Abstract
Aims
Renal denervation has been shown to lower blood pressure in sham-controlled trials and represents a device-based treatment option for hypertension. We sought to project clinical event reductions after radiofrequency renal denervation using a novel modelling approach.
Methods and results
The Global SYMPLICITY Registry is a global, prospective all-comer registry to evaluate safety and efficacy after renal denervation. For this analysis, change in office systolic blood pressure from baseline was calculated from reported follow-up in the Global SYMPLICITY Registry. Relative risks for death and other cardiovascular events as well as numbers needed to treat for event avoidance were obtained for the respective blood pressure reductions based on previously reported meta-regression analyses for the full cohort and high-risk subgroups including type 2 diabetes, chronic kidney disease, resistant hypertension, and high basal cardiovascular risk. Average baseline office systolic blood pressure and reduction estimates for the full cohort (N = 2651) were 166±25 and −14.8 ± 0.4 mmHg, respectively. Mean reductions in blood pressure ranged from −11.0–−21.8 mmHg for the studied high-risk subgroups. Projected relative risks ranged from 0.57 for stroke in the resistant hypertension cohort to 0.92 for death in the diabetes cohort. Significant absolute reductions in major adverse cardiovascular events over 3 years compared with the projected control (8.6 ± 0.7% observed vs. 11.7 ± 0.9% for projected control; P < 0.01) were primarily due to reduced stroke incidence. The robustness of findings was confirmed in sensitivity and scenario analyses.
Conclusion
Model-based projections suggest radiofrequency renal denervation for patients with uncontrolled hypertension adds considerable clinical benefit across a spectrum of different cohort characteristics.
Keywords: hypertension, renal denervation, relative risk reduction, outcome assessment, cardiovascular risk
Introduction
Uncontrolled hypertension remains the leading preventable cause of death globally.1 Treatment strategies that rely on lifestyle modification and chronic drug therapy have not achieved adequate blood pressure control in populations worldwide2,3 due to several factors including lack of patient awareness, socio-economic barriers to care, clinical inertia against guideline recommendations, and non-adherence to prescribed medication.4,5 Percutaneous catheter-based renal denervation has recently been shown to lower both office and 24-hour systolic and diastolic blood pressures in multiple prospective, randomized, sham-controlled trials including nearly 700 patients with uncontrolled hypertension in both the presence and absence of concomitant antihypertensive drug therapy,6–9 and therefore represents an adjuvant to traditional multiple drug therapy.10 Procedural safety, long-term preservation of renal function and low incidence of renal vascular complications following the procedure have also been documented.11–13 Despite the strong correlation between blood pressure and cardiovascular risk,14–16 questions remain about the magnitude of the effects of renal denervation therapy on cardiovascular outcomes. No large outcome trial of device-based treatments for hypertension has been performed to date.17 The ongoing prospective open labelled Global SYMPLICITY Registry has enrolled close to 3000 real-world patients treated with radio frequency renal denervation and most have been enrolled to three years post-procedure.18,19 We previously reported similar blood pressure reduction among various high-risk subgroups of the Global SYMPLICITY Registry.18 In the present analysis, we estimate clinical event reductions in high-risk groups based on the Global SYMPLICITY Registry-observed data and evidence from a published meta-regression analysis.
Methods
Global SYMPLICITY Registry
Details of the design of the international, prospective, single-arm Global SYMPLICITY Registry (NCT01534299) have previously been published.20 Patients with uncontrolled hypertension or conditions associated with sympathetic nervous system activation were enrolled according to local guidelines. The institutional review board or ethics committee at each enrolling site approved the registry, the study design adhered to the Declaration of Helsinki and all patients provided written informed consent. All patients were treated with the Symplicity renal denervation system (Medtronic, Santa Rosa, CA, USA) using either the single electrode Symplicity Flex™ or the multi-electrode Symplicity Spyral™ catheter to accomplish radiofrequency ablation of the renal nerves. Patients were followed at 3-, 6-, 12-, 24- and 36-months post-procedure. Adverse event occurrence, including death, stroke, myocardial infarction, were recorded at each follow-up and were independently adjudicated by the Clinical Events Committee (Cardiovascular Research Foundation, New York, NY, USA).
