non-infectious and infectious etiologies (bacteria, virus, fungal, ectoparasite) with bacterial cultures often resulting as normal skin flora/culture-negative.(10) Each skin specimen was obtained using a flocked swab and placed in an individual transport vial containing skim milk, tryptone, glucose, glycerin broth (STGGB).(11)
Sample Transport to Central Laboratory
Skin swabs for culture were transported in a STGGB media placed in a sealed bag and inside a cold box with icepacks, as described previously.(11) These prepared samples were then frozen at −20C and transported to a central laboratory (MBN Clinical Laboratories) in Kampala, Uganda for plating. Samples were stored at −80C if not plated immediately upon arrival.
Skin Swab Culture and Streptococcal Grouping
The media and swab were fully thawed and then vigorously vortexed for about a minute. Fifty-microliters were then transferred onto 5% sheep blood agar, spread with a 10-uL plastic loop, and incubated at 35°C in 5% CO2 for up to 48 hours. Plates were checked for beta-hemolytic colonies. These colonies were sub-cultured onto sheep blood agar until pure colonies were isolated. Pure beta-hemolytic colonies underwent Lancefield grouping into groups A,B,C,D,F or G based on the Oxoid Streptococcal grouping Kit (Code TSMX3981E, Oxoid, Cambridge, UK) following the manufacturer’s instructions. Furthermore, all the beta-haemolytic streptococci were also species identified by PCR for detection of any of Groups A, B, or C using the GenoType BC Gram Positive test (Hain Lifescience Nehren, Germany). About 10 pure colonies were emulsified in PCR water and DNA was extracted from the colonies using boiling method. Multiplex PCR was then performed on the GT thermocycler followed by nucleic acid reverse hybridization of amplicons on to species-specific probes fixed on a nitrocellulose membrane. PCR analysis for Staphylococcus aureus was outside the scope of this study.
Antibiotic Susceptibility Testing
Antibiotic susceptibility testing was performed on PCR-confirmed GAS bacterial isolates. Purified GAS colonies were selected for antimicrobial susceptibility testing (AST). The AST panel included ceftriaxone, penicillin, erythromycin, vancomycin, chloramphenicol, tetracycline, and ofloxacin. The standard disc diffusion method was used to test susceptibility of the GAS to the antimicrobials. A 0.5 McFarland bacterial suspension was prepared and inoculated on sheep blood Mueller-Hinton (MH) agar plates and incubated in 5% CO2 for 24 hours at 37°C. Zone diameters were read and interpreted following the criteria of the Clinical Laboratory Standards Institute.(12)
Data Collection & Analysis
Clinical data were captured on paper, entered into an electronic REDCap® database, and analyzed with Stata/SE 15.0 (College Station, Texas, US). We organized the reporting of skin conditions using an approach similar to recent publications of school-based skin survey data.(13, 14) Utilizing the clinical diagnoses rendered in Phase Two, we report the number of different types of infectious skin conditions diagnosed and the point prevalence with 95% confidence interval (CI) in the population. Pearson’s chi-squared tests were used to compare differences in prevalence by gender (male, female), age group (4–8 years, 9–12 years, 13–16 years, 17–20 years), study site, and region (Northern vs. Western Uganda). Differences were considered significant at a p-value of <0.05.
Ethics & Regulatory Approval
Institutional review board approval was obtained from Makerere University School of Medicine Research and Ethics Committee (final approval given by Uganda National Council for Science and Technology), Case Western Reserve University, and the University of California, San Francisco. An informational letter was sent home with all students informing parents of the study, and informational sessions were held for parents to obtain more information. Following this, an informed consent/assent form was sent home with all children, and only those returning these forms, and providing their assent (>8 years) were enrolled in the study.
