Abstract
MacroH2A has established tumour suppressive functions in melanoma and other cancers, but an unappreciated role in the tumour microenvironment. Using an autochthonous, immunocompetent mouse model of melanoma, we demonstrate that mice devoid of macroH2A variants exhibit increased tumour burden compared with wild-type counterparts. MacroH2A-deficient tumours accumulate immunosuppressive monocytes and are depleted of functional cytotoxic T cells, characteristics consistent with a compromised anti-tumour response. Single cell and spatial transcriptomics identify increased dedifferentiation along the neural crest lineage of the tumour compartment and increased frequency and activation of cancer-associated fibroblasts following macroH2A loss. Mechanistically, macroH2A-deficient cancer-associated fibroblasts display increased myeloid chemoattractant activity as a consequence of hyperinducible expression of inflammatory genes, which is enforced by increased chromatin looping of their promoters to enhancers that gain H3K27ac. In summary, we reveal a tumour suppressive role for macroH2A variants through the regulation of chromatin architecture in the tumour stroma with potential implications for human melanoma.
Subject terms: Melanoma, Mechanisms of disease
Filipescu et al. report that macroH2A deficiency in cancer-associated fibroblasts leads to altered chromatin looping and elevated inflammatory gene expression, thereby affecting immune cell function and limiting the anti-tumour response in melanoma.
Main
Histone variant chromatin incorporation has profound consequences within the cell, and its deregulation plays an important role in cancer1. Among the H2A variants, macroH2A has a distinct carboxy-terminal non-histone macro domain2. Vertebrate genomes contain two macroH2A genes: H2AFY and H2AFY2. H2AFY (also known as MACROH2A1) produces alternative splice isoforms, macroH2A1.1 and macroH2A1.2, whereas H2AFY2 (also known as MACROH2A2) produces macroH2A2 (refs. 3,4). Although macroH2A generally associates with condensed chromatin and transcriptionally inactive genes (see ref. 5 for review), its role in distinct cell types, tissues or disease states is complex1,6–9. Notably, the function of macroH2A variants during tumorigenesis in vivo remains poorly understood.
Mice deficient for both H2afy and H2afy2, referred to as double KO (dKO) herein, lack manifest developmental abnormalities or cancer development during ageing10. However, macroH2A regulates cellular states. For example, macroH2A variants impede reprogramming of somatic cells towards pluripotency11–13 and mediate gene expression in response to stimuli, such as pro-inflammatory signals7,14–16 or oncogene-induced senescence8.
Melanoma incidence is rising and remains clinically challenging to treat17. We have previously shown that macroH2A expression is lost in advanced human melanoma and, functionally, macroH2A depletion from melanoma cells promoted enhanced tumour growth and metastatic colonization18. Accordingly, overexpression of macroH2A2 induced tumour cell dormancy and suppressed the growth of disseminated cancer cells into overt metastasis19. Furthermore, high macroH2A levels correlate with favourable prognosis in lung cancer and in colon cancer20,21. Collectively, these data provide support for a tumour suppressive role for macroH2A.
Epigenetic regulation of the melanoma tumour microenvironment (TME) remains poorly characterized. Here we investigate the consequences of macroH2A deficiency on autochthonous BRAFV600E/PTEN-deficient melanomas. We report an unappreciated level of intratumoral heterogeneity and an impaired anti-tumour immune response in dKO animals. This phenotype is driven by cancer-associated fibroblasts (CAFs), which accumulate in the TME and express high levels of inflammatory genes through increased promoter–enhancer interactions. Our study highlights a unique role for macroH2A histones in the TME by limiting the pro-inflammatory properties of the tumour stroma.
Results
MacroH2A suppresses autochthonous melanoma growth
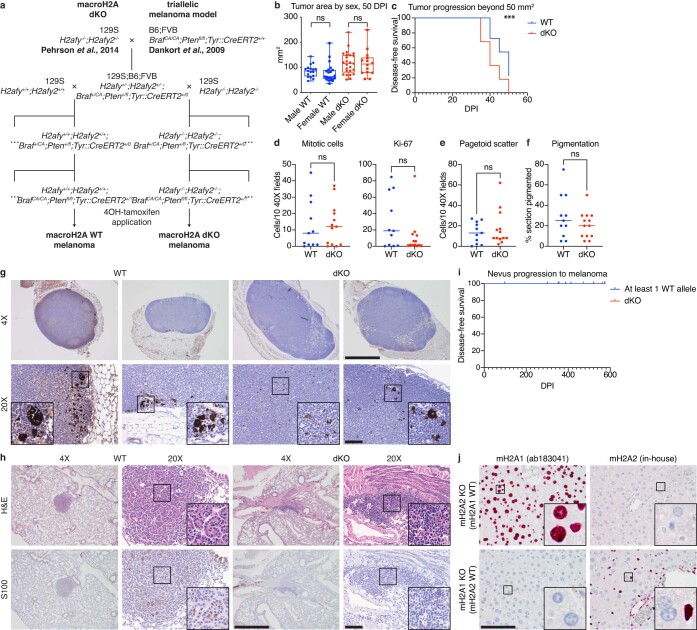
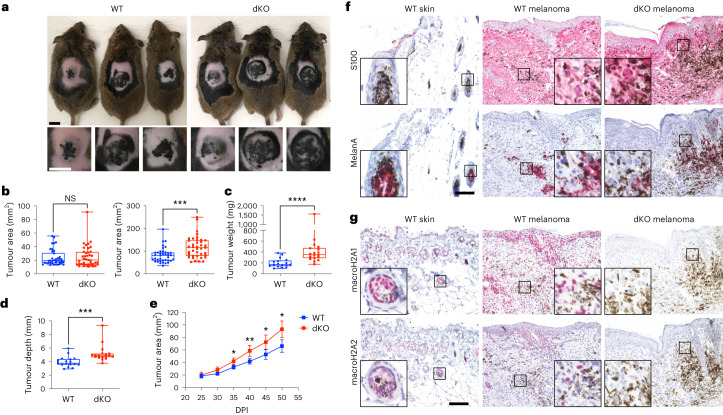
We crossed mice constitutively deficient for macroH2A histones (H2afy and H2afy2 dKO strain)10 to the BrafCAPtenflTyr-CreERT2 triallelic melanoma strain22 (Extended Data Fig. 1a) and initiated tumours through the topical application of 4-hydroxytamoxifen in wild-type (WT) mice and in dKO mice. Although the tumour area was similar at 25 days post-induction (DPI), by 50 DPI, dKO tumours acquired a significant >40% increase in area and a twofold increase in weight versus WT tumours. The increase was independent of sex (Fig. 1a–c and Extended Data Fig. 1b) and involved increased vertical growth (Fig. 1d). The dKO tumours displayed accelerated development (Fig. 1e) and progressed beyond 50 mm2 significantly earlier than WT tumours (Extended Data Fig. 1c).
Extended Data Fig. 1. Characterization of macroH2A WT and dKO murine melanomas.
a) Breeding strategy to obtain WT and dKO mice used for melanoma induction. b) Tumor area in males and females, nWT-M = 17, nWT-F = 21, ndKO-M = 25, ndKO-F = 14. No significant inter-sex differences were observed within genotypes (Kruskal-Wallis with Dunn’s multiple comparisons test). Box plot whiskers represent min-max range, box plot limits – 25th to 75th percentiles, center line – median. c) Kaplan-Meier analysis of disease-free survival. Events represent melanoma growth beyond an area of 50 mm2, nWT = 22, ndKO = 22. P-value = 0.0009, log-rank (Mantel-Cox) test. d) Immunohistochemical scoring of H3S10ph and Ki-67 at 50 DPI in ten random fields per tumor. e) Pagetoid spread of melanocytic cells into epidermal structures scored in ten epidermis-containing fields per tumor on H&E-stained sections. f) Relative pigmented area of the tumor, estimated on a 2X magnification ensemble view of H&E-stained sections. d-f, nWT = 11, ndKO = 13, center line represents median, significance determined using a Mann-Whitney two-tailed test. Exact P-values are provided as numerical source data. g) Tumor-draining axillary lymph nodes stained for S100 (reddish-brown NovaRed substrate color). h) Lung sections of tumor-bearing mice at 50 DPI. g-h, 4X (scale bar = 1 mm) and 20X (scale bar = 100 μm) magnification shown; inserts shown at 2.5X additional magnification. Staining and analysis were performed in 2 animals per genotype with similar results. i) Kaplan-Meier analysis of disease-free survival following nevus induction, nat least 1 WT allele = 7, ndKO = 6. Progression to melanoma, defined as radial and/or vertical lesion growth, was not observed during the lifespan of the mice. j) Validation of antibody specificity for macroH2A variants in immunohistochemistry, using liver of either macroH2A1 or macroH2A2 single KO mice. Scale bar = 100 μm, inserts shown at 5X additional magnification. Experiment was performed twice with similar results.
Fig. 1. MacroH2A loss in the melanoma TME promotes primary tumour growth.
a, Macroscopic appearance of BRAFV600E/PTEN-deficient autochthonous melanomas in macroH2A WT mice and in dKO mice at 50 DPI. b, Comparison of tumour area across genotypes at the indicated time points. nWT = 38, ndKO = 39. P = 0.7962 at 25 DPI, P = 0.0002 at 50 DPI. c, Measured weight of resected tumours at the end point (50 DPI). nWT = 17, ndKO = 18. P < 0.0001. d, Average tumour depth calculated from the tumour area and volume at the end point (50 DPI). nWT = 17, ndKO = 18. For b–d, significance was determined using Mann–Whitney two-tailed test. Box plot whiskers represent the minimum to maximum range, the box plot limits the 25th to 75th percentiles, and the centre line the median. P = 0.0001. e, Tumour growth kinetics between 25 and 50 DPI. nWT = 22, ndKO = 22. Mean and 95% confidence interval error bars are shown. P values adjusted for multiple comparisons: *P < 0.05, **P < 0.01, Mann–Whitney two-tailed test. Exact P values are provided as numerical source data. f, Immunohistochemical characterization of normal dorsal skin and representative tumours in a. Antigens indicated are stained pink (Vector Red substrate). g, Immunohistochemical analysis as in f, but demonstrating macroH2A1 and macroH2A2 staining in normal skin and in WT and dKO melanoma. For f and g, insets are shown at additional ×4 magnification. Staining was repeated on nWT skin = 2, nWT melanoma = 7, ndKO melanoma = 6 mice with similar results. Scale bars, 100 μm (f,g) or 1 cm (a). NS, not significant.
Histology analyses revealed melanocytic lesions with a pigmented epicentre, loss of pigmentation on the margins and depth, and invasion through subcutaneous muscle as determined by S100 staining (Fig. 1f). MelanA staining was present in pigmented foci within tumours and normal melanocytes in the hair follicle, which suggested that most transformed cells lost melanocytic antigens (Fig. 1f). Differences were not observed in proliferation markers, melanocytic spread into the epidermis (pagetoid scatter) or pigmentation between genotypes (Extended Data Fig. 1d–f). Besides melanophages or isolated disseminated tumour cells in lymph nodes (Extended Data Fig. 1g), or rare non-pigmented S100-negative lung lesions (Extended Data Fig. 1h), we did not detect overt metastases at 50 DPI. Furthermore, PtenWT dKO mice developed nevi following BRAFV600E induction22, but did not proliferate or transform during the lifetime of the animals (Extended Data Fig. 1i). MacroH2A1, present throughout normal skin, was retained in melanomas, whereas macroH2A2, detected primarily at low levels in the hair follicle in normal skin, was focally present in the tumour (Fig. 1g and Extended Data Fig. 1j). Altogether, the results indicate that macroH2A loss promotes tumour growth, which may occur through mechanisms distinct from melanocytic hyperproliferation.
dKO melanomas deregulate anti-tumour immunity genes
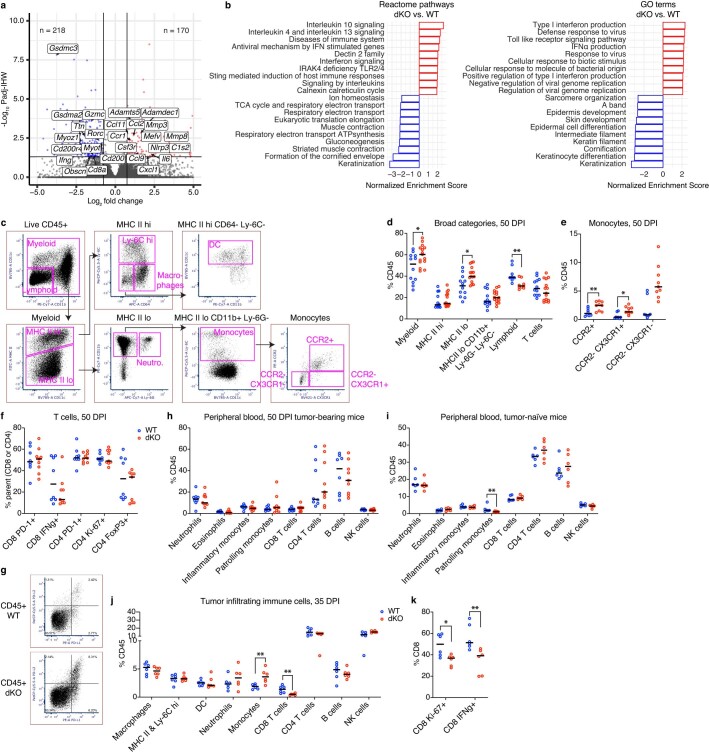
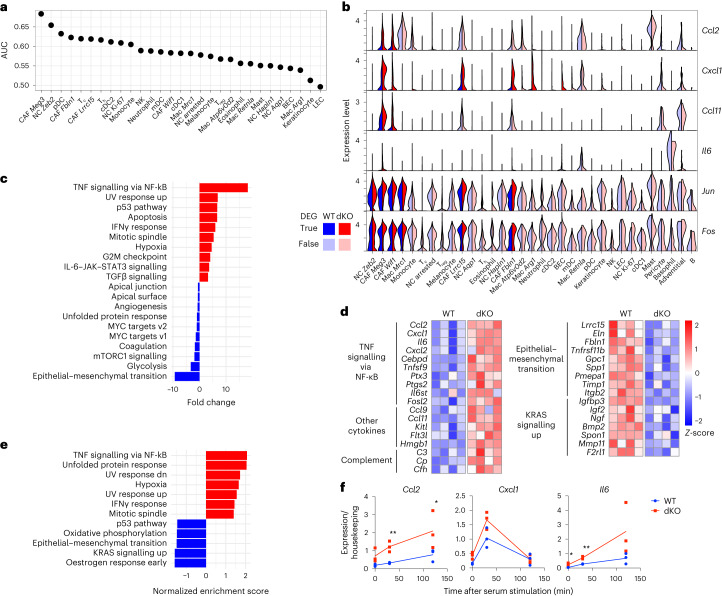
Transcriptomic profiling of bulk tumours at 50 DPI highlighted 170 upregulated genes and 218 downregulated genes (Extended Data Fig. 2a and Supplementary Table 1). Gene set enrichment analysis (GSEA) terms that were up in dKO samples were primarily associated with immune function (Fig. 2a and Extended Data Fig. 2b). The pro-inflammatory cytokines Ccl2, Cxcl1, Ccl9 and Il6 (Fig. 2b), produced by multiple cell types to attract monocytes and myeloid progenitors into tumours23–26, were upregulated. Monocytes and neutrophils recruited by these cytokines and signalling though the G-CSF receptor (Csf3r) may limit the anti-tumour immune response23–25,27–29. Also upregulated were matrix metalloproteinases (Fig. 2b), which have numerous functions, including proteolysis-mediated activation of secreted chemokines30,31.
Extended Data Fig. 2. Murine macroH2A-deficient melanomas harbor a dysfunctional immune microenvironment.
a) Volcano plot of differential gene expression in dKO vs. WT murine melanomas analyzed by RNA-seq. Significant DEGs are colored: red = up in dKO and blue = down in dKO. X and Y axes are zoomed in to depict genes in Fig. 2b. Independent hypothesis weighted Wald test P-values adjusted for multiple comparisons, computed by DESeq2. b) GSEA analysis of Reactome pathways and GO terms in dKO vs. WT murine melanomas. Top 10 significant pathways shown. c) Gating strategy used to delineate tumor-infiltrating myeloid cell subtypes by flow cytometry, shown in a WT tumor. d) Broad immune cell categories not shown in Fig. 2 at 50 DPI, nWT = 12, ndKO = 15 animals except for lymphoid population (sum of T, B and NK cells) where nWT = 8 and ndKO = 11 animals. e) Relative abundance of monocyte subpopulations identified by CCR2 and CX3CR1 expression. nWT = 8 and ndKO = 9 animals. f) Analysis of additional markers of T cell functionality. nWT = 8 and ndKO = 9 animals. g) PD-L1 and PD-L2 staining of CD45+ cells infiltrating WT and dKO melanomas. h) Quantification of indicated populations of non-overlapping peripheral blood immune cell populations at 50 DPI, determined by flow cytometry, nWT = 8, ndKO = 9 animals. i) As in (h) in tumor-naïve mice, nWT = 6, ndKO = 6 animals. j) Quantification of indicated non-overlapping tumor-infiltrating immune cell populations at 35 DPI. k) Proliferative status and anti-tumor activity of CD8 + T cells in (j), assessed as percentage of Ki-67 positivity and interferon-gamma production, respectively. j-k, nWT = 6, ndKO = 6 animals. d-f and h-k, Mann-Whitney two-tailed test P-values: * < 0.05, ** < 0.01, center line represents median. Exact P-values are provided as numerical source data. Non-significant differences are not labeled.
Fig. 2. MacroH2A-deficient melanomas deregulate genes associated with an immune anti-tumour response, and accumulate monocytes and dysfunctional CD8+ T cells.
a, GSEA of hallmark pathways performed on bulk RNA-seq of triallelic melanomas at 50 DPI. dKO versus WT comparison. The top ten significant (Benjamini–Hochberg adjusted P < 0.05) pathways are shown. Exact P values are provided in Supplementary Table 1. b, Heatmap of DEGs in WT and dKO melanomas (bulk tumour), grouped under selected top gene enrichment terms defined using Homer analysis. Each column represents an independent tumour. Expression values are normalized row-wise as Z-scores. c, Quantification of indicated non-overlapping tumour-infiltrating immune cell populations at 50 DPI, determined by flow cytometry. nWT = 12, ndKO = 15 except for the natural killer (NK) cell population, for which nWT = 8 and ndKO = 11. DC, dendritic cells; MHC, major histocompatibility complex. d, Proliferative status of CD8+ T cells in c assessed as a percentage of Ki-67 positivity by flow cytometry. e, Expression of PD-1 ligands on immune cells assessed as a percentage of PD-L1 or PD-L2 positivity by flow cytometry. For d and e, nWT = 8, ndKO = 9. For c–e, Mann–Whitney two-tailed test P values shown: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, with exact P values provided as numerical source data. The centre line represents the median. Non-significant differences are not labelled. f, GSEA of hallmark pathways performed on RNA-seq of CD8+ T cells sorted by flow cytometry from melanomas at 50 DPI. dKO cells versus WT comparison. g, Heatmap of DEGs in WT and dKO melanoma-infiltrating sorted CD8+ T cells, grouped under selected top gene enrichment terms defined using Homer analysis. Each column represents target cells from an independent tumour. Expression values are normalized row-wise as Z-scores.
