Abstract
Background:
After a pilot project in 2014–15 Zimbabwe introduced the human papillomavirus (HPV) vaccine nationally in 2018 for girls aged 10–14 years through a primarily school-based vaccination campaign with two doses administered at 12-month intervals. In 2019, a first dose was delivered to a new cohort of girls in grade 5 of girls age 10 years if out-of-school (OOS), along with a second dose to the 2018 multiple cohorts. Additional effort was made to identify and mobilize OOS girls by Village Health Workers (VHWs) in the community. Zimbabwe reported 1,569,905 doses of HPV vaccine administered during the 2018 and 2019 campaigns. This analysis evaluated the cost of Zimbabwe’s national HPV vaccine introduction.
Methods:
A retrospective, incremental, ingredients-based cost analysis from the provider perspective was conducted in 2018 and 2019. Financial and economic cost data were collected at district and health facility levels using a two-stage cluster sampling approach and four cost dimensions: program activity, resource input, payer, and administrative level. Costs are presented in 2020 US$ in total and per dose.
Results:
The total weighted costs for combined district and health facility administrative levels were US$ 828,731 (financial) and US$ 2,060,943 (economic). For service delivery, the total weighted cost per dose was US$ 0.16 (financial) and US$ 0.59 (economic). The program activities with the largest share of total weighted financial cost were training (37% of total) and service delivery (30%), while the largest shares of total weighted economic costs were service delivery (45%) and training (19%). Efforts by VHWs to reach OOS girls resulted in an additional US$ 2.99 in financial cost per dose and US$ 7.79 in economic cost per dose.
Conclusion:
The service delivery cost per dose was lower than that documented in the pilot program cost analysis in Zimbabwe and studies elsewhere, reflecting a campaign delivery approach that spread fixed costs over a large vaccination cohort. The additional cost of reaching OOS girls with the HPV vaccine was documented for the first time in low- and middle-income countries, which may provide information on potential costs for other countries.
Keywords: Vaccine introduction, Human papillomavirus, Cost, Cost analysis, Zimbabwe
1. Background
Human papillomavirus is associated with cervical cancer, which resulted in an estimated 604,127 (3.1%) new cases and over 341,831 (3.3%) deaths worldwide in 2020 [1]. To prevent human papillomavirus infection and reduce cervical cancer cases and deaths, the World Health Organization (WHO) recommends two doses of human papillomavirus (HPV) vaccines separated by a minimum of six months for girls aged 9–14 years; countries should determine the most feasible delivery approach to optimize coverage and consider vaccinating multiple cohorts when possible [2,3].
In 2019, of a total population of almost 7.6 million women in Zimbabwe, an estimated 4.59 million women (60%) are at risk (aged 15 years or older) of developing cervical cancer [4-6]. In 2020, cervical cancer was the most frequent cancer among women, with 3043 (18.9%) new cases, and was the leading cause of cancer-related mortality (1976 deaths, 18.5%) among people of either sex [6]. In 2020, the age-standardized incidence rate for cervical cancer in Zimbabwe was 61.7 per 100,000 [6]. Zimbabwe’s national cancer prevention and control strategy and National Health Plan prioritize the use of HPV vaccine to prevent cervical cancer while also encouraging improvement of screening and treatment capacity [7,8].
Zimbabwe received funding from Gavi, The Vaccine Alliance to conduct an HPV vaccine pilot program in two districts targeting 10-year-old girls in 2014–2015. Zimbabwe’s pilot program financial cost for service delivery ranged from US$ 3.17 to US$ 4.62 per dose across districts, and economic cost ranged from US$ 10.39 to US$ 10.83 per dose (all inflated to 2020 US$) [9,10]. Based on the pilot program, Zimbabwe received additional Gavi funding in 2017 for nationwide HPV vaccine introduction, targeting multiple cohorts of girls in the first year of the program (all girls age 10–14 years, both in and out-of-school [OOS]) with a bivalent HPV vaccine. Vaccination began in May 2018 as a primarily school-based vaccination campaign, with 768,018 first doses administered (administrative data) [11]. A second dose of HPV vaccine was administered one year later, in May 2019, through a similar school-based vaccination campaign, while a first HPV vaccine dose to girls in grade 5 and OOS girls age 10 concurrently administered. An OOS girl was defined as a girl not currently attending school. In total, the 2019 HPV vaccination campaign administered 801,887 doses (administrative data) [11]. In August 2019 a two-stage cluster vaccination coverage survey was conducted in three districts of Zimbabwe [12]. The first dose uptake was from 88% to 94% and two-dose coverage was between 75% and 86% in the three surveyed districts [12]. Moving forward, Zimbabwe is planning to continue vaccinating girls in grade 5 with 2 doses at a 12-month interval with the focus of minimizing dropout and adding the HPV vaccine into a routine program [12].
In both campaign years, implementation of the HPV vaccination program took place at both district and health facility administrative levels. Each campaign lasted 5 days, with staff at the district level organizing teams of health workers to deliver HPV vaccinations. When schools were within walking distance of health facilities, health facility staff would administer HPV vaccine in schools. Health facilities also had HPV vaccines available during and after the campaign to vaccinate girls who missed vaccination sessions in schools [13]. In some cases, HPV vaccine was provided at outreach points (e.g., church, market). Village health workers (VHWs) were tasked with identifying and mobilizing OOS girls in the community for HPV vaccination; the costs of this strategy have not been previously reported in the literature. UNESCO Institute for Statistics estimates that most girls in Zimbabwe aged 9 to 13 years attend school, with attendance rates ranging from 96% (9-year-olds) to 87% (13-year-olds) [14].
While the costs of adding HPV vaccination to the existing immunization program via small pilot programs has been studied [9,15-20], little is known about the actual costs of introducing a nationwide HPV vaccination program. The pilot programs were reported to be expensive (average cost per dose, financial $9.70 and economic $22.16, inflated to 2020 US$), but it was assumed that costs would decrease as countries introduced HPV vaccine nationally due to economies of scale [10,15]. The nationwide introduction of HPV vaccine in Zimbabwe provided an opportunity to gather comprehensive information on the cost of new vaccine introduction, as well as to fill gaps in the literature on the cost of vaccinating multiple cohorts and special populations (e.g., OOS girls). Although this cost analysis originally aimed to present introduction costs at all administrative levels, planned data collection at the national and provincial levels was discontinued due to local restrictions put in place in response to the global COVID-19 pandemic. However, cost data obtained from district and health facility levels, particularly around service delivery and reaching OOS girls, provide valuable information related to real-world implementation of HPV vaccination programs nationally. This study documents the incremental resource needs for national HPV vaccination introduction at district and health facility administrative levels and estimates the cost of resources used, particularly the additional costs for reaching OOS girls.
