Abstract
Aims
Standardized data definitions are essential for monitoring and assessment of care and outcomes in observational studies and randomized controlled trials (RCTs). The European Unified Registries for Heart Care Evaluation and Randomized Trials (EuroHeart) project of the European Society of Cardiology aimed to develop contemporary data standards for atrial fibrillation/flutter (AF/AFL) and catheter ablation.
Methods and results
We used the EuroHeart methodology for the development of data standards and formed a Working Group comprising 23 experts in AF/AFL and catheter ablation registries, as well as representatives from the European Heart Rhythm Association and EuroHeart. We conducted a systematic literature review of AF/AFL and catheter ablation registries and data standard documents to generate candidate variables. We used a modified Delphi method to reach a consensus on a final variable set. For each variable, the Working Group developed permissible values and definitions, and agreed as to whether the variable was mandatory (Level 1) or additional (Level 2). In total, 70 Level 1 and 92 Level 2 variables were selected and reviewed by a wider Reference Group of 42 experts from 24 countries. The Level 1 variables were implemented into the EuroHeart IT platform as the basis for continuous registration of individual patient data.
Conclusion
By means of a structured process and working with international stakeholders, harmonized data standards for AF/AFL and catheter ablation for AF/AFL were developed. In the context of the EuroHeart project, this will facilitate country-level quality of care improvement, international observational research, registry-based RCTs, and post-marketing surveillance of devices and pharmacotherapies.
Keywords: Data standards, Data variables, Atrial fibrillation, Atrial flutter, Catheter ablation, EuroHeart
Graphical Abstract
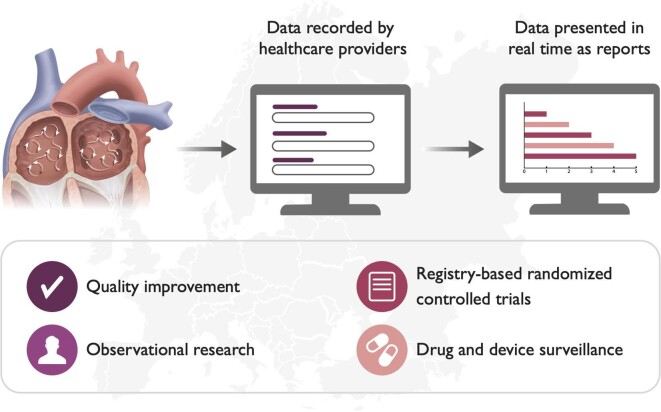
Graphical Abstract.
EuroHeart 2022 data standards for atrial fibrillation/flutter and catheter ablation
Introduction
Atrial fibrillation (AF) and atrial flutter (AFL) are the most frequently sustained cardiac arrhythmias in adults and pose a major healthcare and economic burden on individuals and societies.1,2 About 3% of the adult population have AF/AFL and it is estimated that one out of three European individuals over the age of 55 years will be affected by AF/AFL during their lifetime.3–5 There are differences in the management and outcomes of patients with AF/AFL within and among countries, depending not only on variability in resources and competence but also on an almost universal lack of monitoring of standards of care in the broad AF/AFL populations.6–8 Currently, information on methods for AF/AFL diagnosis, treatment and outcomes is based on randomized controlled trials (RCTs) and limited information from scientific reports from registries, administrative databases and electronic health care records (EHRs).1 Notably, comparisons of data over time and between studies or countries are compounded by variations in the data variables collected and their definitions.9
The adoption of standardised data variables and definitions is necessary for reliable assessment and comparison of quality of care within and among countries, international observational studies, RCTs, and post-marketing surveillance of devices and pharmacotherapies.10 Currently, there are no pan-European data standards for AF/AFL and catheter ablation that reflect contemporary clinical practice guidelines and are applicable to European healthcare systems. The 2004 American College of Cardiology (ACC)/American Heart Association (AHA) Key Data Elements and Definitions for AF provide a catalogue of data variables that describe care delivery and outcomes for patients with AF.9 However, whilst the document defines a comprehensive list of 149 data variables, there is no hierarchical specification as to which variables may be of greater importance—potentially limiting their utility in clinical practice.9 Moreover, those data standards were developed in accordance with the North American clinical practice guidelines and have not since been updated. The 2004 Cardiology Audit and Registration Data Standards (CARDS) project, which was the first European initiative to develop data standards for cardiovascular disease, focussed on acute coronary syndrome (ACS), percutaneous coronary intervention (PCI), and clinical electrophysiology, but not AF/AFL.11
The European Unified Registries for Heart Care Evaluation and Randomized Trials (EuroHeart) is a project initiated and supported by the European Society of Cardiology (ESC) that aims to improve the quality of cardiovascular care through the capture of individual patient data using standardized variables and a common IT infrastructure (Figure 1).12 The aim of EuroHeart is to collect longitudinal data to provide information about the trajectory of patient care and outcomes over time and has published international data standards for ACS, PCI, and heart failure.13,14 Here, we present the EuroHeart data standards for AF/AFL and catheter ablation, which were developed in collaboration with the European Heart Rhythm Association (EHRA) of the ESC.
Figure 1.
European Unified Registries for Heart Care Evaluation and Randomized Trials data standards and aims of the European Unified Registries for Heart Care Evaluation and Randomized Trials project. EuroHeart, European Unified Registries for Heart Care Evaluation and Randomized Trials
Methods
EuroHeart methodology: the five-step process
We adopted the EuroHeart five-step process for the development of data standards for cardiovascular diseases.15 This includes the (i) identification of clinical cardiovascular domains for data standards development; (ii) conduction of a systematic literature review to identify candidate variables and definitions; (iii) selection and prioritisation of variables and definitions by a domain-specific Working Group; (iv) validation of the variables and definitions by a domain-specific Reference Group; and (v) implementation of the developed data standards into the EuroHeart online IT platform.16
EuroHeart data standards
In EuroHeart, there are three categories of variables: Level 1 to 3.16 Level 1 variables are ‘mandatory’. They are relevant to the public reporting of quality of care, risk stratification, case-mix adjustment, and outcome evaluation. EuroHeart provides clinical definitions for the Level 1 variables and implements them on the EuroHeart IT platform to facilitate their collection. Level 2 variables are additional measures that may prove useful in selected areas or circumstances, but which do not need to be universally available. They complement quality assessment and may have a role in observational or randomized clinical research. Level 2 variables are defined in the EuroHeart data standard documents but are not routinely implemented on the EuroHeart IT platform. Finally, the EuroHeart platform also allows the addition of a third set of variables (Level 3) that can be centre- or country-specific and required for national or local studies or a specific quality improvement project. Level 3 variables are not defined or programmed by EuroHeart.
Data Science Group, Working Group, and Reference Group composition
Under the auspice of EuroHeart, a Data Science Group was established in 2019. The group comprised a project chair (C.P.G.), two medical experts (G.B. and S.A.), and a project manager.
A Working Group for the development of the 2022 EuroHeart data standards for AF/AFL and catheter ablation was formed including 23 members representing 11 countries. The domain-specific Working Group comprised representatives from EHRA, selected AF/AFL and catheter ablation registry experts and members from the EuroHeart Data Science Group. Names of the members of the Working Group and their affiliations are provided in the Appendix (Table A1).
