Abstract
Background
The northwestern border of Thailand is an area of low seasonal malaria transmission. Until recent successful malaria elimination activities, malaria was a major cause of disease and death. Historically the incidences of symptomatic Plasmodium falciparum and Plasmodium vivax malaria were approximately similar.
Methods
All malaria cases managed in the Shoklo Malaria Research Unit along the Thailand-Myanmar border between 2000 and 2016 were reviewed.
Results
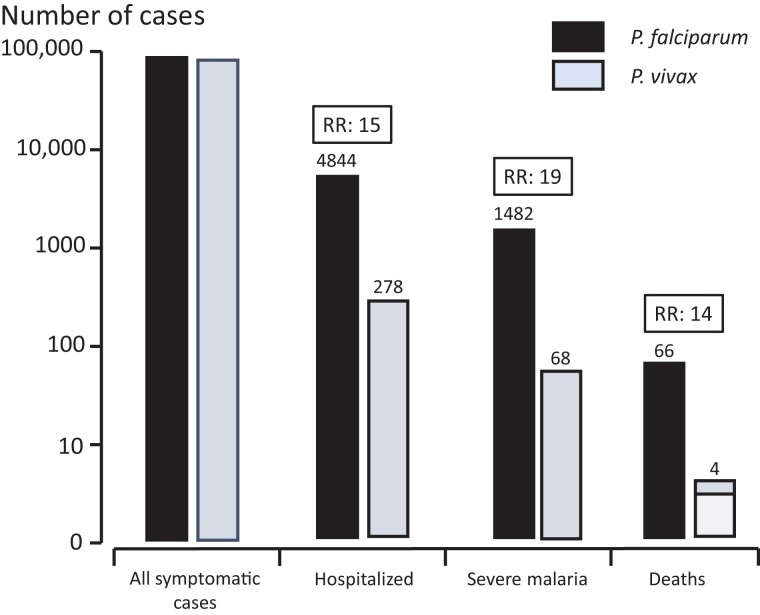
There were 80 841 consultations for symptomatic P. vivax and 94 467 for symptomatic P. falciparum malaria. Overall, 4844 (5.1%) patients with P. falciparum malaria were admitted to field hospitals, of whom 66 died, compared with 278 (0.34%) with P. vivax malaria, of whom 4 died (3 had diagnoses of sepsis, so the contribution of malaria to their fatal outcomes is uncertain). Applying the 2015 World Health Organization severe malaria criteria, 68 of 80 841 P. vivax admissions (0.08%) and 1482 of 94 467 P. falciparum admissions (1.6%) were classified as severe. Overall, patients with P. falciparum malaria were 15 (95% confidence interval, 13.2–16.8) times more likely than those with P. vivax malaria to require hospital admission, 19 (14.6–23.8) times more likely to develop severe malaria, and ≥14 (5.1–38.7) times more likely to die.
Conclusions
In this area, both P. falciparum and P. vivax infections were important causes of hospitalization, but life-threatening P. vivax illness was rare.
Keywords: Plasmodium vivax, Plasmodium falciparum, severe malaria, epidemiology
Among 94 467 patients with Plasmodium falciparum and 80 841 with Plasmodium vivax malaria, patients with P. falciparum were 15 times more likely to require hospital admission, 19 times more likely to develop severe malaria, and 14 times more likely to die.
Early in the 20th century, Plasmodium vivax was known as “benign tertian malaria” to differentiate it from the commonly fatal “malignant tertian” malaria caused by Plasmodium falciparum [1]. In the 1920s, early in the era of malaria therapy for neurosyphilis [2, 3], the lethal potential of P. falciparum was soon evident, so artificial infection with the safer P. vivax malaria became the therapy of choice. Even so, malaria therapy showed that patients who were already severely debilitated by neurosyphilis could die as a result of any of the human malaria infections—even Plasmodium malariae [4]. It was also recognized that P. vivax generally caused fever at lower parasite densities than P. falciparum [5].
The early descriptions of malaria from military and malaria therapy experiences, which created the “textbook” descriptions, referred usually to previously unexposed (ie non-immune) adults [6, 7]. They are more relevant today to malaria in travelers and less so to malaria in endemic areas where the population is exposed repeatedly to malaria infections. In low-transmission areas, malaria occurs at all ages, whereas in higher-transmission settings, with the acquisition of disease controlling immunity, malaria illness is largely confined to younger children. It is estimated that >90% of the deaths from severe malaria in the world are in African children. Nearly all are attributable to P. falciparum [8]. In higher-transmission settings, where asymptomatic patent malaria parasitemia is very common, it is difficult to distinguish malaria as the cause of illness from other illnesses with coincidental malaria parasitemia [9–11]. Even in low-transmission areas, a significant proportion of the community has asymptomatic parasitemia [12]. The mortality rate directly attributable to malaria is consequently overestimated—often by a substantial amount [13].
In recent years, as the global burden of malaria and the geographic extent of malaria-endemic areas have decreased, the number of reports of severe vivax malaria has increased markedly. It is unclear whether this represents a genuine rise, increased recognition, a lower threshold for the diagnosis, incorrect attribution, or selective reporting. The prevalence of severe P. vivax infections has varied markedly both across and within geographic regions [14–20]. Clinical manifestations reported in severe P. vivax malaria are similar to those reported in severe P. falciparum malaria [14]. They include pulmonary edema, severe anemia, shock, hypoglycemia, hepatic or renal dysfunction, neurologic dysfunction (cerebral malaria or multiple convulsions), and severe thrombocytopenia [15]. Extremes of age, comorbid conditions, and chloroquine resistance have been associated with an increased risk of severe vivax malaria [17–20]. As in the earlier malaria therapy experience, already debilitated patients are at greatest risk [3, 4].
