Highlights
-
•
Rice bran protein-tannic acid emulsions were treated with ultrasound.
-
•
Ultrasonic treatment improved microstructure and properties of emulsions.
-
•
The best emulsion properties were obtained at an ultrasonic power of 400 W.
Keywords: Ultrasonic treatment, Rice bran protein, Tannic acid, Emulsion
Abstract
Rice bran protein (RBP)-tannic acid (TA) complex was prepared and the RBP-TA emulsions were subjected to ultrasonic treatment with different powers. Ultrasonic treatment has a positive effect on improving the properties of RBP-TA emulsion. This study investigated the influence of different ultrasonic power levels on the physicochemical properties, microstructure, rheological properties, and stability of emulsions containing RBP-TA. Under the ultrasonic treatment of 400 W, the particle size, zeta potential, and adsorbed protein content of the RBP-TA emulsion were 146.86 nm, −20.7 eV, and 61.91%, respectively. At this time, the emulsion had the best emulsifying properties, apparent viscosity, energy storage modulus and loss modulus. In addition, the POV and TBARS values of RBP-TA emulsions were 6.12 and 7.60 mmol/kg, respectively. The thermal, salt ion, pH and oxidative stability of the emulsions were investigated, and it was shown that ultrasonic treatment was effective in improving the stability of RBP-TA emulsions.
1. Introduction
Emulsions are multiphase systems in which immiscible liquids are uniformly dispersed as droplets in a continuous phase. Such systems are increasingly employed as delivery vehicles for a wide range of functional ingredients, including vitamins, minerals, and bioactive compounds [1]. Emulsion stability can be achieved through the incorporation of emulsifiers, which typically consist of amphiphilic molecules with both hydrophilic and hydrophobic properties [2]. These emulsifiers act by reducing the interfacial tension at the oil/water interface. In particular, proteins exhibit inherent emulsifying capabilities, adsorbing to the interface, envelop oil or air droplets, and stabilizing the dispersion due to their amphiphilic properties [3].
Rice bran protein (RBP) demonstrates exceptional properties, distinguished by noteworthy biological activity, digestibility, and absorptivity. Such traits stem primarily from its distinctive amino acid composition and abundance of bioactive peptides, which serve various crucial physiological roles in the human body, including antioxidant effects and cholesterol reduction. Compared to soy protein and other proteins, RBP contains all essential amino acids necessary for the human body, with amino acid proportions that closely match human needs. In particular, it has relatively high levels of lysine and tryptophan, making it a high-quality protein source. Moreover, RBP offers certain environmental benefits in addition to its nutritional value. The production process of RBP is environmentally friendly, with minimal impact on the environment. RBP is an economical protein source that is hypoallergenic and has attracted considerable attention for use in health and non-allergenic food applications. It also has good functional properties that offer good potential for the use of RBP as an emulsifier in emulsions [4]. On the one hand it has limited potential to inhibit lipid oxidation in emulsions. Previous studies have shown that plant-based polyphenols can act as effective antioxidants, but they are usually not located on the surface of the oil droplets where lipid oxidation usually occurs [5]. On the other hand, with the increasing use of protein-based emulsifiers, many studies on the structure–function approach have identified the formation and interfacial behavior of food emulsifiers based on their physicochemical properties, as well as the limitations of using natural emulsifiers [6]. Therefore, polyphenols have been added to improve the antioxidant and emulsifying properties of the complexes. Chen et al. [7] investigated the non-covalent binding between SPI and tea polyphenols, revealing that the incorporation of tea polyphenols significantly improved both the solubility and emulsifying capabilities of SPI. Tannic acid (TA), a polyphenol naturally present in numerous plants, exhibits various physicochemical properties and physiological activities due to its abundant hydroxyl groups. The formation of protein-TA complexes has been shown to induce alterations in physicochemical properties, thereby enhancing the functional properties of proteins and stabilizing emulsion systems [8]. Although the addition of TA improves the emulsion stability, the emulsions are prone to unstable phenomena such as flocculation and agglomeration due to changes in external environmental conditions and their own properties.
Ultrasonic treatment is an energy efficient and versatile technology that has been widely used in extraction, freezing, thawing, modification, salt production and other types of food processing. Ultrasound creates cavitation bubbles in the system, and the bursting of the bubbles generates energy, and this energy can affect the substances in the liquid. Under the effect of cavitation, the oil droplets of the emulsion are broken and emulsified, and the particle size of the droplets is reduced to make the emulsion more stable and uniform. In addition, ultrasonic emulsification has higher energy efficiency compared to other methods [9]. Zhao et al. [10] observed that ultrasonic treatment improved the solubility, foaming and emulsifying properties of Perilla frutescens proteins. In addition, another study reported a decrease in the mean particle size and an increase in the stability of coconut milk protein emulsion system after ultrasonic treatment [11]. The emulsifying properties of ultrasound have been widely used in the preparation of protein-stabilized emulsions [12]. During ultrasonic waves, the force generated by the bursting of air bubbles can break up large emulsions into small emulsion droplets. Studies have also reported the application of ultrasonic treatment to various oil emulsions in the preparation of plant and animal proteins. In emulsified systems containing a combination of emulsion protein isolates and aphoric collagen [13], increasing the ultrasonic power or the duration of ultrasonic treatment significantly reduced the titer of the oil phase.
Therefore, this investigation focused on subjecting the oil-in-water emulsions stabilized by RBP-TA complexes to ultrasonic treatment to evaluate the effect of different ultrasonic power on the emulsion properties. To gain insight into the emulsion microstructure, the emulsions were analyzed using techniques such as optical microscopy, laser confocal microscopy, and atomic force microscopy. Furthermore, the physicochemical and interfacial properties of the emulsions were derived from measurements of particle size, zeta potential, emulsification properties, interfacial protein content, and rheological properties. In addition, the potential applications of RBP-TA emulsions in various industrial settings can be supported by evaluating their resistance to thermal, ionic, pH, and oxidative challenges. This evaluation aims to provide a theoretical basis for the stability of these emulsions.
2. Materials and methods
2.1. Materials
Low-temperature defatted rice bran flour and corn oil were purchased from Beidahuang Agricultural Group Co Ltd (Harbin, Heilongjiang, China); TA was purchased from Sigma-Aldrich Co. (Shanghai, China). All other chemical agents were of analytical grade.
2.2. Preparation of RBP-TA complex
The extraction of RBP was performed with reference to the method of Wang et al [14]. The RBP (10 mg/mL) was dissolved in deionized water and stirred for 2 h to prepare RBP solution. TA was added to the RBP solution, in which the concentration of TA was 1 mg/mL, and the pH of the solution was adjusted to 7, and the solution was stirred at room temperature for 2 h. Then it was transferred to dialysis at 4 °C for 24 h to remove unbound polyphenols. The obtained sample was freeze-dried (RBP-TA complex).