The Reference Group included 42 international experts in AF/AFL and catheter ablation representing 24 ESC-member countries, patient representatives, and data analysts. The Group reviewed the selected variables and provided their feedback. Names of members of the Reference Group members are provided in the Acknowledgments.
Systematic literature review and other data sources
A systematic literature review was conducted by the EuroHeart Data Science Group. The scope of the review was to identify potential variables from existing registries and data standard documents related to AF/AFL and/or catheter ablation. Using Embase and Medline, we included peer-reviewed documents that defined at least one data variable relevant to AF/AFL and/or catheter ablation between 1 January 2016 and 15 April 2021 (Appendix Table A2). The included studies were reviewed by two independent authors (G.B. and S.A.) who identified potential data variables relevant to AF/AFL and extracted these variables alongside their definitions. Disagreements were resolved through discussion. These variables were added to other variables from existing clinical practice guidelines, quality indicators, and the data dictionaries of existing AF and catheter ablation registries, and formed the basis for the voting process.1,8,9,17–20
Consensus development and selection of variables and definitions
The Working Group decided that the scope of the EuroHeart AF/AFL registry was to capture the continuum of AF, AFL, and catheter ablation care delivery across the range of healthcare settings (e.g. in-patient and out-patient) and clinical contexts (e.g. new onset and prevalent AF/AFL). It was agreed that the data standards should be broadly applicable and enable interoperability between clinical registries, quality-of-care improvement projects, EHRs, and clinical trial protocols.
From the outset, the goal of the Working Group was to strike a balance between developing a focused yet comprehensive data standards document. The Working Group reviewed the candidate variables derived from the literature and decided on the inclusion of the variables and whether they were Level 1 or Level 2 using a modified Delphi method. Two rounds of voting were conducted, and each Working Group member was asked whether the proposed variables should be included as Level 1, included as Level 2, or excluded. An agreement of 75% was needed to include the variable in the dataset. The candidate variables from the systematic review that didn't meet the inclusion criteria during the first voting round but were deemed important by the Working Group in the subsequent meetings were voted upon in a second voting round.
To standardise the voting, the EuroHeart criteria for data standards (importance, evidence base, validity, reliability, feasibility, and applicability) were shared with the Working Group in the initial phases of the process.16 Once the final dataset was selected, the Working Group developed permissible values and definitions for the variables. The work of the Working Group was accomplished during eight virtual peer-to-peer meetings and extensive written correspondence.
Review of the data standards
Following the modified Delphi voting, the data standards were shared with an external Reference Group that reviewed and provided feedback on the proposed data variables, definitions, and permissible values. Narrative and written comments were considered by the Data Science Group and Working Group with variables modified accordingly.
Implementation into the EuroHeart IT platform
The final set of data variables, definitions, and permissible values were transferred to the Registry Technology Group of the EuroHeart project as a basis for programming into the EuroHeart IT platform. All Level 1 variables were implemented into the IT platform. For each variable, the Data Science Group provided the IT team with specifications for data entry and permissible ranges for numerical variables to facilitate consistent data entry.
Results
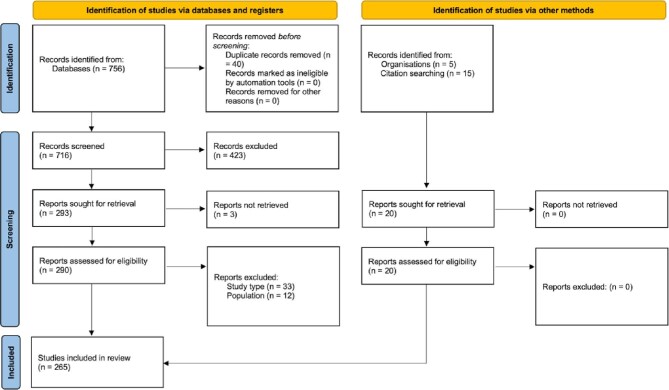
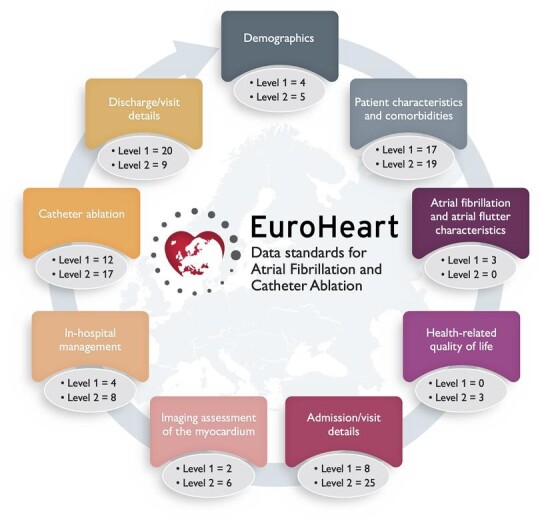
The literature review retrieved 776 articles, of which 265 (34%) articles met the inclusion criteria and were included as the basis for extracting 376 candidate variables (Appendix Figure A1). The modified Delphi process resulted in the selection of 162 variables in the EuroHeart AF/AFL and catheter ablation data standards across nine sections of AF/AFL care (Graphical abstract). This comprised 70 Level 1 (mandatory) variables (58 for AF/AFL and 12 for catheter ablation) and 92 Level 2 (additional) variables (75 for AF/AFL and 17 for catheter ablation) (Supplementary material online, Table S1–S2).
Demographics
The demographics section collects patient details such as identification number, date of birth, and sex (Supplementary material online, Table S1). Notably, the section is fully aligned with the other EuroHeart data standards (ACS, PCI, and heart failure) in order to minimise the burden of data collection for time-independent variables for patients enrolled in >1 EuroHeart registry. Whilst this section captures patient-identifiable information to allow linkage with other data sources (e.g. death registry), these data are kept locally and not shared with the EuroHeart Data Science Group to maintain patient confidentiality. At the centre- or country level, the national unique identification number may be used in the EuroHeart registries. For the countries without unique identification numbers, the EuroHeart IT platform generates for each enrolled patient a unique code that can be used across the different EuroHeart registries.
Patient characteristics and comorbidities
The Level 1 variables in this section collect data about patient sex, anthropometric characteristics (e.g. weight and height), and past medical history (e.g. diabetes mellitus and prior stroke) at the time of hospital admission or outpatient visit (Supplementary material online, Table S1). The selection criteria for the Level 1 variables in this section were determined by variables frequently used in AF/AFL and catheter ablation studies and those important for risk adjustment such that biased estimates of associations when evaluating variations in performance and treatment may be minimized. Many of the variables in this section are also readily available in average medical health records including the components of the CHA2DS2-VASc score (congestive heart failure, hypertension, age, diabetes mellitus, stroke/transient ischaemic attack/thromboembolism history, vascular disease history, and sex).21 Additional characteristics and comorbidities were selected as Level 2 variables (Supplementary material online, Table S2).
Atrial fibrillation and atrial flutter characteristics
This section collects information about the duration and type of AF and/or AFL at the time of clinical encounter and is important for disease characterisation (Supplementary material online, Table S1). Many patients will likely have received a diagnosis of AF and/or AFL prior to initial registration. However, the registry aims to capture and permit the inclusion of patients with a variety of AF/AFL states, including new-onset and established arrhythmia.
