Abstract
Objective:
Evidence regarding effectiveness of interleukin-1 receptor antagonism in Multisystem Inflammatory Syndrome in Children (MIS-C) is lacking. We characterized variation in initial treatment with anakinra and evaluated cardiovascular outcomes associated with adding anakinra to standard initial therapy.
Methods:
We conducted a retrospective cohort study of MIS-C cases in a U.S. surveillance registry November 2020-December 2021. Day 0 was the first calendar day of immunomodulatory treatment. Factors associated with initial anakinra use (days 0–1) were identified. We compared cases ages 2–20 years receiving intravenous immunoglobulin (IVIG) and glucocorticoids vs. anakinra plus IVIG and/or glucocorticoids (days 0–1), using inverse probability weighting to balance severity. Primary outcomes were vasopressor requirement (day 3) and impaired left ventricular ejection fraction (days 3–4). The secondary outcome was 50% reduction in C-reactive protein (day 3).
Results:
Among 1516 MIS-C cases (44 sites), 193 (13%) received anakinra alone or with other immunomodulators as initial treatment (range 0–74% by site). Site accounted for 59% of residual variance in anakinra use. After balancing severity, initial treatment with anakinra plus IVIG and/or glucocorticoids (N=121) vs. IVIG and glucocorticoids (N=389) was not associated with significant differences in vasopressor requirement (25.6% vs. 20.1%; RR 1.27, 95% CI [0.88–1.84]), ventricular dysfunction (33.7% vs. 25.7%; RR 1.31, 95% CI [0.98–1.75]), or C-reactive protein reduction.
Conclusions:
We identified substantial variation in initial anakinra use in a real-world population of children with MIS-C, but no average short-term improvement in cardiovascular outcomes associated with early addition of anakinra to IVIG and/or glucocorticoids compared to IVIG and glucocorticoids alone.
Keywords: Multisystem inflammatory syndrome in children, Anakinra, Interleukin-1, Treatment Outcomes, Epidemiology
INTRODUCTION
The primary drivers of morbidity in multisystem inflammatory syndrome in children (MIS-C) are features of distributive and cardiogenic shock. Evidence of aberrant cytokine signaling has prompted empiric use of cytokine inhibitors, of which the Interleukin-1 receptor antagonist (IL-1Ra) anakinra is the most commonly used in the United States (U.S.) (1,2). IL-1 α/β signaling promotes secretion of C-reactive protein (CRP) and other acute phase reactants, fever, lymphocyte proliferation, endothelial cell activation, and production of other cytokines, including IL-6 and tumor necrosis factor (TNF)-α (3). It is thus reasonable to hypothesize that IL-1 inhibition could improve cardiovascular outcomes of MIS-C by reducing vasoplegia and myocardial injury driven by dysregulated inflammation.
Published experience with anakinra use in children with MIS-C is limited primarily to single-center case series, which are subject to publication bias (4–9). In guidelines released by the American College of Rheumatology (ACR), there was moderate consensus that high-dose anakinra (>4 mg/kg/day) should be considered for MIS-C refractory to intravenous immunoglobulin (IVIG) and glucocorticoids, in patients with features of macrophage activation syndrome (MAS), and in those with relative contraindications to standard therapy (10). An estimated 22% of MIS-C cases in the United States (U.S.) from October 2020–July 2021 received cytokine inhibitors (11). In an international meta-analysis, the pooled proportion of children receiving cytokine inhibitors was 27%, but with considerable heterogeneity (12).
Despite empiric use of cytokine inhibitors in the management of MIS-C, there remains little data on their effectiveness to define optimal use, and thus there is potential for considerable practice variability. The objectives of this study were to 1) describe variation in and factors associated with anakinra use in MIS-C across 44 U.S. pediatric hospitals and 2) compare cardiovascular outcomes of initial treatment with anakinra plus IVIG and/or glucocorticoids vs. IVIG and glucocorticoids alone.
METHODS
Study Design:
This was a retrospective cohort study using The Overcoming COVID-19 registry, a U.S. public health surveillance network for children and adolescents hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-related illness funded by the Centers for Disease Control and Prevention (CDC). The surveillance protocol was approved by the central institutional review board at Boston Children’s Hospital, determined to meet requirements for public health surveillance as defined in 45 CFR 46.102(I)(2), and granted a waiver of informed consent. Trained study personnel at each site abstracted medical record data into standardized case report forms.
Study Population:
All MIS-C cases meeting the CDC case definition from sites that contributed ≥10 cases from November 2020 through December 2021 were considered for inclusion (13). Cases were adjudicated by principal investigators at each site and the coordinating center. Documentation of a positive SARS-CoV-2 reverse transcriptase polymerase chain reaction or antibody test was required. We excluded patients with prior systemic glucocorticoid use within 7 days prior to admission, baseline immunosuppressant use, or immune dysregulation disorders.
Study Measures:
Treatment Groups:
We defined initial therapy as immunomodulatory treatments received on days 0–1, with day 0 being the first calendar day any immunomodulatory treatment was received and day 1 being the next calendar day to ensure exposure assessment occurred over at least 24 hours. We first categorized all MIS-C cases according to whether they received anakinra as initial therapy, either alone or in combination with other immunomodulators. We then categorized cases according to initial therapy with anakinra plus IVIG and/or glucocorticoids vs. IVIG and glucocorticoids. Glucocorticoid dosing was recorded with pulse doses defined as 10–30 mg/kg of intravenous methylprednisolone or equivalent. Because obesity may influence treatment and outcomes, we excluded children <2 years of age in whom standardized obesity classification is not possible.
Outcomes:
To assess treatment variation, we calculated the proportion of cases that received anakinra alone or in combination with immunomodulators on days 0–1, excluding cases that received TNFα or IL-6 inhibitors on days 0–1. To compare initial therapy, primary outcomes included any vasopressor requirement on day 3 of treatment and reduced left ventricular ejection fraction (LVEF) <55% on days 3–4 of treatment. As only calendar day of treatment (not time) was available, day 3 was selected to ensure outcome assessment occurred >24 hours after the first dose of anakinra in the treated group and coincided with the expected time course of improvement in cardiovascular function described in prior reports (14). A range of day 3–4 was used for LVEF due to variable timing and frequency of echocardiograms, the earliest of which was analyzed. As a secondary outcome, we evaluated 50% reduction of baseline CRP levels by day 3.
