Abstract
Mild traumatic brain injury (mTBI) is a major cause of morbidity and mortality with a poorly understood pathophysiology. Animal models have been increasingly utilized to better understand mTBI and recent research has identified visual deficits in these models that correspond to human literature. While visual impairment is being further characterized within TBI, the implications of impaired vision on behavioral tasks commonly utilized in animal models has not been well described thus far. Visual deficits may well confound behavioral tests that are believed to be isolated to cognitive functioning such as learning and memory. We utilized a mouse model of repetitive mTBI (rmTBI) to further characterize visual deficits using an optomotor task, electroretinogram, and visually evoked potential, and located likely areas of damage to the visual pathway. Mice were tested on multiple behavioral metrics, including a touchscreen conditional learning task to better identify the contribution of visual dysfunction to behavioral alterations. We found that rmTBI caused visual dysfunction resulting from damage distal to the retina that likely involves pathology within the optic nerve. Moreover, loss of vision led to poorer performance of rmTBI animals on classic behavioral tests such as the Morris water maze that would otherwise be attributed solely to learning and memory deficits. The touchscreen conditional learning task was able to differentiate rmTBI induced learning and memory dysfunction from visual impairment and is a valuable tool for elucidating subtle changes resulting from TBI.
Keywords: animal model, behavior, ERG, TBI, vision
Introduction
Mild traumatic brain injury (mTBI), including concussion, is a major cause of morbidity and mortality worldwide.1-3 Potential long-term sequelae of mTBI/concussion include but are not limited to neurocognitive impairment and alterations to mental health in diffuse neural circuits,4–8 the incidence of which increase with repetitive mild TBI (rmTBI).9-11 Animal models designed to simulate recurrent concussion have increasingly been used to explore mechanisms of rmTBI to elucidate pathophysiology and develop therapies.
The clinical relevance of animal models of human pathologies relies on recapitulating specific associated behaviors and evaluating the underlying biological changes in the relevant tissues. For example, rodent literature has extensively used the Morris water maze (MWM) task to explore spatial learning and memory and has demonstrated a strong relationship between hippocampal function and MWM performance.12–15 However, the MWM task also requires mice to see visual cues positioned around the pool in order to learn their spatial location and navigate to the platform. In fact, many commonly deployed behavioral tasks require an intact visual system. Despite this, the explicit interrogation of the role of visual systems in TBI models is rarely undertaken. A significant vision impairment may compromise task performance and be misinterpreted as a failure in cognitive abilities, rather than a combination of learning, memory, and visual defects.
Prior studies have focused primarily on the mechanisms underlying visual dysfunction after rmTBI rather than its influence on behavior.16–20 It is therefore necessary to both further explore visual changes resulting from rmTBI and to re-examine behavioral metrics used to demonstrate the clinical relevance of animal models. In order to do so, we performed two sets of experiments: one in which adult mice underwent rmTBI and performed a comprehensive battery of behavioral tests along with electroretinogram (ERG) and visual-evoked potential (VEP) to evaluate anxiety and motor, visual and cognitive function21–27 and a second using a visual cognitive task before and after rmTBI.28-30 Together, these experiments allowed a behavioral differentiation between deficits in visual function and learning/memory.
Methods
Further description of methods can be found in Supplementary Information S1.
Animals
We used 64 male 8-week-old C57BL/6 mice from Jackson Laboratories, fed ad libitum apart from touchscreen testing and housed in a reverse dark/light cycle such that all behavioral testing was conducted during the dark cycle to optimize performance. Mice that underwent the Touchscreen behavioral task (total of 24 animals) were food restricted only for the duration of that task. Food was administered and logged every day of the behavioral test. Bodyweights were tracked and kept in a special care notebook in the housing facility. Any mouse that lost 20% of its bodyweight was removed from the study. No mice had to be removed from the study due to weight loss. Water was provided freely to all animals throughout the experiments. All experiments were approved by the Boston Children's Hospital Institutional Animal Care and Use Committee and complied with the NIH Guide for the Care and Use of Laboratory Animals. Timeline of experiments is shown in Figure 1.
FIG. 1.
Experiment timeline. Two separate cohorts of animals underwent repetitive mild traumatic brain injury (rmTBI) or sham injury, behavioral testing, and sacrifice at the times outlined above.
Repetitive mild TBI
Mice were divided into two groups and subsequently randomized to undergo closed-head, repetitive mild traumatic brain injury (rmTBI) or sham injury. Group 1 (rmTBI, n = 11; sham n = 10) was injured at 8 weeks and Group 2 (rmTBI, n = 13; sham, n = 11) was injured when mice had passed initial touchscreen training as described below, which was between 12-14 weeks of age. Mice underwent rmTBI as previously reported.28–35 In brief, mice were anesthetized with 4% isoflurane in oxygen for 45 sec until the toe-pinch response was absent. Anesthetized mice were placed on a delicate tissue (Kimwipe, Irving, TX) that was grasped on both sides to create tensions without breaking the Kimwipe before the injury. The head was placed directly under a copper guide tube centered over the bregma. Mice were held by the tail as the impact was delivered to the dorsal skull. A 54 g metal bolt (12.75 mm diameter) was dropped through the guide tube from a 107 cm height, resulting in a rotational acceleration of the head through the Kimwipe. Mice received one injury per day for 5 consecutive days. Sham animals received exposure to the isoflurane only. All animals recovered in room air post-injury and were monitored for signs of distress before being returned to their cage. Loss of consciousness was recorded for each animal as the time from removal of anesthesia until they righted themselves.
Classic behavioral tests: Group 1
Locomotor activity and elevated plus maze (EPM) was measured at 4 weeks, 16 weeks, and 28 weeks post-injury. Prior research has demonstrated behavioral deficits from this injury model at these specific time-points and extending up to a year after injury.28,30,34 Locomotor boxes tracked total movement (rmTBI and sham, n = 8) and was run to establish exploratory behavior within sham and rmTBI mice, as lack of exploratory behavior would confound elevated plus maze results. The elevated plus maze is an established metric for impulsive behavior, with interventions that increase impulsivity leading to more time spent in the open arms and decision zone, and less time in the closed arms of the maze. Percent time in closed arms, open arms, and decision zone was calculated.
The rotarod is an established behavioral test for assessing gross locomotor and vestibular function that is known to be disrupted following TBI, with injured animals demonstrating decreased latency to fall.34–37 Rotarod consisted of a habituation day and 2 testing days of four trials per day averaged for one result per day. Latency to fall was recorded.
Group 1 performed the MWM at 28 weeks post-injury (rmTBI and sham n = 8). We chose not to repeat the MWM at different time-points using the same mice in order to avoid pre-training prior to the final evaluation. A white, opaque, circular tub (60 cm depth × 83 cm diameter) was filled with water to a depth of 29 cm at approximately 25°C. Four visible, distinct shapes were placed in each of the four quadrant inner walls of the tub to distinguish the quadrants. A white platform (10 cm diameter) was placed in one quadrant, 1 cm below the water. Two hidden sub-trials per day were run with the platform just out of sight on Days 1-4 of MWM testing for a total of eight sub-trials. Mice were started from a randomly generated quadrant for each sub-trial and sub-trials were spaced at minimum 20 min apart. The two sub-trials of each day were averaged together and the average was reported, with a possible maximum of 90 sec. Two probe trials were run where the platform was removed and mice were allowed 60 sec within the pool, probe 1 on Day 4 following the hidden trials and probe 2 on Day 5 prior to the visible trials. Software (Noldus Ethovision 11.5) was used to track the path of each mouse. A peri-platform area was drawn around the platform during probe trials set to 20% of the total area of the maze. This was done because the platform is close to the center of the pool and thus traditional quadrants would hemi-sect the area searched by a mouse that remembered the platform location.38 Visible trials were run on the last day and involved placement of a prominent red and white circular reflector, 8.26 cm in diameter, on the platform above the water level, highlighting the location of the platform. Latency to the platform was timed and Ethovision was used to track the total distance traveled to the platform.
