Abstract
Drought stress as a result of rapidly changing climatic conditions has a direct negative impact on crop production especially wheat which is the 2nd staple food crop. To fulfill the nutritional demand under rapidly declining water resources, there is a dire need to adopt a precise, and efficient approach in the form of different amendments. In this regard, the present study investigated the impact of nano-biochar (NBC) and brassinosteroids (BR) in enhancing the growth and productivity of wheat under different drought stress conditions. The field study comprised different combinations of amendments (control, NBC, BR, and NBC + BR) under three irrigation levels (D0, D1 and D2). Among different treatments, the synergistic approach (NBC + BR) resulted in the maximum increase in different growth and yield parameters under normal as well as drought stress conditions. With synergistic approach (NBC + BR), the maximum plant height (71.7 cm), spike length (17.1), number of fertile tillers m–2 (410), no. of spikelets spike–1 (19.1), no. of grains spike–1 (37.9), 1000 grain weight (37 g), grain yield (4079 kg ha–1), biological yield (10,502 kg ha–1), harvest index (43.5). In the case of physiological parameters such as leaf area index, relative water contents, chlorophyll contents, and stomatal conductance were maximally improved with the combined application of NBC and BR. The same treatment caused an increase of 54, 10, and 7% in N, P, and K contents in grains, respectively compared to the control treatment. Similarly, the antioxidant response was enhanced in wheat plants under drought stress with the combined application of NBC and BR. In conclusion, the combined application of NBC and BR caused a significant increase in the growth, physiological and yield attributes of wheat under drought stress.
Subject terms: Biotechnology, Plant sciences, Environmental sciences, Chemistry
Introduction
Wheat growth and productivity are hampered due to stress possessed by drought conditions in arid and semiarid areas1,2. A negative effect can be seen in the plant’s photosynthetic machinery, especially in stomatal conductance, thylakoid electron transport, Calvin cycle, and CO2 assimilation3–6. Drought stress also disturbs the balance of the production of reactive oxygen species and antioxidant production system which causes the production and accumulation of ROS which ultimately leads to disruption and disorganization of cell membrane lipids and DNA strands7–11.
While plants have developed protective mechanisms including physiological, biochemical, and morphological against water scarcity3,12 during their evolution process e.g. enhancing signaling pathways of phytohormones in response to abiotic stress13–17. The production of brassinosteroids (BR) which are polyhydroxylated steroidal hormones and play many physiological and morphogenesis processes starting from seed germination to flowering and senescence of plants18. Moreover, abiotic stress is also controlled via BR application19 like (1) enhancing the activity of antioxidative enzymes20 ultimately reducing the production of superoxide anion21 (2) abscisic acid accumulation is reduced by (BR) application22 although this abscisic acid causes the closure of stomata under drought stress20,23 and (3) osmotic permeability of roots are being increased for more water uptake.
Nano-biochar (NBC) also mitigates the negative effect of drought stress on plant growth and yield e.g. sorghum, maize, and wheat24–26. This activity also helps in more water retention hence lower demand of the number of soil irrigation27,28 increasing the nutrient use efficiency29,30, stimulating the activity of gibberellins and auxins and regulation of BR31, and enhancing the stomatal conductance, chlorophyll contents, cytotoxicity, and K+ contents in leaf32. This carbon-rich and cost-effective component is made through the process of pyrolysis of organic residues in the absence of oxygen33,34, resulting in highly porous and aromatic carbon contents35. It was worth seeing that this carbon-enriched compound stays longer in the soil as compared to many other organic residues such as compost hence, this mitigates and competes the climate change through carbon sequestration36,37. In upcoming years there was a dire need in growing crops worldwide to fulfill the food requirements of humans and animals38. Wheat holds a most important place in global food security39–41, which contributes nearly 40% towards total world food demand. During 2019, its global production was 757.4 million tons42.
Many high-yielding wheat cultivars have been introduced for increasing wheat productivity43,44, and these cultivars also uptake high amounts of mineral nutrients45, so appropriate techniques are also needed for the betterment of nutrient uptake to sustain the availability of limited resources. For example, the nitrogen use efficiency of wheat does not exceed 33% globally42, and other nutrient use efficiency does not exceed 50%. Under drought stress, these efficiencies also decrease considerably46 and this condition results in the decline in wheat productivity. Although many studies have investigated the sole positive effect of BR and NBC, the combined effect of both of these compounds has not yet been investigated so far.
Based on the above discussion, the present study hypothesized that the application of BR and NBC alone or in combination could enhance the growth, physiological, and yield attributes of wheat grown under different drought stress conditions. The objective of the present study was to investigate the impact of BR and NBC, alone or combined on the growth, physiological, biochemical, and yield attributes of wheat under different drought stress conditions.
Materials and methods
Soil analysis
Soil samples were taken from experimental plots through auger, before sowing, and placed in tagged polyethylene bags. These bags were shifted to Soil and Water Testing Laboratory, Regional Agriculture Research Institute Bahawalpur. Various physicochemical parameters were measured using standard methods. The soil sandy loam with pH = 7.22, electric conductivity = 2.54 dS m–1, organic matter = 0.90%, nitrogen = 1.57 mg g–1, available phosphorus = 6.63 mg kg–1 and available potassium = 115 mg kg–1. Weather measurement was noted after the experiment from the observatory unit which showed an average precipitation of 15.50 mm and a temperature of 28.17 °C during the growing season.
Field experiment
A field experiment was conducted at the agronomic research area of UCA & ES, The Islamia University of Bahawalpur to study the effect of nano-biochar (NBC) and brassinosteroids (BR) on wheat under drought stress. The experiment was arranged as a randomized complete block design (RCBD) with the factorial arrangement, having four replications in 10 cm apart lines. Faisalabad 2008 cultivar, obtained from RARI (Regional Agriculture Research Institute Bahawalpur) was sown in a 15 m2 plot subjected to drought stress at tillering (D1) and drought stress at anthesis (D2) stages and the plots receiving normal irrigation were considered as control treatment (D0). Three treatments T0 = control, T1 = NBC (Nano-biochar) T2 = BR (Brassinosteroids), and T3 = NBC + BR (co-application of nano-biochar and BR) were applied to the plots. For Brassinosteroids treatment, 24-epibrassinolide (C28H48O6 MW = 480.7) was purchased from Sigma-Aldrich. Brassinosteroids (120 mg L–1) were applied twice (tillering and anthesis stages) through foliar spray while nano-biochar (0.75% w/w) was incorporated in the soil at the time of sowing. Nano-biochar was obtained from Shanghai Hainuo Carbon Industry Co., Ltd China. Three-acre inches per irrigation water was applied as per schedule except during respective tillering and anthesis stages of the treatments to induce the drought stress excluding the control plots. The control plots received four irrigations in total using the flood irrigation method. Tube well water with pH = 6.5 and EC = 886 µS cm–1 was used for irrigation purposes. Fertilizer was applied @ 120 kg N and 80 kg P2O5 per hectare, using urea and diammonium phosphate (DAP), respectively.
Growth and yield parameters
Various yield and growth-related parameters were determined through the procedures discussed below. The number of fertile tillers was counted in per square meter from each plot. Fifteen plants were selected randomly from each treatment plot at the time of harvesting and their spike length and plant height were measured with measuring tape and then averaged. Spikes were then separated from each tiller to record the number of spikelets spike–1 and 1000 grain weight after manual threshing. At the time of harvesting, a manual method was used to cut the crop for reducing any loss. The harvested crop was tied in bundles and their biological yield was recorded with a weighing balance for each treatment.