Cohort characteristics and subgroup identification
Analyses were performed on registry follow-up data collected through May 2019. In addition to the full study cohort, the following high-risk subgroups were specified: resistant hypertension, defined as baseline office systolic blood pressure >150 mmHg despite prescription of ≥3 anti-hypertensive medication classes; history of type 2 diabetes mellitus; chronic kidney disease defined as baseline estimated glomerular filtration rate <60 ml/min/1.73 m2; and high atherosclerotic cardiovascular disease risk at baseline >20%, calculated based on each patient's office systolic blood pressure, antihypertensive medications, serum cholesterol, and diabetic and smoking status.21
Risk analysis
A stepwise calculation approach was applied to compare reported events in Global SYMPLICITY Registry to calculated ‘control’ rates assuming office systolic blood pressure had remained stable at baseline levels (Supplementary material online, Figure S1). First, changes in office systolic blood pressure from baseline were averaged from the office systolic blood pressure changes observed at 6-, 12-, 24-, and 36-months follow-up. Observed event rates at 36 months were obtained from Global SYMPLICITY Registry data, including a combined major adverse cardiac events endpoint, defined as the three-point composite of non-fatal stroke, non-fatal myocardial infarction, and cardiovascular death. Next, relative risks for death, cardiovascular death, myocardial infarction and stroke were obtained for the observed average blood pressure reductions from a meta-regression analysis of randomized trials of office systolic blood pressure reductions in hypertensive patients (see supplementary materials).15 To project ‘control’ event rates, the observed events in the renal denervation-treated patients were divided by the calculated relative risks. The absolute difference between calculated control group events and observed renal denervation group events was subsequently used to calculate the numbers needed to treat to avoid the respective clinical events over a 3-year follow-up.
For each of these outcomes of interest, we calculated results first via the established deterministic approach. Probabilistic results were then calculated to reflect parameter uncertainty in the observed clinical event rates, blood pressure changes, and the relative risks from the published meta-regression. Distributions of mean event rates and blood pressure changes were obtained by calculating 2500 bootstrap samples of the Global SYMPLICITY Registry patient-level data. For the relative risk functions derived from the published meta-regression, the source report provided distributional information.15 Distributions for projected control events, events avoided, and numbers needed to treat were then calculated based on these input distributions using second-order Monte Carlo simulation (n = 5000 simulations). Using the distributions of mean event rates for the treated and control groups, we calculated the probability that the mean treated event rate would be worse than the mean control event rate—and considered a probability of <0.05 as a threshold that renal denervation was significantly better than the control. See supplementary materials for additional details.
Sensitivity analyses
Several sensitivity analyses were performed to explore the robustness of the study findings. First, results were recalculated for a lower effect size of 10 mmHg, in line with recent data from the ON-MED randomized trial, where this average effect size was reported vs. sham control through 36-month follow-up.22 Second, risk functions derived from recently analysed data of The Blood Pressure Lowering Treatment Trialists Collaboration (BPLTTC) were obtained and applied to the full cohort analysis to explore the effect of this different set of risk equations onto the analysis outcomes.
For all analyses, actual event rates and predicted distributions of the event parameter means are reported as mean ± standard deviation. All statistical analyses were performed with JMP 15 (SAS Institute, Cary, NC, USA).
Results
As of May 2019, 2651 patients were enrolled in the Global SYMPLICITY Registry from 196 centers in 45 countries, with a median follow up of 3 years. Median atherosclerotic cardiovascular disease risk score at baseline was 19.8% (Interquartile range: 9%–37%, N = 1485). Baseline demographics for the full registry cohort and for the high-risk subgroups in the supplementary materials are shown in Table1. Patients averaged over 60 years of age, were mostly male and had been diagnosed with hypertension on average 16 ± 12 years prior to enrolment. Most patients had a history of cardiovascular disease and were prescribed an average of 4.6 antihypertensive drug classes at the time of inclusion.
Table 1.
