Summary
Long noncoding RNA (lncRNA) plays crucial roles in the development of gastric cancer (GC); however, studies of their mechanisms of action are needed to determine their clinical value. The aim of this study is to explore the effects and mechanisms of THUMPD3-AS1 in GC. Elevated levels of THUMPD3-AS1 were observed in GC and demonstrated a significant positive correlation with poor prognosis. Functionally, THUMPD3-AS1 promoted GC cell proliferation, migration, invasion, and epithelial-mesenchymal transition (EMT) and induced tumor growth in vivo. THUMPD3-AS1 exerts its regulatory function on BCAT1 through competitive binding with miR-1297. Further investigations confirmed that both THUMPD3-AS1 and miR-1297 interact with BCAT1. These findings suggest that THUMPD3-AS1 promotes GC invasion and EMT by regulating the miR-1297/BCAT1 pathway, indicating that THUMPD3-AS1 may serve as a biomarker and therapeutic target for GC.
Subject areas: Biological sciences, Molecular biology, Bioinformatics, Cancer
Graphical abstract

Highlights
-
•
THUMPD3-AS1 promotes the growth and metastasis of GC in vitro and in vivo
-
•
THUMPD3-AS1 tumorigenic effect was partially reversed by overexpression of miR-1297
-
•
THUMPD3-AS1, miR-1297, and BCAT1 three loci have binding sites for each other in GC
Biological sciences; Molecular biology; Bioinformatics; Cancer
Introduction
Gastric cancer (GC) is a kind of malignant tumor with high incidence worldwide, which is a serious threat to human health. The absence of obvious clinical symptoms in the initial stages of GC usually caused delayed diagnosis and prognosis. Meanwhile, recurrence and metastasis are also the major causes of death in patients.1,2,3 At present, the clinical diagnosis of GC still relies on serum markers, but the detection of markers lacks specificity and sensitivity, resulting in poor efficacy. Therefore, it is urgent to mine corresponding targeted genes, explore potential molecular mechanisms, and identify biomarkers.4 In recent years, a large number of studies have found abnormal expression of long noncoding RNA (lncRNA) in tumor tissues,5,6,7,8 in which a considerable part of lncRNA molecules are closely related to the occurrence and development of GC.9,10,11 lncRNA,12,13,14 as a functional RNA molecule, does not encode proteins, but influences the expression of protein-coding genes by recruiting or isolating gene regulatory proteins, and expresses specific gene expression patterns during cancer development.15,16 A lot of research has shown that lncRNA has been shown to play a key role in cancer regulation of cell proliferation, migration, and invasion, and induction of epithelial-mesenchymal transformation (EMT),17,18,19 thereby participating in the regulation of apoptosis, promoting immune escape, and affecting the occurrence and development of cancer. lncRNA THUMPD3-AS1 is currently a very hot research site..20,21,22 Studies show that THUMPD3-AS1 is involved in the occurrence and development of lung cancer and colorectal cancer, and is closely related to lymphatic metastasis and other risk factors. However, its specific role and mode of action in GC are still not completely clear. Therefore, exploring the mechanism of action and exploring related studies in GC will further help to discover the gene targets, and provide different strategies and directions for clinical diagnosis and treatment. In this study, a large number of bioinformatics methods were used to screen relevant lncRNAs, and the role of THUMPD3-AS1 in GC progression through the competitive endogenous RNA (ceRNA) mechanism was further explored through in vivo and in vitro model validation. The results of this study lay the foundation for elucidating the underlying mechanism of GC progression and identifying potential target genes, which may be more helpful in identifying biomarkers and therapeutic targets for GC diagnosis, leading to more effective GC treatment therapies and better patient prognosis and survival.
Results
Differentially expressed lncRNAs in GC
Significantly differentially expressed lncRNAs based on recent GC data were obtained (|logFC| > 1, FDR <0.05). A total of 1043 differentially expressed lncRNAs and 2841 differentially expressed genes were obtained (Figures 1A and 1B). The differentially expressed lncRNAs and genes can significantly distinguish normal samples from cancer samples (Figure 1C). Based on a corrected p value of <0.05 and expression change of >2-fold, candidate genes were identified. As shown in a heatmap (Figure 1C), the differentially expressed lncRNAs in cancer tissues were significantly upregulated.
Figure 1.
Differentially expressed lncRNAs in gastric cancer
Histogram, volcano plots, and heatmaps are shown.
(A) Histogram.
(B) Volcano plots.
(C) Heatmaps.
Identification of candidate ceRNA pairs in GC
According to known lncRNAs in databases and miRNA targets of genes, overlap in target miRNA sets for each differentially expressed lncRNA-gene pair was evaluated. The results uncovered top-ranked lncRNAs and interacting genes. THUMPD3-AS1 was highly ranked among lncRNAs (Figure 2A) and BRCA1, RACGAP1, and others were top-ranked among mRNAs with significant differences (Figure 2B). A boxplot displays the number of candidate ceRNAs that were significantly associated with each gene or lncRNA (Figure 2C) and the p values for all candidate ceRNAs that were significantly related to the gene or lncRNA were obtained (Figure 2D). For candidate lncRNA-gene pairs with significant correlations determined by the hypergeometric test, Pearson correlation coefficients in cancer samples and normal samples were calculated for all differentially expressed genes and lncRNAs (Figure 2E). There were 4457 significantly correlated pairs in cancer samples and 11097 significantly correlated pairs in normal samples. Sixty-four lncRNA-gene pairs with significant correlations in cancer samples were identified as candidates by the random perturbation method for subsequent analyses.
Figure 2.
Identification of candidate ceRNA pairs in gastric cancer
(A) lncRNAs with significant expression differences.
(B) mRNAs with significant expression differences.
(C) Boxplot of lncRNAs in ceRNA networks.
(D) Boxplot of mRNAs in ceRNA networks.
(E) Pearson correlation coefficients for ceRNA pairs.
Prognosis and functional enrichment analyses of candidate ceRNA pairs
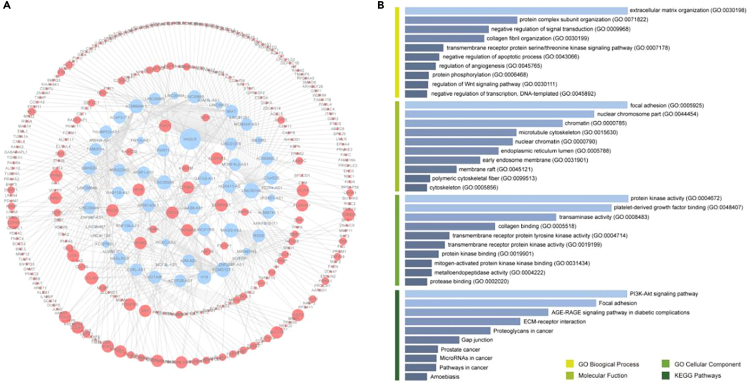
We performed a survival analysis of 2298 candidate lncRNA-gene pairs in GC to examine associations with prognosis. As a result, 460 ceRNA pairs related to survival were obtained. HAGLR had the highest number of connections in the functional protein association network, indicating its central role in protein interactions and signaling pathways (Figure 3A). The genes in the ceRNA network were subjected to functional enrichment analyses. Candidate ceRNA pairs were mainly enriched in extracellular matrix tissue, focal adhesions, PI3K-AKT, and other signaling pathways, indicating that they are related to stromal tissue remodeling and tumor cell migration (Figure 3B).