Leaf area index (LAI)
The total leaf area was measured by randomly selecting fifteen plants from every subplot and then the average was taken out. Hence, LAI was calculated by using the formula given by Watson47.
Harvest index (%)
It was calculated for each plot by using the following formula:
Physiological parameters
Leaf chlorophyll contents
Leaf chlorophyll contents were measured by using a UV/VIS spectrophotometer. Chlorophyll content was measured by using Arnon’s method48. Fresh leaves of 0.1 g were grounded and placed in 80% acetone overnight. After that sample was centrifuged for 5 min at 10,000 rpm. The absorbance was measured at 645 nm and 663 nm wavelength and chlorophyll was measured by the given formula:
V is the supernatant volume and W is the fresh weight.
Relative water contents (%)
The third leaf from the top (fully expanded youngest leaf) of ten plants of each treatment was used to determine the leaf’s relative water content (RWC). Immediately after cutting at the base of the lamina, leaves were sealed within plastic bags and quickly transferred to the lab. Fresh weight (FW) was determined within 2 hours after the excision of leaves. Then turgid weight (TW) was obtained after soaking leaves in distilled water for 16–18 h at room temperature. After soaking, leaves were quickly and carefully blotted dry with tissue paper to calculate the turgid weight. Dry weight (DW) was obtained after oven during the leaf samples for 72 h at 70 °C. Relative water content was calculated by using the following formula49
where FW = fresh weight, DW = dry weight, TW = turgid weight.
Leaf stomatal conductance (mmol of H2O m-2 s-1)
Stomatal resistance/conductance measurements were made with an automatic porometer MK-3 (Delta-T Devices, Burwell Cambridge, England) Hertford, Herts, England).
Grain quality parameters
NPK was measured for assessing the grain quality as per the method described by Wolf50.
Antioxidant activities
Leaf (1 g) was ground in liquid nitrogen to get the enzyme extract. The obtained powder was added to 50 mM phosphate buffer (10 mL) at pH 7.0 and was then mixed with 1 mM ethylene diamine tetraacetic acid (EDTA) and 1% polyvinylpyrrolidone (PVP). The whole mixture was spun at 13,000 × g for 20 min at 4 °C. The resulting supernatant was used for the enzyme assay. H2O2 decomposition rate at 240 nm indicated the catalase (CAT) activity as proposed by Hwang et al.51. The CAT activity (U/mg protein) was estimated from the molar absorption coefficient of 40 mm–1 cm–1 for H2O2. Peroxidase (POD) activity was recorded as per the method given by Kar and Mishra52. The reaction mixture consisted of 10 μL of crude enzyme extract, 10 μL of 100 mM H2O2, 160 μL of 50 mM sodium acetate (pH 5.0), and 20 μL of 100 mM guaiacol. Absorbance was recorded at 450 nm. Superoxide dismutase (SOD) enzyme activity was observed through the measurement of 50% inhibition of the rate nitro blue tetrazolium chloride reduction53. The reaction mixture contained 130 mM methionine, 0.75 mM NBT, 0.05 M phosphate buffer (pH 7.0), 0.02 mM riboflavin, and 300 μL enzyme extract. The reaction mixture and blank were exposed to fluorescent light for 7 min and absorbance was taken at 560 nm.
Statistical analysis
The collected data regarding various parameters were analyzed statistically through a two-way analysis of variance (ANOVA) using Statistix 8.1 software53. The difference among mean values was determined using the least significant difference (LSD) test at a 0.05 probability level. Microsoft Excel 2016 was used for the preparation of graphs and the calculation of means and standard error values.
Ethics approval and consent to participate
The seeds variety (Faisalabad 2008 cultivar) was obtained from RARI (Regional Agriculture Research Institute), Bahawalpur, Pakistan. All the experiments were performed in accordance with relevant guidelines and regulations".
Results
Growth and yield attributes
Statistical analysis of data shows significant differences in plant height as the result of different treatments and drought stress levels (Table 1). Maximum plant height of 71.7 cm was recorded in D0 (Control) whereas statistically lowest plant height (54.99 cm) was obtained in D2 (Drought stress at anthesis stage). Treatment T3 (NBC + BR) resulted in the maximum plant height (71.7 cm) and it was 30.4% more in comparison to the control treatment. In relation to the interaction of both factors under study, a statistically significant (p ≤ 0.001) interaction was recorded (Table 2).
Table 1.
Effect of nano-biochar (NBC) and brassinosteroids (BR) on plant height (cm), spike length (cm), no. of fertile tillers m–2, no. of spikelets spike–1, no. of grains spike–1, 1000 grain weight (g), grain yield (kg ha–1), biological yield (kg ha–1) and harvest index of wheat under drought stress.
| Control | NBC | BR | NBC + BR | |
|---|---|---|---|---|
| Plant height (cm) | ||||
| Normal irrigation | 63.8d* | 66.7c | 68.7b | 71.7a |
| Drought stress at tillering stage | 59.5e | 58.6e | 59.1e | 59.1e |
| Drought stress at anthesis stage | 54.9f | 55.3f | 55.8f | 56.1f |
| Spike length (cm) | ||||
| Normal irrigation | 14.1e | 14.6 cd | 15.5b | 17.1a |
| Drought stress at tillering stage | 11.2i | 11.8 h | 13.5f | 14.3de |
| Drought stress at anthesis stage | 12.4g | 14.1e | 14.8c | 15.7b |
| No. of fertile tillers m–2 | ||||
| Normal irrigation | 402c | 405b | 410a | 410a |
| Drought stress at tillering stage | 310i | 325g | 342e | 342e |
| Drought stress at anthesis stage | 315h | 323g | 337f | 345d |
| No. of spikelets spike–1 | ||||
| Normal irrigation | 16.7ef | 18.6b | 17.7c | 19.1a |
| Drought stress at tillering stage | 15.6hi | 16.4f | 16.8e | 17.1d |
| Drought stress at anthesis stage | 13.7j | 15.5i | 15.8h | 16.0g |
| No. of grains spike–1 | ||||
| Normal irrigation | 33bc | 34b | 37a | 38a |
| Drought stress at tillering stage | 29e | 31d | 33bc | 34b |
| Drought stress at anthesis stage | 27f | 29e | 32cd | 33bc |
| 1000 grain weight (g) | ||||
| Normal irrigation | 35.3b | 35.8b | 36.7a | 37.0a |
| Drought stress at tillering stage | 32.2d | 32.8d | 33.6c | 33.7c |
| Drought stress at anthesis stage | 25.5f | 25.9f | 27.0e | 27.1e |
| Grain yield (kg ha–1) | ||||
| Normal irrigation | 3707d | 3854c | 3976b | 4079a |
| Drought stress at tillering stage | 2949h | 3196g | 3309f | 3353e |
| Drought stress at anthesis stage | 2457l | 2650k | 2826j | 2920i |
| Biological yield (kg ha–1) | ||||
| Normal irrigation | 8513d | 9209c | 10313b | 10502a |
| Drought stress at tillering stage | 7113h | 7794g | 8445f | 8542e |
| Drought stress at anthesis stage | 6735l | 7246k | 8024j | 8316i |
| Harvest index | ||||
| Normal irrigation | 43.5a | 41.9b | 38.6h | 38.8g |
| Drought stress at tillering stage | 41.5c | 40.5d | 39.2f | 39.3e |
| Drought stress at anthesis stage | 36.5j | 36.6i | 35.2k | 35.1l |
*Means with various letters are significantly different according to the least significant difference (LSD) test at 0.05 probability level.