Optomotor visual acuity task: Group 1
The optomotor visual acuity task (OPT) allows rapid testing of sub-cortical visual acuity and was used to assess behavioral threshold visual acuity in Group 1 at 28 weeks post injury (PI) (CerebralMechanics Inc.).36 Vertical sine wave gratings were projected around a testing arena (OptoMotry; CerebralMechanics). If the mouse's head tracked the cylinder rotation it was judged that the animal could see the grating. The OPT value is reported as the highest spatial frequency which elicited head tracking of the waves. Higher values correspond to tighter gratings, indicating better visual acuity. Experimenters were blinded to the injury status.
Electroretinogram (ERG) with visual evoked potential (VEP) test: Group 1
Physiological measurements from ERG and paired VEP were used to assess the activity of retinal neurons and cortical response to a visual stimulus, respectively, in a subset of animals from Group 1 (seven rmTBI, six sham) at 28 weeks post-injury. At the time of testing, mice were anesthetized with a ketamine/xylazine mixture and placed on the instrument's monitored heating pad for the duration of the procedure, as is standard practice to ensure stability of electrodes during monitoring.22-24,39-41 The eyes were moistened with eye lubricant and for the ERG, a LED light stimulator/electrode was placed against the cornea of the eye. For the VEP, a set of electrodes were placed subcutaneously, one under the scalp in correspondence of the occipital region (V1) of the brain, one in the snout as a reference, and the third placed at the base of the tail to serve as the ground electrode. A protocol of three different tests were run; a dark-adapted protocol that elicited and measured an a-wave, b-wave, oscillatory potentials (OPs), and VEP with stimulus of 1 cd sec/m2, a dark-adapted protocol that elicited an a-wave, b-wave, and c-wave stimulus of 150 cd sec/m2, and a light-adapted white stimulus of 20 cd sec/m2 on top of 40 cd sec/m2 of green background to elicit an a-wave, b-wave, and photopic negative response (PhNR; Table 1).
Table 1.
ERG Components and VEP and their Respective Cell Populations
| Test | Measured output | Cell populations/pathways | Potential causes of measured disruption (if applicable) |
|---|---|---|---|
| ERG | a-wave (amplitude, latency) | Photoreceptors (rods, cones) | Damage to rods/cones; phototoxicity58 |
| b-wave (amplitude, latency) | Muller cells ON-Bipolar cells |
Ischemia Intracellular signaling within retina41,49–53 |
|
| c-wave (amplitude, latency) | Pigmented epithelium function Retinal glial cells |
Retinal inflammation, oxidative stress54,55 | |
| Oscillatory potentials (1, 2, 3) | Amacrine cells | Glutamate toxicity56,57 | |
| Photopic negative response (PhNR) | Retinal ganglion cells | Optic nerve atrophy, lens damage21,26,27,46-48 | |
| VEP | Calculated as N1-P2 | Entire visual pathway from retina to visual cortex | Impaired signal transmission distal to retina21–25,42,43 |
Measured outputs from the ERG and paired VEP with contributing cell populations and known causes of alterations. VEP recorded from the occipital region of the brain corresponding to V1 allowed us to determine whether visual inputs were able to travel from the retina to the lateral geniculate, arrive to V1, and elicit neuronal activity intracortically. Disruptions anywhere along this pathway would lead to a decreased recorded VEP signal.
ERG, electroretinogram; VEP, visual-evoked potential; ON, optic nerve.
Behavioral tests: Group 2
Group 2 underwent an iteration of the MWM consisting only of visible trials, where the platform was marked with the large red circular reflector, 3.4 cm × 3.4 cm protruding 4 cm above the platform, for each of the trials. There were two sub-trials per trial over 4 days for a total of eight sub-trials and four trials. Trials were run in the manner described above. Latency to the platform was timed. (rmTBI, n = 13; sham, n = 11).
The forced swim test (FST) was used to test ability and motivation to swim 16-weeks post-injury in Group 2 (rmTBI, n = 5; sham, n = 4) as the final behavioral task. Total distance, speed, time mobile, and time immobile were recoded for each time bin. The immobility was determined as lack of detectable movement.
Touchscreen conditional learning task
The Bussey-Saksida touchscreen system (Lafayette Instrument, Lafayette, IN) was used to conduct the touchscreen task which evaluated learning and memory before and after rmTBI in Group 2. The test consisted of a learning period and then a testing window. Mice were food restricted throughout this task.
The system consists of a chamber with a screen containing two target touchspots and a reward delivery system. A light was illuminated on the screen and the mouse was rewarded if the correct touchspot was chosen within the allotted time. Failure to touch any point on the screen within the allotted time was defined as an omitted trial. The system recorded the number of correct, incorrect, and omitted trials.
The pre-injury training phase began when Group 2 mice were 8 weeks of age, and consisted of gradually decreasing allotments of time (response window [RW], 30, 10, 4, and 2 sec) for which the mouse was shown the light stimulus and was required to select the correct touchspot. Mice had to respond correctly 80% of the 60-trial session for 2 consecutive days to progress to the next phase. After achieving this on the 2-sec training phase, the variable window task, comprised of 60 runs per day for 4 consecutive days, with RW randomly alternating between 2 sec, 1 sec, 0.5 sec, and 0.2 sec, was run. The number of correct, incorrect, and omitted responses was recorded for each trial.
After injury or sham injury, mice entered into post-injury training, identical to the pre-injury training. Mice unable to achieve the 80% needed to progress past each training phase after 10 days (600 trials) were labeled as “Failed.” Mice were again given the variable window task after either failing or completing the 2-sec phase.
Euthanasia and optic nerve collection
All mice in the study were euthanized via cardiac perfusion. Both brain and optic nerve were removed. Additional mice underwent the same injury paradigm and optic nerves were harvested for total Sham n = 20, rmTBI = 20. Optic nerves were examined under a microscope following 24 hours in 4% paraformaldehyde (PFA) in PBS. Diameters from proximal and distal optic nerves from both eyes were measured and averaged for each mouse.
Statistical analysis
All statistical analysis was performed using STATA. Differences between two groups were analyzed using unpaired two-tailed t-tests. Differences between more than two groups were analyzed using analysis of variance (ANOVA) with Bonferroni correction. Multivariate analysis of variance (MANOVA) significance was explored with post hoc one-way ANOVA. Simple regression was used when correlating variables. Statistical sentences are reported as mean ± SD; behavioral data are graphed as mean ± standard error of the mean. A value of p < 0.05 after Bonferroni correction was used to determine significance.
Results
Group 1
Repetitive mTBI leads to behavioral changes in anxiety, gross locomotion, and locomotor function
We examined behavioral tasks that quantify overall locomotion, swimming ability, and exploratory behavior, to understand if these behaviors might contribute to changes to more complex behavioral tasks such as the visible MWM. The locomotor task was run to establish exploratory behavior as lack of exploratory behavior could result in injured mice not seeking new components of their environment, such as the marked platform in the MWM.37,42,43 Within Group 1, rmTBI mice traveled further than sham mice at 16 weeks (p = 0.0165) and 28 weeks PI (p = 0.0147), indicating that rmTBI mice did not explore less than sham mice. Repetitive mTBI mice were quicker to fall off the rotarod than sham mice at 28 weeks post-injury (p = 0.009). There were no differences in time mobile between sham and rmTBI mice in the forced swim task (rmTBI mice spent more time in the open arm of the elevated plus maze than sham mice at 16 weeks post-injury (p = 0.0115; Fig. 2).