One meta-analysis estimated that 1.1% of persons with symptomatic P. vivax infections had severe malaria (11 658 of 10 590 970 total cases). The case fatality was reported as 5% in patients with ≥1 severe manifestation [14]. Very few reports have come from the countries of the Greater Mekong subregion, where severe P. vivax malaria is considered rare [16]. These marked geographic differences, the lack of detailed prospective clinical studies, and the difficulty in establishing causality leave substantial uncertainty over the true incidence, prevalence, and outcomes of severe vivax malaria. Our retrospective review of all patients admitted to clinics and hospitals at the Shoklo Malaria Research Unit (SMRU) on the northwestern border of Thailand over a 16-year period was performed to provide a comparative assessment of prevalence and severity of illness caused by these 2 main malaria species.
METHODS
Study Population
This observational study was conducted by SMRU, which has operated malaria clinics and inpatient facilities along the northwestern Thailand-Myanmar border since 1986. The epidemiology of malaria in this region of hill forest and low seasonal malaria transmission has been studied in detail and reported elsewhere [21, 22]. The patient population comprised migrant workers and displaced persons of all ages of Burman and Karen ethnicities. Until 2012, patient numbers in the transmission season were very high, with nearly 200 consultations each day at one health clinic (50% with confirmed malaria). Before 2010, malaria was diagnosed based on either a P. falciparum specific rapid diagnostic test or a malaria smear, whereby parasite counts were provided for P. falciparum, and P. vivax infection was noted but not quantitated. After 2010, P. vivax parasite densities were quantitated. All patients with >4% P. falciparum parasitemia (hyperparasitemia) were admitted. Otherwise, patients were hospitalized at the physician’s discretion. All had a malaria smear performed. Pulse oximetry was not available before 2009, and was not performed routinely until 2015.
Three days of oral chloroquine (25 mg base/kg) was given for P. vivax malaria, and 3 days of oral artemisinin combination therapy (eg, mefloquine-artesunate, artemether-lumefantrine, or dihydroartemisinin-piperaquine) was given for P. falciparum malaria. As second-line treatment in P. falciparum malaria, or for treatment failure, 7-days of quinine or artesunate combined with doxycycline or clindamycin was given [23]. Primaquine radical cure for P. vivax was not prescribed routinely during the study period. Patients with >4% parasitemia were given artesunate (oral or intravenous, depending on the patient’s clinical condition), and completed 7 days of treatment with artemisinin combination therapy [23]. Other medical management included anticonvulsants to treat seizures, blood transfusions, oral or intravenous antibiotics, and intravenous fluids. Positive pressure ventilation and renal replacement therapies were not available. Full blood cell counts and biochemical and microbiology investigations were not available routinely before 2010.
Study Methods
All patients attending SMRU outpatient clinics had an electronic data entry. Anonymized data from October 2000 to December 2016 provided the total number of outpatient consultations with a malaria diagnosis. For the same period, records of patients admitted to the SMRU hospitals with P. falciparum or P. vivax malaria were obtained from the inpatient electronic database [24]. To account for hospitalizations for post-delivery complications unrelated to malaria, post-partum women were defined as those from 1 calendar day up to <6 weeks from the date of delivery. Dates, age, sex, weight, medical history, presenting symptoms and their duration, vital signs, clinical examination, results of diagnostic tests performed, treatment, and discharge diagnoses were extracted.
Severe malaria diagnoses were based on the current broad World Health Organization (WHO) classification [25] and also analyzed using the stricter research definition, which excludes prostration or convulsions as severe malaria criteria [26] (Supplementary Table 1). Severe anemia was defined as in the WHO classification: hemoglobin ≤5 g/dL or hematocrit ≤15% in children <12 years old (<7 g/dL and <20%, respectively, in adults). Chest radiography was not available on site. Pulmonary edema was diagnosed clinically if pulse oximetry was <92% on room air or—if oximetry was unavailable—if the respiratory rate was elevated for age and chest examination findings were abnormal. If the patient was visibly jaundiced, the serum total bilirubin was assumed to be >50 µmol/L (>3 mg/dL). If a discharge diagnosis of renal failure was recorded, the patient hospital record was reviewed to determine the basis for the diagnosis. Parasite density thresholds for P. vivax were not used in the severity definitions [26].
Statistical Analysis
Comparisons were made using χ2 or Fisher exact or Student t or nonparametric K-sample tests, as appropriate. Multivariable generalized linear modeling was used to assess the effects of age, sex, pregnancy and postpartum status, malaria species, and the presence of concomitant disease on whether the WHO severe malaria criteria were met [25, 26] for P. vivax compared with P. falciparum. Statistical analysis was performed using Stata 15.1 software (StataCorp).
Ethical Review
Ethical approval was given by the Ethics Committee at the Faculty of Tropical Medicine, Mahidol University (TMEC 17–049), the Oxford Tropical Research Ethics Committee (OXTREC 28-09), and the Tak Community Advisory Board (20170729/TCAB-11).