Table 2.
Analysis of variance (ANOVA) of different parameters affected by different treatments under different drought stress conditions.
| Variable | Drought | Treatment | Drought × treatment |
|---|---|---|---|
| Degree of freedom | 2 | 3 | 6 |
| Plant height | *** | *** | *** |
| Spike length | *** | *** | *** |
| No. of fertile tillers m–2 | *** | *** | *** |
| No. of spikelets spike–1 | *** | *** | *** |
| No. of grain spike–1 | *** | *** | NS |
| 1000 grain weight | *** | *** | NS |
| Grain yield | *** | *** | *** |
| Biological yield | *** | *** | *** |
| Harvest index | *** | *** | *** |
| Leaf area index | *** | *** | *** |
| Relative water contents | *** | *** | *** |
| Stomatal conductance | *** | *** | *** |
| Chlorophyll contents | *** | *** | *** |
| Nitrogen contents in grains | *** | *** | NS |
| Phosphorus contents in grains | *** | *** | *** |
| Potassium contents in grains | *** | *** | NS |
| Ascorbate peroxidase | *** | *** | NS |
| Catalase | *** | *** | *** |
| Peroxidase | *** | *** | ** |
| Superoxide dismutase | *** | *** | *** |
where NS non-significant at p ≤ 0.05, ** = significant at p ≤ 0.01 and *** = significant at p ≤ 0.001.
A significant difference in spike length was recorded under different treatments and drought stress levels (Table 1). Spikes with more length were recorded in D0 (17.08) and spikes with minimum length were reported in D1 (11.17). Plots receiving T3 (NBC + BR) resulted in the maximum spike length of (17.08). Statistically significant (p ≤ 0.001) interactive effect of both factors was reported on wheat spike length (Table 2).
A significant difference in the number of fertile tillers was recorded according to treatments and drought stress levels (Table 1). The highest number of tillers were recorded in D0 (410) as BR or NBC + BR were applied and minimum at D1 (310) at the control treatment (T0). The maximum number of fertile tillers was recorded in plots receiving T3 (NBC + BR). Statistically non-significant (p ≤ 0.05) interactive effect of both factors was reported on wheat tillers (Table 2).
Both treatment and drought stress levels had a significant impact on the number of spikelets spike–1. The maximum number of spikelets spike–1 (19.06) was recorded with the application of T3 under D0 while that of the minimum (13.67) with T0 under D2. Graph represented that T3 (NBC + BR) resulted in the maximum number of spikelets spike–1. Statistically significant (p ≤ 0.001) interactive effect of both factors was reported on wheat spikelets (Table 2).
The number of grains spike–1 was significantly controlled by both factors i.e. treatments and drought stress levels. The maximum number of grains spike–1 were recorded in D0 (38) @ T3 and the minimum as drought stress was applied at the anthesis stage i.e. D2 (27). However, this was mitigated by the combined application of NBC and BR which resulted in a 22% recovery (33).
Similarly, drought stress at tillering stage resulted in 29.2 grains spike–1 but NBC and BR applications showed a promising increase of 6% and 13% respectively while the T3 (NBC + BR) recovered 16%. Non-significant interaction was reported between treatment and drought stress (Table 2).
A significant difference in 1000 grain weight was recorded according to treatments and drought stress levels. 1000 grains with more weight were recorded in D0 (37) and the minimum was reported at D2 (25.5) @ control treatment (T0). Plots receiving T3 (NBC + BR) resulted in the maximum weight of grain. Non-significant interactive effect of both factors was reported on wheat 1000 grain weight (Table 2).
A significant difference in grain yield was recorded according to treatments and drought stress levels. Plot having control treatment showed maximum grain yield at D0 (3707 kg ha–1) but as drought stress was applied this value reduced to 2949 kg ha–1 (20%) and 2457 kg ha–1 (33%) at D1 and D2 respectively. Promising responses of 8% and 12% recovery were observed after T1 and T2 application at D1. Similarly, at D2, 8% and 15% gain was seen after NBC and BR incorporation. However, an 18% loss could be reduced as NBC and BR co-applied (T3). A significant interactive effect was found between both factors with maximum grain yield (Table 2).
A significant difference in biological yield was recorded according to treatments and drought stress levels. The biological yield was maximum at D0 (10,502 kg ha–1) and minimum reported at D2 (6735 kg ha–1). Plot received T3 (NBC + BR) reduced the 20% drought stress effect at the tillering stage and 23% at anthesis stage. A significant interactive effect was found between both factors with maximum biological yield (Table 2).
A significant difference has been observed in the harvest index according to treatments and drought stress levels. Drought stress at tillering stage caused a decrease in the harvest index from 43.5 to 41.5 while drought stress at anthesis stage dipped to 36.5. The maximum HI values were recorded in plots receiving T3 (NBC + BR). A statistically significant interactive effect of both factors was reported on the wheat harvest index (Table 2).
Physiological and biochemical attributes
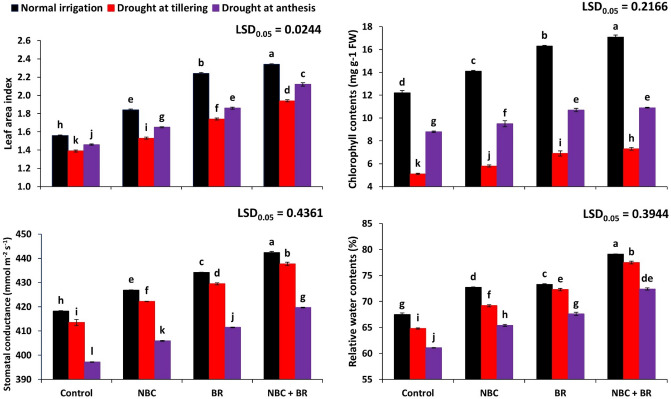
A significant difference has been observed in leaf area index (LAI) according to treatments and drought stress levels (Fig. 1). Drought stress at tillering stage significantly decreased LAI from 1.56 to 1.39 while at anthesis stage, it decreased up to 1.46. Plots receiving T3 (NBC + BR) resulted in the maximum LAI (2.34) of wheat plants. Statistically significant (p ≤ 0.001) interactive effect of both factors was reported on wheat LAI (Table 2). A significant difference has been observed in stomatal conductance according to treatments and drought stress levels (Fig. 1). Drought stress at tillering stage resulted in the minimum stomatal conductance (397.2). The combined application of NBC + BR resulted in the maximum stomatal conductance (442.4) under normal conditions while it was 419.7 under drought stress at the anthesis stage. Statistically significant (p ≤ 0.001) interactive effect of both factors was reported on wheat stomatal conductance (Table 2).
Figure 1.
Effect of nano-biochar (NBC) and brassinosteroids (BR) on wheat leaf area index, chlorophyll contents, relative water contents, and stomatal conductance under drought stress. Bars with different letters are significantly different according to the least significant difference (LSD) test at a 0.05 probability level.