FIG. 2.
Repetitive mild traumatic brain injury (rmTBI) leads to behavioral changes in anxiety, gross locomotion, and locomotor function. (A) Within Group 1, rmTBI mice traveled further than sham mice as measured by locomotor activity at 16 weeks (p = 0.0165; sham = 3541 ± 784 cm; rmTBI = 4583 ± 745 cm) and 28 weeks post injury (p = 0.0147; sham = 2,383 ± 1093 cm; rmTBI = 3750 ± 856 cm), indicating an overall increase in exploratory behavior after rmTBI. (B) Repetitive mTBI mice were quicker to fall off the rotarod than sham mice at 28 weeks post-injury (p = 0.009; sham = 20.59 ± 3.95 sec; rmTBI = 15.42 ± 2.76 sec). (C) There were no differences in time mobile between sham and rmTBI mice in the forced swim task. (D) Repetitive mTBI mice spent more time in the open arm of the elevated plus maze than sham mice at 16 weeks post-injury (p = 0.0115; sham = 6.23 ± 3.20%; rmTBI = 30.67 ± 21.95%). Data are graphed as mean ± standard error of the mean. *p < 0.05; **p < 0.01.
Repetitive mTBI leads to impaired performance in the hidden MWM without impaired swim ability
There was a significant difference in the hidden MWM between Group 1 rmTBI mice and sham [MANOVA, F(1,11) = 5.49, p = 0.011] (Fig. 3). Group 1 rmTBI mice were slower to find the hidden platform in the water maze at 28 weeks post-injury on hidden trials 3 (p = 0.001) and 4 (p = 0.007) but not on hidden trials 1 or 2. During probe trials, sham mice spent more time in the peri-platform area than rmTBI mice (p = 0.02) during the first probe trial only, indicating that sham animals remembered the platform location but began searching for a new platform location during the second trial, after failing to find the platform in the original location.44,45 There was not a significant difference between sham and rmTBI groups in either swim speed or distance traveled during probe trials (p > 0.05), indicating injured animals were both as able and as motivated to swim as well as sham animals.
FIG. 3.
Repetitive mild traumatic brain injury (rmTBI) leads to impairment in the hidden trials Morris water maze (MWM). (A) Group 1: There was a significant difference in the hidden MWM between Group 1 rmTBI mice and sham [MANOVA, F(1,11) = 5.49, p = 0.011]. with rmTBI slower to find the hidden platform in the water maze at 28 weeks post-injury on hidden trials 3 (p = 0.001; sham = 23 ± 15.02 sec; rmTBI = 53.2 5 ± 14.42 sec) and 4 (p = 0.007; sham = 11.06 ± 5.14 sec; rmTBI = 34.25 ± 20.06 sec) only. (B) Diagram of the MWM with peri-platform zone labeled along with platform and quadrants. Peri-platform zone is 20% of the area of the pool. (C) Heatmap tracings of probe trials reveal that sham animals appear to spend more time around platform location. Quadrants are marked by bisecting lines and probe location on prior hidden trials is marked by black circle in left lower quadrant. Data are graphed as mean ± standard error of the mean. *p < 0.05; **p < 0.01.
Repetitive mTBI leads to impaired visual acuity in the optomotor task
The optomotor task (OPT) was used to evaluate behavioral visual function in Group 1 mice at 28 weeks PI. While sham mice were able to track gratings at a visual acuity consistent with that in the literature,36,46 rmTBI mice were almost completely unable to see even the lowest frequency of gratings presented across their visual field, indicating near blindness (Fig. 4).
FIG. 4.
Repetitive mild traumatic brain injury (rmTBI) results in decreased visual acuity bilaterally as measured by the optomotor task. The threshold spatial frequency at which the mouse could track a moving stimulus (cycles-per-degree, cpd) represents optomotor visual acuity task (OPT) acuity. Sham control mice exhibited an OPT acuity around 0.364 c/deg, similar to what has been reported for C57BL6 control mice. In terms of Snellen equivalents, or “20/20 vision,” this is equal to 20/1648 vision, a normal value for the myopic mouse.36,46 In contrast, rmTBI mice showed a strong deficit in the OPT, being almost unable to track the lowest spatial frequencies and had a visual acuity of 0.039 cpd equal to 20/15,384 vision (p < 0.0001; sham = 0.364 ± 0.02 cpd; rmTBI = 0.039 ± 0.053 cpd) indicating near blindness. Data are graphed as mean ± standard error of the mean. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Repetitive mTBI leads to minimal deficits in retinal function measured by ERG and decreased visual cortical function measured by VEP. ERG with the VEP was run on Group 1 mice at 28 weeks post-injury
Using ERG along with the VEP in response to flash stimuli after dark adaptation allowed us to evaluate activity of different discrete cell types in the retina and transduction to the visual cortex (Table 1).21,26,27,41,47–59
The mean amplitude of major ERG components revealed a decrease in OP3 in rmTBI mice (p = 0.049). There was a significant loss of VEP in rmTBI (p = 0.0007; Fig. 5), indicating that rmTBI induced major damages in signal transmission from the retina to the visual cortex, potentially caused by damage to the both the main and accessory optic tract.60
FIG. 5.
Repetitive mild traumatic brain injury (rmTBI) disrupts visual pathway signaling distal to the retina as measured by electroretinogram (ERG) and visually evoked potential (VEP) test. (A) Repetitive mTBI led to decreased latency to a-wave peak at 1 cd sec/m2 (p < 0.001; sham = 17.25 ± 1.97 msec; rmTBI = 14.14 ± 2.10 msec) and at 150 cd sec/m2 (p = 0.011; sham = 21.00 ± 1.26 msec; rmTBI = 19.54 ± 1.42 msec) without changes in a-wave amplitude. Repetitive mTBI led to a decrease in oscillatory potential 3 (OP3; p = 0.049; sham = 27.90 ± 11.45 uV; rmTBI = 20.64 ± 6.01 msec). Repetitive mTBI did not change photopic negative response (PhNR) or b-wave or c-wave amplitude or time to peak. (B) Tracings of ERG components of sham and rmTBI animals overlain. VEP responses in rmTBI mice were dramatically impaired. While Sham control mice exhibited a clear response to flash stimuli with well-defined P1 and N1 peaks, rmTBI mice showed a decreased P1 amplitude (p = 0.007; sham = 11.56 ± 7.14 uV; rmTBI = -0.50 ± 12.51 uV) and N1 response (p = 0.0025; sham = -33.78 ± 17.17 uV; rmTBI = -11.50 ± 19.30 uV). *p < 0.05.
VEP predicts rotarod performance
Vestibular and visual function are closely linked in both humans and animals.61-63 Simple regression revealed a negative correlation between VEP and latency to fall on rotarod (coefficient = -0.14, p = 0.04, adjusted R2 = 0.46).
Repetitive mTBI leads to gross morphological changes in the optic nerve at 7 months
Repetitive mTBI resulted in decreased averaged diameter of the optic nerve (p < 0.0001, sham = 0.43 ± 0.03 mm; rmTBI = 0.29 ± 0.03 mm; Fig. 6).
FIG. 6.
Optic nerves (ONs) are grossly abnormal and have decreased diameters following repetitive mild traumatic brain injury (rmTBI). (A) Prior to removal, optic nerves were visualized by dissecting away the superior orbit and frontal bone of skull. ONs were subsequently dissected out following perfusions and diameters were measured at proximal and distal ends of the nerves. (B) Grossly, ONs were translucent and less distinct from surrounding tissue in rmTBI animals and ON diameter was significantly reduced following rmTBI (p < 0.05).
