Abstract
Mass vaccination has led poliomyelitis to become a rare disease in a large part of the world, including Western Europe. However, in the past 20 years wild polioviruses imported from countries where polio is endemic have been responsible for outbreaks in otherwise polio-free European countries. We report on the characterization of poliovirus isolates from a large outbreak of poliomyelitis that occurred in Albania in 1996 and that also spread to the neighboring countries of Yugoslavia and Greece. The epidemics involved 145 subjects, mostly young adults, and caused persisting paralysis in 87 individuals and 16 deaths. The agent responsible for the outbreak was isolated from 74 patients and was identified as wild type 1 poliovirus by both immunological and molecular methods. Sequence analysis of the genome demonstrated the involvement of a single virus strain throughout the epidemics, and genotyping analysis showed 95% homology of the strain with a wild type 1 poliovirus strain isolated in Pakistan in 1995. Neutralization assays with both human sera and monoclonal antibodies were performed to analyze the antigenic structure of the epidemic strain, suggesting its peculiar antigenic characteristics. The presented data underline the current risks of outbreaks due to imported wild poliovirus and emphasize the need to improve vaccination efforts and also the need to implement surveillance in countries free of indigenous wild poliovirus.
The World Health Organization (WHO) program Global Eradication of Poliomyelitis by the Year 2000 has made great progress, and paralytic poliomyelitis has become a rare disease in the Americas, Western Europe, and many other regions of the world (9, 28). Nonetheless, imported wild polioviruses from areas where polio is endemic have caused outbreaks in countries formerly reported to be polio free and in which specific subpopulations either show a weak general immunity, such as in Finland in 1984 and 1985 (7), or lack immunity, such as in The Netherlands in 1978 (2) and 1992 (19), Bulgaria (30), and Romania (25). The reemergence of poliomyelitis has also been reported in countries where political and economic changes have made it difficult to maintain immunization programs, such as some regions of the former Soviet Union in the 1990s (17, 20, 27). In Albania, the latest large outbreak of poliomyelitis occurred in 1978, when 71 cases associated with either wild type 1 or type 3 poliovirus strains were reported. Routine vaccination with a three-dose regimen of the oral poliovirus vaccine was reinstated for newborns in 1980, and the vaccination coverage rate was estimated to be high (92 to 95%) until 1990. Virological surveillance of acute flaccid paralysis (AFP) was conducted in Albania from 1980 through 1995; a total of 93 AFP cases were reported, and 11 of them were classified as vaccine-associated poliomyelitis (VAPP) (5). The isolation of only Sabin poliovirus strains from these patients and from their healthy contacts suggested that circulation of wild poliovirus in Albania during this period was very limited, if it occurred at all (5). However, seroepidemiological investigations performed in Albania in 1980 through 1990 and with Albanian immigrants in Italy in 1991 showed that 15 to 30% of the surveyed adult population was susceptible to poliovirus, suggesting the existence of gaps in the immunization programs (4, 23).
The apparent lack of circulation of wild poliovirus in Albania was likely due to the absence of virtually any contact with other countries until 1990. The opening of Albania’s borders to foreigners in 1991 exposed the population to the risk of imported poliovirus from areas where polio is endemic due to the settlement of people from Western Europe or the passage of people to Western Europe.
A large outbreak of paralytic poliomyelitis with a wild type 1 poliovirus occurred in Albania in May 1996 and involved 145 subjects, mostly adults. An epidemiological description of the poliomyelitis outbreak has been reported elsewhere (21). The outbreak was preceded by national immunization days (NIDs), targeting more than 97% of children less than 6 years of age (approximately 350,000 subjects).
In this paper, we report a detailed characterization of poliovirus isolates and the results of serological analyses conducted with patients. Cases of polio recorded in the neighboring countries of Greece and the Federal Republic of Yugoslavia are also described.
MATERIALS AND METHODS
Patients and virus isolation.
AFP was reported in a total of 145 Albanian subjects between April and November 1996. Twenty-four cases of poliomyelitis were reported between August and October 1996 in Yugoslavia, in the region of Kosovo, and involved unvaccinated children. Five cases were also reported among unvaccinated Gypsy children in three distinct regions of Greece between the end of June and September 1996.
Stools and rectal swabs were obtained from 99 and 26 Albanian AFP patients, respectively. For approximately 32% of the patients, samples were collected within 48 h after admission to the hospital, and for 50% of the patients sample collection was effected within 2 weeks from the time of onset of symptoms. Venous blood was drawn from 120 patients, and cerebrospinal fluid was obtained from 3 patients.
HEp-2 and RD cell monolayers grown in Eagle’s minimal essential medium (MEM) supplemented with 10% fetal calf serum (FCS) were inoculated with 0.2 ml of 10% stool suspensions in Hank’s balanced salt solution, rectal swab collection medium, or cerebrospinal fluid and were incubated at 37°C for 7 days or until the development of a cytopathic effect (CPE). A total of three blind passages were performed before a culture was considered negative. Isolates from CPE-positive cultures were identified by a microneutralization assay with serum pools and type-specific poliovirus antisera (National Institute of Public Health and the Environment, Bilthoven, The Netherlands) performed by standard methods recommended by WHO (29).
Polioviruses isolated from the first patient with polio occurred in Yugoslavia, and the patients from Greece were also included in the study.
Characterization of poliovirus isolates.
