Abstract
Background:
Post-acute care outcomes for patients with cancer <65 with multiple payers are largely unknown.
Objective:
Describe the population and outcomes of younger adults discharged to skilled nursing facility (SNF) and those discharged home or with home health care six months following hospitalization.
Design:
Descriptive cohort analysis.
Setting/Subjects:
Using a linkage between the Colorado All Payers Claims Database and the Colorado Central Cancer Registry, we studied patients <65 with stage III or IV advanced cancer between 2012 and 2017.
Measurements:
Receipt of cancer treatment, 30-day readmission, death, and hospice use. Groups of interest were compared by patient demographics and disease characteristics using chi-square tests. Logistic regression was used to describe unadjusted and adjusted outcome rates among discharge setting. Kaplan-Meier method was used to estimate survival by discharge destination.
Results:
Three percent of patients were discharged to SNF, 79.0% to home, and 18.0% to home health care. SNF discharges were less likely to receive cancer treatment. Among decedents, 39.0%, 51.0%, and 58.0% of SNF, home, and home health care discharges received hospice, respectively. Patients with Medicaid were more likely to be discharged to an SNF. Black/Hispanic patients were more likely to have Medicaid and received less radiation and hospice care, irrespective of discharge location. Those who were discharged to SNF were more likely to receive radiation compared to White patients.
Conclusions:
Younger patients with cancer discharged to SNF were unlikely to receive cancer treatment and hospice care before death. Racial disparities exist in cancer treatment receipt and hospice use warranting further investigation.
Keywords: cancer, disparities, palliative care, post-acute care
Introduction
Hospitalized older adults with advanced cancer discharged to a skilled nursing facility (SNF) are unlikely to receive further cancer treatment, have high hospital readmission rates, and have minimal hospice use.1 SNF care is one part of the post-acute care (PAC) continuum that includes long-term acute care hospitals, inpatient rehabilitation facilities, and home health care. Forty-four percent of hospitalized patients used PAC services in 2018 at a cost of $60 billion, a trend in part driven by shorter hospital stays.2 Patients who discharge to SNF are typically older, are more medically complex, and have higher hospital readmission rates than those strong enough to discharge home.3 Among patients using a PAC facility within the last 30 days of life, about half returned to a hospital before death.4
Hospitalized older adults with advanced cancer and functional impairment have higher symptom burden, longer hospital stay,s and worse survival, and those discharged to PAC facilities experience symptom burden similar to patients with cancer discharged to hospice.4,5 Readmissions are high for those who are discharged to an SNF, where only 21.0% received subsequent chemotherapy and nearly a third were readmitted within 30 days.1 The need for SNF-level care indicates significant functional impairment. Based on what is known about functional decline in advanced cancer, even with intensive rehabilitation, the progressive functional decline may not be reversible for older adults with lower reserve.6,7
Therefore, the low rates of cancer treatment in patients discharged to SNF, who are not functionally able to manage at home, are consistent with current American Society of Clinical Oncology (ASCO) guidelines.8 However, these findings also suggest that many older adults with cancer are cycling between SNFs and hospitals in the final months of life and are at high risk for being “rehabbed to death.”9
The PAC outcomes of younger adults with advanced cancer in SNFs are unknown due to a lack of available population-based data for younger patients with multiple payers. Prior studies have demonstrated that younger patients with cancer, who have Medicaid insurance, are more likely to receive aggressive care and have low rates of hospice use before death.10,11 Evaluations of racial and ethnic disparities have been mixed with some studies showing that Black and Hispanic patients have increased health care utilization and receive aggressive care near the end of life.10,12,13
These studies have not described the outcomes of this population in PAC where they may have additional support from home health care or a, SNF stay. Conversely, there may be additional barriers Black/Hispanic patients experience in PAC, which may contribute to poor outcomes (e.g., bias, structural racism, language). In this context, we leverage an existing linkage between the Colorado All Payers Claims Database (APCD) and the Colorado Central Cancer Registry (CCCR) to describe outcomes of hospitalized adults <65 with advanced cancer, who are discharged to home, home with home health care, or to SNF. A major limitation of secondary data sources is the lack of documented functional status.
However, in this descriptive study, we use discharge location as a surrogate for functional status and examine outcomes across discharge locations. We describe racial and ethnic differences in health care utilization, receipt of future cancer treatment and hospice use near the end of life. One advantage of this longitudinal linkage is that it allows us to study data from multiple payers and settings. Because of linkage to the cancer registry, time and stage at diagnosis and date of death can be accurately ascertained.