A significant difference has been observed in chlorophyll contents according to treatments and drought stress levels (Fig. 1). Drought stress at tillering stage caused a significant decrease in chlorophyll contents from 12.2 to 5.1 while drought stress at anthesis stage decreased it to 8.8. Plots receiving T3 (NBC + BR) resulted in the maximum chlorophyll contents (16.3) under normal conditions. Statistically significant (p ≤ 0.001) interactive effect of both factors was reported on wheat chlorophyll contents (Table 2). A significant difference has been observed in relative water contents according to treatments and drought stress levels (Fig. 1). A similar decrease with drought stress at tillering and anthesis stages. The maximum relative water contents (79.1) were recorded in plots receiving T3 (NBC + BR). Statistically significant (p ≤ 0.001) interactive effect of both factors was reported on wheat relative water contents (Table 2).
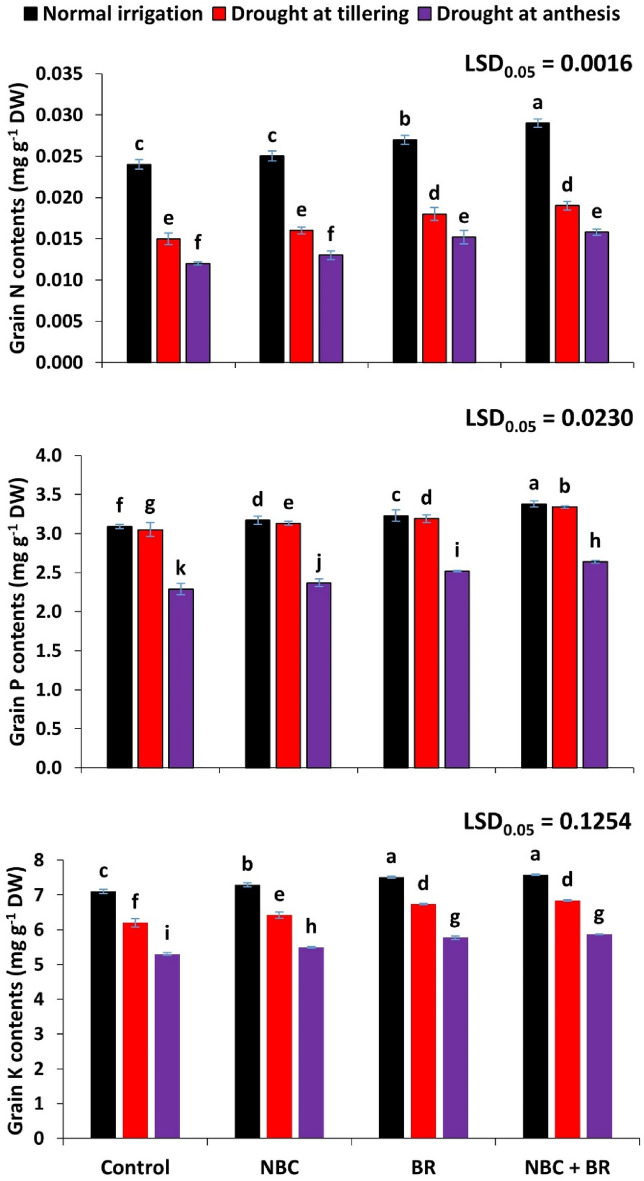
Drought stress and treatments had a significant effect on the nitrogen contents of wheat grain (Fig. 2). Statistically maximum N contents were reported at D0 (0.029 mg g–1) at T3 (NBC + BR) and the lowest contents were recorded in D2 (0.012) at T0. Drought stress at the anthesis stage caused a 50% reduction in nitrogen uptake which was recovered (31%) at co-application of NBC + BR (0.026).
Figure 2.
Effect of nano-biochar (NBC) and brassinosteroids (BR) wheat grain nitrogen (N), phosphorous (P), and potassium (K) contents under drought stress. Bars with different letters are significantly different according to the least significant difference (LSD) test at a 0.05 probability level.
Phosphorous content showed a significant effect at drought stress and different amendments (Fig. 2). Statistically maximum P contents were reported at D0 (3.38) at T3 (NBC + BR) and the lowest contents were recorded in D2 (2.29 mg g–1) at T0 (control). Drought stress caused a 25% reduction in P content at D2 which was mitigated through NBC and BR application. Results showed that 15% P content was recovered at D2 as NBC and BR co-applied (T3).
Results regarding K accumulation in grains indicated that enhanced accumulation occurred in the control treatment of T1, T2 and T3 (Fig. 2). Drought stress at tillering stage caused a 12% loss while drought stress at anthesis stage caused a 25%. These losses were seen mitigated by 10% at D1 and D2 after the co-application of NBC + BR. Drought stress and treatments had a significant effect on the potassium contents of wheat grain.
Statistically significant (p ≤ 0.001) interactive effect of both factors was reported on phosphorus contents in grains while it was statistically non-significant (p ≤ 0.05) in case of the both nitrogen and potassium contents in grains of wheat (Table 2).
Antioxidant response
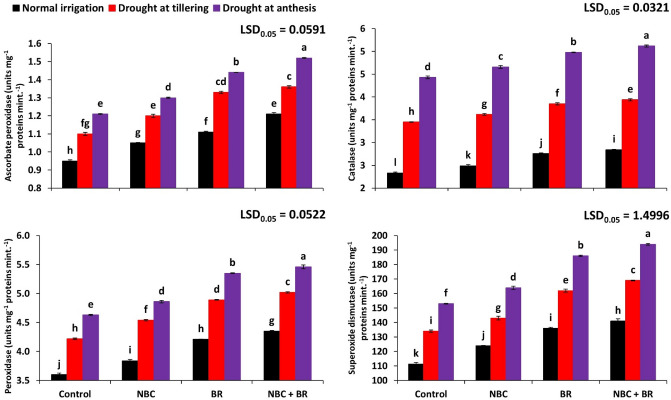
Statistical analysis of ascorbate peroxidase showed that APX activity was significantly controlled by various treatments and drought stress levels during the study (Fig. 3). The maximum rate of APX activity was recorded in D2 at treatment T3 (1.52) and the minimum in D0 (0.95) at T0. The regression graph of drought stress showed the coefficient of regression 97% and 96% at D1 and D2 which indicated the reliability of the study at the field level. Catalase, peroxidase, and superoxide dismutase activities were significantly decreased under drought stress at tillering and anthesis stages (Fig. 3). Maximum CAT, POD, and SOD were recorded at T3 when NBC and BR were co-applied. We recorded 15, 17, and 26% increases in CAT, POD, and SOD respectively under the co-application of NBC and BR at D2 as compared to their control treatments. Similar responses were also recorded at D1 as 14%, 19%, and 26% improvements were seen in CAT, POD, and SOD in respective to the control. Interestingly, the same trend was seen during the control treatment after the application of NBC and BR. The regression coefficient of above 94% in CAT, POD, and SOD showed the reliability of the experiment as well. In the case of the interactive effect of both factors, a statistically significant (p ≤ 0.001) interaction was noted in the case of catalase, peroxidase, and superoxide dismutase while it was statistically non-significant (p ≤ 0.05) in case of the ascorbate peroxidase of wheat (Table 2).
Figure 3.
Effect of nano-biochar (NBC) and brassinosteroids (BR) on ascorbate peroxidase, catalase, peroxidase, and superoxide dismutase activities of Wheat under drought stress. Bars with different letters are significantly different according to the least significant difference (LSD) test at a 0.05 probability level.