2. Methods
A program cost analysis using an incremental, retrospective, bottom-up, ingredients-based design was conducted from the provider perspective [21]. The provider perspective includes costs to government and donors while excluding patient out-of-pocket costs and other health system costs. The cost analysis includes financial and economic costs for all HPV vaccine introduction activities. Financial costs were defined as direct expenditures by government and donors for HPV vaccine introduction activities [22-24]. Economic costs were defined as financial costs plus opportunity costs, including time for existing personnel and in-kind resources (e.g., volunteer time and donated goods) [22-24].
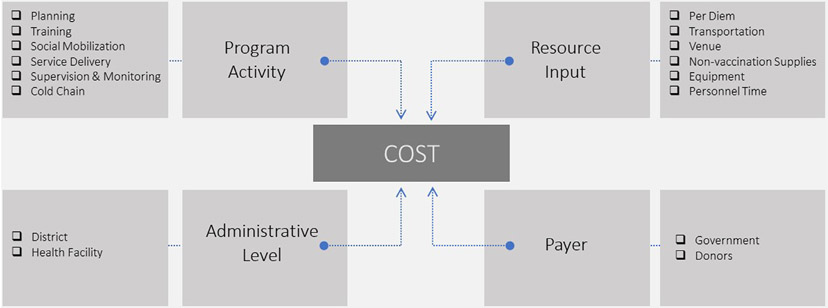
All costs were collected and analyzed by program activity, resource input, administrative level, and payer (Fig. 1). Program activity categories were service delivery, planning, training, social mobilization, supervision and monitoring, and cold chain. Program activities were further grouped into investment costs that were expected to last longer than one year (planning, training, social mobilization, and cold chain capital) and recurrent costs that were expected to be repetitive in nature (service delivery, supervision and monitoring, and cold chain) [15,23-24]. Investment and recurrent program activity categories followed the WHO Cervical Cancer Control and Costing (C4P) Tool, which was used to cost many pilot HPV vaccination programs [15,23-24], and were also informed by the EPIC costing guidance [22]. Further information on investment and recurrent cost breakdown by program activity and resource input is provided in the supplementary material. For each program activity, data included date of activity, length of activity in hours, staff participation, and resources used. Each program activity is comprised of a combination of resource inputs, which were per diem, transportation, venue, non-vaccination supplies, equipment, and personnel time. Costs were categorized by administrative level where the activity occurred (district or health facility). Payer was defined as the organization of the disbursing agent or custodian of funds that directly made the monetary payment for the good or service. For capital goods, the payer was defined as the resource owner.
Fig. 1.
Diagram of cost dimensions for a nationwide HPV vaccination program in Zimbabwe.
Capital goods procured and used were assigned a default discount rate for the national HPV vaccination program of 5% and ULYs were estimated using WHO-CHOICE [25]. An annualization factors table was consulted to identify the annuity factor [21]. Each capital cost item was collected with the following components: type of capital item and the amount of time or space (e.g., in case of shared equipment such as cold chain) used for the program. Using the data collected on the type of capital items, the unit cost was obtained from the UNICEF supply catalog [26] or previously collected data from the HPV vaccination pilot project in Zimbabwe [9]. The number of working days per year was estimated at the national level (n = 222) and was used to estimate the total cost of capital items, as well as personnel costs, with the exception of the cold chain items of refrigerators and freezers that are assumed to run 365 days per year. For capital items that are estimated to last greater than one year, a share of the cost was apportioned to the program cost analysis.
The study timeframe and analytic horizon were concurrent with the vaccination program from the first activity at district or health facility administrative levels (in January 2018) through the end of the 2019 vaccination campaign (June 2019).
The cost analysis excludes all costs incurred at the national and provincial levels including procured HPV vaccine and vaccination supplies (e.g., syringes and safety boxes). Additional exclusions from the analysis include resources devoted to this or other evaluations, routine immunization operations related to planning and implementation of the HPV vaccine national introduction, overhead costs, surveillance and surveillance materials, cold chain energy costs and maintenance, building space, office equipment and existing office supplies, and the value of time for officials, invited speakers, and politicians.
The EPIC immunization costing studies were used as a guide to create a nationally representative sample of districts and health facilities with a two-stage cluster sampling approach to allow inferences to be made about other subnational units [22,27-28]. Districts were selected using probability proportional to size (PPS) sampling based on the volume of HPV vaccine doses delivered in 2018 as the size variable. The final sample included 30 out of 62 total districts in Zimbabwe. Within the selected districts, health facilities were stratified by urban or rural status and simple random sampling was used to select two health facilities per district, one urban and one rural if possible. The final sample included 60 health facilities (52 rural and 8 urban) out of 1780 total health facilities in the sampling frame.
Trained data collectors interviewed program staff from sampled districts and health facilities in January and February of 2020. Interviewed program staff included anyone knowledgeable about the HPV program or HPV expenditures and was not limited to a single respondent but often included all program staff available (e.g., human resources, procurement, program manager, cold chain staff). Data collection questionnaires were built using Open Data Kit software and collected on tablets. Three data collection questionnaires were administered: one at the district level and two at the health facility level (one for the health facility itself and one to a VHW). VHW questionnaires were specifically focused on additional costs for reaching OOS girls through social mobilization and were administered to VHWs selected purposively by data collectors at sampled health facilities [29]. Data were collected from VHW interviews conducted by trained data collectors for all cost dimensions, with the exception that social mobilization was the only program activity cost collected.
The data collection questionnaires used at district and health facility levels included screening questions to facilitate consistent inclusion or exclusion of costs specific to the introduction of the HPV vaccine into the immunization program. These screening questions focused on vaccine logistics, cold chain capital items, and waste disposal and management. Outside of screening questions, resources were not assessed for slackness in this analysis and capital items collected were assigned an opportunity cost.