2.3. Preparation and ultrasonic treatment of RBP-TA stabilized O/W emulsions
The RBP-TA complex was used as an emulsifier dissolved in deionized water at a concentration of 20 mg/mL. RBP-TA was mixed with corn oil at a water–oil ratio of 8:2, and homogenized at a high speed of 13,000 rpm for 2 min (Ultra-Turrax T18, Angi Co., Shanghai, China), and the crude emulsion was obtained. The crude emulsion was treated with Scientz-II D ultrasound generator (Scientz Biotechnology Co., Ltd., Ningbo, China), with the pulse working time set to 4 s, the intermittent time set to 2 s, and the frequency set to 20 kHz. The ultrasound power was adjusted to 0, 100, 200, 300, 400, and 500 W. The temperature was controlled by an ice bath throughout the process to obtain the RBP-TA emulsions under different power ultrasound treatments.
2.4. Determination of particle size and zeta potential of RBP-TA emulsion
The determination of particle size and zeta potential of RBP-TA emulsions after ultrasonic treatment at different powers levels was carried out according to Wang et al.[15] The particle size distribution curves and zeta potentials of the diluted RBP-TA emulsions were determined using a nanoparticle size potentiostat (Zetasizer Nano ZS, Malvern Instruments Ltd., UK), and the volumetric mean diameter of the emulsions was calculated (D43).
2.5. Microstructure of RBP-TA emulsion
2.5.1. Optical microscope observation of RBP-TA emulsion
The RBP-TA emulsion after ultrasonic treatment at different powers was diluted to 10-fold with deionized water and dropped onto slides for observation with the BX53 microscopic imaging system (Olympus Co., Tokyo, Japan). The magnification was 20 ×. Ten images were taken for each sample, and the image with universality was selected. No image processing software was used for image enhancement or modification.
2.5.2. CLSM observation of RBP-TA emulsion
RBP-TA emulsion staining was followed by CLSM observation. The aqueous phase was stained with a 1% (w/v) Nile blue solution, and the oil phase was stained with a 0.1% (w/v) Nile red solution. To stain 1 mL of emulsion, a sequential addition of 25 μL of Nile blue solution and 20 μL of Nile red solution was added sequentially. Following the addition, the mixture was allowed to stand undisturbed for a duration of 30 min to allow the staining process to occur.
2.5.3. AFM observation of RBP-TA emulsion
The AFM observation was carried out by referring to the method of Wang et al. [16]. An appropriate amount of emulsion was diluted and 10 μL drops were taken onto the mica sheet for uniform distribution and air-dried. The scanning frequency was 1.0 Hz and the scanning area was 1.5 × 1.5 μm. The resulting images were processed using Nanoscope software.
2.6. Determination of emulsifying properties of RBP-TA emulsion
The emulsifying properties of RBP-TA included two indexes, emulsification activity index (EAI) and emulsion stability index (ESI). The method was carried out with reference to the study of Wang et al. [17], where 0.1% (w/v) dodecyl sulfuric acid was added to 50 μL of RBP-TA emulsion, and the absorbance of the sample was measured at 500 nm, and the EAI and ESI were calculated according to the following equations:
| (1) |
| (2) |
where A0 represents the absorbance measurement at 0 min. DF refers to the dilution factor (1 0 0). C denotes the protein concentration in the pre-emulsion measured in grams per milliliter (g/mL). θ represents the oil volume fraction of the emulsion in volume per volume (v/v). L indicates the optical path length set at 1 cm. A10 corresponds to the absorbance recorded at 10 min.
2.7. Determination of interfacial protein content of RBP-TA emulsion
Fresh RBP-TA emulsions were centrifuged at 18,000 × g for 30 min to isolate proteins adsorbed to the interface from those that remained non-adsorbed. The resulting mixture was then filtered and analyzed using the method described by Wang et al [15] to determine and differentiate adsorbed and non-adsorbed proteins present in RBP emulsions.
2.8. Determination of rheological properties of RBP-TA emulsion
To investigate the rheological properties of the RBP-TA emulsion under different ultrasonic power, the AR2000 rotational dynamic shear rheometer (TA Instruments, UK) was selected to evaluate the apparent viscosity, storage modulus (G'), and loss modulus (G'') of the fresh emulsion. Apparent viscosity of the emulsion was measured over a shear rate range of 0.01 to 100 s−1, while G' and G'' were measured over a frequency range of 0.1 to 10 Hz.
2.9. Determination of emulsion stability of RBP-TA emulsion
2.9.1. Determination of thermal stability of RBP-TA emulsion
The fresh RBP-TA emulsion (5 mL) was placed in a glass test tube and heated at 70, 80, and 90 °C for 30 min. The change in D43 of the RBP-TA emulsion was then evaluated and documented.
2.9.2. Determination of salt ion stability of RBP-TA emulsion
The fresh RBP-TA emulsion (5 mL) was placed in a glass test tube, followed by the addition of NaCl solution at concentrations of 100, 200, and 300 mM (0.5 mL each). The mixtures were then stored at 25 °C for 2 h. The change in D43 value of the RBP-TA emulsion was then determined and recorded.
2.9.3. Determination of pH stability of RBP-TA emulsion
The fresh RBP-TA emulsion (5 mL) was transferred to a glass test tube, and the pH of the emulsion was adjusted to 3, 5, 7, and 9 using either a 0.1 M NaOH solution or a 0.1 M HCL solution. The emulsion was then stored at 25 °C for 2 h, and the change in D43 of the RBP-TA emulsion was measured and documented.
2.9.4. Determination of oxidative stability of RBP-TA emulsion
The effect of different ultrasonic powers on the oxidative stability of RBP-TA emulsions was investigated at 25 °C by determining the peroxide value (POV) and Thiobarbituric Acid Reactive Substances (TBARS) values. POV was determined according to the official method Cd 8–53 of the American Oil Chemists Society (AOCS), while TBARS was determined according to the study of Ym et al. [18].
2.10. Statistical analysis
All experiments were conducted in triplicate and the results presented represent the mean values. The data was analyzed using Origin 2018 software to generate figures, while SSPS 26.0 was utilized for performing single factor ANOVA analysis and calculating standard deviation using Duncan's test (p < 0.05), and the differences were considered significant with p < 0.05.