Pearson correlation
Pearson correlation was calculated among different growth, physiological, biochemical, and antioxidant activities of wheat under different treatments and drought stress levels (Table 3). Generally, the different growth, yield, physiological, and biochemical attributes of wheat plants were significantly and positively correlated with each other (Table 3). Growth parameters such as plant height, had a negative but significant correlation with ascorbate peroxidase, catalase, peroxidase, and superoxide dismutase while spike length has non-significant relation with ascorbate peroxidase, catalase, peroxidase, and superoxide dismutase. Biochemical parameters such as nitrogen, phosphorus, and potassium contents in grains samples of wheat had a negative but significant correlation with ascorbate peroxidase, catalase, peroxidase, and superoxide dismutase. The biological yield had a negative and non-significant correlation with ascorbate peroxidase, peroxidase, and superoxide dismutase but a significant and positive correlation with catalase. Grain yield, harvest index, and 1000 grain weight had a negative but significant correlation with ascorbate peroxidase, catalase, peroxidase, and superoxide dismutase.
Table 3.
Pearson correlation among different parameters.
| AOX | BY | CAT | CC | TGW | GY | HI | K | LAI | N | NFT | NGS | NSPS | P | PH | POD | RWC | SC | SL | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BY | − 0.1824 ns | ||||||||||||||||||
| CAT | 0.8628*** | − 0.5875*** | |||||||||||||||||
| CC | − 0.2438 ns | 0.8096*** | − 0.4503** | ||||||||||||||||
| TGW | − 0.5801*** | 0.7640*** | − 0.8948*** | 0.4107* | |||||||||||||||
| GY | − 0.4958** | 0.9142*** | − 0.8468*** | 0.6750*** | 0.9399*** | ||||||||||||||
| HI | − 0.8214*** | 0.1839 ns | − 0.8548*** | − 0.0271 ns | 0.7356*** | 0.5650*** | |||||||||||||
| K | − 0.5000** | 0.8749*** | − 0.8533*** | 0.5704*** | 0.9702*** | 0.9854*** | 0.6154*** | ||||||||||||
| LAI | 0.3002 ns | 0.8488*** | − 0.0989 ns | 0.7419*** | 0.3552* | 0.5787*** | − 0.3169 ns | 0.5217** | |||||||||||
| N | − 0.4715** | 0.9281*** | − 0.8071*** | 0.7894*** | 0.8651*** | 0.9709*** | 0.4645** | 0.9315*** | 0.6363*** | ||||||||||
| NFT | − 0.4661** | 0.8907*** | − 0.7474*** | 0.8819*** | 0.7389*** | 0.9092*** | 0.3802* | 0.8474*** | 0.6308*** | 0.9482*** | |||||||||
| NGS | − 0.0523 ns | 0.9333*** | − 0.4926** | 0.6665*** | 0.7273*** | 0.8537*** | 0.1808 ns | 0.8375*** | 0.8221*** | 0.8282*** | 0.7916*** | ||||||||
| NSPS | − 0.2655 ns | 0.9044*** | − 0.6689*** | 0.6218*** | 0.8473*** | 0.9287*** | 0.4137* | 0.9091*** | 0.6752*** | 0.8867*** | 0.8081*** | 0.8814*** | |||||||
| P | − 0.3795* | 0.6884*** | − 0.7417*** | 0.2078 ns | 0.9475*** | 0.8461*** | 0.6708*** | 0.9049*** | 0.3564* | 0.7365*** | 0.5605*** | 0.7162*** | 0.8193*** | ||||||
| PH | − 0.5675*** | 0.8731*** | − 0.8409*** | 0.7489*** | 0.8592*** | 0.9365*** | 0.4962** | 0.9033*** | 0.5609*** | 0.9527*** | 0.8830*** | 0.7559*** | 0.8564*** | 0.7267*** | |||||
| POD | 0.9718*** | − 0.2872 ns | 0.9202*** | − 0.3334* | − 0.6595*** | − 0.5958*** | − 0.8504*** | − 0.5875*** | 0.1999 ns | − 0.5844*** | − 0.5705*** | − 0.1642 ns | − 0.3760* | − 0.4541** | − 0.6530*** | ||||
| RWC | 0.1616 ns | 0.8304*** | − 0.3216 ns | 0.4560** | 0.6512*** | 0.7340*** | 0.0987 ns | 0.7392*** | 0.8136*** | 0.6949*** | 0.5877*** | 0.8653*** | 0.8536*** | 0.7442*** | 0.6146*** | 0.0468 ns | |||
| SC | − 0.0553 ns | 0.8548*** | − 0.5181** | 0.4108* | 0.8224*** | 0.8396*** | 0.3114 ns | 0.8672*** | 0.7056*** | 0.7704*** | 0.6284*** | 0.8791*** | 0.8954*** | 0.8953*** | 0.7185*** | − 0.1520 ns | 0.9545*** | ||
| SL | 0.2338 ns | 0.7752*** | − 0.0931 ns | 0.8321*** | 0.2466 ns | 0.5270** | − 0.3036 ns | 0.4408** | 0.9102*** | 0.6224*** | 0.6932*** | 0.7320*** | 0.6076*** | 0.1831 ns | 0.5107** | 0.1302 ns | 0.6836*** | 0.5380*** | |
| SOD | 0.9720*** | − 0.2703 ns | 0.9231*** | − 0.2726 ns | − 0.6790*** | − 0.5905*** | − 0.8720*** | − 0.5937*** | 0.2315 ns | − 0.5633*** | − 0.5270** | − 0.1588 ns | − 0.3729* | − 0.4938** | − 0.6435*** | 0.9900*** | 0.0387 ns | − 0.1763 ns | 0.179 ns |
where AOX Ascorbate peroxidase; BY Biological yield; CAT Catalase; CC = Chlorophyll contents; TGW 1000 grain weight; GY Grain yield; HI Harvest index; K Potassium contents in grains; LAI Leaf area index; N Nitrogen contents in grains; NFT No. of fertile tillers; NGS No. of grains spike–1; NSPS No. of spikelets spike-1; P Phosphorus contents in grains; PH Plant height; POD Peroxidase; RWC Relative water contents; SC Stomatal conductance; SL Spike length; SOD Superoxide dismutase; NS non-significant at p ≤ 0.05, * = significant at p ≤ 0.05, ** = significant at p ≤ 0.01 and *** = significant at p ≤ 0.001.
Discussion
Water scarcity affects plant height and growth negatively54,55. Under drought stress plant height can be increased by supplying such soil fixation and growth regulating agents that benefit both crop and soil physical and chemical health. Maximum plant height was achieved by adding nano-biochar (NBC) and brassinosteroids (BR) under normal irrigation, the increase in plant height was due to the positive influence of both agents. This study is supported by Raza et al.56 that biochar and plant growth-promoting regulators help in promoting plant height.
Spike length plays a vital role in determining half of the yield-determining attributes greater the spike length more will be the crop yield ultimately as increased spike length produces an increased number of spikelets spike–1 which promotes higher grain formation. Like other growth and development stages of crop water availability affects spike length and to attain maximum spike length crop yield and growth-enhancing amendments are required with normal irrigation. Drought stress had a serious negative relation with wheat spike length. For eliminating the negative impact of drought stress, BR and NBC treatments were tested which showed an increase in spike length by treatment having both NBC and BR. This study is supported by the statements of Almeselmani et al.57 where a 16.61% increase in spike length was observed.
The number of fertile tillers determines the crop yield. Grain production and count increase with the increase in fertile tillers population. Drought stress at any stage of crop growth and development restricts the tiller fertility thus lowering grain count and weight. An increase in fertile tillers was recorded as the result OF NBC + BR under control irrigation. According to Ramraj et al.58 exogenous applications of BR increase the number and degree of fertile tillers and spikes respectively whereas59 biochar increases crop growth and yield attributes.