Methods
Data source
The data source was a linkage between the APCD and the CCCR. Colorado APCD is a database that includes health care insurance claims and dates of service from commercial health insurance plans, Medicare, and Colorado's Medicaid program (Health First Colorado).14 The CCCR is Colorado's statewide database of cancer diagnoses and includes cancer site, stage at diagnosis, initial treatment, and month and year of diagnosis.15 The data also include patient demographic characteristics such as age, sex, race, ethnicity, marital status, and patient geographic information. The Colorado APCD and CCCR were linked using probabilistic linkage methods achieving an overall linkage rate of 93.0%, with close to 99.0% linkage rate for Medicaid and Medicare beneficiaries.16
Sample selection
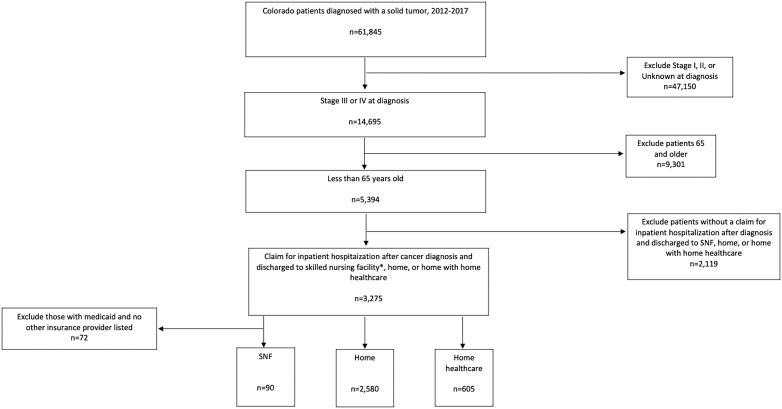
We selected patients with a newly diagnosed solid tumor cancer from 2012 to 2017 (n = 61,845). Solid tumors selected were as follows: colorectal, pancreas, bladder, lung, breast, prostate, melanoma, ovarian, kidney, thyroid, esophageal, gastric, uterine, liver, and oropharyngeal cancer. We limited our sample to patients with American Joint Committee on Cancer, 7th Edition Stage III or IV (n = 14,695) tumors, and selected those younger than age 65 (n = 5394). We defined the index hospitalization as the first inpatient hospitalization claim of patients with cancer in our study period, who were discharged to an SNF, home, or home with home health care (n = 3275) (Fig. 1).
FIG. 1.
CONSORT diagram.
We excluded 72 patients who were discharged to a, SNF and only had Medicaid listed as their primary insurance provider, with no secondary insurance provider. Facilities in the APCD licensed as a “Skilled Nursing Facility” can have both SNF beds, covered by Medicare or private insurance, and long-term care beds, covered by Medicaid. Colorado Medicaid does not provide a skilled or rehabilitation benefit for its beneficiaries so patients with only Medicaid insurance residing at a facility were presumed to be receiving custodial care and not SNF care, and thus were excluded from our sample. The Colorado Multiple Institutional Review Board approved this study.
Groups
Analyses were conducted among patients in three groups based on the discharge destination from an inpatient hospitalization: (1) SNF (n = 90), (2) home (n = 2580), and (3) home with home health care (n = 605). Discharge destination was determined by discharge status and discharge facility information recorded on the inpatient hospitalization claim.
Outcomes
Claims in the 6 months after hospital discharge were used to obtain the following outcome measures: receipt of cancer treatment, hospice use, emergency department (ED) visits, 30-day readmission, and receipt of cancer treatment within 14 days before death. The National Quality Forum has endorsed indicators of overly aggressive end of life care to include the following: hospitalizations, ED visits, or intensive care unit stays within the last month of life, chemotherapy less than or equal to two weeks before death, and late or absent hospice referrals.17
CPT codes, the Healthcare Common Procedure Coding System (HCPCS) codes, ICD-9 and ICD-10 procedure and diagnosis codes, and National Drug Codes (NDCs) were used to identify treatment received, including intravenous (IV) chemotherapy, radiation, and IV targeted therapy. We defined IV targeted therapy as the receipt of one of the following drugs: bevacizumab, cetuximab, everolimus, panitumumab, ramucirumab, and ziv-aflibercept. Hospice use was measured among decedents during the six-month follow-up period (Appendix Table A1). Registry data included the last date of contact and vital status information. For deceased patients, the last date of contact was used as their date of death. For the cohort discharged to SNF, we evaluated predictors of health outcomes in the six-month follow-up period and five-year survival.
Control variables
We included patient demographics, tumor characteristics at diagnosis, prior health conditions, and payer as control variables in our analyses. Cancer registry data were used to determine patient sex, age at diagnosis, race/ethnicity, marital status, year of diagnosis, primary cancer site, and stage at diagnosis. Race/ethnicity data in the CCCR are entered at the hospital by a trained certified tumor registrar who abstracts data from medical charts. These data are checked for quality at the CCCR as part of regular quality control audits to assure quality data. Specific health conditions of interest were identified using the Centers for Medicare and Medicaid Services' Chronic Conditions Data Warehouse Algorithm and included hypertension, diabetes, chronic kidney disease, chronic obstructive pulmonary disease (COPD), heart disease, heart failure, stroke, depression, cognitive disorder, tobacco use, alcohol use, and drug use.18
These conditions were used in the analysis as the sum of comorbidities. We were unable to perform analysis on separate racial and ethnic groups because sample sizes were too small to disaggregate. As a result, Black and Hispanic patients were combined for analysis and their outcomes compared to White patients.
Statistical analysis
The three patient groups of interest were compared by patient demographics and disease characteristics using chi-square tests. Logistic regression models were estimated separately to estimate unadjusted and adjusted rates of receipt of anticancer treatment, death, hospice use, ED visit, and 30-day readmission during the 6-month follow-up period by discharge group and race. We present average predicted probabilities (predictive margins) so rates from unadjusted and adjusted models are comparable and easier to interpret. In addition, we present marginal effects to easily interpret the change in predicted probabilities by discharge destination. Kaplan-Meier curves were used to estimate survival by discharge destination. All statistical analyses were completed using SAS 9.4 (SAS Institute, Cary, NC) and Stata 16.
