Abstract
a) Objectives
Urinalysis is one of the most common laboratory screening tests to detect problems in the renal and urinary system; however, they cannot detect atypical cells (Atyp.Cs). The Sysmex UF-5000, a fully automated urine particle analyzer, can detect Atyp.Cs via its Atyp.C parameter. This study aimed to compare the clinical value of the Atyp.C parameter with that of urine sediment microscopy.
b) Method
A total of 471 leftover urine samples were submitted to the Department of Clinical Laboratory at the University of Tokyo Hospital for urinalysis by manual sediment microscopy examination and UF-5000 Atyp.C analysis.
c) Result
Of 471 submitted samples, 117 were positive for Atyp.Cs by urine sediment and 354 samples were negative. The histological subtypes of the Atyp.Cs included 105 cases of suspected urothelial carcinoma cells, 10 suspected squamous carcinoma cells, and 2 of suspected adenocarcinoma cells. The Atyp.C values for the Atyp.C-positive and -negative groups were 2.64 ± 0.69 and 0.38 ± 0.16, respectively. The optimal Atyp.C cutoff value determined by the receiver operating characteristic curve analysis was 0.4/μL. The area under the curve was 0.856, with a sensitivity of 79.5% and specificity of 85.1%. Atyp.C values of the UF-5000 showed high predictive performance for Atyp.C-positive specimens identified by urine sediment microscopy.
d) Conclusions
This study shows that a combination of UF-5000 analysis and microscopic examination of urine sediment improves Atyp.C detection in urine sediment analysis. These results suggest that Atyp.C measured by UF-5000 could be a useful screening parameter in routine testing of urine samples.
Keywords: Atypical cells, Fully automated urine particle analyzer, UF-5000, Urine sediment, Urinalysis
Graphical abstract
Highlights
-
•
The optimal UF-5000 Atyp.C cutoff value for microscopic atypical cells was 0.4/μL.
-
•
The Atyp.C AUC was 0.856, with a sensitivity of 79.5% and specificity of 85.1%.
-
•
UF-5000 Atyp.C showed high predictive performance for microscopic atypical cells.
-
•
UF-5000 Atyp.C could be a useful screening parameer in urine testing.
Abbreviations
- Atyp.C
atypical cell
- EC
Epithelial cell
- HPF
High power field
- RBC
Red blood cell
- WBC
White blood cell
- ROC
Receiver operating characteristic curve
1. Introduction
Urinalysis is a major screening test in clinical laboratories and is essential for diagnosing and monitoring of renal and urinary system diseases [1]. Recent advancements in automated urine particle analyzers have reduced the number of microscopic examinations, shortened the turnaround time, improved accuracy and reliability, and reduced costs [[2], [3], [4]]. According to their measurement principles, urine particle analyzers are broadly classified into flow cytometry and digital image-based types [5]. Flow cytometry analyzers show good correlation for blood cells with microscopy, which is the golden standard of urine sediment analysis [6,7]. Besides, bacterial count by flow cytometry analyzers shows good diagnostic performance for urinary tract infection [8]. Some studies have suggested it could be used for ruling out urinary tract infection [9]. However, until now, no parameters indicated an atypical cell (Atyp.C) count [10,11], which relies at present on microscopic examination of urine sediment by specially trained staff [12].
The UF-5000 fully automated urine particle analyzer is a flow cytometry-based analyzer that uses a blue semiconductor laser [13]. The specific staining of cellular nucleic acids with special fluorescent stains and analysis of signal information using a new analysis technique have enabled the estimation of nucleic acid content in cells, which are classified as epithelial cells (ECs) or Atyp.Cs. The UF-5000 measures the Atyp.C count parameter as Atyp.C. If microscopic examination of urine sediment is performed, it is believed that Atyp.C values can be measured with high accuracy in routine testing.
This study aimed to compare the clinical usefulness of the UF-5000's Atyp.C values with that of urine sediment microscopy.
2. Materials and methods
2.1. Specimens
The samples were 471 leftover urine test specimens that had been submitted for urine analysis by the Department of Urology to the Department of Clinical Laboratory at the University of Tokyo Hospital between April 2015 and November 2016. Urine samples were tested within 4 h of collection. In total, 117 samples were found to be positive and 354 samples were negative for Atyp.Cs by microscopic examination of the urine sediment. All 117 Atyp.C-positive samples were finally defined as cancer via histological analysis. Among the 117 Atyp.C-positive samples, 34 were negative for occult blood (–or ±) and 83 were positive (≥1+) (Table 1). The histological subtypes of Atyp.Cs included 105 cases of suspected urothelial carcinoma cells, 10 of suspected squamous carcinoma cells, and 2 of suspected adenocarcinoma cells. Among the 353 Atyp.C-negative samples, 215 were negative for occult blood, and 138 samples were positive.