Group 2
All mice can learn the touchscreen task
Prior to rmTBI, all mice successfully learned the touchscreen task and advanced through all training stages (Table 2). There was no significant difference between mice randomized to the rmTBI and sham groups (p > 0.05). In the variable-window task, mice overall answered fewer trials correctly, answered more trials incorrectly, and omitted more trials when the RW was shorter [F(9, 219) = 24.16, p < 0.0001; Table 3].
Table 2.
Pre-Injury (All Mice): Ability to Progress
| Training phase(s) | Number of trials | Correct (%) | Incorrect (%) | Omitted (%) |
|---|---|---|---|---|
| 30 | 400.2 ± 120.0 | 69.6 ± 9.22 | 23.42 ± 5.13 | 6.96 ± 6.73 |
| 10 | 155.3 ± 53.0*** | 86.83 ± 6.19*** | 9.54 ± 3.34*** | 3.63 ± 4.93 |
| 4 | 170.0 ± 92.9 | 84.32 ± 7.38 | 6.79 ± 3.22 | 8.88 ± 5.66** |
| 2 | 357.3 ± 154.4*** | 79.7 ± 3.27 | 6.26 ± 2.51 | 14.08 ± 3.06** |
All animals were able to learn the touchscreen conditional learning task before injury (training phase). Twenty-four animals were trained in total and randomly assigned to sham injury or repetitive mild traumatic brain injury following training. The minimum possible number of trials needed to progress is 120, as mice needed to demonstrate 80% correct response rate over 2 days with 60 trials/day. Data are shown as mean ± standard deviation. Statistically significant difference of each phase from the preceding phase are shown (i.e., a significance marker within 10-sec phase indicates difference from 30-sec phase).
p < 0.05; **p < 0.01; ***p < 0.001.
Table 3.
Pre-Injury (All Mice): Baseline Variable Test
| Testing phase (sec) | Correct (%) | Incorrect (%) | Omitted (%) |
|---|---|---|---|
| 2 | 79.74 ± 9.14 | 7.55 ± 4.00 | 12.63 ± 8.92 |
| 1 | 66.70 ± 8.30*** | 9.96 ± 5.40 | 23.58 ± 8.74** |
| 0.5 | 50.64 ± 11.77*** | 14.82 ± 5.11*** | 34.68 ± 12.65** |
| 0.2 | 37.00 ± 8.41*** | 21.27 ± 6.58*** | 41.44 ± 11.82 |
Pre-injury baseline variable window test for all mice following touchscreen training task. All 24 mice underwent the variable window test, consisting of trials with varying windows of time of 2 sec, 1 sec, 0.5 sec, or 0.2 sec randomly alternating. Pre-injury training on the touchscreen task. Overall, mice were less likely to respond correctly and more likely to respond incorrectly or omit trials during trials with a shorter response window in the variable window test; notably, mice did not answer more trials incorrectly until response window was under 1 sec, but started omitting more trials at when limited to under 2 sec. Mice omitted the same number of trials, around 40%, at the two shortest response windows of 0.5 and 0.2 sec. Statistically significant difference of each phase from the preceding phase are shown (i.e., a significance marker within 10-sec phase indicates difference from 30-sec phase).
p < 0.05; **p < 0.01; ***p < 0.001.
Sham animals re-learn touchscreen task more rapidly than initial training
Animals who received sham injury progressed through the touchscreen task more rapidly than initial learning (Table 3 in Supplementary Information S1), indicating they remembered the task after the week of sham injury.
Repetitive mTBI slows or prevents progression through the touchscreen task
After injury, rmTBI mice were less able to progress through the touchscreen training than sham mice. Seven of the 13 rmTBI animals failed the 30-sec task, and one additional mouse failed the 4-sec task. All sham animals were able to progress through the entire task (Fig. 7). For further analysis, rmTBI mice were divided into those that progressed past the 30-sec task (Injured/Progressed, I/P) and those that failed (Injured/Failed, I/F). I/P mice performed worse than sham mice, but better than I/F mice on the 30-sec phase (Fig. 8; Table S4 in Supplementary Information S1).
FIG. 7.
Post-injury touchscreen. A significant percent of repetitive mild traumatic brain injury (rmTBI) animals were unable to re-learn the touchscreen conditional learning task after injury, despite having adequately learned the task initially. Seven of 13 animals failed at the 30-sec phase, defined as unable to reach 2 consecutive days of 80% correct responses within 10 days. One additional mouse failed the 4-sec phase.
FIG. 8.
Repetitive mild traumatic brain injury (rmTBI) leads to more incorrect and more omitted trials in the touchscreen conditional learning task. Repetitive mTBI mice were divided into two groups based on ability to progress past post-injury 30-sec training phase, Injured/Progressed (I/P) and Injured/Failed (I/F). (A) At all phases, I/P responded to fewer trials correctly than Sham. This was driven predominantly by an increased number of incorrect trials (B), apart from the 2-sec training phase where I/P animals omitted more trials than Sham (C). Within the 30-sec phase, I/P mice responded to more trials correctly than I/F, responded to fewer trials incorrectly, and omitted fewer trials. *p < 0.05; **p < 0.01; ***p < 0.001 between sham and I/P mice. #p < 0.05; ##p < 0.01; ###p < 0.001 between I/P and I/F mice.
Repetitive mTBI impairs performance on the touchscreen variable testing task
In the post-injury touchscreen variable window test, compared to sham mice, I/P mice answered fewer trials correctly when the RW was 2 sec, 1 sec, and 0.5 sec, more trials incorrectly when the RW was 1 sec, and omitted more trials when the RW was 2 sec and 1 sec. I/P mice answered more trials correctly and omitted fewer trials than I/F mice across all RWs, and answered fewer trials incorrectly when RW was 1 sec and 0.2 sec (Fig. 9; Table S5 in Supplementary Information S1).
FIG. 9.
Repetitive mild traumatic brain injury (rmTBI) results in impaired performance in the post-injury variable window test of the touchscreen task. Injured mice were separated into two groups as in Figure 8 (Injured/Progressed [I/P) and Injured/Failed [I/F]). (A) Within 2-sec trials, I/P responded to fewer trials correctly than sham, and I/F animals responded to fewer trials correctly than I/P. (B) I/P responded to more trials incorrectly than sham within 1-sec trials. (C) I/P omitted more trials than sham within 2-sec and 1-sec trials, but omitted fewer trials than I/F in all trials. *p < 0.05; **p < 0.01; ***p < 0.001 between sham and I/P mice. #p < 0.05; ##p < 0.01; ###p < 0.001 between I/P and I/F mice.
Repetitive mTBI leads to deficits in the visible MWM. Group 2 mice underwent the visible MWM after the touchscreen task
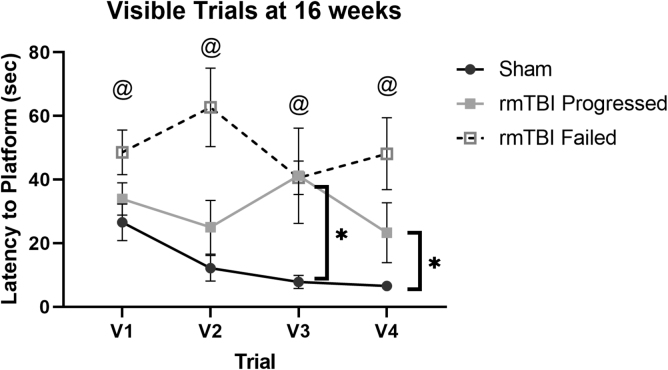
Injured animals were separated during analysis as described above (I/P, I/F). I/P mice were slower to platform than sham on visible trials 3-4 (Fig. 10; Sham vs. I/P, p < 0.05) despite swimming faster during the task (Sham = 16.1 ± 6.9 cm/sec, I/P = 18.9 ± 5.83 cm/sec, p = 0.027). I/F mice were slower to platform on all visible trials compared to sham performance (Fig. 10; Sham vs. I/F, p < 0.05) with no difference in swim speed (p > 0.05). There was no difference between I/P mice and I/F mice in latency to platform or swim speed (Fig. 10; I/P vs. I/F, p > 0.05).