Results
Patient characteristics
Our study population consisted of 3275 adults with stage III or IV advanced solid tumor cancer (Table 1). The average age of patients was 54 years and 72% identified as White Non-Hispanic, 17.0% Hispanic, and 6.0% Black. The 5.0% of patients categorized as “other” or “unknown” were included in our regression analysis with White patients. Three percent of patients discharged to an SNF, 79.0% discharged home, and 18.0% discharged home with home health care (Table 2). Patients who discharged to SNF had more comorbidities and were less often married or partnered.
Table 1.
Descriptives by Discharge Group
| Category | Value | Overall n (%) | SNF n (%) | Home n (%) | Home health n (%) |
|---|---|---|---|---|---|
| Overall | 3275 (100.0) | 90 (2.7) | 2580 (78.7) | 605 (18.4) | |
| Sex | Male | 1651 (50.4) | 44 (48.9) | 1298 (50.3) | 309 (51.1) |
| Female | 1624 (49.6) | 46 (51.1) | 1282 (49.7) | 296 (48.9) | |
| Age | 21–30 | 54 (1.6) | 0 (0.0) | 47 (1.8) | 7 (1.2) |
| 31–40 | 196 (6.0) | 0 (0.0) | 169 (6.6) | 27 (4.5) | |
| 41–50 | 575 (17.6) | 3 (3.3) | 482 (18.7) | 90 (14.9) | |
| 51–60 | 1573 (48.0) | 47 (52.2) | 1216 (47.1) | 310 (51.2) | |
| 61–64 | 877 (26.8) | 40 (44.4) | 666 (25.8) | 171 (28.3) | |
| Race/ethnicity | White/non-Hispanic | 2357 (72.0) | 75 (83.3) | 1833 (71.0) | 449 (74.2) |
| Hispanic | 553 (16.9) | 13 (14.4) | 450 (17.4) | 90 (14.9) | |
| Black | 201 (6.1) | 2 (2.2) | 163 (6.3) | 36 (6.0) | |
| Other | 121(3.7) | 0 (0.0) | 100 (3.9) | 21 (3.5) | |
| Unknown | 43 (1.3) | 0 (0.0) | 34 (1.3) | 9 (1.5) | |
| Marital status | Not married or partnered | 1701 (51.9) | 75 (83.3) | 1286 (49.8) | 340 (56.2) |
| Married or partnered | 1563 (47.7) | 15 (16.7) | 1284 (49.8) | 264 (43.6) | |
| Unknown | 11 (0.3) | 0 (0.0) | 10 (0.4) | 1 (0.2) | |
| Primary payer | Medicare | 132 (4.0) | 14 (15.6) | 95 (3.7) | 23 (3.8) |
| Medicaid | 1834 (56.0) | 42 (46.7)a | 1430 (55.4) | 362 (59.8) | |
| Commercial | 1186 (36.2) | 22 (24.4) | 972 (37.7) | 192 (31.7) | |
| Medicare Advantage | 123 (3.8) | 12 (13.3) | 83 (3.2) | 28 (4.6) | |
| Lung cancer | Stage III | 212 (6.5) | 6 (6.7) | 170 (6.6) | 36 (6.0) |
| Stage IV | 570 (17.4) | 25 (27.8) | 458 (17.8) | 87 (14.4) | |
| N/A | 2493 (76.1) | 59 (65.6) | 1952 (75.7) | 482 (79.7) | |
| Colorectal cancer | Stage III | 221 (6.7) | 2 (2.2) | 147 (5.7) | 72 (11.9) |
| Stage IV | 377 (11.5) | 11 (12.2) | 289 (11.2) | 77 (12.7) | |
| N/A | 2677 (81.7) | 77 (85.6) | 2144 (83.1) | 456 (75.4) | |
| Breast cancer | Stage III | 207 (6.3) | 2 (2.2) | 182 (7.1) | 23 (3.8) |
| Stage IV | 163 (5.0) | 4 (4.4) | 127 (4.9) | 32 (5.3) | |
| N/A | 2905 (88.7) | 84 (93.3) | 2271 (88.0) | 550 (90.9) | |
| Pancreatic cancer | Stage III | 46 (1.4) | 0 (0.0) | 39 (1.5) | 7 (1.2) |
| Stage IV | 174 (5.3) | 6 (6.7) | 146 (5.7) | 22 (3.6) | |
| N/A | 3055 (93.3) | 84 (93.3) | 2395 (92.8) | 576 (95.2) | |
| Other cancer | Stage III | 422 (12.9) | 5 (5.6) | 358 (13.9) | 59 (9.8) |
| Stage IV | 883 (27.0) | 29 (32.2) | 664 (25.7) | 190 (31.4) | |
| N/A | 1970 (60.2) | 56 (62.2) | 1558 (60.4) | 356 (58.8) | |
| Prior visit with oncologist | No | 2109 (64.4) | 65 (72.2) | 1715 (66.5) | 329 (54.4) |
| Yes | 1166 (35.6) | 25 (27.8) | 865 (33.5) | 276 (45.6) | |
| Sum of comorbidities | 0 | 708 (21.6) | 6 (6.7) | 586 (22.7) | 116 (19.2) |
| 1 | 787 (24.0) | 13 (14.4) | 633 (24.5) | 141 (23.3) | |
| 2 | 713 (21.8) | 20 (22.2) | 565 (21.9) | 128 (21.2) | |
| 3 | 488 (14.9) | 18 (20.0) | 377 (14.6) | 93 (15.4) | |
| 4 | 265 (8.1) | 11 (12.2) | 197 (7.6) | 57 (9.4) | |
| 5 or more | 314 (9.6) | 22 (24.4) | 222 (8.6) | 70 (11.6) |
Comorbidities of interest are prior hypertension, diabetes, chronic kidney disease, chronic obstructive pulmonary disease, heart disease, heart failure, stroke, depression, cognitive disease, tobacco use, alcohol use, or drug use.