The number of spikelets spike–1, the number of grains spike–1, and 1000 grain weight are directly related to crop yield. Drought stress causes a reduction in all these attributes thus producing low yield. The number of spikelets spike–1 is reduced under drought stress due to the death of floret sets at the terminal and basal ends whereas the number of grains spike–1 was lowered due to the dehydration of the pollen grains60. 1000 grain weight was also determined significantly by drought stress as the maximum 1000 grain weight was obtained under normal irrigation as floret sets and pollen grain development was boosted which led to a higher 1000 grain weight. NBC + BR application resulted in a higher number of grains spike–1, 1000 grain weight, and the number of spikelets spike–1. According to Wang et al.59, biochar application increases the number of spikelets spike–1 in wheat, the number of grains, and 1000 grain weight in rice and wheat respectively.
The final aim of crop production is to gain maximum grain yield. The grain yield of the crop depends upon several yield attributes and unfortunately drought stress harmed those yield attributes. The occurrence of drought stress at critical growth stages is harmful as reported by Raza et al.61. Drought stress at anthesis stage causes maximum loss. In this study, an increase in grain yield was reported by NBC + BR application under no drought stress. The biological yield represents the dry accumulation by the crop during the entire season. Biological yield and drought stress relation are reported as same as others BY increases with decrease or elimination of drought stress and vice versa.
The crop plant portioning ability of photosynthates towards economical parts is determined by Harvest Index. An increase in the harvest index reflects an improvement in crop growth and development. The lowest harvest index was reported under drought stress at anthesis stages as it lowered grain production and yield whereas NBC and BR application combined under normal irrigation resulted in an increased harvest index. Improvement in grain yield to biomass (HI) due to the improved plant biomass as the result of BR application was stated by Hnilicka et al.62.
Among plant growth and development-promoting nutrients63, nitrogen is the most important and commonly used and required nutrient. Phosphorous and Potassium are also among other nutrients required by plants regularly64–66. Under drought stress, potassium is required by the plants for maintaining the turgidity and osmotic potential whereas under low moisture uptake of P and K is restricted. Combined application of NBC and BR significantly increased NPK contents of grains in wheat. This is because biochar increased organic matter in the soil and improved water retention of sandy loam soil50 which leads to an increase in NPK uptake.
From the results stated above, it is obvious that water stress increased the secretion of ROS in wheat. This overproduction might be to mitigate the prevailing drought stress as stated by56,57. At D1 and D2, APX, CAT, POD, and SOD production was enhanced compared to respective control treatments. Biochar concentrations increased the antioxidant activities in the wheat plants by improving cell growth, and soil–plant water relationship67–69. Nanoparticles increased the POD and APX activity to mitigate the water scarcity situation as reported by70–73. Correlation analysis showed a linear relationship among treatments and recommended the usage of brassinosteroids for increasing stomatal conductance, leaf area index, relative water contents, and chlorophyll contents and for ameliorating the effect of drought stress.
Conclusions
The results showed that the combined application of brassinosteroids (BR) and nano-biochar (NBC) had an ameliorating impact against drought stress and a synergistic impact on the growth, yield, physiological, and biochemical attributes of wheat. Drought stress significantly reduced the growth, yield, physiological, and biochemical attributes of wheat. This stress was ameliorated with the application of BR and NBC alone or combined. The combined application of BR and NBC had a significant and synergistic impact on growth (plant height, spike length, and no. of spikelets spike–1), yield (no. of fertile tillers m–2, grain yield, biological yield, harvest index), physiological (leaf area index, relative water contents, stomatal conductance, and chlorophyll contents) and biochemical attributes (catalase, peroxidase, superoxide dismutase, and phosphorus contents in grains) while non-significant with no. of grains spike–1, 1000 grain weight, nitrogen, and potassium contents in grains and ascorbate peroxidase. In conclusion, the combined application of BR and NBC could ameliorate the negative impacts of drought on growth, yield, physiological, and biochemical attributes of wheat under field conditions. To authenticate the efficacy of tested amendments, more field and laboratory trials involving different crops under different climatic conditions are needed in the future.
Acknowledgements
The authors extend their appreciation to the Researchers supporting project number (RSPD2023R1091), King Saud University, Riyadh, Saudi Arabia.
Author contributions
Conceptualization, M.A.S.R., M.A.I., A.D., R.I., M.U.A., F.M., S.A., B.A., R.M.I., M.N.M. and M.H.R.; Formal analysis, M.A.S.R., M.A.I., A.D., R.I., M.U.A., F.M., S.A., B.A., R.M.I., M.N.M., and M.H.R.; Funding acquisition, M.S.A, M.S.E, F.G, M.H.R., R.M.I. and M.N.M..; Investigation, M.A.I. and R.I.; Methodology, M.A.S.R., M.A.I., A.D., R.I., M.U.A., F.M., S.A., R.M.I., M.N.M., and M.H.R.; Project administration; M.S.A, M.S.E, F.G, M.A.S.R.; Software, M.A.S.R., M.A.I., A.D., R.I., M.U.A., F.M., S.A., B.A., R.M.I., M.N.M., and M.H.R.; Supervision, M.A.S.R.; Validation, M.A.S.R., M.A.I., A.D., R.I., M.S.A, M.S.E, F.G, M.U.A., F.M., S.A., B.A., R.M.I., M.N.M., and M.H.R.; Visualization, M.A.S.R., M.A.I., A.D., R.I., M.U.A., F.M., S.A., B.A., R.M.I., M.N.M., and M.H.R.; Writing – original draft, S.A., B.A.; Writing – review & editing, M.A.S.R., M.S.A, M.S.E, F.G, M.A.I., A.D., R.I., M.U.A., F.M., S.A., B.A., R.M.I., M.N.M., and M.H.R. All the authors read and approved the final manuscript.
Funding
Researchers supporting project number (RSPD2023R1091).
Data availability
All data generated or analyzed during this study are included in this submitted article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Muhammad Aown Sammar Raza, Email: aown.sammar@iub.edu.pk.
Muhammed Habib ur Rahman, Email: mhabibur@uni-bonn.de.