FIG. 10.
Repetitive mild traumatic brain injury (rmTBI) results in impairment in a visible Morris water maze that is different between Injured/Progressed (I/P) mice vs. Injured/Failed (I/F) mice. Mice that received rmTBI were divided into I/P and I/F groups based on touchscreen performance. I/P mice are slower than sham to find the visible platform on Days 3 and 4. I/F mice are slower than shame to reach the visible platform on all visible trials (p < 0.05). *p < 0.05 between sham and I/P mice. @p < 0.05 between sham and I/F mice.
Visible trial performance predicts omitted trials on the touchscreen 30-sec task regardless of injury severity
All visible trials of the MWM were summed for an aggregate metric of performance to assess functional visual capacity; a mouse that could see the platform would navigate quicker than a mouse that could not see it.15,64,65 Within the touchscreen task, mice may have omitted trials because they were unable to see the cue. A multiple regression controlling for loss of consciousness, as a marker for severity of injury, revealed that the aggregate latency to visible platform in the MWM was related to number of omitted trials in the 30-sec touchscreen task (coefficient = 3.10, p = 0.020, adjusted R2 = 0.409) but was not related to loss of consciousness (p = 0.054; Fig. 11).
FIG. 11.
Aggregate time required for a mouse to reach the visible platform in the MWM predicted number of omitted trials in at 30-sec in the touchscreen task (coefficient = 0.04, p = 0.0006, adjusted R2 = 0.397). Visible trials were summed to create a metric for overall visual performance. Thirty-second trial omitted trials were chosen as mice that were unable to see the cue starting the task would have failed to respond to the trial entirely, resulting in trial omission.
Discussion
Here, we found that a reliable model of rmTBI elicits clear damage to multiple cortical and subcortical brain regions impacting not only locomotor function, anxiety behaviors, and cognition, but also visual processing, strongly suggesting possible interactions between the primary sensory modality deficits and higher function behavioral performance.
Notably, visual impairment was correlated with locomotor dysfunction on the rotarod, a task that relies on vestibular function. The vestibulo-ocular reflex (VOR) maintains balance via positional and spatial feedback to stabilize during movement66 and is disrupted after TBI in both humans and animals.62,63,67-69 Tasks that require rapid balance adjustment such as the rotarod are strongly affected by damage to the VOR.61 The relationship between dysfunction in vision and vestibular function establishes the sensitivity of the VOR to damage after our model of rmTBI.
Repetitive mTBI-induced visual pathway dysfunction was distal to the retina as evidenced by the almost complete erosion of VEP without notable ERG changes, indicating a deficit in retinal signal transduction which has been noted in a similar model of rmTBI.18 Proposed mechanisms for such transduction damage include traumatic optic neuropathy concurrent with loss of retinal ganglion cells (RCG)19,20,70 and widespread cortical and sub-cortical axonal injury.71–75 Both of these mechanisms are likely contributory to visual dysfunction noted here. The axons of retinal ganglion cells make up the optic nerve, which was atrophied here similarly to other models of TBI19,20,76 and may be explained by the reduction in number and demyelination of axons that occurs after TBI.77-79 The results of this study, in conjunction with prior research, implicate both optic neuropathy and white and gray matter damage as key mediators of impaired visual function following rmTBI.
It is important to examine the behavioral implications of this damage in addition to elucidating the underlying pathology. Our model of rmTBI, like many others, partially relies on behavioral testing paradigms to support the external validity of the model regarding clinical phenotypes. For example, the Morris water maze, a metric for spatial learning and memory, requires the mouse to see visual cues to spatially orient itself and find the platform. Injured mice were slower to navigate to both the hidden and visible platforms than sham, suggesting the visual system may be a moderator of the relationship between brain injury and MWM performance as impaired visual processing limits the ability of the animal to orient itself. Importantly, injured animals demonstrated more exploratory behavior in the locomotor task, were as mobile in the FST, and did not swim slower during the MWM. Mice were therefore motivated to escape the MWM and able to navigate it, further implicating visual dysfunction as the primary pathology underlying impaired performance. In the setting of injuries such as TBI, it is therefore necessary to either develop methods to control for visual damage or utilize tasks that can distinguish visual impairment from learning, memory, and other cognitive deficits.
The touchscreen learning task allows separation between visual processing and cognitive impairments. Repetitive mTBI resulted in more incorrect and omitted trials indicating concurrent deficits in both learning/memory and in visual function. Separating injured mice into two groups based on performance on the 30-sec task allowed distinction between mice that failed to see and thus omitted trials, and mice that could see trials but responded incorrectly, suggesting that vision was preserved enough for this group to see and respond to the stimulus. The increase in omitted trials during shorter training phases reveals subtler visual deficits still elucidated by this task. By selectively evaluating differences in incorrect trials between groups, we could reasonably well isolate impairments in learning from visual deficits. Importantly, mice that omitted more trials on the 30-sec task were slower to find the visible platform in the MWM regardless of injury severity. This further supports omissions in the touchscreen task as a metric for visual impairment, as well as implicates visual dysfunction as the underlying variable between omissions in touchscreen and latency on the visible MWM.
There are limitations to this study. We were not able to measure all outcomes in all animals; a complete assessment of the impact of visual deficits across the spectrum of behavioral tests is necessary. Our ERG with VEP protocol utilized only one stimulus intensity each for measurement of VEP, c-wave, and PhNR. More robust testing with a broader range of stimuli including pattern stimuli may reveal more subtle differences previously noted by Tzekov and colleagues after TBI.76 We selected a relatively strict passing criteria (80%) to advance onto subsequent touchscreen phases; a less stringent criteria would likely have resulted in more injured mice progressing past the 30-sec re-training phase.
Our model of rmTBI resulted in widespread functional impairment in the key cortical circuits controlling learning and memory, vision, and anxiety behaviors. As visual impairments have been reported after TBI in humans, including acute symptoms like photosensitivity and blurry vision,70,80–83 and long-term visual dysfunction in the majority of high-risk populations,84 future work across species should be devoted to better characterize the impact of loss of proper visual processing on higher cognitive and emotional functions.
Supplementary Material
Acknowledgments
We would like to thank Lois Smith, MD, PhD, for her insightful contributions to this manuscript.
Authors' Contributions
Nicholas Morriss: Conceptualization, methodology, investigation, data interpretation, writing, final draft approval, revision review and editing.
Grace Conley: Conceptualization, methodology, investigation, data interpretation, writing, final draft approval, revision review and editing.
Nathaniel Hodgson: Methodology, investigation, data interpretation, writing, final draft approval.
Masen Boucher: investigation, writing, data interpretation, final draft approval.
Sara Ospina-Mora: investigation, final draft approval.
Michela Fagiolini: Methodology, data interpretation, writing, final draft approval, revisions.
Mark Puder: Methodology, data interpretation, writing, final draft approval, revisions.