Patients discharged to SNF and whose primary payer was Medicaid had to have Medicare or Commercial coverage listed in their secondary insurance to be included in this study. Those with ONLY Medicaid have been excluded.
SNF, skilled nursing facility.
Table 2.
Outcomes at Six Months by Discharge Destination
| N | Unadjusted rates |
Adjusteda
rates |
|||||
|---|---|---|---|---|---|---|---|
| SNF n (%) | Home n (%) | Home health n (%) | SNF | Home | Home health | ||
| Patients, n | 3275 | 90 | 2580 | 605 | 90 | 2580 | 605 |
| 30 days readmit | 3275 | 30 (33.3) | 775 (30.0) | 135 (22.3) | 28.5 | 30.4 | 21.7 |
| Death | 3275 | 45 (50.0) | 470 (18.2) | 121 (20.0) | 39.8 | 18.5 | 19.3 |
| Hospiceb | 636 | 17 (37.8) | 238 (50.6) | 72 (59.5) | 38.9 | 50.9 | 58.1 |
| Hospice LOS <3 daysb | 636 | 8 (17.8) | 166 (35.3) | 48 (39.7) | 21.3 | 34.9 | 39.4 |
| Chemotherapy | 3275 | 22 (24.4) | 1229 (47.6) | 251 (41.5) | 25.7 | 47.5 | 41.8 |
| Radiation | 3275 | 13 (14.4) | 595 (23.1) | 139 (23.0) | 12.6 | 23.3 | 22.4 |
| Targeted treatment | 3275 | 1 (1.1) | 155 (6.0) | 36 (3.0) | 1.3 | 5.9 | 6.1 |
| ED visit | 3275 | 24 (26.7) | 833 (32.3) | 209 (34.6) | 24.2 | 32.7 | 33.4 |
| Chemotherapy before deathc | 2178 | 21 (21.0) | 1193 (36.7) | 252 (30.9) | 20.8 | 36.6 | 31.2 |
| Radiation before deathc | 2178 | 17 (17.3) | 755 (22.4) | 158 (20.0) | 16.0 | 22.4 | 20.0 |
| Targeted chemotherapy before deathc | 2178 | 5 (6.2) | 229 (8.4) | 59 (8.6) | 7.5 | 8.3 | 8.7 |
Adjusted for age, sex, race, stage at diagnosis, primary payer, and comorbidities.
Only among those who died in six-month follow-up.
Death at any point, not limited to six-month follow-up period.
ED, emergency department; LOS, length of stay.
Patient outcomes by discharge destination
Home
Seventy-nine percent of younger adults discharged home after their hospitalization. Thirty percent were readmitted within 30 days of discharge and 18% had died at 6-month follow-up. Only half received hospice care before death. Forty-eight percent of patients received chemotherapy, 23.0% received radiation, and 8.0% received targeted therapy after discharge home. Thirty-seven percent, 22.0%, and 8.0% of patients received chemotherapy, radiation, or targeted therapy within 14 days of death. These outcomes were similar when adjusted for covariates.
Home with home health care
Eighteen percent of patients discharged home with home health care. Twenty-two percent were readmitted within 30 days, 35.0% had an ED visit, and 20.0% had died at 6-month follow-up. Sixty percent of deceased patients received hospice care before death and 40.0% had a hospice length of stay (LOS) less than three days. Forty-two percent of patients received chemotherapy after hospital discharge and about a third received chemotherapy within 14 days of death when adjusted for covariates.
Skilled nursing facility
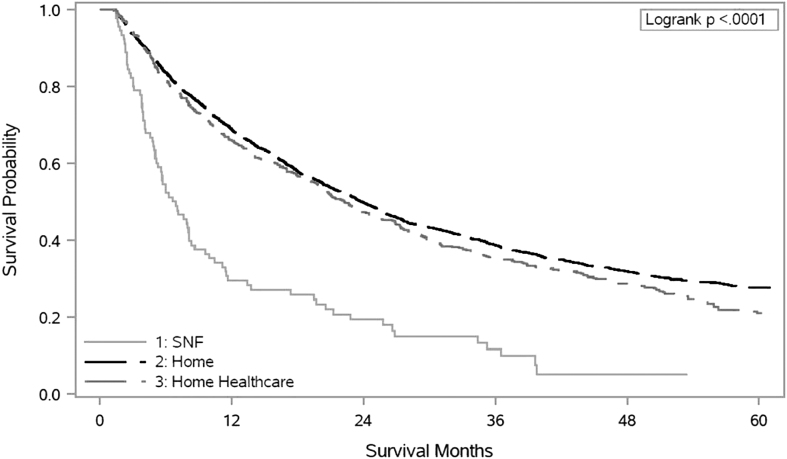
Three percent of younger hospitalized adults with advanced cancer discharged to an SNF. Thirty-three percent were readmitted within 30 days. When adjusting for covariates, 21.0%, 16.0%, and 7.5% of SNF discharges received IV chemotherapy, radiation therapy, and IV targeted therapy, respectively, after hospitalization. Forty percent of SNF discharges died within six months of hospitalization compared to 18.5% and 19.0% of patients discharged home and home with home health care, respectively (Fig. 2). Only 39.0% of younger adults received hospice care before death and approximately half had a hospice LOS less than three days.