Health-related quality of life
Patient reported outcome measures (PROMs), such as symptom burden, functional status, and health-related QoL (HRQoL) are important prognostic elements which inform patients, healthcare providers, and policymakers about a patient's self-perceived morbidity.22 Numerous generic and disease-specific HRQoL tools are available [e.g. European QoL-5 dimensions (EQ-5D) and AF effect on QoL (AFEQT)].23–25 However, HRQoL is a difficult concept to measure, and the assessment is often a complex undertaking requiring multiple measures to capture subjectivity and multidimensionality.26 As such and given the concerns about the feasibility of measuring HRQoL routinely in practice, the EuroHeart AF/AFL and catheter ablation data standards include variables about whether the HRQoL was assessed, which tool was used and the results of the measurement as Level 2 variables (Supplementary material online, Table S2). With appropriate resources and copyright license agreement, specific HRQoL questionnaires may be implemented into the registry as Level 3 variables.
Admission/visit details
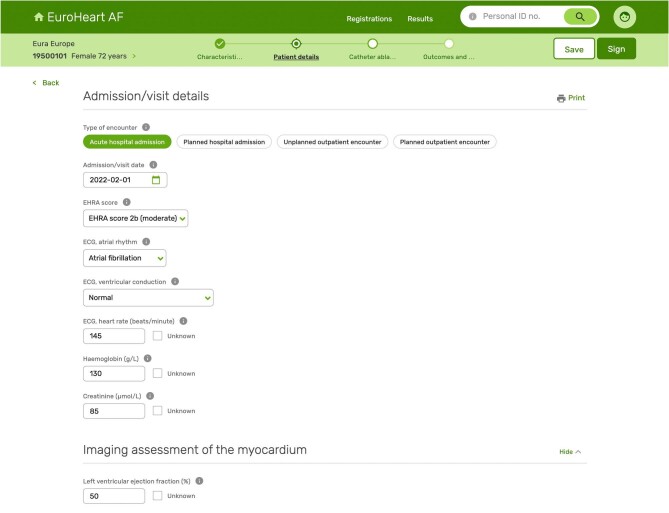
The admission/visit details section captures information about the type (in-patient or out-patient) and context (acute or planned) of the clinical encounter, as well as details about patient's current symptoms (EHRA score), biomarker levels (including haemoglobin, creatinine), and clinical findings at the time of assessment (such as ECG parameters) (Figure 2). These variables are often readily available and have prognostic value for individualised risk assessment and for tailoring of treatment strategies (Supplementary material online, Table S1).27,28 Medical therapy prior to the clinical encounter is part of the admission/visit details section and is included as Level 2 variables because of concerns among the Working Group members about the feasibility of collecting such information (Supplementary material online, Table S2).
Figure 2.
European Unified Registries for Heart Care Evaluation and Randomized Trials IT platform and collection form for atrial fibrillation/flutter and catheter ablation. EuroHeart, European Unified Registries for Heart Care Evaluation and Randomized Trials.
Imaging assessment of the myocardium
In this section, the Level 1 variables comprise the findings of the most recent evaluation of the left ventricular ejection fraction and the left atrial volume (Supplementary material online, Table S1). Given their prognostic significance and importance for AF/AFL management, these variables were selected as Level 1 by the Working Group.29,30 Other variables such as diastolic dysfunction, hypertrophic cardiomyopathy and right atrial volume were included as Level 2 variables (Supplementary material online, Table S2).
In-hospital management
The in-hospital management section includes information about the delivery of patient care during hospital admission or clinic visit. Here, the Level 1 variables capture data about cardioversion (including electrical or pharmacologic approaches) and left atrial appendage closure/exclusion (Supplementary material online, Table S1). In addition, other variables relevant to the management of the patient during the hospital stay were included at Level 2 (Supplementary material online, Table S2).
Catheter ablation
Presently, catheter ablation for AF and AFL is the most common cardiac ablation procedure in Europe.31 This section collects information about AF/AFL catheter ablation including procedural indication, approach and lesion location, as well as energy source and the outcome of the ablation (Supplementary material online, Table S1). Variables that collect greater detail about the procedure are provided as Level 2 (Supplementary material online, Table S2).
Discharge/visit details
The discharge/visits section collects information about the outcomes of care (including peri-procedural and in-hospital events), the hospital discharge date and information such as ECG findings at the time of discharge and planned treatment strategies (i.e. rhythm vs. rate control). For risk stratification, the CHA2DS2-VASc score is automatically calculated to allow the utilisation of collected data in predicting stroke risk (Supplementary material online, Table S1).21
The final part of the section is dedicated to the medications prescribed at the time of discharge from hospital. Many of the medications in this section are recommended by the current ESC guidelines for the diagnosis and management of AF and tally with the ESC quality indicators for AF.1,17 For medications commonly prescribed to patients with AF/AFL (e.g. oral anticoagulants), information not only about the class of drug, but also about the generic name and the dose is captured (Supplementary material online, Table S1–S2).32,33
Discussion
The recording of standardised and high-quality data underpins the process by which clinical care may be evaluated and improved.10 A lack of monitoring of care processes and outcomes, compounded by the absence of contemporary internationally defined data standards, has contributed to variability in the detection, management, and outcomes of AF/AFL across Europe.6,7 This has also resulted in inefficiently delivered cardiovascular studies.34 By means of a structured methodology, and in collaboration with EHRA, the EuroHeart project of the ESC has defined a catalogue of 70 Level 1 and 92 Level 2 AF/AFL and catheter ablation variables with accompanying definitions and permissible values. These data standards have been implemented in the interactive EuroHeart IT platform to facilitate efficient longitudinal data collection and quality improvement, observational and registry-based randomized research and post-marketing surveillance of devices and pharmacotherapies. These structured data standards may also be implemented by other registration systems, case report forms, or EHRs and will facilitate national and international collaborations on quality development and scientific studies in patients with AF/AFL.
It is recognised that AF/AFL has become a major public health problem.3 It is estimated that approximately 3% of the adult population has AF/AFL, with the incidence and prevalence estimated to increase with an accompanying surge in clinical and public health costs and novel approaches to its detection.35 However, the true prevalence of AF/AFL is unknown, probably due to estimates derived from populations with varying risk and owing to asymptomatic AF/AFL often being undiagnosed.3,36 The emergence of novel medications (e.g. direct oral anticoagulants) and treatment strategies for AF/AFL has improved outcomes, but patients with AF/AFL are still at high risk of mortality, morbidity and poor HRQoL.37 The growing health burden of AF/AFL shapes the need to develop systems for continuous capture of AF/AFL data and to monitor the quality of care. Such systems may also be used to deliver high-quality, yet cost-effective research particularly if accompanied by an integrated IT platform that facilitates data collection, analysis and reporting. Until now, the lack of harmonized data standards for AF/AFL and catheter ablation has limited the opportunity to combine data from different registries and thus, conduct of international comparative analyses and large-scale collaborations.