Afebrile participants with skin bacterial infection received topical antibiotics and were re-evaluated in 2 days by the study nurse and changed to oral antibiotics for 7 days if not improved; febrile participants with skin bacterial infection were treated with oral antibiotics for 7 days and re-evaluated by the study nurse. Participants diagnosed with fungal infection received topical antifungals with the exception of tinea capitis for which griseofulvin was given with instruction to follow-up at the local health center. Benzyl benzoate was given for scabies infestation. Follow-up at the local health center was advised for the remainder of conditions diagnosed. There was no cost to the student or family for evaluation, microbiology testing, or treatment that was provided by the study. Participants were sent home with a note alerting the family of an identified infection and the treatment plan, as well as contact information for the study nurse.
Results
Absenteeism was low, with greater than 95% attendance on the day of skin screening at each school. All children attending school on the days of skin screening participated in the study, resulting in a total of 3265 participants across 3 schools with 343 in Northern Uganda A, 352 in Northern Uganda B, and 2570 in Western Uganda. 51% of the 3265 participants (1677) were female. There was a difference in age for the 3 schools (p<0.05) and by region (p<0.05), with teenagers representing a larger proportion of participants at both Northern Ugandan primary schools. (See Table 1 for study participant demographics). Based on clinical diagnosis in all 3265 participants, we observed a prevalence of 32% (1050) for children with ≥ 1 fungal skin infection, 1.8% (60) for children with ≥ 1 bacterial skin infection, 0.9% (30) for children with ≥ 1 viral skin infection, and 0.2% (7) for children with ≥ 1 ectoparasitic infestation. Tinea capitis was the most common infectious skin condition, affecting just under one-third of the study population with a prevalence of 28% (934). See Table 2 for point prevalence with 95% CI of all infectious skin conditions.
Table 1:
Demographics of primary schoolchildren
| Study Site | Northern Uganda A n=343 | Northern Uganda B n=352 | Western Uganda n=2570 | Total n= 3265 |
|---|---|---|---|---|
| Female | 180 (53%) | 162 (46%) | 1335 (52%) | 1677 (51%) |
| Age (years) | ||||
| 4–8 | 52 (15%) | 47 (13%) | 894 (35%) | 993 (30%) |