FIG. 2.
Overall survival, censored at five years, by discharge group.
Outcomes by payer and race/ethnicity
Payer source by race and ethnicity type is shown in Table 3. Of the White patients in this cohort, 43.0% had private commercial insurance, 49.0% had Medicaid, and 8.0% had either Medicare or Medicare Advantage (MA) insurance. Eighteen percent, 75.0%, and 7.0% of Hispanic patients had private commercial insurance, Medicaid, and either Medicare or MA, respectively. Of the Black patients in this cohort, 19.0% had private commercial insurance, 73.0% had Medicaid, and 8.0% had Medicare or MA. Younger adults who discharged to an SNF were more likely to have Medicaid. Of SNF patients whose primary payer was Medicaid, 62.0% were dual eligible, and 38.0% had private secondary insurance.
Table 3.
Payer Source by Race and Ethnicity
| Private n (%) | Medicare n (%) | Medicare advantage n (%) | Medicaid n (%) | |
|---|---|---|---|---|
| White, NH (N = 2357) | 1005 (42.6) | 109 (4.6) | 84 (3.5) | 1160 (49.2) |
| Hispanic (N = 553) | 102 (18.4) | 13 (2.3) | 24 (4.3) | 414 (74.8) |
| Black (N = 201) | 39 (19.4) | 3 (1.4) | 13 (6.5) | 146 (72.6) |
| Other (N = 121) | 33 (27.2) | 5 (4.1) | 2 (1.6) | 81 (66.9) |
| Unknown (N = 43) | 7 (16.2) | 2 (4.6) | 1 (2.3) | 33 (76.7) |
| Total (N = 3275) | 1186 | 132 | 123 | 1834 |
NH, non-Hispanic
When adjusting for covariates, there were significant disparities between White and Black/Hispanic patients for radiation and hospice use regardless of discharge location, as shown in Table 4. Eighteen percent of Black/Hispanic patients received radiation treatment compared to 24.5% of White patients (p = 0.000). Only 43.0% of Black/Hispanic decedents received hospice care compared to 55.0% of White decedents (p = 0.008). Interactions between race and ethnicity and discharge destination were not significant, except for the outcome of radiation. Marginal effects for the outcome of radiation for Black/Hispanic patients discharged to SNF, home, and home with home health care were 19.9%, −8.3%, and −0.2%, respectively (p-value = 0.006).
Table 4.
Outcomes at Six Months by White vs. Black and Hispanic Patients
| N | Unadjusted rates |
Adjusteda
rates |
|||
|---|---|---|---|---|---|
| White n (%) | Black and Hispanic n (%) | White | Black and Hispanic | ||
| Patients, n | 3275 | 2400 | 875 | 2400 | 875 |
| 30 days readmit | 3275 | 670 (27.9) | 270 (30.9) | 27.9 | 30.9 |
| Death | 3275 | 464 (19.3) | 172 (19.7) | 19.2 | 20.0 |
| Hospiceb | 636 | 252 (54.3) | 75 (43.6) | 54.7 | 42.6 |
| Hospice LOS <3 daysb | 636 | 165 (35.6) | 57 (33.1) | 37.0 | 29.7 |
| Chemotherapy | 3275 | 1122 (46.8) | 380 (43.4) | 46.6 | 43.9 |
| Radiation | 3275 | 591 (24.6) | 156 (17.8) | 24.5 | 18.2 |
| Targeted treatment | 3275 | 132 (5.5) | 60 (6.9) | 5.6 | 6.5 |
| ED visit | 3275 | 733 (30.5) | 333 (38.1) | 31.6 | 35.0 |
| Chemotherapy before deathc | 2178 | 573 (35.7) | 189 (33.0) | 35.3 | 34.2 |
| Radiation before deathc | 2178 | 359 (22.4) | 114 (19.9) | 22.0 | 20.8 |
| Targeted chemotherapy before deathc | 2178 | 139 (8.7) | 43 (7.5) | 8.7 | 7.5 |
djusted for age, sex, discharge destination, stage at diagnosis, primary payer, and comorbidities.
Only among those who died in six-month follow-up.
Death at any point, not limited to six-month follow-up period.
Discussion
This study describes the PAC outcomes of hospitalized adults <65 with advanced cancer in Colorado. Younger adults rarely discharged to SNF, but those who did, were unlikely to receive further cancer treatment, had high rates of readmission, and had high mortality with low hospice utilization. Half of all SNF discharges had died at six-month follow-up. Most patients discharged home after hospitalization and also had high readmission rates, and approximately half subsequently received chemotherapy. About 20% of patients discharged home with home health care and 60% of decedents in this cohort received hospice care. Black/Hispanic patients were more likely to have Medicaid as their primary insurance and received less radiation and hospice care irrespective of discharge location; those who discharged to SNF, however, were more likely to receive radiation compared to White patients.
Our findings highlight an important knowledge gap regarding rehabilitative gains that patients with advanced cancer experience during their SNF stay. Minimum Data Set—ADL scores have been developed to assess changes in ADL self-performance, but studies evaluating functional change have primarily focused on long-term care populations and not PAC patients.19–21 Previous research has shown a significant correlation between functional status and survival, hospitalization, and institutionalization for older adults with cancer.22–27 Half the SNF discharges died within six months, emphasizing the need to better support SNFs in caring for patients with serious illness and improving identification of those most likely to benefit from care in this setting.