3. Results and discussion
3.1. Particle size and zeta potential of RBP-TA emulsion
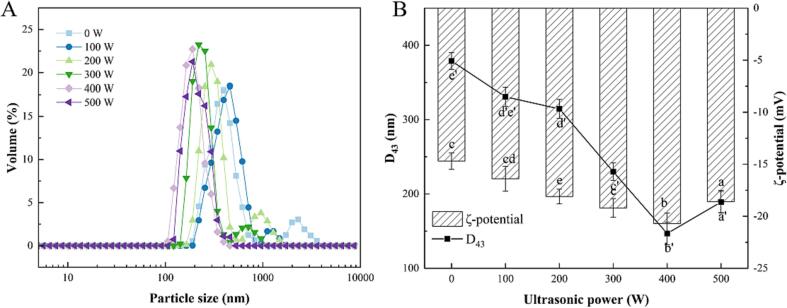
Particle size is a commonly used method to measure the size or quantity of a substance, which has an important reference value in studying emulsion properties. The effect of ultrasonic treatment on the particle size distribution curve and D43 of RBP-TA emulsion is shown in Fig. 1. As the ultrasound power increased, the peak position of particle size distribution curve was shifted to the left. The bimodal distribution curve changed to a unimodal one, indicating that the ultrasonic treatment facilitated uniform dispersion of the emulsion droplets. Ultrasonic treatment promoted a leftward shift in the particle size distribution curve of the emulsion and a decrease in the droplet size, which could be attributed to the ultrasound-induced turbulence and cavitation effects that broke up the emulsion droplets. The D43 of the RBP-TA emulsions gradually decreased and then increased with the increase in ultrasonic power compared to that of the untreated emulsions, with the smallest D43 of the emulsions being 146.86 nm at 400 W. The change in particle size may be attributed to the mechanical vibration and shear force generated by ultrasound, which reduced the force between RBP-TA complexes in the emulsions and caused them to break into smaller droplets. This finding was consistent with the observation of Li et al. [19] that ultrasonic treatment of hemp seed protein emulsions disrupted some larger insoluble protein aggregates, resulting in more uniform dispersion of hemp seed proteins within the emulsions. The particle size distribution curve still showed a single-peak distribution, despite the increase in D43 to 189.10 nm as the ultrasonic power continued to increase. This phenomenon can be attributed to excessive ultrasonic power, which can cause instability in the RBP-TA emulsion, resulting in an increase in particle size. However, excessive application of ultrasonic power may yield contrasting effects. In general, excessive ultrasonic power has the potential to produce certain thermal effects, leading to the reaggregation of emulsion droplets and the formation of insoluble aggregates.
Fig. 1.
The particle size distribution (A), the D43, zeta potential (B) of the RBP-TA emulsion. Different letters represent significant differences at p < 0.05.
The zeta potential is used as an indicator to evaluate the stability of an emulsion. The effect of ultrasonic treatment on the zeta potential of the RBP-TA emulsion is shown in Fig. 1B. Ultrasonic treatment increased the absolute zeta potential, indicating an increase in emulsion stability. Notably, as the ultrasonic power increased, there was an initial rise followed by a subsequent decrease in the absolute zeta potential of the RBP-TA emulsion. It reached a value of 20.7 eV at 400 W. This phenomenon can be attributed to the reduction in droplet size induced by ultrasonic treatment, which increased the spatial repulsion between droplets and prevents emulsion flocculation [20]. However, excessive application of ultrasonic power may produce opposite effects.
3.2. Microstructure of RBP-TA emulsion
3.2.1. Optical microscope images of RBP-TA emulsion
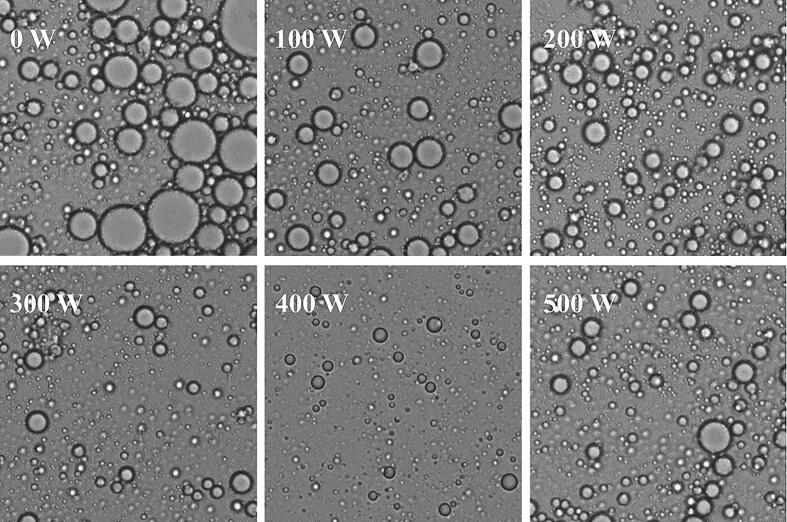
Optical microscopy is a device that uses optical principles to magnify and image objects, serving as a means of evaluating the microstructure of emulsions. The impact of ultrasonic treatment on the RBP-TA emulsion is shown in Fig. 2. The droplet size of the untreated RBP-TA emulsion was found to be the largest, while a significant reduction in droplet size was observed for the RBP-TA emulsion after ultrasonic treatment at 100 W. As the ultrasonic power increased, there was a gradual reduction in droplets size within the RBP-TA emulsion and an increase in dispersion compared to the untreated emulsion. The samples exhibit significant differences. Under the ultrasonic power of 400 W, the RBP-TA emulsion demonstrates the smallest droplet size and highest dispersion. A possible reason for this phenomenon was that the ultrasonic treatment promoted the dispersion of RBP-TA complexes in the emulsion. The high-frequency vibration weakened the inter-particle forces between the emulsifier particles, leading to an increase in the inter-particle distance and favorable dispersion. In addition, ultrasonic treatment induced high-frequency vibrations and cavitation effects within the emulsion, which facilitated the disruption of emulsion droplets, thereby improving dispersion. This was also proved by Li et al. on the wheatolin-Rutin stabilized emulsion [21].
Fig. 2.
The optical microscope images of RBP-TA emulsion.