Among the downregulated genes, those associated with muscle and keratinization (Fig. 2b and Extended Data Fig. 2b) indicated displacement of normal epidermal and subcutaneous muscle structures in the dKO samples. Also downregulated were gasdermins, mediators of pyroptosis, an immunogenic form of cell death32, together with the CD200 axis, which inhibits myeloid cell function33,34. Importantly, dKO tumours downregulated markers of effector CD8+ cells, including Cd8a, Ifng and Gzmc. Overall, the transcriptomics data suggest that there is an impaired anti-tumour immune response in dKO animals.
Tumour-infiltrating immune cell dysfunction in dKO mice
We immunophenotyped tumours at 50 DPI (Extended Data Fig. 2c) and observed expansion of classical (CCR2+) and non-classical (CCR2−CX3CR1+) monocytes in dKO samples (Fig. 2c and Extended Data Fig. 2d,e). These cells, often termed mononuclear phagocyte-like myeloid-derived suppressor cells, inhibit T cell function and have negative prognostic value in cancer35. Accordingly, there was a significant reduction in the relative abundance of CD8+ T cells (Fig. 2c) and their proliferation (Fig. 2d). Other CD8 cell markers and their counterparts in CD4+ cells were not affected (Extended Data Fig. 2f). Increased PD-1 ligands in dKO immune cells (Fig. 2e and Extended Data Fig. 2g), probably stemming from the myeloid compartment to which PD-L2 is mainly restricted36, further indicated immunosuppression37.
By sorting CD8+ T cells and profiling them using RNA sequencing (RNA-seq) (Supplementary Table 2), we found downregulation of the G2/M checkpoint and E2F targets, as well as genes associated with response to interferons and viral infection (Fig. 2f,g). Upregulated genes highlighted a pathological CD8+ T cell phenotype, including the RORγt receptor (Rorc), IL-17F and IL-23R, which are markers of TC17 cell polarization. This response can be driven by monocyte-derived IL-1β (the receptors of which are upregulated in the dKO CD8+ population; Fig. 2g), IL-6 and IL-23 (which signal though the upregulated JAK–STAT pathway)38 (Fig. 2f), and is associated with impaired anti-tumour activity39–41. Finally, TNF signalling (upregulated in dKO mice; Fig. 2g) in activated melanoma-infiltrating CD8+ T cells triggers their death42.
We did not observe significant changes in the relative proportions of immune cells in peripheral blood, regardless of tumour-bearing status (Extended Data Fig. 2h,i), which suggested that immunophenotypic changes were TME-specific. The increase in monocytes and decrease in CD8+ T cell abundance and functionality was recapitulated at 35 DPI (Extended Data Fig. 2j,k), which signified that the dKO immunophenotype is not a consequence of tumour size. Together, our data indicate that without macroH2A, increased pro-tumour inflammatory signals in the TME inhibit immune-mediated tumour cell killing, which facilitates tumour growth.
Melanocyte dedifferentiation in dKO tumours
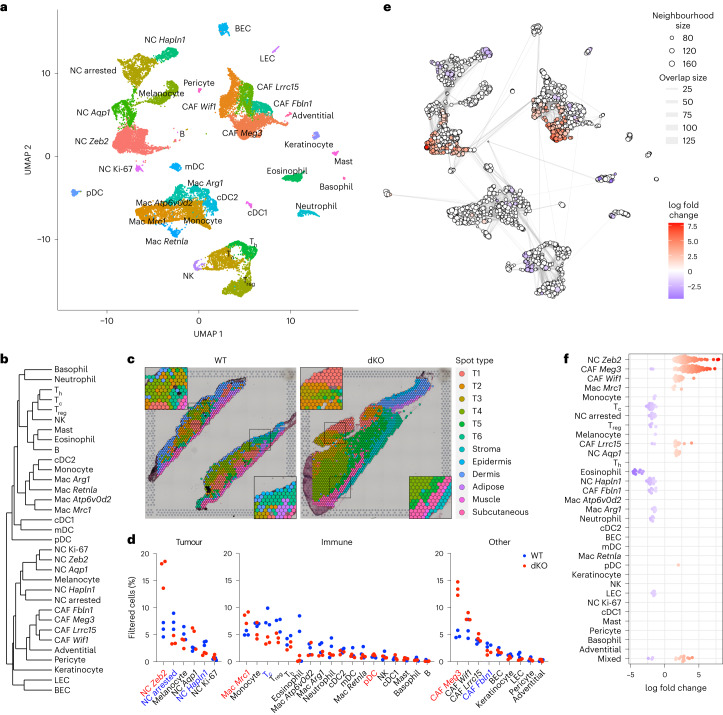
This melanoma model lacks macroH2A in a constitutive manner; thus, the dKO phenotype could stem from several cell types. We performed single cell RNA-seq (scRNA-seq) of three WT and three dKO melanomas, generating a dataset of ~24,000 high-quality cells. We identified 33 cell clusters, including melanocytes, immune cells and CAFs, as well as rarer cell populations (Fig. 3a). Cell types and states represented by each cluster were annotated on the basis of expression of known lineage markers (Extended Data Fig. 3a), similarity (Fig. 3b) or the most significant cluster-specific genes43 (Supplementary Table 3). We performed spatial transcriptomics (ST) to visualize the distribution of tumour populations (spot clusters T1–T6) compared with normal regions (for example, epidermis) in their native tissue context (Fig. 3c and Supplementary Table 4). ST confirmed the identities we ascribed using scRNA-seq (Extended Data Fig. 3b) and the increased tumour area and invasiveness of the dKO tumours (Fig. 3c) as observed above (Fig. 1).
Fig. 3. scRNA-seq identifies dKO-associated remodelling of the NC-derived and immune compartments.
a, Dimension-reduced representation using uniform manifold approximation and projection (UMAP) of cell clusters in WT and dKO melanomas profiled by scRNA-seq at 35 DPI. Dots correspond to single cells from three independently processed tumours per genotype, coloured by cluster identity. b, Phylogenetic tree showing the degree of cell type and state similarity based on distances between clusters in principal component analysis space. See Supplementary Table 3 for a description of the cell-type acronyms used. c, Distribution of annotated spot types derived from ST analysis, overlaid on WT and dKO tumour histology. Insets are shown at ×2 magnification. d, Relative cell frequencies across clusters in individual melanomas profiled by scRNA-seq. Values shown are normalized to the total number of high-quality cells per sample included in the analysis. Names in colour represent clusters with significant differences between WT and dKO frequencies (two-tailed unpaired t-test < 0.05); red indicates more abundant in dKO, whereas blue indicates more abundant in WT. P values are provided as numerical source data. e, UMAP representation of differential abundance analysis performed using Milo. Cells are grouped into overlapping neighbourhoods based on their k-nearest neighbour graph position, depicted as circles proportional in size to the number of cells contained, coloured by the log fold change of abundance between genotypes. The graph edge thickness is proportional to the number of cells shared between adjacent neighbourhoods. f, Bee swarm plot of significant differences in e showing distributions of abundance log fold changes between dKO and WT samples in neighbourhoods belonging to the indicated clusters as in a. For e and f, neighbourhoods with significant differential abundance at a 5% false discovery rate are coloured. In f, non-significant neighbourhoods are not shown.
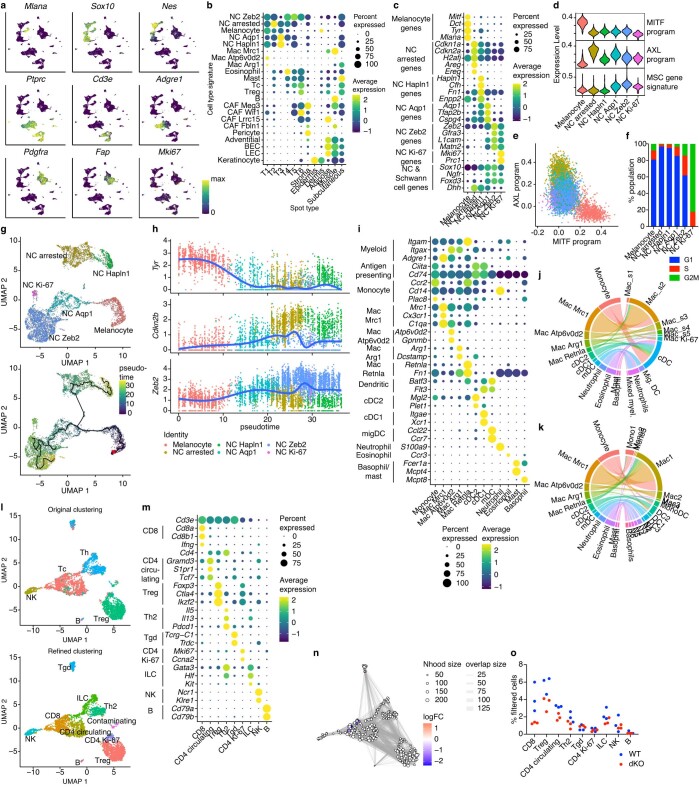
Extended Data Fig. 3. Details of scRNA-seq analysis to annotate components of the melanoma TME.
a) Expression of marker genes of major cell lineages expected in the TME, shown on UMAP plots. b) Distribution of cell type signatures derived from murine melanoma scRNA-seq in spot types of the murine melanoma ST dataset. c) Selected cluster-specific markers used to annotate neural crest (NC) cell types/states. d) Violin plots of indicated gene expression signatures measured across NC clusters. e) Scatter plot of antagonistic MITF and AXL-driven gene expression signatures identified in human melanoma, across NC clusters in (d). f) Distribution of predicted cell cycle stages across NC clusters in (d). g) Pseudotime analysis of reclustered NC cells. Top, original neural crest clusters after re-integration, dimensionality reduction and UMAP embedding. Bottom, cell trajectories in pseudotime anchored in the melanocyte cluster (red dot). h) Representation of expression profiles of key genes across pseudotime in NC clusters. i) As in (c), for myeloid cells. j) Circos plots showing correspondence between myeloid cell clusters identified and myeloid cell identities in a published scRNA-seq datasets of murine subcutaneous sarcoma52. k) As in (j) in murine lung adenocarcinoma53. l) UMAP plot of lymphoid cells after re-integration, dimensionality reduction and UMAP embedding, labeled according to their original clusters (top) and annotations generated by reclustering (bottom). m) Selected markers used to annotate lymphoid cell types/states following reclustering in (l). n) UMAP representation of differential abundance analysis of cells in (l) performed with Milo. Cells are grouped into overlapping neighborhoods based on their K-nearest neighbor graph position, depicted as circles proportional in size to the number of cells contained, colored by log fold change of abundance between genotypes. Graph edge thickness is proportional to the number of cells shared between adjacent neighborhoods. o) Relative abundance across lymphoid clusters as annotated in (l) and (m), shown as percentage of total viable cells in each tumor.
In the tumour compartment, melanocytes represented only ~5% of cells (Fig. 3d); however, ~25% of cells expressed the neural crest (NC) cell marker Sox10 while lacking melanocyte markers or a MITF gene signature derived from a human melanoma scRNA-seq analysis44 (Extended Data Fig. 3c–e). These ‘NC’ clusters (Fig. 3a) expressed genes associated with developmental precursors of mouse melanocytes and Schwann cells45, including Ngfr (NC), Foxd3 (migratory NC and Schwann cell precursors (SCPs)) and Dhh (SCPs) (Extended Data Fig. 3c), and occupied distinct tissue niches in the ST dataset (Extended Data Fig. 3b). The NC arrested cluster displayed a transcriptional profile (Extended Data Fig. 3c) and cell cycle distribution (Extended Data Fig. 3f) that was consistent with growth arrest. The NC clusters Hapln1 and Aqp1 expressed melanoma dissemination and NC cell migration genes. Finally, the NC Zeb2 and NC Ki-67 clusters were characterized by the transcription factor (TF) Zeb2, which is involved in the proliferation of melanoma cells before and following dissemination46, along with Gfra3, which is associated with a NC stem cell signature and residual disease in human melanoma47. The NC arrested and Aqp1 clusters expressed high levels of an AXL-driven programme associated with melanoma invasion44, whereas the Zeb2 and Ki-67 clusters expressed the highest levels of a melanocyte stem cell signature48 (Extended Data Fig. 3d,e).
To dissect the relationship between NC cells and melanocytes, we performed cell trajectory analysis (Extended Data Fig. 3g,h). One branch incurred a growth suppressive programme in the NC arrested cluster, whereas a second branch dedifferentiated towards a state resembling the migratory and stem-like stages of NC development (NC Aqp1 and Zeb2/Ki-67 clusters; Extended Data Fig. 3h), which are associated with poor prognosis in human melanoma49. This trajectory was supported by the ST data. As expected, melanocytes were present in the dermis and in T1 spots replacing normal dermis (Fig. 3c and Extended Data Fig. 3b). NC arrested cells were most abundant in T2 spots, whereas migratory NC Aqp1 and Hapln1 cells defined T3 spots. Meanwhile, T4–T6 spots harboured the bulk of NC Zeb2 cells that invaded subcutaneous structures (Fig. 3c and Extended Data Fig. 3b). Relative to the total, dKO tumours were enriched in NC Zeb2 cells to the detriment of NC arrested and Hapln1 cells (Fig. 3d). This result was confirmed using an unbiased approach that leveraged local cell abundance changes across conditions (Fig. 3e,f). Together, these data suggest that macroH2A deficiency promotes dedifferentiation in the NC compartment.
Pro-tumour features of myeloid cells in the dKO TME
We identified multiple clusters of mononuclear phagocytes including macrophages (Mac), several of which expressed genes associated with pro-tumour subtypes, including CD206 (Mrc1), Arg1, Retnla, Fn1 and C1qa50,51 (Fig. 3a,b and Extended Data Fig. 3i). By annotating against two mouse tumour model scRNA-seq datasets, we found that Mac Mrc1, which accumulated in the dKO (Fig. 3d), was fully encompassed by Mac_s2, tumour-enriched macrophages that become depleted following immune checkpoint blockade52, and by Mac1, the human counterpart of which is associated with poor prognosis in patients with lung adenocarcinoma53 (Extended Data Fig. 3j,k). These similarities reinforce the finding that immunosuppressive myeloid cells accumulate in dKO tumours.
Lymphoid clusters recapitulated the decrease in cytotoxic T (Tc) cells in dKO tumours (Fig. 3d), as observed by flow cytometry (Fig. 2c). Through reclustering, we refined lymphoid cells such that the Tc cell cluster split into a Cd4-positive CD4 circulating cluster (Extended Data Fig. 3l,m) and a bona fide CD8 cluster negative for Cd4, which was locally depleted in dKO tumours (Extended Data Fig. 3n). Overall, scRNA-seq corroborated our bulk RNA-seq and immunophenotyping data, providing support for the presence of increased immunosuppressive myeloid and decreased Tc cell infiltration (Extended Data Fig. 3o) in dKO melanomas.
CAFs produce pro-inflammatory signals in the dKO TME
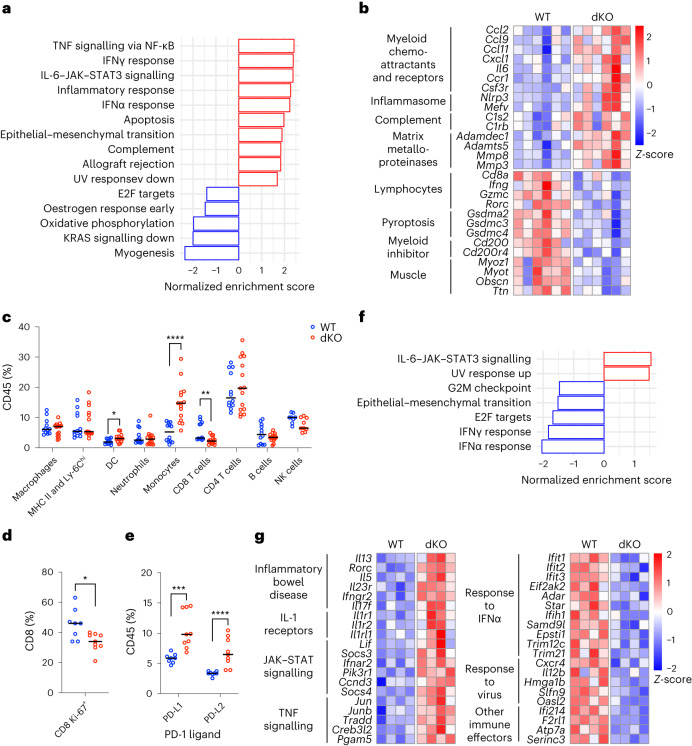
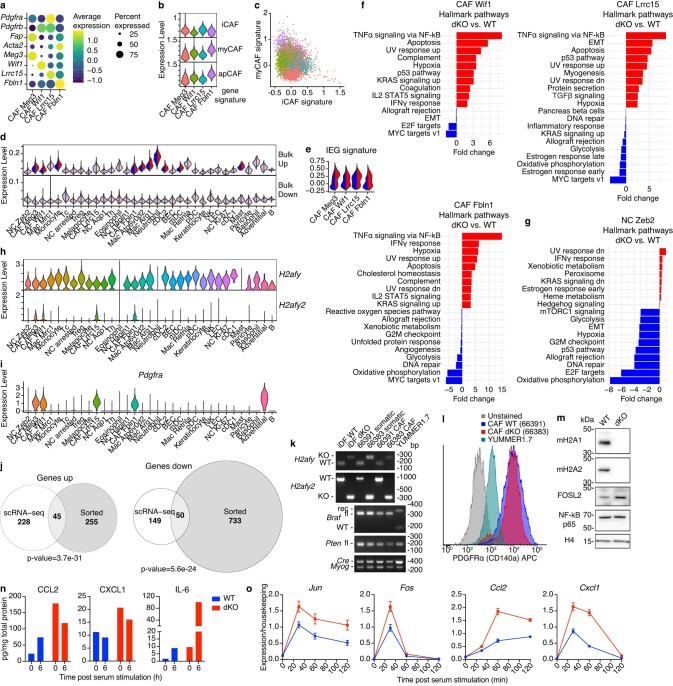
We identified four CAF clusters (Extended Data Fig. 4a), which lacked distinction between inflammatory (iCAF), myofibroblastic (myCAF) and antigen-presenting CAFs (apCAF) reported in pancreatic cancer54 (Extended Data Fig. 4b,c). This result suggests that there is distinct CAF origin or functional specialization across tumours. The CAF Meg3 cluster exhibited an almost threefold increase in dKO tumours (Fig. 3d), and with NC Zeb2, this cluster was the most relatively enriched cell type (Fig. 3e,f). By computationally assessing the weight of each cluster, CAF Meg3 was highlighted as the top driver of the dKO transcriptional profile (Fig. 4a). Moreover, we found a significant upregulation of the ‘dKO tumour up’ signature, which consisted of all upregulated genes in the bulk RNA-seq dataset (Supplementary Table 2), across all dKO CAF clusters, as well as a subset of myeloid clusters (Extended Data Fig. 4d). Importantly, the upregulated cytokines in the bulk RNA-seq data (for example, Ccl2, Cxcl1, Ccl11 and Il6) were significantly overexpressed in dKO CAF clusters (Fig. 4b). We additionally found upregulation of other immediate-early genes (for example, Jun and Fos), which was indicative of signal response pathway activation. This finding was confirmed by comparing our data to a signature comprising 139 immediate-early genes55 (Extended Data Fig. 4e). GSEA revealed upregulation of inflammatory pathways, led by ‘TNFα signalling via NF-κB’ across all CAF clusters (Fig. 4c and Extended Data Fig. 4f), whereas NC Zeb2, the next highest cluster (Fig. 4a) did not (Extended Data Fig. 4g). Together with the increased CAF Meg3 prevalence (Fig. 3d), these data suggest that CAFs are the primary source of the abovementioned cytokines in the TME and promote the dKO immunophenotype. Of note, whereas H2afy expression was readily detected across clusters, H2afy2 was limited to CAFs (Extended Data Fig. 4h), which suggested that its loss contributes to a CAF-specific phenotype.