Figure 3.
Results of a prognostic analysis and enrichment analysis of candidate ceRNA pairs
(A) Prognostic value of candidate ceRNA pairs.
(B) GO and KEGG pathway enrichment analyses of candidate ceRNA pairs.
Expression and prognostic value of THUMPD3-AS1-BCAT1 in tissues and cell lines
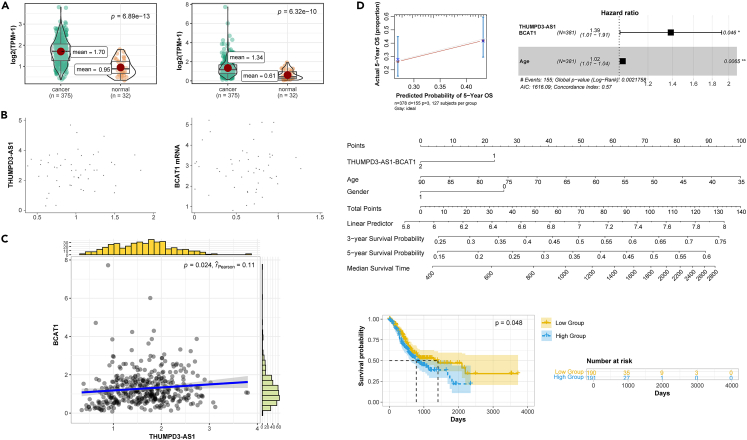
THUMPD3-AS1-BCAT1 is an important pair of candidate ceRNAs. The expression level of the THUMPD3-AS1 pair in the tumor group was significantly higher than that in the normal group (Figure 4A). A dot plot demonstrated that THUMPD3-AS1 and BCAT1 exhibited a general upward trend in 50 GC tissues (Figure 4B). A series of analyses revealed that THUMPD3-AS1-BCAT1, an important pair of ceRNAs, is highly expressed in cancer, with significantly correlated expression. A correlation analysis revealed that the expression levels of BCAT1 and THUMPD3-AS1 were significantly positively correlated, and the distributions of expression levels were both unimodal (Figure 4C). The relationship between THUMPD3-AS1-BCAT1 and overall survival was analyzed based on a multivariate Cox regression analysis. The actual 5-year overall survival and predicted 5-year overall survival were significantly positively correlated, indicating that the model was highly effective (Figure 4D). The risk rate of the BCAT1 and THUMPD3-AS1 treatment group was 1.4 times that of the control group (Figure 4D). A survival curve analysis of The Cancer Genome Atlas (TCGA) data revealed that patients with high expression of THUMPD3-AS1 had significantly lower survival rates compared to those with low expression. This suggests that the expression of THUMPD3-AS1 is significantly associated with patient survival (Figure 4D).
Figure 4.
Expression levels of THUMPD3-AS1-BCAT1 in tissues and cell lines and its prognostic value
(A) Boxplot (TCGA) of the differential expression of THUMPD3-AS1 and BCAT1 in gastric cancer tissues.
(B) Scatterplot of the differential expression in tissues.
(C) Correlation analysis of THUMPD3-AS1 and BCAT1 expression levels in gastric cancer tissues.
(D) Prognostic analysis based on TCGA data.
Effects of THUMPD3-AS1 on cell viability and invasion
THUMPD3-AS1 was differentially expressed in five cell lines, including GES-1, AGS, and HGC27, with the weakest expression in GES-1 (Figure 5A). In an overexpression experiment of THUMPD3-AS1-siRNA, miR1297 expression was significantly upregulated in the transfection group but significantly lower compared to the vehicle control and negative control groups (Figure 5B). Conversely, during the overexpression of THUMPD3-AS1, the vehicle control and negative control groups exhibited higher expression levels than the transfection group. An analysis of THUMPD3-AS1 inhibition revealed that cell viability in the transfection group was notably higher than that in the vehicle control and negative control groups from 0 to 72 h. As shown in Figure 5C, cell viability was increased in the overexpression group and decreased in the inhibition group compared to the control group. Following the overexpression of THUMPD3-AS1, the cell wound healing rate in the transfection group exhibited a notable increase compared to both the control and negative groups. Additionally, the number of cells migrating through the membrane and the expression levels of associated genes were significantly higher in the transfection group than in the control and negative groups. Conversely, upon inhibition, the wound healing rate of cells in the transfection group decreased substantially compared to the control and negative groups, with a corresponding reduction in the number of cells migrating through the membrane and the expression levels of related genes (Figure 5D).
Figure 5.
Effect of THUMPD3-AS1 on cell viability and invasion
(A) Expression of THUMPD3-AS1 in different cell lines.
(B) THUMPD3-AS1-siRNA inhibition/overexpression assays.
(C) Comparison of cell viability.
(D) Alterations in migration, invasion, and levels of invasion- and EMT-related genes (MMP-2, MMP-9, TIMP-3; E-cadherin, N-cadherin, and Vimentin) after the inhibition and overexpression of THUMPD3-AS1.
In vivo validation
Upon transfection with MNK45 (an inhibitor of THUMPD3-AS1), a notable reduction in both tumor volume and weight was observed in comparison to the control group (Figure 6A). In solid tumor specimens, the dimensions of the tumors in the treatment group were markedly smaller than those in the control group (Figure 6B). Furthermore, the levels of TIMP3 and E-cadherin in the transfection group were notably elevated compared to other protein levels, whereas the concentrations of the remaining proteins were consistently lower in the transfection group relative to the control group (Figure 6C).
Figure 6.
In vivo validation results
(A) Tumor volume and weight.
(B) Tumor comparison.
(C) Detection of protein levels (western blotting).