For the rapid differentiation of the wild and Sabin-like poliovirus strains isolated, the PCR amplification and restriction fragment length polymorphism (RFLP) analysis method described by Balanant and coworkers (1) was initially adopted. Sequencing of the VP1-2A junction genomic region after PCR amplification (10) was subsequently used. The primers used for reverse transcription and PCR amplification of viral RNA were as follows: Jun A (antisense; bp 3493 to 3514; 5′-AAGAGGTCTCTATTCCACATGA-3′) and Jun S (sense; bp 3209 to 3228; 5′-GTGGTCAATGATCACAACCC-3′). The PCR products were purified with a Centricon 100 concentrator (Amicon) and were sequenced by the Taq dyedeoxy terminator cycle method with an automated sequencer (Applied Biosystems). Each amplified product was sequenced from both ends with the primers used in the PCRs. The 150-bp sequence of the VP1-2A junction region was aligned with the corresponding sequences of poliovirus type 1 reference strains or field isolates reported in the EMBL Data Bank and in databases available at the Centers for Disease Control and Prevention, Atlanta, Ga. (kindly supplied by Olen Kew), the National Institute of Public Health and the Environment (Bilthoven, The Netherlands), and the National Public Health Institute (Helsinki, Finland).
A dendrogram of sequence relatedness among the Albanian poliovirus strains and strains isolated in other countries was constructed by the method of cluster analysis with the PHYLIP program (version 3.5c; University of Washington).
Stool samples negative at isolation for poliovirus and other enteroviruses were analyzed directly by PCR with primers specific for the 5′ noncoding region (5′NCR) common to all enteroviruses: PVM 1-1 (bp 160 to 178) (5′-CAAGCACTTCTGTTTCCCC-3′), PVM 1-2 (bp 577 to 594) (5′-ATTGTCACCATAAGCAGC-3′), and primers specific for the polio VP1-2A junction. This was followed by sequencing of the amplified products. Sequences of the 5′NCR of Sabin-like poliovirus isolates were analyzed in detail for the presence of mutations.
For 20 samples, poliovirus characterization was confirmed by enzyme-linked immunosorbent assay with cross-absorbed type-specific polyclonal antibodies (26).
Determination of poliovirus antibody in sera from patients.
Neutralizing antibodies to poliovirus in serum were titrated in microcultures of HEp-2 cells by using wild reference strains (poliovirus type 1 Mahoney, poliovirus type 2 MEF-1, and poliovirus type 3 Leon) as described previously (16). Acute- and convalescent-phase sera from each patient were tested in the same run. The antibody titer was expressed as the highest serum dilution that completely inhibited the CPE. Titers of <1:8 were considered negative.
Poliovirus type-specific immunoglobulin M (IgM) determination was performed with sera from 70 AFP patients by an IgM antibody-capture enzyme-linked immunosorbent assay as described previously (15).
Antigenic analysis.
The antigenic properties of representative epidemic strains isolated from patients with poliomyelitis in Albania, Greece, and Yugoslavia were analyzed by a neutralization index test with monoclonal antibodies (MAbs) as described previously (3). Two sets of poliovirus-neutralizing MAbs, raised against reference type 1 Mahoney or Sabin strains, were used. MAbs raised against epidemic type 1 poliovirus strains isolated in India in 1989 and in Israel in 1988 were also used. Fifty microliters of each MAb properly diluted in serum-free MEM was mixed with the same volume of serial 10-fold-diluted virus suspensions in 96-well microtiter plates. After incubation at 37°C for 2 h, 100 μl of MEM supplemented with 2% FCS was added to each well, which contained 2 × 104 HEp-2 cells. The plates were then scored for CPE after 5 days of incubation at 37°C in a CO2 atmosphere. The neutralization index was defined as the difference in the log virus titers in the absence and the presence of each antibody. Values equal to or higher than 2 logs were considered positive.
To detect possible abnormal antigenic properties of wild poliovirus type 1 isolates recognized by human sera, two different assays were used. In the first assay, a panel of sera collected from 10 Albanian paralytic patients in 1996, sera collected from 6 Albanian subjects long before the outbreak (2 children and 4 adults), and sera collected from 10 oral polio vaccine (OPV)-immunized Italian children were examined by a microneutralization assay as described above. Neutralizing titers against Sabin, wild type 1 Mahoney, and an epidemic virus (strain 3864Alb96) were compared. In the second assay, selected sera from patients were tested in a plaque reduction neutralization assay, with excess antibody, against the two references and the epidemic strains. Sera at a 1:20 dilution were incubated with 107 PFU of each virus at 37°C for 2 hours. Virus-antibody mixtures were then serially (10-fold) diluted, and 200 μl of each dilution was inoculated onto HEp-2 cell monolayers in 35-mm dishes. After binding at 37°C for 1 h, the monolayers were washed and overlaid with 3 ml of MEM containing 2% agar (Gibco) and 2% FCS. After 48 h of incubation at 37°C in a CO2 atmosphere, the cells were stained with neutral red and the plaques were counted.
RESULTS
The occurrence of an unusually wide cluster of AFP cases in Albania involving adults was reported to the Laboratory of Virology at the Istituto Superiore di Sanità in Rome, Italy, at the end of July 1996; concurrently, clinical specimens from the first eight patients and two poliovirus isolates were forwarded for virological investigations. A nationwide active surveillance of AFP cases was then implemented, and further samples were made available for laboratory analysis by the end of August through November 1996. A total of 145 cases of AFP were reported in 27 of 36 districts of Albania. Males represented 70% of the 145 patients with AFP.
Early paralytic cases.
The first poliovirus-positive AFP case was reported in a 1-year-old child who developed paralysis 10 days after being vaccinated during the NID in Albania on 9 April 1996. A poliovirus type 1 strain was isolated from stools and was characterized as Sabin-like by both RFLP analysis and sequencing of the 5′NCR region of the virus genome, showing a G→A480, one of the substitutions known to be associated with the loss of the Sabin type 1 attenuated phenotype (12). Since no wild-type poliovirus strain could be isolated from the patient’s fecal samples, this subject, classified as having type 1 VAPP, therefore was not likely involved in the outbreak.
Wild type 1 poliovirus was isolated from the stools of a 30-year-old man who developed paralysis on 23 May 1996 and who may represent the actual index patient for the epidemics. Although a vaccination history was not available for this patient, his serum showed high titers of antibody against all poliovirus types.