RESULTS
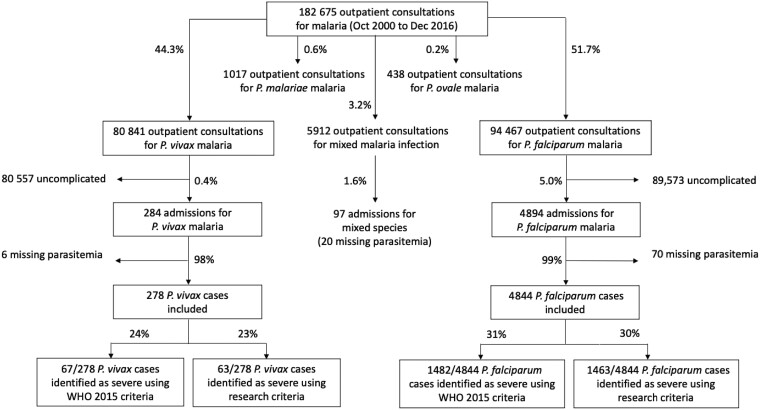
Between October 2000 and December 2016, there were 80 841 consultations for P. vivax malaria, 94 467 for P. falciparum, 1017 for P. malariae, and 438 for Plasmodium ovale. The vast majority of patients (80 557 [99.6%] with P. vivax and 89 573 [95%] with P. falciparum) had uncomplicated illness. Mixed-species infections were documented in 5912 (3.2%) of the 182 675 symptomatic malaria infections. A total of 175 308 cases were included in this study; 278 patients (0.34%) were hospitalized with a diagnosis of severe P. vivax malaria, and 4844 (5.0%) were hospitalized for severe P. falciparum malaria (Figure 1). Thus, symptomatic P. falciparum infections were 15 (95% confidence interval [CI], 13.2–16.8) times more likely than P. vivax infections to require hospital admission (P < .001) (Figure 2). Parasite densities in patients hospitalized with P. vivax infections ranged from 16/µL to 189 028/µL (geometric mean, 2284/µL), and in those with P. falciparum infection they ranged from 16/µL to 2 409 007/µL (geometric mean,161 231/µL) (Table 1).
Figure 1.
Flow diagram of malaria cases included. Excluded cases are not enclosed in an outlined box. Abbreviations: P. falciparum, P. malariae, P. ovale, and P. vivax, Plasmodium falciparum, malariae, ovale, and vivax; WHO, World Health Organization.
Figure 2.
The relative risks (RRs) of hospitalization, severe malaria, and death for Plasmodium falciparum compared with Plasmodium vivax. In 3 of the 4 fatalities with P. vivax malaria (white box) the primary diagnosis was sepsis, and the causal role of malaria was uncertain.
Table 1.
Characteristics of Patients Admitted to Shoklo Malaria Research Unit Hospitals Between 2000 and 2016 With Plasmodium vivax or Plasmodium falciparum Malaria
| Characteristic | Values by Patient Age Group, Median (Range)a | ||||||
|---|---|---|---|---|---|---|---|
| 0–28 d | 29 d to <1 y | 1–5 y | 6–10 y | 11–15 y | >15 y | Total | |
| Plasmodium vivax | |||||||
| All records, no. (%)b | 11 (4) | 45 (16) | 64 (23) | 20 (7) | 10 (4) | 128 (46) | 278 (100) |
| Male sex, no. (%)c | b5 (45) | 24 (53) | 33 (52) | 11 (55) | 6 (60) | 26 (20) | 105 (38) |
| Weight, kg | 3.4 (1.5 to 4.4) [1] | 6.5 (2.6–9.7) | 10.0 (6.3–18) [1] | 16.5 (11–28) | 32.5 (25–49) | 49 (30–71) [1] | 26 (1.5–71) [3] |
| Temperature, °C | 38.5 (36.5–39.8) | 37.8 (36.3–40.0) | 38.8 (36.5–40.7) | 38.0 (36.0–40.3) | 38.2 (37.4–40.1) | 38.0 (35.9–41.2) | 38.2 (35.9–41.2) |
| Pulse rate, beats/min | 148 (90–180) | 140 (90–188) | 131 (98–199) | 126 (84–160) | 98 (72–120) | 96 (66–148) | 119 (66–199) |
| Respirations/min | 48 (30–80) | 46 (20–76) | 39 (20–90) | 32 (22–48) | 26 (20–38) | 28 (14–52) | 32 (14–90) |
| Hematocrit, % | 36 (29–47) [2] | 27 (12–50) [2] | 30 (9–44) [2] | 29 (11–40) [4] | 33 (12–43) [2] | 33 (3–56) [8] | 32 (3–56) [20] |
| Met severe anemia definition, no. (%)c,d | 0 | 2 (4) | 2 (3) | 2 (10) | 1 (10)d | 11 (9)d | 18 (6) |
| Parasitemia, geometric mean (range), parasites/µL | 9871 (256–189 028) | 1738 (16–125 600) | 2719 (16–143 184) | 1350 (48–58 027) | 1713 (32–35 168) | 2257 (16–105 504) | 2284 (16–189 028) |
| Plasmodium falciparum | |||||||
| All records, no. (%)b | 2 (0.04) | 88 (2) | 1156 (24) | 888 (18) | 613 (13) | 2097 (43) | 4844 (100) |
| Male sex, no. (%)c | 1 (50) | 43 (49) | 615 (53) | 520 (59) | 404 (66) | 1447 (69) | 3030 (63) |
| Weight, kg | 2.5 (2.0–2.9) | 7.0 (2.6–11) | 12 (5.0–25) [1] | 19 (8–50) | 33 (16–58) [1] | 50 (20–84) [6] | 31 (2–84) [8] |
| Temperature, °C | 36.9 (35.8–38.0) | 37.8 (34.8–40.3) | 38.1 (35.5–40.9) | 38.2 (35.5–41.5) | 38.1 (35.0–41.5) | 38.0 (34.7–41.6) [3] | 38.0 (34.7–41.6) [3] |
| Heart rate, beats/min | 139 (120–158) | 140 (100–190) | 132 (60–200) | 120 (64–200) | 110 (64–160) [1] | 100 (60–160) [35] | 114 (60–200) [36] |
| Respirations/min | 39 (34–44) | 44 (26–90) | 36 (14–80) | 30 (14–60) | 28 (18–52) [1] | 26 (10–62) [35] | 28 (10–90) [36] |
| Hematocrit, % | 40 (26–54) | 26 (9–45) | 29 (6–61) [9] | 33 (9–50) [5] | 36 (8–54) [1] | 37 (5–77) [23] | 34 (5–77) [38] |
| Met severe anemia definition, no. (%)c,d | 0 | 12 (14) | 64 (6) | 18 (2) | 9 (1)d | 64 (3)d | 167 (3) |
| Parasitemia, geometric mean (range), parasites/µL | 9655 (128–728 229) | 124 644 (160–1 311 264) | 145 311 (16–1 962 375) | 208 660 (16–2 409 007) | 199 228 (32–2 101 537) | 145 580 (16–1 512 850) | 161 231 (16–2 409 007) |
Data represent median (range) unless otherwise indicated. Numbers in brackets indicate number of missing values.