| 9–12 | 149 (43%) | 163 (46%) | 1549 (60%) | 1861 (57%) |
| 13–16 | 140 (41%) | 142 (40%) | 125 (5%) | 407 (13%) |
| 17–20 | 2 (0.6%) | 0 | 0 | 2 (0.06%) |
| Unknown | 0 | 0 | 2 (0.08%) | 2 (0.06%) |
Table 2:
Point prevalence (95% confidence interval, CI) of skin infections based on clinical diagnosis
| Northern Uganda A | Northern Uganda B | Western Uganda | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Children | 343 | 352 | 2570 | 3265 | ||||
| N | Prevalence (95% CI) | N | Prevalence (95% CI) | N | Prevalence (95% CI) | N | Prevalence (95% CI) | |
| Children with ≥ 1 fungal skin infection | ||||||||
| Total | 114 | 33 (28–38)% | 109 | 31 (26–36)% | 827 | 32 (30–34)% | 1050 | 32 (31–34)% |
| Tinea capitis | 101 | 29 (25–34)% | 70 | 20 (16–24)% | 763 | 30 (28–31)% | 934 | 28 (27–30)% |
| - with pustules/crust | 7 | 2.0 (0.5–3.5)% | 4 | 1.1 (0.03–2.2)% | 24 | <1% | 35 | <1% |
| Tinea corporis | 21 | 6.1 (3.6–8.7)% | 24 | 6.8 (4.2–9.5)% | 75 | 2.9 (2.2–3.6)% | 120 | 3.7 (3.0–4.3)% |
| Tinea faciei | 0 | 0 | 1 | <1% | 8 | <1% | 9 | <1% |
| Tinea pedis | 1 | <1% | 2 | <1% | 43 | 1.7 (1.2–2.2)% | 46 | 1.4 (1.0–1.8)% |
| Tinea unguium | 0 | 0 | 1 | <1% | 0 | 0 | 1 | <1% |
| Interdigital toeweb | 1 | <1% | 1 | <1% | 25 | 1.0 (0.6–1.4)% | 27 | <1% |
| Pityriasis versicolor | 6 | 1.8 (0.4–0.3.1)% | 24 | 6.8 (4.2–9.5)% | 2 | <1% | 32 | 1.0 (0.6–1.3)% |
| Children with ≥ 1 bacterial skin infection | ||||||||
| Total | 9 | 2.6 (0.9–4.3)% | 6 | 1.7 (0.4–3)% | 45 | 1.8 (1.2–2.3)% | 60 | 1.8 (1.4–2.3)% |
| Impetigo | 1 | <1% | 2 | <1% | 14 | <1% | 17 | <1% |
| Folliculitis/Furunculosis, non-scalp | 4 | 1.2 (0.03–2.3% | 2 | <1% | 17 | <1% | 23 | <1% |
| Scalp folliculitis | 3 | <1% | 2 | <1% | 10 | <1% | 15 | <1% |
| Cellulitis | 1 | <1% | 0 | 0 | 3 | <1% | 4 | <1% |
| Pitted keratolysis | 0 | 0 | 0 | 0 | 3 | <1% | 3 | <1% |
| Children with ≥ 1 viral skin infection | ||||||||
| Total | 0 | 0 | 1 | <1% | 29 | 1.1 (0.7–1.5)% | 30 | <1% |
| Verruca vulgaris | 0 | 0 | 1 | <1% | 16 | <1% | 17 | <1% |
| Molluscum contagiosum | 0 | 0 | 0 | 0 | 10 | <1% | 10 | <1% |
| Herpes simplex virus, oral labialis | 0 | 0 | 0 | 0 | 3 | <1% | 4 | <1% |
| Children with ≥ 1 ectoparasite infestation | ||||||||
| Total | 0 | 0 | 1 | <1% | 6 | <1% | 7 | <1% |
| Scabies | 0 | 0 | 1 | <1% | 5 | <1% | 6 | <1% |
| Tungiasis | 0 | 0 | 0 | 0 | 1 | <1% | 1 | <1% |
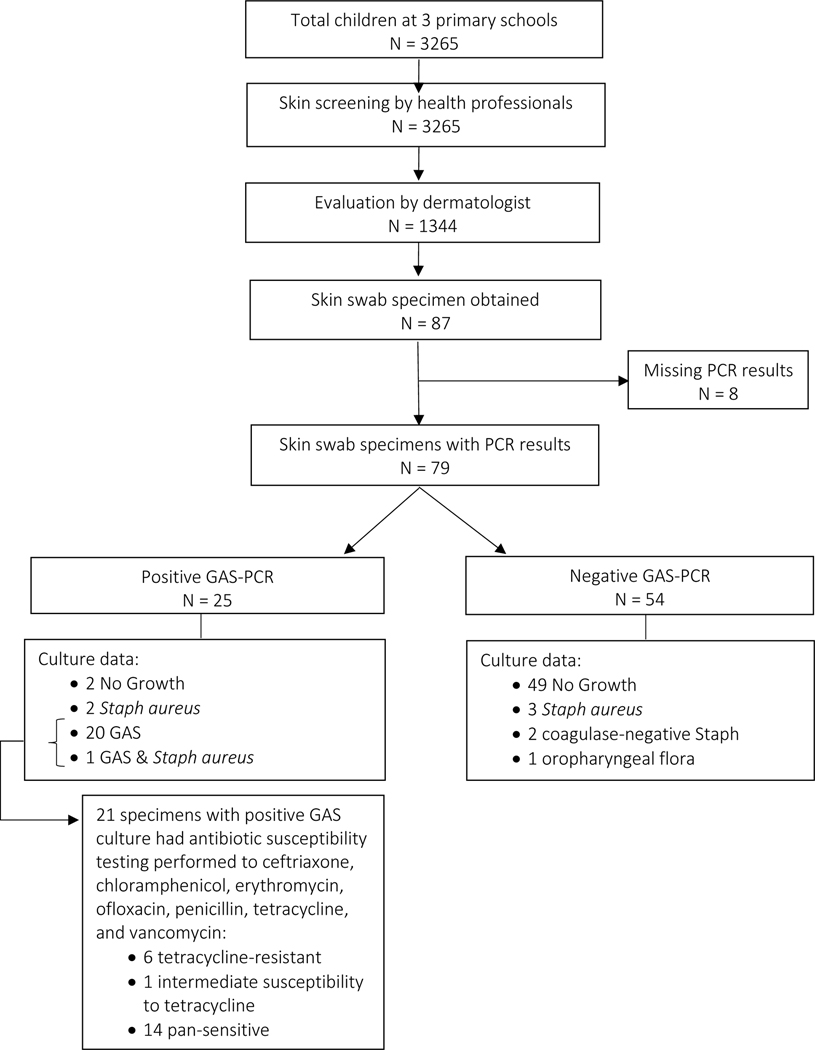