The third patient, whose illness was reported on 26 May 1996, was a 2-year-old child from northern Albania who had participated in the polio NIDs conducted in April and May 1996 after having previously received four doses of OPV. Both Sabin-like type 1 and wild type 1 polioviruses were isolated from the patient’s stools. The presence of wild type 1 poliovirus was relevant for the diagnosis of epidemic poliomyelitis.
Wild type 1 poliovirus was isolated from two further adult patients whose illnesses were reported in June 1996. A type 3 Sabin-derived strain, carrying a U→C472 reversion, was isolated from a 3.5-month-old child, reported in June 1996, who had received one dose of OPV 10 days before the onset of paralysis and was classified as having VAPP. The number of patients reported to be excreting wild type 1 poliovirus increased rapidly by the end of July.
Case classification and virological findings.
On the basis of the WHO criteria for the definition of poliomyelitis, 74 patients were confirmed to have poliomyelitis (Table 1), with wild type 1 poliovirus being isolated from all of them, while 64 subjects, from which wild type 1 poliovirus was not isolated, were defined as having an illness compatible with polio (Table 1). For 43 of these patients, clinical samples were made available only late after the onset of the disease or rectal swabs instead of stools were collected, which reduced the probability of isolation. Samples from 20 AFP subjects were not supplied. Five patients from whom poliovirus was not isolated were excluded because they eventually recovered from their illnesses. The 16 deaths recorded occurred in subjects aged 13 to 37 years during the first half of the outbreak and were due to respiratory failure.
TABLE 1.
Grouping of AFP cases according to WHO criteria for case definition, Albania, 1996
| Status at clinical follow-up | No. of patientsa
|
||||||
|---|---|---|---|---|---|---|---|
| Confirmed to have polio
|
Illness
compatible with polio
|
No polio
|
Total | ||||
| Wild type 1 epidemic strain | VAPP | Negative or Sabin strain positive | No sample was available | Negative or Sabin strain positive | No sample was available | ||
| Residual paralysis | 53 | 2 | 28b | 6 | 0 | 0 | 89 |
| Dead | 3 | 0 | 3 | 10 | 0 | 0 | 16 |
| Missing | 18 | 0 | 14c | 3 | 0 | 0 | 35 |
| Recovered | 0 | 0 | 0 | 0 | 4 | 1 | 5 |
| Total | 74 | 2 | 45 | 19 | 4 | 1 | |
Among the 145 subjects in the three groups, 76 were confirmed to have polio, and 64 had an illness compatible with polio; 5 subjects were excluded, having recovered from illness.
Sabin poliovirus type 1 or 3 was isolated from three patients.
Sabin poliovirus type 1 or 3 was isolated from four patients.
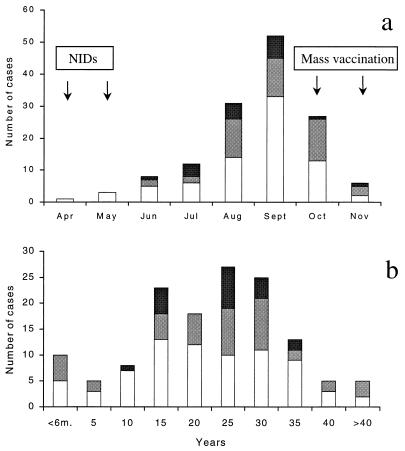
The distribution of confirmed cases of polio and cases of illness compatible with polio per month is shown in Fig. 1a. The epidemic peaked in September 1996, and the last case occurred on 23 November 1996.
FIG. 1.
(a) Distribution of confirmed cases of polio (□) and
illness compatible with polio (
, samples available;
 , samples not available) by month
of onset of paralysis in Albania in 1996. The times of NIDs and mass
vaccination campaigns are indicated. (b) Age distribution of patients
confirmed to have polio and with illness compatible with polio in
Albania in 1996.
, samples not available) by month
of onset of paralysis in Albania in 1996. The times of NIDs and mass
vaccination campaigns are indicated. (b) Age distribution of patients
confirmed to have polio and with illness compatible with polio in
Albania in 1996.
The ages of patients ranged between 2 months and 52 years, with 78% of them being 11 to 35 years old (Fig. 1b). The incidence of poliomyelitis was low among young children, indicating the efficacies of the NIDs. On the contrary, a relatively high incidence was shown among infants aged less than 6 months. With only one exception, these occurred late during the epidemic, among children who were not born at the time of the NIDs (Table 2). These children were exposed to the circulating wild virus during the peak of the epidemic, and even though some of them had been at least partially vaccinated in the following months, they apparently were immunologically unprotected.
TABLE 2.
Epidemiological and virological findings of paralytic poliomyelitis in children younger than age 6 months, Albania, 1996
| Patient | Age | No. of OPV doses | Date (mo/day/yr) of onset of paralysisa | Isolated virusb | Region of Albania | Follow-up | Case definition |
|---|---|---|---|---|---|---|---|
| 3909Alb96 | 3.5 mo | 1 | 06/14/1996c | P3, SL revertant | Has | Residual paralysis | Poliomyelitis |
| 3994Alb96 | 26 days | 0 | 09/01/1996 | Negatived | Fier | Residual paralysis | Polio compatible |
| 3967Alb96 | 5 mo | 1 | 09/13/1996 | P1, wild | Kukes | Missing | Poliomyelitis |
| 3993Alb96 | 2 mo | 1 | 09/15/1996e | P2, SL revertant | Tirana | Residual paralysis | Polio compatible |
| 4029Alb96 | 53 days | 0 | 09/19/1996 | P1, wild | Kukes | Residual paralysis | Poliomyelitis |
| 4035Alb96 | 4 mo | 2 | 09/20/1996 | P2, SL revertant | Lac | Missing | Polio compatible |
| 3989Alb96 | 40 days | 0 | 09/23/1996 | P1 wild | Lac | Residual paralysis | Poliomyelitis |
| 4024Alb96 | 3.5 mo | 0 | 10/02/1996 | Negatived | Shkoder | Missing | Polio compatible |
| 4055Alb96 | 4.5 mo | 1 | 10/13/1996 | Negatived | Mat | Missing | Polio compatible |
| 4075Alb96 | Unknown | 2 | 10/20/1996 | Negatived | Librazhd | Residual paralysis | Polio compatible |
The time lapse between vaccination and the onset of paralysis was 10 days for patient 3909Alb96 and 6 days for patient 4055Alb96.