Extended Data Fig. 4. Characterization of CAF clusters in the melanoma TME and their in vitro counterparts.
a) Dot plot of markers used to annotate CAFs, and CAF cluster specific genes. b) Violin plots of murine pancreatic cancer CAF population signatures measured across murine melanoma CAF clusters. c) Scatter plot of inflammatory and myocyte-like CAF gene expression signatures identified in pancreatic cancers across murine melanoma CAF clusters. d) Gene signatures derived from differentially expressed genes in the bulk RNA-seq dataset, calculated across clusters and genotypes. Significance defined as Wilcoxon rank sum test adjusted P-value < 0.05, cluster-average log2 fold change > 0.02 or < -0.02. Exact P-values are provided in Table 3. e) Violin plot of an immediate early gene (IEG) signature in CAFs. Significant dKO vs. WT differences are present within each cluster (Wilcoxon rank sum test adjusted P-value < 0.05, cluster-average log2 fold change > 0.1). Exact P-values are provided in Table 3. f) Significant Hallmark pathways in GSEA analysis of dKO vs. WT CAF clusters. g) As in f, for the NC Zeb2 cluster. h) Violin plots of macroH2A gene expression in WT melanoma. i) Violin plot of Pdgfra expression in the melanoma TME. j) Intersections of DEGs across single-cell and bulk RNA-seq modalities in CAFs. DEGs in the scRNA-seq dataset were determined by grouping all CAF clusters as one, prior to contrasting by genotype. P-values of Fisher’s exact test shown. k) Genotyping of cultured CAFs and immortalized dermal fibroblasts (iDFs) compared to somatic DNA. YUMMER1.7 cells168 are used as a reference for Braf allele recombination. Experiment was performed twice with similar results. l) Flow cytometric detection of PDGFRα on cultured CAFs. YUMMER1.7 is used as a negative control. m) Western blot showing deletion of macroH2A and accumulation of FOSL2 in dKO cultured CAFs. Histone H4 and NF-κB p65 are used as loading controls. Experiment was performed twice with similar results. n) Protein levels of indicated cytokines in serum-starved and stimulated cultured CAFs, measured by multiplexed bead-capture assay. o) Expression normalized to housekeeping controls of indicated immediate-early and cytokine genes determined by RT-qPCR in iDFs from WT and dKO mice at indicated time after serum stimulation. Mean of 3 PCR replicates shown, error bars represent SEM.
Fig. 4. Pro-inflammatory signals in dKO tumours originate from CAFs.
a, Prioritization of the contribution of each cell cluster to gene expression changes in dKO versus WT samples using Augur, a method that measures the separation in gene expression space between cells in each cluster as a function of genotype. AUC, area under the curve. b, Genes of interest with significant upregulation in dKO samples in clusters highlighted in bold colours (Wilcoxon rank-sum test adjusted P < 0.05). P values are provided in Supplementary Table 3. c, Significant hallmark pathways in a GSEA of dKO versus WT samples performed in the CAF Meg3 cluster. d, Heatmap of DEGs in CAFs sorted by flow cytometry from WT and dKO melanomas at 50 DPI, grouped under selected top gene enrichment terms defined using Homer analysis. Each column represents CAFs from an independent tumour. Expression values are normalized row-wise as Z-scores. e, Significant hallmark pathways in GSEA of sorted CAFs as in d. f, Expression normalized to housekeeping controls of indicated cytokine genes determined by reverse transcription-qPCR in cultured CAFs isolated from WT and dKO tumours at the indicated times following serum stimulation. Line represents the mean of three independently performed experiments shown. Ratio paired two-tailed t-test P values shown: *P < 0.05, **P < 0.01. Exact P values are provided as numerical source data. Non-significant differences are not labelled.
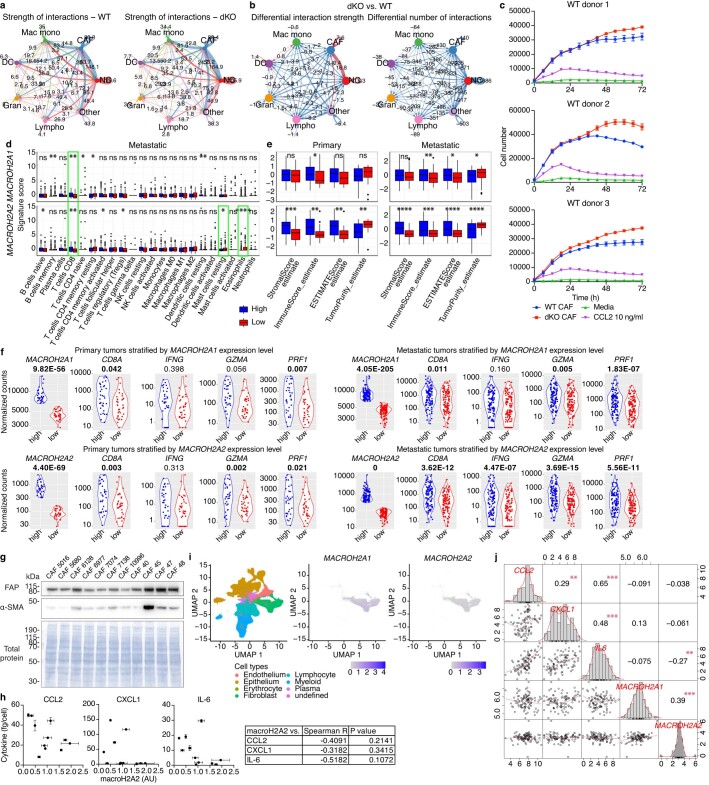
Next, we sorted CAFs from WT and dKO tumours by flow cytometry on the basis of CD140a (Pdgfra) expression (Extended Data Fig. 4i). RNA-seq analyses confirmed the upregulation of cytokines, among others, and activation of the TNF–NF-κB pathway (Fig. 4d,e and Supplementary Table 5). Differentially expressed genes (DEGs) in the sorted CAFs significantly overlapped with those identified by scRNA-seq in the combined mesenchymal populations (Extended Data Fig. 4j). We established pure cultures of sorted WT and dKO CAFs (Extended Data Fig. 4k–m). Given the inducible nature of cytokines, we stimulated primary CAF cultures with serum56–58, followed by quantitative PCR (qPCR) and Luminex-based quantitation (Fig. 4f and Extended Data Fig. 4n). dKO CAFs expressed higher levels of Ccl2, Cxcl1 and Il6 at baseline, which were further induced after serum stimulation. In immortalized dermal fibroblasts (iDFs) derived from non-tumour bearing WT skin and dKO skin11, similar results were observed (Extended Data Fig. 4o), which highlighted a conserved cell-intrinsic mechanism.
Conserved macroH2A-dependent cytokine regulation in CAFs
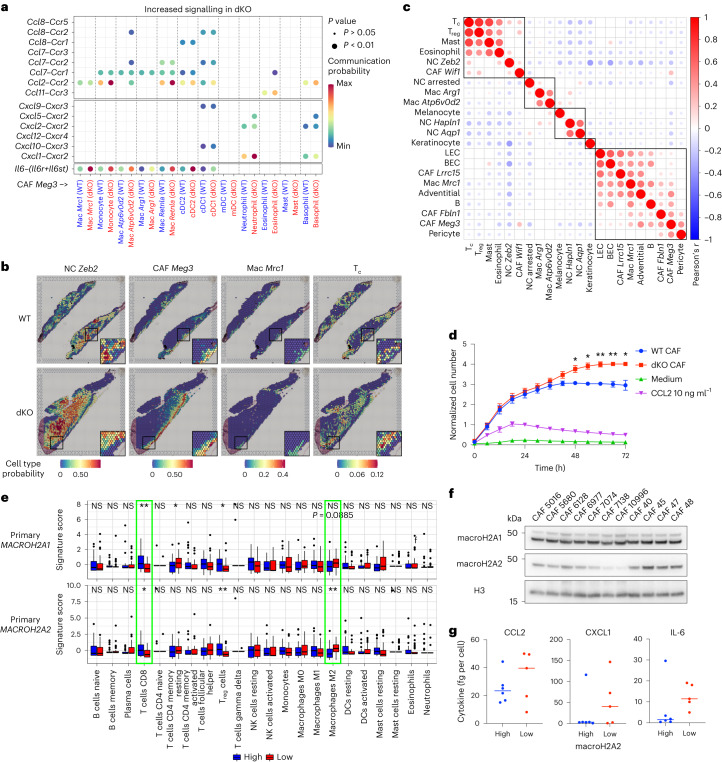
CAFs can recruit immunosuppressive myeloid cells by secreting CCL2, IL-6 and CXCL1 (reviewed in ref. 59). Thus, we performed ligand–receptor analysis of the scRNA-seq dataset. CAFs had the most prolific outgoing interactions with other cell types both in the WT and dKO samples (Extended Data Fig. 5a), as previously described60. Differential analysis showed a generalized decrease in the number of interactions in the dKO samples, but an increase in the strength of communication from CAFs to NC cells (Extended Data Fig. 5a). Importantly, dKO CAFs increased signalling interactions to the mononuclear phagocyte lineage through the CCL2 and IL-6 pathways, and to neutrophils and basophils through CXCL1 (Fig. 5a). Our ST data revealed that CAF Meg3 cells formed a distinct layer (Fig. 5b), partially overlapping with subcutaneous spot types (Extended Data Fig. 3b). Mac Mrc1-enriched spots were enriched at the tumour periphery (Fig. 5b), and correlation analysis revealed a significant positive association between Mac Mrc1 and Meg3, Fbln1 and Lrrc15 CAF subtypes (Fig. 5c), which suggested proximity. Importantly, these cell types shared a significant negative correlation with Tc cells, a characteristic consistent with local T cell exclusion (Fig. 5b,c), which was probably driven by Mrc1+ myeloid cells. We confirmed the chemoattractant properties of CAFs in vitro by measuring the migration of WT bone-marrow-derived monocytes towards WT or dKO CAFs through Transwell assays. MacroH2A dKO CAFs displayed significantly higher monocyte recruitment at later time points (Fig. 5d and Extended Data Fig. 5c), which bolstered our finding of increased monocyte-derived cells in the dKO TME.
Extended Data Fig. 5. MacroH2A regulates pro-inflammatory signals in mouse and human melanoma CAFs.
a) Ligand-receptor analysis of cell signaling performed with CellChat110 summarizing interaction strength between broad cell types. Arrows depicting signaling direction are colored according to emitting cell type. Arrow weight is proportional to interaction strength. b) Differential interaction strength and number between WT and dKO cell type pairs. Arrows are colored according to direction of change (WT – blue, dKO – red) and weight represents amplitude of change. c) Transwell assay measuring the migration of CMFDA-labeled WT bone marrow-derived monocytes towards unlabelled WT or dKO CAFs. Average cell counts of 3 technical replicates and error bars representing SEM are shown for WT and dKO CAF conditions. No replicates are performed for negative and positive controls. Individual experiments using different monocyte donors shown. d) Comparison of deconvoluted immune cell type scores142 between TCGA metastatic melanoma tumors with MACROH2A1/2 high and low expression levels. n = 123 biologically independent samples per category. e) As in (d) for estimated immune, stromal and tumor purity scores143. nprimary = 35, nmetastatic = 123 biologically independent samples per category. d-e, Wilcoxon rank-sum test P-values adjusted for multiple comparisons shown: * < 0.05, ** < 0.01, *** < 0.001, **** < 0.0001. Exact P-values are provided as numerical source data. Box plot center line represents the median, box plot limits – 25th to 75th percentiles, whiskers extend from the box limit to the most extreme value no further than 1.5 * inter-quartile range from the box limit, any data beyond whiskers is plotted as individual points. f) Expression of genes associated with anti-tumor cytotoxic activity in human primary and metastatic melanoma samples from the TCGA cohort, segregated by MACROH2A1 or MACROH2A2 gene expression levels. High and low terciles are compared. Independent hypothesis weighted Wald test P-values adjusted for multiple comparisons, computed by DESeq2, shown. g) Detection of CAF markers by western blot in a panel of 11 human melanoma primary CAF cultures. Membrane stain for total protein shown for loading control. h) Correlation between macroH2A2 protein levels and indicated cytokine secretion in CAF cultures from (g). n = 11 biologically independent samples. Error bars correspond to SEM of 3 technical replicates of blot-based quantification for macroH2A2 and up to 3 dilutions each in 2 technical replicates for cytokine ELISA. Spearman correlation statistics, calculated on the average values without considering individual technical replicates, are shown. i) UMAP plots of a human pan-cancer scRNA-seq dataset60, together with macroH2A gene expression in fibroblasts. j) Correlation analysis between pseudobulk expression levels of selected cytokines and macroH2A genes in CAFs from (i). Pearson correlation coefficients and significance shown above the diagonal, pairwise scatter plots shown below. Histograms of individual gene expression comprise the diagonal. P-values shown: ** < 0.01, *** < 0.001. Exact P-values are provided as numerical source data.
Fig. 5. CAFs are the source of pro-inflammatory signals in the dKO TME.
a, Comparison of signalling probability along CCL, CXCL and IL-6 pathways leveraging scRNA-seq data from CAF Meg3 cells to myeloid cell clusters. Dots represent significant ligand–receptor interaction pairs with increased signalling in the dKO. The dot colour represents communication probability, the dot size represents the P value of one-sided permutation test, and the absence of a dot signifies a null probability of signalling. Exact P values are provided as numerical source data. b, Prediction of the spatial localization of indicated scRNA-seq cell clusters in WT and dKO melanoma by label transfer onto ST data. Insets are shown at ×2 magnification. c, Correlation analysis of cell-type scores for all scRNA-seq clusters detected in SC data, based on the combined set of WT and dKO spots. Dots shown correspond to significant correlations (two-tailed t-test adjusted for multiple comparisons, adjusted P < 0.05), heatmap colour corresponds to Pearson’s correlation coefficient. Exact P values are provided as numerical source data. Black squares represent hierarchical clusters of cell types based on correlation coefficients. d, Transwell assay measuring the migration of CMFDA-labelled WT bone-marrow-derived monocytes towards unlabelled WT or dKO CAFs. Monocyte counts are normalized to the CCL2 condition at 24 h. Summary of three independent experiments using different monocyte donors shown. Error bars represent s.e.m. Two-tailed t-test P values shown: *P < 0.05, **P < 0.01. Exact P values are provided as numerical source data. Non-significant differences are not labelled. e, Comparison of deconvoluted immune cell type scores between TCGA primary melanoma tumours with MACROH2A1 and MACROH2A2 high and low expression levels. Wilcoxon rank-sum test P values adjusted for multiple comparisons shown: *P < 0.05, **P < 0.01. Exact P values are provided as numerical source data. N = 35 biologically independent samples per category. The box plot centre line represents the median, the box plot limits indicate the 25th to 75th percentiles, the whiskers extend from the box limit to the most extreme value no further than 1.5× the inter-quartile range from the box limit, any data beyond whiskers are plotted as individual points. f, MacroH2A1 and macroH2A2 levels in a panel of 11 human melanoma CAF lines. Histone H3 was used as a control for total histone content. g, Indicated cytokine levels in CAF lines in f, stratified according to macroH2A2 levels along the median. Nhigh = 6, nlow = 5 biologically independent samples. The western blot was repeated three times.
Next, we predicted immune cell abundance in macroH2A high and low tumours from the melanoma cohort of The Cancer Genome Atlas (TCGA SKCM). MacroH2A1low and macroH2A2low primary and metastatic samples were associated with significantly reduced CD8 T cell scores (Fig. 5e and Extended Data Fig. 5d). In primary tumours, M2 (pro-tumour) macrophages were significantly associated with macroH2A2low and trended for macroH2A1low tumours (Fig. 5e). Some myeloid subtypes were negatively correlated with macroH2A2 in metastatic tumours (Extended Data Fig. 5d), although macroH2Alow tumours appeared overall depleted of immune cells (Extended Data Fig. 5e), which affected our ability to detect relative increases in immune subtypes. We also identified key Tc cell marker genes, CD8A and IFNG, and components of the tumour cytolytic activity score, GZMA and PRF1 (ref. 61), as significantly downregulated in macroH2Alow tumours (Extended Data Fig. 5f). These correlations highlight anti-tumour immunity dysfunction in human macroH2Alow melanomas.
Examining human melanoma-derived primary CAF cultures (Extended Data Fig. 5g) revealed homogenous levels of macroH2A1 protein, whereas macroH2A2 spanned almost an order of magnitude (Fig. 5f and Extended Data Fig. 5h). MacroH2A2low CAFs secreted more CCL2, CXCL1 and IL-6 when stimulated (Fig. 5g). Although not significant owing to the low sample size (Extended Data Fig. 5h), we analysed a large pan-cancer scRNA-seq dataset60 comprising over 56,000 CAFs across 98 samples. The majority of CAFs had no detectable MACROH2A2 counts (Extended Data Fig. 5i), so pseudobulk expression values per tumour were calculated. Whereas CCL2, CXCL1 and IL6 were positively correlated to each other (Extended Data Fig. 5j), the negative correlation between IL6 and MACROH2A2 was significant (Extended Data Fig. 5j). This result suggests that the relationship between macroH2A2 and inflammatory signalling is conserved in human CAFs.