Table 1.
Presence of atypical cells in collected specimens in relation to the presence of occult blood (n = 470).
| Occult blood | Urine sediment atypical cell assessment |
|
|---|---|---|
| Negative | Positive | |
| – | 168 | 17 |
| +− | 47 | 17 |
| 1+ | 43 | 13 |
| 2+ | 38 | 18 |
| 3+ | 56 | 52 |
| 4+ | 1 | 0 |
| Total | 353 | 117 |
2.2. Analyzer
The fully automated UF-5000 (Sysmex Corporation, Kobe, Japan) is a flow cytometry-based urine particle analyzer with a blue semiconductor laser. The aspirated urine sample is divided into channels that classify components as with nucleic acids (CR channel) and without nucleic acids (SF channel). In the CR channel, nucleic acids are specifically stained, and the laser light is projected through the liquid stream containing the stained cells to obtain various types of signal information that reflects the size and stainability, nucleic acid content, and intensity of birefringence coupled with the complexity of the internal structure of particles. Particles classified as Atyp.C are mainly those with high nucleic acid content detected in the CR channel.
2.3. Microscopic examination of urine sediment
The urine sediment was examined based on the guidelines of the Japanese Association of Medical Technologists using optical microscopy (OLYMPUS, Tokyo, Japan) [7]. Atyp.Cs was confirmed by double-checking by laboratory technicians, who are certified for urinalysis. The Atyp.Cs per high-power field (HPF) were counted using a method devised for this study, and the samples were stratified into groups as follows: 1+ (C0), 1–10 cells; 2+ (C1), 11–30 cells; and 3+ (C2), >30 cells.
2.4. Statistical analysis
JMP 14.2.0 (SAS Institute Inc.) was used for the statistical analysis of the obtained data. One-way analysis of variance was performed to compare Atyp.C values (based on Atyp.C counts) obtained in the Atyp.C-positive and Atyp.C-negative groups. Receiver operating characteristic (ROC) curve analysis was used to determine the predictive performance and optimal cutoff for Atyp.Cs. The associations between the UF-5000's Atyp.C cutoff values and the counts of red blood cells (RBCs), white blood cells (WBCs), renal tubular ECs, urothelial cells, squamous ECs, and intracytoplasmic inclusion-bearing cells determined by urine sediment microscopy were evaluated. The relevance of the measured values of each parameter obtained from the UF-5000 was also evaluated.