Covariates:
We considered demographics (age, sex, race and ethnicity, insurance type, social vulnerability index); calendar period, categorized as pre-Delta (November 2020 – May 2021) vs. Delta variant predominance (June 2021 – December 2021); other clinical characteristics (body mass index classification based on CDC percentile-for-age for children ages 2–19 and standard categories for adults over 19, one or more underlying conditions, day of illness); and laboratory characteristics on admission (CRP, platelet count, neutrophil/lymphocyte ratio, albumin, ferritin, and creatinine). Impairment in estimated glomerular filtration rate (eGFR) was used to quantify renal dysfunction. We also assessed severity of illness indicators within the first 24 hours of admission, including positive pressure ventilation (non-invasive or invasive ventilation), intensive care unit (ICU) admission, baseline LVEF <55%, and any vasopressor requirement. Vasopressor use was additionally tested as an ordinal variable classified by the pediatric Sequential Organ Failure Assessment (pSOFA) score (15). We considered those requiring either invasive mechanical ventilation or vasopressors to have life-threatening illness.
Statistical analysis:
To characterize treatment variation, we calculated the proportion of MIS-C cases that received anakinra as initial therapy (days 0–1) by site. We used mixed effects logistic regression models to identify factors associated with receipt of anakinra, with a random intercept for site. Age, sex, and severity indicators were included a priori. Additional covariates were added using a forward selection procedure and retained based on likelihood ratio tests or evidence of confounding, defined as >10% change in other coefficients. Ferritin was categorized according to the cutoff (>684 ng/mL) in the 2016 classification criteria for macrophage activation syndrome (MAS) in systemic juvenile arthritis (16). Patterns of missingness were assessed. Complete case analysis was employed in all primary analyses, except for ferritin, for which a missing category was included due to the large proportion of missing values (28%) and potential for non-random missingness (Supplementary Methods). The intraclass correlation coefficient was used to quantify residual variance accounted for by site. In a secondary analysis, we also estimated marginal (population-average) effects of each factor on the likelihood of initial anakinra use via generalized estimating equations with an exchangeable correlation structure.
To compare treatment outcomes, we estimated risk ratios using modified (robust) Poisson regression models with site-level random effects. To account for confounding by indication, we performed inverse probability of treatment weighting (IPTW) using propensity scores to balance covariates and indicators of disease severity at baseline across treatment groups (Supplementary Methods). Covariates were included in the propensity model based on clinical judgement, published literature (2,17), and identification of confounders or variables predictive of the outcome (18). To ensure cases had reasonable likelihood of receiving either treatment, we restricted comparator groups to the common (overlapping) region of the propensity distributions and trimmed extreme propensity scores (<10% probability of receiving either treatment) (19), prior to re-estimating propensity and inverse probability weights (20,21). Balance of covariates after IPTW was assessed using standardized mean differences (SMD) and kernel density plots. We tested further adjustment for pSOFA vasopressor scores and initial LVEF as a continuous measure in the weighted outcome models (22). The primary analysis compared initial treatment with anakinra plus IVIG and/or glucocorticoids (anakinra group) vs. IVIG and glucocorticoids. In a secondary analysis, we restricted the anakinra group to those who received both IVIG and glucocorticoids, and also tested further adjustment for initial use of pulse dose intravenous methylprednisolone. With an estimated convenience sample of 120 anakinra treated vs. 360 untreated cases and a 30–40% probability of each outcome, we calculated 80% power to detect a risk difference of 12–13% at a significance level of 0.05 in the absence of confounders (Mantel-Haenszel test).
We conducted several sensitivity analyses. To ensure results were robust to treatment of missing day 3–4 echocardiographic data, we compared estimates using complete case analysis to last observation carried forward. To evaluate the sensitivity of results to the chosen interval for outcome assessment, we shifted the vasopressor outcome by one calendar day in either direction. Furthermore, rather than restricting LVEF outcome assessment to days 3–4, we compared time to first normal LVEF ≥55% by treatment group among those with abnormal LVEF at treatment initiation using IPTW Cox proportional hazards regression, censoring observations at discharge. We also conducted propensity score matching using k-nearest neighbor caliper matching with replacement (k=2, caliper width of 0.1). Lastly, we calculated e-value bias statistics to evaluate the magnitude of unmeasured confounding necessary to change our conclusions. All statistical analyses were conducted using STATA version 16.0 (College Station, TX).
RESULTS
Variation in initial treatment patterns
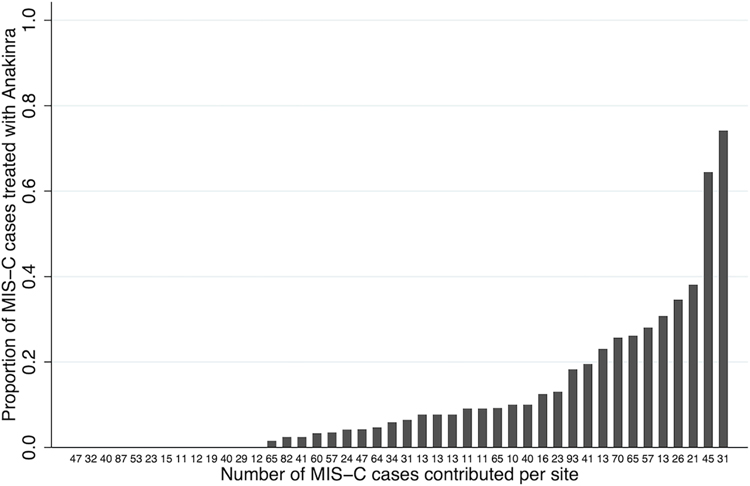
Among 1516 MIS-C cases from 44 sites, 193 (13%) received anakinra with or without other immunomodulators as initial therapy, 964 (64%) received IVIG and glucocorticoids, 239 (16%) received IVIG alone, and a minority received glucocorticoids alone (4%) or no immunomodulators (4%). The 99 cases that received TNF inhibitors, comprised almost exclusively by two sites, and 7 cases receiving tocilizumab, were not analyzed further (Supplemental Table 1). The majority (98%) of initial glucocorticoid administration was intravenous, most commonly methylprednisolone (93%). The proportion of cases per site that received anakinra as initial therapy varied between 0–74% with a median of 6% (Figure 1) and was not associated with the number of cases contributed (ρ 0.02, p=0.92).
Figure 1.
Bar graph representing the proportion of MIS-C cases contributed by each site that received anakinra alone or in combination with other immunomodulators as initial treatment on days 0–1, in ascending order. Each bar is labeled on the x-axis with the total number of MIS-C cases contributed by that site.
Of the 193 cases receiving anakinra as initial therapy, 161 (83%) received it with IVIG and glucocorticoids, 17 (9%) with IVIG only, 13 (7%) with glucocorticoids only, and 2 (1%) without other immunomodulators.
Of 964 cases treated initially with IVIG and glucocorticoids, 126 (13%) subsequently received anakinra on day 2 or later (median day 2.5, IQR [2–4]) (Supplemental Table 1). The median initial dose of anakinra used in all cases was 4 mg/kg/day (range 0.2–20 mg/kg/day), with a median of 6 total days of anakinra use (IQR [4–9]), including use after hospital discharge.