Costs were collected and categorized by program activity, resource input and payer for both administrative levels. The total economic cost across activities at each level was calculated as the sum of financial costs plus the sum of opportunity costs. Cost calculations were further categorized as investments versus recurrent costs. Cost per dose was calculated by dividing the total cost by the number of HPV doses administered in the 2018–2019 campaigns (1,569,905 doses) [11]. To calculate the cost per OOS girl of VHW outreach efforts, the number of doses given to OOS girls in 2018 (12,878 doses) was collected from the Ministry of Health and Childcare (MOHCC); however, the number of doses given to OOS girls in 2019 was unable to be obtained from MOHCC and was therefore estimated using the percentage of girls vaccinated in 2018 who were OOS (1.68%) applied to the number of doses administered in 2019 for all girls, resulting in an estimated 13,472 doses administered to OOS girls in 2019. To calculate the total additional cost per dose given to an OOS girl, the total additional cost of VHW social mobilization efforts for OOS girls was divided by the total number of OOS girls who received an HPV vaccine dose. VHW questionnaire cost data were analyzed separately from the total cost of the HPV national vaccination program to understand the cost of the additional effort made to reach only OOS girls.
Sample weights were applied to all data, which allowed for inference to similar units (i.e., districts and health facilities) and to calculate the total weighted cost per administrative level. Costs from sampled districts were multiplied by district sample weights and from sampled health facilities by both their respective district and health facility sample weights. Health facility weights were applied to data collected from VHWs associated with those facilities. All results presented in the main text are weighted costs. Two-sided 95% confidence intervals of total costs and cost per dose were calculated assuming costs were normally distributed and the selected health facilities were independent at their administrative levels.
Data were analyzed using Microsoft Power BI (Version 2.87.762.0). Unit cost per dose was calculated for each cost dimension and presented separately for financial and economic costs. Costs were collected from January 2018 through June 2019 as either local currency (Real Time Gross Settlement [RTGS]) or US$. Costs collected in RTGS were converted into US$ using the month when the cost was incurred [30] and then all US$ were inflated using the US consumer price index [31] and presented in 2020 US$.
3. Results
For the nationwide HPV vaccination program in 2018 and 2019, the total combined financial and economic costs were US$ 828,731 and US$ 2,060,943, respectively (Table 1). Using the administrative number of doses delivered (1,569,905), the financial cost was US$ 0.53 (95% C.I. US$ 0.37–US$ 0.69) per dose and the economic cost was US$ 1.31 (95% C.I. US$ 0.99–US$ 1.64) per dose. Seventy-four percent of total financial costs (US$ 611,900; 95% C.I. US$ 425,617–US$ 798,184) and 77% of total economic costs (US$ 1,594,863; US$ 1,238,406–US$ 1,951,319) were spent between January 2018 and June 2018 (attributable to the 2018 vaccination) (Table 2). Twenty-six percent of total financial costs (US$ 216,831; 95% C.I. US$ 127,708–US$ 305,954) and 23% of total economic costs (US$ 466,081; 95% C.I. US$ 293,819–US$ 638,342) were spent between July 2018 and June 2019 (attributable to the 2019 vaccination). By administrative level, the total financial cost at the district level was US$ 626,531 (95% C.I. US$ 444,565–US$ 808,498) and the economic cost was US$ 933,373 (95% C.I. US$ 685,880–US$ 1,180,866) (Table 3). The total financial cost at the health facility level was US$ 202,200 (95% C.I. US$ 48,373–US$ 356,028) and the economic cost was US$ 1,127,571 (95% C.I. US$ 728,528–US$ 1,526,613). Rural health facilities accounted for 22% of the total financial costs (US$ 180,480; 95% C.I. US$ 28,531–US$ 332,430) and 50% of total economic costs (US$ 1,025,920; 95% C.I. US$ 636,436–US$ 1,415,404); while urban health facilities represented 3% (US$ 21,720; 95% C.I. US$ 0–US$ 47,543) of total financial costs and 5% of total economic costs (US$ 101,651; 95% C.I. US$ 25,272–US$ 178,029).
Table 1.
Costs associated with a nationwide HPV vaccination program in Zimbabwe (2020 US$) (January 2018–June 2019).±
| FINANCIAL |
ECONOMIC |
|||||
|---|---|---|---|---|---|---|
| Total Weighted Cost (95% C.I.) |
% of Total | Financial Cost per Dose*** (95% C.I.) |
Total Weighted Cost (95% C.I.) |
% of Total | Economic Cost per Dose*** (95% C.I.) |
|
| Investment * | $406,260 ($251,456–$561,063) |
49 | $0.26 ($0.16–$0.36) |
$813,978 ($567,662–$1,060,295) |
40 | $0.52 ($0.36–$0.68) |
| Recurrent ** | $422,472 ($270,272–$574,672) |
51 | $0.27 ($0.17–$0.37) |
$1,246,965 ($895,076–$1,598,854) |
60 | $0.79 ($0.57–$1.02) |
| TOTAL | $828,731 ($573,892–$1,083,571) |
100 | $0.53 ($0.37–$0.69) |
$2,060,943 ($1,554,092–$2,567,794) |
100 | $1.31 ($0.99–$1.64) |
Abbreviations: C.I. = confidence interval.
Not including additional cost of reaching out-of-school (OOS) girls.
Investment costs are expected to last longer than one year and include the activity categories of planning, training, and social mobilization materials.
Recurrent costs are expected to be repetitive in nature. This includes program activities of service delivery, supervision & monitoring, and cold chain.
Using the number of doses administered = 1,569,905.
Table 2.
Weighted cost by year (2020 US$) (January 2018–June 2019).±
| Time Period | Number of Doses Administered |
FINANCIAL |
ECONOMIC |
||
|---|---|---|---|---|---|
| Total Weighted Cost (95% C.I.) |
% of Total | Total Weighted Cost (95% C.I.) |
% of Total | ||
| January 2018–June 2018 | 768,018 | $611,900 ($425,617–$798,184) |
74 | $1,594,863 ($1,238,406–$1,951,319) |
77 |
| July 2018–June 2019 | 801,887 | $216,831 ($127,708–$305,954) |
26 | $466,081 ($293,819–$638,342) |
23 |
| TOTAL | 1,569,905 | $828,731 ($573,892–$1,083,571) |
100 | $2,060,943 ($1,554,092–$2,567,794) |
100 |
Abbreviations: C.I. = confidence interval.
Not including additional cost of reaching out-of-school (OOS) girls.
Table 3.