Percentage of total records.
Percentage of age group.
The age threshold used is 12 years. Severe anemia is defined as hemoglobin ≤5 g/dL or hematocrit ≤15% in children <12 years old (<7 g/dL and <20%, respectively, in adults).
More than half of the patients hospitalized with malaria were children ≤15 years of age (54% for P. vivax and 57% for P. falciparum; P = .4). Significantly more infants and young children ≤5 years of age were admitted with P. vivax malaria (43%) than with P. falciparum malaria (26%) (relative risk [RR], 1.7 [95% CI, 1.5–1.9]; P < .001) (Table 1).
In adults (aged >15 years) hospitalized for suspected severe malaria, more women were admitted with P. vivax (102 of 128 [80%]) than with P. falciparum malaria (650 of 2097 [31%]) (RR, 2.5 [95% CI, 2.3–2.9]; P < .001) (Table 1). Pregnant and post-partum women comprised more than half of the women admitted for P. vivax (58 of 102 [57%]), and significantly less (123 of 650 [19%]) for P. falciparum. Many of the post-partum P. vivax patients admitted had reasons for admission that were unrelated to malaria. In hospitalized P. vivax malaria cases, the presenting mean hematocrit was 4.2% lower in pregnant women (95% CI, 1.6%–6.9%; P = .002) and 7.3% lower in post-partum women (95% CI 3.0–11.5; P = .001) than for nonpregnant or non post-partum patients. Chronic disease (including malnutrition, hypertension, cirrhosis, and alcohol dependence) was much more common in patients hospitalized with P. vivax than in those hospitalized with P. falciparum (RR, 6.8 [95% CI, 2.9–16.1]; P < .001) (Table 2). Patients with P. vivax malaria were hospitalized for a shorter period (median [interquartile range], 3 [2–4] days) than those with P. falciparum (4 [4–6] days); P < .001) (Table 2).
Table 2.
Characteristics of Hospitalized Malaria Patients Who Did or Did Not Meet World Health Organization (2015) Broad Severe Malaria Criteria
| Plasmodium vivax | Plasmodium falciparum | |||||
|---|---|---|---|---|---|---|
| Clinical Characteristic | Patients, No. (%)a | P Value | Patients, No. (%)a | P Value | ||
| WHO Criteria Not Met (n = 210) |
WHO Criteria Met (n = 68) |
WHO Criteria Not Met (n = 3362) |
WHO Criteria Met (n = 1482) |
|||
| Infectious diagnosis present | 50 (24) | 20 (29) | .35 | 137 (4) | 110 (7) | <.001 |
| Chronic disease present | 4 (2) | 3 (4) | .25 | 8 (0.2) | 10 (0.7) | .02 |
| Post-partum womenb | 10/127 (8) | 7/46 (15) | .16 | 5/1215 (0.4) | 3/599 (0.5) | .72 |
| Prostration | 25 (12) | 16 (24) | .02 | 355 (11) | 469 (32) | <.001 |
| Convulsionsc | 0 (0) | 4 (6) | .003 | 0 (0) | 33 (2) | <.001 |
| Age, y | ||||||
| <1 | 48 (23) | 8 (12) | .10d | 55 (2) | 35 (2) | <.001d |
| 1–5 | 48 (23) | 16 (24) | 709 (21) | 447 (30) | ||
| 6–10 | 14 (7) | 6 (9) | 616 (18) | 272 (18) | ||
| 11–15 | 8 (4) | 2 (3) | 473 (14) | 140 (9) | ||
| >15 | 92 (44) | 35 (53) | 1509 (45) | 588 (40) | ||
| Parasitemia, geometric mean (95% CI), parasites/µL | 2576 (32–46 472) | 1576 (16–31 149) | .12 | 147 606 (3768–425 030) | 196 986 (2304–976 162) | <.001 |
| Duration of hospital stay, median (IQR; range) d | 3 (2–4; 1–32) | 2 (2–4; 1–26) | .90 | 4 (3–6; 1–34) | 5 (4–6; 1–31) | <.001 |
| Intravenous artesunate | 31 (15) | 13 (19) | .39 | 655 (19) | 677 (46) | <.001 |
| Blood transfusion | 26 (12) | 19 (28) | .002 | 323 (10) | 317 (21) | <.001 |
Abbreviations: CI, confidence interval; IQR, interquartile range; WHO, World Health Organization.
Data represent no. (%) of patients unless otherwise indicated.