MacroH2A-regulated genes are enriched in super-MCDs
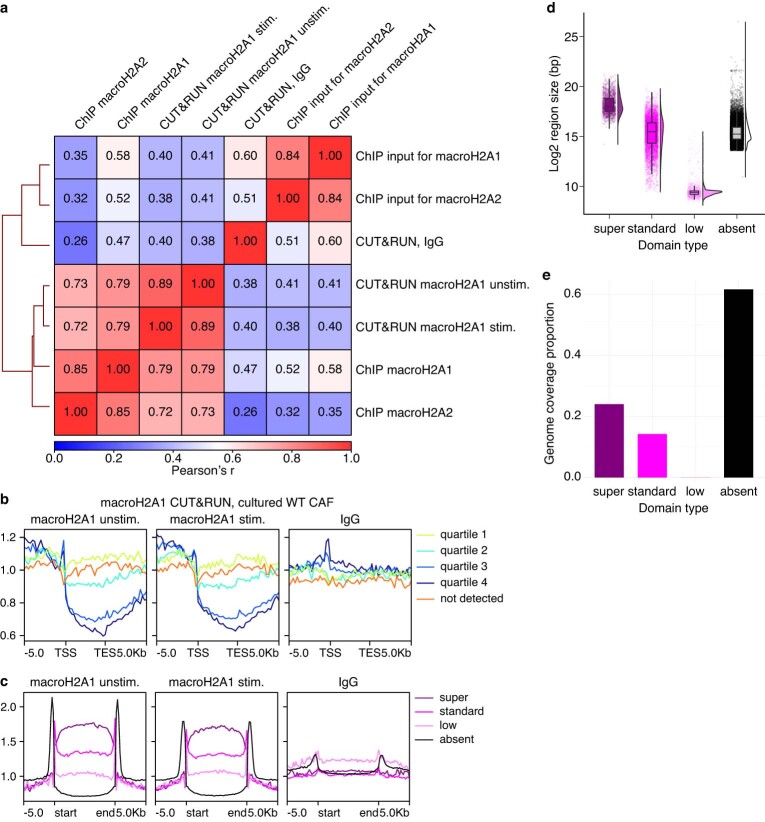
CUT&RUN analyses of macroH2A1 was performed in WT cultured CAFs (Extended Data Fig. 6a). As previously reported62, macroH2A was excluded from the bodies of highly expressed genes and retained at lowly expressed ones (Extended Data Fig. 6b). We identified macroH2A1 chromatin domains (MCDs)62, which we partitioned into ‘super’ and ‘standard’ classes on the basis of enrichment and size (Extended Data Fig. 6c–e). Genome-wide, macroH2A1 was enriched at significant DEGs compared with a control set of static genes with matched expression (Fig. 6a). These DEGs also significantly overlapped MCDs (Fig. 6b). Notably, the 39 significantly upregulated inflammatory genes (Supplementary Table 5) showed higher average macroH2A1 occupancy compared with static genes (Fig. 6c).
Extended Data Fig. 6. Genome-wide macroH2A2 occupancy in cultured WT CAFs.
a) Correlation of genome-wide enrichment of macroH2A between ChIP-seq and CUT&RUN methodologies. ChIP-seq and associated inputs were previously generated in dermal fibroblasts62, CUT&RUN was performed in serum-starved unstimulated and serum-stimulated WT CAFs, with associated IgG control. Pearson’s correlation coefficients shown, conditions are clustered based on Euclidean distance. b) Metagene profile of macroH2A CUT&RUN signal in cultured WT CAFs before and after serum stimulation across genes binned into quartiles according to their expression levels. Genes not detected are either not expressed or not mappable. n = 3000 randomly selected genes within each group. c) Metagene profile of macroH2A CUT&RUN signal in cultured WT CAFs before and after serum stimulation at different classes of MCDs and genomic regions where MCDs are absent. d) Size distributions of regions in (c). Box plot center line represents the median, box plot limits – 25th to 75th percentiles, whiskers extend from the box limit to the most extreme value no further than 1.5 * inter-quartile range from the box limit. c-d, nsuper = 1560, nstandard = 5440, nlow = 1556, nabsent = 23615 regions. e) Proportion of the genome comprised within different classes of MCDs.
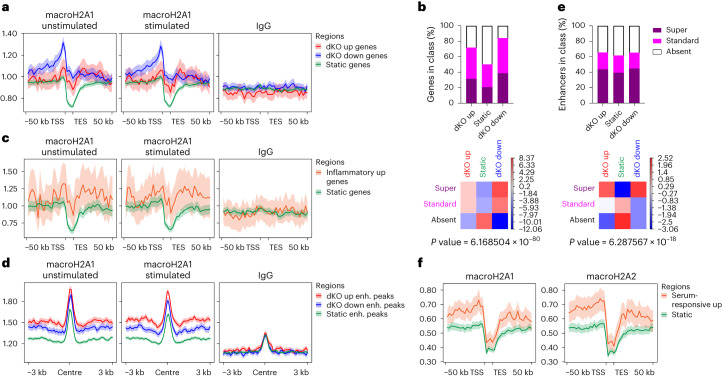
Fig. 6. MacroH2A-sensitive genes and enhancers occupy highly enriched macroH2A chromatin domains.
a, Metagene profile of macroH2A1 CUT&RUN signals in cultured WT CAFs before and after serum stimulation at genes differentially up or down or static genes of matched expression levels in dKO versus WT sorted CAFs. ndKO up = 357, ndKO down = 884, nStatic = 3,708. TES, transcription end site; TSS, transcription start site. b, Top, percentage of overlap between DEGs and MCDs. Bottom, deviation from random distribution shown as a heatmap of Chi-square test residuals, together with the associated P value. c, As in a, but at inflammatory genes upregulated in dKO sorted CAFs and static genes of matched expression levels. nInflammatory up = 39, nStatic = 385. d, Average profile of macroH2A CUT&RUN signals in cultured WT CAFs before and after serum stimulation centred around ATAC peaks located in enhancers (enh.) that gain, lose or maintain static H3K27ac levels in dKO versus WT tumours. ndKO up = 6,659, ndKO down = 5,211, nStatic = 18,961. Note the signal pattern at the centre of the ATAC peak, which is probably due to a bias of CUT&RUN for accessible chromatin sites. e, As in b, but for overlap between enhancers with peaks shown in d and MCDs. f, Average profile of macroH2A1 and macroH2A2 signals in dermal fibroblasts62 analysed by ChIP–seq at genes hyperinduced by serum stimulation in the absence of macroH2A and static genes of matched expression levels. nSerum-responsive up = 139, nStatic = 695. For a, c, d and f, mean signal value and 95% confidence interval as determined by bootstrap analysis are shown.
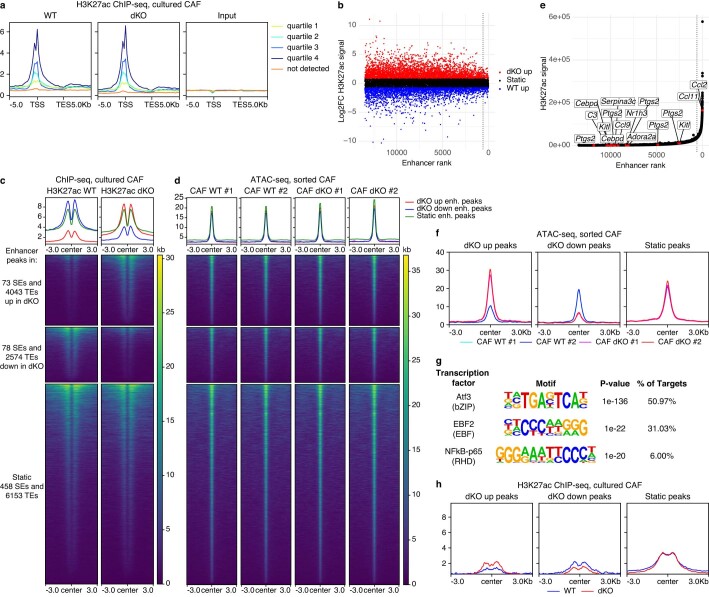
Intergenic enrichment of macroH2A suggested that it may regulate cis-regulatory elements. Traditional enhancers (TEs) and superenhancers (SEs) are emerging regulators of NF-κB-driven inflammatory gene transcription63–67. Moreover, macroH2A regulates specific promoter–enhancer contacts68 and suppresses a subset of enhancers69. Therefore, we performed chromatin immunoprecipitation with sequencing (ChIP–seq) for H3K27ac, which marks active promoters (Extended Data Fig. 7a) and enhancers, in serum stimulated, cultured CAFs. The dKO CAFs revealed increased H3K27ac levels at 4,043 TEs and 73 SEs and decreased H3K27ac at 2,574 TEs and 78 SEs (Extended Data Fig. 7b–d). Similar to DEGs, differentially activated enhancers (DAEs) were significantly enriched in macroH2A and preferentially located in super-MCDs (Fig. 6d,e). Moreover, several significantly upregulated inflammatory genes were located within 50 kb of TEs and SEs that gained H3K27ac (Extended Data Fig. 7e).
Extended Data Fig. 7. Enhancer and chromatin accessibility analysis in the absence of macroH2A in CAFs.
a) Metagene profile of H3K27ac levels in serum-stimulated cultured CAFs across genes binned into quartiles according to their expression levels. Genes not detected are either not expressed or not mappable. n = 3000 randomly selected genes within each group. b) Changes in H3K27ac signal at all detected enhancers in cultured CAFs depicted by MA plot. TEs and SEs are separated by a vertical dashed line. Enhancers with a log2 fold change > 0.75 are shown in color for the respective genotype. c) Heatmap of H3K27ac ChIP signal in serum-stimulated cultured CAFs centered around ATAC peaks located in enhancers that gain, lose or maintain static H3K27ac levels in dKO vs. WT, ndKO up = 6659, ndKO down = 5211, nStatic = 18961. Number of TEs and SEs noted for each cluster. d) ATAC-seq signals at peaks in (c). e) Hockey plot highlighting TEs and SEs gaining H3K27ac in dKO within 50 kb of upregulated inflammatory genes (red) among all TEs and SEs ranked by H3K27ac signal in cultured CAFs. f) Average profile of ATAC-seq signal at differentially accessible regions in dKO vs. WT sorted CAFs, ndKO up = 667, ndKO down = 668, nStatic = 3000 randomly selected non-changing peaks. g) Top 3 hits of HOMER TF motif analysis of regions of increased accessibility in dKO vs. WT sorted CAFs. Fisher Exact test P-values shown. h) H3K27ac ChIP signal in serum-stimulated cultured CAFs at ATAC-seq regions defined in (j). The same scale was used as in (g) for comparison.
Chromatin accessibility, however, was only minimally affected in the dKO CAFs (667 up and 668 down of >140,000 detected peaks; Extended Data Fig. 7f). Nonetheless, motif analysis of the dKO up peaks detected using assay for transposase-accessible chromatin with sequencing (ATAC–seq) highlighted significant enrichment of TFs involved in inflammatory signalling, including NF-κB (Extended Data Fig. 7g). The differentially accessible regions displayed concordant changes in H3K27ac (Extended Data Fig. 7h) but remained small compared with DAEs (Extended Data Fig. 7c).
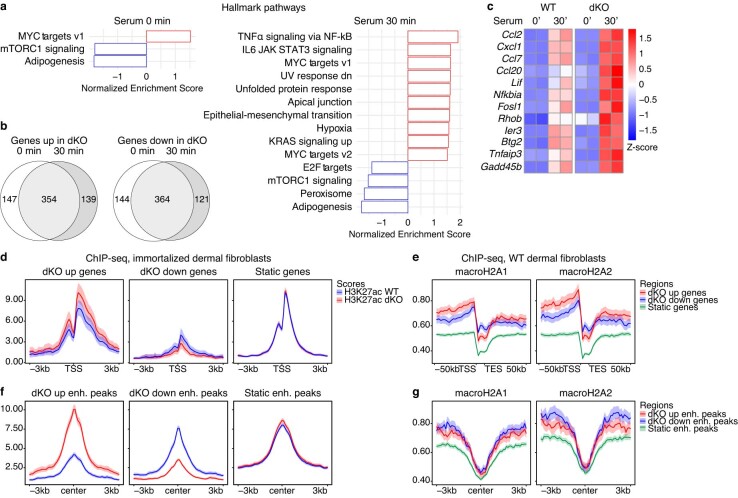
We next utilized iDFs to query whether macroH2A regulates inducible genes beyond CAFs. We performed RNA-seq before and after serum stimulation and found that TNF signalling through the NF-κB pathway was significantly upregulated in the dKO samples following stimulation (Extended Data Fig. 8a and Supplementary Table 6). The upregulated serum-responsive genes (n = 139; Extended Data Fig. 8b,c) were preferentially enriched in macroH2A1 and macroH2A2, as measured in WT DFs62, relative to static genes (Fig. 6f). Generally, DEGs and DAEs were enriched in macroH2A variants in WT fibroblasts compared with static regions, regardless of the change in direction (Extended Data Fig. 8d–g). Thus, macroH2A variants may act as both repressors and activators, as previously reported7,8,68,70–72.
Extended Data Fig. 8. Transcriptomic and enhancer analysis in the absence of macroH2A in iDFs.
a) Significant Hallmark pathways in GSEA analysis of dKO vs. WT performed in iDFs prior to (0 min) and post serum stimulation (30 min). b) Venn diagrams representing the extent of overlap between pre- and post- serum stimulation in genes up- and downregulated by macroH2A deficiency in iDFs. c) Heatmap of a subset of inflammatory genes hyper-induced by serum stimulation in the absence of macroH2A. d) Average profile of H3K27ac ChIP signal in serum-stimulated WT vs. dKO iDFs at promoters of all differentially expressed and static genes of matched expression levels following serum stimulation, ndKO up = 494, ndKO down = 485, nStatic = 2937. e) Average profile of macroH2A1 and macroH2A2 signal62 in dermal fibroblasts at genes described in (d). f) As in (d), for enhancer H3K27ac peaks differentially enriched in H3K27ac, ndKO up = 1438, ndKO down = 1764, nStatic = 7037. g) Average profile of macroH2A1 and macroH2A2 signal in dermal fibroblasts at regions described in (f). d-g, mean signal value and 95% CI as determined by bootstrap shown.
MacroH2A loss leads to rewired chromatin looping
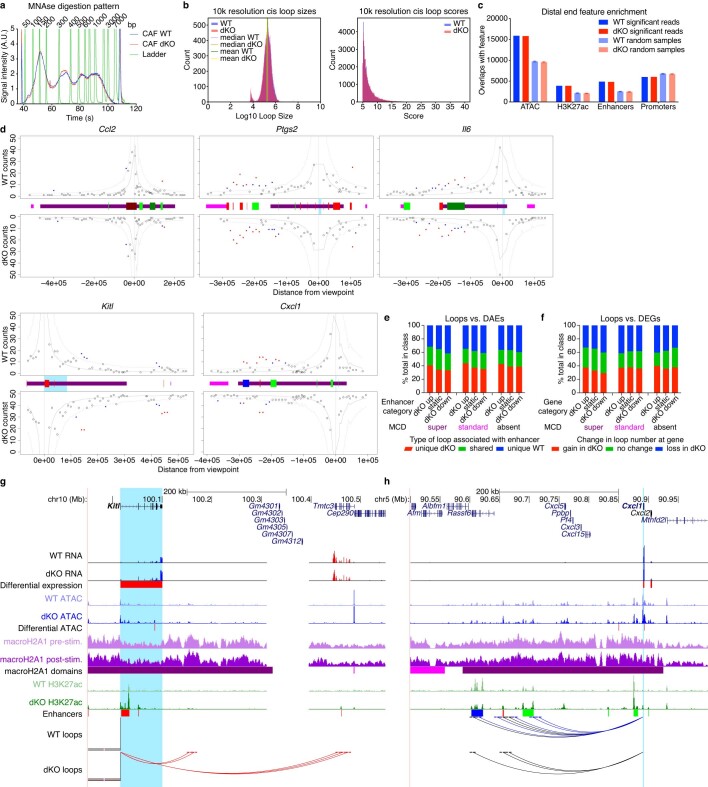
Emerging evidence suggests that macroH2A regulates 3D genome organization68,73–76. To assess this possibility, and to annotate functional promoter–enhancer pairs, we performed Micro-C77 coupled with promoter capture (pcMicro-C)78 in CAFs (Extended Data Fig. 9a). At 10 kb resolution, we identified a similar number of promoter-originating loops in WT and dKO CAFs, with similar size and score distributions (Fig. 7a and Extended Data Fig. 9b). Distal loop ends were enriched for open chromatin and active enhancers (Extended Data Fig. 9c), which validated the functional nature of the contacts.
Extended Data Fig. 9. pcMicro-C analysis in WT and dKO CAFs.
a) Size distribution of MNAse-digested double cross-linked input chromatin for Micro-C. b) Distribution of sizes and scores of chromatin loops called at 10 kb resolution, retained for further analysis. c) Enrichment of indicated functional elements at the distal end of loops, compared to a random distribution. Number of overlaps of significantly called interactions with genomic features are shown in solid color for n = 1 biological sample. Random regions of the same length and count of the significant regions are chosen, and the overlaps with genomic features are counted. The operation is permuted n = 100 times and the average number of overlaps is shown in light color. Error bars represent 95% confidence intervals. d) Bait map plots depicting all called interactions for the bait located at the indicated gene promoter. Dotted lines represent significance thresholds. Only highly significant loops (passing threshold = 5, red dots) are considered. Colored bars represent enhancers and MCDs as in (g). e) Proportions of enhancers associated with changes in chromatin looping, according to changes in their H3K27ac level and location within MCDs. f) Proportions of genes associated with net gain or loss of loops, according to changes in their expression level and location within MCDs. g) UCSC browser screenshots of the Kitl locus and its chromatin environment. Bars under RNA-seq and ATAC-seq tracks indicate significantly up- (red) or downregulated (blue) genes or accessible regions in dKO vs. WT sorted CAFs. Bars under macroH2A CUT&RUN tracks indicate ‘super’ (purple) and “standard’ (magenta) macroH2A chromatin domains. Below H3K27ac tracks, bright and dark bars indicate TEs and SEs, respectively; red, blue, and green denote gain, loss, and no change, respectively, of H3K27ac level in dKO vs. WT CAFs. Chromatin loops at 10 kb resolution, originating at the promoter of the highlighted gene, are shown in red if specific for the dKO, blue for the WT, and black if shared. h) as in e, for the Cxcl1 locus.