Weighted cost by administrative level (2020 US$) (January 2018–June 2019).±
| Administrative Level* | FINANCIAL |
ECONOMIC |
||
|---|---|---|---|---|
| Total Weighted Cost (95% C.I.) |
% of Total** | Total Weighted Cost (95% C.I.) |
% of Total** | |
| District | $626,531 ($444,565–$808,498) |
76 | $933,373 ($685,880–$1,180,866) |
45 |
| Health Facility | $202,200 ($48,373–$356,028) |
24 | $1,127,571 ($728,528–$1,526,613) |
55 |
| Rural | $180,480 ($28,531–$332,430) |
22 | $1,025,920 ($636,436–$1,415,404) |
50 |
| Urban | $21,720 ($0–$47,543) |
3 | $101,651 ($25,272–$178,029) |
5 |
| TOTAL | $828,731 ($573,892–$1,083,571) |
$2,060,943 ($1,554,092–$2,567,794) |
||
Abbreviations: C.I. = confidence interval.
Not including additional cost of reaching out-of-school (OOS) girls.
Administrative level costs only reflect activities at that level and are not combined across administrative levels.
Total is only reflective of costs incurred at the district and health facility levels. Totals may occasionally sum to more than 100 percent due to rounding.
Among total financial costs, investment costs were 49% amounting to US$ 406,260 (95% C.I. US$ 251,456–US$ 561,063) in total (US$ 0.26 per dose; 95% C.I. US$ 0.16–US$ 0.36), while recurrent costs were 51% amounting to US$ 422,472 (95% C.I. US$ 270,272–US$ 574,672) in total (US$ 0.27 per dose; 95% C.I. US$ 0.17–US$0.37). Among total economic costs, investment costs made up 40% amounting to US$ 813,978 (95% C.I. US$ 567,662–US$ 1,060,295) in total (US$ 0.52 per dose; 95% C.I. US$ 0.36–US$ 0.68), while recurrent costs made up 60% amounting to US$ 1,246,965 (95% C.I. US$ 895,076–US$ 1,598,854) in total (US$ 0.79 per dose; 95% C.I. US$ 0.57–US$ 1.02).
The program activity with the largest total financial cost for district and health facility administrative levels combined was training (US$ 310,626; 95% C.I. US$ 203,004–US$ 418,249; 37%), followed by service delivery (US$ 244,523; 95% C.I. US$ 162,701–US$ 326,345; 30%) (Table 4). For financial costs of training, 89% of total training funds were spent in the first year (2018) while 11% were spent in the second year (2019). For economic costs, the program activity with the largest total cost was service delivery (US$ 929,674; 95% C.I. US$ 630,119–US$ 1,229,228; 45%), followed by training (US$ 381,528; 95% C.I. US$ 255,894–US$ 507,163; 19%).
Table 4.
Weighted cost by program activity (2020 US$) (January 2018–June 2019).±
| Program Activity | FINANCIAL |
ECONOMIC |
||
|---|---|---|---|---|
| Total Weighted Cost (95% C.I.) |
% of Total | Total Weighted Cost (95% C.I.) |
% of Total | |
| Training | $310,626 ($203,004–$418,249) |
37 | $381,528 ($255,894–$507,163) |
19 |
| Service Delivery | $244,523 ($162,701–$326,345) |
30 | $929,674 ($630,119–$1,229,228) |
45 |
| Supervision & Monitoring | $168,166 ($59,684–$276,648) |
20 | $299,769 ($146,731–$452,808) |
15 |
| Social Mobilization | $49,919 ($14,587–$85,251) |
6 | $202,570 ($132,969–$272,172) |
10 |
| Planning | $45,714 ($0–$97,821) |
6 | $229,880 ($127,629–$332,130) |
11 |
| Cold Chain | $9783 ($1317–$18,248) |
1 | $17,522 ($5635–$29,409) |
1 |
| TOTAL | $828,731 ($573,892–$1,083,571) |
100 | $2,060,943 ($1,554,092–$2,567,794) |
100 |
Abbreviations: C.I. = confidence interval.
Not including additional cost of reaching out-of-school (OOS) girls.
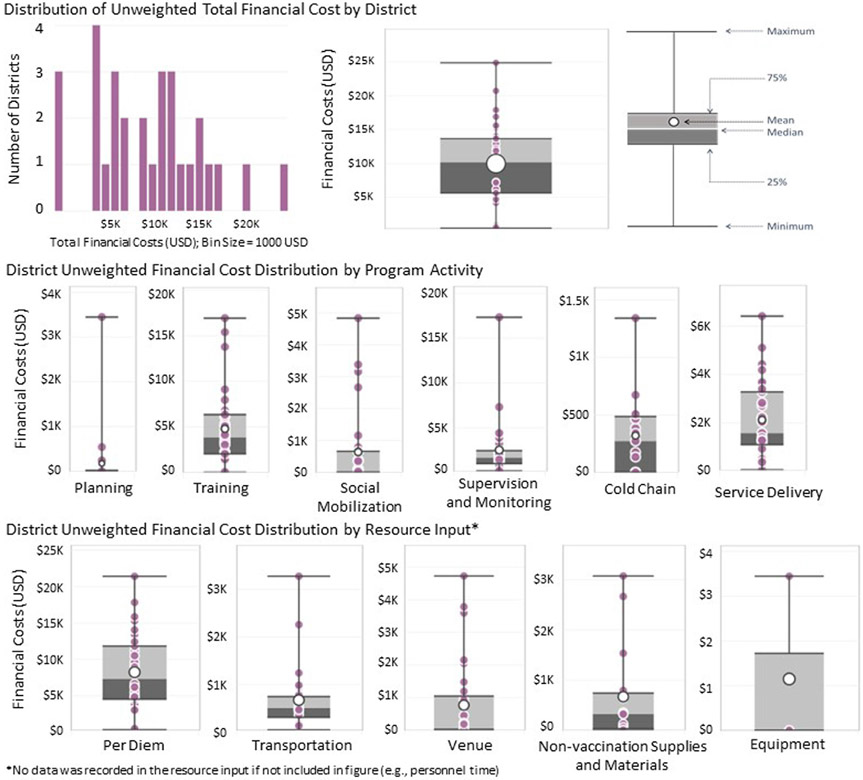
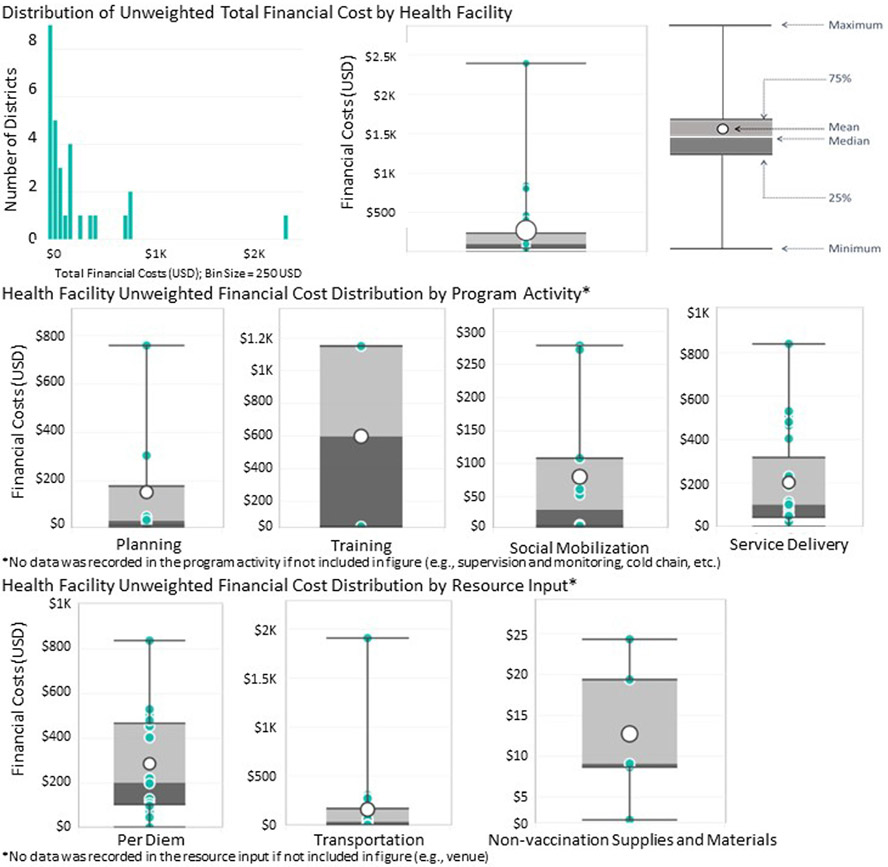