The denominators for post-partum women represent the number of female patients aged >15 years.
The WHO 2015 malaria guidelines [25] criterion for convulsions is >2 convulsions in 24 hours. It does not specify that convulsions must be accompanied by a low Glasgow Coma Scale score.
Univariable ordered logistic regression analysis was used to determine the relationship between age groups and whether or not WHO criteria were met.
Deaths
There were 70 deaths in total, 66 with P. falciparum and 4 with P. vivax infection. One P. vivax malaria death occurred in a 54-year old man with Gram-negative meningitis; Escherichia coli grew in an immediate post-mortem cerebrospinal fluid culture. The parasite density was 1 in 500 white blood cells (16/µL). This suggests that the parasitemia was incidental to the fatal bacterial meningitis. Two other P. vivax deaths occurred in patients with low parasite densities (<3300/µL), leukocytosis, and a clinical diagnosis of sepsis, although blood cultures were negative. Both patients were anemic and required blood transfusions. The fourth P. vivax malaria death occurred in a malnourished woman (HIV and tuberculosis negative) in the third trimester of pregnancy [27]. She experienced acute respiratory distress 67 hours after starting treatment with artesunate-mefloquine [28]. The overall mortality rate in patients hospitalized for malaria was 4 of 278 (1.4%) for P. vivax and 66 of 4844 (1.4%) for P. falciparum malaria, although only 1 of the P. vivax deaths was clearly related to malaria. Analyzed as a proportion of total outpatient confirmed malaria cases, the risk of death associated with symptomatic P. falciparum malaria was therefore ≥14 times greater (95% CI, 5.1–38.7) than for P. vivax malaria (Figure 2).
Severe Malaria Admissions
Fewer hospitalized patients with P. vivax (68 of 278 [24%]) than with P. falciparum (1482 of 4844 [31%]) met the WHO criteria for severe malaria (Table 2 and Supplementary Table 1). Post-partum and pregnant women comprised 55% (6 of 11) of the severe anemia cases with P. vivax malaria and 16% (10 of 64) of those with P. falciparum malaria (RR, 5.7 [95% CI, 2.0–16.7]; P = .001) (Table 3). Impaired consciousness was present in 13% (9 of 68) of the P. vivax cases. Pulmonary edema was a more common manifestation of severity in P. vivax (27 of 68 [40%]) than in P. falciparum malaria (167 of 1482 [11%]) (RR, 3.2 [95% CI, 2.3–4.5]; P < .001). Severe anemia was also more common among the patients with severe vivax malaria (18 of 68 [26%]) than among those with severe falciparum malaria (167 of 1482 [11%]) (RR, 1.8 [95% CI, 1.1–2.7]; P = .01) (Table 3). The proportions of patients with prostration or convulsions [25] were similar between P. vivax and P. falciparum malaria. (Supplementary Table 1).
Table 3.
Hospitalized Malaria Cases Classified as Severe by the World Health Organization (2015) Broad Severe Malaria Criteria
| Clinical Characteristica | Patients, No. (%) | |||||
|---|---|---|---|---|---|---|
| Plasmodium vivax | Plasmodium falciparum | |||||
| Age ≤15 y (n = 32) | Age >15 y (n = 36) | Total (n = 68) | Age ≤15 y (n = 894) | Age >15 y (n = 588) | Total (n = 1482) | |
| Impaired consciousness | 7 (22) | 2 (6) | 9 (13) | 66 (7) | 117 (20) | 183 (12) |
| Prostration | 7 (22) | 9 (26) | 16 (24) | 220 (25) | 249 (42) | 469 (32) |
| Convulsions | 1 (3) | 3 (8) | 4 (6) | 23 (3) | 10 (2) | 33 (2) |
| Shock | 0 | 4 (11) | 4 (11) | 6 (4) | 28 (5) | 34 (5) |
| Jaundiceb | 0 | 0 | 0 | 29 (3) | 82 (15) | 111 (8) |
| Significant bleeding | 0 | 0 | 0 | 0 | 0 | 0 |
| Pulmonary edema | 14 (44) | 13 (36) | 27 (40) | 123 (14) | 44 (7) | 167 (11) |
| Hypoglycemia | 2 (15) | 2 (11) | 4 (13) | 41 (6) | 15 (4) | 56 (5) |
| Metabolic acidosisc | NA | NA | NA | NA | NA | NA |
| Severe anemiab,d | 7 (22) | 11 (31) | 18 (26) | 103 (12) | 64 (11) | 167 (11) |
| Renal impairmentc | 0 | 0 | 0 | 1 (0.1) | 7 (1) | 8 (0.5) |
| Hyperparasitemia | NA | NA | NA | 607 (68) | 277 (47) | 884 (60) |
Abbreviation: NA, not available.
Nineteen cases with P. vivax and 601 with P. falciparum met ≥2 World Health Organization severity criteria [25].
For P. vivax, parasite density thresholds were not used in the severity definitions of jaundice or anemia.
Laboratory testing was not available. For renal impairment, the discharge diagnosis was used as a proxy.
Severe anemia is defined as hemoglobin ≤5 g/dL or hematocrit ≤15% in children <12 years old (<7 g/dL and <20%, respectively, in adults).
Applying the stricter research criteria for severe malaria [26], the proportions of P. vivax (63 vs 67 of 278) and P. falciparum cases (1463 vs 1482 of 4844) classified as severe malaria were largely unchanged (Supplementary Tables 2 and 3). Based on the broad WHO criteria [25], the overall proportion of severe malaria was 0.84/1000 cases (95% CI, 0.7–1.1) P. vivax cases (68 of 80 841) compared with 15.7/1000 cases (14.9–16.5) P. falciparum cases (1482 of 94 467). Thus, for outpatients presenting with malaria, the risk of severe malaria was 19 (95% CI, 14.7–23.8) times greater in P. falciparum than in P. vivax infections (P < .001).