Leo Mejia: Methodology, data interpretation, revision, final draft approval.
Jianhua Qiu: Data interpretation, revision, final draft approval.
William Meehan: Conceptualization, methodology, supervision, investigation, data interpretation, writing, final draft approval, revision, funding acquisition.
Rebekah Mannix: Conceptualization, methodology, supervision, investigation, data interpretation, writing, final draft approval, revision, funding acquisition.
Funding Information
The experiments described here were funded, in part, by the National Football League through the NFL LONG study and the National Football League Players Association. Part of the work was performed in the Animal Behavioral and Physiology Core at Boston Children's Hospital (CHB IDDRC, 1U54HD090255).
Author Disclosure Statement
Dr. Meehan receives royalties from: 1) ABC-Clio publishing for the sale of his books, Kids, Sports, and Concussion: A Guide for Coaches and Parents and Concussions; 2) Springer International for the book Head and Neck Injuries in Young Athlete; and 3) Wolters Kluwer for working as an author for UpToDate. His research is funded, in part, by philanthropic support from the National Hockey League Alumni Association through the Corey C. Griffin Pro-Am Tournament and a grant from the National Football League. Dr. Mannix is funded in part by a grant from the National Football League.
For the other authors, no competing financial interests exist.
Supplementary Material
References
- 1. Rutland-Brown, W., Langlois, J.A., Thomas, K.E., and Xi, Y.L. (2006). Incidence of traumatic brain injury in the United States, 2003. J. Head Trauma Rehabil. 21, 544–548. [DOI] [PubMed] [Google Scholar]
- 2. Langlois, J.A., Rutland-Brown, W., and Wald, M.M. (2006). The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 21, 375–378. [DOI] [PubMed] [Google Scholar]
- 3. Torres, D.M., Galetta, K.M., Phillips, H.W., Dziemianowicz, E.M., Wilson, J.A., Dorman, E.S., Laudano, E., Galetta, S.L., and Balcer, L.J. (2013). Sports-related concussion: anonymous survey of a collegiate cohort. Neurol. Clin. Pract. 3, 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Manley, G., Gardner, A.J., Schneider, K.J., Guskiewicz, K.M., Bailes, J., Cantu, R.C., Castellani, R.J., Turner, M., Jordan, B.D., Randolph, C., Dvorak, J., Hayden, K.A., Tator, C.H., McCrory, P., and Iverson, G.L. (2017). A systematic review of potential long-term effects of sport-related concussion. Br. J. Sports Med. 51, 969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meehan, W.P., 3rd and Micheli, L.J. (2011). Concussion results in deficits in neurocognitive functioning. Preface. Clin. Sports. Med. 30, xvii–xviii. [DOI] [PubMed] [Google Scholar]
- 6. Catena, R.D., van Donkelaar, P., and Chou, L.S. (2009). Different gait tasks distinguish immediate vs. long-term effects of concussion on balance control. J. Neuroeng. Rehabil. 6, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iverson, G.L., Gaetz, M., Lovell, M.R., and Collins, M.W. (2004). Cumulative effects of concussion in amateur athletes. Brain Inj. 18, 433–443. [DOI] [PubMed] [Google Scholar]
- 8. Rice, S.M., Parker, A.G., Rosenbaum, S., Bailey, A., Mawren, D., and Purcell, R. (2018). Sport-related concussion and mental health outcomes in elite athletes: a systematic review. Sports Med. 48, 447–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khurana, V.G. and Kaye, A.H. (2012). An overview of concussion in sport. J. Clin. Neurosci. 19, 1–11. [DOI] [PubMed] [Google Scholar]
- 10. McAllister, T. and McCrea, M. (2017). Long-term cognitive and n europsychiatric consequences of repetitive concussion and head-impact exposure. J. Athl. Train. 52, 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Macciocchi, S.N., Barth, J.T., Alves, W., Rimel, R.W., and Jane, J.A. (1996). Neuropsychological functioning and recovery after mild head injury in collegiate athletes. Neurosurgery 39, 510–514. [PubMed] [Google Scholar]
- 12. Floyd, C.L., Golden, K.M., Black, R.T., Hamm, R.J., and Lyeth, B.G. (2002). Craniectomy position affects morris water maze performance and hippocampal cell loss after parasagittal fluid percussion. J. Neurotrauma 19, 303–316. [DOI] [PubMed] [Google Scholar]
- 13. Logue, S.F., Paylor, R., and Wehner, J.M. (1997). Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav. Neurosci. 111, 104–113. [DOI] [PubMed] [Google Scholar]
- 14. Dong, Z., Bai, Y., Wu, X., Li, H., Gong, B., Howland, J.G., Huang, Y., He, W., Li, T., and Wang, Y.T. (2013). Hippocampal long-term depression mediates spatial reversal learning in the Morris water maze. Neuropharmacology 64, 65–73. [DOI] [PubMed] [Google Scholar]
- 15. Redish, A.D. and Touretzky, D.S. (1998). The role of the hippocampus in solving the Morris water maze. Neural Comput. 10, 73–111. [DOI] [PubMed] [Google Scholar]
- 16. Yue, J.K., Levin, H.S., Suen, C.G., Morrissey, M.R., Runyon, S.J., Winkler, E.A., Puffer, R.C., Deng, H., Robinson, C.K., Rick, J.W., Phelps, R.R.L., Sharma, S., Taylor, S.R., Vassar, M.J., Cnossen, M.C., Lingsma, H.F., Gardner, R.C., Temkin, N.R., Barber, J., Dikmen, S.S., Yuh, E.L., Mukherjee, P., Stein, M.B., Cage, T.A., Valadka, A.B., Okonkwo, D.O., and Manley, G.T.; TRACK-TBI Investigators. (2019). Age and sex-mediated differences in six-month outcomes after mild traumatic brain injury in young adults: a TRACK-TBI study. Neurol. Res. 41, 609–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vest, V., Bernardo-Colon, A., Watkins, D., Kim, B., and Rex, T.S. (2019). Rapid repeat exposure to subthreshold trauma causes synergistic axonal damage and functional deficits in the visual pathway in a mouse model. J. Neurotrauma 36, 1646–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Desai, A., Chen, H.Z., and Kim, H.Y. (2020). Multiple mild traumatic brain injuries lead to visual dysfunction in a mouse model. J. Neurotrauma 37, 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tzekov, R., Ferro, A., Mouzon, B., Biggins, D., Spinelli, R., Mullan, M., Mullan, M., and Crawford, F. (2013). Optic nerve and inner retina damage after repeated mild traumatic brain injury in a mouse model. Invest. Ophth. Vis. Sci. 54. [Google Scholar]
- 20. Tzekov, R., Dawson, C., Orlando, M., Mouzon, B., Reed, J., Evans, J., Crynen, G., Mullan, M., and Crawford, F. (2016). Sub-chronic neuropathological and biochemical changes in mouse visual system after repetitive mild traumatic brain injury. PLoS One 11, e0153608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benchorin, G., Calton, M.A., Beaulieu, M.O., and Vollrath, D. (2017). Assessment of murine retinal function by electroretinography. Bio Protoc 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pinto, L.H., Invergo, B., Shimomura, K., Takahashi, J.S., and Troy, J.B. (2007). Interpretation of the mouse electroretinogram. Doc. Ophthalmol. 115, 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chou, T.H. and Porciatti, V. (2012). The Bioelectric Field of the Pattern Electroretinogram in the Mouse. Invest. Ophth. Vis. Sci. 53, 8086–8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chou, T.H., Toft-Nielsen, J., and Porciatti, V. (2018). High-throughput binocular pattern electroretinograms in the mouse. Methods Mol. Biol. 1695, 63–68. [DOI] [PubMed] [Google Scholar]