3. Results
3.1. Comparison of Atyp.C values
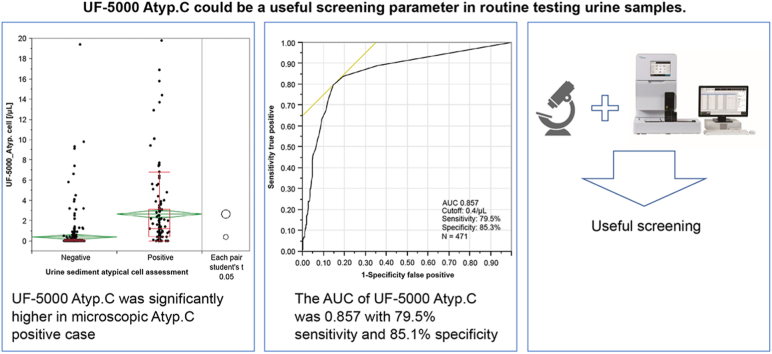
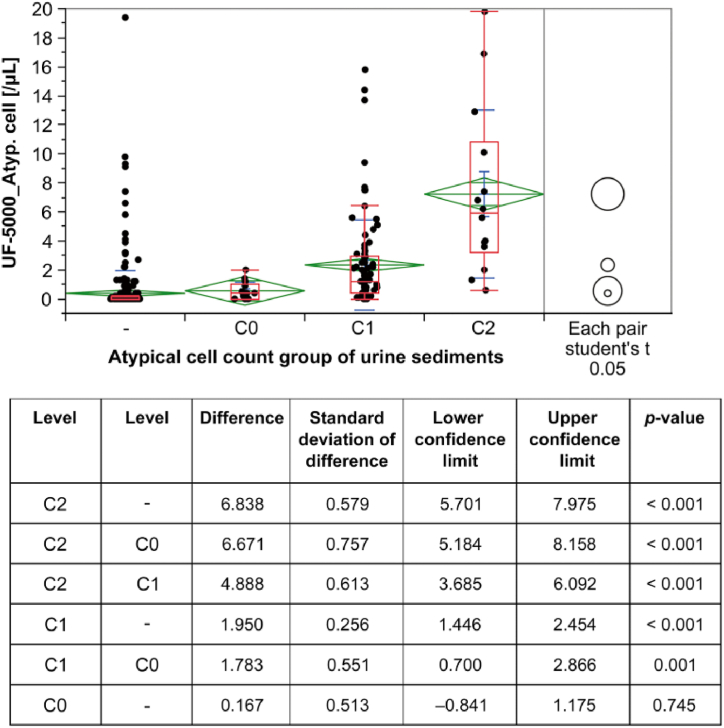
The Atyp.C values of the Atyp.C-positive and -negative sample cohorts were 2.64 ± 0.69 and 0.38 ± 0.16, respectively (Fig. 1). A comparison of sample categories reflecting increasing numbers of Atyp.C (C0, C1, and C2)—investigated by automated urinalysis and sediment microscopy—confirmed a correlation between increased Atyp.C values and increased numbers of Atyp.Cs (Fig. 2). According to the histological classification of Atyp.Cs, the Atyp.C value was lower in the group with suspected squamous carcinoma cells than in the groups with Atyp.Cs of other histological types (Table 2).
Fig. 1.
Comparison of Atyp.C values in Atyp.C-negative and Atyp.C-positive sample cohorts. Atyp.C, atypical cell.
Fig. 2.
Atyp.C values of different Atyp.C count groups.
Table 2.
Differences in Atyp.C values according to different cancer types.
| Suspected urothelial carcinoma | Suspected squamous cell carcinoma | Suspected adenocarcinoma | ||
|---|---|---|---|---|
| N | 105 | 10 | 2 | |
| Mean | 2.880 | 0.270 | 2.250 | |
| Minimum | 0.000 | 0.000 | 0.600 | |
| Maximum | 19.800 | 1.000 | 3.900 | |
| Median | 1.400 | 0.150 | 2.250 | |
| True positive | 87 | 4 | 2 | |
| False negative | 18 | 6 | 0 |
| Cancer type | Cancer type | Difference | Standard deviation of difference | Lower confidence limit | Upper confidence limit | p-value |
|---|---|---|---|---|---|---|
| UC | SCC | 2.607 | 1.230 | 0.170 | 5.044 | 0.036* |
| AC | SCC | 1.980 | 2.879 | −3.723 | 7.683 | 0.493 |
| UC | AC | 0.627 | 2.653 | −4.629 | 5.883 | 0.814 |
AC, adenocarcinoma; SCC, squamous cell carcinoma; UC, urothelial carcinoma.
A comparison of Atyp.C values obtained by automated detection on the UF-5000 and Atyp.C counts obtained by manual sediment microscopy confirmed a correlation between increased Atyp.C values and increased numbers of the four different Atyp.C count groups. – (<1 cell), C0 (1–10 cells), C1 (11–30 cells), and C2 (>30 cells).
Atyp.C, atypical cell.