The EuroHeart AF/AFL and catheter ablation data standards extend the literature by providing a contemporary international perspective for AF/AFL management and incorporate the processes of AF care proposed by the ESC guidelines.1 Despite the development of harmonized definitions for AF in the US, the ACC/AHA Key Data Elements and Definitions for AF have not been fully adopted by the ACC's AFib Ablation Registry and the AHA's Get With the Guidelines (GWTG) AF Registry, resulting in heterogeneity in the methods by which the AF variables are defined and collected.9,18,19 In addition, these efforts provide a North American perspective to AF care which may differ from Europe's, stressing the need for pan-European data standards for AF/AFL.9,18,19
Although the EuroHeart AF/AFL data standards share some similar variables with those defined by the ACC and the AHA, there are important differences. First, the EuroHeart variables are harmonised across various cardiovascular domains including ACS, PCI, heart failure and transcatheter aortic valve implantation (TAVI). This provides means for data pooling from different resources when conducting research studies. Second, the EuroHeart AF/AFL and catheter ablation data standards have two levels of variables based on importance, with only the mandatory variables implemented onto an IT platform. By contrast, the ACC/AHA data standards have not established a hierarchy based on the importance of the variables and mandate the collection of around 150–200 variables for every patient (compared to 70 Level 1 variables in the EuroHeart AF/AFL and catheter ablation registry).9,18,19 For Europe, the CARDS project in 2004 defined data variables for clinical electrophysiology, but these variables have since then not been updated to reflect the advances in catheter ablation and medical management for AF/AFL.11
The EuroHeart data standards for AF/AFL and catheter ablation have been developed in collaboration with EHRA and with the involvement of AF/AFL experts and registry leaders from 11 countries. A Reference Group involved members from 24 countries to provide feedback in collaboration with EHRA, the ESC Working Group on Thrombosis, the ESC Patient Forum, the Association of Cardiovascular Nursing and Allied Professions (ACNAP), the ESC Committee for Young Cardiovascular Professionals, the Working Group on Myocardial and Pericardial Diseases Young in Career Group, and the EURObservational Research Programme (EORP). The level of engagement of international experts highlights the mission of EuroHeart, which is to provide means for capturing structured data to improve the quality of patient care and to support the generation of representative observational data.12
Individual patient data collected by participating EuroHeart centres will be stored in the country of collection under the responsibility of national or regional registry centres, according to the local law and data protection regulations. Signed informed consent will not be required for data collection for quality development in most countries. Only de-identified and aggregated data (i.e. no individual patient data) may be shared by participating countries for pre-defined statistical analysis. For prospective registry-based RCTs or drug and device monitoring initiatives, ethical approval, and informed consent from patients will be required as for any other clinical investigation.
We recognise the limitations of the EuroHeart methodology for data standards development. Despite basing the data standards on the results from a systematic review, the final variables, definitions, and permissible values were selected by a Working Group based on expert opinion and this may incur bias. Nonetheless, we feel that the inclusiveness of the Working Group (which comprised experts from many European countries with experience in national and international registries, quality improvement projects and observation and randomized research) provided a robust and transparent framework. We also formed an international Reference Group, including patient representatives and computer scientists, to ‘check and challenge’ and therefore enhance the robustness and validity of our approach. Even so, future Working Groups may benefit from being wider and inclusive of pharmacists, primary care physicians, health service researchers, and quality improvement specialists. The EuroHeart data standards for AF/AFL and catheter ablation have been implemented into the EuroHeart IT platform as a basis for continuous longitudinal data entry. However, the data standards have not been translated into computer-based language structures [e.g. Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT)].38 Standardized computer-based language structures are important for the electronic exchange of data between IT systems (e.g. registries and EHRs), but the linkage of variables and data ontologies is currently beyond the scope of this project. The data standards proposed in this document are based on the evidence available at the time of development. Future updates may be required as new evidence becomes available.
Conclusions
This document presents the first EuroHeart data standards for AF/AFL and catheter ablation which have been developed in collaboration with the EHRA of ESC using a standardized methodology. In total, 162 variables have been proposed as either mandatory (Level 1) or additional (Level 2), with the Level 1 variables been implemented into the EuroHeart IT platform. Once adopted, the data standards will facilitate country-level quality of care improvement, observational and registry-based randomized research and post-marketing surveillance of devices and pharmacotherapies.
Supplementary Material
Acknowledgment
We thank the European Heart Rhythm Association (EHRA), ESC Working Group on Thrombosis, ESC Patient Forum, Association of Cardiovascular Nursing and Allied Professions (ACNAP), ESC Committee for Young Cardiovascular Professionals, Working Group on Myocardial and Pericardial Diseases Young in Career Group, and EURObservational Research Programme (EORP) for their collaboration during the development of the EuroHeart AF/AFL and catheter ablation data standards.
We thank the members of the Reference Group for their expertise when reviewing the EuroHeart AF/AFL and catheter ablation data standards: Ayan Abdrakhmanov (Kazakhstan), Mervat Aboulmaaty (Egypt), Carlo de Asmundis (Belgium), Angelo Auricchio (Switzerland), Panayiotis Avraamides (Cyprus), Amitava Banerjee (United Kingdom), Christian Camm (United Kingdom), José Manuel Rubio Campal (Spain), Yengi Umut Celikyurt (Turkey), Harry Crijns (Netherlands), Gheorghe Andrei Dan (Romania), Natasja DeGroot (Netherlands), Rodrigue Garcia (France), Charles Guenancia (France), Jose M Guerra Ramos (Spain), Moti Haim (Israel), Łukasz Januszkiewicz (Poland), Oskars Kalējs (Latvia), Arvid Kauppi (Sweden), Małgorzata Lelonek (Poland), Konstantinos Letsas (Greece), Helena Malmborg (Sweden), Jacques Mansourati (France), Paweł Matusik (Poland), Erkin M Mirrakhimov (Kyrgyzstan), Pierre Ollitrault (France), Emin Evren Özcan (Turkey), Roberto De Ponti (Italy), Lidija Poposka (North Macedonia), Helmut Pürerfellner (Austria), Mariusz Pytkowski (Poland), Michiel Rienstra (Netherlands), Christopher Aldo Rinaldi (United Kingdom), Mark Sammut (Malta), Javier Garcia Seara (Spain), Michael D Spartalis (Italy), Nuria Basterra Sola (Spain), Noémi de Stoutz (Switzerland), Vassil Traykov (Bulgaria), Zakirov Nodir Uzuevich (Uzbekistan), Graziana Viola (Italy), Mihailo Vukmirović (Montenegro).
We also thank Catherine Reynolds (EuroHeart Project Manager, Leeds Institute of Cardiovascular & Metabolic Medicine, University of Leeds, Leeds, United Kingdom), Charlotta Elfström and Lan Vu Thi (EuroHeart Project Managers, Uppsala Clinical Research Center, Uppsala, Sweden) for their support and contribution to the EuroHeart project. We thank Ida Björkgren (Uppsala Clinical Research Center, Uppsala, Sweden) for the editorial support.
Appendix
Figure A1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart for the literature review for the development of the European Unified Registries for Heart Care Evaluation and Randomized Trials atrial fibrillation/flutter and catheter ablation data standards. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; EuroHeart, European Unified Registries for Heart Care Evaluation and Randomized Trials.
Table A1.