DISCUSSION
Although P. vivax malaria was very common along the Thailand-Myanmar border, severe vivax malaria was rare, as it is elsewhere in the Greater Mekong subregion. Nearly all malaria deaths in this region result from P. falciparum. The relatively low mortality rate of severe falciparum malaria in this series (4.4%) is explained by the high proportion of cases with prostration or otherwise uncomplicated hyperparasitemia, both of which carry a relatively good prognosis, and prompt treatment with artesunate [26, 29]. Our findings contrast with observations from India and the island of New Guinea, the 2 areas reporting high caseloads of severe P. vivax malaria [30–34]. In India, the transmission of P. vivax and P. falciparum is generally low and unstable, as it is in the Greater Mekong subregion [21, 22].
Symptomatic malaria occurs at all ages, and severe P. vivax malaria has been reported extensively [31–34]. A 2021 systematic review of 162 studies from India reported that 29.3% of patients hospitalized with P. vivax infections had severe malaria [31]. This is similar to the proportion for P. falciparum malaria in our study and considerably exceeds the proportion observed for P. vivax. The case-specific mortality rate of acute vivax malaria on the Thailand-Myanmar border is >100 times lower than reported from Bikaner in Northwest India, 6 times lower than reported from Manaus in Brazil [34], and half of that reported from Papua in Indonesia [35] (Table 4). It is unclear whether there are genuinely more severe P. vivax cases in India, suggesting greater P. vivax virulence there, or unusual susceptibility, or whether there is an ascertainment bias in the diagnoses. In contrast, the island of New Guinea has markedly higher P. vivax transmission than in other malaria-endemic areas of the world. Young children are affected particularly, and frequent P. falciparum and multiple relapsing P. vivax infections result in severe anemia and an increased risk of death [30].
Table 4.
Comparison of Reported Severe Vivax Malaria Cases From 4 Locations
| Plasmodium vivax Malaria Outcome | Mae Sot, Thailand (Current Report) | Manaus, Brazil [34]a | Bikaner, India [32]a | Papua, Indonesia [35] |
|---|---|---|---|---|
| Total consultations, no. | 80 841 | 10 283 | 843 | 293 763 |
| Hospitalizations, no. (%) | 278 (0.34) | 316 (3) | 462 (55) | 3495 (1) |
| WHO severity criteria fulfilled, no. (% of hospitalizations) | 67 (24) | 40 (12.6) | 157 (34) | 845 (24) |
| Mortality rate, deaths/1000 cases | 0.05 | 0.3 | 6.1 | 0.12 |
Abbreviation: WHO, World Health Organization.
The Manaus and Bikaner consultations were at tertiary reference hospitals.
Although some patients with acute P. vivax malaria did require hospitalization in our series, the majority (75%) did not have severe malaria, and their prognosis was very good. Pregnancy and the postpartum period were particular risk factors for P. vivax–associated severe anemia hospitalizations. This reflects both the cumulative impact of recurrent P. vivax malaria and the higher risk of anemia in this population generally [36]. Severe anemia (associated with both species of malaria) and otherwise uncomplicated hyperparasitemia (in P. falciparum malaria) both have a relatively good prognosis, provided that there is ready access to diagnosis and treatment (with artesunate) and that blood transfusions can be given [26, 29, 36]. Even with acute pulmonary edema in P. vivax malaria (which carries a high mortality rate in P. falciparum malaria), all but 1 patient survived [26, 27, 29, 37].
Several factors contribute to the “severe P. vivax malaria” reporting differences between malaria regions. These relate to the criteria used, definitions applied, and use of proxy indicators to determine severity. For example, severe thrombocytopenia is often used as a severity criterion and accounts for a large proportion of reported “severe vivax” cases [15, 31]. This is not a severity criterion for falciparum malaria [26, 29]. Many severe P. vivax case patients have severe anemia (approximately 20% in a meta-analysis [15] and nearly 30% in the current series). The anemia criterion for severe falciparum malaria requires concomitant parasitemia (parasite density, 10 000/µL) to improve specificity [26], but in many reported severe vivax cases, the associated parasite count is not specified. The jaundice criterion for severe falciparum malaria requires a concomitant parasite density of 100 000/µL, which is very unusual in P. vivax malaria, whereas jaundice with any parasite density is defined as severe P. vivax malaria [25, 26]. This reduces diagnostic specificity, as some patients with uncomplicated malaria may develop transient cholestatic jaundice.
Other possible contributors to the diagnosis of severe P. vivax malaria are preexisting conditions (such as often undiagnosed or unconfirmed chronic diseases or coexisting infections [38]), which may cause or predispose to vital organ dysfunction and for which the malaria parasitemia is coincidental rather than a cause of severe illness or death. The patient with fatal Gram-negative meningitis in this series is a probable example of coincidental P. vivax infection. Within malaria-endemic areas, misdiagnosis of severe malaria is very common. It is estimated that approximately one-third of African children with diagnosed severe P. falciparum malaria, even in specialist research centers, are misdiagnosed [13]. Other factors, such as being very young or old, pregnant, or post-partum, also increase the likelihood of hospitalization. In older and frail adults, and in those debilitated by chronic diseases, acute malaria illness (caused by any species) can prove fatal.