The unweighted median financial cost per sampled district was US$ 10,165 (US$ 457–US$ 24,871; Fig. 2). For health facilities, the unweighted median financial cost per sampled health facility was US$ 101 (US$ 0–US$ 2,390; Fig. 3).
Fig. 2.
District Unweighted financial cost distribution (2020 US$) (January 2018–June 2019).
Fig. 3.
Health Facility Unweighted Financial Cost Distribution (2020 US$) (January 2018–June 2019).
School-based vaccination accounted for more than 98% (US$ 241,744; 95% C.I. US$ 159,837–US$ 323,650) of the total financial costs for service delivery and more than 76% (US$ 707,088; 95% C.I. US$ 516,850–US$ 897,326) of the total economic costs (Table 5). For service delivery, total financial costs at health facility sites were US$ 1001 (95% C.I. US$ 0–US$ 2059) and at outreach sites were US$ 1778 (95% C.I. US$ 0–US$ 4537), which combined represented less than 2% of total financial costs for service delivery. For service delivery, total economic costs at health facility sites were 23% (US$ 216,219; 95% C.I. US$ 42,936–US$ 389,502) and at outreach sites were less than 1% (US$ 6367; 95% C.I. US$ 0–US$ 12,783) of total economic costs for service delivery. Outreach sites specified during data collection were shopping centres, markets, shops, and churches. Total financial costs of service delivery via all approaches was US$ 0.16 (95% C.I. US$ 0.1–US$ 0.21) per dose and total economic cost was US$ 0.59 (95% C.I. US$ 0.4–US$ 0.78) per dose. The resource input with the largest total financial cost for combined district and health facility administrative levels was per diem (US$ 619,892; 95% C.I. US$ 438,851–US$ 800,933); 75%) (Table 6). For economic costs, the resource input with the largest total cost was personnel time (US$ 841,456; 95% C.I. US$ 549,901–US$ 1,133,011; 41%).
Table 5.
Weighted cost of service delivery per delivery approach (2020 US$) (January 2018–June 2019).±
| Service Delivery Approach |
FINANCIAL |
ECONOMIC |
||
|---|---|---|---|---|
| Service Delivery Total Weighted Costs (95% C.I.) |
% of Total Service Delivery Costs |
Service Delivery Total Weighted Costs (95% C.I.) |
% of Total Service Delivery Costs |
|
| School | $241,744 ($159,837–$323,650) |
98.9 | $707,088 ($516,850–$897,326) |
76.0 |
| Outreach | $1778 ($0–$4537) |
0.7 | $6367 ($0–$12,783) |
0.7 |
| Health Facility | $1001 ($0–$2059) |
0.4 | $216,219 ($42,936–$389,502) |
23.3 |
| TOTAL | $244,523 ($162,701–$326,345) |
100 | $929,674 ($630,119–$1,229,228) |
100 |
Abbreviations: C.I. = confidence interval.
Not including additional cost of reaching out-of-school (OOS) girls.
Table 6.
Weighted cost by resource input (2020 US$) (January 2018–June 2019).±
| Resource Input | FINANCIAL |
ECONOMIC |
||
|---|---|---|---|---|
| Total Weighted Costs (95% C.I.) |
% of Total | Total Weighted Costs (95% C.I.) |
% of Total | |
| Per diem * | $619,892 ($438,851–$800,933) |
75 | $619,892 ($438,851–$800,933) |
30 |
| Transportation | $145,930 ($27,728–$264,132) |
17 | $279,762 ($153,217–$406,308) |
14 |
| Venue | $38,831 ($11,545–$66,116) |
4 | $244,230 ($136,529–$351,931) |
11 |
| Non-vaccination supplies and materials | $24,070 ($3,224–$44,917) |
3 | $72,946 ($34,527–$111,366) |
3 |
| Personnel time | $0** ($0–$0) |
0** | $841,456 ($549,901–$1,133,011) |
41 |
| Equipment | $9 ($0–$26) |
<1 | $2657 ($0–$7,828) |
<1 |
| TOTAL | $828,731 ($573,892–$1,083,571) |
100 | $2,060,943 ($1,554,092–$2,567,794) |
100 |
Abbreviations: C.I. = confidence interval.