Relationship between THUMPD3-AS1 and miR-1297
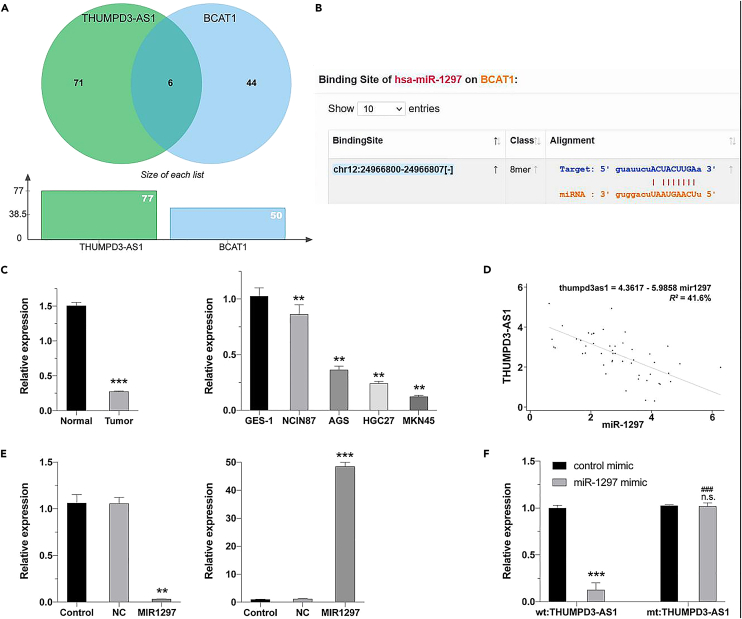
As determined by the hypergeometric test, the target miRNA sets of THUMPD3-AS1 and BCAT1 had significant overlap (Figure 7A), and miRDB predicted that THUMPD3-AS1 had a miR-1297 binding site (Figure 7B). In 50 clinical samples, miR-1297 levels were significantly lower in tumor tissues than in paracancerous tissues, further verifying that this locus acts as a tumor suppressor in GC tissue; however, the latter was significantly lower than the former in analyses of the overexpression and inhibition of THUMPD3-AS1 expression (Figure 7C). The expression levels of THUMPD3-AS1 and miR-1297 in 50 GC tissues were negatively correlated (Figure 7D). In the THUMPD3-AS1 overexpression experiment, miR-1297 levels were significantly higher in the transfection group than in the vehicle control group and the negative control group (Figure 7E). A dual luciferase reporter assay showed that derivative THUMPD3-AS1 affected downstream gene transcription and translation and that luciferase levels decreased at 560 nm. Additionally, THUMPD3-AS1 had a binding site for miR-1297 (Figure 7F).
Figure 7.
Relationship between THUMPD3-AS1 and miR-1297 expression
(A) Hypergeometric test results for the identification of target miRNAs of THUMPD3-AS1 and BCAT1.
(B) Online prediction results using miRDB.
(C) Expression levels of miR-1297 in tissues and cell lines.
(D) Expression levels of THUMPD3-AS1 and miR-1297 in tissues were negatively correlated.
(E) Effect of the overexpression and inhibition of THUMPD3-AS1 on miR-1297 levels.
(F) Results of a dual-luciferase reporter assay.
Relationship between BCAT1 mRNA and miR-1297
In terms of the binding relationship between BCAT1 mRNA and miR-1297 (Figure 8A), in 50 GC tissues, BCAT1 mRNA levels were significantly higher in tumor tissues than in paracancerous tissues (Figure 8B). The expression levels of THUMPD3-AS1 and BCAT1 mRNA in tissues were positively correlated (Figure 8C), while the expression level of miR-1297 exhibited a negative correlation with the BCAT1 mRNA expression in tissues (Figure 8D). BCAT1 mRNA levels were lowest in the GES-1 cell line, highest in the MKN45 cell line, and significantly higher in the MKN45 cell line than in the NCIN87 cell line (Figure 8E). When miR-1297 was overexpressed, BCAT1 expression was evaluated at the mRNA and protein levels. BCAT1 mRNA levels in the transfection group were significantly lower than those in the vehicle control group and the negative control group (Figure 8F). When miR-1297 was inhibited, BCAT1 mRNA levels in the transfection group were significantly higher than those in the vehicle control group and the negative control group (Figure 8G). Derivative BCAT1 affected the transcription and translation of downstream genes, and luciferase activity decreased at 560 nm, while the mutant had no effect on transcription and translation. These findings indicated that BCAT1 had a binding site for miR-1297 (Figure 8H).
Figure 8.
Relationship between BCAT1 mRNA and miR-1297 levels
(A) Online prediction using TargetScan and miRDB.
(B) BCAT1 mRNA expression in tissues.
(C) THUMPD3-AS1 levels were positively correlated with mRNA expression levels of BCAT1 in tissues.
(D) miR-1297 levels were negatively correlated with BCAT1 mRNA expression levels in tissues.
(E) Expression levels of BCAT1 mRNA in different cell lines.
(F) Effect of the overexpression of miR-1297 on BCAT1 mRNA and protein levels.
(G) Effect of the inhibition of miR-1297 on BCAT1 mRNA and protein levels.
(H) Dual-luciferase reporter assay.
Effect of the THUMPD3-AS1/miR-1297 axis on the viability, invasion, and migration of GC cells
When THUMPD3-AS1 was inhibited, BCAT1 mRNA and protein expression levels were notably higher in the control and negative groups than in the THUMPD3-AS1-siRNA group. Its expression in the THUMPD3-AS1-siRNA+miR-1297 inhibition group was slightly lower than that in the THUMPD3-AS1-siRNA group (Figure 9A). When THUMPD3-AS1 was overexpressed, BCAT1 mRNA and protein expression levels were notably lower in the negative control group than in the overexpression group. The expression was low in the THUMPD3-AS1overexpression+miR-1297 mimic group (Figure 9B). The cell viability, wound healing rate, and number of cells that migrated through the membrane were considerably lower in the THUMPD3-AS1-siRNA group than in the THUMPD3-AS1-siRNA+miR-1297 inhibition group (Figure 9C). When THUMPD3-AS1 was overexpressed, the cell viability, wound healing rate, and number of cells that migrated through the membrane were notably higher than those in the mimic group (Figure 9D).
Figure 9.
Effects of the THUMPD3-AS1/miR-1297 axis on the activity, invasion, and migration ability of gastric cancer cells
(A) Effect of THUMPD3-AS1 inhibition on BCAT1 mRNA and protein levels in cells (control group, negative group, THUMPD3-AS1-siRNA group, THUMPD3-AS1-siRNA+miR-1297 inhibition group).
(B) Effect of THUMPD3-AS1 overexpression on BCAT1 mRNA and protein levels in cells (negative group, THUMPD3-AS1 overexpression group, THUMPD3-AS1 overexpression + miR-1297 mimics group).
(C) Effects of THUMPD3-AS1 inhibition on cell viability, migration, and invasion (vehicle control group, negative control group, THUMPD3-AS1-siRNA group, THUMPD3-AS1-siRNA+miR-1297 inhibition group).
(D) Effect of THUMPD3-AS1 overexpression on cell migration and invasion (negative control group, THUMPD3-AS1 overexpression group, THUMPD3-AS1 overexpression+miR-1297 mimics group).