3.2. Atyp.C values and ROC curves for Atyp.Cs
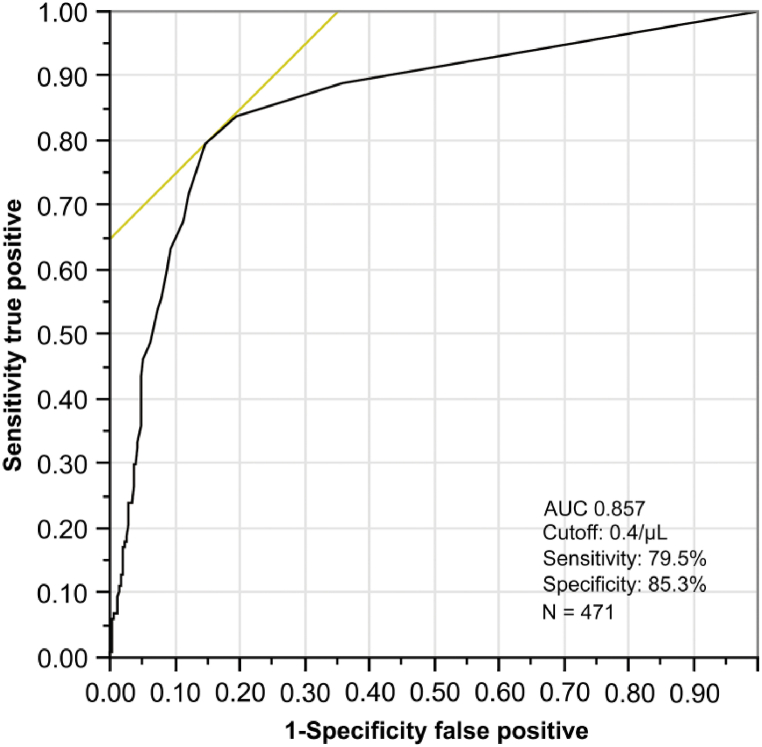
The optimal cutoff value of Atyp.C determined by ROC analysis was 0.4/μL. The area under the curve was 0.856, the sensitivity was 79.5%, and the specificity was 85.1% (Fig. 3).
Fig. 3.
Predictive power of the Atyp.C parameter.
The receiver operating characteristic curve (ROC) for UF-5000 Atyp.C counts in comparison with manual sediment microscopy of 471 samples, resulting in an area under the curve of 0.857. Urine specimens were considered positive for Atyp.Cs if Atyp.C ≥ 0.4/μL.
Atyp.C, atypical cell.
3.3. Factors responsible for Atyp.C values ≥ 0.4/μL in the Atyp.C-negative group
We analyzed different urine sediment components (RBCs, WBCs, squamous ECs, urothelial cells, renal tubular ECs, and intracytoplasmic inclusion-bearing cells) in 52 samples of the Atyp.C-negative group with Atyp.C values ≥ 0.4/μL. Table 3 shows the mean Atyp.C values of the negative or positive results for the sediment components by urine sediment microscopy. Herein, 0–1/HPF for RBCs and 1–4/HPF for WBCs were defined as negative in the results of urine sediment microscopy. For other components, 0–1/HPF was defined as negative in the urine sediment microscopy results. The highest mean Atyp.C value by the UF-5000 was 2.95 when WBCs were negative in urine sediment microscopy. The lowest mean Atyp.C value by the UF-5000 was 0.84 when no intracytoplasmic inclusion-bearing cells were detected by urine sediment microscopy. The mean values were high (range, 2.47–2.95 cells/μL) when respective results by urine sediment microscopy were negative, except in cases with negative results for intracytoplasmic inclusion-bearing cells.
Table 3.
Mean UF-5000 Atyp.C value of the microscopic Atyp.C-negative group when the samples are positive and negative for individual urine sediment components.
| Results from urine sediment microscopy | Mean Atyp.C value from UF-5000 | p-value | |
|---|---|---|---|
| Red blood cells | Positive | 1.07 | 0.169 |
| Negative*1 | 2.79 | ||
| White blood cells | Positive | 1.81 | 0.256 |
| Negative*1 | 2.95 | ||
| Squamous cells | Positive | 1.61 | 0.293 |
| Negative*2 | 2.75 | ||
| Urothelial cells | Positive | 1.63 | 0.684 |
| Negative*2 | 2.47 | ||
| Renal tubular epithelial cells | Positive | 0.83 | 0.156 |
| Negative*2 | 2.76 | ||
| Intracytoplasmic inclusion-bearing cells | Positive | 4.01 | 0.006* |
| Negative*2 | 0.84 | ||
Negative*1: 0–1/HPF and 1–4/HPF.
Negative*2: 0–1/HPF.
4. Discussion
Previous studies have compared the usefulness of the Atyp.C parameter with that of cytological and histological findings. To the best of our knowledge, this study is the first to compare the clinical usefulness of Atyp.C values with that of microscopic examination of urine sediment. We examined 117 Atyp.C-positive cases determined by urine sediment, which were finally defined as cancer via histological analysis. Our findings showed that Atyp.Cs could be detected using the UF-5000 Atyp.C parameter when the number of Atyp.Cs was high; however, they were difficult to detect when the number was low. As regards the relationship between the Atyp.C values and tissue classification, the Atyp.C value tended to be low when the sample contained squamous cell carcinoma cells. Thus, re-examination rules for detecting such cells must be established.