Factors associated with anakinra as initial therapy in any combination with other immunomodulators
Indicators of illness severity, including respiratory support or vasopressors within 24 hours of admission, as well as moderate-severe renal impairment (eGFR<40), were independently associated with anakinra use with any initial therapy (Table 1). Site of care accounted for 59% of residual variance after adjustment for individual characteristics, indicating substantial variation by site. The proportion of missing ferritin values also varied substantially by site (range 0–73%), but neither elevated nor missing ferritin was independently associated with anakinra. Similarly, in the marginal model, baseline respiratory support and vasopressor requirements were significantly associated with anakinra use, but not ferritin (Supplemental Table 2).
Table 1.
Factors associated with receipt of anakinra with initial therapy for MIS-C
| Adjusted OR | [95% CI] | p-value | |
|---|---|---|---|
|
| |||
| Age (years) | 1.0 | [0.9, 1.0] | 0.22 |
| Female sex | 0.7 | [0.4, 1.1] | 0.10 |
| Body mass index classification | |||
| Healthy weight | - | ||
| Overweight | 1.1 | [0.6, 2.3] | 0.73 |
| Obese | 1.5 | [0.9, 2.6] | 0.12 |
| Social vulnerability index | |||
| Lowest | - | ||
| Medium-Low | 1.4 | [0.7, 2.8] | 0.32 |
| Medium-High | 1.9 | [0.9, 3.9] | 0.08 |
| Highest | 1.7 | [0.8, 3.5] | 0.17 |
| Severity indicators within 24 hours of admission | |||
| Respiratory support | |||
| None | - | ||
| Supplemental oxygen only | 2.2 | [1.2, 3.9] | 0.01 |
| Non-invasive positive pressure ventilation | 4.0 | [1.2, 14.0] | 0.03 |
| Invasive mechanical ventilation | 8.9 | [3.3, 24.2] | <0.01 |
| Any vasopressor requirement | 2.3 | [1.3, 4.3] | 0.01 |
| Initial left ventricular ejection fraction <55% | 1.4 | [0.8, 2.3] | 0.24 |
| Laboratory characteristics at admission | |||
| Ferritin level | |||
| ≤684 ng/mL | - | ||
| >684 ng/mL | 0.9 | [0.5, 1.5] | 0.59 |
| Missing | 0.7 | [0.3, 1.3] | 0.22 |
| Estimated glomerular filtration rate | |||
| Normal (≥90 mL/min/1.73 m2) | - | ||
| Mild-moderate impairment (45–89) | 1.3 | [0.7, 2.4] | 0.37 |
| Moderate-severe impairment (<45) | 2.6 | [1.2, 5.5] | 0.02 |
| Unknown/missing | 0.9 | [0.1, 8.8] | 0.96 |
| Upper quartile of neutrophil:lymphocyte ratio | 1.7 | [1.0, 2.8] | 0.06 |
| Platelet count (natural log) | 0.7 | [0.4, 1.0] | 0.07 |
| Albumin | 0.7 | [0.4, 1.1] | 0.12 |
Results from mixed effects multivariable logistic regression model (N=1125, age ≥2 years with complete data) with random intercept for site. Days of illness at presentation, presence of a pre-existing condition, race and ethnicity, insurance status, alanine transaminase levels, C-reactive protein, calendar period (pre- vs. post-Delta predominance), and ICU admission were tested in the model and did not meet criteria for inclusion.
Outcomes associated with anakinra plus IVIG and/or glucocorticoids as initial therapy compared to IVIG and glucocorticoids alone
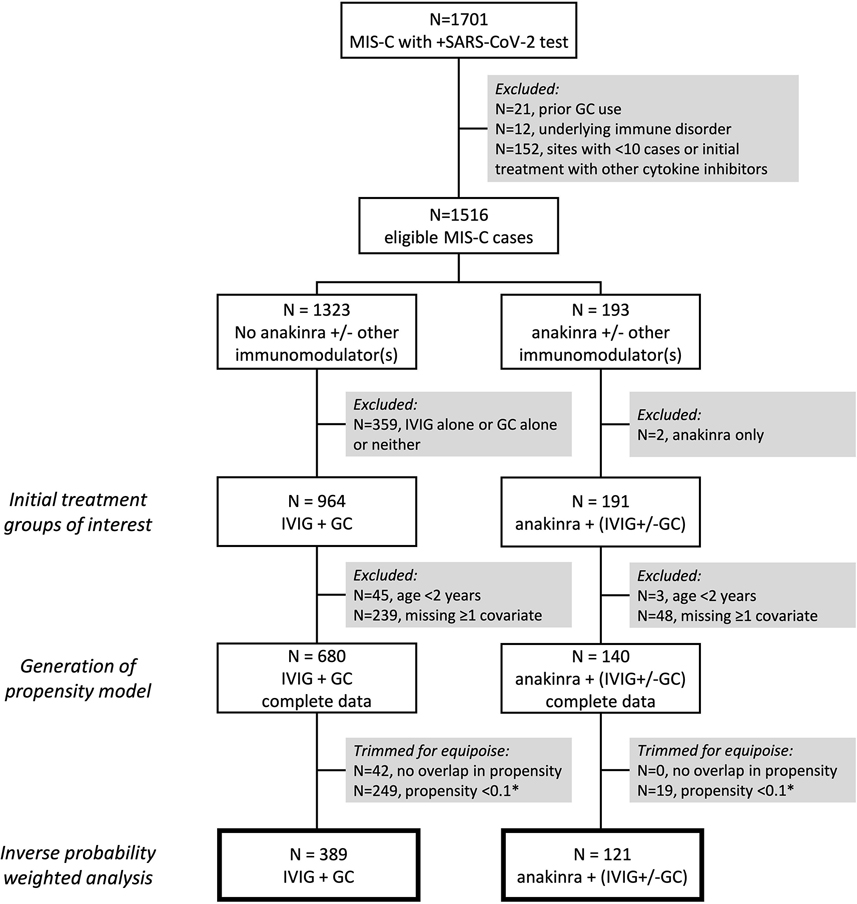
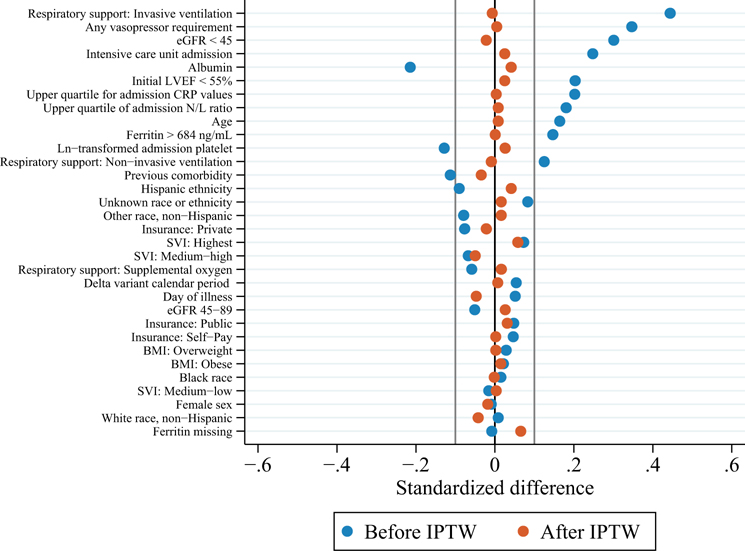
A total of 820 observations with complete data were used to generate the propensity model, including 140 cases who received anakinra plus IVIG and/or glucocorticoids on days 0–1 and 680 who received IVIG and glucocorticoids (Figure 2). Baseline characteristics and severity indicators in the propensity model prior to weighting are shown in Supplemental Table 3. There were no substantial differences in treatment assignment or baseline characteristics between included cases compared to those excluded for ≥1 missing covariates (Supplemental Table 4). Partial overlap in propensity scores was observed between treatment groups (Supplemental Figure 1). Restricting cases to the region of overlap resulted in loss of 42 cases from the IVIG and glucocorticoid group only. Further trimming of cases with too low of a predicted probability (<10%) of receiving anakinra resulted in loss of 19 cases from the anakinra group and 249 cases from the IVIG and glucocorticoid group, but no cases had to be trimmed for too low of a probability of receiving only IVIG and glucocorticoids (Figure 2). Covariate balance was achieved in