Discussion
GC distant metastasis is an important cause of high recurrence rate and mortality.23,24 Therefore, in-depth exploration of the invasion and metastasis mechanism of gastric cancer and identification of key molecules in it have important potential application value in gastric cancer research.25,26,27 As a key regulatory factor of cancer, lncRNAs regulate the expression of cancer-related genes at different levels. Convincing evidence indicates that lncRNAs play an important role in the pathogenesis of GC and have great value for GC diagnosis and treatment.8,27,28,29 These abnormal lncRNAs are involved in various behaviors of GC cells, including proliferation, apoptosis, metastasis, drug resistance, etc. In this study, our main objective was to identify and validate an important lncRNA THUMPD3-AS1 and investigate its regulatory mechanisms for gastric cancer cell invasion and EMT in the context of gastric cancer by using clinical tissue samples and extensive biological analysis.
Although the function of lncRNA THUMPD3-AS1 in tumors is unknown,30,31 it has been reported to be significantly elevated in a variety of cancer tissues, and studies have shown that THUMPD3-AS1 enhances the proliferation and invasiveness of colorectal, non-small-cell lung, and hepatocellular carcinoma cells.32,33 These results suggest that THUMPD3-AS1 is commonly highly expressed in tumors with proto-oncogene characteristics. However, another study showed that lncRNA THUMPD3-AS1 levels were significantly reduced in GC tissues and cells. However, this study was an independent study, using Kaplan-Meier and multivariate Cox regression analysis, and no extensive bioinformatics screening was performed. Also, extensive in vivo and in vitro validation has not been performed, and further studies are needed to clarify these conflicting results. In this study, we complemented and improved on the previous study by screening the relevant lncRNA by bioinformatics methods and validated by extensive in vivo and in vitro models. Mining and analysis of TCGA data showed that the expression level of THUMPD3-AS1 was significantly higher in gastric cancer tissues compared with normal tissues and positively correlated with invasive metastasis of gastric cancer. We confirmed these findings by examining the levels of THUMPD3-AS1 in 50 gastric cancer tissues and found that the levels were higher in cancer tissues than in paracancerous tissues. Functional experiments showed that THUMPD3-AS1 promotes the growth and metastasis of gastric cancer both in vitro and in vivo. The present findings suggest that THUMPD3-AS1 may function through regulation of BCAT1, as it acts as a “sponge” for hsa-miR-1297 in cells.
It was shown that exogenous overexpression of miR-1297 in GC inhibits cell proliferation and induces apoptosis,34 and inhibition of miR-1297 promotes cell proliferation and reduces apoptosis in vitro. circ_PGPEP1, a sponge of miR-1297, promotes GC progression by regulating E2F3, and also by downregulating CDC6 expression to inhibit GC cell proliferation and promote apoptosis.18,24,35,36 Other studies suggest that miR-1297 may act as an antitumor molecule in lung cancer, pancreatic cancer,14,37 and colorectal cancer.38 Indeed, miR-1297 showed low expression levels in GC tissues. Dual luciferase reporter gene assays showed that THUMPD3-AS1 bound to miR-1297 and that the tumorigenic effect of THUMPD3-AS1 in GC could be partially reversed by miR-1297 overexpression.27,32 BCAT1 is involved in the regulation of gastric cancer cell growth as a key catalase, and its overexpression was associated with TNM staging, local invasion, lymph node involvement, and metastasis. In the present study, a dual luciferase reporter assay indicated that BCAT1 bound to miR-1297. miR-1297 overexpression resulted in significantly lower BCAT1 levels in the transfected group than in the drug-loaded control and negative control groups. In contrast, when miR-1297 was inhibited, BCAT1 levels were significantly higher than those of the control group. BCAT1 is predicted to be a target of miR-1297.8,12 Previous reports have shown that BCAT1 overexpression promotes the proliferation of colorectal cancer,39,40 lung cancer,41 hepatocellular carcinoma,19,42 and head and neck squamous cell carcinoma for proliferation, invasion, and metastasis. This study confirmed the correlation between the expression levels of miR-1297 and BCAT1 in gastric cancer and highlighted their effects on cancer cell migration and invasion.
This research represents the pioneering investigation into the clinical importance and roles of THUMPD3-AS1, miR-1297, and BCAT1 in GC. The three loci have binding sites for each other, further suggesting that THUMPD3-AS1 promotes tumor metastasis via the regulation of mRNA. We preliminarily conclude that the highly expressed lncRNA THUMPD3-AS1 regulates the expression of BCAT1 in the tumor by specifically reducing the expression of miR-1297, thereby promoting the invasion and EMT of GC cells.
Limitations of the study
There are some limitations to our study at this time. We performed some bioinformatics analyses and cellular experiments, but the number of clinical samples is small and prospective studies are lacking. Future studies will involve collecting more clinical samples for more detailed analysis of THUMPD3-AS1, BCAT1, and miR-1297. The study should pay attention to expanding clinical samples and increasing in vivo and in vitro validation experiments, as well as refining the acquisition of clinical tissues, paying attention to the reproducibility of validation and the timeliness and accuracy of data acquisition. This study did not carry out large-scale follow-up clinical expression research and clinical practice research, which involves a large amount of bioinformatics, and we will expand the research targets in the later stage, to further discover and validate the expression of the relevant molecules, so that the study will provide a better basis for subsequent molecular screening and mechanisms. We will expand the study population in the future to further discover and verify the expression of relevant molecules, so as to better provide ideas for the screening of subsequent molecules and mechanism research.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| nerve calmodulin (N-cadherin) antibody | Merck | C9848; RRID: AB_476894 |
| epithelial calmodulin (E-cadherin) antibody | Merck | A1978; RRID: AB_476692 |
| glyceraldehyde-3-phosphate dehydrogenase antibody | Merck | HPA004812; RRID: AB_1078369 |
| β-actin antibody | Merck | MAB374; RRID: AB_2107445 |
| Biological samples | ||
| Gastric cancer tissue and normal paracancerous tissue | The Fourth Hospital of Hebei Medical University | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Fet al bovine serum | Merck | F8687 |
| DMEM medium | Merck | D6429-500ML |
| Streptomycin | Merck | 3810-74-0 |
| Basement membrane, Transwell | Merck | CLS6571 |
| CCK8 | MCE | HY-K0301 |
| Trypsin | thermofisher | 78442 |
| Penicillin and streptomycin | thermofisher | 15140148 |