Not including additional cost of reaching out-of-school (OOS) girls.
Per Diem includes travel allowance.
Personnel time was US$ 0 in financial costs because no contracted personnel for the HPV vaccination program were found in the data analysis. Therefore, all personnel time was an economic cost.
The MOHCC was the payer of the largest share of total financial costs (77%; US$ 209,832) followed by the WHO (14%; US$ 37,969), with all other payers individually accounting for less than 3%. The largest payer of total economic costs was also the MOHCC, accounting for 69% (US$ 310,011).
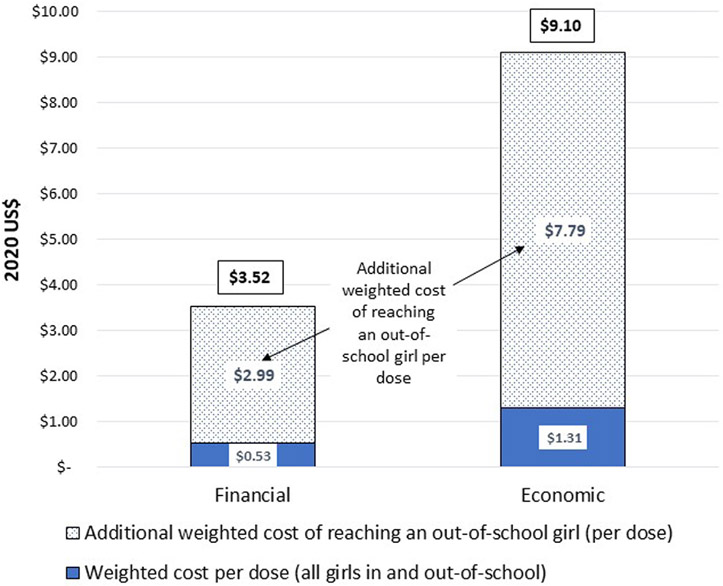
The 2018–2019 HPV vaccination campaign reached a lower proportion of OOS girls compared to their estimated proportion in the vaccine-eligible population based on data from the UNESCO Institute for Statistics [14]. An estimated 1,543,555 doses (98.3%) of vaccine were administered to girls attending school, while an estimated 26,350 doses (1.7%) were administered to OOS girls. Data collected from VHWs indicated additional total financial cost of reaching OOS girls was US$ 78,578 and the additional total economic cost was US$ 205,157. This resulted in an additional US$ 2.99 financial cost per dose and US$ 7.79 economic cost per dose (Fig. 4), giving a total financial cost per dose for OOS girls of US$ 3.52 and total economic cost per dose for OOS girls of US$ 9.10.
Fig. 4.
Additional Weighted Cost to reaching Out-of-School girls per dose (2020 US$) (January 2018–June 2019) *. *Estimated number of doses delivered to out-of school girls = 26,350.
4. Discussion
Zimbabwe’s delivery approach (annual campaign to multiple cohorts that included overlapping first and second dose delivery) is different than those described in previously published HPV vaccination cost studies [9,15-20]; therefore, the results are not directly comparable. In addition, this cost analysis only includes district and health facility costs; analysis did not include national and provincial level costs due to COVID-19 pandemic disruptions. Therefore, the total costs presented in this analysis cannot be compared to other countries’ pilot programs or national studies. Nevertheless, Zimbabwe’s findings can be compared to other findings in the context of scale efficiencies, service delivery costs at district and health facility levels, and the costs of reaching OOS girls [9,15-20].
One comparison that can be made is across service delivery cost per dose. Zimbabwe’s service delivery cost per dose was lower via the nationwide HPV vaccination campaign reported here (financial US$ 0.16 and economic US$ 0.59) than via the initial pilot program (financial cost per dose ranged from US$ 3.17 to US$ 4.62 across districts and economic cost per dose ranged from US$ 10.39 to US$ 10.83 (inflated to 2020 US$) [9,10]. This finding supports economic theory and prior study hypotheses that HPV national vaccination programs would find a lower service delivery cost per dose as compared to pilot programs due to a larger target population (i.e., scale efficiencies) [15]. While the pilot program reached only 10-year-old girls, the national vaccination campaign targeted all girls 10–14 years old, and girls in grade 5 (if OOS, girls age 10). When HPV vaccines are delivered to more girls, scale efficiencies are realized, a finding commonly seen in HPV costing studies [9,15]. When Zimbabwe transitions back to a single cohort (girls in grade 5), as planned for future HPV vaccine delivery, the service delivery cost per dose will likely increase, due to moving from a larger target population to a smaller target population and therefore fixed costs spread across fewer girls.
Outside of Zimbabwe, the financial service delivery costs of HPV vaccine programs in the published literature range from costs of US$ 1.98 per dose in Vietnam (school-based delivery) to US$ 2.57 per dose in Uganda (school-based delivery, inflated to 2020 US$) [10,16-17]. The economic costs range from US$ 2.54 per dose in Vietnam (school-based delivery) to US$ 4.74 per dose in Peru (school-based delivery, inflated to US$ 2020) [10,16-17]. These studies all look at service delivery costs in the HPV pilot programs, which may be higher than in national vaccination programs due to fixed costs being spread over smaller target populations.
By program activity, the highest financial cost was for training (US$ 310,626; 37%). An investment in training of this level of effort will likely not take place again in Zimbabwe in the near future; this amortization of training investment costs over multiple years is demonstrated by 89% of total financial costs being spent for training in 2018 and only 11% spent in 2019 [22]. This training investment cost is not comparable to those found in other studies, because it only includes the cost of training at the district and health facility levels, excluding the national or provincial level investments in training activities. Two other in-depth studies from the Zimbabwe HPV national vaccination program identified challenges around training (lack of clarity on reporting and varied information) that lead to misunderstandings and gaps in knowledge [13,29]. This programmatically suggests that a future investment in training may be important, though likely a lower recurring investment [13,29].