the remaining N=510 cases (121 anakinra group vs. 389 IVIG and glucocorticoids) after inverse probability weighting (Figure 3). In the weighted population, 85% of cases in both treatment groups had ≥1 severity indicator, 59% in the anakinra group vs. 58% in the IVIG and glucocorticoid group required invasive ventilation or vasopressors at baseline, and mean baseline LVEF was 54% (SD 11–12) in both groups (Table 2). Of those receiving glucocorticoids (N=499), the median dose was 2 mg/kg/day methylprednisolone [IQR 1–8], including 24% treated with pulse dose methylprednisolone. IPT weight and propensity score distributions are shown in Supplemental Figure 2 and Supplemental Figure 3, respectively.
Figure 2.
Flow diagram of selection criteria and sample sizes at each stage of the propensity weighted analysis. Boxes with thick borders indicate the final sample sizes in the inverse probability of treatment weighted estimates of the average treatment effect (ATE) of anakinra plus intravenous immunoglobulin (IVIG) and/or glucocorticoids (GC) compared to IVIG and GC alone. *Cases with a <10% predicted probability of receiving either treatment were removed to preserve clinical equipoise; in this cohort, only those with <10% probability of receiving anakinra needed to be removed, as no cases had <10% probability of receiving only IVIG and GC.
Figure 3.
Standardized mean differences in individual covariates between the anakinra plus IVIG and/or glucocorticoid initial treatment group (N=121) and the IVIG and glucocorticoid initial treatment group (N=389), before and after inverse probability of treatment weighting. By convention, standardized differences less than 0.1 indicate adequate balance. eGFR = estimated glomerular filtration rate; LVEF = left ventricular ejection fraction; CRP = C-reactive protein; N/L ratio = neutrophil:lymphocyte ratio; SVI = social vulnerability index
Table 2.
Distribution of baseline covariates before and after inverse probability weighting
| Before weighting | After weighting | |||||
|---|---|---|---|---|---|---|
| Anakinra | Anakinra | |||||
| IVIG+GC N=389 |
IVIG±GC N=121 |
SMD | IVIG+GC Σwt=387.6 |
IVIG±GC Σwt=122.7 |
SMD | |
|
| ||||||
| Age (years), mean (SD) | 9.9 (4.1) | 10.6 (4.1) | 0.17 | 10.1 (4.1) | 10.1 (4.1) | 0.01 |
| Female sex, n (%) | 140 (36%) | 43 (36%) | −0.01 | 139 (36%) | 43 (35%) | −0.02 |
| Delta predominance | 234 (60%) | 76 (63%) | 0.05 | 237 (61%) | 75 (61%) | 0.01 |
| Days of illness at admission | 4.9 (2.8) | 5.0 (3.0) | 0.05 | 4.9 (2.8) | 4.8 (3.0) | −0.05 |
| Days of illness at treatment day 0† | 5.5 (2.8) | 5.7 (2.9) | 0.06 | 5.6 (2.8) | 5.5 (2.9) | −0.01 |
| Race and ethnicity | ||||||
| Asian or Pacific Islander | 6 (2%) | 4 (3%) | 0.11 | 7 (2%) | 2 (1%) | −0.02 |
| Black, non-Hispanic | 113 (29%) | 36 (30%) | 0.02 | 112 (29%) | 35 (29%) | 0.00 |
| Hispanic ethnicity, any race | 95 (24%) | 25 (21%) | −0.09 | 92 (24%) | 31 (25%) | 0.04 |
| Other race, non-Hispanic | 11 (3%) | 2 (2%) | −0.08 | 10 (3%) | 3 (3%) | 0.02 |
| Unknown | 21 (5%) | 9 (7%) | 0.08 | 25 (6%) | 8 (7%) | 0.02 |
| White, non-Hispanic | 143 (37%) | 45 (37%) | 0.01 | 142 (37%) | 42 (35%) | −0.04 |
| Insurance status | ||||||
| Private | 156 (40%) | 44 (36%) | −0.08 | 152 (39%) | 47 (38%) | −0.02 |
| Self-Pay | 7 (2%) | 3 (2%) | 0.05 | 8 (2%) | 3 (2%) | 0.00 |
| Public | 219 (56%) | 71 (59%) | 0.05 | 221 (57%) | 72 (59%) | 0.03 |
| Unknown | 7 (2%) | 3 (2%) | 0.05 | 7 (2%) | 1 (1%) | −0.05 |
| Social Vulnerability Index | ||||||
| Lowest | 79 (20%) | 25 (21%) | 0.01 | 79 (20%) | 24 (20%) | −0.02 |
| Medium-Low | 99 (25%) | 30 (25%) | −0.02 | 98 (25%) | 31 (26%) | 0.00 |
| Medium-High | 108 (28%) | 30 (25%) | −0.07 | 103 (26%) | 30 (24%) | −0.05 |
| Highest | 103 (26%) | 36 (30%) | 0.07 | 108 (28%) | 37 (30%) | 0.06 |
| Body mass index classification | ||||||
| Healthy weight | 188 (48%) | 56 (46%) | −0.04 | 183 (47%) | 57 (46%) | −0.02 |
| Overweight | 54 (14%) | 18 (15%) | 0.03 | 56 (14%) | 18 (14%) | 0.00 |
| Obese | 147 (38%) | 47 (39%) | 0.02 | 149 (39%) | 48 (39%) | 0.02 |
| Previous comorbidity | 152 (39%) | 54 (45%) | −0.11 | 157 (41%) | 52 (42%) | −0.03 |