| PCR | MCE | HY-K0501A |
| Phosphate buffer | thermofisher | 003002 |
| Critical commercial assays | ||
| Dual luciferase activity kit | abcam | ab246539 |
| BCA Kit | Merck | BCA1-1KT |
| CCK8 kit | MCE | HY-K0301 |
| Trizol kits, fluorescence quantification kits | Merck | MAK043 |
| Reverse transcription kits | thermofisher | K1691 |
| Deposited data | ||
| TCGA Database | This paper, mendeley Date | http://cancergenome.nih.gov/ |
| TarBase database (version 6.0), miRTarBase database (version 7.0) | This paper |
https://doi.org/10.17632/69ckvtv5xh.1 http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=tarbasev8%2Findex |
| miRecords (version 4.0) DIANA-LncBase v2.0 Database |
This paper, mendeley Date |
https://doi.org/10.17632/69ckvtv5xh.1 http://mirecords.biolead.org |
| Experimental models: Cell lines | ||
| Gastric cancer cell line AGS | Beijing qualityard biotechnology Co., Ltd. | QYC0007 |
| SGC7901 | Beijing qualityard biotechnology Co., Ltd. | QYC0008 |
| HGC-27 | Beijing qualityard biotechnology Co., Ltd. | QYC0003 |
| Experimental models: Organisms/strains | ||
| BALB/c naked mouse | SLAC Laboratory Animal Center | KY023 |
| Oligonucleotides | ||
| hsa-miR-1297 PCR: Upstream primers: TTCAAGTAATTCAGGTGGTCGT Downstream primers: GTCGTATCCAGTGCAGGGT |
Merck | HLMIR0167 |
| U6 Upstream primers: 5′-CTCGCTTCGGCAGCACA-3′ Downstream primers: 5′-AACGCTTCACGAATTTGCGT-3′ THUMPD3-AS1 Upstream primers: ATTCTGTCCCTGACCGTCT Downstream primers: GTTCTCTTCCTGTTTCCACAC BCAT1 Upstream primers: TGTATCGCTCTGCTGTGAGG Downstream primers: CAGTTCCAATGAATGTAGGACG |
Merck | SAB1402701 |
| β-actin: Upstream primers: GGTCATCACCATTGGCAA Downstream primers: GAGTTGAAGGTAGTTTCGTGGA |
Merck | A5316 |
| Software and algorithms | ||
| cox regression coefficient | This paper | N/A |
| column line graph | This paper | N/A |
| Differential expression analysis | This paper | N/A |
| hypergeometric test | This paper | N/A |
| Pearson correlation | This paper | N/A |
| enrichment analysis | This paper | N/A |
| t-test | This paper | N/A |
| log-rank test | This paper | N/A |
| Other | ||
| Related sequence data, analysis and resources | This paper |
https://doi.org/10.17632/69ckvtv5xh.1 https://data.mendeley.com/datasets/69ckvtv5xh/1 |
| 96-well cell culture plate | MCE | HY-K0301 |
| CO2 incubator | MCE | Z331732 |
| Alcohol lamp, micro pipette | MCE | PSHT004S5 |
| ELISA | MCE | MAK159 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Bibo Tan (tanbibo@hebmu.edu.cn.).
Materials availability
Requests for materials should be directed to the corresponding authors.
Experimental model and subject details
Clinical samples
In this study, we included a total of 50 patients with gastric cancer, aged 40–75 years, with no restriction on gender, ancestry, race or ethnicity or socioeconomic information, with surgical treatment dates from January 2019 to June 2022, with matched gastric and paracancerous tissue samples, none of whom had received radiotherapy or chemotherapy, and no other cancer co-morbidities. During surgery, tumor tissues and paracancerous tissues were collected, immediately placed in liquid nitrogen, and then transferred to a refrigerator at −80°C for storage. All enrolled patients gave informed consent before this study, and the study protocol was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University (Ethics No. 2019MEC039).
For animal studies
We selected 20 BALB/c nude mice (Cell Resource Center, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, China), sexed as males, to avoid female breeding, with an average body weight of about 2 kg, age of about 4–5 weeks old, and a controlled body length of 6–8 cm, which were well-proportioned and well-grown. The nude mice were placed in a specific pathogen-free environment at 23°C–25°C. Weekly subcutaneous injections were performed to establish xenograft tumors and measure the size of the tumors, and after 4 weeks, we handled and removed the tumors to determine their size and weight, and fixed the xenograft tumors with formalin for follow-up studies. All procedures followed the guidelines for the care and use of laboratory animals established by the Fourth Hospital of Hebei Medical University, in accordance with the regulations of the Animal Protection and Use Committee of Hebei Medical University, and were also approved by the Medical Ethics Committee of the Fourth Hospital of Hebei Medical University.
For cell lines
Human GC cell lines AGS, SGC7901, HGC-27(Beijing qualityard biotechnology Co., Ltd.) were purchased from the ATCC and routinely checked for mycoplasma contamination using the Vazyme Mycoplasma detection kit. Nude mice were purchased from SLAC Laboratory Animal Center. primers were designed based on the sequences of THUMPD3-AS1, miR-1297, BCAT1, U6 (internal control) and β-actin (internal control) and synthesized by Merck. Fet al bovine serum, Dulbecco’s modified Eagle’s medium (DMEM), streptomycin, β-actin antibody, recombinant basement membrane and Transwell were purchased from Merck. CCK-8 reagent, trypsin, penicillin and streptomycin, and phosphate-buffered saline (PBS) were purchased from MCE and Dual luciferase reporter assay kits and BCA kits were purchased from abcam and Merck. N-cadherin, E-cadherin and glyceraldehyde-3-phosphate dehydrogenase antibodies were purchased from Merck.
Method details
Culture conditions
Cells were meticulously cultured in DMEM supplemented with 10% foet al bovine serum, 100 U/mL of penicillin, and 100 mg/mL of streptomycin. The carefully regulated incubation environment maintained a consistent 5% CO2-saturated humidity and a stable temperature of 37°C to facilitate optimal cell growth and proliferation. Upon attaining a confluent monolayer, the medium was carefully removed, and the cells were thoroughly rinsed twice with PBS. Subsequently, the cells were digested with 0.25% trypsin. Upon observing an increase in intercellular space, the trypsin was aspirated, and medium was added to prepare a single-cell suspension. Experiments were performed when cells reached 60-80% confluency.
Bioinformatics analysis
The GC dataset from The Cancer Genome Atlas (TCGA) database (http://cancergenome.nih.gov/) was used. Gene expression data were obtained from TCGA using the R package “TCGAbiolinks”. The R package DESeq2 was employed to identify differentially expressed genes based on the original read count data. Genes with a corrected p-value of <0.05 and fold change of >2 were considered significantly differentially expressed. A differential expression analysis, functional enrichment analysis, and correlation analysis to determine the prognostic value were performed.
CCK8 assay
Cells in each group were retrieved and placed in an incubator for 24, 48, and 72 h separately. The culture was interrupted by discarding the medium and adding 100 μL of medium that contained CCK-8 reagent. The cells were then returned to the incubator for 2 h. The OD value at 450 nm was measured using a microplate reader. Six parallel wells were prepared in each group, and the experiment was repeated thrice.