The literature from both HPV vaccine pilot programs and national programs have highlighted challenges of reaching OOS girls [19] and alluded to concerns about inequity in access to vaccination due to the lack of a dedicated strategy to reach them [20]. During Zimbabwe’s HPV vaccine pilot program, VHWs anecdotally reported mobilizing OOS girls to receive vaccination in schools; however, incremental costs to reach OOS girls were not collected or studied [9,32]. The Zimbabwe HPV vaccination coverage survey that was conducted following the national introduction found that less than 2% of girls did not attend school and given this small number was unable to power the survey to measure vaccination coverage of 2-doses for OOS girls [12]. The additional cost of reaching an OOS girl in Zimbabwe during the national program was an additional US$ 2.99 per dose in financial cost and US$ 7.79 per dose in economic cost; this is the first such estimate of costs to reach OOS girls with HPV vaccination in any setting. The magnitude of additional costs indicates the need for further studies to assess the effectiveness of this approach, as well as the cost and effectiveness of other approaches for reaching OOS girls.
4.1. Limitations
These cost analysis findings have several limitations. First, respondents allocated a percentage of each resource input to the corresponding HPV program activity, and it is possible that program staff could have under- or overestimated the costs due to the inherent difficulty with estimating shared costs [22]. In many cases respondents reported 100% allocation to the program activity; however, in some cases, respondents reported that resources were only partially allocated to the respective program activity. It is unknown how any inaccuracies in these allocation estimates may have affected this analysis.
Some standard cost data, such as personnel salaries, the costs of vehicles, and the cost of equipment and cold chain were drawn from existing data sources rather than collected from respondents [9,26]. Every effort was made to collect the cost of equipment procured and used; however, very few equipment costs were allocated specifically to the HPV vaccination program and hence economic costs of existing equipment used for HPV vaccination may be underestimated in this analysis.
Weighting by volume of doses delivered was not used in this cost analysis methodology to estimate unit costs and the simple mean approach used may lead to an upward bias in unit costs [22].
The number of doses delivered to OOS girls in the 2019 campaign was estimated based on the percent of doses delivered to OOS girls in 2018. It is unknown how this may have affected the analysis.
The two-sided confidence interval provided a range of uncertainty to the estimates, however, they were based on the normality and independence assumptions, which may not be true and could lead to under- or over-estimation of confidence intervals.
This analysis was conducted using a retrospective design where respondents were asked to recall information from the past at a time when Zimbabwe’s economy was dealing with hyperinflation [30]. While efforts were made to collect costs in the currency in which it was expended, the use of two currencies, US$ and RTGS, during the study time-period likely biased both data collectors and respondents as they were trying to recall past costs. Additionally, a monthly exchange rate from the Reserve Bank of Zimbabwe [30] was used; however, media sources speculated that unofficial exchange rates in the marketplace were exponentially higher. Furthermore, currency inflation in Zimbabwe was rampant at the time of our study. We chose to use the US inflation rate in calculating 2020 prices to limit additional stochasticity in the cost estimates. These factors (respondent recall, hyperinflation period, and using Reserve Bank of Zimbabwe exchange rates) likely caused an underestimate of costs in this analysis.
5. Conclusions
In Zimbabwe’s nationwide HPV vaccination program, which implemented a unique delivery approach to multiple cohorts including overlapping first and second dose delivery annually, the service delivery cost per dose was lower than what has been documented in other pilot programs. The service delivery cost per dose will likely increase as the target population transitions from multiple cohorts to a single grade-based cohort. The additional cost of reaching OOS girls with the HPV vaccine was documented for the first time in low- and middle-income countries; this provides a better understanding of the potential additional costs to reach this population for other countries with high levels of school attendance. Further studies should be considered to assess costs and effectiveness of various strategies to reach OOS girls to ensure equitable access to HPV vaccination.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the time and collaboration of those who participated in consultations and data collection for this analysis, including: Zimbabwe Ministry of Health and Child Care (The Secretary for Health and Child Care, the EDC Director, EPI Manager and Staff, Ministry of Primary and Secondary Education), the Zimbabwe National Statistics Agency, World Health Organization (Zimbabwe Country Office, AFRO, and IST) and John Snow International, Zimbabwe.
Source(s) of support
This work was supported by Gavi, the Vaccine Alliance [“Evaluation of Human Papillomavirus (HPV) Vaccine National Introduction in Low-and-Lower-Middle Income Countries” - Contract No. ME 9422 12 20].
Footnotes
CRediT authorship contribution statement
Anna Hidle: Conceptualization, Methodology, Software, Validation, Formal analysis, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization, Supervision. Timothy Brennan: Conceptualization, Methodology, Software, Validation, Formal analysis, Resources, Data curation, Writing – original draft, Writing – review & editing. Julie Garon: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. Qian An: Conceptualization, Methodology, Validation, Formal analysis, Writing – original draft, Writing – review & editing. Anagha Loharikar: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Funding acquisition. Joan Marembo: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. Portia Manangazira: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. Nelly Mejia: Methodology, Formal analysis, Writing – original draft, Writing – review & editing, Supervision, Project administration. Taiwo Abimbola: Conceptualization, Methodology, Validation, Writing – original draft, Writing – review & editing, Supervision, Project administration.
Disclaimers
The findings represent the personal views of the authors and not the official position of the U.S. Centers for Disease Control and Prevention or the Zimbabwe Ministry of Health and Child Care.
Human subjects
The evaluation protocol was determined to be not human subjects research and therefore exempt from institutional review board (IRB) review by the CDC Center for Global Health Associate Director for Science.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This article was published as part of a supplement supported by Centers for Disease Control and Prevention Global Immunization Division. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or World Health Organization. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.01.024.