Of the 1344 participants who screened positive for an abnormal skin finding in Phase 1, 87 were determined to have clinical signs of bacterial infection (crust, pus, pustule as defined in study protocol) and had a skin swab specimen collected for microbiology analysis. Of these 87 specimens, 79 had PCR results available. 25 of 79 specimens (32%) were positive for GAS by PCR. By site, the prevalence of GAS skin infection, as determined by PCR, was Northern Uganda A: 1.2% (4 of 343), Northern Uganda B: 0.9% (3 of 352), Western Uganda: 0.7% (18 of 2570). The overall study prevalence was 0.8% (25 of 3265).
For the 79 specimens analyzed by PCR, the clinical diagnoses were as follows: tinea capitis with pustules/crust (39%), impetigo (22%), folliculitis/furunculosis, non-scalp (20%), scalp folliculitis (18%), scabies (1%). GAS PCR-positive skin specimens were more likely to be associated with a clinical diagnosis of impetigo (p<0.05). (See Table 3). Of the 17 impetigo cases, 13 (76%) were located on the lower extremity (leg or foot), 3 (18%) on the head/neck, and 1 at an unspecified site. No impetigo cases were associated with scabies or eczematous dermatitis; the study prevalence of each was low: scabies: 0.18% (6 of 3265) and eczematous dermatitis: 1.8% (57 of 3265). Of the 15 non-scalp folliculitis cases, 12 (80%) were located on the lower extremity, 4 (27%) on head/neck, 1 on the upper extremity (arm), 1 on the back, and 1 at an unspecified site. By definition, all tinea capitis and scalp folliculitis cases were located on the scalp.
Table 3.
Group A streptococcus (GAS) polymerase chain reaction (PCR) status by clinical diagnosis for 79 skin specimens
| Impetigo | Folliculitis/Furunculosis, non-scalp | Tinea capitis with pustules/crust | Scalp folliculitis | Scabies | ||
|---|---|---|---|---|---|---|
| GAS PCR+ | 14 (82%) | 6 (38%) | 4 (13%) | 1 (7%) | 0 | p<0.05 |
| GAS PCR− | 3 (18%) | 10 (62%) | 27 (87%) | 13 (93%) | 1 | |
| Total, n=79 | 17 | 16 | 31 | 14 | 1 |
Of the 25 specimens with GAS identified by PCR, 21 had cultures that grew GAS. For these 21 GAS+ culture specimens, AST was performed. Six of 21 (29%) specimens demonstrated resistance to tetracycline (inhibition zone 15–18mm). One specimen demonstrated intermediate susceptibility to tetracycline (inhibition zone 22mm). The remaining 14 specimens (66%) were pan-sensitive. See Figure 1 for summary of participants’ skin evaluation and microbiology results.