Real-time fluorescent quantitative RT-PCR
Total RNA extraction was carried out from tumour tissues and cells from each group after transfection. According to the instructions provided with the reverse transcription kit, 2mg of total RNA was added to establish 20mL reaction system,and cDNAwas obtained by reverse transcription. A real-time quantitative PCR system was established using the correspondingprimers for target genes and GoTaq qPCR Master Mix. The TaqMan Real-Time PCR Kit was used with β-actin and U6 as internal controls. To guarantee the accuracy and reliability ofthe results, the final relative expression levels were calculated using the 2-ΔΔCt method, and the experiment was conducted three times to ensure accuracy. Average values wereobtained from the replicates. The primers used in the study were listed in the Table S1.
Detection of protein expression by western blotting
The total protein from each group of samples was extracted by the ABC method, followed by the preparation of the electrophoresis gel and SDS-PAGE (initial voltage 80 V, increased to 180 V after proteins entered the separation gel, and continued until the front of the sample reached the bottom of the separation gel). After electrophoresis, the membrane was transferred (150mA, 2h), and the transferred membrane was placed in a blocking solution, gently rocking at room temperature for 1 hour. The membranes were placed in hybridisation bags, which were sealed after air bubbles were driven out and were placed on a shaker at 4°C for overnight shaking. Then, the hybridisation bag was cut open, and the membranes were rinsed three times before they were placed in the hybridisation bag containing the secondary antibody and incubated at room temperature for 2h with gentle shaking. Subsequently, the membranes were placed in the colour developing solution for reaction, after which they were rinsed with distilled water and air-dried. Images were obtained, and grey values and relative expression levels were analysed.
Detection of cell migration ability by scratch test
Five horizontal lines were marked on the backside of a 6-well plate. After 48 hours of transfection, 5 ×105 cells from each group were collected and seeded into a 6-well plate. After an overnight incubation, perpendicular scratch wounds were created on the cells using a 200 μL pipette tip along the previously marked lines. The scratched cells were washed with PBS and incubated in serum-free culture medium for 48 hours. Upon completion of the incubation period, the cells were visually examined and photographed using an inverted microscope. For each group, three replicate wells were utilized. Images obtained were analyzed through NIH's ImageJ software (Bethesda, MD, USA). Observations were made on cell migration across scratch boundaries, as well as cell morphology, at 24-, 48-, and 72-hours post-scratch.
Transwell detection of cell invasion ability
Transwell invasion chambers were placed in a clean, sterile 24-well plate. After pipetting, the Transwell chambers were placed in a 37°C incubator for incubation and hydration. After 2 hours, cells in logarithmic growth phase were carefully removed, washed three times with buffer, and adjusted to a density of 8.0×104 cells/mL. Then, 500μL of cell suspension was added to each well, and the Transwell chambers were placed into the pre-treated 24-well plate. 500μL of DMEM containing 10% fet al bovine serum was added to each well, and the plate was incubated for 24-48 hours. After incubation, the Transwell chambers were gently removed, and the cells were fixed with paraformaldehyde and stained with crystal violet. The number of cells that migrated through the membrane was counted under a microscope, and the invasive capacity of cells was evaluated by determining the average number of cells that crossed the membrane.
Dual luciferase reporter assay
The comprehensive analysis of bioinformatics tools, including StarBase v2.0, miRcode, and RNAhybrid, enabled the identification of potential binding sites for THUMPD3-AS1. To validate these predictions, a specific primer for miR-1297 was designed and used to amplify a fragment that was subsequently inserted into a luciferase reporter plasmid, creating the THUMPD3-AS1-Wt reporter vector. Moreover, a mutant version of the predicted miR-1297 fragment was generated, and the resulting THUMPD3-AS1-Mut reporter vector was constructed. The plasmid and miR-1297 mimics were transfected into HGC-27 cells, and luciferase activity was detected in 48 h. The regulatory relationship with BCAT1 was verified by the same method.
Quantification and statistical analysis
The experimental data were obtained from three independent experiments and presented as mean ± standard deviation. Statistical analyses were performed using SPSS 21.0. For normally distributed measurement data, the mean ± standard deviation was used to express the results. The t-test was used to compare two groups, while one-way ANOVA was used to compare multiple groups. All statistical tests were two-way, and p-values less than 0.05 were considered statistically significant.
Acknowledgments
We thank Yong Li, Liqiao Fan, Jiaxiang Cuiand Wenbo Liu for the helpful discussion. This work is supported by grants from the Hebei Provincial Science and Technology Department’s project of introducing foreign intelligence (Project No. 7002011); The 14th Five-Year Plan for Clinical Medicine Innovation Research Team Support Program of Hebei Medical University (Project No. 2022LCTD-A13); Medical Science Research Project of Hebei Provincial Health Care Commission (Project No. 20230123).
Ethics approval: Our study was reviewed and approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University (ethics number: 2019MEC039).
Author contributions
Z.Z. wrote the paper, B.W. and L.Y. made important revisions to the paper; W.L., L.F., and J.C. collected the literature; B.T. approved the final version of the paper for publication. B.T. carried out the high-throughput sequencing experiments and performed the bioinformatics analysis. Z.Z. and B.T. confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We worked to ensure sex balance in the selection of non-human subjects. We worked to ensure diversity in experimental samples through the selection of the cell lines. We worked to ensure diversity in experimental samples through the selection of the genomic datasets.
Published: August 18, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107673.
Supplemental information
Data and code availability
-
•
The primary research molecules in this paper were derived from the gastric cancer dataset generated from the existing TCGA database (http://cancergenome.nih.gov/) without associated sequencing and generation of new proteins and crystal structures, and the main original protein blot images have been preserved in Figures 5, 6, 8, and 9 and in Mendeley Data (https://doi.org/10.17632/69ckvtv5xh.1) and are publicly available from the date of publication. The DOI is listed in the key resources table.
-
•
This paper does not report original code.