P1, P2, and P3, poliovirus types 1, 2, and 3, respectively; SL, Sabin-like.
The patient had VAPP.
Inadequate samples were available.
Onset of paralysis on the same day as receipt of vaccine.
Origins of epidemic strains.
Overall, 74 poliovirus isolates from the 125 AFP patients who were analyzed were characterized as wild type 1 polioviruses. Sabin-like polioviruses of different serotypes were identified in nine patients.
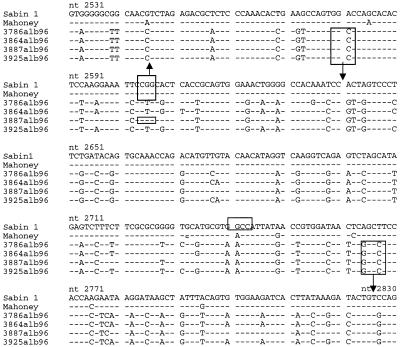
Intratypic differentiation of isolates was initially performed by the RFLP method (1), which allowed the two isolates from the patient reported in April and May 1996 to be identified as Sabin-derived poliovirus. However, no PCR amplification could be obtained with the epidemic strains tested with the standard UC1-UG1 pair of primers in the VP1 capsid protein region (bp 2401 to 2880) which had previously proven to be rather conserved among polioviruses, suggesting the occurrence of a modified poliovirus variant. Amplification was instead obtained by pairing UC1 with T1G5 (bp 2485 to 2504), indicating a mismatch of UG1, as also confirmed by sequencing (Fig. 2). In addition, the generation of two new HaeIII restriction sites by mutations at positions 2581 and 2764 to 2767 (Fig. 2) together with the suppression of the HaeIII site at positions 2603 to 2606 (common to the Sabin and Mahoney strains) by a mutation at position 2605 yielded a migration pattern closely similar to that of Sabin 1 poliovirus. Although the HaeIII site at positions 2603 to 2606 was conserved in one isolate, the small molecular size of the digested fragment together with the presence of the new HaeIII site (positions 2579 to 2582) did not change the electrophoretic migration pattern, thus yielding Sabin-like results also for this strain. Because rapid characterization of wild isolates by RFLP analysis was not feasible, all further strains were directly investigated by PCR amplification and sequencing of the region coding for the VP1-2A junction.
FIG. 2.
Comparison of the VP1 sequences (nucleotides [nt] 2531 to 2830) of representative wild type 1 poliovirus isolates from Albania and the Sabin and Mahoney reference strains. HaeIII restriction enzyme sites are indicated. □, HaeIII sites; ↑, new site; ↓, suppressed site.
Strain genealogy.
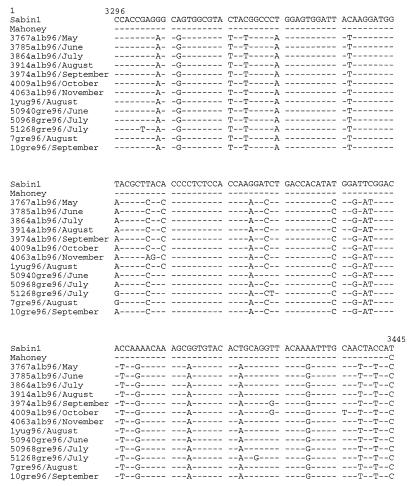
The genealogy of the Albanian epidemic strains was analyzed by alignment of the sequences of the VP1-2A junction, the 5′NCR (bp 391 to 540), and the VP1-coding region (bp 2531 to 2830). A 99 to 100% homology was found between wild type 1 poliovirus strains isolated at different intervals during the Albanian outbreak, indicating the involvement of a single virus genotype in the outbreak. The sequences of isolates from the late phase of the epidemic presented few additional mutations in the VP1-2A junction (Fig. 3) and in the other regions sequenced (data not shown) compared to the sequences of the isolates from the early phase of the epidemics, possibly due to intra- and interhuman virus passage. Comparison of the sequences in the VP1-2A region also showed that a type 1 poliovirus with a similar genotype was responsible for the poliomyelitis cases that occurred in Yugoslavia and Greece (Fig. 3).
FIG. 3.
Sequence alignment of the VP1-2A junction of wild type 1 poliovirus strains from Albania isolated in different months during the epidemics, wild type 1 strains isolated in Yugoslavia and Greece, and Sabin and Mahoney reference strains. The strains 1yug96 (Kosovo, Yugoslavia), 50940gre96 (Attica, Greece), 50968gre96 (Attica, Greece), 51268gre96 (Crete, Greece), 7gre96 (Thessalonica, Greece), and 10gre96 (Thessalonica, Greece) were from the regions indicated in parentheses.