There is a critical lack of palliative care delivery in SNFs that places adults with cancer at risk for receiving burdensome transitions of care and aggressive treatment near the end of life.28,29 The sickest patients in our study, who were functionally debilitated to need SNF care, were also the patients who had the lowest hospice use, highest mortality, and highest 30-day readmission rate. They would benefit from palliative care, which has been shown to help with shared decision making, enhance communication, and reduce low-value care.30–33 Palliative care delivery models studied in nursing homes have focused on long-term care populations and have been challenging to scale, replicate, and achieve diffusion.34–36
Misaligned health policies in acute and PAC likely contribute to poor outcomes. The Prospective Payment System provides fixed reimbursement to hospitals for care delivered. This incentivizes hospitals to diagnose and treat patients faster and have shorter hospital stays, which can lead to patients being discharged “sicker and quicker.”37 This payment structure disincentivizes hospitals from keeping patients admitted longer to communicate prognosis and better understand values and preferences for care.
Even if patients understand their poor prognosis and wish to discharge home with hospice, those with limited support and/or high care needs may still be forced to discharge to an SNF due to lack of robust home and community-based services. The Hospital Readmissions Reduction Program is a Medicare value-based program that penalizes hospitals for 30-day readmissions, for specific medical conditions; however cancer is not a condition targeted by the policy. Since the implementation of this Program, readmissions have declined across all insurance types, although patients with Medicaid continue to have higher readmission rates than those with Medicare.38
These results also add to a growing body of literature demonstrating racial disparities in cancer care.39–43 Black/Hispanic patients received less radiation before death than White patients regardless of discharge location; however, discharge to SNF resulted in an increased receipt of radiation therapy for this population. Low rates of cancer treatment in the SNF cohort are likely reflective of the functional impairment that led to a skilled need for rehabilitation. ASCO recommends against the use of chemotherapy in patients with solid tumors, who have an Eastern Cooperative Oncology Group performance status score ≥3.8
Radiation therapy, however, can be used both for disease-modifying therapy or to provide symptom relief near the end of life.44 This has important implications as our study only included adults with advanced cancer for whom palliative radiation may be indicated. We hypothesize that Black/Hispanic patients may be more likely to receive radiation after SNF discharge because of increased care coordination and transportation resources available. Seventy-three percent of Black/Hispanic patients had Medicaid as their primary insurer and the majority who discharged to SNF were dual eligible. Social determinants of health, such as food and housing insecurity, transportation needs, and lack of paid sick leave, disproportionately disadvantage Medicaid and dual-eligible beneficiaries, who in our study were more likely to be Black and Hispanic.45,46
Limitations
Some limitations are notable. We used a secondary claims database that does not include measures of functional status, social support, or patient care goals, which might influence outcomes. The APCD is limited to Colorado and only captures some insurance plans limiting generalizability of these findings across state lines. Categorizing Black and Hispanic patients into one group is not ideal; however, our small sample size limited our ability to further disaggregate this group.
Our dataset included 17.0% of patients who identified as Hispanic and 6.0% who identified as Black, which is reflective of the Colorado population in general. In addition, this study estimates associations and not causality. We considered matching estimators and inverse propensity score weighting, but these methods rely on the assumption that all confounders are observed, which we also acknowledge as a limitation. The APCD does not include information on uninsured patients who are more likely to experience worse cancer outcomes than those captured in the database.
Conclusion
Younger adults with advanced cancer are rarely discharged to SNFs, but those who do are at high risk for poor outcomes. Policy gaps contribute to the churn of patients between care settings near the end of life, placing them at high risk of being “rehabbed to death.”9,47 Targeted payment reform should be investigated to better align financial incentives for hospitals and SNFs. Palliative care delivery models that transition with patients between these silos have the potential to enhance communication and support in-the-moment decision making. Understanding experiences and outcomes of different racial and ethnic populations after hospital discharge will be necessary to begin to address disparities.
Appendix Table A1.
Codes for Treatment and Hospice Variables
| Variable | ICD-9 diagnosis codes | ICD-9 procedure codes | HCPCS codes | Revenue codes | NDCs |
|---|---|---|---|---|---|
| Radiotherapy | 92.21–92.29 | 76370, 76950, 77014, 77261–77263, 77280, 77285, 77290, 77295, 77299–77301, 77305, 77310, 77315, 77321, 77326–77328, 77331–77334, 77336, 77338, 77370–77373, 77399, 77401–77499, 77520, 77522, 77523, 77525, 77750–77799, 0073T, 0082T, 0083T, 0182T, 0520F, G0173, G0174, G0178, G0242, G0243, G0251, G0256, G0261, G0273, G0274, G0338-G0340 | 0330, 0333, 0339, 0342, 0344 | ||
| Chemotherapy | V58.1, V58.11, V58.12 | 00.10, 99.25, 99.28 | 96400–96599, C8953–C8955, C9127, C9205, C9213, C9215, C9235, C9257, C9414, C9418, C9425, C9427, C9431, C9432, C9440, J8510, J8520, J8521, J8530, J8600, J8610, J8700, J8705, J8999–J9999, Q0083–Q0085, S0177, S9329–S9331, 51720, 61517, 0519F, S0116, Q2024, C9025, C9296, J7527 | ||
| Bevacizumab | C9257, J9035, S0116, Q2024 | 50242006001, 50242006101 | |||
| Cetuximab | J9055 | 66733094823, 66733095823 | |||
| Everolimus | J7527 | 00078056651, 00078056751, 00078059451, 00078062051, 00078062651, 00078062751, 00078062851 | |||
| Panitumumab | J9303 | 55513095401, 55513095501, 55513095601, 59703095601 | |||
| Ramucirumab | C9025, J9308 | 00002766901, 00002767801 | |||
| Ziv-aflibercept | C9296, J9400 | 00024584001, 00024584003, 00024584101 | |||
| Variable | CO APCD bill type code | ||||
| Hospice use | 81-Special Facility, Hospice (non-hospital based) 82-Special Facility, Hospice (hospital based) |
||||
CO APCD, Colorado All Payers Claims Database; HCPCS, Healthcare Common Procedure Coding System; NDC, National Drug Code.