-
•
Other original western blot images will be shared by the lead contact upon request. Any additional in-formation required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Alsina M., Arrazubi V., Diez M., Tabernero J. Current developments in gastric cancer: from molecular profiling to treatment strategy. Nat. Rev. Gastroenterol. Hepatol. 2023;20:155–170. doi: 10.1038/s41575-022-00703-w. [DOI] [PubMed] [Google Scholar]
- 2.López M.J., Carbajal J., Alfaro A.L., Saravia L.G., Zanabria D., Araujo J.M., Quispe L., Zevallos A., Buleje J.L., Cho C.E., et al. Characteristics of gastric cancer around the world. Crit. Rev. Oncol. Hematol. 2023;181 doi: 10.1016/j.critrevonc.2022.103841. [DOI] [PubMed] [Google Scholar]
- 3.Thrift A.P., Wenker T.N., El-Serag H.B. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat. Rev. Clin. Oncol. 2023;20:338–349. doi: 10.1038/s41571-023-00747-0. [DOI] [PubMed] [Google Scholar]
- 4.Conti C.B., Agnesi S., Scaravaglio M., Masseria P., Dinelli M.E., Oldani M., Uggeri F. Early Gastric Cancer: Update on Prevention, Diagnosis and Treatment. Int. J. Environ. Res. Public Health. 2023;20:2149. doi: 10.3390/ijerph20032149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Röcken C. Predictive biomarkers in gastric cancer. J. Cancer Res. Clin. Oncol. 2023;149:467–481. doi: 10.1007/s00432-022-04408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anbiyaiee A., Ramazii M., Bajestani S.S., Meybodi S.M., Keivan M., Khoshnam S.E., Farzaneh M. The function of LncRNA-ATB in cancer. Clin. Transl. Oncol. 2023;25:1–9. doi: 10.1007/s12094-022-02848-1. [DOI] [PubMed] [Google Scholar]
- 7.Yang M., Lu H., Liu J., Wu S., Kim P., Zhou X. lncRNAfunc: a knowledgebase of lncRNA function in human cancer. Nucleic Acids Res. 2022;50:D1295–D1306. doi: 10.1093/nar/gkab1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li D., Xia L., Huang P., Wang Z., Guo Q., Huang C., Leng W., Qin S. Heterogeneity and plasticity of epithelial-mesenchymal transition (EMT) in cancer metastasis: Focusing on partial EMT and regulatory mechanisms. Cell Prolif. 2023;56 doi: 10.1111/cpr.13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X., Song J., Wang X., Sun D., Liu Y., Jiang Y. LncRNA LINC00460: Function and mechanism in human cancer. Thorac. Cancer. 2022;13:3–14. doi: 10.1111/1759-7714.14238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sur S., Ray R.B. Emerging role of lncRNA ELDR in development and cancer. FEBS J. 2022;289:3011–3023. doi: 10.1111/febs.15876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang H., Hu Y., Weng M., Liu X., Wan P., Hu Y., Ma M., Zhang Y., Xia H., Lv K. Hypoxia inducible lncRNA-CBSLR modulates ferroptosis through m6A-YTHDF2-dependent modulation of CBS in gastric cancer. J. Adv. Res. 2022;37:91–106. doi: 10.1016/j.jare.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z., Cao L., Zhou S., Lyu J., Gao Y., Yang R. Construction and Validation of a Novel Pyroptosis-Related Four-lncRNA Prognostic Signature Related to Gastric Cancer and Immune Infiltration. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.854785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang P., Chen C., Zhang J., Yu X. LncRNA CRYM-AS1 Inhibits Gastric Cancer Progression via Epigenetically Regulating CRYM. Ann. Clin. Lab. Sci. 2022;52:249–259. doi: 10.21203/rs.3.rs-593172/v1. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z., Ma Y., Pan Y., Zhu H., Yu C., Sun C. MiR-1297 suppresses pancreatic cancer cell proliferation and metastasis by targeting MTDH. Mol. Cell. Probes. 2018;40:19–26. doi: 10.1016/j.mcp.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Kong W., Yin G., Zheng S., Liu X., Zhu A., Yu P., Zhang J., Shan Y., Ying R., Jin H. Long noncoding RNA (lncRNA) HOTAIR: Pathogenic roles and therapeutic opportunities in gastric cancer. Genes Dis. 2022;9:1269–1280. doi: 10.1016/j.gendis.2021.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCabe E.M., Rasmussen T.P. lncRNA involvement in cancer stem cell function and epithelial-mesenchymal transitions. Semin. Cancer Biol. 2021;75:38–48. doi: 10.1016/j.semcancer.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Hashemi M., Moosavi M.S., Abed H.M., Dehghani M., Aalipour M., Heydari E.A., Behroozaghdam M., Entezari M., Salimimoghadam S., Gunduz E.S., et al. Long non-coding RNA (lncRNA) H19 in human cancer: From proliferation and metastasis to therapy. Pharmacol. Res. 2022;184 doi: 10.1016/j.phrs.2022.106418. [DOI] [PubMed] [Google Scholar]
- 18.Tan Y., Liu L., Zhang X., Xue Y., Gao J., Zhao J., Chi N., Zhu Y. THUMPD3-AS1 Is Correlated with Gastric Cancer and Regulates Cell Function through miR-1252-3p and CXCL17. Crit. Rev. Eukaryot. Gene Expr. 2022;32:69–80. doi: 10.1615/CritRevEukaryotGeneExpr.2022042848. [DOI] [PubMed] [Google Scholar]
- 19.Ding Y., Wang X., Lu S., Lai A., Xie B., He X., Liu Q. BCAT1, as a prognostic factor for HCC, can promote the development of liver cancer through activation of the AKT signaling pathway and EMT. J. Mol. Histol. 2023;54:25–39. doi: 10.1007/s10735-022-10108-3. [DOI] [PubMed] [Google Scholar]
- 20.Anbiyaiee A., Ramazii M., Bajestani S.S., Meybodi S.M., Keivan M., Khoshnam S.E., Farzaneh M. The function of LncRNA-ATB in cancer. Clin. Transl. Oncol. 2023;25:1–9. doi: 10.1007/s12094-022-02848-1. [DOI] [PubMed] [Google Scholar]
- 21.Li D., Xu M., Wang Z., Huang P., Huang C., Chen Z., Tang G., Zhu X., Cai M., Qin S. The EMT-induced lncRNA NR2F1-AS1 positively modulates NR2F1 expression and drives gastric cancer via miR-29a-3p/VAMP7 axis. Cell Death Dis. 2022;13:84. doi: 10.1038/s41419-022-04540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia F., Wang Y., Xue M., Zhu L., Jia D., Shi Y., Gao Y., Li L., Li Y., Chen S., et al. LncRNA KCNQ1OT1: Molecular mechanisms and pathogenic roles in human diseases. Genes Dis. 2022;9:1556–1565. doi: 10.1016/j.gendis.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pu Y., Wei J., Wu Y., Zhao K., Wu Y., Wu S., Yang X., Xing C. THUMPD3-AS1 facilitates cell growth and aggressiveness by the miR-218-5p/SKAP1 axis in colorectal cancer. Cell Biochem. Biophys. 2022;80:483–494. doi: 10.1007/s12013-022-01074-4. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y., Liu X., Wang L., Zhang Z., Li Z., Li M. Circ_PGPEP1 Serves as a Sponge of miR-1297 to Promote Gastric Cancer Progression via Regulating E2F3. Dig. Dis. Sci. 2021;66:4302–4313. doi: 10.1007/s10620-020-06783-5. [DOI] [PubMed] [Google Scholar]