Names, countries and affiliations of the European Unified Registries for Heart Care Evaluation and Randomized Trials atrial fibrillation/flutter and catheter ablation Working Group members. EuroHeart, European Unified Registries for Heart Care Evaluation and Randomized Trials
| Name | Country | Affiliation |
|---|---|---|
| Suleman Aktaa | United Kingdom | • Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, UK• Leeds Institute for Data Analytics, University of Leeds, Leeds, UK• Department of Cardiology, Leeds Teaching Hospitals NHS Trust, Leeds, UK• EuroHeart Data Science Group |
| Gorav Batra | Sweden | • Department of Medical Sciences, Cardiology and Uppsala Clinical Research Center, Uppsala University, Uppsala, Sweden• EuroHeart Data Science Group |
| A. John Camm | United Kingdom | • St George's University, London, UK |
| Barbara Casadei | United Kingdom | • Division of Cardiovascular Medicine, NIHR Oxford Biomedical Research Centre, University of Oxford, Oxford, UK |
| Francisco Costa | Portugal | • Cardiology Department, Centro Hospitalar de Lisboa Ocidental EPE Hospital de Santa Cruz, Lisboa, Portugal |
| Luigi Di Biase | Italy | • Division of Cardiology, Department of Medicine, Albert Einstein College of Medicine/Montefiore Medical Center, New York, USA |
| David Duncker | Germany | • Hannover Heart Rhythm Center, Department of Cardiology and Angiology, Hannover Medical School, Hannover, Germany |
| Laurent Fauchier | France | • Service de Cardiologie, Center Hospitalier Universitaire Trousseau et Faculté de Médecine, Université de Tours, Tours, France |
| Nikolaos Fragakis | Greece | • 3rd Cardiology Department, Hippokration General Hospital, Aristotle University Medical School, Thessaloniki, Greece |
| Lars Frost | Denmark | • Department of Cardiology, Regional Hospital Central Jutland, Silkeborg, and Department of Clinical Medicine, Aarhus University, Aarhus, Denmark |
| Chris P Gale | United Kingdom | • Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, UK• Leeds Institute for Data Analytics, University of Leeds, Leeds, UK• Department of Cardiology, Leeds Teaching Hospitals NHS Trust, Leeds, UK• EuroHeart Data Science Group |
| Ziad Hijazi | Sweden | • Department of Medical Sciences, Cardiology and Uppsala Clinical Research Center, Uppsala University, Uppsala, Sweden |
| Tord Juhlin | Sweden | • Department of Cardiology, Skåne University Hospital, Lund, Sweden |
| José Luis Merino | Spain | • Arrhythmia and Robotic Electrophysiology Unit, Hospital Universitario La Paz, Idipaz, Universidad Autonoma, Madrid, Spain |
| Lluis Mont | Spain | • Hospital Clinic Barcelona, University of Barcelona, Barcelona, Spain• Institut de Recerca Biomèdica August Pi Sunyer (IDIBAPS), Barcelona, Spain• CIBER cardiovascular, Madrid, Spain |
| Jens Cosedis Nielsen | Denmark | • Department of Cardiology, Aarhus University Hospital and Department of Clinical Medicine, Aarhus University, Aarhus, Denmark |
| Jonas Oldgren | Sweden | • Department of Medical Sciences, Cardiology and Uppsala Clinical Research Center, Uppsala University, Uppsala, Sweden |
| Anna Polewczyk | Poland | • Department of Physiology, Patophysiology and Clinical Immunology, Collegium Medicum of The Jan Kochanowski University, Kielce, Poland• Department of Cardiac Surgery, Świętokrzyskie Center of Cardiology, Kielce, Poland |
| Tatjana Potpara | Serbia | • School of Medicine, University of Belgrade and Intensive Arrhythmia Care, Cardiology Clinic, Clinical Center of Serbia, Belgrade, Serbia |
| Frederic Sacher | France | • Electrophysiology and Ablation Unit, Bordeaux University Hospital (CHU), LIRYC Institute, Bordeaux, France |
| Philipp Sommer | Germany | • Clinic for Electrophysiology, Herz- und Diabeteszentrum NRW, Ruhr-Universität Bochum, Bad Oeynhausen, Germany |
| Roland Tilz | Germany | • Department of Rhythmology, University Heart Center Luebeck, Lübeck, Germany |
| Lars Wallentin | Sweden | • Department of Medical Sciences, Cardiology and Uppsala Clinical Research Center, Uppsala University, Uppsala, Sweden |
Table A2.
Terms used for the systematic review of the literature for the development of the EuroHeart atrial fibrillation/flutter and catheter ablation data standards
| Database: Embase < 1996 to 2021 Week 18 > Search strategy: | |
|---|---|
| 1. | atrial fibrillation/or atrial flutter/or heart atrium fibrillation/or heart atrium flutter/(170 921) |
| 2. | [(atrial or atrium or auricular) adj1 fibrillation].ti,ab. (138 321) |
| 3. | [(atrial or atrium or auricular) adj1 flutter].ti,ab. (8499) |
| 4. | (AF or Afib or AFl).ti,ab. (88 427) |
| 5. | ablation catheter/or radiofrequency ablation/or thermal ablation delivery device/or ablation therapy/or catheter ablation/or radiofrequency catheter ablation/(88 873) |
| 6. | or/1–5 (274 341) |
| 7. | register/(113 700) |
| 8. | disease registry/(18 055) |
| 9. | registry.ti,ab. (218 444) |
| 10. | registries.ti,ab. (43 352) |
| 11. | register*.ti,ab. (295 098) |
| 12. | database*.ti,ab. (808 323) |
| 13. | exp cohort analysis/(813 073) |
| 14. | exp longitudinal study/(162 841) |
| 15. | exp prospective study/(733 419) |
| 16. | exp follow-up/(1 745 593) |
| 17. | cohort .tw. (1 220 221) .tw. (1 220 221) |
| 18. | (random or placebo or placebo or single blind or single blind or double blind or double blind or triple blind or triple blind ).ti,ab. (1 733 043) ).ti,ab. (1 733 043) |
| 19. | or/7–18 (5 407 181) |
| 20. | exp common data elements/(432) |
| 21. | electronic data interchange/(780) |
| 22. | exp nomenclature/(47 033) |
| 23. | [data adj3 (element* or field* or variable*)].ti,ab. (35 573) |
| 24. | [data adj3 (harmoni? * or standard* or defin*)].ti,ab. (42 723) |
| 25. | data aggregation/(300) |
| 26. | big data/(4123) |
| 27. | data base/(239 963) |
| 28. | clinical data repository/(1283) |
| 29. | data extraction/(20 169) |
| 30. | data consistency/(456) |
| 31. | data collection method/(5531) |
| 32. | data interoperability/(405) |
| 33. | or/20–32 (386 130) |
| 34. | (random sample or random digit or random digit or random effect or random effect or random survey or random regression).ti,ab. not exp randomized controlled trial/(113 617) or random survey or random regression).ti,ab. not exp randomized controlled trial/(113 617) |
| 35. | news/or exp historical article/or Anecdotes asTopic/(10) |
| 36. | (book or conference paper or review).pt. not exp randomized controlled trial/(2 955 489) |
| 37. | meta-analysis/(236 278) |
| 38. | ‘systematic review’/or ‘review’/(2 470 783) |
| 39. | conference abstract/(1 477 113) |
| 40. | case report/or case study/(2 026 757) |
| 41. | (editorial or letter or comment*).ti. (230 585) |
| 42. | (animal not human not human ).sh,hw. (3 072 660) ).sh,hw. (3 072 660) |
| 43. | (animals/not humans/) or exp Animals, Laboratory/or exp Animal Experimentation/or exp Models, Animal/or exp Rodentia/or (rat or rats or mouse or mice).ti. (3 735 892) |
| 44. | or/34–43 (10 454 131) |
| 45. | 6 and 19 and 33 (4567) |
| 46. | 45 not 44 (3569) |
| 47. | limit 46 to English language (3537) |
| 48. | limit 47 to dd = 20 160 101-current (688) |
Table A2.