Authors' Contributions
All listed authors made substantive intellectual contributions to this study. All authors designed research, interpreted data, and wrote the article. E.M. and M.P. conducted statistical analysis.
Funding Information
This research was supported by the National Palliative Care Research Center Kornfeld Scholar Award and by the National Cancer Institute grant number P30CA046934, University of Colorado Cancer Center Core Support Grant. Analyses were completed by the Population Health Shared Resource (P30CA046934). Dr. Marcelo Perraillon received support from an R01CA229551 from the NCI.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Singh S, Eguchi M, Min SJ, et al. Outcomes of patients with cancer discharged to a skilled nursing facility after acute care hospitalization. J Natl Compr Canc Netw 2020;18(7):856–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Medicare Payment Advisory Commission; 2020. Report to the Congress: Medicare Payment Policy. [Google Scholar]
- 3. Burke RE, Juarez-Colunga E, Levy C, et al. Rise of post-acute care facilities as a discharge destination of US hospitalizations. JAMA Intern Med 2015;175(2):295–296. [DOI] [PubMed] [Google Scholar]
- 4. Lage DE, Caudry DJ, Ackerly DC, et al. The care continuum for hospitalized medicare beneficiaries near death. Ann Intern Med 2018;168(10):748–750. [DOI] [PubMed] [Google Scholar]
- 5. Lage DE, El-Jawahri A, Fuh CX, et al. Functional impairment, symptom burden, and clinical outcomes among hospitalized patients with advanced cancer. J Natl Compr Canc Netw 2020;18(6):747–754. [DOI] [PubMed] [Google Scholar]
- 6. Singh S, Molina E, Meyer E, et al. Post-acute care outcomes and functional status changes of adults with new cancer discharged to skilled nursing facilities. J Am Med Dir Assoc 2022;23(11):1854–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu MA, Keeney T, Papaila A, et al. Functional status and survival in older nursing home residents with advanced non-small-cell lung cancer: A SEER-medicare analysis. JCO Oncol Pract 2022;18(6):e886–e895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schnipper LE, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: The top five list for oncology. J Clin Oncol 2012;30(14):1715–1724. [DOI] [PubMed] [Google Scholar]
- 9. Flint LA, David DJ, Smith AK. Rehabbed to death. N Engl J Med 2019;380(5):408–409. [DOI] [PubMed] [Google Scholar]
- 10. Yang A, Goldin D, Nova J, et al. Racial disparities in health care utilization at the end of life among new jersey medicaid beneficiaries with advanced cancer. JCO Oncol Pract 2020;16(6):e538–e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin 2008;58(1):9–31. [DOI] [PubMed] [Google Scholar]
- 12. Mack JW, Chen K, Boscoe FP, et al. High intensity of end-of-life care among adolescent and young adult cancer patients in the New York State Medicaid Program. Med Care 2015;53(12):1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guadagnolo BA, Liao KP, Giordano SH, et al. Variation in intensity and costs of care by payer and race for patients dying of cancer in Texas: An analysis of registry-linked medicaid, medicare, and dually eligible claims data. Med Care 2015;53(7):591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Center for Improving Value in Health Care. Colorado All Payer Claims Database. Available from: https://www.civhc.org/get-data/co-apcd-info/ [Last accessed: August 12, 2021].
- 15. Colorado Department of Public Health & Environment. Colorado Central Cancer Registry. Available from: https://cdphe.colorado.gov/center-for-health-and-environmental-data/registries-and-vital-statistics/colorado-central-cancer [Last accessed: August 22, 2021].
- 16. Perraillon MC, Liang R, Sabik LM, et al. The role of all-payer claims databases to expand central cancer registries: Experience from Colorado. Health Serv Res 2021;57(3):703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Earle CC, Landrum MB, Souza JM, et al. Aggressiveness of cancer care near the end of life: Is it a quality-of-care issue? J Clin Oncol 2008;26(23):3860–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Center for Medicare and Medicaid Services. Chronic Conditions Data Warehouse. Available from: https://www.ccwdata.org/web/guest/condition-categories [Last accessed: August 12, 2021].