3.2.2. CLSM images of RBP-TA emulsion
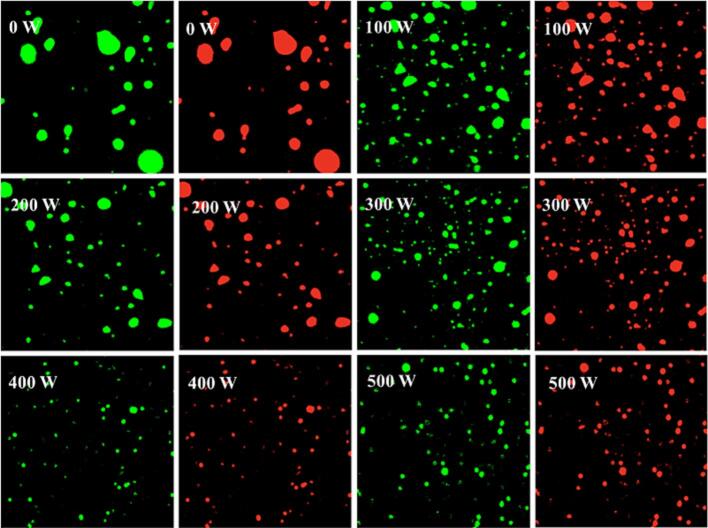
The effect of ultrasonic treatment at different powers on RBP-TA emulsion is shown in Fig. 3, where the green color represents the aqueous phase (RBP-TA) and the red color represents the oil phase. Compared to the untreated samples, ultrasonic treatment at 100 W significantly reduced the droplet size in RBP-TA emulsion without producing excessively large droplets. This indicated that ultrasound played a role in improving emulsion homogeneity. Moreover, increasing ultrasonic power (200–400 W) further enhanced the formation and stability of RBP-TA emulsion. Ultrasonic treatment was most effective at 400 W, which formed RBP-TA emulsions with uniformly dispersed droplets. This indicated that the physical effects produced by the ultrasonic treatment effectively broke up the emulsion droplets and reduced their size [22]. However, as the ultrasonic power continued to increase (500 W), the stability of the RBP-TA emulsion may be affected and an increase in droplet size occurred. This could be attributed to excessive heat and vibration generated by the ultrasonic treatment destabilizing the RBP-TA emulsions, and a certain degree of flocculation occurred in the emulsion.
Fig. 3.
The CLSM images of RBP-TA emulsion.
3.2.3. AFM images of RBP-TA emulsion
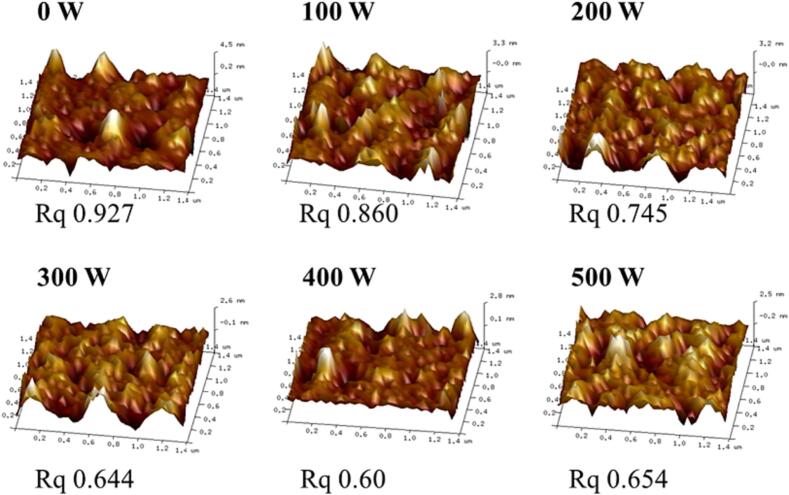
AFM is a commonly used means of determining the apparent morphology of emulsions, and the AFM images of the RBP-TA emulsions are shown in Fig. 4. The surface roughness (Rq) of the untreated RBP-TA emulsion was 0.927. The Rq of the RBP-TA emulsion decreased with increasing ultrasonic power during the ultrasonic treatment, reaching a minimum value of 0.60 at 400 W. This can be attributed to the fact that the ultrasonic treatment broke up the particles within the emulsion, leading to their redistribution and ultimately reducing the surface roughness. This was also demonstrated in the study by Wang et al.[23], where AFM images showed that ultrasonic treatment at 450 W resulted in the best dispersion and lowest surface roughness of the RBP-TA emulsion. However, further increase of ultrasonic power up to 500 W resulted in an increase of Rq to 0.654, possibly due to changes in protein structure caused by excessive ultrasound. According to Xiong et al. [24], excessive ultrasonic treatment can cause changes in the molecular structure of proteins, resulting in a reduction of their emulsifying properties. Therefore, it is important to use moderate in ultrasonic treatment to ensure optimal improvement of emulsion properties. Moderate ultrasonic treatment, as suggested by the study, has been shown to be capable of significantly improving emulsion properties.
Fig. 4.
The AFM images of the RBP-TA emulsion.
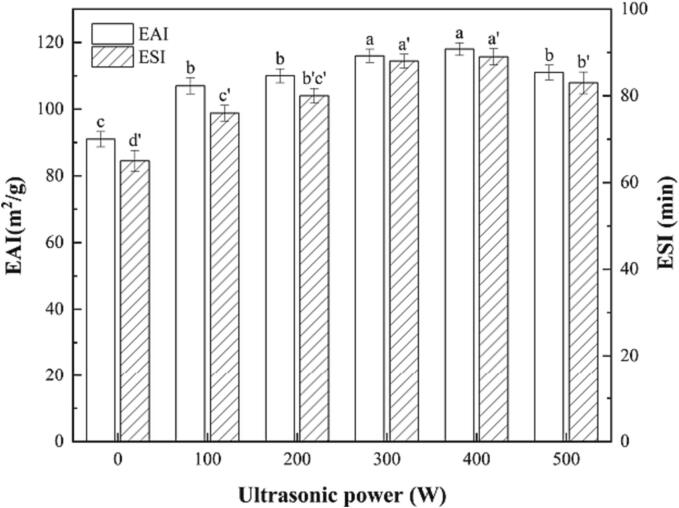
3.3. Emulsifying properties of RBP-TA emulsion
The emulsifying properties are one of the key indicators for evaluating RBP-TA emulsions, which are directly related to the emulsion formation and storage. The effects of different ultrasonic powers on the EAI and ESI of RBP-TA emulsions are shown in Fig. 5. It can be seen that the emulsifying properties of RBP-TA emulsions showed changes after ultrasonic treatment. With the increase of ultrasonic power, the trend of EAI showed an initial increase and then a decrease. The maximum EAI of RBP-TA emulsion was 118 m2/g under the ultrasonic treatment of 400 W. This result was mainly because the high-frequency vibration of ultrasonic waves to make the molecules in the water and oil mixtures to obtain the energy, which accelerated the molecular movement and reduced the distance between molecules to improve the EAI of the emulsion. The EAI of the emulsion showed negligible changes with increasing ultrasonic power, which is consistent with the results reported by Zhang et al [25]. With increasing of ultrasonic power, the ESI of the RBP-TA emulsion showed a trend of first increasing and then decreasing, with the maximum point occurring at 400 W. The reason for the observed trend in emulsifying properties could be explained as follows: at low ultrasonic power, the energy provided by the ultrasonic waves may be insufficient to fully emulsify the oil–water mixtures, resulting in a lower ESI. As the ultrasonic power increased and energy was gradually enhanced, the tension at the oil–water interface was broken leading to full emulsification and a gradual increase in ESI. Similar results were found by Sui et al.[26]. It appears that there is no significant difference in emulsifying properties between RBP-TA emulsions treated with ultrasound at 300 W and 400 W. This may suggest that the ultrasound power of 300 W is already effective in enhancing the emulsifying properties of RBP-TA emulsions. Further increasing the power may not have a notable impact on improving the emulsifying performance.