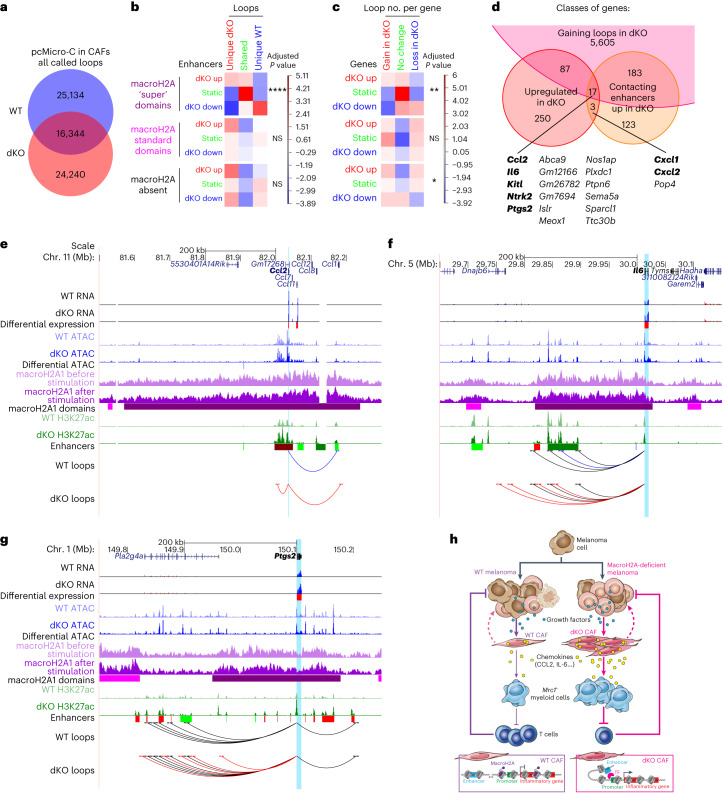
Fig. 7. DAEs and DEGs acquire changes in chromatin looping in dKO tumours.
a, Extent of overlap between chromatin loops detected in WT and dKO CAFs after serum stimulation at 10 kb resolution. b, Chi-square test of independence evaluating the association between changes in H3K27ac level at enhancers and gains or losses of loops to these enhancers in the absence of macroH2A. Combinations of loop and enhancer status are stratified according to the position of enhancers with respect to MCDs. c, As in b, but for changes in gene expression and in the total number of loops per gene. For b and c, P values adjusted for multiple comparisons shown: *P < 0.05, **P < 0.01, ****P < 0.0001. Exact P values are provided as numerical source data. d, Overlap between genes upregulated, in contact with enhancers gaining H3K27ac, and with net loop gains in dKO tumours. Genes in bold are associated with inflammatory signalling pathways according to HOMER analysis. e, University of California Santa Cruz (UCSC) genome browser screenshots of the Ccl2 locus and its chromatin environment showing indicated transcriptomic and epigenomic features. Bars under RNA-seq and ATAC–seq tracks indicate significantly upregulated (red) or downregulated (blue) genes or accessible regions in dKO versus WT sorted CAFs. Bars under macroH2A CUT&RUN tracks indicate ‘super’ (purple) and ‘standard’ (magenta) macroH2A chromatin domains. Below H3K27ac tracks, bright and dark bars indicate TEs and SEs, respectively; red, blue and green denote gain, loss and no change, respectively of H3K27ac level in dKO versus WT CAFs. Chromatin loops, originating at the promoter of the highlighted gene, are shown in red if specific for the dKO, blue for the WT, and black if shared. f, As in e, but for the Il6 locus. g, As in e, but for the Ptgs2 locus. h, Model of the impact of macroH2A loss on the melanoma TME. In the absence of macroH2A, inflammatory genes in CAFs become intrinsically hyperinducible owing to increased enhancer activity and promoter–enhancer looping. This leads to an increased production of pro-inflammatory cytokines by CAFs, which in turn attract Mrc1+ myeloid cells with a pro-tumour phenotype. Accumulating myeloid cells inhibit CD8+ T-cell-mediated tumour cell killing, which results in increased tumour size in dKO animals. CAF-driven inflammatory signalling could also promote melanoma dedifferentiation through mechanisms that are yet to be determined (dashed lines). Illustration in h by Jill K. Gregory, reproduced with permission from © Mount Sinai Health System.
A comparison of loop coordinates revealed moderate overlap between WT and dKO CAFs (Fig. 7a), which suggested that there was genome-wide reorganization of promoter contacts. However, these changes were also driven by small shifts in the distal ends of loops to an adjacent 10 kb bin (Extended Data Fig. 9d). By examining the activity of enhancers located at distal loop ends, we found that gain of H3K27ac in the dKO samples was more frequently associated with unique dKO loops (and loss of H3K27ac with unique WT loops) compared with static enhancers (Fig. 7b and Extended Data Fig. 9e). This association in the dKO samples was highly significant for enhancers within super-MCDs (Fig. 7b), which suggested that macroH2A instructs functional looping properties of the chromatin fibres it is highly enriched in. Similarly, by associating changes in the total number of loops originating in each gene with DEGs, the overlap reached the highest significance and level of directional correlation in macroH2A super-MCDs (Fig. 7c and Extended Data Fig. 9f).
Because loop changes often did not match those in gene or enhancer activity (Fig. 7b,c), we intersected genes upregulated in dKO CAFs with bona fide target genes of enhancers gaining H3K27ac, as determined by looping data. Although only 20 genes fit these stringent criteria, 17 had a net increase in the number of loops in dKO CAFs, and 7 were part of inflammatory signalling (Fig. 7d), with significant enrichment for NF-κB targets (Supplementary Table 7). Loci such as Ccl2, Il6, Cxcl1, Ptgs2 (the predominant prostaglandin-endoperoxide synthase in CAFs) and Kitl (which encodes Kit ligand/stem cell factor) were located within super-MCDs (Fig. 7e–g and Extended Data Fig. 9g,h). Ccl2 and Il6 gained loops to a hyperacetylated enhancer within their respective super-MCDs, whereas Ptgs2 and Kitl gained loops to more distal hyperacetylated enhancers. Cxcl1 displayed a net loss of loops in dKO CAFs but maintained contact with an enhancer-gaining H3K27ac. Altogether, these results demonstrate that macroH2A represses inflammatory genes in CAFs by restricting enhancer contacts and/or activity.
Discussion
Our study of a macroH2A-deficient melanoma mouse model revealed an unappreciated role for this histone variant in the TME. By profiling macroH2A-dependent chromatin looping, we identified widespread changes in the promoter–enhancer interaction landscape. Together with changes in enhancer activity, this finding suggests that macroH2A may enforce the position of enhancers relative to nuclear compartments or the ability of enhancers to interact with their cognate promoters. Accordingly, previous studies have suggested that macroH2A affects 3D chromatin organization at several scales, including stabilizing nucleosome–DNA contacts to limit the mobility of the chromatin fibre73,76, associating with the boundaries of lamina-associated domains and promoting heterochromatin74,75, blocking BRD4 binding at macroH2A-bound enhancers69, and changing contact frequency of promoter–enhancer pairs, the activity of which is affected by macroH2A1 or macroH2A2 depletion68. Interestingly, however, changes in chromatin accessibility were minimal, which suggests that macroH2A loss does not affect chromatin remodelling, as previously reported79.
In our model, macroH2A-dependent regulatory mechanisms converged on a small set of inflammatory genes, which underscores a role for macroH2A in limiting inflammatory signalling in vivo. Notably, CAFs hijack these inflammatory genes as a mechanism of tumour immune escape80–83, and our studies suggest that dKO CAFs promote an immunosuppressive environment that leads to increased melanoma burden (Fig. 7h).
MacroH2A deficiency in cancer promotes tumour growth through multiple mechanisms1. Our previous report that macroH2A blocks the proliferative and metastatic capacity of melanoma cells18 aligns with the increased size of primary tumours observed in dKO mice. Here, we revealed that macroH2A suppresses dedifferentiation along the NC lineage towards a state associated with advanced disease and poor prognosis44,47,49, immune evasion and immunotherapy resistance84–87. We uncovered a conserved role for macroH2A in mouse and human melanoma CAFs, which produce increased cytokines when macroH2A levels are low. This phenotype appears CAF intrinsic, although we cannot exclude the possibility that a hyperinducible response to stimuli occurs in other macroH2A-deficient cells of the TME. Furthermore, given that macroH2A loss decreases the frequency of the CAF Fbln1 cluster (Fig. 3d), which expresses myofibroblast markers (Extended Data Fig. 4a), and downregulates the myofibroblast-associated genes Lrrc15 and Fbln1 (refs. 88,89) in sorted CAFs (Fig. 4d), we propose that the increased inflammation observed is a consequence of skewed dermal fibroblast polarization towards iCAFs at the expense of myCAFs54. Inflammatory signalling was among the first identified microenvironmental cues that induce melanoma dedifferentiation84,90,91, which raised the possibility that CAFs not only recruit immunosuppressive myeloid cells but may also promote melanocyte dedifferentiation. Such crosstalk occurs in colorectal cancer, in which Ptgs2 expression in CAFs drives the expansion of tumour-initiating stem cells in a paracrine manner92. We speculate that the convergence of these mechanisms would predict poor response of macroH2Alow tumours to immunotherapy, with potential to stratify patients.
Methods
Mouse melanoma model
All animal experiments received previous ethical and technical approval from the Icahn School of Medicine at Mount Sinai (ISMMS) Institutional Animal Care and Use Committee (protocol number LA11-00122). Humane end points for tumour induction studies were inability to breathe, eat, drink or move normally, behavioural abnormalities, body condition score <3, tumour size >1,000mm3, tumour ulceration and loss of body weight greater than 20%. Maximal tumour size was not exceeded before the end point of experiments in this study. Mus musculus macroH2A WT and dKO inducible melanoma strains were generated by breeding B6;FVB-Tg(Tyr-cre/ERT2)13Bos Braftm1Mmcm Ptentm1Rdp(ref. 22) and 129S6.Cg-Macroh2a2tm1.1Peh Macroh2a1tm1Peh(ref. 10) or 129S6/SvEvTac (Taconic) mice. Besides decreased body mass and reduced reproductive efficiency, the 129S dKO strain lacks spontaneous disease phenotypes10. The BrafCAPtenflTyr-CreERT2 strain (C57BL/6-FVB background) develops spatially and temporally inducible22, polyclonal melanomas arising in quiescent amelanotic precursors and subsequent expansion of pigmented melanocytes93,94. Mice were housed in a facility with specific pathogen-free health status, in individually ventilated cages at 21–22 °C and 39–50% relative humidity, on a 7:00–19:00 light cycle, with free access to food and water. Genotyping was performed as previously described10,95. Mouse lines were maintained on a mixed background: on average, WT and dKO mice are predicted to have a 75% 129S background, with the remainder a mix of B6 and FVB present in the original triallelic melanoma strain. Cre-positive BrafCA/CAPtenfl/fl and Cre-positive BrafCA/CAPtenwt/wt mice of both sexes were used for melanoma and nevus induction, respectively. Differences in tumour growth between sexes were tested for and found to be non-significant (Extended Data Fig. 1b). For tumour growth and histology experiments, Cre-mediated recombination of Braf and Pten alleles was induced in 7–11-week-old mice through the application of 1 μl of 5 mM 4-hydroxytamoxifen (70% Z-isomer, Sigma H6278, dissolved in ethanol) on the medial dorsal skin 24 h after hair removal with depilatory cream. Tumour length and width were measured from 25 DPI onwards using calipers, and the area was calculated assuming an elliptical tumour shape. Tumour thickness was approximated from the end point tumour area and weight, assuming an elliptic cylinder shape and tissue density = 1. Tumours were collected no later than 50 DPI before reaching a humane end point (tumour length over 1 cm, presence of ulceration). Five WT and 10 dKO male and 7 WT and 5 dKO female mice aged 9–10 weeks at induction were used for tumour immunophenotyping at 50 DPI. Three WT and 5 dKO male and 5 WT and 4 dKO female mice aged 9–10 weeks at induction were used for peripheral blood immune cell counts at 50 DPI. Two WT and 1 dKO male and 4 WT and 5 dKO female mice aged 10–11 weeks at induction were used for tumour immunophenotyping at 35 DPI. Two WT and 3 dKO male and 4 WT and 3 dKO female mice aged 7 weeks were used for peripheral blood immune cell counts in the tumour-naive setting. Two females and 2 males each for WT and dKO mice aged 9 weeks at induction were used for CD8 T cell sorting at 50 DPI for RNA-seq. Two females and 2 males each for WT and dKO mice aged 10 weeks at induction were used for CAF sorting at 50 DPI for RNA-seq. Two WT and 2 dKO females aged 10 weeks at induction were used for CAF sorting at 50 DPI for ATAC–seq. One WT and 1 dKO male aged 13 weeks at induction were used for CAF sorting at 50 DPI for culturing. Females were used for scRNA-seq to avoid the potential impact of sex-specific transcripts on integration. Pairs of WT and dKO age-matched females were induced at 10, 12 and 9 weeks in 3 independent experiments. One WT and 1 dKO female were induced at 9 weeks and processed at 35 DPI for ST analyses.
Histology
Resected tissue was fixed in neutral buffered formalin for 24 h, paraffin embedded and sectioned at the ISMMS Biorepository and Pathology Core. Antigens were retrieved in citrate buffer (pH 6) in a domestic pressure cooker for 5 min. Immunodetection was performed using ImmPRESS polymer, ImmPACT Vector Red and NovaRed kits (Vector). Primary antibodies are listed in Supplementary Table 8. Ten random ×40 objective fields within the tumour were scored by board-certified dermatopathologists at ISMMS (M.S.G. and N.S.V.) for mitotic cells (H3S10ph) and Ki-67. Pagetoid scatter was measured as the number of melanoma cells within the epidermis across ten ×40 fields. The degree of pigmentation was estimated on sections stained with haematoxylin and eosin as the fraction of tissue area containing pigment using a ×2 objective.
Tumour dissociation
Resected BRAFV600E/PTEN-deficient melanomas were cut into 1–2 mm fragments. For immunophenotyping and CD8+ T cell sorting, tumour fragments were digested in RPMI containing 400 U ml–1 collagenase IV (Gibco), 100 U ml–1 hyaluronidase (Sigma) and 100 μg ml–1 DNAse (Roche) at 37 °C for 1 h96, aspirated 5 times through a 14 G needle, and filtered through a 70 μm cell strainer. Immune cells were enriched by centrifugation through a discontinuous 40/90 Percoll (GE Healthcare Life Sciences) gradient97. For scRNA-seq and CAF sorting, tumour fragments were digested using a Tumour Dissociation kit, mouse (Miltenyi) in DMEM using the soft/medium protocol according to the manufacturer’s instructions. For scRNA-seq specifically, red blood cell lysis was performed in ACK (ammonium–chloride–potassium) buffer for 5 min on ice, followed by a wash in 1× DPBS containing 0.04% BSA.
Flow cytometry
Before intracellular staining for IFNγ, TNF and FOXP3, cells were stimulated with 100 ng ml–1 PMA (Sigma-Aldrich) and 0.5 μg ml–1 ionomycin (Sigma-Aldrich) in the presence of 10 μg ml–1 brefeldin A (Sigma-Aldrich) for 4 h. Staining was performed as previously described97 using the fluorophore-conjugated antibodies listed in Supplementary Table 8. Samples were acquired and sorted on LSRFortessa and FACSAria SORP cytometers (BD Biosciences), respectively, running FacsDiVa (v.8.0.2; BD Biosciences) at the ISMMS Flow Cytometry Core. Data were analysed using FCS Express (v.7.12; De Novo Software). Cell population frequencies were compared using a Mann–Whitney test in Prism (v.9.5.1) software (GraphPad).
RNA extraction
Snap-frozen tumours were disrupted in QIAzol reagent (Qiagen) by milling with zirconia beads (Fisher). Sorted cells were centrifuged and resuspended in QIAzol. Cultured cells were lysed directly in their culture vessel. After the addition of chloroform (Sigma) and centrifugation, RNA was isolated from the aqueous phase using an RNEasy Mini (tumours and cultured cells) or Micro (sorted cells) kit (Qiagen).
RNA-seq library preparation
For tumours (n = 6 WT, 6 dKO), poly-A enrichment was carried out on 1 μg total RNA with a NEBNext Poly(A) mRNA Magnetic Isolation Module (NEB) followed by library preparation with a NEXTFLEX Rapid Directional RNA-seq kit (PerkinElmer). For sorted CAFs (n = 4 WT, 4 dKO), NEXTFLEX poly(A) beads 2.0 and a NEXTFLEX Rapid Directional RNA-seq kit 2.0 (PerkinElmer) were used, starting from 80 ng RNA isolated from 67,000–200,000 cells. For iDFs (two experimental replicates each of WT and dKO before and after stimulation), the same protocol was applied starting from 1 μg RNA. For CD8+ T cells (n = 4 WT, 4 dKO), 5 ng RNA from 5,000 sorted cells was used as input for a NEBNext Single Cell/Low Input RNA Library Prep kit for Illumina (NEB). Libraries were sequenced in 75 bp single-end mode on NextSeq 500 systems (Illumina).
RNA-seq analysis
Reads were quasi-mapped to the Gencode M25 (GRCm38.p6) gene set using salmon (v.1.2.1)98, and DEGs were called using DESeq2 (v.1.36.0)99 by filtering for an independent hypothesis weighting100 adjusted P value of <0.05 and a log2 fold change of >0.75 or < –0.75. GSEA was performed with the fgsea (v.1.22.0) package101 on the entire expressed gene set pre-ranked based on the Wald test statistic computed using DESeq2. Significant pathways were reported using an adjusted P value cutoff of 0.05 and ordered on normalized enrichment scores. Gene ontology enrichment was performed using HOMER (v.4.10)102. Heatmaps were generated for visualization purposes using counts normalized with the variance stabilization transformation of DESeq2 and corrected for library preparation batch or sex-specific effects when applicable using limma (v.3.54.0)103.
Reverse transcription-qPCR
A First Strand cDNA Synthesis kit (OriGene), FastStart Universal SYBR Green Master (Rox) mix (Roche) and primers listed in Supplementary Table 7 were used for RT-qPCR. Amplification was performed on a CFX384 instrument (Bio-Rad), and target genes were quantified relative to Hprt and Sdha housekeeping genes using CFX Manager (v.3.1) software (Bio-Rad).
scRNA-seq library preparation
Droplet-based scRNA-seq (Chromium, 10x Genomics) was applied to single-cell suspensions of BRAFV600E/PTEN-deficient melanomas from 3 WT and 3 dKO mice; 6 × 103 cells were loaded per well for each of the 6 samples, and partitioning and library preparation were performed according to the manufacturer’s protocol for the 3′ v.3 chemistry.
scRNA-seq analysis
Filtered gene–barcode matrices generated using cellranger (v.7.0.1) with the mm10-2020-A transcriptome (10x Genomics) were further analysed using Seurat (v.4.0)104. Low-quality cells (fewer than 500 unique molecular identifiers or 250 detected genes, or at least 20% mitochondrial transcripts) were removed, and data were normalized using SCTransform (v.2), regressing out cell cycle scores (determined using the CellCycleScoring function) and mitochondrial ratio. Datasets were integrated using reciprocal principal component analysis on the first 50 principal components with an alignment strength of ten. Within this combined dataset, clusters were determined using a variety of resolutions and annotated using top cluster-specific genes conserved between WT and dKO cells, as well as known lineage genes for expected cell types. At final resolution (0.6), selected to avoid overclustering while distinguishing rare cell types, five clusters with a low number of significant genes, also displaying a low nuclear transcriptome complexity, were considered to represent low-quality cells and were discarded. The least abundant cluster, characterized by genes of multiple lineages, probably contained doublets and was also discarded. dKO versus WT DEGs were identified within each cluster on the RNA slot using the FindMarkers function in Seurat. GSEA was performed using SCPA (v.1.5.1)105. Reclustering of related cell types was performed by subsetting the Seurat object to the relevant clusters, then performing normalization, integration and clustering as described above. Pseudotime trajectories for NC lineage clusters were calculated using Monocle 3 (v.1.3.1)106, ordering cells in pseudotime under the assumption that they shared a common precursor and transformation initiated in the highest Tyr-expressing cluster. Unbiased cell identity mapping to existing scRNA-seq datasets was performed by subsetting Seurat objects to myeloid cell clusters, then determining the highest similarity in terms of gene expression to the reference cell types using singleR (v.1.10.0)107. Local changes in cell abundance were profiled using miloR (v.1.5.0)108 with the following parameters for the entire dataset: 50 principal components, 40 nearest neighbours, sampling proportion 0.1 and sampling refinement algorithm. Thirty nearest neighbours and a sampling proportion of 0.2 were used for the lower number of cells in the lymphoid reclustered dataset. Cell-type prioritization to evaluate the contribution to changes in gene expression between WT and dKO samples was performed using Augur (v.1.0.3)109 using default parameters except for a minimum cell number of 50. Cell signalling through ligand–receptor interaction analysis was performed using CellChat (v.1.6.1)110 using the comparison workflow on a merged object.