| Upper quartile for N/L ratio | 130 (33%) | 51 (42%) | 0.18 | 138 (36%) | 44 (36%) | 0.01 |
| Upper quartile for CRP | 126 (32%) | 51 (42%) | 0.20 | 134 (35%) | 43 (35%) | 0.00 |
| Platelet count (natural log) | 5.0 (0.5) | 4.9 (0.5) | −0.13 | 5.0 (0.5) | 5.0 (0.5) | 0.03 |
| Albumin (g/dL) | 3.3 (0.6) | 3.1 (0.6) | −0.21 | 3.2 (0.6) | 3.3 (0.6) | 0.04 |
| eGFR (mL/min/1.73 m2) | ||||||
| ≥90 | 195 (50%) | 49 (40%) | −0.19 | 186 (48%) | 59 (48%) | −0.01 |
| 45–89 | 138 (35%) | 40 (33%) | −0.05 | 137 (35%) | 45 (37%) | 0.03 |
| <45 | 56 (14%) | 32 (26%) | 0.30 | 64 (17%) | 19 (16%) | −0.02 |
| Ferritin (ng/mL) | ||||||
| ≤684 | 210 (54%) | 57 (47%) | −0.14 | 203 (52%) | 61 (50%) | −0.04 |
| >684 | 133 (34%) | 50 (41%) | 0.15 | 138 (36%) | 44 (36%) | 0.00 |
| Missing | 46 (12%) | 14 (12%) | −0.01 | 47 (12%) | 18 (14%) | 0.07 |
| Severity of illness indicators within 24 hours of admission * | ||||||
| Respiratory support | ||||||
| None | 172 (44%) | 35 (29%) | −0.32 | 159 (41%) | 50 (41%) | −0.01 |
| Supplemental oxygen | 172 (44%) | 50 (41%) | −0.06 | 170 (44%) | 55 (45%) | 0.02 |
| Non-invasive ventilation | 20 (5%) | 10 (8%) | 0.13 | 23 (6%) | 7 (6%) | −0.01 |
| Mechanical ventilation | 25 (6%) | 26 (21%) | 0.44 | 37 (10%) | 11 (9%) | −0.01 |
| Any vasopressor requirement | 212 (54%) | 86 (71%) | 0.25 | 225 (58%) | 72 (58%) | 0.03 |
| Intensive care unit admission | 289 (74%) | 102 (84%) | 0.35 | 298 (77%) | 96 (78%) | 0.00 |
| Initial LVEF <55% | 176 (45%) | 67 (55%) | 0.20 | 185 (48%) | 60 (49%) | 0.03 |
| Initial LVEF (continuous)ǂ | 55.0 (11.6) | 51.9 (11.2) | −0.28 | 54.5 (11.9) | 53.9 (10.8) | −0.05 |
Distribution of covariates included in the propensity score model (except where indicated) before and after inverse probability weighting. IVIG = intravenous immunoglobulin; GC = glucocorticoid; SMD = standardized mean difference; Σwt = sum of weights; LVEF = left ventricular ejection fraction; eGFR = estimated glomerular filtration rate; N/L ratio = neutrophil:lymphocyte ratio; CRP = C-reactive protein
Before weighting, 325 (84%) in the IVIG+GC group vs. 110 (91%) in the anakinra group had at least one illness severity indicator (positive pressure ventilation, vasopressor requirement, ICU admission, or impaired LVEF) (SMD 0.22). After weighting, 85% in both treatment groups had at least one severity indicator (SMD −0.02).
Shown to evaluate balance only; days of illness at admission timestamp rather than calendar day of treatment initiation was selected for inclusion in the propensity model.
Among N=447 cases with quantitative measures of initial LVEF available. Means are shown to evaluate balance only; LVEF was not included as a continuous measure in the propensity model. Minimum values of LVEF were 20% in the IVIG+GC group vs. 22% in the anakinra treated group before weighting.
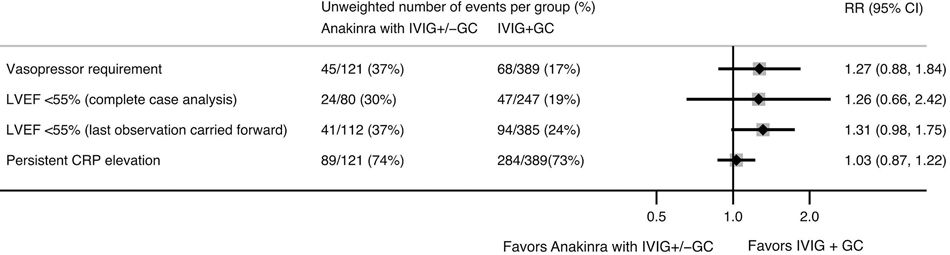
Compared to initial therapy with IVIG and glucocorticoids, anakinra plus IVIG and/or glucocorticoids was not associated with significant differences in risk of vasopressor use on day 3 (25.6% in anakinra group vs. 20.1% IVIG and glucocorticoids alone; RR 1.27, 95% CI [0.88–1.84]) or left ventricular dysfunction on days 3–4 (33.7% vs. 25.7%; RR 1.31, 95% CI [0.98–1.75]) in the weighted analyses (Figure 4). Additional adjustment in weighted outcome models for baseline LVEF as a continuous measure and pSOFA vasopressor score did not significantly change results. There was also no significant difference in CRP reduction by 50% by day 3 (Figure 4).
Figure 4.
Relative risks (RR) of clinical outcomes in children with MIS-C receiving anakinra as initial therapy (with intravenous immunoglobulin [IVIG] and/or glucocorticoids [GC]) compared to IVIG and GC alone, estimated using inverse probability weighted models with site-level random effects. Clinical outcomes were assessed on day 3 of treatment, except for left ventricular ejection fraction (LVEF), which was assessed on day 3–4, using either complete case analysis (N=327 LVEF recorded on day 3–4) or last observation carried forward (N=497). The direction of the average treatment effect for C-reactive protein (CRP) is represented as failure to achieve 50% reduction in CRP from admission.