Fig. 5.
The Emulsifying properties of the RBP-TA emulsion. Different letters represent significant differences at p < 0.05.
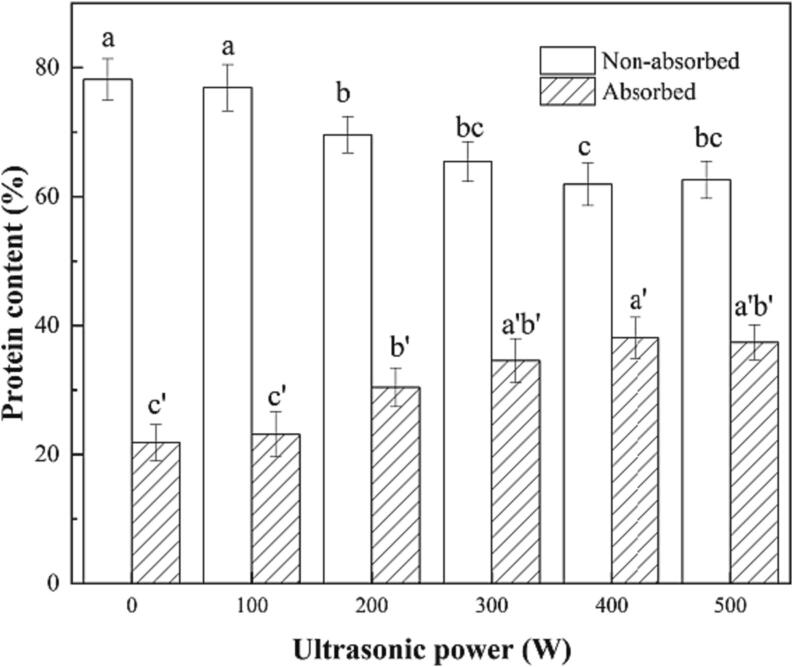
3.4. Interfacial protein content of RBP-TA emulsion
The interfacial protein content is an important indicator to evaluate the impact of ultrasonic treatment at different power levels on emulsion performance. The effect of ultrasonic power on the interfacial protein content of the RBP-TA emulsion is shown in Fig. 6. With increasing ultrasonic power, the absorbed protein content initially decreased and then increased. Specifically, at a power level of 400 W, the absorbed protein content reached its lowest value of 61.91% compared to the non-absorbed proteins. This phenomenon can be attributed to the effects of the increased ultrasonic power on the particles of the emulsion system. The strong vibrations induced by the ultrasonic waves promoted the interaction between the adsorbed proteins, thus reducing their content. The energy generated by the ultrasonic waves disrupted the protein layers at the oil–water interface, causing some proteins to detach or rearrange, resulting in a decrease in the content of interfacial proteins. However, as the ultrasonic power continued to increase, the temperature of the emulsion system also increased. This increase in temperature enhances the energy between the interfacial protein molecules, facilitating their reabsorption at the oil–water interface. Consequently, beyond a certain point (400 W), a dynamic equilibrium was established in the interaction between the adsorbed interfacial proteins, resulting in their lowest content at that particular power level [27].
Fig. 6.
The interface protein distribution of the RBP-TA emulsion. Different letters represent significant differences at p < 0.05.
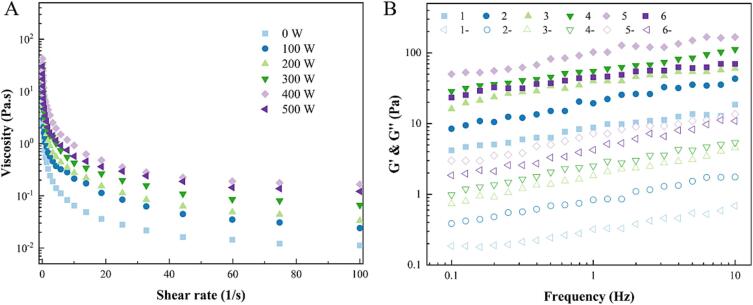
3.5. Rheological properties of RBP-TA emulsion
The apparent viscosity of the RBP-TA emulsions is shown in Fig. 7A. Based on the observations made, it was evident that the apparent viscosity of all the RBP-TA emulsions decreased as the shear rate increased. This indicated that the RBP-TA emulsions exhibit shear thinning behavior, meaning their viscosity decreased with increasing shear rate. In addition, the apparent viscosity of the emulsions displayed a trend of initially increasing and then decreasing as the ultrasonic power was increased. This suggested that there was an optimal ultrasonic power level that maximizes the apparent viscosity of emulsion. Beyond this optimum point, further increased in ultrasonic power may lead to a decrease in the apparent viscosity of the emulsion. The maximum apparent viscosity was observed at 400 W. It has been observed that the apparent viscosity of RBP-TA emulsion is higher than that of the untreated RBP-TA emulsion. This phenomenon may be related to the effect of ultrasonic treatment on the interfacial layer of RBP-TA emulsions, where an increase in the adsorbed protein content resulted in a thickening of the interfacial layer of the RBP-TA emulsions, which altered the speed of droplet movement, thereby increasing the apparent viscosity [28].The effect of ultrasonic treatment power on emulsion G' and G'' is also a key index for evaluating the rheological properties of emulsions. The trends of RBP emulsions G' and G'' with frequency (0.1–10 Hz) are shown in Fig. 7B. The G' and G'' of all RBP-TA emulsions had similar trends, and G' was always higher than G''. Meanwhile, both G' and G'' of RBP-TA emulsions showed an increasing trend with increasing ultrasonic power, implying that the ultrasonic treatment played a positive role in contributing to the improvement of the network structure and viscoelasticity of the emulsions. This phenomenon is similar to the study of Li et al. [29], where high-intensity ultrasonic treatment improved the rheological properties and stability of myofibrillar protein emulsions. Therefore, it can be concluded that 400 W ultrasonic treatment promoted the rheological properties of RBP-TA emulsions.
Fig. 7.
The Viscosity (A), G' and G“ (B) of the RBP-TA emulsion. Where 1 ∼ 6 represent the storage modulus (G') of the RBP-TA emulsion treated with ultrasound ranging from 0 to 500 W, and 1-∼6- represent the loss modulus (G'') of the RBP-TA emulsion treated with ultrasound ranging from 0 to 500 W.