Figure 1:
Flow chart of the school-based skin survey conducted at three primary schools in Northern Uganda (Gulu) and Western Uganda (Mbarara). PCR, polymerase chain reaction. GAS, Group A streptococcus.
Discussion
Herein, we report prevalence data on infectious skin conditions and characterize GAS skin infection in schoolchildren in urban Northern and Western Uganda. In our study, the overall prevalence of GAS skin infection was 0.8%, as defined by identification of GAS by PCR in specimens obtained from skin lesions with pus, pustules, or crust.
Our point prevalence of 1.8% for bacterial skin infection (as determined by clinical diagnosis) falls within the prevalence range of 1.3% to 12.7% reported from schoolchildren in rural, mixed rural/urban, and urban areas of East Africa.(13, 15–20) These studies utilized clinical diagnosis to determine the presence or absence of bacterial skin infection; microbiology data were not reported.
Our low prevalence of GAS skin infection may be a reflection of an urban population’s better access to water, sanitation, and hygiene.(21) Impetigo is more common in rural areas(2), and GAS skin infection is influenced by hygiene factors.(22) Another consideration is the potential impact of the Onchocerciasis Control Program, which utilizes mass drug administration of ivermectin twice yearly to households in endemic areas. In other world regions, impetigo is driven by scabies and when scabies is treated, both scabies and impetigo rates decline.(23, 24) If a similar relationship exists in Northern Uganda where onchocerciasis is endemic, a recent round of ivermectin prophylaxis could have treated scabies and impetigo before our study was conducted. Furthermore, measurements of point prevalence do not account for seasonal weather patterns. In monsoonal climates, GAS is more common in dry seasons, compared with wet ones.(25) If the same is true in Uganda, then an assessment during the dry seasons might identify a higher prevalence of GAS skin infection.
In Northern Uganda, where the subclinical definite RHD prevalence is 1.1%(7), we found the point prevalence of GAS skin infection to be 1.2% at one school and 0.9% at the other. While the prevalence of GAS skin infection is not nearly as high as in the Pacific region, where the strongest linkage between GAS skin infection and RHD exists, these data suggest that skin infection could plausibly be contributing to RHD, by contributing to the overall GAS burden in African communities. Future work is needed to help elucidate this potential connection.
The observation that about one-third of GAS skin specimens were resistant to tetracycline is consistent with other studies demonstrating tetracycline-resistance in the majority of GAS isolates from referral healthcare settings in sub-Saharan Africa(26–29) and another school-based study in East Africa.(30) Clinicians should be aware of tetracycline-resistant GAS in Ugandan communities, particularly as doxycycline is a common treatment for bacterial skin infections in East Africa, given its dosing, general tolerability, and widespread availability.
We utilized a two-phase skin examination workflow that proved to be an efficient method for integrating dermatology expertise within a large study population, nearly identical to an approach used in a larger study of 13,019 schoolchildren in Cote d’Ivoire.(14) For population-based studies in settings where dermatologists are not available or other human resource constraints exist, a modified skin examination of exposed areas (head & neck, lower & upper extremities) may be appropriate, given that we found the anatomic distribution of GAS skin infections to be primarily in exposed areas.
Differences in GAS PCR result based on clinical diagnosis are not surprising. Of specimens submitted for GAS PCR, 4 of 31 (13%) cases of tinea capitis with pustules/crust and 1 of 14 (7%) cases of scalp folliculitis were GAS PCR positive. This finding could represent asymptomatic scalp carriage of GAS(9) or true GAS skin infection. As mentioned earlier, tinea capitis with pustules/crust would not typically lead a dermatologist to perform a bacterial culture, and folliculitis is understood to have a variety of etiologies with negative bacterial cultures commonplace.