Antimalarial resistance increases P. vivax recurrence rates, especially when anti-relapse treatment is not given, and it thereby increases the prevalence and severity of anemia. Chloroquine resistance may be a contributor to reported severe P. vivax malaria in India, although the levels of resistance reported to date in South Asia and the Greater Mekong subregion are low [39].
This study had several limitations. This was a retrospective evaluation, so procedures varied in time and place, and missing inpatient records were not included and outcomes were sometimes not known for patients referred to tertiary hospitals. Full blood cell counts were routinely available only in the latter half of the study period. Results of biochemical investigations were not available. Pulmonary edema could not be confirmed with chest radiography, and pulse oximetry was not routinely available. Investigations to exclude alternative diagnoses limited our ability to differentiate causation between severe P. vivax and other common infections.
In conclusion, in northwest Thailand along the Myanmar border, P. vivax malaria is common, but severe infections and death resulting from P. vivax are rare. Further investigation is needed to determine why there are differences in the apparent proportions of severe P. vivax malaria across different geographic regions, transmission settings, and relapse intervals.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Cindy S Chu, Shoklo Malaria Research Unit, Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Mae Sot, Thailand; Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom.
Marie Stolbrink, Shoklo Malaria Research Unit, Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Mae Sot, Thailand.
Daniel Stolady, Shoklo Malaria Research Unit, Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Mae Sot, Thailand.
Makoto Saito, Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom; Division of Infectious Diseases, Advanced Clinical Research Center, Institute of Medical Science, University of Tokyo, Tokyo, Japan.
Candy Beau, Shoklo Malaria Research Unit, Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Mae Sot, Thailand.
Kan Choun, Shoklo Malaria Research Unit, Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Mae Sot, Thailand.
Tha Gay Wah, Shoklo Malaria Research Unit, Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Mae Sot, Thailand.
Ne Mu, Shoklo Malaria Research Unit, Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Mae Sot, Thailand.
Klay Htoo, Shoklo Malaria Research Unit, Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Mae Sot, Thailand.
Be Nu, Shoklo Malaria Research Unit, Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Mae Sot, Thailand.
Arunrot Keereevijit, Shoklo Malaria Research Unit, Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Mae Sot, Thailand.
Jacher Wiladpaingern, Shoklo Malaria Research Unit, Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Mae Sot, Thailand.
Verena Carrara, Shoklo Malaria Research Unit, Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Mae Sot, Thailand; Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom; Faculty of Medicine, Institute of Global Health, University of Geneva, Geneva, Switzerland.
Aung Pyae Phyo, Shoklo Malaria Research Unit, Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Mae Sot, Thailand.
Khin Maung Lwin, Shoklo Malaria Research Unit, Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Mae Sot, Thailand.
Christine Luxemburger, Shoklo Malaria Research Unit, Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Mae Sot, Thailand.
Stephane Proux, Shoklo Malaria Research Unit, Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Mae Sot, Thailand.
Prakaykaew Charunwatthana, Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand; Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand.
Rose McGready, Shoklo Malaria Research Unit, Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Mae Sot, Thailand; Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom.
Nicholas J White, Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom; Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand.
François Nosten, Shoklo Malaria Research Unit, Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Mae Sot, Thailand; Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom.
Notes
Acknowledgments. The results from this study reflect more than 30 years of humanitarian field work performed by the Shoklo Malaria Research Unit (SMRU) healthcare providers, medical staff, and the laboratory, data, logistic, and administrative teams. These efforts contribute directly to the SMRU academic outputs. Many thanks to all who supported malaria diagnosis, treatment, and management from 2000 to 2016. The authors especially thank the patients for placing their trust in SMRU.
Financial support. This work is supported by the Wellcome Trust (grant 089179 to C. S. C., R. M., N. J. W., and F. N.). For the purpose of open access, the authors have applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission.
References
- 1. Craig CF. The classification of the malarial plasmodia. Boston Med Surg J 1909; 160:677–9. [Google Scholar]
- 2. Wagner-Jauregg J. Über die einwirkung der malaria auf die progressive paralyse. Psychiatr Neurol Wochenschr 1918; 21/22:132–4. [Google Scholar]
- 3. Snounou G, Pérignon JL. Malariotherapy—insanity at the service of malariology. Advances Parasitol 2013; 18:223–55. [DOI] [PubMed] [Google Scholar]
- 4. Swellengrebel NH, De Buck A. Malaria in the Netherlands. Amsterdam,the Netherlands: Scheltema & Holkema, 1938. [Google Scholar]
- 5. Kitchen SF. Symptomatology: general considerations. In: Boyd MF, ed. Malariology. Philadelphia, PA and London: W. B. Saunders, 1949:966–94. [Google Scholar]
- 6. Mowrey FH. Statistics of malaria. In: Internal medicine in World War II. Washington, DC: Office of the Surgeon General, Department of the Army, 1963:449–63. [Google Scholar]
- 7. Manson-Bahr P. War malaria and its treatment. Br Med J 1944; 2:350–1. [Google Scholar]
- 8. World Health Organization . World malaria report 2022. Geneva,Switzerland: World Health Organization, 2022. Available at: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022. Accessed 13 March 2023. [Google Scholar]