3.6. Emulsion stability of RBP-TA emulsion
3.6.1. Thermal stability of RBP-TA emulsion
To investigate the difference in stability of emulsions at different processing and storage temperatures, the D43 of emulsions was studied under heating conditions of 70–90 °C. The D43 values of emulsions under heating conditions of 70–90 °C are shown in Table 1. Within this temperature range, the D43 values of the emulsions gradually increased with increasing temperature, indicating reduced stability at higher temperatures. This phenomenon can be attributed to polymer degradation within the emulsion and droplets coalescence. These changes are likely to be accelerated at elevated temperatures, resulting in reduced overall stability [30]. Furthermore, when emulsions were compared at identical temperatures, a negative correlation between ultrasonic power and D43 value was observed until reaching a minimum point at 400 W. Subsequently, with further increased in ultrasonic power, there was a tendency for the D43 value to increase again. This behavior may be explained by the increased shear forces exerted on the particles within the emulsion due to higher ultrasonic power levels, thus promoting particle agglomeration and formation of smaller particles. In addition, excessive ultrasonic power could enhance inter-particle interactions within the emulsion facilitating particle aggregation.
Table 1.
Thermal stability of RBP-TA emulsions.
| Ultrasonic power (W) | 25 ℃ | 70 ℃ | 80 ℃ | 90 ℃ |
|---|---|---|---|---|
| 0 | 378.93 ± 11.66 d | 559.92 ± 11.34c | 669.12 ± 18.24b | 754.86 ± 14.16 a |
| 100 | 330.66 ± 13.44 d | 526.20 ± 13.83c | 648.97 ± 19.86b | 722.54 ± 14.55 a |
| 200 | 314.46 ± 19.03 d | 499.32 ± 19.31c | 605.59 ± 19.28b | 697.29 ± 11.26 a |
| 300 | 229.97 ± 16.48 d | 440.26 ± 16.68c | 573.89 ± 10.37b | 673.34 ± 10.78 a |
| 400 | 146.86 ± 11.95 d | 379.98 ± 10.7c | 508.61 ± 19.55b | 642.31 ± 12.08 a |
| 500 | 189.12 ± 14.13 d | 424.06 ± 18.36c | 567.67 ± 16.45b | 682.19 ± 14.27 a |
Different letters represent significant differences at p < 0.05.
3.6.2. Salt ion stability of RBP-TA emulsion
Salt ion stability is an important indicator of emulsion stability, and the effect of ultrasonic treatment on the salt ion stability of RBP-TA emulsion is shown in Table 2. The effect of different concentrations of NaCl (0–300 mM) on the D43 of the emulsions had a certain regularity. The D43 of the emulsion gradually increased with the increase of salt ion concentration. The increase of salt ion concentration may cause the emulsion stability to decrease, and the reason for this phenomenon may be related to the role of salt ions in the emulsion. Salt ions can be used as electrolytes in emulsions to regulate the surface charge of emulsions. However, when the concentration of salt ions is gradually increased, the pH of the emulsion may deviate from neutral, leading to hydrolysis, polymerization and other reactions of the substances in the emulsion, thus reducing the stability of the emulsion [31]. Meanwhile, increasing the ultrasonic power reduced the degree of particle aggregation in the emulsion and increased the adsorbed protein content at the emulsion interface, which improved the salt ion resistance of the emulsion, indicating that moderate ultrasonication was beneficial for improving the stability of the emulsion [32].
Table 2.
Salt ion stability of RBP-TA emulsions.
| Ultrasonic power (W) | 100 mM | 200 mM | 300 mM |
|---|---|---|---|
| 0 | 549.25 ± 19.53c | 739.96 ± 14.96b | 948.35 ± 11.06 a |
| 100 | 517.01 ± 19.62c | 665.59 ± 10.25b | 932.94 ± 18.86 a |
| 200 | 483.89 ± 13.38c | 626.80 ± 13.14b | 922.41 ± 10.72 a |
| 300 | 440.33 ± 16.77c | 584.26 ± 14.17b | 889.60 ± 19.75 a |
| 400 | 380.98 ± 16.54c | 525.16 ± 14.93b | 832.43 ± 15.32 a |
| 500 | 440.33 ± 11.84c | 593.18 ± 16.08b | 885.60 ± 11.36 a |
Different letters represent significant differences at p < 0.05.
3.6.3. pH stability of RBP-TA emulsion
The pH stability of emulsions is critical to prevent problems such as coagulation, phase separation, and creaming. It is necessary to study the pH stability of emulsions. The pH stability of RBP-TA emulsions at different ultrasonic power is shown in Table 3. At pH 3 and 5, the D43 of the RBP-TA emulsion increased significantly compared to the neutral condition, which was related to the proximity of the system pH to the protein isoelectric point. On the one hand, the proteins would lose their natural structure and function under these two pH conditions. On the other hand, an excessively low pH reduced the intermolecular electrostatic repulsion, which promoted the attractive interactions of proteins and resulted in the formation of additional protein aggregates. The trend of emulsions pH stability was more pronounced in the case of varying ultrasonic power. The emulsion pH stability increased with increasing ultrasonic power, a phenomenon consistent with the study of Zhu et al [33].
Table 3.
pH stability of RBP-TA emulsions.
| Ultrasonic power (W) | 3 | 5 | 7 | 9 |
|---|---|---|---|---|
| 0 | 966.14 ± 19.04 a | 692.61 ± 12.71b | 378.93 ± 11.66 d | 519.69 ± 12.72c |
| 100 | 944.99 ± 12.79 a | 653.65 ± 12.09b | 330.66 ± 13.44 d | 472.58 ± 16.14c |
| 200 | 902.72 ± 13.69 a | 638.62 ± 15.26b | 314.46 ± 19.03 d | 452.97 ± 13.48c |
| 300 | 875.21 ± 11.38 a | 591.23 ± 12.56b | 229.97 ± 16.48 d | 405.6 ± 13.39c |
| 400 | 821.22 ± 10.54 a | 535.23 ± 13.75b | 146.86 ± 11.95 d | 360.27 ± 17.75c |
| 500 | 871.39 ± 10.76 a | 580.05 ± 15.70b | 189.17 ± 14.13 d | 399.49 ± 19.94c |
Different letters represent significant differences at p < 0.05.