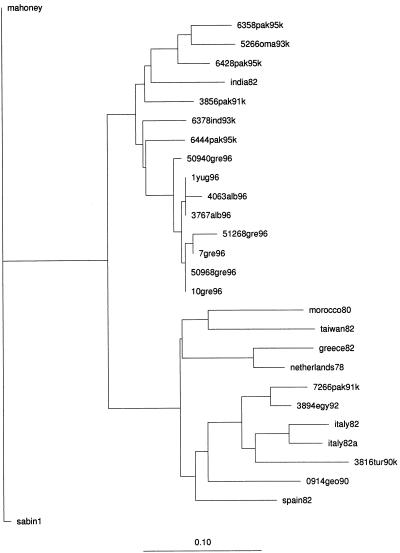
The dendrograms presented in Fig. 4 were derived from sequence alignments of the VP1-2A genomic regions of the Albanian epidemic strain and representative epidemic strains from other geographic regions. The highest degree of homology (95%) was found with a wild type 1 poliovirus strain isolated in Pakistan in 1995 (6444 Pak 95 K) (10, 13).
FIG. 4.
Dendrogram summarizing sequence relatedness among wild type 1 polioviruses isolated in Albania, Yugoslavia, and Greece and recent wild type 1 polioviruses isolated worldwide across the interval from nucleotides 3216 to 3445 (VP1-2A region). The PHYLIP distance method (University of Washington) was used.
Abnormal antigenic properties of wild type 1 poliovirus.
The similarities of the Albanian outbreak to those in Finland in 1985 (8) and Israel in 1988 (22) (mostly adults vaccinated many years earlier and infected with a new poliovirus variant) led us to investigate a possible discordance between the antigenicity of the Albanian strain and the immunity induced by OPV. Neutralizing titers against a representative Albanian the isolate obtained in 1996, the reference wild Mahoney strains, and the vaccine Sabin type 1 polioviruses were determined in sera from patients infected with wild poliovirus from the epidemics, contemporary patients with non-polio-associated meningoencephalitis, and Albanian and Italian OPV vaccinees with no previous exposure to wild poliovirus. Sera from poliomyelitis patients showed similar neutralizing titers against all three viruses. On the contrary, control OPV vaccinees had higher neutralizing titers against the Mahoney and Sabin type 1 strains than against the epidemic strain (2.19 to 2.39 versus 1.63 log10; P = 0.019 and 0.006, respectively). Also, in plaque reduction neuralization assays, sera from OPV vaccinees reduced the infectivity of the epidemic strain 40- to 100-fold less than they reduced the infectivities of reference Mahoney and Sabin type 1 strains, whereas sera from patients infected with wild virus reacted with the three viruses equally. Altogether, these data suggest that antibodies elicited by immunization with OPV were not fully reactive with the Albanian wild strain.
The antigenicities of the epidemic strains were investigated in further detail by a neutralization test with a panel of well-characterized type 1 poliovirus-specific MAbs (3) (Table 3). None of the isolates was recognized by the Sabin-specific MAb, MAb 1o (antigenic site IIIb, N-AgIIIB, including residues 58 to 60 of VP3) (14), confirming the nonvaccine origins of the strains. However, two Mahoney-specific MAbs, Io and Id (N-AgIIIB), failed to neutralize all isolates except strains 3767Alb96 and 3788Alb96, both of which reacted with MAb Id. Most of the isolates from Albania and Yugoslavia, but not those from Greece, reacted with the other Mahoney-specific MAbs, MAbs Ia and Ic (Also N-AgIIIB). Among the isolates tested, all except the early epidemic isolate 3767Alb96 showed higher reactivities with MAbs raised against wild type 1 isolated in India and Israel. The analysis of the amino acid sequence in the region of the VP3 capsid protein around amino acids 59 and 60 showed a Mahoney-like amino acid Thr60 for most of the isolates from Albania, Yugoslavia, and Greece. The only exceptions were strains 50968gre96 and 7gre96 from Greece, which showed an Ala59-to-Val substitution in VP3; however, this change apparently did not produce any different neutralization patterns for these strains. A Glu71-to-Arg substitution in N-AgIIIA (11) was shared by all investigated strains except for the early isolate 3767Alb96.
TABLE 3.
Neutralization of polioviruses with MAbs
| Poliovirus strain | NI of the MAbs neutralizing the following type 1
straina:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| K, MAb Ib | Sabin, MAb 1o | Mahoney
|
India, MAb In 37 | Israel
|
||||||
| MAb Io | MAb Ia | MAb Ic | MAb Id | MAb Is 10 | MAb Is 15 | MAb Is 17 | ||||
| Sabin 1 | 3.25 | 5.00 | 0.00 | 1.75 | 1.75 | 1.75 | 1.50 | 0.00 | 0.75 | 0.50 |
| Mahoney | 4.25 | 0.50 | 3.00 | 4.25 | 3.00 | 3.50 | 3.50 | 0.25 | 0.00 | 0.75 |
| 3767Alb96 | 4.25 | 0.00 | 0.50 | 4.00 | 4.00 | 3.75 | 0.50 | 1.00 | 1.25 | 0.75 |
| 3786Alb96 | 5.25 | 0.00 | 0.00 | 2.75 | 1.75 | 1.75 | 2.00 | 3.00 | 4.75 | 2.25 |
| 3788Alb96 | 6.25 | 0.50 | 0.50 | 4.00 | 2.75 | 2.00 | 2.25 | 4.00 | 5.00 | 2.00 |
| 1you96/August | 6.50 | 0.00 | 0.50 | 2.75 | 2.00 | 0.50 | 2.00 | 1.00 | 4.50 | 4.25 |
| 50940gre96/June | 4.00 | 1.00 | 0.00 | 2.00 | 1.00 | 1.00 | 1.00 | 2.00 | 4.00 | 1.00 |
| 50968gre96/July | 6.50 | 0.00 | 1.25 | 0.00 | 0.50 | 0.00 | 0.25 | 4.00 | 6.00 | 0.00 |
| 51268gre96/July | 3.50 | 0.00 | 1.00 | 1.00 | 0.50 | 0.50 | 0.50 | 2.50 | 4.50 | 0.00 |
| 7gre96/August | 2.00 | 0.00 | 0.00 | 0.50 | 2.00 | 0.00 | 0.00 | 2.00 | 2.50 | 0.00 |
| 10gre96/September | 1.50 | 0.00 | 0.00 | 1.50 | 1.00 | 1.00 | 0.50 | 2.00 | 3.00 | 1.00 |
The neutralization index (NI) was calculated as the log virus difference in the presence or absence of a fixed MAb concentration. A positive result is a neutralization index of ≥2.00. MAbs that neutralize both Sabin and Mahoney type 1 viruses were designated K. In, MAb produced against the wild type 1 strain circulating during the 1989 outbreak in India; Is, MAb produced against the wild type 1 strain circulating during the outbreak in Israel in 1988.