Continue
| Database: Ovid MEDLINE(R) ALL < 1946 to 14 April, 2021 > Search strategy: | |
|---|---|
| 1. | atrial fibrillation/or atrial flutter/or heart atrium fibrillation/or heart atrium flutter/(67 610) |
| 2. | [(atrial or atrium or auricular) adj3 fibrillation].ti,ab. (81 992) |
| 3. | [(atrial or atrium or auricular) adj3 flutter].ti,ab. (7729) |
| 4. | (AF or Afib or AFl).ti,ab. (48 290) |
| 5. | ablation catheter/or radiofrequency ablation/or thermal ablation delivery device/or ablation therapy/or catheter ablation/or radiofrequency catheter ablation/(37 948) |
| 6. | or/1–5 (138 125) |
| 7. | register/(6643) |
| 8. | registry.ti,ab. (132 710) |
| 9. | registries.ti,ab. (29 290) |
| 10. | register*.ti,ab. (235 428) |
| 11. | database*.ti,ab. (562 321) |
| 12. | exp cohort analysis/(2 315 930) |
| 13. | exp longitudinal study/(156 422) |
| 14. | exp prospective study/(619 714) |
| 15. | cohort .tw. (737 597) .tw. (737 597) |
| 16. | (random or placebo or placebo or single blind or single blind or double blind or double blind or triple blind or triple blind ).ti,ab. (1 401 762) ).ti,ab. (1 401 762) |
| 17. | or/7–16 (4 315 836) |
| 18. | exp common data elements/(135) |
| 19. | (data adj3 (harmoni? * or standard* or defin*)).ti,ab. (31 306) |
| 20. | [data adj3 (element* or field* or variable*)].ti,ab. (28 880) |
| 21. | Electronic Data Processing/(13 375) |
| 22. | Routinely Collected Health Data/(67) |
| 23. | Database Management Systems/(7719) |
| 24. | Data Collection/(91 381) |
| 25. | Data Warehousing/(196) |
| 26. | [data adj3 (element* or field* or variable*)].ti,ab. (28 880) |
| 27. | [data adj3 (harmoni? * or standard* or defin*)].ti,ab. (31 306) |
| 28. | data aggregation/(61) |
| 29. | big data/(2050) |
| 30. | data collection method/(91 381) |
| 31. | or/18–30 (170 745) |
| 32. | (random sample or random digit or random digit or random effect or random effect or random survey or random regression).ti,ab. not exp randomized controlled trial/(95 146) or random survey or random regression).ti,ab. not exp randomized controlled trial/(95 146) |
| 33. | news/or exp historical article/or Anecdotes asTopic/(614 505) |
| 34. | (book or conference paper or review).pt. not exp randomized controlled trial/(2 953 576) |
| 35. | ‘Systematic Review’/(189 020) |
| 36. | meta-analysis/(155 515) |
| 37. | case report/or case study/(2 256 717) |
| 38. | (editorial or letter or comment*).ti. (213 914) |
| 39. | (animal not human not human ).sh,hw. (4 933 342) ).sh,hw. (4 933 342) |
| 40. | (animals/not humans/) or exp Animals, Laboratory/or exp Animal Experimentation/or exp Models, Animal/or exp Rodentia/or (rat or rats or mouse or mice).ti. (5 963 387) |
| 41. | or/32–40 (11 660 750) |
| 42. | 6 and 17 and 31 (240) |
| 43. | 42 not 41 (190) |
| 44. | limit 43 to English language (182) |
| 45. | limit 44 to dt = 20 160 101-current (68) |
Notes
Developed in collaboration with the European Heart Rhythm Association (EHRA) of the European Society of Cardiology
Contributor Information
Gorav Batra, Department of Medical Sciences, Cardiology and Uppsala Clinical Research Center, Uppsala University, 751 85 Uppsala, Sweden.
Suleman Aktaa, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds Institute for Data Analytics, University of Leeds and Department of Cardiology, Leeds Teaching Hospitals NHS Trust, Leeds LS1 3EX, UK.
A John Camm, St George's University, London SW17 0RE, UK.
Francisco Costa, Cardiology Department, Centro Hospitalar de Lisboa Ocidental EPE Hospital de Santa Cruz, 1449-005 Lisboa, Portugal.
Luigi Di Biase, Division of Cardiology, Department of Medicine, Albert Einstein College of Medicine/Montefiore Medical Center, New York City, NY 10467, USA.
David Duncker, Hannover Heart Rhythm Center, Department of Cardiology and Angiology, Hannover Medical School, 30625 Hannover, Germany.
Laurent Fauchier, Service de Cardiologie, Center Hospitalier Universitaire Trousseau et Faculté de Médecine, Université de Tours, 37044 Tours, France.
Nikolaos Fragakis, 3rd Cardiology Department, Hippokration General Hospital, Aristotle University Medical School, 54124 Thessaloniki, Greece.
Lars Frost, Department of Cardiology, Regional Hospital Central Jutland, Silkeborg, and Department of Clinical Medicine, Aarhus University, 8200 Aarhus, Denmark.
Ziad Hijazi, Department of Medical Sciences, Cardiology and Uppsala Clinical Research Center, Uppsala University, 751 85 Uppsala, Sweden.
Tord Juhlin, Department of Cardiology, Skåne University Hospital, 221 85 Lund, Sweden.
José L Merino, Arrhythmia and Robotic Electrophysiology Unit, Hospital Universitario La Paz, IdiPaz, Universidad Autonoma, 28046 Madrid, Spain.
Lluis Mont, Hospital Clinic, Universitat de Barcelona, Institut de Recerca Biomèdica August Pi Sunyer (IDIBAPS), 08036 Barcelona, Spain; CIBER cardiovascular, 28029 Madrid, Spain.
Jens C Nielsen, Department of Cardiology, Aarhus University Hospital and Department of Clinical Medicine, Aarhus University, 8200 Aarhus, Denmark.
Jonas Oldgren, Department of Medical Sciences, Cardiology and Uppsala Clinical Research Center, Uppsala University, 751 85 Uppsala, Sweden.
Anna Polewczyk, Department of Physiology, Patophysiology and Clinical Immunology, Collegium Medicum of The Jan Kochanowski University, 25-369 Kielce, Poland; Department of Cardiac Surgery, Department of Cardiac Surgery Świętokrzyskie Center of Cardiology, Kielce, Poland.
Tatjana Potpara, School of Medicine, University of Belgrade and Intensive Arrhythmia Care, Cardiology Clinic, Clinical Center of Serbia, 11000 Belgrade, Serbia.
Frederic Sacher, Electrophysiology and Ablation Unit, Bordeaux University Hospital (CHU), LIRYC Institute, 33600 Bordeaux, France.
Philipp Sommer, Clinic for Electrophysiology, Herz- und Diabeteszentrum NRW, Ruhr-Universität Bochum, 32545 Bad Oeynhausen, Germany.
Roland Tilz, Department of Rhythmology, University Heart Center Luebeck, 23538 Lübeck, Germany.