In Japan, reporting the results of urine particle analyzer tests alone for all requested samples is considered unacceptable because of reliability issues. Therefore, all clinical laboratories use a combination of the results from urine particle analyzers and microscopic examination of urine sediment according to their scheme of operation for routine testing [14].
A detailed microscopic examination of urine sediment is conducted to detect atypical bladder cancer cells [15]. Despite the demand for urine particle analyzers to detect Atyp.Cs, the analyzers did not have an Atyp.C-related parameter until now. Hematuria has been suggested as a screening marker for bladder cancer [16,17]. Although >80% of patients with bladder cancer have hematuria, if we include both gross and microscopic hematuria, only 13%–28% of the patients with asymptomatic gross hematuria as their chief complaint are diagnosed with bladder cancer [18]. Although hematuria is often observed in specimens that show Atyp.Cs in urine sediment microscopy, the bleeding is intermittent and not persistent. Therefore, samples may not always be positive for occult blood in dipstick tests. In this study, we detected Atyp.Cs in 34 (29%) patients with an occult blood score <1+. Furthermore, diseases other than bladder cancer can present with hematuria [19]. Thus, hematuria is a sign of the potential presence of Atyp.Cs, but it is not a satisfactory screening marker [20].
In this study, the UF-5000 is a urine particle analyzer equipped with a parameter called Atyp.C that indicates the presence of Atyp.Cs [21]. UF-5000 analyzes the area of signal waveforms, which was absent in earlier devices based on flow cytometry. The side fluorescence signal waveform area obtained using this new technology reflects the nucleic acid contents of cells. This allows the detection of cells with higher nucleic acid contents than those with normal cell contents, as is the case in Atyp.Cs. Additionally, the particle classification accuracy is improved by combining signal information, such as the forward-scattered light signal width, which reflects the particle length, and the side-scattered light signal waveform area, which reflects size information, considering the complexity of internal cellular structures. Using the new information gathered by the UF-5000, urine components can be assessed using an approach in which samples suspected to contain Atyp.Cs because of their Atyp.C value undergo a microscopic examination of the urine sediment, which differs from the approach used with conventional urine particle analyzers [22,23]. For example, when screening for urinary tract tumors during health checkups, it would be a great advantage if detailed examinations were based on Atyp.C values in addition to hematuria, which is conventionally used.
According to Okumura et al. [24], if the malignant cells in urine were present in small numbers, were small, or showed necrosis or degeneration, the urine cytology test result may be negative more often than the urine sediment microscopy result. Since the detection of Atyp.Cs in urine sediment is highly significant, the detection of Atyp.Cs at the screening stage would be an advantage. Therefore, we suggest that Atyp.C-positive samples should be examined by urine sediment microscopy before cytology tests.
Regarding comparisons between the results of cytology tests and Atyp.C values, Chunyun et al. [25] examined 163 specimens from patients suspected with urothelial carcinoma and found that 67 (41.1%) specimens were positive for cancer by cytology and 59 (36.2%) specimens were positive by UF-5000 analysis. Tınay et al. [26] broadly classified patients into risk groups based on a diagnosis of bladder cancer and performed cytology tests and UF-5000 analysis on 27 patients in the low-risk group and 47 patients in the high-risk group. The concordance between the results of the two tests was 96.3% and 76.9% in the low- and high-risk groups, respectively. Additionally, Ozgur presented a case in which Atyp.Cs were detected by microscopy when the Atyp.C value was >1/mL [27]. The results of our study suggest that the Atyp.C value increases with an increase in intracytoplasmic inclusion-bearing cells and severe leukocyturia, but why this occurs has not been explained. Although leukocyturia suggests a urinary tract infection, determining whether there is an underlying disorder in the urinary tract infection, such as urinary tract calculi or a urinary tract tumor is clinically important, and urine sediment analysis is indispensable for this purpose. The appearance of intracytoplasmic inclusion-bearing cells is relevant not only because of its association with RNA virus infection but also because such cells are often present in the urine of patients with cystitis, pyelonephritis, post-urinary diversion complications, tubular disorders, and urinary tract tumors [28]. As the microscopic examination of urinary sediment is essential for specimens containing cells with intracellular inclusions, the high Atyp.C values in samples containing cells with intracellular inclusions will not increase the number of specimens that require microscopic examination of the urine sediment. Instead, such samples could be selected as appropriate for urine sediment microscopy that would improve the quality of the urine sediment results.