Detailed cluster annotation
In addition to those described in the text, the following genes were used as markers during cluster annotation. Melanocytes were identified through the expression of genes associated with pigment production, such as Mitf, Mlana and Dct45. The NC arrested cluster expressed high levels of the cell cycle inhibitors p21(CIP1) (encoded by Cdkn1a) and p19(ARF) (encoded by Cdkn2a), the EGF-like ligands amphiregulin (Areg) and epiregulin (Ereg), and the histone variant H2A.J (H2afj). Amphiregulin expression is associated with BRAFV600E-induced senescence in melanocytes111 and H2A.J accumulates in senescent fibroblasts112. Another NC cluster expressed Hapln1, an ECM crosslinker the downregulation of which in aged fibroblasts promotes melanoma dissemination113,114. The NC Aqp cluster was characterized by aquaporin 1 (Aqp1) and Tfap2b, two factors that orchestrate NC cell migration115,116. The monocyte cluster was characterized by Ccr2, Ms4a4c and Plac8 expression, markers of tissue monocytes shown to transcriptionally resemble peripheral blood monocytes117. Mac Atp6v0d2 selectively expressed Atp6v0d2 and Gpnmb, which are associated with lysosomal function and phagocytosis118,119, and probably corresponds to melanophages118. We identified conventional type 2 dendritic cells (cDC2) through CD301b (Mgl2) and Plet1 expression120,121, their type 1 counterparts (cDC1) through CD103 (Itgae) and Xcr1(ref. 122), and a mature, migratory subtype (mDC) expressing Ccl22 and Ccr7(ref. 122). Mast cells and basophils were distinguished by mutually exclusive expression of Mcpt4andMcpt8(ref. 123). After reclustering of lymphoid cells, the CD4 circulating cluster expressed genes consistent with a non-effector phenotype, including Gramd3, a marker of circulating T cells124, S1pr1, a positive regulator of T cell emigration from peripheral organs125, and TCF1 (Tcf7), expressed in naive and memory but not effector T cells126. The T helper 2 (Th2) cell population comprised Cd4-positive cells expressing Il5 and Il13, Tγδ cells expressed γδ T cell receptor genes Tcrg-C1 and Trdc, and innate lymphoid cells in the ILC cluster expressed Gata3 and Hlf while being negative for Cd3e and Ncr1(ref. 127). We identified a prototypical CAF cluster expressing the highest levels of Pdgfra and Fap128 and CAF-specific lincRNA Meg3(refs. 129,130). A second fibroblast cluster expressed Wif1, an emerging marker in papillary dermis131–133. Two clusters with high levels of the myofibroblast marker Acta2 expressed Lrrc15 and Fbln1, respectively, both genes associated with immunoregulatory myofibroblast populations in pancreatic cancer and in breast cancer88,89.
ST analysis
Tissue was prepared according to 10x Genomics Visium V1 Slide–3′ Spatial guidelines. Tumours were frozen in a bath of isopentane and liquid nitrogen, stored at –80 °C in a sealed container, then embedded in OCT. Tissue was sectioned at temperatures of –20 °C for the cryostat chamber and –10 °C for the specimen head at 10 μm thickness. An optimal permeabilization time of 18 min was determined using a Visium Spatial Tissue Optimization kit (10x Genomics). Sequencing data were mapped using spaceranger (v.2.0.0) using the mm10-2020-A transcriptome (10x Genomics) to generate spatial gene expression matrices, which were processed according to the Seurat spatial vignette (https://satijalab.org/seurat/articles/spatial_vignette.html). We integrated data normalized with SCTransform (v.2) across the two ST samples. For label transfer, we then integrated this dataset on the first 30 principal components with scRNA-seq data normalized using SCTransform (v.2). The probabilistic distribution of cell types identified by scRNA-seq in each ~50 μm spot of the spatial array was calculated using the TransferData function. To assess cell-type colocalization within the tissue134, we determined significant Pearson’s correlations between cell-type scores across each array spot with the corr.test function of the psych (v.2.3.3) package (https://CRAN.R-project.org/package=psych), using the Holm method for multiple comparison P value adjustment.
CAF cultures
Primary mouse CAFs were sorted from tumours at 50 DPI based on PDGFRα (also known as CD140a) expression and cultured in DMEM supplemented with 10% FBS and penicillin–streptomycin at 37 °C and 5% CO2. Cells were split at a ratio of 1:2–1:5 when fully confluent for no more than 15 total passages from the time of sorting. For cytokine gene or protein induction studies, cells were grown to confluency then deprived of serum in DMEM with 0.5% FBS for 24 h, followed by stimulation with 10% FBS for the indicated amount of time. Primary human melanoma CAFs were obtained from the NCI Patient-Derived Models Repository (PDMR135, NCI-Frederick, Frederick National Laboratory for Cancer Research, Frederick, MD, https://pdmr.cancer.gov/) and the Aplin laboratory136,137. All human-derived cell cultures were stripped of any patient identifiers before they were provided to our laboratory, and their use is not subjected to Institutional Review Board approval. Ethics information relevant to their collection is provided in the noted references. Cells were maintained on plates coated with Cultrex Basement Membrane Extract, PathClear (Bio-Techne) in Advanced DMEM/F12 (Gibco) supplemented with 5% heat-inactivated FBS, 2 mM l-glutamine (Gibco), 10 ng ml–1 recombinant human EGF (Gibco), 400 ng ml–1 hydrocortisone (Sigma), 24 μg ml–1 adenine (Sigma), 100 μg ml–1 Primocin and 25 μg ml–1 Plasmocin (Invivogen); 10 μM Y-27632 dihydrochloride (Tocris) was added to the medium during the initial expansion of cultures. For serum starvation before cytokine induction, hydrocortisone, adenine and EGF were omitted, and the serum concentration was reduced to 0.25%. After 24 h, starvation medium was replaced with complete human CAF medium without Y-27632 dihydrochloride. Supernatant was collected, centrifuged to remove cells and debris, and flash-frozen. Cells were counted using a Guava Muse Cell Analyzer Count & Viability kit (Luminex).
Protein quantitation
Western blotting was performed as previously described62 using the antibodies listed in Supplementary Table 8. For cytokine quantitation in mouse samples, total protein was extracted using RIPA buffer and normalized to the lowest sample concentration. Analytes of interest were quantified using a Mouse Cytokine Array/Chemokine Array 31-Plex by Eve Technologies. For cytokine quantitation in human CAFs, supernatant was diluted 1:5, 1:10 and 1:20 with assay diluent and subjected to ELISA in duplicate against human CCL2, IL-6 (BioLegend) and CXCL1 (R&D Systems) according to the manufacturers’ instructions. Data points within range of the standard curve were normalized by cell number and averaged for each sample.
Monocyte Transwell migration assay
Monocyte migration was performed as previously described138 but with modifications. In brief, 7.5 × 104 WT or dKO CAFs were seeded per well of a 24-well cell culture insert companion plate (Corning), in triplicate, allowed to grow for 24 h reaching confluency, then starved for 24 h as described above. Monocytes were isolated from femur and tibia bone marrow of tumour-naive WT 129S mice using a MojoSort Mouse Monocyte Isolation kit (BioLegend) and labelled with 3 μM CMFDA green dye in serum-free RPMI 1640 medium for 30 min at 37 °C. After washing with R10 medium (RPMI 1640 supplemented with 10% heat-inactivated FBS, 20 mM HEPES, 0.5 mM sodium pyruvate, 1% penicillin–streptomycin, 1× MEM amino acids without l-glutamine, 1× MEM non-essential amino acids, Gibco), unbound dye was allowed to diffuse out by another incubation for 30 min at 37 °C in R10. Next, 1 × 105 monocytes in 300 μl R10 were added to a FluoroBlok insert with 3 μm pore size (Corning), which functioned as the upper chamber. Concomitantly, starvation medium on CAFs in the companion plate, which functioned as a lower chamber, was replaced with 800 μl R10. One well each of R10 and R10 supplemented with 10 ng ml–1 recombinant mouse CCL2 (BioLegend) were used as negative and positive controls, respectively. Plates were imaged for green widefield fluorescence at the Z position corresponding to the CAF layer, every 6 h for a 72 h time course, in a Cytation 7 automated microscope (Agilent). Image stitching, background subtraction, segmentation and cell count were performed using the onboard Gen5 (v.3.12) software (Agilent). To account for differences in monocyte reactivity among donor mice, cell numbers were normalized to the number of monocytes migrated at 24 h in the CCL2-positive control.
TCGA data analysis
TCGA melanoma cohort (SKCM)139 RNA-seq raw counts and sample annotations were downloaded using the TCGAbiolinks (v.2.25.3) package140. Gene counts were normalized using the TMM method from the edgeR (v.3.40.1) package141 to account for biases arising from library size and gene length. Primary and metastatic lesions were separately analysed, and comparisons for TME composition and gene expression were performed between the first and third tercile of MACROH2A1 and MACROH2A2 expression. Deconvolution of immune populations142 and tumour/stroma/immune microenvironmental composition143 was performed using the IOBR (v.0.99.9) package144. Differential gene expression analysis was performed using DESeq2 (ref. 99) with raw counts as input.
Human CAF scRNA-seq reanalysis
scRNA count matrix of 855,271 high-quality cells and associated metadata annotations from a previous study60 (GSE210347) were reanalysed. Counts were scaled using the Seurat LogNormalize function with a scale factor of 10,000. The top 2,000 most highly variable genes were identified using the vst selection method of FindVariableFeatures. The RunFastMNN function from the SeuratWrappers (v.0.3.0) package was utilized to perform batch correction using the ‘SampleID’ metadata column. The top 30 principal components were used in the downstream analysis. To investigate the correlation between MACROH2A1–MACROH2A2 and IL6–CXCL1–CCL2 expression, we generated pseudo-bulk counts from fibroblasts. In brief, the filtered Seurat object containing annotated fibroblasts was converted into a SingleCellExperiment (v.1.12) object using the raw counts per cell. The function aggregate.Matrix was used to sum and collapse the raw counts of each cell by their ‘SampleID’ annotation. Samples with <100 cells and non-tumour tissue were filtered out. Collapsed raw counts were normalized using the vst function from DESeq2 and the Pearson’s correlation between genes was calculated using the chart.Correlation function from the PerformanceAnalytics (v.2.0.4) package.
ATAC–seq library preparation
5 × 104 sorted CAFs per sample were processed for ATAC–seq as previously described145. The optimal number of library amplification cycles was determined146. Libraries were subjected to double-sided size selection using SPRIselect beads (Beckman Coulter) at ratios of 0.55 and 1.2× before sequencing on a NextSeq 500 (Illumina) in 75 bp paired-end mode.
ATAC–seq analysis
Read pairs were merged and adapters removed using NGmerge (v.0.3)147, followed by alignment to the mm10 assembly with bowtie2 (v.2.4.1)148. Low-quality (MAPQ < 30), mitochondrial genome and duplicated reads were removed using samtools (v.1.9)149, and genome coverage calculation for visualization purposes on the UCSC genome browser was performed using deepTools (v.3.5.1)150 excluding blacklisted regions151. WT and dKO replicates were concatenated using samtools merge to generate a master bam file. MACS2 (v.2.1.0)152 was used to identify significant peaks in the master bam file using the parameters –nomodel –nolambada –keepdup all –slocal 10,000. Quantification of reads in significant peaks for all samples was preformed using bedtools (v.2.29.2)153 multicov. Differential peak analysis was preformed using DEseq2 (1.30.1)99 (adjusted P value of <0.05 using the Benjamini and Hochberg procedure). For plotting static peaks, a set of 3,000 peaks with P adjusted of >0.05 and absolute log2 fold change of ≤0.2 were randomly selected.
ChIP–seq
Approximately 8 × 106 cultured CAFs or iDFs after 30 min of serum stimulation were single-crosslinked and processed for ChIP154 for H3K27ac (antibody 13-0045, Epicypher, lot number 20120001-28, 4 μg per reaction). Sequencing was performed on a NextSeq 500 in 75 bp single-end mode.
ChIP–seq analysis
Adapters were trimmed using Trimmomatic (v.0.36)155, and alignment, read filtering and genome coverage calculation were performed as for ATAC–seq. The bam files of WT and dKO samples were concatenated into a master bam file, which was used to call significant peaks with matching input controls using MACS2 (v.2.1.0)152. Cutoff values for q-value significance were determined post-hoc, testing several q-values on the basis of the signal-to-background ratio. Quantification of reads in significant peaks for all samples was performed using bedtools (v.2.29.2)153 multicov. TEs and SEs were called on the basis of H3K27ac enrichment using the ROSE algorithm (rank ordering of super-enhancers) (v.0.11)156,157 with a stitching distance of 12.5 kb and a TSS exclusion zone size of 2.5 kb. The ROSE algorithm was also used to extract H3K27ac levels at TEs and SEs for WT and dKO samples individually. The average H3K27ac signal value across all elements was calculated for each sample and further used for normalization between samples. The log2 fold change ratio of the normalized signal (–0.75 < log2 fold change < 0.75) was used to call differential TEs and SEs. All other enhancers were considered static.
CUT&RUN
2 × 105 short-term cultured CAFs before or after 30 min of serum stimulation were processed for CUT&RUN158 for macroH2A1 (antibody ab37264, Abcam, lot number GR278020-1, 1 μg per reaction). Cells were permeabilized with 0.0085% digitonin and incubated with antibody. DNA was cleaved with the CUTANA pAG-MNase (Epicypher). Released DNA was processed using a NEBNext Ultra II Library Preparation kit for Illumina (NEB), including a 14-cycle amplification step. Libraries were sequenced and reads were processed as for ATAC–seq.
CUT&RUN analysis
MacroH2A1 enrichment determined by CUT&RUN was benchmarked by correlation analysis with published macroH2A1 ChIP–seq data in dermal fibroblasts62. Enrichment at the level of read pileups had a correlation coefficient of 0.79 (Extended Data Fig. 6a). MacroH2A1 chromatin domains were called separately for stimulated and unstimulated samples using SEACR (v.1.3)159 without a control in stringent mode with a threshold of 0.05, and significant peaks were merged using bedtools (v.2.29.2)153 if within 25 kb distance. Merged peak and alignment files for unstimulated and stimulated cells were then concatenated to generate master files for the ROSE algorithm156,157. A stitching distance of 12.5 kb, no TSS exclusion and normalization to element size were used in ROSE to rank macroH2A1 domains. The equivalent of SEs called by ROSE based on the macroH2A1 signal were termed super-MCDs. The equivalent of TEs was then divided into groups of standard and low macroH2A1 signals using a cutoff above 3,780 (signal density × length units) determined post-hoc on the basis of the macroH2A1 enrichment-to-background ratio. Most domains with low macroH2A1 signal were also below 1.5 kb in length, represented less than 0.5% of the genome, showed higher enrichment of IgG over the macroH2A signal, and were therefore excluded from further analysis. For comparing macroH2A1 enrichment levels, static genes were selected as follows: for each differentially expressed gene, all genes within 25% of its averaged transcripts per million (TPM) level in WT samples were identified, and 10 (inflammatory up genes comparison) or 3 (all DEG comparison) genes that were neither differentially expressed nor less than 1 kb in length were randomly selected, added to a running list, and duplicates were removed.
pcMicro-C
Micro-C was performed using a Dovetail Micro-C kit (Cantata Bio) according to the manufacturer’s protocol. Chromatin from 1 × 106 WT and dKO cultured CAFs, crosslinked after serum stimulation, was digested with 3 μl MNase to obtain a mononucleosomal fraction within specifications. Each chromatin preparation was subjected to proximity ligation and subsequent library preparation in duplicate. Next, 400 ng of each library was pooled and subjected to promoter capture using a Dovetail Target Enrichment kit and a Mouse Pan Promoter Panel (Cantata Bio). The manufacturer’s protocol was adjusted by halving the streptavidin bead elution volume to enable the use of the entire post-capture material for the amplification reaction and performing only seven PCR cycles. The resulting 4-plex library was sequenced on a NextSeq 500 high output lane in a 75 bp paired-end configuration.
Chromatin looping analysis
Interaction calling and significance thresholding was performed based on the workflow developed by Dovetail Genomics (https://micro-c.readthedocs.io/en/latest/) and the CHiCAGO tool recommendations160. Sequenced reads were aligned using BWA (v.0.7.17-r1188)161 and filtered using PairTools (v.1.0.2)162 and SAMTools (v.1.9)149 to identify non-duplicated read pairs. The technical replicates were merged, and significant interactions (chromatin loops) were called using CHiCAGO (v.1.2.0)163 with default parameters at 10 kb resolution160. Significant Interactions were defined as those with a CHiCAGO score of ≥5 per condition. For visualization on the UCSC genome browser, output loop files were converted from the WashU EpiGenome Browser interactBED format to the bigInteract format using BEDTools (v.2.30.0)153,164 and UCSC-Utils (v.2.9)165,166. Bait map plots depicting all called interactions per bait were generated using the plotBaits function native to CHiCAGO. Histograms showing the enrichment of functional elements (ATAC–seq peaks and H3K27ac peaks) at the distal end of loops were generated as a default output of the CHiCAGO pipeline. Interactions were defined as shared if both ends overlapped between conditions, and unique otherwise. Promoters and enhancers at proximal and distal ends of loops, respectively, were divided into classes on the basis of the type of macroH2A1 domain they overlapped with—super, standard and absent—and the nature of the loops underlying them—shared, WT-specific or dKO-specific. For the enhancer-specific association, enhancers were further divided on the basis of the direction of their change in H3K27ac levels by macro level to generate a count matrix of changes in enhancer activity versus changes in interactions, stratified by macroH2A1 level. We performed a Chi-square test of independence to determine associations between enhancer and looping deregulation in the absence of macroH2A. The mouse promoter panel uses multiple baits to capture alternative promoters of the same gene; therefore, we summed loop counts for all baits associated with the same gene name for overlap with gene expression changes. We compared the loop counts per gene in dKO versus WT samples and further stratified genes on the basis of their differential expression status and macroH2A1 occupancy. We performed a Chi-square test of independence to determine associations between gene expression and looping deregulation in the absence of macroH2A.