Upon limiting the anakinra group to those who received concomitant initial treatment with both IVIG and glucocorticoids (n=96), there was similarly no difference compared to IVIG and glucocorticoids (n=310) in vasopressor requirement (RR 1.30, 95% CI [0.84–2.01]) or ventricular dysfunction (RR 1.34, 95% CI [0.96–1.85]). Further adjustment for initial use of pulse dose methylprednisolone (42% in anakinra group vs. 23% IVIG and glucocorticoids) yielded similar results (RR 1.30 [0.85–1.98] and RR 1.33 [0.95–1.87] for vasopressor use and ventricular dysfunction, respectively).
Among cases with abnormal LVEF at treatment initiation (N=84 anakinra plus IVIG and/or glucocorticoids; N=191 IVIG and glucocorticoids), median time to first normal LVEF was 3 days (IQR [2–5], range 1–11). There was no significant difference in time to LVEF normalization in the anakinra group vs. IVIG and glucocorticoids alone (HR 0.78 for LVEF normalization after weighting, 95% CI [0.52–1.17]). Uncensored individuals with prolonged LVEF recovery (≥6 days) in both anakinra treated (12/61) and untreated (16/135) groups had lower initial LVEF (mean 43% vs. 49%, p<0.01), older age (mean 12.5 vs. 10.2 years (p<0.01), and a higher frequency of severe renal impairment (39% vs. 20%, p<0.01); no other severity indicators or clinical features (obesity, ferritin, troponin when available, sociodemographic characteristics) were associated with prolonged LVEF recovery.
Of those treated initially with IVIG and glucocorticoids, 42/389 (11%) received anakinra as rescue therapy on day 2 or later. Anakinra rescue therapy was associated with a higher frequency of positive pressure ventilation within 24 hours of admission (21% vs. 10%, p=0.03) and higher baseline ferritin (median 599 [IQR 420–1325] vs. 528 [284–907], p=0.04).
Sensitivity Analyses
The likelihood of missing day 3/4 LVEF outcome data did not differ by treatment group, but baseline respiratory support was associated with greater completion of day 3/4 echocardiograms (Supplemental Table 5). Complete case analysis vs. last observation carried forward yielded similar point estimates for ventricular dysfunction (Figure 4). Shifting vasopressor outcome assessment by one calendar day earlier or later did not change conclusions (data not shown). Similarly, the propensity matched analysis (N=100 in the anakinra plus IVIg and/or glucocorticoid group vs. N=143 in the IVIG and glucocorticoid group, representing 38 sites) did not demonstrate a significant association between anakinra use and outcomes of vasopressor requirement (RR 1.10, 95% CI [0.63–1.93]) or ventricular dysfunction (RR 1.25, 95% CI [0.77–2.03]). Assuming the true effect of anakinra is a 20% or 10% risk reduction, an unmeasured confounder would need to have a minimum strength of association on the risk ratio scale of 2.65 or 2.26, respectively, with both treatment and outcome to yield the null effect observed in the primary analysis of vasopressor use.
DISCUSSION
In this real-world epidemiologic study, there was substantial variation in use of anakinra as initial treatment for MIS-C across pediatric centers in the U.S. Practice variation enabled identification of cases with reasonable likelihood to have received initial treatment with either anakinra plus standard therapy or IVIG and glucocorticoids alone. In these children with MIS-C, in which nearly 60% presented with life-threatening illness, we did not observe any significant associations between early anakinra use and short-term vasopressor requirement, ventricular dysfunction, or CRP reduction to support routine addition of anakinra to initial therapy with IVIG or glucocorticoids.
Our findings contrast with a number of case series describing clinical improvement following early treatment with anakinra and good outcomes of early aggressive therapy for severe MIS-C cases, though none directly compared outcomes against standard therapy with IVIG and glucocorticoids (23). There may be several reasons for this observation. First, IVIG and glucocorticoids may be effective for controlling inflammation such that we cannot detect effects of targeted therapies when used concurrently. Previous studies suggested that adding glucocorticoids to IVIG promotes faster recovery of cardiac function in MIS-C (2,24). Moreover, a recent study proposed IVIG targets activated neutrophils expressing IL-1β in MIS-C and a similar syndrome called Kawasaki disease, providing a basis for efficacy of IVIG, although further validation is necessary (25). Second, it is possible that cardiovascular dysfunction and CRP elevation in MIS-C are driven by IL-1 to a lesser degree than in other hyperinflammatory states such as MAS and Kawasaki disease. The distinct immune profiles of these conditions cautions against a one-size-fits-all approach to treatment (6,26). Conversely, recent studies in small groups of children with MIS-C and vaccine-induced myocarditis described a high prevalence of anti-IL-1Ra autoantibodies corresponding to reduced free IL-1Ra, which authors postulate may contribute to hyperinflammation (27,28). Thus, if anti-IL-1Ra autoantibodies contribute to MIS-C pathophysiology, it is possible that the average anakinra dose of 4 mg/kg/day in this study was insufficient to detect an effect. Larger doses up to 10 mg/kg/day have been reported in case series describing clinical benefit of anakinra for MIS-C (8,23). A greater understanding of the role of IL-1 and autoantibodies in MIS-C can inform the design of future studies of the effectiveness of IL-1 inhibition.
Current ACR guidelines recommend anakinra for treatment intensification but make no specific recommendations regarding initial treatment with anakinra, with the exception of MAS or contraindications to standard therapy (10). These recommendations were based largely on descriptive series (5,29,30), clinical experience of the expert panel, and extrapolation from experience using anakinra for other hyperinflammatory conditions, including IVIG-resistant Kawasaki disease (31). In our cohort, local practices sometimes diverged from ACR guidelines and favored early treatment with cytokine inhibitors, often in children with more life-threatening presentations and multi-organ dysfunction. The frequency of early anakinra use ranged from none at some sites to three-quarters of patients at others, and nearly all TNF inhibitor use was accounted for by two sites, which may reflect ways in which institutions operationalized local multidisciplinary treatment standards, as previously described (23,32,33). While greater illness severity and local context were both important factors in receipt of anakinra, we do not know whether specific clinical presentations such as fulminant myocardial dysfunction or suspected MAS prompted early anakinra use and how this differed by site. As many features of MAS overlap with MIS-C, it was not possible to classify a specific subgroup of patients with MAS in this registry. Of note, although race, ethnicity, and socioeconomic disadvantage have been associated with disproportionately higher rates of MIS-C (34,35), as well as more severe presentations (36), they were not significant predictors of early anakinra use to suggest that differential cytokine inhibitor use drives disparities in outcomes. Data from our epidemiologic study provide additional evidence to support current ACR recommendations to reserve cytokine inhibitors for treatment intensification in most hospitalized children with MIS-C. Per consensus guidelines, given the favorable safety profile of anakinra, empiric use in the setting of suspected MAS or refractory disease may be indicated, and our findings are not intended to supersede clinical judgement in this regard.