- 25. Porciatti, V. (2007). The mouse pattern electroretinogram. Doc. Ophthalmol. 115, 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pasmanter, N., and Petersen-Jones, S.M. (2020). A review of electroretinography waveforms and models and their application in the dog. Vet. Ophthalmol. 23, 418–435. [DOI] [PubMed] [Google Scholar]
- 27. Tanimoto, N., Muehlfriedel, R.L., Fischer, M.D., Fahl, E., Humphries, P., Biel, M., and Seeliger, M.W. (2009). Vision tests in the mouse: functional phenotyping with electroretinography. Front. Biosci. (Landmark Ed.) 14, 2730–2737. [DOI] [PubMed] [Google Scholar]
- 28. Mannix, R., Berglass, J., Berkner, J., Moleus, P., Qiu, J., Andrews, N., Gunner, G., Berglass, L., Jantzie, L.L., Robinson, S., and Meehan, W.P., 3rd (2014). Chronic gliosis and behavioral deficits in mice following repetitive mild traumatic brain injury. J. Neurosurg. 121, 1342–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mannix, R., Meehan, W.P., Mandeville, J., Grant, P.E., Gray, T., Berglass, J., Zhang, J., Bryant, J., Rezaie, S., Chung, J.Y., Peters, N.V., Lee, C., Tien, L.W., Kaplan, D.L., Feany, M., and Whalen, M. (2013). Clinical correlates in an experimental model of repetitive mild brain injury. Ann. Neurol. 74, 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meehan, W.P., 3rd, Zhang, J., Mannix, R., and Whalen, M.J. (2012). Increasing recovery time between injuries improves cognitive outcome after repetitive mild concussive brain injuries in mice. Neurosurgery 71, 885–0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ma, G., Liu, C., Hashim, J., Conley, G., Morriss, N., Meehan, W.P., Qiu, J., and Mannix, R. (2019). Memantine mitigates oligodendrocyte damage after repetitive mild traumatic brain Injury. Neuroscience 421, 152–161. [DOI] [PubMed] [Google Scholar]
- 32. Mei, Z., Qiu, J., Alcon, S., Hashim, J., Rotenberg, A., Sun, Y., Meehan, W.P., 3rd, and Mannix, R. (2018). Memantine improves outcomes after repetitive traumatic brain injury. Behav. Brain Res. 340, 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Robinson, S., Berglass, J.B., Denson, J.L., Berkner, J., Anstine, C.V., Winer, J.L., Maxwell, J.R., Qiu, J., Yang, Y., Sillerud, L.O., Meehan, W.P., 3rd, Mannix, R., and Jantzie, L.L. (2017). Microstructural and microglial changes after repetitive mild traumatic brain injury in mice. J. Neurosci. Res. 95, 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mannix, R., Berkner, J., Mei, Z., Alcon, S., Hashim, J., Robinson, S., Jantzie, L., Meehan, W.P., 3rd, and Qiu, J. (2017). Adolescent mice demonstrate a distinct pattern of injury after repetitive mild traumatic brain injury. J. Neurotrauma 34, 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu, X., Qiu, J., Alcon, S., Hashim, J., Meehan, W.P., 3rd, and Mannix, R. (2017). Environmental enrichment mitigates deficits after repetitive mild traumatic brain injury. J. Neurotrauma 34, 2445–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prusky, G.T., Alam, N.M., Beekman, S., and Douglas, R.M. (2004). Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest. Ophthalmol. Vis. Sci. 45, 4611–4616. [DOI] [PubMed] [Google Scholar]
- 37. Szentes, N., Tekus, V., Mohos, V., Borbely, E., and Helyes, Z. (2019). Exploratory and locomotor activity, learning and memory functions in somatostatin receptor subtype 4 gene-deficient mice in relation to aging and sex. Geroscience 41, 631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kolarik, B.S., Shahlaie, K., Hassan, A., Borders, A.A., Kaufman, K.C., Gurkoff, G., Yonelinas, A.P., and Ekstrom, A.D. (2016). Impairments in precision, rather than spatial strategy, characterize performance on the virtual Morris water maze: a case study. Neuropsychologia 80, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perlman, I. (1995). The electroretinogram: ER, in: Webvision: The Organization of the Retina and Visual System. H. Kolb, R, Nelson, E. Fernandez, and B Jones. (eds). https://webvision.med.utah.edu (Last accessed August 17, 2021).
- 40. Schechner, R., Gdalon, M., Cohen, D., Meyer, E., Zonis, S., and Perlman, I. (1987). Recovery of the electroretinogram in rabbits after argon-laser photocoagulation. Invest. Ophth. Vis. Sci. 28, 1605–1613. [PubMed] [Google Scholar]
- 41. Noell, W.K. (1954). The origin of the electroretinogram. Am J Ophthalmol 38, 78-90. [DOI] [PubMed] [Google Scholar]
- 42. Tatem, K.S., Quinn, J.L., Phadke, A., Yu, Q., Gordish-Dressman, H., and Nagaraju, K. (2014). Behavioral and locomotor measurements using an open field activity monitoring system for skeletal muscle diseases. J. Vis. Exp. 51785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pellow, S. and File, S.E. (1986). Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 24, 525–529. [DOI] [PubMed] [Google Scholar]
- 44. Blokland, A., Geraerts, E., and Been, M. (2004). A detailed analysis of rats' spatial memory in a probe trial of a Morris task. Behav. Brain Res. 154, 71–75. [DOI] [PubMed] [Google Scholar]
- 45. Vorhees, C.V. and Williams, M.T. (2006). Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 1, 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hamm, R.J., Pike, B.R., O'Dell, D.M., Lyeth, B.G., and Jenkins, L.W. (1994). The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J. Neurotrauma 11, 187–196. [DOI] [PubMed] [Google Scholar]
- 47. Asi, H. and Perlman, I. (1992). Relationships between the rlectroretinogram a-wave, B-wave and oscillatory potentials and their application to clinical-diagnosis. Doc. Ophthalmol. 79, 125–139. [DOI] [PubMed] [Google Scholar]
- 48. Asi, H., Leibu, R., and Perlman, I. (1992). Frequency-domain analysis of the human corneal electroretinogram. Clin. Vis. Sci. 7, 9–19. [Google Scholar]
- 49. Tomiyama, Y., Fujita, K., Nishiguchi, K.M., Tokashiki, N., Daigaku, R., Tabata, K., Sugano, E., Tomita, H., and Nakazawa, T. (2016). Measurement of electroretinograms and visually evoked potentials in awake moving mice. PLoS One 11, e0156927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brown, K.T. and Watanabe, K. (1962). Isolation and identification of a receptor potential from the pure cone fovea of the monkey retina. Nature 193, 958. [DOI] [PubMed] [Google Scholar]
- 51. Nilsson, S.E. (1971). Human retinal vascular obstructions. A quantitative correlation of angiographic and electroretinographic findings. Acta Ophthalmol. (Copenh.) 49, 111–133. [PubMed] [Google Scholar]
- 52. Furukawa, T. and Hanawa, I. (1955). Effects of some common cations on electroretinogram of the toad. Jpn. J. Physiol. 5, 289–300. [DOI] [PubMed] [Google Scholar]
- 53. Sillman, A.J., Ito, H., and Tomita, T. (1969). Studies on the mass receptor potential of the isolated frog retina. I. General properties of the response. Vis. Res. 9, 1435–1442. [DOI] [PubMed] [Google Scholar]