In conclusion, this study shows that a combination of UF-5000 analysis and microscopic examination of urine sediment improves Atyp.C detection in urine sediment analysis. These results suggest that Atyp.C measured by UF-5000 could be a useful screening parameter in routine testing of urine samples.
Funding statement
This research was supported by Sysmex Corporation. However, sponsors have no involvement in the development of protocols, data collection, data analysis, or literature writing.
Ethics approval statement
This study was approved by the Ethics Committee of the Graduate School of Medicine and the Faculty of Medicine of the University of Tokyo (3333-99). This study was conducted as a contract research for Sysmex Corporation.
Patient consent statement
We took informed consent from the participants by opt-out.
Permission to reproduce material from other sources
Not Applicable.
Clinical trial registration
Not Applicable.
Declaration of competing interest
This research was conducted in collaboration with Sysmex Corporation. The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Data availability
The authors do not have permission to share data.
References
- 1.Urinalysis C.L.S.I. Clinical and Laboratory Standards Institute; PA: 2009. Approved Guideline–Third Edition. CLSI Document GP16-A3 Waye. [Google Scholar]
- 2.Shayanfar N., Tobler U., von Eckardstein A., Bestmann L. Automated urinalysis: first experiences and a comparison between the Iris iQ200 urine microscopy system, the Sysmex UF-100 flow cytometer and manual microscopic particle counting. Clin. Chem. Lab. Med. 2007;45:1251–1256. doi: 10.1515/CCLM.2007.503. [DOI] [PubMed] [Google Scholar]
- 3.Delanghe J. New screening diagnostic techniques in urinalysis. Acta Clin. Belg. 2007;62:155–161. doi: 10.1179/acb.2007.026. [DOI] [PubMed] [Google Scholar]
- 4.Zaman Z. Automated urine screening devices make urine sediment microscopy in diagnostic laboratories economically viable. Clin. Chem. Lab. Med. 2015;53(Supplement 2):s1509–s1511. doi: 10.1515/cclm-2015-0476. [DOI] [PubMed] [Google Scholar]
- 5.Oyaert M., Delanghe J. Progress in automated urinalysis. Ann Lab Med. 2019;39:15–22. doi: 10.3343/alm.2019.39.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enko D., Stelzer I., Böckl M., Derler B., Schnedl W.J., Anderssohn P., et al. Comparison of the diagnostic performance of two automated urine sediment analyzers with manual phase-contrast microscopy. Clin. Chem. Lab. Med. 2020;58:268–273. doi: 10.1515/cclm-2019-0919. [DOI] [PubMed] [Google Scholar]
- 7.Tantisaranon P., Dumkengkhachornwong K., Aiadsakun P., Hnoonual A. A comparison of automated urine analyzers cobas 6500, UN3000-111b and iRICELL 3000 with manual microscopic urinalysis. Pract Lab Med. 2021;24 doi: 10.1016/j.plabm.2021.e00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hua W., Fei-Fei H., Jian-Xun W., Zhi Y., Yan-Qiu H., Zhi-De H., Wen-Qi Z. Accuracy of the Sysmex UF-5000 analyzer for urinary tract infection screening and pathogen classification. PLoS One. 2023:18. doi: 10.1371/journal.pone.0281118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alenkaer L.K., Pedersen L., Szecsi P.B., Bjerrum P.J. Evaluation of the Sysmex UF-5000 fluorescence flow cytometer as a screening platform for ruling out urinary tract infections in elderly patients presenting at the Emergency Department. Scand. J. Clin. Lab. Investig. 2021;81:379–384. doi: 10.1080/00365513.2021.1929441. [DOI] [PubMed] [Google Scholar]
- 10.Manoni F., Tinello A., Fornasiero L., Hoffer P., Temporin V., Valverde S., et al. Urine particle evaluation: a0020comparison between the UF-1000i and quantitative microscopy. Clin. Chem. Lab. Med. 2010;48:1107–1111. doi: 10.1515/CCLM.2010.233. [DOI] [PubMed] [Google Scholar]
- 11.Erdman P., Anderson B., Zacko J.C., Taylor K., Donaldson K. The accuracy of the Sysmex UF-1000i in urine bacterial detection compared with the standard urine analysis and culture. Arch. Pathol. Lab Med. 2017;141:1540–1543. doi: 10.5858/arpa.2016-0520-OA. [DOI] [PubMed] [Google Scholar]