- 25.Hu J., Chen Y., Li X., Miao H., Li R., Chen D., Wen Z. THUMPD3-AS1 Is Correlated With Non-Small Cell Lung Cancer And Regulates Self-Renewal Through miR-543 And ONECUT2. OncoTargets Ther. 2019;12:9849–9860. doi: 10.2147/OTT.S227995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang M., Cao C., Li X., Gu Q., Xu Y., Zhu Z., Xu D., Wei S., Chen H., Yang Y., et al. Five EMT-related genes signature predicts overall survival and immune environment in microsatellite instability-high gastric cancer. Cancer Med. 2023;12:2075–2088. doi: 10.1002/cam4.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian L., Li N., Lu X.C., Xu M., Liu Y., Li K., Zhang Y., Hu K., Qi Y.T., Yao J., et al. Enhanced BCAT1 activity and BCAA metabolism promotes RhoC activity in cancer progression. Nat. Metab. 2023;5:1159–1173. doi: 10.1038/s42255-023-00818-7. [DOI] [PubMed] [Google Scholar]
- 28.Bi B., Deng G.F., Duan Y.M., Huang Z.J., Chen X.Y., Zhang C.H., He Y.L. Retrospective analysis of risk factors for distant metastasis of early-onset gastric cancer during the perioperative period. Front. Oncol. 2023;13 doi: 10.3389/fonc.2023.1003977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z., Wang J., Wang Z., Xu Y. Towards an optimal model for gastric cancer peritoneal metastasis: current challenges and future directions. EBioMedicine. 2023;92 doi: 10.1016/j.ebiom.2023.104601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Q., Wang C., Yang Y., Xu R., Li Z. LncRNA and its role in gastric cancer immunotherapy. Front. Cell Dev. Biol. 2023;11 doi: 10.3389/fcell.2023.1052942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li G.S., Huang H.Q., Liang Y., Pang Q.Y., Sun H.J., Huang Z.G., Dang Y.W., Yang L.J., Chen G. BCAT1: A risk factor in multiple cancers based on a pan-cancer analysis. Cancer Med. 2022;11:1396–1412. doi: 10.1002/cam4.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhuo Z.L., Xian H.P., Sun Y.J., Long Y., Liu C., Liang B., Zhao X.T. Long noncoding RNA ZNFX1-AS1 promotes the invasion and proliferation of gastric cancer cells by regulating LIN28 and CAPR1N1. World J. Gastroenterol. 2022;28:4973–4992. doi: 10.3748/wjg.v28.i34.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei F., Wang Y., Zhou Y., Li Y. Long noncoding RNA CYTOR triggers gastric cancer progression by targeting miR-103/RAB10. Acta Biochim. Biophys. Sin. 2021;53:1044–1054. doi: 10.1093/abbs/gmab071. [DOI] [PubMed] [Google Scholar]
- 34.Lee Y.E., Lee J., Lee Y.S., Jang J.J., Woo H., Choi H.I., Chai Y.G., Kim T.K., Kim T., Kim L.K., Choi S.S. Identification and Functional Characterization of Two Noncoding RNAs Transcribed from Putative Active Enhancers in Hepatocellular Carcinoma. Mol. Cells. 2021;44:658–669. doi: 10.14348/molcells.2021.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mei J., Liu G., Li R., Xiao P., Yang D., Bai H., Hao Y. LncRNA SNHG6 knockdown inhibits cisplatin resistance and progression of gastric cancer through miR-1297/BCL-2 axis. Biosci. Rep. 2021;41 doi: 10.1042/BSR20211885. BSR20211885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X., Zhang M., Guo Q., Hu X., Zhao Z., Ni L., Liu L., Wang X., Wang Z., Tong D., et al. MicroRNA-1297 inhibits proliferation and promotes apoptosis in gastric cancer cells by downregulating CDC6 expression. Anti Cancer Drugs. 2019;30:803–811. doi: 10.1097/CAD.0000000000000776. [DOI] [PubMed] [Google Scholar]
- 37.Li X., Zhang Q., Yang Z. Knockdown of hsa_circ_0058124 inhibits the proliferation of human lung cancer cells by up-regulation of miR-1297. Artif. Cells Nanomed. Biotechnol. 2020;48:584–593. doi: 10.1080/21691401.2020.1725537. [DOI] [PubMed] [Google Scholar]
- 38.Chen P., Wang B.L., Pan B.S., Guo W. MiR-1297 regulates the growth, migration and invasion of colorectal cancer cells by targeting cyclo-oxygenase-2. Asian Pac. J. Cancer Prev. 2014;15:9185–9190. doi: 10.7314/apjcp.2014.15.21.9185. [DOI] [PubMed] [Google Scholar]
- 39.Ye J., Yan Y., Xin L., Liu J., Tang T., Bao X. Long non-coding RNA TMPO-AS1 facilitates the progression of colorectal cancer cells via sponging miR-98-5p to upregulate BCAT1 expression. J. Gastroenterol. Hepatol. 2022;37:144–153. doi: 10.1111/jgh.15657. [DOI] [PubMed] [Google Scholar]
- 40.Yu M., Zhao Q., Li J., Xu F., Zhang Z., Liu Y., Dai L., Zhang B., Zhang J., Zhang J. BCAT1 promotes lung adenocarcinoma progression through enhanced mitochondrial function and NF-κB pathway activation. J. Zhejiang Univ. - Sci. B. 2022;23:760–769. doi: 10.1631/jzus.B2100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palanca-Ballester C., Hervas D., Villalba M., Valdes-Sanchez T., Garcia D., Alcoriza-Balaguer M.I., Benet M., Martinez-Tomas R., Briones-Gomez A., Galbis-Caravajal J., et al. Translation of a tissue epigenetic signature to circulating free DNA suggests BCAT1 as a potential noninvasive diagnostic biomarker for lung cancer. Clin. Epigenetics. 2022;14:116. doi: 10.1186/s13148-022-01334-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H., Wang F., Ouyang W., Jiang X., Wang Y. BCAT1 overexpression regulates proliferation and c-Myc/GLUT1 signaling in head and neck squamous cell carcinoma. Oncol. Rep. 2021;45:52. doi: 10.3892/or.2021.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The primary research molecules in this paper were derived from the gastric cancer dataset generated from the existing TCGA database (http://cancergenome.nih.gov/) without associated sequencing and generation of new proteins and crystal structures, and the main original protein blot images have been preserved in Figures 5, 6, 8, and 9 and in Mendeley Data (https://doi.org/10.17632/69ckvtv5xh.1) and are publicly available from the date of publication. The DOI is listed in the key resources table.
-
•
This paper does not report original code.
-
•
Other original western blot images will be shared by the lead contact upon request. Any additional in-formation required to reanalyze the data reported in this paper is available from the lead contact upon request.