Our study’s high prevalence of fungal skin infections, particularly tinea capitis, is consistent with data from other school-based prevalence studies of skin conditions in East Africa.(13, 15–20) Pityriasis versicolor was more prevalent in Northern Uganda (p<0.05), likely due to the adolescent age of primary school students which predisposes to the condition. Northern Uganda is a post-conflict region where the local population is still experiencing substantial economic hardship and thus children are often delayed in schooling. Interdigital toeweb infection, which includes dermatophyte and candidal fungal infections, tended to be more prevalent in Western Uganda (p=0.07) and may be related to the observation that many schoolchildren were wearing damp stockings and socks.
Beyond the aforementioned considerations with interpretation of point prevalence data, our study has additional limitations. Our study was not designed to draw conclusions regarding the clinical drivers of GAS skin infection in Uganda. Further work will be needed in this area. Second, S. aureus is an important causative agent of bacterial skin infection, particularly bacterial folliculitis and furunculosis, but pertinent studies were outside the scope of this study. Finally and perhaps most importantly, the lack of uniform definitions for bacterial skin infections and GAS skin infection was a major challenge for this study. The broadest definition of pyoderma we found was “any variant of superficial bacterial skin infection (e.g. impetigo, impetigo contagiosa, ecthyma, folliculitis, “impetigo of Bockhart”, furuncle, carbuncle, tropical ulcer, etc.).”(3) However, some authors distinguish folliculitis from pyoderma, infected wounds from tropical ulcer from impetigo, tropical ulcer from pyoderma(19), and abscess from pyoderma.(15) “Skin sore” has become synonymous with impetigo in the literature, but this description also applies to the clinical presentation of ecthyma, as well as a myriad of skin conditions that present with disruption of the skin barrier, such as herpes simplex virus, deep fungal infections, atypical mycobacterial infections, and non-infectious ulcers. Even when microbiology data are available, our study highlights the clinical-pathological discrepancies that can arise. For example, were the GAS PCR-positive cases of tinea capitis and scalp folliculitis appropriate to include as GAS skin infections when they could represent asymptomatic carriage? Should the criteria for GAS skin infection include a stipulation that an alternative explanation for signs of bacterial infection be excluded? With the research momentum around impetigo and pyoderma as related to GAS, achieving consensus on the case definition of GAS skin infection and which clinical diagnoses comprise superficial bacterial skin infections should be a priority to ensure consistent methodology in future studies and facilitate appropriate interpretation of data.
In conclusion, future studies are needed to evaluate the role of GAS skin infection in the development of RHD in Ugandan communities. This study contributes school-based community prevalence data on infectious skin diseases, identifies tetracycline-resistant GAS in Ugandan communities, suggests that modified skin examination of exposed anatomic locations may be appropriate for population-based GAS skin infection studies, and underscores the need for clear case definitions of GAS skin infection.
Acknowledgements:
We would like to thank the enthusiastic and hardworking Ugandan health professionals who helped to make this study possible.
Source of Funding:
This study was funded by philanthropic support from Karp Family Foundation and Gift of Life International to the Children’s National Health System, Washington, D.C. Aileen Chang was supported by NIH Research Training Grant # R25 TW009343 funded by the Fogarty International Center; the National Institute of Mental Health; the National Health, Lung and Blood Institute; and the Office of Research on Women’s Health, as well as the University of California Global Health Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the University of California Global Health Institute. Emmy Okello is a DELTAS/THRiVE fellow under grant DEL-15–001/07742/Z/15/Z.