Serological investigation.
The presence of neutralizing antibodies to reference poliovirus strains was investigated in the sera of 124 patients involved in the outbreak. Paired serum specimens were collected from only 48 patients, and seroconversion versus type 1 poliovirus was shown by 15 poliomyelitis patients from whom wild type 1 poliovirus was isolated. Seroconversion to type 1 virus supported infection with the epidemic virus in five further patients with paralysis who were negative for virus isolation and in a patient missing at follow-up. High titers of antibody against poliovirus type 1 were present in the sera of all patients confirmed to have polio and patients with an illness compatible with polio. Antibodies to poliovirus types 1, 2, and 3 were exhibited by 97, 93.5, and 86.3% of the 124 AFP patients, respectively; and no significant difference was found for any polio type among subjects confirmed to have polio, subjects with an illness compatible with polio, and subjects without paralysis and negative for wild virus isolation.
The sera from 70 subjects were assayed for IgM antibodies. Only two subjects showed IgM antibody to either poliovirus type 2 or 3, whereas 21 of 44 patients confirmed to have polio and positive for wild virus isolation had IgM antibodies against type 1 poliovirus. IgM antibodies were also found in six patients with persisting paralysis and who were negative for virus isolation. If seroconversion and the presence of IgM to poliovirus type 1 are considered, at least 11 patients acknowledged to have an illness compatible with polio may be included in the group confirmed to have polio and would raise the total number of patients with confirmed polio to 85 or more.
DISCUSSION
A large outbreak of paralytic poliomyelitis caused by wild type 1 poliovirus occurred in Albania from May through November 1996, 15 years after the apparent interruption of indigenous wild poliovirus transmission. The outbreak started soon after the NIDs, and the temporal association between vaccination and the appearance of poliomyelitis cases was initially misleading in defining the etiology of the disease.
Partial genomic sequencing of the VP1-2A region showed 99 to 100% homology among the strains isolated during the epidemics, thus demonstrating the involvement of a unique genotype throughout the outbreak. This genotype showed 95% homology with a wild type 1 poliovirus isolated in Pakistan in 1995. The sequence relationship, however, does not prove conclusively that the virus was imported directly into Albania from Pakistan. The possibility that the virus had been circulating in the country for a long time and that it was sustained by the weakened herd immunity cannot be excluded.
The highest incidence rate was found among young adults, suggesting a major failure in immunization practices before the year 1980. A minor peak in the incidence was, however, also found among infants younger than 6 months of age who were born after the NIDs and before the mass vaccination campaigns began in October 1996. This may be at least partially due to the lack of maternal antibodies. However, since some of these patients were vaccinated only according to the routine schedule, this suggested that during an outbreak routine immunization is less effective than mass vaccination. In fact, the Albanian epidemic was successfully interrupted by two mass vaccination campaigns targeted to persons aged 0 to 50 years. The limited number of cases which occurred in the 1- to 5-year-old age group provides evidence for the effectiveness of NIDs targeted to children up to 5 years of age, although improvements in the cold chain for routine immunization services since 1994 may have played an important role in protecting this age group (6). Children born between 1986 and 1991 also appeared to be protected by routine OPV immunization programs, which have been improved since 1980.
Several factors created the conditions for a nationwide epidemic transmission of imported wild type 1 poliovirus. The accumulation of a large susceptible population, despite the reportedly high vaccination coverage, might have been the result of the lack of adequate cold chain maintenance before 1994, making vaccination with OPV less effective. Moreover, the OPV supplied before 1980 was not stabilized; it was administered once or twice per year, allowing children to miss vaccinations or receive only incomplete vaccination.
The large and abrupt population movement following the opening of borders to foreigners in 1991 favored the importation of wild virus from countries where polio is endemic. Poor sanitation and water supplies probably had a role in the spread of the virus through the population (18). On the other hand, we cannot exclude the possibility that the outbreak may have also been favored by a change in the antigenic structure of the epidemic virus. The peculiar antigenic characteristics of the type 1 poliovirus strains from Albania, Yugoslavia, and Greece are in fact suggested by their patterns of reactivity with MAbs raised against either the Sabin type 1 or the Mahoney type 1 poliovirus strains. Although none of the isolates was neutralized by a Sabin-specific MAb, thus confirming their wild origin, Mahoney-reactive MAbs Io and Id, targeted to amino acids 58 to 60 of VP3, also failed to neutralize most strains. Mahoney-specific MAbs Ia and Ic, also targeted to this antigenic site, showed neutralizing activity against the strains isolated in Albania and Yugoslavia but were far less reactive with strains from Greece. These findings further suggest that high variability can be shown by closely related poliovirus strains at the epitope level (8, 22). Despite the differences in epitopes, however, OPV was able to stop the epidemic.
Neutralizing antibody titers to poliovirus types 2 and 3, in addition to serotype 1, were high overall among Albanian patients with a diagnosis of AFP, which is apparently in contrast to the hypothesis that the poor vaccination history may have been a major factor in the outbreak. However, it is reasonable to assume that both the vaccination of more than 97% of children less than 6 years old just prior to the outbreak and the low sanitation standards in Albania have led a large number of nonimmunized individuals to be in contact with the polio vaccine strains.