- 19. Ursem C, Diaz-Ramirez LG, Boscardin J, et al. Changes in functional status associated with radiation for prostate cancer in older veterans. J Geriatr Oncol 2021;12(5):808–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murray KS, Prunty M, Henderson A, et al. Functional status in patients requiring nursing home stay after radical cystectomy. Urology 2018;121:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carpenter GI, Hastie CL, Morris JN, et al. Measuring change in activities of daily living in nursing home residents with moderate to severe cognitive impairment. BMC Geriatr 2006;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kenis C, Decoster L, Bastin J, et al. Functional decline in older patients with cancer receiving chemotherapy: A multicenter prospective study. J Geriatr Oncol 2017;8(3):196–205. [DOI] [PubMed] [Google Scholar]
- 23. Giebel CM, Sutcliffe C, Stolt M, et al. Deterioration of basic activities of daily living and their impact on quality of life across different cognitive stages of dementia: A European study. Int Psychogeriatr 2014;26(8):1283–1293. [DOI] [PubMed] [Google Scholar]
- 24. Han L, Allore H, Murphy T, et al. Dynamics of functional aging based on latent-class trajectories of activities of daily living. Ann Epidemiol 2013;23(2):87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Greysen SR, Stijacic Cenzer I, Auerbach AD, et al. Functional impairment and hospital readmission in Medicare seniors. JAMA Intern Med 2015;175(4):559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meddings J, Reichert H, Smith SN, et al. The impact of disability and social determinants of health on condition-specific readmissions beyond medicare risk adjustments: A cohort study. J Gen Intern Med 2017;32(1):71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Millan-Calenti JC, Tubio J, Pita-Fernandez S, et al. Prevalence of functional disability in activities of daily living (ADL), instrumental activities of daily living (IADL) and associated factors, as predictors of morbidity and mortality. Arch Gerontol Geriatr 2010;50(3):306–310. [DOI] [PubMed] [Google Scholar]
- 28. Tucker-Seeley RD, Abel GA, Uno H, et al. Financial hardship and the intensity of medical care received near death. Psychooncology 2015;24(5):572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA 2016;315(3):284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363(8):733–742. [DOI] [PubMed] [Google Scholar]
- 31. Gomes B, Calanzani N, Curiale V, et al. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev 2013;2013(6):CD007760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gomez-Batiste X, Caja C, Espinosa J, et al. The Catalonia World Health Organization demonstration project for palliative care implementation: Quantitative and qualitative results at 20 years. J Pain Symptom Manage 2012;43(4):783–794. [DOI] [PubMed] [Google Scholar]
- 33. Colligan EM, Ewald E, Ruiz S, et al. Innovative oncology care models improve end-of-life quality, reduce utilization and spending. Health Aff (Millwood) 2017;36(3):433–440. [DOI] [PubMed] [Google Scholar]
- 34. Tyler DA, Shield RR, Miller SC. Diffusion of palliative care in nursing homes: Lessons from the culture change movement. J Pain Symptom Manage 2015;49(5):846–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lester PE, Stefanacci RG, Feuerman M. Prevalence and description of palliative care in US nursing homes: A descriptive study. Am J Hosp Palliat Care 2016;33(2):171–177. [DOI] [PubMed] [Google Scholar]
- 36. Zarowitz BJ, Resnick B, Ouslander JG. Quality clinical care in nursing facilities. J Am Med Dir Assoc 2018;19(10):833–839. [DOI] [PubMed] [Google Scholar]
- 37. Kosecoff J, Kahn KL, Rogers WH, et al. Prospective payment system and impairment at discharge. The ‘quicker-and-sicker’ story revisited. JAMA 1990;264(15):1980–1983. [PubMed] [Google Scholar]
- 38. Ferro EG, Secemsky EA, Wadhera RK, et al. Patient readmission rates for all insurance types after implementation of the hospital readmissions reduction program. Health Aff (Millwood) 2019;38(4):585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boczar D, Restrepo DJ, Sisti A, et al. Disparity on unplanned readmission in melanoma patients: A National Cancer Database Analysis. Anticancer Res 2019;39(12):6877–6880. [DOI] [PubMed] [Google Scholar]
- 40. Karanth S, Rajan SS, Sharma G, et al. Racial-ethnic disparities in end-of-life care quality among lung cancer patients: A SEER-medicare-based study. J Thorac Oncol 2018;13(8):1083–1093. [DOI] [PubMed] [Google Scholar]
- 41. Miesfeldt S, Murray K, Lucas L, et al. Association of age, gender, and race with intensity of end-of-life care for medicare beneficiaries with cancer. J Palliat Med 2012;15(5):548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang SY, Hall J, Pollack CE, et al. Trends in end-of-life cancer care in the Medicare program. J Geriatr Oncol 2016;7(2):116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tucker-Seeley RD. Social determinants of health and disparities in cancer care for Black People in the United States. JCO Oncol Pract 2021;17(5):261–263. [DOI] [PubMed] [Google Scholar]
- 44. Gunderson L L TJE. Clinical Radiation Oncology. 3rd ed. Elsevier: London; 2012. [Google Scholar]
- 45. Patel MI, Lopez AM, Blackstock W, et al. Cancer disparities and health equity: A policy statement from the American Society of Clinical Oncology. J Clin Oncol 2020;38(29):3439–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hahn RA. Access to Social Determinants of Health and Determinant Inequity for the Black Population in US States in the Early Twenty-First Century. J Racial Ethn Health Disparities 2021;8(2):433–438. [DOI] [PubMed] [Google Scholar]
- 47. Flint LA, David D, Lynn J, et al. Rehabbed to death: Breaking the cycle. J Am Geriatr Soc 2019;67(11):2398–2401. [DOI] [PubMed] [Google Scholar]