- 9. Craig CF. A study of latent and recurrent malarial infection and the significance of intracorpuscular conjugation in the malarial plasmodia. J Infect Dis 1907; 4:108–40. [Google Scholar]
- 10. Bookless AS, Naftalin JM. Typhoid fever complicated by benign tertian malaria. Br Med J 1945; 2:804–5. [PubMed] [Google Scholar]
- 11. Watson JA, Uyoga S, Wanjiku P, et al. Improving the diagnosis of severe malaria in African children using platelet counts and plasma PfHRP2 concentrations. Sci Transl Med 2022; 14:eabn5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Imwong M, Stepniewska K, Tripura R, et al. Numerical distributions of parasite densities during asymptomatic malaria. J Infect Dis 2016; 213:1322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. White NJ, Watson JA, Uyoga S, Williams TN, Maitland KM. Substantial misdiagnosis of severe malaria in African children. Lancet 2022; 400:807. [DOI] [PubMed] [Google Scholar]
- 14. Naing C, Whittaker MA, Nyunt Wai V, Mak JW. Is Plasmodium vivax malaria a severe malaria? A systematic review and meta-analysis. PLoS Negl Trop Dis 2014; 8:e3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rahimi BA, Thakkinstian A, White NJ, Sirivichayakul C, Dondorp AM, Chokejindachai W. Severe vivax malaria: a systematic review and meta-analysis of clinical studies since 1900. Malar J 2014; 13:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Phyo AP, Dahal P, Mayxay M, Ashley EA. Clinical impact of vivax malaria: a collection review. PLoS Med 2022; 19:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Genton B, D’Acremont V, Rare L, et al. Plasmodium vivax and mixed infections are associated with severe malaria in children: a prospective cohort study from Papua New Guinea. PLoS Med 2008; 5:e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tjitra E, Anstey NM, Sugiarto P, et al. Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med 2008; 5:e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Quispe AM, Pozo E, Guerrero E, et al. Plasmodium vivax hospitalizations in a monoendemic malaria region: severe vivax malaria? Am J Trop Med Hyg 2014; 91:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park SY, Park YS, Park Y, et al. Severe vivax malaria in the Republic of Korea during the period 2000 to 2016. Travel Med Infect Dis 2019; 30:108–13. [DOI] [PubMed] [Google Scholar]
- 21. Luxemburger C, Ricci F, Nosten FH, Raimond D, Bathet S, White NJ. The epidemiology of severe malaria in an area of low transmission in Thailand. Trans R Soc Trop Med Hyg 1997; 91:256–62. [DOI] [PubMed] [Google Scholar]
- 22. Luxemburger C, Thwai KL, White NJ, et al. The epidemiology of malaria in a Karen population on the western border of Thailand. Trans R Soc Trop Med Hyg 1996; 90:105–11. [DOI] [PubMed] [Google Scholar]
- 23. Shoklo Malaria Research Unit. Malaria guidelines. 24th ed. Available at: https://www.shoklo-unit.com/resources . Accessed 13 March 2023.
- 24. Shoklo Malaria Research Unit. SMRU annual reports. Available at: https://www.shoklo-unit.com/humanitarian-activities/reports. Accessed 23 January 2023.
- 25. World Health Organization. Guidelines for malaria, 2022. Available at: https://www.who.int/publications/i/item/guidelines-for-malaria. Accessed 13 March 2023.
- 26. World Health Organization . Severe malaria. Trop Med Int Health 2014; 19:7–131. [DOI] [PubMed] [Google Scholar]
- 27. McGready RM, Wongsaen K, Chu CS, et al. Uncomplicated Plasmodium vivax malaria in pregnancy associated with mortality from acute respiratory distress syndrome. Malar J 2014; 13:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saito M, Carrara VI, Gilder ME, et al. A randomized controlled trial of dihydroartemisinin-piperaquine, artesunate-mefloquine and extended artemether-lumefantrine treatments for malaria in pregnancy on the Thailand-Myanmar border. BMC Med 2021; 19:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. White NJ. Severe malaria. Malar J 2022; 21:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poespoprodjo JR, Fobia W, Kenangalem E, et al. Vivax malaria: a major cause of morbidity in early infancy. Clin Infect Dis 2009; 48:1704–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kojom Foko LP, Arya A, Sharma A, Singh V. Epidemiology and clinical outcomes of severe Plasmodium vivax malaria in India. J Infect 2021; 82:231–46. [DOI] [PubMed] [Google Scholar]
- 32. Kochar DK, Das A, Kochar SK, et al. Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg 2009; 80:194–8. [PubMed] [Google Scholar]
- 33. Nayak KC, Kumar S, Gupta BK, et al. Clinical and histopathological profile of acute renal failure caused by falciparum and vivax monoinfection: an observational study from Bikaner, northwest zone of Rajasthan, India. J Vector Borne Dis 2014; 51:40–6. [PubMed] [Google Scholar]
- 34. Siqueira AM, Lacerda MVG, Magalhães BML, et al. Characterization of Plasmodium vivax-associated admissions to reference hospitals in Brazil and India. BMC Med 2015; 13:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Douglas NM, Pontororing GJ, Lampah DA, et al. Mortality attributable to Plasmodium vivax malaria: a clinical audit from Papua, Indonesia. BMC Med 2014; 12:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boel ME, Rijken MJ, Leenstra T, et al. Malaria in the post-partum period; a prospective cohort study. PLoS One 2013; 8:e57890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tan LK, Yacoub S, Scott S, Bhagani S, Jacobs M. Acute lung injury and other serious complications of Plasmodium vivax malaria. Lancet Infect Dis 2008; 8:449–54. [DOI] [PubMed] [Google Scholar]
- 38. Bhattacharya SK, Sur D, Dutta S, et al. Vivax malaria and bacteraemia: a prospective study in Kolkata, India. Malar J 2013; 12:e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Price RN, von Seidlein L, Valecha N, Nosten FH, Baird JK, White NJ. Global extent of chloroquine-resistant Plasmodium vivax: a systematic review and meta-analysis. Lancet Infect Dis 2014; 14:982–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.