Both the timing and the results of virologic analysis indicate that a wild type 1 strain of poliovirus spread from Albania to neighboring countries. All cases that occurred in Yugoslavia and Greece were among unvaccinated or incompletely vaccinated children. In particular, the fact that the five cases that occurred in Greece were among Gypsies who had escaped vaccination stresses the urgency of implementing immunization coverage in high-risk populations to counteract the importation and spread of wild poliovirus strains.
The high standard of the vaccination policy in Italy is the possible reason why no cases were reported in that country, despite the large immigration flow of Albanians during and following the epidemic period.
The Albanian polio outbreak strongly indicates that until global eradication of poliomyelitis is accomplished, supplementary routine immunization and mass vaccination activities that achieve high rates of OPV immunization in all community subpopulations are needed. This appears to be particularly true for the Indian subcontinent countries, where the disease is still endemic and from which many but not all of the poliovirus genotypes responsible for recent epidemics in Europe have originated (17, 19, 20).
In conclusion, the lesson from the 1996 poliomyelitis outbreak in Albania is that the absence of poliomyelitis cases for many years does not preclude the existence of gaps in immunity among the population. High-risk populations in each country should therefore be identified, and special vaccination programs should be provided. Surveillance systems for suspected poliomyelitis cases should also be strengthened in countries where wild viruses have not been isolated for a long time. Timely completion of primary vaccination should also be implemented in areas with high coverage levels.
ACKNOWLEDGMENTS
We thank the epidemiologists of the Public Health Institute, Tirana, Albania, the Centers for Disease Control and Prevention, the Istituto Superior di Sanità and WHO for contributions to the investigation of the outbreak; E. Mezini, V. Gjoni, A. Meculi, and V. Biondi for technical assistance; S. Jankovic, N. Spyrou, and F. Franzidou for providing the poliovirus isolates from Yugoslavia and Greece; E. Medda and M. E. Grandolfo for database management and analysis; and F. M. Ruggeri for critical reading of the manuscript.
This study was partially supported by grants from the Istituto Superiore di Sanità (“Prevention of risk factors of maternal and child health”; art. 12 D.L. 502/92) and WHO (“Characterization of polio and other enteroviruses associated with paralytic disease in Italy, Albania and Malta”; I8/181/211-BH/Jr 1996–1997).
REFERENCES
- 1.Balanant J, Guillot S, Candrea A, Delpeyroux F, Crainic R. The natural genomic variability of poliovirus analyzed by a restriction fragment length polymorphism assay. Virology. 1991;184:645–654. doi: 10.1016/0042-6822(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 2.Bijkerk H. Poliomyelitis in The Netherlands. Dev Biol Stand. 1979;43:195–206. [PubMed] [Google Scholar]
- 3.Crainic R, Couillin P, Blondel B, Cabau N, Boué A, Horodniceanu F. Natural variation of poliovirus neutralization epitopes. Infect Immun. 1983;41:1217–1225. doi: 10.1128/iai.41.3.1217-1225.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamanti E, Ibrahimi B, Dobi V, Dodbiba A. The immunization status against poliomyelitis in Albania, 1986–1990. Bulletin of the University of Tirana, Serial Medical Science. Tirana, Albania: University of Tirana; 1996. [Google Scholar]
- 5.Diamanti, E., B. Ibrahimi, F. Tafaj, E. Mezini, A. Dodbiba, V. Dobi, S. Catone, D. Genovese, P. Simeoni, and L. Fiore. Vaccine, in press. [DOI] [PubMed]
- 6.Evert H. Albania: cold chain report and proposal for improvement. Amsterdam, The Netherlands: Medicin Sans Frontiers; 1992. [Google Scholar]
- 7.Hovi T, Cantell K, Huovilainen A, Kinnunen E, Kuronen T, Lapinleimu K, Pöyry T, Roivanen M, Salama N, Stenvik M, Silander A, Thoden C-J, Salminen S, Weckström P. Outbreak of paralytic poliomyelitis in Finland: widespread circulation of antigenically altered poliovirus type 3 in a vaccinated population. Lancet. 1986;i:1427–1432. doi: 10.1016/s0140-6736(86)91566-7. [DOI] [PubMed] [Google Scholar]
- 8.Hovi T. The outbreak of poliomyelitis in Finland in 1984–1985: significance of antigenic variation of type 3 polioviruses and site specificity of antibody responses in antipolio immunization. Adv Virus Res. 1989;37:243–275. doi: 10.1016/s0065-3527(08)60837-4. [DOI] [PubMed] [Google Scholar]
- 9.Hull H F, Ward N A, Hull B P, Milstien J B, de Quadros C A. Paralytic poliomyelitis: seasoned strategies, disappearing disease. Lancet. 1994;343:1331–1337. doi: 10.1016/s0140-6736(94)92472-4. [DOI] [PubMed] [Google Scholar]
- 10.Kew O M, Mulders M N, Lipskaya G Y, Da Silva E E, Pallansch M A. Molecular epidemiology of poliovirus. Semin Virol. 1995;6:401–414. [Google Scholar]
- 11.Minor P D. Antigenic structure of picornaviruses. Curr Top Microbiol Immunol. 1990;61:122–154. doi: 10.1007/978-3-642-75602-3_5. [DOI] [PubMed] [Google Scholar]