References
- 1.Bukhari SABH, et al. Drought stress alleviation by potassium-nitrate-containing chitosan/montmorillonite microparticles confers changes in Spinacia oleracea L. Sustainability. 2021;13:9903. [Google Scholar]
- 2.Dianatmanesh M, et al. Yield and yield components of common bean as influenced by wheat residue and nitrogen rates under water deficit conditions. Environ. Technol. Innov. 2022;28:102549. [Google Scholar]
- 3.Ali J, et al. Biochemical response of Okra (Abelmoschus esculentus L.) to Selenium (Se) under drought stress. Sustainability. 2023;15:5694. [Google Scholar]
- 4.Sharma S, et al. Regulation of the Calvin cycle under abiotic stresses: An overview. Plant Life Under Chang. Environ. 2020 doi: 10.1016/B978-0-12-818204-8.00030-8. [DOI] [Google Scholar]
- 5.Talaat NB. 24-Epibrassinolide and spermine combined treatment sustains maize (Zea mays L.) drought tolerance by improving photosynthetic efficiency and altering phytohormones profile. J. Soil Sci. Plant Nutr. 2020;20:516–529. [Google Scholar]
- 6.Manaa A, et al. Photosynthetic performance of quinoa (Chenopodium quinoa Willd.) after exposure to a gradual drought stress followed by a recovery period. Biochim. Biophys. Acta (BBA) Bioenerg. 2021;1862:148383. doi: 10.1016/j.bbabio.2021.148383. [DOI] [PubMed] [Google Scholar]
- 7.Afridi MS, et al. New opportunities in plant microbiome engineering for increasing agricultural sustainability under stressful conditions. Front. Plant Sci. 2022;13:1–22. doi: 10.3389/fpls.2022.899464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Afridi MS, et al. Plant microbiome engineering: hopes or hypes. Biology (Basel) 2022;11:1782. doi: 10.3390/biology11121782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Huqail AA, et al. Efficacy of priming wheat (Triticum aestivum) seeds with a benzothiazine derivative to improve drought stress tolerance. Funct. Plant Biol. 2023 doi: 10.1071/FP22140. [DOI] [PubMed] [Google Scholar]
- 10.Asma, et al. Alleviating effects of salicylic acid spray on stage-based growth and antioxidative defense system in two drought-stressed rice (Oryza sativa L.) cultivars. Turk. J. Agric. For. 2023;47:79–99. [Google Scholar]
- 11.Kheirizadeh Arough Y, Seyed Sharifi R, Seyed Sharifi R. Bio fertilizers and zinc effects on some physiological parameters of triticale under water-limitation condition. J. Plant Interact. 2016;11:167–177. [Google Scholar]
- 12.Dola DB, et al. Nano-iron oxide accelerates growth, yield, and quality of Glycine max seed in water deficits. Front. Plant Sci. 2022;13:992535. doi: 10.3389/fpls.2022.992535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azeem M, et al. Salinity stress improves antioxidant potential by modulating physio-biochemical responses in Moringa oleifera Lam. Sci. Rep. 2023;13:1–17. doi: 10.1038/s41598-023-29954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salam A, et al. Nano-priming against abiotic stress: A way forward towards sustainable agriculture. Sustainability. 2022;14:14880. [Google Scholar]
- 15.Saleem A, et al. Iron sulfate (FeSO4) improved physiological attributes and antioxidant capacity by reducing oxidative stress of Oryza sativa L. cultivars in alkaline soil. Sustainability. 2022;14:16845. [Google Scholar]
- 16.Shahzadi E, et al. Silicic and ascorbic acid induced modulations in photosynthetic, mineral uptake, and yield attributes of mung bean (Vigna radiata L. Wilczek) under ozone stress. ACS Omega. 2023 doi: 10.1021/acsomega.3c00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jangid KK, Dwivedi P. Physiological and biochemical changes by nitric oxide and brassinosteroid in tomato (Lycopersicon esculentum Mill.) under drought stress. Acta Physiol. Plant. 2017;39:1–10. [Google Scholar]
- 18.Gruszka D, et al. Barley brassinosteroid mutants provide an insight into phytohormonal homeostasis in plant reaction to drought stress. Front. Plant Sci. 2016;7:1824. doi: 10.3389/fpls.2016.01824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deb A, Grewal RK, Kundu S. Regulatory cross-talks and cascades in rice hormone biosynthesis pathways contribute to stress signaling. Front. Plant Sci. 2016;7:1303. doi: 10.3389/fpls.2016.01303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vardhini BV, Anjum NA. Brassinosteroids make plant life easier under abiotic stresses mainly by modulating major components of antioxidant defense system. Front. Environ. Sci. 2015;2:67. [Google Scholar]
- 21.Li S, Zheng H, Lin L, Wang F, Sui N. Roles of brassinosteroids in plant growth and abiotic stress response. Plant Growth Regul. 2021;93:29–38. [Google Scholar]
- 22.Elahi NN, et al. Foliar application of Gibberellin alleviates adverse impacts of drought stress and improves growth, physiological and biochemical attributes of Canola (Brassica napus L.) Sustainability. 2022;15:78. [Google Scholar]
- 23.Haubrick LL, Assmann SM. Brassinosteroids and plant function: Some clues, more puzzles. Plant Cell Environ. 2006;29:446–457. doi: 10.1111/j.1365-3040.2005.01481.x. [DOI] [PubMed] [Google Scholar]
- 24.Deng B, et al. Drought stress and Acacia seyal biochar effects on sorghum gas exchange and yield: A greenhouse experiment. Agric. Nat. Resour. 2019;53:573–580. [Google Scholar]
- 25.Alharby HF, Fahad S. Melatonin application enhances biochar efficiency for drought tolerance in maize varieties: Modifications in physio-biochemical machinery. Agron. J. 2020;112:2826–2847. [Google Scholar]
- 26.Ullah N, et al. Appraisal for organic amendments and plant growth-promoting rhizobacteria to enhance crop productivity under drought stress: A review. J. Agron. Crop Sci. 2021;207:783–802. [Google Scholar]
- 27.Elkhlifi Z, et al. Potential role of biochar on capturing soil nutrients, carbon sequestration and managing environmental challenges: A review. Sustainability. 2023;15:2527. [Google Scholar]
- 28.Bassouny M, Abbas MHH. Role of biochar in managing the irrigation water requirements of maize plants: The pyramid model signifying the soil hydro-physical and environmental markers. Egypt. J. Soil Sci. 2019;59:99–115. [Google Scholar]
- 29.Javed MA, et al. Positive and Negative Impacts of Biochar on Microbial Diversity BT—Sustainable Agriculture Reviews 61: Biochar to Improve Crop Production and Decrease Plant Stress under a Changing Climate. Springer International Publishing; 2023. pp. 311–330. [Google Scholar]
- 30.Ahmad R, Hadi F, Jan AU, Ditta A. Straw incorporation enhances drought stress tolerance but at the same time increases bioaccumulation of heavy metals under contaminated soil in Oryza sativa L. Sustainability. 2022;14:10578. [Google Scholar]
- 31.French E, Iyer-Pascuzzi AS. A role for the gibberellin pathway in biochar-mediated growth promotion. Sci. Rep. 2018;8:5389. doi: 10.1038/s41598-018-23677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jan AU, Hadi F, Ditta A, Suleman M, Ullah M. Zinc-induced anti-oxidative defense and osmotic adjustments to enhance drought stress tolerance in sunflower (Helianthus annuus L.) Environ. Exp. Bot. 2022;193:104682. [Google Scholar]
- 33.Abdelhafez AA, et al. Eco-friendly production of biochar via conventional pyrolysis: Application of biochar and liquefied smoke for plant productivity and seed germination. Environ. Technol. Innov. 2021;22:101540. [Google Scholar]
- 34.Tolba M, et al. Integrated management of K-additives to improve the productivity of zucchini plants grown on a poor fertile sandy soil. Egypt. J. Soil Sci. 2021;61:355–365. [Google Scholar]
- 35.Ali S, et al. Biochar soil amendment on alleviation of drought and salt stress in plants: A critical review. Environ. Sci. Pollut. Res. 2017;24:12700–12712. doi: 10.1007/s11356-017-8904-x. [DOI] [PubMed] [Google Scholar]