There are several remaining clinical questions our study could not answer. We lacked sufficient sample size to evaluate whether anakinra can supplant IVIG or glucocorticoids as initial treatment. This would be an important future direction, as fluid overload from IVIG in the setting of impaired cardiac function is of concern, and adverse effects of glucocorticoid use in MIS-C has been demonstrated (37), particularly in children with obesity. Obesity is both prevalent in MIS-C and associated with worse outcomes (38), therefore identifying optimal treatment strategies for children with comorbid obesity is necessary. Although it is unknown whether any patients receiving anakinra had relative contraindications to standard therapy, sensitivity analyses restricted to those receiving both IVIG and glucocorticoids suggest this does not explain our results. Secondly, we do not know if there is a subset of critically ill children that would benefit from early anakinra use or what doses may be required to achieve a clinical effect. Anakinra rescue therapy was administered to 11% of children who received IVIG and glucocorticoids initially, particularly those requiring mechanical ventilation. Our study was not designed to evaluate rescue therapy or directly compare step-up to step-down approaches.
Strengths of our study include the large sample size and representation of pediatric hospitals across the U.S. There are, however, several limitations related to the retrospective nature of this analysis and application of real-world data. Our results may not be wholly generalizable, as we had insufficient sample size to stratify by anakinra dose or by life-threatening features to assess heterogeneous treatment effects, including effect modification by level of baseline cardiac dysfunction. Additionally, our data reflects national practices and guidelines in the U.S. and may not be generalizable to other countries (39). Laboratory data were missing in a substantial proportion of cases, particularly ferritin and troponin. Missingness may be non-random if ferritin was checked only for suspected MAS, which could bias estimates toward worse outcomes in the anakinra group. However, we balanced missing ferritin across treatment groups to limit the potential impact of this bias, and the substantial site variation in ferritin collection suggests that practice variability plays an important role in collecting ferritin rather than suspicion of a specific pathophysiologic process. An additional limitation of the surveillance registry is the inability to assess precise timing of therapies and response, as only calendar days were available. Similarly, cardiac enzymes and other measures of myocardial function (e.g. strain) were not captured sufficiently to assess fulminant myocardial injury and its relationship to anakinra usage or potential utility. With the observed effect estimates, we were likely underpowered to detect statistically significant differences. Despite the lower precision of our estimates in this setting, we believe that they are still informative, as accumulation of data from additional analyses in other cohorts would facilitate more precise pooled estimates (40). Lastly, it is important to emphasize this was a retrospective observational study; therefore, associations are hypothesis-generating and should be interpreted cautiously. Although inverse probability weighting is commonly used to address confounding by indication and several severity indicators were included to limit this bias, residual confounding remains a possibility. Mean baseline LVEF was slightly lower in the anakinra group (52% vs. 55%), so while weighting achieved similar LVEF distributions in both treatment groups, other unmeasured indicators of cardiac injury may introduce confounding and could explain why our point estimates appeared to favor IVIG and glucocorticoids. However, any unmeasured confounder would need to have a rather large effect to explain our results.
In summary, we identified substantial variation in use of anakinra as initial treatment for MIS-C in the setting of uncertain effectiveness and rapid development of local standards and treatment protocols. Our observational data do not provide evidence to support routine addition of anakinra to initial treatment with IVIG and glucocorticoids in most children hospitalized with MIS-C. Although the rare incidence of MIS-C impeded assessment of clinical efficacy in a randomized trial, retrospective analyses of real-world clinical experiences can inform future MIS-C treatment guidelines and comparative study designs. Additional understanding of the pathogenesis and treatment outcomes of MIS-C is needed to determine which rational therapies can augment or replace broadly immunomodulating agents and reduce their adverse effects. In addition, the role of targeted cytokine inhibition for patients with contraindications to broad immunomodulation or those requiring adjunctive rescue therapy may warrant evaluation.
Supplementary Material
Funding:
This work was funded by the Centers for Disease Control and Prevention. J.C.C was supported by the National Institutes of Health (NIH) [K23-HL148539]. E.M. and S.K.S.T were supported by NIH R61 HD105593. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Conflicts of Interest: All relevant disclosures are listed on the disclosure forms provided by the authors.
References
- 1.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med 2020;383:334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Son MBF, Murray N, Friedman K, Young CC, Newhams MM, Feldstein LR, et al. Multisystem Inflammatory Syndrome in Children — Initial Therapy and Outcomes. N Engl J Med 2021;385:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaneko N, Kurata M, Yamamoto T, Morikawa S, Masumoto J. The role of interleukin-1 in general pathology. Inflamm Regener 2019;39:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Della Paolera S, Valencic E, Piscianz E, Moressa V, Tommasini A, Sagredini R, et al. Case Report: Use of Anakinra in Multisystem Inflammatory Syndrome During COVID-19 Pandemic. Frontiers in Pediatrics 2021;8:943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, et al. Acute Heart Failure in Multisystem Inflammatory Syndrome in Children in the Context of Global SARS-CoV-2 Pandemic. Circulation 2020;142:429–436. [DOI] [PubMed] [Google Scholar]
- 6.Lee PY, Day-Lewis M, Henderson LA, Friedman KG, Lo J, Roberts JE, et al. Distinct clinical and immunological features of SARS–CoV-2–induced multisystem inflammatory syndrome in children. Journal of Clinical Investigation 2020: 10.1172/JCI141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller J, Cantor A, Zachariah P, Ahn D, Martinez M, Margolis KG. Gastrointestinal Symptoms as a Major Presentation Component of a Novel Multisystem Inflammatory Syndrome in Children That Is Related to Coronavirus Disease 2019: A Single Center Experience of 44 Cases. Gastroenterology 2020;159:1571–1574.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mastrolia MV, Marrani E, Calabri GB, L’Erario M, Maccora I, Favilli S, et al. Fast recovery of cardiac function in PIMS-TS patients early using intravenous anti-IL-1 treatment. Crit Care 2021;25:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mastrolia MV, Marrani E, Maccora I, Pagnini I, Simonini G. The Role of Anti-IL-1 Treatment in MIS-C Patients. Expert Opinion on Biological Therapy 2022;22:1–5. [DOI] [PubMed] [Google Scholar]