Aldo P Maggioni, ANMCO Research Center, Heart Care Foundation, 50121 Florence, Italy.
Lars Wallentin, Department of Medical Sciences, Cardiology and Uppsala Clinical Research Center, Uppsala University, 751 85 Uppsala, Sweden.
Barbara Casadei, Division of Cardiovascular Medicine, NIHR Oxford Biomedical Research Centre, University of Oxford, Oxford OX4 2PG, UK.
Chris P Gale, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds Institute for Data Analytics, University of Leeds and Department of Cardiology, Leeds Teaching Hospitals NHS Trust, Leeds LS1 3EX, UK.
Funding
European Society of Cardiology.
Conflicts of interest:
G.B. reports, outside the submitted work, institutional research grants from Pfizer, expert committee and consulting fees to his institution from Bayer. Honoraria for lectures and scientific advice from AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Pfizer, and Sanofi.
A.J.C., personal fees from Acesion, Allergan, Alta Thera, Arca, Incarda, Menarini, Milestone, Sanofi, Bayer, Daiichi Sankyo, Pfizer, Abbott, Biosense Webster, Biotronik, Boston Scientific, Eli Lilly, Medtronic, Johnson and Johnson.
L.D.B. is a consultant for Biosense Webster, Stereoataxis and Rhythm Management, and has received speaker honoraria/travel from Biosense Webster, St. Jude Medical (now Abbott), Boston Scientific, Medtronic, Biotronik, Atricure, Baylis, and Zoll.
D.D. has received lecture/speaker fees from Abbott, AstraZeneca, Bayer, Bristol Myers Squibb, Boston Scientific, CVRx, Medtronic, Pfizer, and Zoll.
L.F., reports, outside this work, consultant or speaker activities for AstraZeneca, Bayer, BMS/Pfizer, Boehringer Ingelheim, Medtronic, Novartis, Novo, XO, and Zoll.
N.F., reports, outside this work, lecture/speaker fees from Boehringer Ingelheim, Pfizer, Abbot and Janssen.
L.F., reports, outside this work, support by the Health Research Foundation of Central Denmark Region; honoraria from AstraZeneca, BMS, and Pfizer for educational activities, scientific advices and consulting.
Z.H. receives research support from the Swedish Society for Medical Research (S17-0133), the Swedish Heart-Lung Foundation (20 200 722) and Uppsala University Hospital, Sweden. Outside this work, Z.H. has received lecture/consulting fees from Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, and Roche Diagnostics.
T.J., reports, outside this work, lecture/speaker fees from Boehringer-Ingelheim, Pfizer, Bristol-Myers Squibb, Organon, and Bayer.
J.L.M. reports, outside the submitted work, institutional research grants from Abbott, Medtronic and Microport, honoraria for lectures, educational support, and scientific advice from Abbott, Micropotr, and Sanofi.
L.M., reports, outside this work, consultant, lecturer and advisory board fees from Abbott Medical, Medtronic, Boston Scientific, and J&J. L.M. is stockholder of Galgo Medical, S.L.
J.O. has outside present work received fees for consultancy and lectures to his institution from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Novartis, Pfizer, Portola, and Sanofi), and grant support to his institution from Roche Diagnostics.
T.P. reports consultancy (no personal fees) for Bayer and Pfizer.
F.S., reports, outside this work, lecture/speaker fees from Abbott, Bayer, Biosense Webster, Boston Scientific, Inheart, and Microport.
P.S. is advisory board member of Abbott, Biosense Webster, Boston Scientific, and Medtronic.
R.T., reports, outside this work, R.T. is consultant of Boston Scientific, Biotronik and Biosense Webster and received Speaker´s Honoraria from Biosense Webster, Medtronic, Boston Scientific, and Abbot Medical.
A.P.M. reports, outside the submitted work, personal fees for participation in committees of studies sponsored by AstraZeneca, Bayer, Fresenius, Novartis.
L.W. reports, outside the submitted work, institutional research grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, GlaxoSmithKline, Merck&Co, and Roche Diagnostics.
B.C. reports, outside the submitted work, kind support from Roche Diagnostics and iRhythm for clinical studies of atrial fibrillation.
C.P.G. reports, outside the submitted work, consultancy (AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer-Ingelheim, Chiesi, Daiichi Sankyo, Menarini), speaker fees (AstraZeneca, Menarini, Raisio Group, Wondr Medical, Zydus), editorship (Deputy Editor: European Heart Journal Quality of Care and Clinical Outcomes, Oxford University Press), grants (British Heart Foundation, National Institute for Health Research, Horizon 2020, Abbott Diabetes, Bristol Myers Squibb), Advisory Board (Amgen, Bayer, Bristol Myers Squibb, Boehringer-Ingelheim, Chiesi, Daiichi Sankyo, Menarini), leadership (NICE Indicator Advisory Committee, Chair ESC Quality Indicator Committee).
All other authors have nothing to declare.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist Cet al. ESC Scientific Document Group . 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 2. Johnsen SP, Dalby LW, Täckström T, Olsen J, Fraschke A.. Cost of illness of atrial fibrillation: a nationwide study of societal impact. BMC Health Services Research 2017;17:714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rahman F, Kwan GF, Benjamin EJ.. Global epidemiology of atrial fibrillation. Nat Rev Cardiol 2014;11:639–654. [DOI] [PubMed] [Google Scholar]
- 4. Krijthe BP, Kunst A, Benjamin EJ, Lip GYH, Franco OH, Hofman Aet al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 2013;34:2746–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu J, Nadarajah R, Nakao YM, Nakao K, Wilkinson C, Mamas MAet al. Temporal trends and patterns in atrial fibrillation incidence: a population-based study of 3·4 million individuals. The Lancet Regional Health - Europe 2022;17:100386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lip GYH, Laroche C, Boriani G, Dan G-A, Santini M, Kalarus Zet al. Regional differences in presentation and treatment of patients with atrial fibrillation in Europe: a report from the EURObservational Research Programme Atrial Fibrillation (EORP-AF) Pilot General Registry. Europace 2015;17:194–206. [DOI] [PubMed] [Google Scholar]
- 7. Boriani G, Proietti M, Laroche C, Fauchier L, Marin F, Nabauer Met al. EORP-AF Long-Term General Registry Investigators; Steering Committee (National Coordinators) . Contemporary stroke prevention strategies in 11 096 European patients with atrial fibrillation: a report from the EURObservational Research Programme on Atrial Fibrillation (EORP-AF) Long-Term General Registry. EP Europace 2018;20:747–757. [DOI] [PubMed] [Google Scholar]
- 8. Potpara TS, Lip GYH, Dagres N, Crijns HJMG, Boriani G, Kirchhof Pet al. Cohort profile: the ESC EURObservational Research Programme Atrial Fibrillation III (AF III) Registry. Eur Heart J Qual Care Clin Outcomes 2021;7:229–237. [DOI] [PubMed] [Google Scholar]
- 9. McNamara RL, Brass LM, Drozda JP, Go AS, Halperin JL, Kerr CRet al. ACC/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with atrial fibrillation. Circulation American Heart Association; 2004;109:3223–3243. [DOI] [PubMed] [Google Scholar]