- 54. Pepperberg, D.R. and Masland, R.H. (1978). Retinal-induced sensitization of light-adapted rabbit photoreceptors. Brain Res. 151, 194–200. [DOI] [PubMed] [Google Scholar]
- 55. Sparrow, J.R., Hicks, D., and Hamel, C.P. (2010). The retinal pigment epithelium in health and disease. Curr. Mol. Med. 10, 802–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Trakkides, T.O., Schafer, N., Reichenthaler, M., Kuhn, K., Brandwijk, R., Toonen, E.J.M., Urban, F., Wegener, J., Enzmann, V., and Pauly, D. (2019). Oxidative stress increases endogenous complement-dependent inflammatory and angiogenic responses in retinal pigment epithelial cells independently of exogenous complement sources. Antioxidants (Basel) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Duarte, C.B., Ferreira, I.L., Santos, P.F., Carvalho, A.L., Agostinho, P.M., and Carvalho, A.P. (1998). Glutamate in life and death of retinal amacrine cells. Gen. Pharmacol. 30, 289–295. [DOI] [PubMed] [Google Scholar]
- 58. Sucher, N.J., Lipton, S.A., and Dreyer, E.B. (1997). Molecular basis of glutamate toxicity in retinal ganglion cells. Vis. Res. 37, 3483–3493. [DOI] [PubMed] [Google Scholar]
- 59. Organisciak, D.T. and Vaughan, D.K. (2010). Retinal light damage: mechanisms and protection. Prog. Retin. Eye Res. 29, 113–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee, J.Y., Holden, L.A., and Djamgoz, M.B. (1997). Effects of ageing on spatial aspects of the pattern electroretinogram in male and female quail. Vis. Res. 37, 505–514. [DOI] [PubMed] [Google Scholar]
- 61. Jones, S.M., Jones, T.A., Mills, K.N., and Gaines, G.C. (2009). Anatomical and physiological considerations in vestibular dysfunction and compensation. Semin. Hear. 30, 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hoffer, M.E., Gottshall, K.R., Moore, R., Balough, B.J., and Wester, D. (2004). Characterizing and treating dizziness after mild head trauma. Otol. Neurotol. 25, 135–138. [DOI] [PubMed] [Google Scholar]
- 63. Fausti, S.A., Wilmington, D.J., Gallun, F.J., Myers, P.J., and Henry, J.A. (2009). Auditory and vestibular dysfunction associated with blast-related traumatic brain injury. J. Rehabil. Res. Dev. 46, 797–810. [DOI] [PubMed] [Google Scholar]
- 64. Eichenbaum, H., Stewart, C., and Morris, R.G. (1990). Hippocampal representation in place learning. J. Neurosci. 10, 3531–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McDonald, R.J. and White, N.M. (1994). Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav. Neural Biol. 61, 260–270. [DOI] [PubMed] [Google Scholar]
- 66. Peterson, M.D. (2010). A case-oriented approach exploring the relationship between visual and vestibular disturbances and problems of higher-level mobility in persons with traumatic brain injury. J. Head Trauma Rehabil. 25, 193–205. [DOI] [PubMed] [Google Scholar]
- 67. Wallace, B. and Lifshitz, J. (2016). Traumatic brain injury and vestibulo-ocular function: current challenges and future prospects. Eye Brain 8, 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chamelian, L. and Feinstein, A. (2004). Outcome after mild to moderate traumatic brain injury: the role of dizziness. Arch. Phys. Med. Rehabil. 85, 1662–1666. [DOI] [PubMed] [Google Scholar]
- 69. Wright, W.G., Tierney, R.T., and McDevitt, J. (2017). Visual-vestibular processing deficits in mild traumatic brain injury. J. Vestib. Res. 27, 27–37. [DOI] [PubMed] [Google Scholar]
- 70. Sen, N. (2017). An insight into the vision impairment following traumatic brain injury. Neurochem Int 111, 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Maxwell, W.L. (2013). Damage to myelin and oligodendrocytes: a role in chronic outcomes following traumatic brain injury? Brain Sci. 3, 1374–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang, J., Fox, M.A., and Povlishock, J.T. (2013). Diffuse traumatic axonal injury in the optic nerve does not elicit retinal ganglion cell loss. J. Neuropathol. Exp. Neurol. 72, 768–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bennett, R.E., Mac Donald, C.L., and Brody, D.L. (2012). Diffusion tensor imaging detects axonal injury in a mouse model of repetitive closed-skull traumatic brain injury. Neurosci. Lett. 513, 160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Petraglia, A.L., Plog, B.A., Dayawansa, S., Chen, M., Dashnaw, M.L., Czerniecka, K., Walker, C.T., Viterise, T., Hyrien, O., Iliff, J.J., Deane, R., Nedergaard, M., and Huang, J.H. (2014). The spectrum of neurobehavioral sequelae after repetitive mild traumatic brain injury: a novel mouse model of chronic traumatic encephalopathy. J. Neurotrauma 31, 1211–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dikranian, K., Cohen, R., Mac Donald, C., Pan, Y., Brakefield, D., Bayly, P., and Parsadanian, A. (2008). Mild traumatic brain injury to the infant mouse causes robust white matter axonal degeneration which precedes apoptotic death of cortical and thalamic neurons. Exp. Neurol. 211, 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tzekov, R., Quezada, A., Gautier, M., Biggins, D., Frances, C., Mouzon, B., Jamison, J., Mullan, M., and Crawford, F. (2014). Repetitive mild traumatic brain injury causes optic nerve and retinal damage in a mouse model. J. Neuropath. Exp. Neur. 73, 345–361. [DOI] [PubMed] [Google Scholar]
- 77. Soto, I., Oglesby, E., Buckingham, B.P., Son, J.L., Roberson, E.D., Steele, M.R., Inman, D.M., Vetter, M.L., Horner, P.J., and Marsh-Armstrong, N. (2008). Retinal ganglion cells downregulate gene expression and lose their axons within the optic nerve head in a mouse glaucoma model. J. Neurosci. 28, 548–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Soto, A., Perez-Samartin, A.L., Etxebarria, E., and Matute, C. (2004). Excitotoxic insults to the optic nerve alter visual evoked potentials. Neuroscience 123, 441–449. [DOI] [PubMed] [Google Scholar]
- 79. Dratviman-Storobinsky, O., Hasanreisoglu, M., Offen, D., Barhum, Y., Weinberger, D., and Goldenberg-Cohen, N. (2008). Progressive damage along the optic nerve following induction of crush injury or rodent anterior ischemic optic neuropathy in transgenic mice. Mol. Vis. 14, 2171–2179. [PMC free article] [PubMed] [Google Scholar]
- 80. Wang, J., Hamm, R.J., and Povlishock, J.T. (2011). Traumatic axonal injury in the optic nerve: evidence for axonal swelling, disconnection, dieback, and reorganization. J. Neurotrauma 28, 1185–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Vien, L., DalPorto, C., and Yang, D. (2017). Retrograde degeneration of retinal ganglion cells secondary to head trauma. Optom. Vis. Sci. 94, 125–134. [DOI] [PubMed] [Google Scholar]
- 82. Atkins, E.J., Newman, N.J., and Biousse, V. (2008). Post-traumatic visual loss. Rev. Neurol. Dis. 5, 73–81. [PMC free article] [PubMed] [Google Scholar]
- 83. Jacobs, S.M. and Van Stavern, G.P. (2013). Neuro-ophthalmic deficits after head trauma. Curr Neurol Neurosci Rep 13, 389. [DOI] [PubMed] [Google Scholar]
- 84. Frick, K.D. and Singman, E.L. (2019). Cost of military eye injury and vision impairment related to traumatic brain injury: 2001-2017. Mil. Med. 184, e338–e343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.