- 12.Minor P D. The molecular biology of poliovaccines. J Gen Virol. 1992;73:3065–3077. doi: 10.1099/0022-1317-73-12-3065. [DOI] [PubMed] [Google Scholar]
- 13.Mulders M N, van Loon A M, van der Avoort H G A M, Riemerink J H J, Ras A, Bestebroer T M, Drebot M A, Kew O M, Koopmans M P G. Molecular characterization of the wild poliovirus type 3 epidemic in The Netherlands (1992 and 1993) J Clin Microbiol. 1995;33:3252–3256. doi: 10.1128/jcm.33.12.3252-3256.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murdin A D, Wimmer E. Construction of a poliovirus type 1/type 2 antigenic hybrid by manipulation of neutralization antigenic site II. J Virol. 1989;63:5251–5257. doi: 10.1128/jvi.63.12.5251-5257.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nibbeling R, Reimerink J H J, Agboatwala M, Naquib T, Ras A, Pelstra P, van der Avoort H G A M, van Loon A M. A poliovirus type-specific IgM antibody-capture enzyme-linked immunosorbent assay for the rapid diagnosis of poliomyelitis. Clin Diagn Virol. 1994;2:113–126. doi: 10.1016/0928-0197(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 16.Oberhofer T R, Brown G C, Monto A S. Seroimmunity to poliomyelitis in an American community. Am J Epidemiol. 1975;101:333–339. doi: 10.1093/oxfordjournals.aje.a112101. [DOI] [PubMed] [Google Scholar]
- 17.Oblapenko, G., and R. W. Sutter. 1997. Status of poliomyelitis eradication in Europe and in Central Asian Republics of former Soviet Union. J. Infect. Dis. 175(Suppl. 1):S576–S581. [DOI] [PubMed]
- 18.Offerhaus L. Albania: a plundered country. Lancet. 1991;337:44–45. doi: 10.1016/0140-6736(91)91879-y. [DOI] [PubMed] [Google Scholar]
- 19.Oostvogel P M, van Wyngarden J K, van der Avoort H G A M, Mulders M N, Conyn van Spaedonck M A E, Rumke H C, van Steins G, van Loon A M. Poliomyelitis outbreak in unvaccinated community in The Netherlands, 1992–93. Lancet. 1994;344:665–670. doi: 10.1016/s0140-6736(94)92091-5. [DOI] [PubMed] [Google Scholar]
- 20.Patriarca, P. A., R. W. Sutter, and P. M. Oostvogel. 1997. Outbreaks of paralytic poliomyelitis, 1976–1995. J. Infect. Dis. 175(Suppl. 1):S165–S172. [DOI] [PubMed]
- 21.Prevots D R, Ciofi degli Atti M L, Sallabanda A, Diamanti E, Aylward R B, Kakariqqi E, Fiore L, Ylly A, van der Avoort H, Sutter R W, Tozzi A, Panei P, Schinaia N, Genovese D, Oblapenko G, Greco D, Wassilak S G F. Outbreak of paralytic poliomyelitis in Albania, 1996: high attack rate among adults and apparent interruption of transmission following nationwide mass vaccination. Clin Infect Dis. 1998;26:419–425. doi: 10.1086/516312. [DOI] [PubMed] [Google Scholar]
- 22.Slater P E, Orenstein W A, Morag A, Avni A, Handsher R, Green M S, Costin C, Yarrow A, Rishpon S, Havkin O, Ben-Zvi T, Kew O M, Rey M, Epstein I, Swartz T A, Melnick J L. Poliomyelitis outbreak in Israel in 1988: a report with two commentaries. Lancet. 1990;335:1192–1195. doi: 10.1016/0140-6736(90)92705-m. [DOI] [PubMed] [Google Scholar]
- 23.Squarcione S, Germinario C, Iandolo E, Lo Caputo S, Bergamini F, Profeta M L, Greco D, Quarto M, Barbuti S. Seroimmunity to poliomyelitis in an Albanian immigrant population. Vaccine. 1992;10:853–856. doi: 10.1016/0264-410x(92)90049-p. [DOI] [PubMed] [Google Scholar]
- 24.Strebel P M, Sutter R W, Cochi S L, Biellik R J, Brink E W, Kew O M, Pallansch M A, Orenstein W A, Hinman A R. Epidemiology of poliomyelitis in the United States one decade after the last reported case of indigenous wild virus-associated disease. Clin Infect Dis. 1992;14:568–579. doi: 10.1093/clinids/14.2.568. [DOI] [PubMed] [Google Scholar]
- 25.Strebel P M, Aubert-Combiescu A, Ion-Nedelcu N, Biberi-Moroeanu S, Combiescu M, Sutter R W, Kew O M, Pallansch M A, Patriarca P A, Cochi S L. Paralytic poliomyelitis in Romania, 1984–1992: evidence for a high risk of vaccine-associated disease and reintroduction of wild-virus infection. Am J Epidemiol. 1994;140:1111–1124. doi: 10.1093/oxfordjournals.aje.a117211. [DOI] [PubMed] [Google Scholar]
- 26.van der Avoort H G A M, Hull B, Hovi T, Pallansch M A, Kew O M, Crainic R, Woods D J, Mulders M N, van Loon A M. A comparative study of five methods for intratypic differentiation of polioviruses. J Clin Microbiol. 1995;33:2562–2566. doi: 10.1128/jcm.33.10.2562-2566.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wassilak S, Oblapenko G, Dittmann S. Progress in Europe towards the goal of poliomyelitis eradication. Eurosurveillance. 1997;2:39–41. doi: 10.2807/esm.02.05.00163-en. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. Resolution of the 41st World Health Assembly (WHO 41.28) 1988. Global eradication of poliomyelitis by the year 2000. Geneva, Switzerland: World Health Organization; 1988. [Google Scholar]
- 29.World Health Organization. Expanded programme on immunization. Manual for the virological investigation of poliomyelitis. WHO publication no. WHO/EPI/CDC/Polio/90.1. Geneva, Switzerland: World Health Organization; 1990. [Google Scholar]
- 30.World Health Organization. Expanded program on immunization. Poliomyelitis outbreak, Bulgaria. Weekly Epidemiol Rec. 1992;67:336–337. [Google Scholar]