References
- [1].International Agency for Research on Cancer. Cervix uteri cancer fact sheet [Internet]. Global Cancer Observatory; 2020. [cited 2021 FEB 08]. Available from: 23-Cervix-uteri-fact-sheet.pdf (iarc.fr). [Google Scholar]
- [2].World Health Organization. Human papillomavirus vaccines: WHO position paper, May 2017. (Weekly Epidemiologic Record; ). Report No.: 92(19):241–268. [Google Scholar]
- [3].Meeting of the Strategic Advisory Group of Experts on immunization. October 2016 - conclusions and recommendations [Internet]. World Health Organization; 2016. Dec p. 561–84. (Weekly Epidemiologic Record). Report No.: 48, 91. Available from: http://apps.who.int/iris/bitstream/10665/251810/1/WER9148.pdf?ua=1. [Google Scholar]
- [4].Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136(5):E359–86. [DOI] [PubMed] [Google Scholar]
- [5].Bruni L, Barrionuevo-Rosas L, Serrano B, et al. Human papillomavirus and related diseases report in Zimbabwe [Internet]; 2014. [cited 2021 FEB 08]. (ICO Information Centre on HPV and Cancer; ). Available from: http://www.hpvcentre.net/statistics/reports/ZWE.pdf. [Google Scholar]
- [6].International Agency for Research on Cancer. Zimbabwe cancer fact sheet [Internet]. Global Cancer Observatory; 2018. [cited 2021 FEB 08]. Available from:http://gco.iarc.fr/today/data/factsheets/populations/716-zimbabwe-fact-sheets.pdf. [Google Scholar]
- [7].A national cancer prevention and control strategy for Zimbabwe 2014–2018. Harare: Ministry of Health and Child Welfare Zimbabwe; 2013. [cited 2020 Dec 03]. [Google Scholar]
- [8].Zimbabwe national health strategy 2016–2020. Harare: Government of Zimbabwe; 2017. [cited 2020 DEC 03]. Available from: https://zdhr.uz.ac.zw/xmlui/bitstream/handle/123456789/703/National_Health_Strategy_for_Zimbabwe_2016-2020.pdf?sequence=1&isAllowed=y [Google Scholar]
- [9].Hidle A, Gwati G, Abimbola T, Pallas SW, Hyde T, Petu A, et al. Cost of a human papillomavirus vaccination project, Zimbabwe. Bull World Health Organ 2018;96(12):834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Databases, tables and calculations by subject. CPI inflation calculator [Internet]. Washington, DC: United States Bureau of Labor Statistics; 2018. [cited 2021 Feb 21]. Available from: https://www.bls.gov/. [Google Scholar]
- [11].Zimbabwe Ministry of Health and Child Care. Zimbabwe HPV Vaccine Campaign May 2019 Report; May 2019. Unpublished Report.
- [12].LaMontagne DS, Manangazira P, Marembo J, Chigodo C, Zvamashakwe C, Tshuma E, et al. HPV vaccination coverage in three districts in ZIMBABWE following national introduction of 0, 12 month schedule among 10 to 14 year old girls. Vaccine 2021. 10.1016/j.vaccine.2021.07.012. [DOI] [PubMed] [Google Scholar]
- [13].Garon J, Marembo J, Ndlela E, Rupfutse M, Shearley A, Makwabarara E, et al. Nationwide introduction of HPV vaccine in Zimbabwe – experiences with multiple cohort vaccination delivery (Unpublished). n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].UNESCO Institute for Statistics and the World Health Organization. Zimbabwe: Age distribution and school attendance of girls aged 9-13 years; 17 June 2013. [Google Scholar]
- [15].Botwright S, Holroyd T, Nanda S, Bloem P, Griffiths UK, Sidibe A, et al. Experiences of operational costs of HPV vaccine delivery strategies in Gavi-supported demonstration projects. PLoS One 2017;12(10):e018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Levin CE, Van Minh H, Odaga J, Rout SS, Ngoc DNT, Menezes L, et al. Delivery cost of human papillomavirus vaccination of young adolescent girls in Peru, Uganda and Viet Nam. Bull World Health Organ 2013;91(8):585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ngabo F, Levin A, Wang SA, Gatera M, Rugambwa C, Kayonga C, et al. A cost comparison of introducing and delivering pneumococcal, rotavirus and human papillomavirus vaccines in Rwanda. Vaccine 2015;33(51):7357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Quentin W, Terris-Prestholt F, Changalucha J, Soteli S, Edmunds WJ, Hutubessy R, et al. Costs of delivering human papillomavirus vaccination to schoolgirls in Mwanza Region, Tanzania. BMC Med 2012;10(1). 10.1186/1741-7015-10-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hanson CM, Eckert L, Bloem P, Cernuschi T. Gavi HPV programs: application to implementation. Vaccine 2015;3(2):408–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gallagher KE, Howard N, Kabakama S, Mounier-Jack S, Griffiths UK, Feletto M, et al. Lessons learnt from human papillomavirus (HPV) vaccination in 45 low- and middle-income countries. PLoS ONE 2017;12(6):e0177773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Haddix AC, Teutsch SM, Corso PS. Prevention effectiveness: a guide to decision analysis and economic evaluation. Oxford University Press; 2003. [Google Scholar]
- [22].Resch S, Menzies N, Portnoy A, Clarke-Deelder E, O’Keeffe L, Suharlim C, et al. How to cost immunization programs: a practical guide on primary data collection and analysis. Cambridge, MA: immunizationeconomics.org/ Harvard T.H. Chan School of Public Health; 2020. [Google Scholar]
- [23].World Health Organization. WHO Cervical Cancer Prevention and Control Costing (C4P) Tool User Guide: 5 Year Scale-Up, Version 4.0. Geneva, Switzerland; 2016. [Google Scholar]
- [24].World Health Organization. WHO Cervical Cancer Prevention and Control Costing (C4P) Tool User Guide: Demonstration Project Version 2.0. Geneva, Switzerland; 2012. [Google Scholar]
- [25].World Health Organization. WHO ∣ Table: Prices and useful lives of tradable capital goods. Available from: https://www.who.int/choice/cost-effectiveness/inputs/capital_goods/en [accessed December 2020].
- [26].UNICEF Supply Catalogue. Available from: https://supply.unicef.org/ [accessed December 2020].
- [27].Geng F, Suharlim C, Brenzel L, Resch SC, Menzies NA. The cost structure of routine infant immunization services: a systematic analysis of six countries. Health Policy Plan 2017;32(8):1174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rivera-Rodriguez CL, Resch S, Haneuse S. Quantifying and reducing statistical uncertainty in sample-based health program costing studies in low-and middle-income countries. SAGE Open Med. 2018;6:2050312118765602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Garon J, Mukavhi A, Rupfutse M, Bright S, Brennan T, Manangazira P, et al. Multiple cohort HPV vaccination in Zimbabwe: 2018-2019 program feasibility, awareness and acceptability among health, education, and community stakeholders. Vaccine 2021. 10.1016/j.vaccine.2021.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Reserve Bank of Zimbabwe: Consumer Price Index Table - June 2019. [cited 2020 DEC 03]. Available from: https://www.rbz.co.zw/index.php/research/markets/inflation.
- [31].U.S. Bureau of Labor Statistics. Consumer Price Index for All Urban Consumers [cited 2021 JAN 15]. Available from: https://www.bls.gov/.
- [32].Zimbabwe Ministry of Health and Child Care et al. Zimbabwe HPV Vaccination Demonstration Project Cost Analysis; 2016. Unpublished Report.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.