- 12.Fogazzi G.B., Pallotti F., Garigali G. Atypical/malignant urothelial cells in routine urinary sediment: worth knowing and reporting. Clin. Chim. Acta. 2015;439:107–111. doi: 10.1016/j.cca.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Previtali G., Ravasio R., Seghezzi M., Buoro S., Alessio M.G. Performance evaluation of the new fully automated urine particle analyzer UF-5000 compared to the reference method of the Fuchs-Rosenthal chamber. Clin. Chim. Acta. 2017;4:123–130. doi: 10.1016/j.cca.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 14.Morita Y., Shukuya K., Tanaka M., Hisasue T., Sato E., Ono Y., et al. Validation of three automated urine sediment analyzers and the efficient workflow using criteria. JJCLA. 2019;44:602–609. [Google Scholar]
- 15.Fogazzi G.B., Delanghe J. Microscopic examination of urine sediment: phase contrast versus bright field. Clin. Chim. Acta. 2018;487:168–173. doi: 10.1016/j.cca.2018.09.036. [DOI] [PubMed] [Google Scholar]
- 16.Farling K.B. Bladder cancer: risk factors, diagnosis, and management. Nurs. Pract. 2017;42:26–33. doi: 10.1097/01.NPR.0000512251.61454.5c. [DOI] [PubMed] [Google Scholar]
- 17.Lenis A.T., Lec P.M., Chamie K., Mshs M.D. Bladder cancer: a review. JAMA. 2020;324:1980–1991. doi: 10.1001/jama.2020.17598. [DOI] [PubMed] [Google Scholar]
- 18.Editorial Committee of Japanese Clinical Practice Guidelines for Hematuria Diagnosis: Japanese Clinical Practice Guidelines for Hematuria Diagnosis 2013. Life Science Publishing; Tokyo: 2013. [Google Scholar]
- 19.Ingelfinger J.R. Hematuria in adults. N. Engl. J. Med. 2021;385:153–163. doi: 10.1056/NEJMra1604481. [DOI] [PubMed] [Google Scholar]
- 20.Moyer V.A., U.S. Preventive Services Task Force Screening for bladder cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2011;155:246–251. doi: 10.7326/0003-4819-155-4-201108160-00008. [DOI] [PubMed] [Google Scholar]
- 21.Aydin O. Atypical cells parameter in an automated urine analyzer: does it have a future? Anal. Biochem. 2020;600 doi: 10.1016/j.ab.2020.113763. [DOI] [PubMed] [Google Scholar]
- 22.Anderlini R., Manieri G., Lucchi C., Raisi O., Soliera A.R., Torricelli F., et al. Automated urinalysis with expert review for incidental identification of atypical urothelial cells: an anticipated bladder carcinoma diagnosis. Clin. Chim. Acta. 2015;451:252–256. doi: 10.1016/j.cca.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Muto S., Sugiura S., Nakajima A., Horiuchi A., Inoue M., Saito K., et al. Isomorphic red blood cells using automated urine flow cytometry is a reliable method in diagnosis of bladder cancer. Int. J. Clin. Oncol. 2014;19:928–934. doi: 10.1007/s10147-013-0623-9. [DOI] [PubMed] [Google Scholar]
- 24.Okumura M., Yagi S., Tomoda M., Katohno Y., Nakayama K., Yonese J., et al. Morphological features of malignant cells in urine sediments that show negative results in a urine cytology test. Jpn J Technol. 2011;60:704–708. [Google Scholar]
- 25.Ren C., Wang X., Yang C., Li S., Liu S., Cao H. Investigation of Atyp.C using UF-5000 flow cytometer in patients with a suspected diagnosis of urothelial carcinoma: a single-center study. Diagn. Pathol. 2020;15:77. doi: 10.1186/s13000-020-00993-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tınay İ., Şahin B., Saraçoğlu S., Yanılmaz Ö Özgür, Aksu M.B., Ayaş R., et al. “Atypical Cell” parameter in automated urine analysis for the diagnosis of bladder cancer: a retrospective pilot study. Bull Urooncol. 2019;18:17–19. [Google Scholar]
- 27.Aydin O. Atypical cells parameter in Sysmex UN automated urine analyzer: feedback from the field. Diagn. Pathol. 2021;16:9. doi: 10.1186/s13000-021-01068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Japanese Association of Medical Technologists: Examination of Urinary Sediment 2010. 2011. Tokyo. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.