- 10.Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS–CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 3. Arthritis & Rheumatology 2022;74:e1–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller AD, Zambrano LD, Yousaf AR, Abrams JY, Meng L, Wu MJ, et al. Multisystem Inflammatory Syndrome in Children—United States, February 2020–July 2021. Clinical Infectious Diseases 2021:ciab1007. [DOI] [PubMed] [Google Scholar]
- 12.Santos MO, Gonçalves LC, Silva PAN, Moreira ALE, Ito CRM, Peixoto FAO, et al. Multisystem inflammatory syndrome (MIS-C): a systematic review and meta-analysis of clinical characteristics, treatment, and outcomes. Jornal de Pediatria 2021:S0021755721001480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). Emergency preparedness and response: HAN00432. Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19) 2020. Available at: https://emergency.cdc.gov/han/2020/han00432.asp. Accessed June 6, 2022. [Google Scholar]
- 14.Feldstein LR, Tenforde MW, Friedman KG, Newhams M, Rose EB, Dapul H, et al. Characteristics and Outcomes of US Children and Adolescents With Multisystem Inflammatory Syndrome in Children (MIS-C) Compared With Severe Acute COVID-19. JAMA 2021;325:1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matics TJ, Sanchez-Pinto LN. Adaptation and Validation of a Pediatric Sequential Organ Failure Assessment Score and Evaluation of the Sepsis-3 Definitions in Critically Ill Children. JAMA Pediatr 2017;171:e172352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravelli A, Minoia F, Davì S, Horne A, Bovis F, Pistorio A, et al. 2016 Classification Criteria for Macrophage Activation Syndrome Complicating Systemic Juvenile Idiopathic Arthritis: A European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborat: EULAR/ACR CLASSIFICATION CRITERIA FOR MAS. Arthritis & Rheumatology 2016;68:566–576. [DOI] [PubMed] [Google Scholar]
- 17.Abrams JY, Oster ME, Godfred-Cato SE, Bryant B, Datta SD, Campbell AP, et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: a retrospective surveillance study. Lancet Child Adolesc Health 2021;5:323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol 2006;163:1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crump RK, Hotz VJ, Imbens GW, Mitnik OA. Dealing with limited overlap in estimation of average treatment effects. Biometrika 2009;96:187–199. [Google Scholar]
- 20.Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D. Use of Stabilized Inverse Propensity Scores as Weights to Directly Estimate Relative Risk and Its Confidence Intervals. Value in Health 2010;13:273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008;168:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen T-L, Collins GS, Spence J, Daurès J-P, Devereaux PJ, Landais P, et al. Double-adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance. BMC Med Res Methodol 2017;17:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brisca G, Consolaro A, Caorsi R, Pirlo D, Tuo G, Campanello C, et al. Timely Recognition and Early Multi-Step Antinflammatory Therapy May Prevent ICU Admission of Patients With MIS-C: Proposal for a Severity Score. Front Pediatr 2021;9:783745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belhadjer Z, Auriau J, Méot M, Oualha M, Renolleau S, Houyel L, et al. Addition of Corticosteroids to Immunoglobulins Is Associated With Recovery of Cardiac Function in Multi-Inflammatory Syndrome in Children. Circulation 2020;142:2282–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu YP, Shamie I, Lee JC, Nowell CJ, Peng W, Angulo S, et al. Immune response to intravenous immunoglobulin in patients with Kawasaki disease and MIS-C. Journal of Clinical Investigation 2021;131:e147076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Consiglio CR, Cotugno N, Sardh F, Pou C, Amodio D, Rodriguez L, et al. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell 2020;183:968–981.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeifer J, Thurner B, Kessel C, Fadle N, Kheiroddin P, Regitz E, et al. Autoantibodies against interleukin-1 receptor antagonist in multisystem inflammatory syndrome in children: a multicentre, retrospective, cohort study. The Lancet Rheumatology 2022;4:e329–e337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thurner L, Kessel C, Fadle N, Regitz E, Seidel F, Kindermann I, et al. IL-1RA Antibodies in Myocarditis after SARS-CoV-2 Vaccination. N Engl J Med 2022:NEJMc2205667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA 2020;324:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Capone CA, Subramony A, Sweberg T, Schneider J, Shah S, Rubin L, et al. Characteristics, Cardiac Involvement, and Outcomes of Multisystem Inflammatory Syndrome of Childhood Associated with severe acute respiratory syndrome coronavirus 2 Infection. The Journal of Pediatrics 2020;224:141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koné-Paut I, Tellier S, Belot A, Brochard K, Guitton C, Marie I, et al. Phase II Open Label Study of Anakinra in Intravenous Immunoglobulin–Resistant Kawasaki Disease. Arthritis Rheumatol 2021;73:151–161. [DOI] [PubMed] [Google Scholar]
- 32.Cole LD, Osborne CM, Silveira LJ, Rao S, Lockwood JM, Kunkel MJ, et al. IVIG Compared to IVIG Plus Infliximab in Multisystem Inflammatory Syndrome in Children. Pediatrics 2021:e2021052702. [DOI] [PubMed] [Google Scholar]
- 33.Jain PN, Acosta S, Annapragada A, Checchia PA, Moreira A, Muscal E, et al. Comparison of Laboratory and Hemodynamic Time Series Data Across Original, Alpha, and Delta Variants in Patients With Multisystem Inflammatory Syndrome in Children. Pediatric Critical Care Medicine 2022;Publish Ahead of Print. Available at: https://journals.lww.com/10.1097/PCC.0000000000002976. Accessed May 17, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stierman B, Abrams JY, Godfred-Cato SE, Oster ME, Meng L, Yip L, et al. Racial and Ethnic Disparities in Multisystem Inflammatory Syndrome in Children in the United States, March 2020 to February 2021. Pediatric Infectious Disease Journal 2021;40:e400–e406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee EH, Kepler KL, Geevarughese A, Paneth-Pollak R, Dorsinville MS, Ngai S, et al. Race/Ethnicity Among Children With COVID-19–Associated Multisystem Inflammatory Syndrome. JAMA Netw Open 2020;3:e2030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Savorgnan F, Acosta S, Alali A, Moreira A, Annapragada A, Rusin CG, et al. Social and Demographic Disparities in the Severity of Multisystem Inflammatory Syndrome in Children. Pediatric Infectious Disease Journal 2022;41:e256–e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Son MBF, Berbert L, Young C, Dallas J, Newhams M, Chen S, et al. Postdischarge Glucocorticoid Use and Clinical Outcomes of Multisystem Inflammatory Syndrome in Children. JAMA Netw Open 2022;5:e2241622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zachariah P Severity predictors in pediatric SARS-CoV-2 and MIS-C. The Journal of Pediatrics 2021;232:307–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harwood R, Allin B, Jones CE, Whittaker E, Ramnarayan P, Ramanan AV, et al. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): results of a national Delphi process. The Lancet Child & Adolescent Health 2021;5:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernán MA. Causal analyses of existing databases: no power calculations required. Journal of Clinical Epidemiology 2022;144:203–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.