Abbreviated
- GAS
Skin Infections Ugandan Schoolchildren
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Bernard P. Management of common bacterial infections of the skin. Curr Opin Infect Dis. 2008;21(2):122–8. doi: 10.1097/QCO.0b013e3282f44c63. [DOI] [PubMed] [Google Scholar]
- 2.Bowen AC, Mahe A, Hay RJ, Andrews RM, Steer AC, Tong SY, et al. The Global Epidemiology of Impetigo: A Systematic Review of the Population Prevalence of Impetigo and Pyoderma. PLoS One. 2015;10(8):e0136789. doi: 10.1371/journal.pone.0136789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Epidemiology and management of common skin diseases in children in developing countries. 2005. [Google Scholar]
- 4.McDonald M, Currie BJ, Carapetis JR. Acute rheumatic fever: a chink in the chain that links the heart to the throat? Lancet Infect Dis. 2004;4(4):240–5. doi: 10.1016/S1473-3099(04)00975-2. [DOI] [PubMed] [Google Scholar]
- 5.Parks T, Smeesters PR, Steer AC. Streptococcal skin infection and rheumatic heart disease. Curr Opin Infect Dis. 2012;25(2):145–53. doi: 10.1097/QCO.0b013e3283511d27. [DOI] [PubMed] [Google Scholar]
- 6.Okello E, Longenecker CT, Scheel A, Aliku T, Rwebembera J, Mirembe G, et al. Impact of regionalisation of a national rheumatic heart disease registry: the Ugandan experience. Heart Asia. 2018;10(1):e010981. doi: 10.1136/heartasia-2017-010981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaton A, Lu JC, Aliku T, Dean P, Gaur L, Weinberg J, et al. The utility of handheld echocardiography for early rheumatic heart disease diagnosis: a field study. Eur Heart J Cardiovasc Imaging. 2015;16(5):475–82. doi: 10.1093/ehjci/jeu296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The World Bank. School enrollment, primary (% net): The World Bank Group; 2019. [August 12, 2019]. Available from: https://data.worldbank.org/indicator/SE.PRM.NENR?locations=UG&view=chart. [Google Scholar]
- 9.Mastro TD, Farley TA, Elliott JA, Facklam RR, Perks JR, Hadler JL, et al. An outbreak of surgical-wound infections due to group A streptococcus carried on the scalp. N Engl J Med. 1990;323(14):968–72. doi: 10.1056/NEJM199010043231406. [DOI] [PubMed] [Google Scholar]
- 10.McMichael A, Curtis AR, Guzman-Sanchez D, Kelly AP. Folliculitis and Other Follicular Disorders. In: Bolognia J, Schaffer J, Cerroni L, editors. Dermatology: Saunders; 2012. [Google Scholar]
- 11.McDonald M, Towers R, Fagan P, McKinnon M, Benger N, Andrews R, et al. Recovering streptococci from the throat, a practical alternative to direct plating in remote tropical communities. J Clin Microbiol. 2006;44(2):547–52. doi: 10.1128/JCM.44.2.547-552.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. CLSI supplement M1002017. [Google Scholar]
- 13.Hogewoning A, Amoah A, Bavinck JN, Boakye D, Yazdanbakhsh M, Adegnika A, et al. Skin diseases among schoolchildren in Ghana, Gabon, and Rwanda. Int J Dermatol. 2013;52(5):589–600. doi: 10.1111/j.1365-4632.2012.05822.x. [DOI] [PubMed] [Google Scholar]
- 14.Yotsu RR, Kouadio K, Vagamon B, N’Guessan K, Akpa AJ, Yao A, et al. Skin disease prevalence study in schoolchildren in rural Cote d’Ivoire: Implications for integration of neglected skin diseases (skin NTDs). PLoS Negl Trop Dis. 2018;12(5):e0006489. doi: 10.1371/journal.pntd.0006489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferie J, Dinkela A, Mbata M, Idindili B, Schmid-Grendelmeier P, Hatz C. Skin disorders among school children in rural Tanzania and an assessment of therapeutic needs. Trop Doct. 2006;36(4):219–21. doi: 10.1258/004947506778604823. [DOI] [PubMed] [Google Scholar]