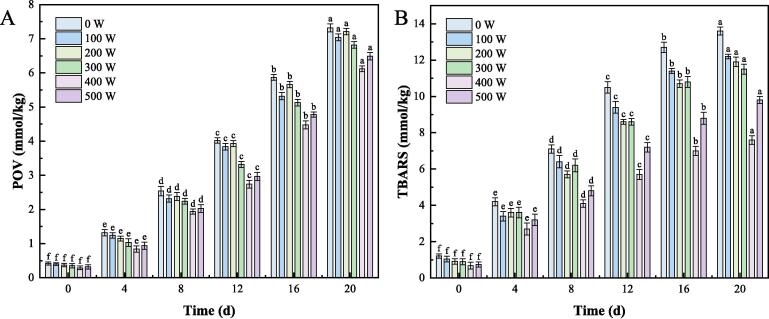
3.6.4. Oxidative stability of RBP-TA emulsion
To investigate the impact of different ultrasonic power levels on the oxidative stability of RBP-TA emulsion, the POV and TBARS values were measured as shown in Fig. 8 A and 7B. The RBP-TA emulsion treated with different ultrasonic power levels was stored at 20 °C for 20 days, and then the POV and TBARS values of the emulsion were determined. During the 20-day storage period, all emulsions showed an increasing trend in POV values. With increasing ultrasonic power, the POV and TBARS values of the RBP-TA emulsion gradually decreased on the 20th day. At an ultrasonic power of 400 W, the POV values of the RBP-TA emulsion were 6.12 and 7.60 mmol/kg, respectively. Regarding the study of the variation of the TBARS values in the emulsion, it was found that the TBARS values showed an increasing trend with time, especially during the storage period (from the 8th to the 12th day), where the increase of the TBARS values was most pronounced. This can be attributed to the decomposition of the initial oxidation products with the prolongation of time, leading to an increase in the TBARS values. Compared to untreated emulsion, the ultrasonically treated RBP-TA emulsion showed improved oxidative stability. This may be due to the increased adsorption proteins content at the emulsion interface, thereby enhancing the rate of free radical scavenging to inhibit lipid oxidation [34]. Furthermore, ultrasound may also alter the structure of RBP, enhancing its ability to bind with TA, thereby increasing the exposure of more antioxidant-friendly moieties [35].
Fig. 8.
Oxidation stability of SPI and SPI-TA emulsions, POV (A) and TABRS (B) Different letters represent significant differences at p < 0.05.
4. Conclusion
The results of our investigation revealed that the properties of emulsions stabilized by RBP-TA complexes were enhanced through ultrasonic treatment. By examining the influence of different ultrasonication power levels on the properties of RBP-TA emulsions, notable improvements were observed in the physicochemical properties, microstructure, rheological properties, and stability of the emulsions as the ultrasonication power increased. Notably, the RBP-TA emulsion exhibited optimized particle size (146.86 nm), zeta potential (-20.7 eV), adsorbed protein content (61.91%), and microstructure when subjected to 400 W ultrasonication. Furthermore, the apparent viscosity and viscoelasticity of the emulsion were enhanced. Importantly, ultrasonic treatment effectively prevented emulsion flocculation and improved the thermal, ionic, pH, and oxidative stability of RBP-TA emulsions. As a result, it can be inferred that ultrasonic treatment significantly enhanced the stability of RBP-TA emulsions, thereby laying a solid theoretical groundwork for their potential application in industrial production.
CRediT authorship contribution statement
Ning Wang: Methodology, Investigation, Writing – original draft. Donghua Wang: Formal analysis. Kaiwen Xing: Investigation, Validation, Data curation. Xiaoyu Han: Writing – review & editing. Shan Gao: Funding acquisition. Tong Wang: Data curation. Dianyu Yu: Supervision. Walid Elfalleh: Methodology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by a project from the Heilongjiang Provincial Scientific Research Institute (Youth Project): Study on Magnetase Continuous Esterification of Rice Bran Oil with High Acid Value as Functional Lipid (CZKYF2023-1-C014).
Contributor Information
Shan Gao, Email: gaoshanzz@aliyun.com.
Tong Wang, Email: wt19952320@163.com.
References
- 1.Dickinson E. Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocolloid. 2009;23(6):1473–1482. [Google Scholar]
- 2.Kim W., Wang Y., Selomulya C. Dairy and plant proteins as natural food emulsifiers. Trends Food Sci. Tech. 2020;105:261–272. [Google Scholar]
- 3.Burger T.G., Zhang Y. Recent progress in the utilization of pea protein as an emulsifier for food applications. Trends Food Sci. Tech. 2019;86:25–33. [Google Scholar]
- 4.Fabian C., Ju Y.-H. A review on rice bran protein: its properties and extraction methods. Crit. Rev. Food Sci. 2011;51(9):816–827. doi: 10.1080/10408398.2010.482678. [DOI] [PubMed] [Google Scholar]
- 5.Huang Y., Li A., Qiu C., Teng Y., Wang Y. Self-assembled colloidal complexes of polyphenol–gelatin and their stabilizing effects on emulsions. Food Funct. 2017;8(9):3145–3154. doi: 10.1039/c7fo00705a. [DOI] [PubMed] [Google Scholar]
- 6.Liu F., Zhang S., Li J., Mcclements D.J., Liu X. Recent development of lactoferrin-based vehicles for the delivery of bioactive compounds: complexes, emulsions, and nanoparticles. Trends Food Sci. Tech. 2018;79:67–77. [Google Scholar]
- 7.Mcclements D.J., Bai L., Chung C. Recent advances in the utilization of natural emulsifiers to form and stabilize emulsions. Annu. Rev. Food Sci. T. 2017;8(1):205–236. doi: 10.1146/annurev-food-030216-030154. [DOI] [PubMed] [Google Scholar]
- 8.Li R., Dai T., Tan Y., Fu G., Wan Y., Liu C., Mcclements D.J. Fabrication of pea protein-tannic acid complexes: impact on formation, stability, and digestion of flaxseed oil emulsions. Food Chem. 2020;310 doi: 10.1016/j.foodchem.2019.125828. [DOI] [PubMed] [Google Scholar]
- 9.Ashokkumar M. Applications of ultrasound in food and bioprocessing. Ultrason. Sonochem. 2015;25:17–23. doi: 10.1016/j.ultsonch.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y.-Y., Wang P., Zou Y.-F., Li K.e., Kang Z.-L., Xu X.-L., Zhou G.-H. Effect of pre-emulsification of plant lipid treated by pulsed ultrasound on the functional properties of chicken breast myofibrillar protein composite gel. Food Res. Int. 2014;58:98–104. [Google Scholar]
- 11.Lu X., Chen J., Zheng M., Guo J., Qi J., Chen Y., Miao S., Zheng B. Effect of high-intensity ultrasound irradiation on the stability and structural features of coconut-grain milk composite systems utilizing maize kernels and starch with different amylose contents. Ultrason. Sonochem. 2019;55:135–148. doi: 10.1016/j.ultsonch.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Cui Q., Wang L., Wang G., Zhang A., Wang X., Jiang L. Ultrasonication effects on physicochemical and emulsifying properties of cyperus esculentus seed (tiger nut) proteins. LWT. 2021;142 [Google Scholar]