Statistics and reproducibility
Information on the number of biologically independent samples analysed and the number of times experiments were performed is included in the figure legends. No assumption of data normality was made, and non-parametric statistical tests were performed except when n = 3 replicates (comparison of cell-type frequency in scRNA-seq data, CAF RT-qPCR) for which data distribution was assumed to be normal but this was not formally tested. All statistical tests performed were two-sided except when noted. No statistical method was used to predetermine sample sizes, but were similar to those reported in previous publications22,97,167. No data were excluded from the analyses, and biological samples were excluded from the study only if sample preparation or data acquisition failed. The experiments were not randomized, and the investigators were not blinded to allocation during experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41556-023-01208-7.
Supplementary information
Bulk tumour RNA-seq.
CD8+ T cell RNA-seq.
scRNA-seq.
ST analysis.
CAF RNA-seq.
iDF RNA-seq.
pcMicro-C.
Reagents.
Acknowledgements
The authors thank the laboratories of J.-C. Marine, J. Pehrson, J. Celebi and M. Bosenberg for reagents and advice; R. Singh for pathology support; I. Russell, L. Munding and M. Elkhawand (Cantata Bio) for technical support of pcMicro-C; M. Spivakov for assistance with Micro-C analysis; the Bernstein and Merad laboratories for discussions and feedback; and J. Gregory for illustrations. This study was supported by an American Skin Association Ping Y. Tai Melanoma Research Grant to D.F.; a Mount Sinai Skin Biology and Diseases Resource-based Center Transition to Independence Minigrant to D.F., funded through NIAMS/NIH SBDRC P30 AR079200; a Melanoma Research Alliance Pilot Award (10.48050/pc.gr.91577), Department of Defense (W81XWH2010803) and NIH/NCI R01 CA154683 and CA218024 to E.B. A.E.A. is supported by NIH/NCI R01 CA182635. A.O.K. is supported by NIH/NCI R01 AI153363-01A1. This work was supported in part through the Oncological Sciences Sequencing Core supported by the Tisch Cancer Institute of the ISMMS Cancer Center Support Grant P30 (CA196521), and Scientific Computing is supported by the Office of Research Infrastructure of the NIH under award number S10OD026880 to ISMMS. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. The authors acknowledge the Center for Advanced Genomics Technology, the Dean’s Flow Cytometry Core, the Biorepository and Pathology Core, The Bioinformatics for Next Generation Sequencing Core, the Center for Comparative Medicine and Surgery, and the Microscopy Core at ISMMS.
Extended data
Source data
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Unprocessed western blots.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Unprocessed gels.
Statistical source data.
Unprocessed western blots.
Statistical source data.
Author contributions
Conceptualization: D.F. and E.B. Investigation: D.F., D.H., N.T., É.H., S.S. and F.G.G. Formal analysis: D.F., D.H., S.C., A.A., M.S.G. and N.S.V. Writing original draft: D.F. and E.B. Writing, review and editing: D.F., D.H., S.C., A.A., D.D., M.S.G., H.S., R.S., A.O.K., M.M. and E.B. Visualization: D.F., S.C., A.A. and D.H. Resources, expertise and methods: É.H., D.D., M.S.G., K.G.B., C.C., A.E.A., H.S., R.S., A.O.K. and M.M. Data curation: D.F. Supervision: E.B. Funding acquisition: D.F. and E.B.
Peer review
Peer review information
Nature Cell Biology thanks Marcus Buschbeck, Daniel Öhlund and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The transcriptomics and epigenomic datasets, including raw and processed sequencing data generated and analysed during the current study, are available in the Gene Expression Omnibus (GEO) repository under accession number GSE200751 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE200751) and in this article’s table files. TCGA melanoma data are publicly available through the NCI Genomic Data Commons (GDC) data portal under project ID TCGA SKCM (https://portal.gdc.cancer.gov/projects/TCGA-SKCM). The human pan-cancer scRNA-seq dataset mined in this study is available in the GEO repository under accession number GSE210347 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE210347). The mouse M25 (GRCm38.p6) genome assembly and gene set used for transcriptomics and epigenomic analyses are available at Gencode (https://www.gencodegenes.org/mouse/release_M25.html). All other data supporting the findings of this study is available from the corresponding authors upon request. Source data are provided with this paper.
Code availability
All packages used for data analysis are publicly available. No custom code was generated for this study. All scripts used for bulk RNA-seq, scRNA-seq, ATAC–seq, ChIP–seq and CUT&RUN data analyses in this study are available from the corresponding authors upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dan Filipescu, Email: dan.filipescu@mssm.edu.
Emily Bernstein, Email: emily.bernstein@mssm.edu.
Extended data
is available for this paper at 10.1038/s41556-023-01208-7.
Supplementary information
The online version contains supplementary material available at 10.1038/s41556-023-01208-7.
References
- 1.Ghiraldini FG, Filipescu D, Bernstein E. Solid tumours hijack the histone variant network. Nat. Rev. Cancer. 2021;21:257–275. doi: 10.1038/s41568-020-00330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bönisch C, Hake SB. Histone H2A variants in nucleosomes and chromatin: more or less stable? Nucleic Acids Res. 2012;40:10719–10741. doi: 10.1093/nar/gks865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pehrson JR, Fried VA. MacroH2A, a core histone containing a large nonhistone region. Science. 1992;257:1398–1400. doi: 10.1126/science.1529340. [DOI] [PubMed] [Google Scholar]
- 4.Costanzi C, Pehrson JR. MACROH2A2, a new member of the MACROH2A core histone family. J. Biol. Chem. 2001;276:21776–21784. doi: 10.1074/jbc.M010919200. [DOI] [PubMed] [Google Scholar]
- 5.Sun Z, Bernstein E. Histone variant macroH2A: from chromatin deposition to molecular function. Essays Biochem. 2019;63:59–74. doi: 10.1042/EBC20180062. [DOI] [PubMed] [Google Scholar]
- 6.Buschbeck M, et al. The histone variant macroH2A is an epigenetic regulator of key developmental genes. Nat. Struct. Mol. Biol. 2009;16:1074–1079. doi: 10.1038/nsmb.1665. [DOI] [PubMed] [Google Scholar]
- 7.Gamble MJ, Frizzell KM, Yang C, Krishnakumar R, Kraus WL. The histone variant macroH2A1 marks repressed autosomal chromatin, but protects a subset of its target genes from silencing. Gene Dev. 2010;24:21–32. doi: 10.1101/gad.1876110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H, et al. MacroH2A1 and ATM play opposing roles in paracrine senescence and the senescence-associated secretory phenotype. Mol. Cell. 2015;59:719–731. doi: 10.1016/j.molcel.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marjanović MP, et al. MacroH2A1.1 regulates mitochondrial respiration by limiting nuclear NAD+ consumption. Nat. Struct. Mol. Biol. 2017;24:902–910. doi: 10.1038/nsmb.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pehrson JR, Changolkar LN, Costanzi C, Leu NA. Mice without macroH2A histone variants. Mol. Cell. Biol. 2014;34:4523–4533. doi: 10.1128/MCB.00794-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaspar-Maia A, et al. MacroH2A histone variants act as a barrier upon reprogramming towards pluripotency. Nat. Commun. 2013;4:1565. doi: 10.1038/ncomms2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasque V, Gillich A, Garrett N, Gurdon JB. Histone variant macroH2A confers resistance to nuclear reprogramming. EMBO J. 2011;30:2373–2387. doi: 10.1038/emboj.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrero MJ, et al. Macrohistone variants preserve cell identity by preventing the gain of H3K4me2 during reprogramming to pluripotency. Cell Rep. 2013;3:1005–1011. doi: 10.1016/j.celrep.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 14.Agelopoulos M, Thanos D. Epigenetic determination of a cell‐specific gene expression program by ATF‐2 and the histone variant macroH2A. EMBO J. 2006;25:4843–4853. doi: 10.1038/sj.emboj.7601364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gamble MJ, Kraus WL. Multiple facets of the unique histone variant macroH2A: from genomics to cell biology. Cell Cycle. 2010;9:2568–2574. doi: 10.4161/cc.9.13.12144. [DOI] [PubMed] [Google Scholar]
- 16.Hussey KM, et al. The histone variant macroH2A1 regulates target gene expression in part by recruiting the transcriptional coregulator PELP1. Mol. Cell. Biol. 2014;34:2437–2449. doi: 10.1128/MCB.01315-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atkins MB, et al. The state of melanoma: emergent challenges and opportunities. Clin. Cancer Res. 2021;27:2678–2697. doi: 10.1158/1078-0432.CCR-20-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapoor A, et al. The histone variant macroH2A suppresses melanoma progression through regulation of CDK8. Nature. 2010;468:1105–1109. doi: 10.1038/nature09590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun D, et al. MacroH2A impedes metastatic growth by enforcing a discrete dormancy program in disseminated cancer cells. Sci. Adv. 2022;8:eabo0876. doi: 10.1126/sciadv.abo0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sporn JC, et al. Histone macroH2A isoforms predict the risk of lung cancer recurrence. Oncogene. 2009;28:3423–3428. doi: 10.1038/onc.2009.26. [DOI] [PubMed] [Google Scholar]
- 21.Sporn JC, Jung B. Differential regulation and predictive potential of macroH2A1 isoforms in colon cancer. Am. J. Pathol. 2012;180:2516–2526. doi: 10.1016/j.ajpath.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dankort D, et al. BrafV600E cooperates with Pten loss to induce metastatic melanoma. Nat. Genet. 2009;41:544–552. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018;15:234–248. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gschwandtner M, Derler R, Midwood KS. More than just attractive: how CCL2 influences myeloid cell behavior beyond chemotaxis. Front. Immunol. 2019;10:2759. doi: 10.3389/fimmu.2019.02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Do HTT, Lee CH, Cho J. Chemokines and their receptors: multifaceted roles in cancer progression and potential value as cancer prognostic markers. Cancers. 2020;12:287. doi: 10.3390/cancers12020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin S, et al. Myeloid-derived suppressor cells promote lung cancer metastasis by CCL11 to activate ERK and AKT signaling and induce epithelial–mesenchymal transition in tumor cells. Oncogene. 2021;40:1476–1489. doi: 10.1038/s41388-020-01605-4. [DOI] [PubMed] [Google Scholar]
- 27.Li J, et al. Tumor cell-intrinsic factors underlie heterogeneity of immune cell infiltration and response to immunotherapy. Immunity. 2018;49:178–193.e7. doi: 10.1016/j.immuni.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karagiannidis I, et al. G-CSF and G-CSFR modulate CD4 and CD8 T cell responses to promote colon tumor growth and are potential therapeutic targets. Front. Immunol. 2020;11:1885. doi: 10.3389/fimmu.2020.01885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karagiannidis I, et al. G-CSF and G-CSFR induce a pro-tumorigenic macrophage phenotype to promote colon and pancreas tumor growth. Cancers. 2020;12:2868. doi: 10.3390/cancers12102868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nissinen L, Kähäri V-M. Matrix metalloproteinases in inflammation. Biochim. Biophys. Acta. 2014;1840:2571–2580. doi: 10.1016/j.bbagen.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Fingleton B. Matrix metalloproteinases as regulators of inflammatory processes. Biochim. Biophys. Acta. 2017;1864:2036–2042. doi: 10.1016/j.bbamcr.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, Xia S, Zhang Z, Wu H, Lieberman J. Channelling inflammation: gasdermins in physiology and disease. Nat. Rev. Drug Discov. 2021;20:384–405. doi: 10.1038/s41573-021-00154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenmalm MC, Cherwinski H, Bowman EP, Phillips JH, Sedgwick JD. Regulation of myeloid cell function through the CD200 receptor. J. Immunol. 2006;176:191–199. doi: 10.4049/jimmunol.176.1.191. [DOI] [PubMed] [Google Scholar]
- 34.Snelgrove RJ, et al. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat. Immunol. 2008;9:1074–1083. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- 35.Hegde S, Leader AM, Merad M. MDSC: markers, development, states, and unaddressed complexity. Immunity. 2021;54:875–884. doi: 10.1016/j.immuni.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Latchman Y, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 37.Cha J-H, Chan L-C, Li C-W, Hsu JL, Hung M-C. Mechanisms controlling PD-L1 expression in cancer. Mol. Cell. 2019;76:359–370. doi: 10.1016/j.molcel.2019.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuang D-M, et al. Tumor-activated monocytes promote expansion of IL-17–producing CD8+ T cells in hepatocellular carcinoma patients. J. Immunol. 2010;185:1544–1549. doi: 10.4049/jimmunol.0904094. [DOI] [PubMed] [Google Scholar]
- 39.Huber M, et al. A Th17‐like developmental process leads to CD8+ Tc17 cells with reduced cytotoxic activity. Eur. J. Immunol. 2009;39:1716–1725. doi: 10.1002/eji.200939412. [DOI] [PubMed] [Google Scholar]
- 40.Ciric B, El-behi M, Cabrera R, Zhang G-X, Rostami A. IL-23 drives pathogenic IL-17-producing CD8+ T cells. J. Immunol. 2009;182:5296–5305. doi: 10.4049/jimmunol.0900036. [DOI] [PubMed] [Google Scholar]
- 41.Chellappa S, et al. CD8+ T cells that coexpress RORγt and T-bet are functionally impaired and expand in patients with distal bile duct cancer. J. Immunol. 2017;198:1729–1739. doi: 10.4049/jimmunol.1600061. [DOI] [PubMed] [Google Scholar]
- 42.Bertrand F, et al. Blocking tumor necrosis factor α enhances CD8 T-cell-dependent immunity in experimental melanoma. Cancer Res. 2015;75:2619–2628. doi: 10.1158/0008-5472.CAN-14-2524. [DOI] [PubMed] [Google Scholar]
- 43.Davidson S, et al. Single-cell RNA sequencing reveals a dynamic stromal niche that supports tumor growth. Cell Rep. 2020;31:107628. doi: 10.1016/j.celrep.2020.107628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tirosh I, et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352:189–196. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raamsdonk CDV, Deo M. Links between Schwann cells and melanocytes in development and disease. Pigment Cell Melanoma Res. 2013;26:634–645. doi: 10.1111/pcmr.12134. [DOI] [PubMed] [Google Scholar]
- 46.Vandamme N, et al. The EMT transcription factor ZEB2 promotes proliferation of primary and metastatic melanoma while suppressing an invasive, mesenchymal-like phenotype. Cancer Res. 2020;80:2983–2995. doi: 10.1158/0008-5472.CAN-19-2373. [DOI] [PubMed] [Google Scholar]
- 47.Rambow F, et al. Toward minimal residual disease-directed therapy in melanoma. Cell. 2018;174:843–855.e19. doi: 10.1016/j.cell.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 48.Belote RL, et al. Human melanocyte development and melanoma dedifferentiation at single-cell resolution. Nat. Cell Biol. 2021;23:1035–1047. doi: 10.1038/s41556-021-00740-8. [DOI] [PubMed] [Google Scholar]
- 49.Diener J, Sommer L. Reemergence of neural crest stem cell‐like states in melanoma during disease progression and treatment. Stem Cell Transl. Med. 2021;10:522–533. doi: 10.1002/sctm.20-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Bohlson SS, O’Conner SD, Hulsebus HJ, Ho M-M, Fraser DA. Complement, C1q, and C1q-related molecules regulate macrophage polarization. Front. Immunol. 2014;5:402. doi: 10.3389/fimmu.2014.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gubin MM, et al. High-dimensional analysis delineates myeloid and lymphoid compartment remodeling during successful immune-checkpoint cancer therapy. Cell. 2018;175:1014–1030.e19. doi: 10.1016/j.cell.2018.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zilionis R, et al. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity. 2019;50:1317–1334.e10. doi: 10.1016/j.immuni.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elyada E, et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 2019;9:1102–1123. doi: 10.1158/2159-8290.CD-19-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu YE, Pan L, Zuo Y, Li X, Hong W. Detecting activated cell populations using single-cell RNA-seq. Neuron. 2017;96:313–329.e6. doi: 10.1016/j.neuron.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 56.Yoshimura T, Leonard EJ. Secretion by human fibroblasts of monocyte chemoattractant protein-1, the product of gene JE. J. Immunol. 1990;144:2377–2383. doi: 10.4049/jimmunol.144.6.2377. [DOI] [PubMed] [Google Scholar]
- 57.Ryseck R-P, Macdonald-Bravo H, Mattei MG, Bravo R. Cloning and sequence of a secretory protein induced by growth factors in mouse fibroblasts. Exp. Cell. Res. 1989;180:266–275. doi: 10.1016/0014-4827(89)90230-9. [DOI] [PubMed] [Google Scholar]
- 58.Iyer VR, et al. The transcriptional program in the response of human fibroblasts to serum. Science. 1999;283:83–87. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- 59.Saw PE, Chen J, Song E. Targeting CAFs to overcome anticancer therapeutic resistance. Trends Cancer. 2022;8:527–555. doi: 10.1016/j.trecan.2022.03.001. [DOI] [PubMed] [Google Scholar]
- 60.Luo H, et al. Pan-cancer single-cell analysis reveals the heterogeneity and plasticity of cancer-associated fibroblasts in the tumor microenvironment. Nat. Commun. 2022;13:6619. doi: 10.1038/s41467-022-34395-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun Z, et al. Transcription-associated histone pruning demarcates macroH2A chromatin domains. Nat. Struct. Mol. Biol. 2018;25:958–970. doi: 10.1038/s41594-018-0134-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonello GB, et al. An evolutionarily conserved TNF-α-responsive enhancer in the far upstream region of human CCL2 locus influences its gene expression. J. Immunol. 2011;186:7025–7038. doi: 10.4049/jimmunol.0900643. [DOI] [PubMed] [Google Scholar]
- 64.Brown JD, et al. NF-κB directs dynamic super enhancer formation in inflammation and atherogenesis. Mol. Cell. 2014;56:219–231. doi: 10.1016/j.molcel.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weiterer S, et al. Distinct IL‐1α‐responsive enhancers promote acute and coordinated changes in chromatin topology in a hierarchical manner. EMBO J. 2020;39:e101533. doi: 10.15252/embj.2019101533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu M, et al. Super enhancer regulation of cytokine-induced chemokine production in alcoholic hepatitis. Nat. Commun. 2021;12:4560. doi: 10.1038/s41467-021-24843-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Higashijima, Y. & Kanki, Y. Potential roles of super enhancers in inflammatory gene transcription. FEBS J. 10.1111/febs.16089 (2021). [DOI] [PubMed]
- 68.Corujo D, et al. MacroH2As regulate enhancer–promoter contacts affecting enhancer activity and sensitivity to inflammatory cytokines. Cell Rep. 2022;39:110988. doi: 10.1016/j.celrep.2022.110988. [DOI] [PubMed] [Google Scholar]
- 69.Ismail WM, et al. MacroH2A histone variants modulate enhancer activity to repress oncogenic programs and cellular reprogramming. Commun. Biol. 2023;6:215. doi: 10.1038/s42003-023-04571-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen H, et al. MacroH2A1.1 and PARP-1 cooperate to regulate transcription by promoting CBP-mediated H2B acetylation. Nat. Struct. Mol. Biol. 2014;21:981–989. doi: 10.1038/nsmb.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lavigne MD, et al. Composite macroH2A/NRF-1 nucleosomes suppress noise and generate robustness in gene expression. Cell Rep. 2015;11:1090–1101. doi: 10.1016/j.celrep.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 72.Recoules, L. et al. The histone variant macroH2A1.1 regulates RNA polymerase II paused genes within defined chromatin interaction landscapes. J. Cell Sci. 10.1242/jcs.259456 (2022). [DOI] [PMC free article] [PubMed]