- 16.Schmeller W, Dzikus A. Skin diseases in children in rural Kenya: long-term results of a dermatology project within the primary health care system. Br J Dermatol. 2001;144(1):118–24. [PubMed] [Google Scholar]
- 17.Walker SL, Lebas E, De Sario V, Deyasso Z, Doni SN, Marks M, et al. The prevalence and association with health-related quality of life of tungiasis and scabies in schoolchildren in southern Ethiopia. PLoS Negl Trop Dis. 2017;11(8):e0005808. doi: 10.1371/journal.pntd.0005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komba EV, Mgonda YM. The spectrum of dermatological disorders among primary school children in Dar es Salaam. BMC Public Health. 2010;10:765. doi: 10.1186/1471-2458-10-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Figueroa JI, Fuller LC, Abraha A, Hay RJ. The prevalence of skin disease among school children in rural Ethiopia--a preliminary assessment of dermatologic needs. Pediatr Dermatol. 1996;13(5):378–81. [DOI] [PubMed] [Google Scholar]
- 20.Murgia V, Bilcha KD, Shibeshi D. Community dermatology in Debre Markos: an attempt to define children’s dermatological needs in a rural area of Ethiopia. Int J Dermatol. 2010;49(6):666–71. doi: 10.1111/j.1365-4632.2009.04284.x. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization, UNICEF. Meeting the MDG drinking water and sanitation target : the urban and rural challenge of the decade. 2006. Accessed 12 Dec 2018. Available from: https://www.who.int/water_sanitation_health/monitoring/jmpfinal.pdf. [Google Scholar]
- 22.Sims Sanyahumbi A, Colquhoun S, Wyber R, Carapetis JR. Global Disease Burden of Group A Streptococcus. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: Basic Biology to Clinical Manifestations. Oklahoma City (OK)2016. [PubMed] [Google Scholar]
- 23.Lawrence G, Leafasia J, Sheridan J, Hills S, Wate J, Wate C, et al. Control of scabies, skin sores and haematuria in children in the Solomon Islands: another role for ivermectin. Bull World Health Organ. 2005;83(1):34–42. doi: /S0042–96862005000100012. [PMC free article] [PubMed] [Google Scholar]
- 24.Romani L, Whitfeld MJ, Koroivueta J, Kama M, Wand H, Tikoduadua L, et al. Mass Drug Administration for Scabies Control in a Population with Endemic Disease. N Engl J Med. 2015;373(24):2305–13. doi: 10.1056/NEJMoa1500987. [DOI] [PubMed] [Google Scholar]
- 25.McDonald MI, Towers RJ, Andrews R, Benger N, Fagan P, Currie BJ, et al. The dynamic nature of group A streptococcal epidemiology in tropical communities with high rates of rheumatic heart disease. Epidemiol Infect. 2008;136(4):529–39. doi: 10.1017/S0950268807008655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camara M, Dieng A, Boye CS. Antibiotic susceptibility of streptococcus pyogenes isolated from respiratory tract infections in dakar, senegal. Microbiol Insights. 2013;6:71–5. doi: 10.4137/MBI.S12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tewodros W, Muhe L, Daniel E, Schalen C, Kronvall G. A one-year study of streptococcal infections and their complications among Ethiopian children. Epidemiol Infect. 1992;109(2):211–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mhalu FS, Hofstad T. Antibiotic susceptibility of group A streptococci in a national consultant hospital in Dar es Salaam: a four year follow-up. East Afr Med J. 1997;74(3):177–8. [PubMed] [Google Scholar]
- 29.Ndiaye AG, Boye CS, Hounkponou E, Gueye FB, Badiane A. Antimicrobial susceptibility of select respiratory tract pathogens in Dakar, Senegal. J Infect Dev Ctries. 2009;3(9):660–6. [DOI] [PubMed] [Google Scholar]
- 30.Braito A, Galgani I, Mohammed MR, Iozzi C, Ame SM, Haji HS, et al. Epidemiology of streptococcus group A in school aged children in Pemba. East Afr Med J. 2004;81(6):307–12. [DOI] [PubMed] [Google Scholar]