- 10. Richesson RL, Krischer J.. Data standards in clinical research: gaps, overlaps, challenges and future directions. J Am Med Inform Assoc 2007;14:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Flynn MR, Barrett C, Cosío FG, Gitt AK, Wallentin L, Kearney Pet al. The Cardiology Audit and Registration Data Standards (CARDS), European data standards for clinical cardiology practice. Eur Heart J 2005;26:308–313. [DOI] [PubMed] [Google Scholar]
- 12. Wallentin L, Gale CP, Maggioni A, Bardinet I, Casadei B.. EuroHeart: European unified registries on heart care evaluation and randomized trials - An ESC project to develop a new it registry system which will encompass multiple features of cardiovascular medicine. Eur Heart J 2019;40:2745–2749. [DOI] [PubMed] [Google Scholar]
- 13. Batra G, Aktaa S, Wallentin L, Maggioni AP, Ludman P, Erlinge Det al. Data standards for acute coronary syndrome and percutaneous coronary intervention: the European Unified Registries for Heart Care Evaluation and Randomised Trials (EuroHeart). Eur Heart J 2022;ehac133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aktaa S, Batra G, Cleland JGF, Coats A, Lund LH, McDonagh Tet al. Data standards for heart failure: the European Unified Registries for Heart Care Evaluation and Randomized Trials (EuroHeart). Eur Heart J 2022;ehac151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aktaa S, Batra G, Wallentin L, Baigent C, Erlinge D, James Set al. European Society of Cardiology methodology for the development of quality indicators for the quantification of cardiovascular care and outcomes. Eur Heart J Qual Care Clin Outcomes 2022;8:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Batra G, Aktaa S, Wallentin L, Maggioni AP, Wilkinson C, Casadei Bet al. Methodology for the development of international clinical data standards for common cardiovascular conditions: European Unified Registries for Heart Care Evaluation and Randomised Trials (EuroHeart). Eur Heart J Qual Care Clin Outcomes 2021;qcab052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arbelo E, Aktaa S, Bollmann A, D'Avila A, Drossart I, Dwight Jet al. Quality indicators for the care and outcomes of adults with atrial fibrillation: task Force for the development of quality indicators in atrial fibrillation of the European Heart Rhythm Association (EHRA) of the European Society of Cardiology (ESC). EP Europace 2021;23:494–495. [DOI] [PubMed] [Google Scholar]
- 18. Masoudi FA, Ponirakis A, de Lemos JA, Jollis JG, Kremers M, Messenger JCet al. Trends in U.S. cardiovascular care. J Am Coll Cardiol 2017;69:1427–1450. [DOI] [PubMed] [Google Scholar]
- 19. Piccini JP, Xu H, Cox M, Matsouaka RA, Fonarow GC, Butler Jet al. Adherence to guideline-directed stroke prevention therapy for atrial fibrillation ss achievable. Circulation 2019;139:1497–1506. [DOI] [PubMed] [Google Scholar]
- 20. Kakkar AK, Mueller I, Bassand J-P, Fitzmaurice DA, Goldhaber SZ, Goto Set al. International longitudinal registry of patients with atrial fibrillation at risk of stroke: Global Anticoagulant Registry in the FIELD (GARFIELD). Am Heart J 2012;163:13–19.e1. [DOI] [PubMed] [Google Scholar]
- 21. Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM.. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 22. Anker SD, Agewall S, Borggrefe M, Calvert M, Cowie MR, Ford Iet al. The importance of patient-reported outcomes: a call for their comprehensive integration in cardiovascular clinical trials. Eur Heart J 2014;35:2001–2009. [DOI] [PubMed] [Google Scholar]
- 23. Andrade JG, Wells GA, Deyell MW, Bennett M, Essebag V, Champagne Jet al. Cryoablation or Drug Therapy for Initial Treatment of Atrial Fibrillation. N Engl J Med 2021;384:305–315. [DOI] [PubMed] [Google Scholar]
- 24. Kotecha D, Bunting K V, Gill SK, Mehta S, Stanbury M, Jones JCet al. Rate Control Therapy Evaluation in Permanent Atrial Fibrillation (RATE-AF) team. effect of digoxin vs bisoprolol for heart rate control in atrial fibrillation on patient-reported quality of life: the RATE-AF randomized clinical trial. JAMA 2020;324:2497–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spertus J, Dorian P, Bubien R, Lewis S, Godejohn D, Reynolds MRet al. Development and Validation of the Atrial Fibrillation Effect on QualiTy-of-Life (AFEQT) questionnaire in patients with atrial fibrillation. Circ Arrhythm Electrophysiol 2011;4:15–25. [DOI] [PubMed] [Google Scholar]
- 26. Haraldstad K, Wahl A, Andenæs R, Andersen JR, Andersen MH, Beisland Eet al. A systematic review of quality of life research in medicine and health sciences. Qual Life Res 2019;28:2641–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wynn GJ, Todd DM, Webber M, Bonnett L, McShane J, Kirchhof Pet al. The European Heart Rhythm Association symptom classification for atrial fibrillation: validation and improvement through a simple modification. Europace 2014;16:965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hijazi Z, Oldgren J, Lindbäck J, Alexander JH, Connolly SJ, Eikelboom JWet al. The novel biomarker-based ABC (age, biomarkers, clinical history)-bleeding risk score for patients with atrial fibrillation: a derivation and validation study. Lancet North Am Ed 2016;387:2302–2311. [DOI] [PubMed] [Google Scholar]
- 29. Henry WL, Morganroth J, Pearlman AS, Clark CE, Redwood DR, Itscoitz SBet al. Relation between echocardiographically determined left atrial size and atrial fibrillation. Circulation 1976;53:273–279. [DOI] [PubMed] [Google Scholar]
- 30. Dries D, Exner D, Gersh B, Domanski M, Waclawiw M, Stevenson L.. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. J Am Coll Cardiol 1998;32:695–703. [DOI] [PubMed] [Google Scholar]
- 31. Holmqvist F, Kesek M, Englund A, Blomström-Lundqvist C, Karlsson LO, Kennebäck Get al. A decade of catheter ablation of cardiac arrhythmias in Sweden: ablation practices and outcomes. Eur Heart J 2019;40:820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MDet al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet North Am Ed 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 33. Dan G-A, Martinez-Rubio A, Agewall S, Boriani G, Borggrefe M, Gaita Fet al. Antiarrhythmic drugs—clinical use and clinical decision making: a consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) Working Group on Cardiovascular Pharmacology, endorsed by the Heart Rhythm Soci. EP Europace 2018;20:731–732an. [DOI] [PubMed] [Google Scholar]
- 34. Camm AJ, Fox KAA.. Strengths and weaknesses of ‘real-world’ studies involving non-vitamin K antagonist oral anticoagulants. Open Heart 2018;5:e000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nadarajah R, Wu J, Frangi AF, Hogg D, Cowan C, Gale C.. Predicting patient-level new-onset atrial fibrillation from population-based nationwide electronic health records: protocol of FIND-AF for developing a precision medicine prediction model using artificial intelligence. BMJ Open 2021;11:e052887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M.. Mass Screening for untreated atrial fibrillation: the STROKESTOP study. Circulation 2015;131:2176–2184. [DOI] [PubMed] [Google Scholar]
- 37. Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH.. Atrial fibrillation. Circ Res 2017;120:1501–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haendel MA, Chute CG, Robinson PN.. Classification, ontology, and precision medicine. N Engl J Med 2018;379:1452–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.