- 36.Ullah N, et al. Integrated effect of algal biochar and plant growth promoting rhizobacteria on physiology and growth of maize under deficit irrigations. J. Soil Sci. Plant Nutr. 2020;20:346–356. [Google Scholar]
- 37.Farid IM, et al. Co-composted biochar derived from rice straw and sugarcane bagasse improved soil properties, carbon balance, and zucchini growth in a sandy soil: A trial for enhancing the health of low fertile arid soils. Chemosphere. 2022;292:133389. doi: 10.1016/j.chemosphere.2021.133389. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, et al. Global irrigation contribution to wheat and maize yield. Nat. Commun. 2021;12:1235. doi: 10.1038/s41467-021-21498-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rafiq M, et al. Improving quantitative and qualitative characteristics of wheat (Triticum aestivum L.) through nitrogen application under semiarid conditions. Phyton (B Aires) 2023;92:1001–1017. [Google Scholar]
- 40.Saini A, et al. Impact of cultivation practices and varieties on productivity, profitability, and nutrient uptake of rice (Oryza sativa L.) and wheat (Triticum aestivum L.) cropping system in India. Agriculture. 2022;12:1678. [Google Scholar]
- 41.Dhillon J, et al. Nitrogen management impact on winter wheat grain yield and estimated plant nitrogen loss. Agron. J. 2020;112:564–577. [Google Scholar]
- 42.Salim N, Raza A. Nutrient use efficiency (NUE) for sustainable wheat production: a review. J. Plant Nutr. 2020;43:297–315. [Google Scholar]
- 43.Tahir O, et al. Evaluation of agronomic performance and genetic diversity analysis using simple sequence repeats markers in selected wheat lines. Sustainability. 2023 doi: 10.3390/su15010293. [DOI] [Google Scholar]
- 44.Lu Y, Yan Z, Li L, Gao C, Shao L. Selecting traits to improve the yield and water use efficiency of winter wheat under limited water supply. Agric. Water Manag. 2020;242:106410. [Google Scholar]
- 45.Zhang L, et al. Tiller development affected by nitrogen fertilization in a high-yielding wheat production system. Crop Sci. 2020;60:1034–1047. [Google Scholar]
- 46.Wang Y, et al. Efficient physiological and nutrient use efficiency responses of maize leaves to drought stress under different field nitrogen conditions. Agronomy. 2020;10:523. [Google Scholar]
- 47.Watson DJ. Comparative physiological studies on the growth of field crops: I. Variation in net assimilation rate and leaf area between species and varieties, and within and between years. Ann. Bot. 1947;11:41–76. [Google Scholar]
- 48.Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barrs H, Weatherley P. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962;15:413. [Google Scholar]
- 50.Wolf B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 1982;13:1035–1059. [Google Scholar]
- 51.Hwang S-Y, Lin H-W, Chern R-H, Lo HF, Li L. Reduced susceptibility to waterlogging together with high-light stress is related to increases in superoxide dismutase and catalase activities in sweet potato. Plant Growth Regul. 1999;27:167–172. [Google Scholar]
- 52.Kar M, Mishra D. Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 1976;57:315–319. doi: 10.1104/pp.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steel RGD, Torrie JH. Principles and Procedures of Statistics, a Biometrical Approach. McGraw-Hill Kogakusha Ltd; 1980. [Google Scholar]
- 54.Yasmeen S, et al. Melatonin as a foliar application and adaptation in lentil (Lens culinaris Medik.) crops under drought stress. Sustainability. 2022;14:16345. [Google Scholar]
- 55.Wahab A, et al. Plants’ physio-biochemical and phyto-hormonal responses to alleviate the adverse effects of drought stress: A comprehensive review. Plants. 2022;11:1620. doi: 10.3390/plants11131620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raza MAS, et al. Drought ameliorating effect of exogenous applied cytokinin in wheat. Pak. J. Agric. Sci. 2020;57:725–733. [Google Scholar]
- 57.Almeselmani M, Deshmukh PS, Sairam RK, Kushwaha SR, Singh TP. Protective role of antioxidant enzymes under high temperature stress. Plant Sci. 2006;171:382–388. doi: 10.1016/j.plantsci.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 58.Ramraj VM, et al. Effects of 28-homobrassinolide on yields of wheat, rice, groundnut, mustard, potato and cotton. J. Agric. Sci. 1997;128:405–413. [Google Scholar]
- 59.Wang Y, Wei Y, Sun J. Biochar application promotes growth parameters of soybean and reduces the growth difference. Commun. Soil Sci. Plant Anal. 2016;47:1493–1502. [Google Scholar]
- 60.Aslam M, et al. Mechanisms of drought resistance. In: Aslam M, Maqbool MA, Cengiz R, et al., editors. Drought Stress in Maize (Zea mays L.) Effects, Resistance Mechanisms, Global Achievements and Biological Strategies for Improvement. Springer; 2015. pp. 19–36. [Google Scholar]
- 61.Raza MAS, et al. Integrating biochar, rhizobacteria and silicon for strenuous productivity of drought stressed wheat. Commun. Soil Sci. Plant Anal. 2021;52:338–352. [Google Scholar]
- 62.Hnilička F, Hniličková H, Martinková J, Bláha L. The influence of drought and the application of 24-epibrassinolide on the formation of dry matter and yield in wheat. Cereal Res. Commun. 2007;35:457–460. [Google Scholar]
- 63.Zafar M, et al. Application of zinc, iron and boron enhances productivity and grain biofortification of Mungbean. Phyton (B. Aires) 2023;92:983–999. [Google Scholar]
- 64.Adnan M, et al. Comparative efficacy of phosphorous supplements with phosphate solubilizing bacteria for optimizing wheat yield in calcareous soils. Sci. Rep. 2022;12:1–17. doi: 10.1038/s41598-022-16035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahmad M, et al. Managing phosphorus availability from organic and inorganic sources for optimum wheat production in calcareous soils. Sustainablity. 2022;14:7669. [Google Scholar]
- 66.Khan J, Saeed I, Fayaz M, Zada M, Jan D. Perceived overqualification? Examining its nexus with cyberloafing and knowledge hiding behaviour: Harmonious passion as a moderator. J. Knowl. Manag. 2023;27:460–484. [Google Scholar]
- 67.Parveen A, et al. Promotion of growth and physiological characteristics in water-stressed Triticum aestivum in relation to foliar-application of salicylic acid. Water. 2021;13:1316. [Google Scholar]
- 68.Mustafa F, et al. Validation of gosat and oco-2 against in situ aircraft measurements and comparison with carbontracker and geos-chem over Qinhuangdao, China. Remote Sens. 2021;13:899. [Google Scholar]
- 69.Shahzaman M, et al. Remote sensing indices for spatial monitoring of agricultural drought in South Asian countries. Remote Sens. 2021;13:2059. [Google Scholar]
- 70.Arough YK, Sharifi RS, Sedghi M, Barmaki M. Effect of zinc and bio fertilizers on antioxidant enzymes activity, chlorophyll content, soluble sugars and proline in triticale under salinity condition. Not. Bot. Horti Agrobot. Cluj-Napoca. 2016;44:116–124. [Google Scholar]
- 71.Chiahi N, Bouloudenine M, Daira NH, Guerfi N, Brinis L. L ’ impact des nanoparticules ZnO sur les paramètres physiologiques et biochimiques chez le blé dur (Triticum turgidum) J. New Sci. 2016;27:1549–1558. [Google Scholar]
- 72.Rahman, M. H. et al. Multi‑model projections of future climate and climate change impact uncertainty assessment for cotton production in Pakistan. Agric. For. Meteorol.253, 94–113 (2018).
- 73.Habib, M. U. et al. Impact of in-field soil heterogeneity on biomass and yield of winter triticale in an intensively cropped hummocky landscape under temperate climate conditions. Precis. Agric.11, 1–27 (2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this submitted article.