- 13.Lad V.N., Murthy Z.V.P. Enhancing the stability of oil-in-water emulsions emulsified by coconut milk protein with the application of acoustic cavitation. Ind. Eng. Chem. Res. 2012;51(11):4222–4229. [Google Scholar]
- 14.Wang W., Wang R., Yao J., Luo S., Wang X., Zhang N., Wang L., Zhu X. Effect of ultrasonic power on the emulsion stability of rice bran protein-chlorogenic acid emulsion. Ultrason. Sonochem. 2022;84 doi: 10.1016/j.ultsonch.2022.105959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang N., Wang T., Yu Y., Xing K., Qin L., Yu D. Dynamic high-pressure microfluidization assist in stabilizing hemp seed protein-gum arabic bilayer emulsions: rheological properties and oxidation kinetic model. Ind. Crop Prod. 2023;203 [Google Scholar]
- 16.Wang T., Wang N., Dai Y., Yu D., Cheng J. Interfacial adsorption properties, rheological properties and oxidation kinetics of oleogel-in-water emulsion stabilized by hemp seed protein. Food Hydrocolloid. 2023;137 [Google Scholar]
- 17.Wang T., Wang N., Yu Y., Yu D., Xu S., Wang L. Study of soybean protein isolate-tannic acid non-covalent complexes by multi-spectroscopic analysis, molecular docking, and interfacial adsorption kinetics. Food Hydrocolloid. 2023;137 [Google Scholar]
- 18.Meng Y., Liang Z., Zhang C., Hao S., Han H., Du P., Li A., Shao H., Li C., Liu L. Ultrasonic modification of whey protein isolate: implications for the structural and functional properties. LWT. 2021;152 [Google Scholar]
- 19.Li N., Wang T., Yang X., Qu J., Wang N., Wang L., Yu D., Han C. Effect of high-intensity ultrasonic treatment on the emulsion of hemp seed oil stabilized with hemp seed protein. Ultrason. Sonochem. 2022;86 doi: 10.1016/j.ultsonch.2022.106021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fioramonti S.A., Martinez M.J., Pilosof A.M.R., Rubiolo A.C., Santiago L.G. Multilayer emulsions as a strategy for linseed oil microencapsulation: effect of ph and alginate concentration. Food Hydrocolloid. 2015;43:8–17. [Google Scholar]
- 21.Li C., Wang Q., Zhang C., Lei L., Lei X., Zhang Y., Li L., Wang Q., Ming J. Effect of simultaneous treatment combining ultrasonication and rutin on gliadin in the formation of nanoparticles. J. Food Sci. 2022;87(1):80–93. doi: 10.1111/1750-3841.15993. [DOI] [PubMed] [Google Scholar]
- 22.Leong T.S.H., Martin G.J.O., Ashokkumar M. Ultrasonic encapsulation - a review. Ultrason. Sonochem. 2017;35:605–614. doi: 10.1016/j.ultsonch.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Wang T., Wang N., Li N., Ji X., Zhang H., Yu D., Wang L. Effect of high-intensity ultrasound on the physicochemical properties, microstructure, and stability of soy protein isolate-pectin emulsion. Ultrason. Sonochem. 2022;82 doi: 10.1016/j.ultsonch.2021.105871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong Y., Li Q., Miao S., Zhang Y., Zheng B., Zhang L. Effect of ultrasound on physicochemical properties of emulsion stabilized by fish myofibrillar protein and xanthan gum. Innov. Food Sci. Emerg. 2019;54:225–234. [Google Scholar]
- 25.Zhang Q., Tu Z., Xiao H., Wang H., Huang X., Liu G., Liu C., Shi Y., Fan L., Lin D. Influence of ultrasonic treatment on the structure and emulsifying properties of peanut protein isolate. Food Bioprod. Process. 2014;92(1):30–37. [Google Scholar]
- 26.Sui X., Bi S., Qi B., Wang Z., Zhang M., Li Y., Jiang L. Impact of ultrasonic treatment on an emulsion system stabilized with soybean protein isolate and lecithin: its emulsifying property and emulsion stability. Food Hydrocolloid. 2017;63:727–734. [Google Scholar]
- 27.Sun Y., Zhong M., Zhao X., Song H., Wang Q., Qi B., Jiang L. Structural and interfacial characteristics of ultrasonicated lipophilic-protein-stabilized high internal phase pickering emulsions. LWT. 2022;158 [Google Scholar]
- 28.Ren Z., Li Z., Chen Z., Zhang Y., Lin X., Weng W., Yang H., Li B. Characteristics and application of fish oil-in-water pickering emulsions structured with tea water-insoluble proteins/κ-carrageenan complexes. Food Hydrocolloid. 2021;114 [Google Scholar]
- 29.Li K., Fu L., Zhao Y., Xue S., Wang P., Xu X., Bai Y. Use of high-intensity ultrasound to improve emulsifying properties of chicken myofibrillar protein and enhance the rheological properties and stability of the emulsion. Food Hydrocolloid. 2020;98 [Google Scholar]
- 30.Mohammadian M., Salami M., Emam-Djomeh Z., Momen S., Moosavi-Movahedi A.A. Gelation of oil-in-water emulsions stabilized by heat-denatured and nanofibrillated whey proteins through ion bridging or citric acid-mediated cross-linking. Int. J. Biol. Macromol. 2018;120:2247–2258. doi: 10.1016/j.ijbiomac.2018.08.085. [DOI] [PubMed] [Google Scholar]
- 31.Taha A., Ahmed E., Hu T., Xu X., Pan S., Hu H. Effects of different ionic strengths on the physicochemical properties of plant and animal proteins-stabilized emulsions fabricated using ultrasound emulsification. Ultrason. Sonochem. 2019;58 doi: 10.1016/j.ultsonch.2019.104627. [DOI] [PubMed] [Google Scholar]
- 32.Pongsawatmanit R., Harnsilawat T., Mcclements D.J. Influence of alginate, ph and ultrasound treatment on palm oil-in-water emulsions stabilized by β-lactoglobulin. Colloids Surf A Physicochem Eng Asp. 2006;287(1–3):59–67. [Google Scholar]
- 33.Zhu Z., Zhao C., Yi J., Cui L., Liu N., Cao Y., Decker E.A. Ultrasound improving the physical stability of oil-in-water emulsions stabilized by almond proteins. J. Sci. Food Agr. 2018;98(11):4323–4330. doi: 10.1002/jsfa.8958. [DOI] [PubMed] [Google Scholar]
- 34.Hu J.-N., Zheng H., Chen X.-X., Li X., Xu Y.u., Xu M.-F. Xu, Synergetic effects of whey protein isolate and naringin on physical and oxidative stability of oil-in-water emulsions. Food Hydrocolloid. 2020;101:105517. [Google Scholar]
- 35.Niu B., Shao P., Sun P. Ultrasound-assisted emulsion electrosprayed particles for the stabilization of β-carotene and its nutritional supplement potential. Food Hydrocolloid. 2020;102 [Google Scholar]