- 73.Chakravarthy S, Patel A, Bowman GD. The basic linker of macroH2A stabilizes DNA at the entry/exit site of the nucleosome. Nucleic Acids Res. 2012;40:8285–8295. doi: 10.1093/nar/gks645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fu Y, et al. MacroH2A1 associates with nuclear lamina and maintains chromatin architecture in mouse liver cells. Sci. Rep. 2015;5:17186. doi: 10.1038/srep17186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Douet J, et al. MacroH2A histone variants maintain nuclear organization and heterochromatin architecture. J. Cell Sci. 2017;130:1570–1582. doi: 10.1242/jcs.199216. [DOI] [PubMed] [Google Scholar]
- 76.Kozlowski M, et al. MacroH2A histone variants limit chromatin plasticity through two distinct mechanisms. EMBO Rep. 2018;19:e44445. doi: 10.15252/embr.201744445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krietenstein N, et al. Ultrastructural details of mammalian chromosome architecture. Mol. Cell. 2020;78:554–565.e7. doi: 10.1016/j.molcel.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee BH, Wu Z, Rhie SK. Characterizing chromatin interactions of regulatory elements and nucleosome positions, using Hi-C, Micro-C, and promoter capture Micro-C. Epigenetics Chromatin. 2022;15:41. doi: 10.1186/s13072-022-00473-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang EY, et al. MacroH2A allows ATP-dependent chromatin remodeling by SWI/SNF and ACF complexes but specifically reduces recruitment of SWI/SNF. Biochemistry. 2008;47:13726–13732. doi: 10.1021/bi8016944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-κB-dependent manner. Cancer Cell. 2010;17:523. doi: 10.1016/j.ccr.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 81.Yang X, et al. FAP promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3–CCL2 signaling. Cancer Res. 2016;76:4124–4135. doi: 10.1158/0008-5472.CAN-15-2973. [DOI] [PubMed] [Google Scholar]
- 82.Kumar V, et al. Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell. 2017;32:654–668.e5. doi: 10.1016/j.ccell.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cho H, Kim J-H, Jun C-D, Jung D-W, Williams DR. CAF-derived IL6 and GM-CSF cooperate to induce M2-like TAMs–response. Clin. Cancer Res. 2019;25:894–895. doi: 10.1158/1078-0432.CCR-18-3344. [DOI] [PubMed] [Google Scholar]
- 84.Landsberg J, et al. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature. 2012;490:412–416. doi: 10.1038/nature11538. [DOI] [PubMed] [Google Scholar]
- 85.Tsoi J, et al. Multi-stage differentiation defines melanoma subtypes with differential vulnerability to drug-induced iron-dependent oxidative stress. Cancer Cell. 2018;33:890–904.e5. doi: 10.1016/j.ccell.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pérez-Guijarro E, et al. Multimodel preclinical platform predicts clinical response of melanoma to immunotherapy. Nat. Med. 2020;26:781–791. doi: 10.1038/s41591-020-0818-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee JH, et al. Transcriptional downregulation of MHC class I and melanoma de-differentiation in resistance to PD-1 inhibition. Nat. Commun. 2020;11:1897. doi: 10.1038/s41467-020-15726-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krishnamurty AT, et al. LRRC15+ myofibroblasts dictate the stromal setpoint to suppress tumour immunity. Nature. 2021;611:148–154. doi: 10.1038/s41586-022-05272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bartoschek M, et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat. Commun. 2018;9:5150. doi: 10.1038/s41467-018-07582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoek KS, et al. In vivo switching of human melanoma cells between proliferative and invasive states. Cancer Res. 2008;68:650–656. doi: 10.1158/0008-5472.CAN-07-2491. [DOI] [PubMed] [Google Scholar]
- 91.Huang F, Santinon F, González REF, Rincón SVdel. Melanoma plasticity: promoter of metastasis and resistance to therapy. Front. Oncol. 2021;11:756001. doi: 10.3389/fonc.2021.756001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roulis M, et al. Paracrine orchestration of intestinal tumorigenesis by a mesenchymal niche. Nature. 2020;580:524–529. doi: 10.1038/s41586-020-2166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Köhler C, et al. Mouse cutaneous melanoma induced by mutant BRaf arises from expansion and dedifferentiation of mature pigmented melanocytes. Cell Stem Cell. 2017;21:679–693.e6. doi: 10.1016/j.stem.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 94.Moon H, et al. Melanocyte stem cell activation and translocation initiate cutaneous melanoma in response to UV exposure. Cell Stem Cell. 2017;21:665–678.e6. doi: 10.1016/j.stem.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meeth K, Wang JX, Micevic G, Damsky W, Bosenberg MW. The YUMM lines: a series of congenic mouse melanoma cell lines with defined genetic alterations. Pigment Cell Melanoma Res. 2016;29:590–597. doi: 10.1111/pcmr.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao F, et al. Paracrine Wnt5a-β–catenin signaling triggers a metabolic program that drives dendritic cell tolerization. Immunity. 2018;48:147–160.e7. doi: 10.1016/j.immuni.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Salmon H, et al. Expansion and activation of CD103+ dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity. 2016;44:924–938. doi: 10.1016/j.immuni.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods. 2017;14:417–419. doi: 10.1038/nmeth.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ignatiadis N, Klaus B, Zaugg JB, Huber W. Data-driven hypothesis weighting increases detection power in genome-scale multiple testing. Nat. Methods. 2016;13:577–580. doi: 10.1038/nmeth.3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Korotkevich, G. et al. Fast gene set enrichment analysis. Preprint at bioRxiv10.1101/060012 (2021).
- 102.Heinz S, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ritchie ME, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hao Y, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573–3587.e29. doi: 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bibby JA, et al. Systematic single-cell pathway analysis to characterize early T cell activation. Cell Rep. 2022;41:111697. doi: 10.1016/j.celrep.2022.111697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cao J, et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature. 2019;566:496–502. doi: 10.1038/s41586-019-0969-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aran D, et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 2019;20:163–172. doi: 10.1038/s41590-018-0276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dann E, Henderson NC, Teichmann SA, Morgan MD, Marioni JC. Differential abundance testing on single-cell data using k-nearest neighbor graphs. Nat. Biotechnol. 2022;40:245–253. doi: 10.1038/s41587-021-01033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Squair JW, Skinnider MA, Gautier M, Foster LJ, Courtine G. Prioritization of cell types responsive to biological perturbations in single-cell data with Augur. Nat. Protoc. 2021;16:3836–3873. doi: 10.1038/s41596-021-00561-x. [DOI] [PubMed] [Google Scholar]
- 110.Jin S, et al. Inference and analysis of cell–cell communication using CellChat. Nat. Commun. 2021;12:1088. doi: 10.1038/s41467-021-21246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pommer M, Kuphal S, Bosserhoff AK. Amphiregulin regulates melanocytic senescence. Cells. 2021;10:326. doi: 10.3390/cells10020326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Contrepois K, et al. Histone variant H2A.J accumulates in senescent cells and promotes inflammatory gene expression. Nat. Commun. 2017;8:14995. doi: 10.1038/ncomms14995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kaur A, et al. Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov. 2019;9:64–81. doi: 10.1158/2159-8290.CD-18-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ecker BL, et al. Age-related changes in HAPLN1 increase lymphatic permeability and affect routes of melanoma metastasis. Cancer Discov. 2019;9:82–95. doi: 10.1158/2159-8290.CD-18-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McLennan R, et al. Neural crest cells bulldoze through the microenvironment using Aquaporin 1 to stabilize filopodia. Development. 2019;147:dev185231. doi: 10.1242/dev.185231. [DOI] [PubMed] [Google Scholar]
- 116.Schmidt M, et al. The transcription factors AP-2β and AP-2α are required for survival of sympathetic progenitors and differentiated sympathetic neurons. Dev. Biol. 2011;355:89–100. doi: 10.1016/j.ydbio.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 117.Calcagno DM, et al. The myeloid type I interferon response to myocardial infarction begins in bone marrow and is regulated by Nrf2-activated macrophages. Sci. Immunol. 2020;5:eaaz1974. doi: 10.1126/sciimmunol.aaz1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baranska A, et al. Unveiling skin macrophage dynamics explains both tattoo persistence and strenuous removal. J. Exp. Med. 2018;215:1115–1133. doi: 10.1084/jem.20171608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li B, et al. The melanoma‐associated transmembrane glycoprotein Gpnmb controls trafficking of cellular debris for degradation and is essential for tissue repair. FASEB J. 2010;24:4767–4781. doi: 10.1096/fj.10-154757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kumamoto Y, et al. CD301b+ dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity. 2013;39:733–743. doi: 10.1016/j.immuni.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wagner C, et al. Differentiation paths of Peyer’s patch LysoDCs are linked to sampling site positioning, migration, and T cell priming. Cell Rep. 2020;31:107479. doi: 10.1016/j.celrep.2020.03.043. [DOI] [PubMed] [Google Scholar]
- 122.Maier B, et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature. 2020;580:257–262. doi: 10.1038/s41586-020-2134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ugajin T, et al. Basophils preferentially express mouse mast cell protease 11 among the mast cell tryptase family in contrast to mast cells. J. Leukoc. Biol. 2009;86:1417–1425. doi: 10.1189/jlb.0609400. [DOI] [PubMed] [Google Scholar]
- 124.Swarnalekha N, et al. T resident helper cells promote humoral responses in the lung. Sci. Immunol. 2021;6:eabb6808. doi: 10.1126/sciimmunol.abb6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Evrard M, et al. Sphingosine 1-phosphate receptor 5 (S1PR5) regulates the peripheral retention of tissue-resident lymphocytes. J. Exp. Med. 2021;219:e20210116. doi: 10.1084/jem.20210116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhao D-M, et al. Constitutive activation of Wnt signaling favors generation of memory CD8 T cells. J. Immunol. 2009;184:1191–1199. doi: 10.4049/jimmunol.0901199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kobayashi T, et al. Homeostatic control of sebaceous glands by innate lymphoid cells regulates commensal bacteria equilibrium. Cell. 2019;176:982–997.e16. doi: 10.1016/j.cell.2018.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sahai E, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer. 2020;20:174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vafaee F, Colvin EK, Mok SC, Howell VM, Samimi G. Functional prediction of long non-coding RNAs in ovarian cancer-associated fibroblasts indicate a potential role in metastasis. Sci. Rep. 2017;7:10374. doi: 10.1038/s41598-017-10869-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tsang M, et al. Insights into fibroblast plasticity: cellular communication network 2 is required for activation of cancer-associated fibroblasts in a murine model of melanoma. Am. J. Pathol. 2019;190:206–221. doi: 10.1016/j.ajpath.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 131.Philippeos C, et al. Spatial and single-cell transcriptional profiling identifies functionally distinct human dermal fibroblast subpopulations. J. Invest. Dermatol. 2018;138:811–825. doi: 10.1016/j.jid.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tabib T, et al. Myofibroblast transcriptome indicates SFRP2hi fibroblast progenitors in systemic sclerosis skin. Nat. Commun. 2021;12:4384. doi: 10.1038/s41467-021-24607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Solé-Boldo L, et al. Single-cell transcriptomes of the human skin reveal age-related loss of fibroblast priming. Commun. Biol. 2020;3:188. doi: 10.1038/s42003-020-0922-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Qi J, et al. Single-cell and spatial analysis reveal interaction of FAP+ fibroblasts and SPP1+ macrophages in colorectal cancer. Nat. Commun. 2022;13:1742. doi: 10.1038/s41467-022-29366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sun H, et al. Comprehensive characterization of 536 patient-derived xenograft models prioritizes candidates for targeted treatment. Nat. Commun. 2021;12:5086. doi: 10.1038/s41467-021-25177-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Capparelli C, Rosenbaum S, Berger AC, Aplin AE. Fibroblast-derived neuregulin 1 promotes compensatory ErbB3 receptor signaling in mutant BRAF melanoma. J. Biol. Chem. 2015;290:24267–24277. doi: 10.1074/jbc.M115.657270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Capparelli C, et al. ErbB3 targeting enhances the effects of MEK inhibitor in wild-type BRAF/NRAS melanoma. Cancer Res. 2018;78:5680–5693. doi: 10.1158/0008-5472.CAN-18-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kersten K, et al. Spatiotemporal co-dependency between macrophages and exhausted CD8+ T cells in cancer. Cancer Cell. 2022;40:624–638.e9. doi: 10.1016/j.ccell.2022.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.The Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell. 2015;161:1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mounir M, et al. New functionalities in the TCGAbiolinks package for the study and integration of cancer data from GDC and GTEx. PLoS Comput. Biol. 2019;15:e1006701. doi: 10.1371/journal.pcbi.1006701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Robinson MD, McCarthy DJ, Smyth G. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Newman AM, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yoshihara K, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 2013;4:2612. doi: 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zeng D, et al. IOBR: multi-omics immuno-oncology biological research to decode tumor microenvironment and signatures. Front. Immunol. 2021;12:687975. doi: 10.3389/fimmu.2021.687975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Corces MR, et al. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat. Methods. 2017;14:959–962. doi: 10.1038/nmeth.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Buenrostro JD, Wu B, Chang HY, Greenleaf WJ. ATAC‐seq: a method for assaying chromatin accessibility genome‐wide. Curr. Protoc. Mol. Biol. 2015;109:21.29.1–21.29.9. doi: 10.1002/0471142727.mb2129s109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gaspar JM. NGmerge: merging paired-end reads via novel empirically-derived models of sequencing errors. BMC Bioinformatics. 2018;19:536. doi: 10.1186/s12859-018-2579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Danecek P, et al. Twelve years of SAMtools and BCFtools. Gigascience. 2021;10:giab008. doi: 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ramírez F, et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 2016;44:W160–W165. doi: 10.1093/nar/gkw257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Amemiya HM, Kundaje A, Boyle AP. The ENCODE blacklist: identification of problematic regions of the genome. Sci. Rep. 2019;9:9354. doi: 10.1038/s41598-019-45839-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gaspar, J. M. Improved peak-calling with MACS2. Preprint at bioRxiv10.1101/496521 (2018).
- 153.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fontanals-Cirera B, et al. Harnessing BET inhibitor sensitivity reveals AMIGO2 as a melanoma survival gene. Mol. Cell. 2017;68:731–744.e9. doi: 10.1016/j.molcel.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Whyte WA, et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153:307–319. doi: 10.1016/j.cell.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lovén J, et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153:320–334. doi: 10.1016/j.cell.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Skene PJ, Henikoff JG, Henikoff S. Targeted in situ genome-wide profiling with high efficiency for low cell numbers. Nat. Protoc. 2018;13:1006–1019. doi: 10.1038/nprot.2018.015. [DOI] [PubMed] [Google Scholar]
- 159.Meers MP, Tenenbaum D, Henikoff S. Peak calling by sparse enrichment analysis for CUT&RUN chromatin profiling. Epigenetics Chromatin. 2019;12:42. doi: 10.1186/s13072-019-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Freire-Pritchett P, et al. Detecting chromosomal interactions in capture Hi-C data with CHiCAGO and companion tools. Nat. Protoc. 2021;16:4144–4176. doi: 10.1038/s41596-021-00567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. Preprint at arXiv10.48550/arxiv.1303.3997 (2013).
- 162.Open2C et al. Pairtools: from sequencing data to chromosome contacts. Preprint at bioRxiv10.1101/2023.02.13.528389 (2023).
- 163.Cairns J, et al. CHiCAGO: robust detection of DNA looping interactions in capture Hi-C data. Genome Biol. 2016;17:127. doi: 10.1186/s13059-016-0992-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dale RK, Pedersen BS, Quinlan AR. Pybedtools: a flexible Python library for manipulating genomic datasets and annotations. Bioinformatics. 2011;27:3423–3424. doi: 10.1093/bioinformatics/btr539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kent WJ, Zweig AS, Barber G, Hinrichs AS, Karolchik D. BigWig and BigBed: enabling browsing of large distributed datasets. Bioinformatics. 2010;26:2204–2207. doi: 10.1093/bioinformatics/btq351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Raney BJ, et al. Track data hubs enable visualization of user-defined genome-wide annotations on the UCSC Genome Browser. Bioinformatics. 2014;30:1003–1005. doi: 10.1093/bioinformatics/btt637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 168.Wang J, et al. UV‐induced somatic mutations elicit a functional T cell response in the YUMMER1.7 mouse melanoma model. Pigment Cell Melanoma Res. 2017;30:428–435. doi: 10.1111/pcmr.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bulk tumour RNA-seq.
CD8+ T cell RNA-seq.
scRNA-seq.
ST analysis.
CAF RNA-seq.
iDF RNA-seq.
pcMicro-C.
Reagents.
Data Availability Statement
The transcriptomics and epigenomic datasets, including raw and processed sequencing data generated and analysed during the current study, are available in the Gene Expression Omnibus (GEO) repository under accession number GSE200751 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE200751) and in this article’s table files. TCGA melanoma data are publicly available through the NCI Genomic Data Commons (GDC) data portal under project ID TCGA SKCM (https://portal.gdc.cancer.gov/projects/TCGA-SKCM). The human pan-cancer scRNA-seq dataset mined in this study is available in the GEO repository under accession number GSE210347 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE210347). The mouse M25 (GRCm38.p6) genome assembly and gene set used for transcriptomics and epigenomic analyses are available at Gencode (https://www.gencodegenes.org/mouse/release_M25.html). All other data supporting the findings of this study is available from the corresponding authors upon request. Source data are provided with this paper.
All packages used for data analysis are publicly available. No custom code was generated for this study. All scripts used for bulk RNA-seq, scRNA-seq, ATAC–seq, ChIP–seq and CUT&RUN data analyses in this study are available from the corresponding authors upon request.