Baseline demographics for GSR cohort and sub-groups
| % or mean ± SD | All Patients (N = 2651)* | rHTN† (n = 1821) | Type 2 DM+ (n = 1007) | CKD‡ (n = 630) | High ASCVD Risk§ (n = 737) |
|---|---|---|---|---|---|
| Age (years) | 61 ± 12 | 61 ± 12 | 64 ± 10 | 65 ± 12 | 69 ± 8 |
| Male gender (%) | 57.8 | 57.5 | 59.3 | 52.2 | 64.0 |
| Body Mass Index (kg/m2) | 31 ± 6 | 31 ± 6 | 32 ± 6 | 31 ± 6 | 31 ± 5 |
| eGFR(mL/min/1.73 m2) | 76 ± 25 | 76 ± 25 | 72 ± 25 | 45 ± 13 | 72 ± 24 |
| History of cardiac disease (%) | 46.9 | 47.9 | 57.3 | 56.5 | 57.1 |
| Atrial fibrillation (%) | 12.5 | 11.7 | 13.7 | 17.1 | 16.4 |
| Diabetes, type 2 (%)+ | 38.1 | 40.7 | 100 | 47.9 | 57.8 |
| Obstructive sleep apnea (%) | 11.5 | 11.9 | 15.1 | 12.9 | 13.9 |
| Current smoking (%) | 9.8 | 10.3 | 8.5 | 9.4 | 10.3 |
| Office systolic BP (mmHg) | 166 ± 25 | 175 ± 20 | 165 ± 23 | 164 ± 26 | 167 ± 24 |
| Office diastolic BP (mmHg) | 90 ± 17 | 93 ± 16 | 86 ± 16 | 84 ± 17 | 85 ± 15 |
| 24-hr mean systolic BP (mmHg) | 154 ± 18 | 157 ± 18 | 155 ± 18 | 154 ± 19 | 153 ± 17 |
| 24-hr mean diastolic BP (mmHg) | 87 ± 14 | 88 ± 15 | 83 ± 13 | 82 ± 14 | 82 ± 12 |
| Number of AH meds | 4.6 ± 1.4 | 4.7 ± 1.2 | 4.7 ± 1.3 | 4.8 ± 1.3 | 4.7 ± 1.3 |
Original dataset18 included 1 additional patient who subsequently revoked consent and whose data were stricken from the database; Groups are not mutually exclusive. †rHTN, Resistant hypertension defined as oSBP >150 mmHg and prescription of ≥3 antihypertensive drug classes; +Type 2 DM, Based on physician reporting; ‡CKD: Chronic kidney disease defined as eGFR <60 mL/min/1.73 m2; §High ASCVD risk defined as baseline score >20%.
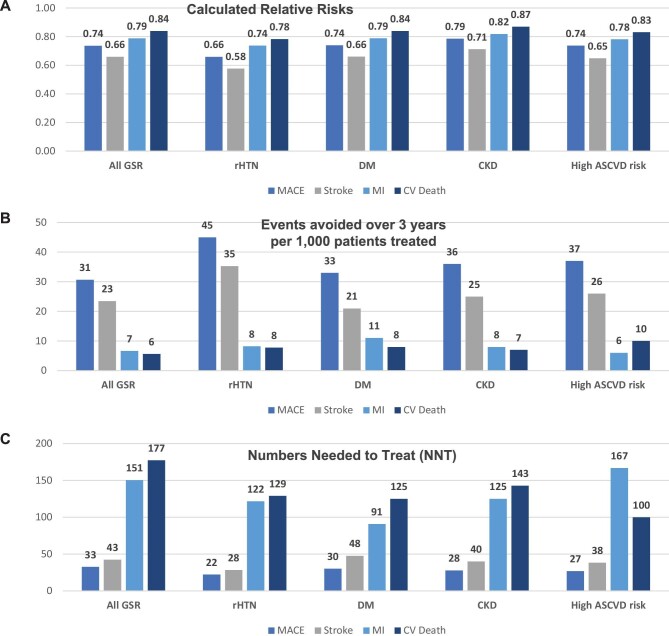
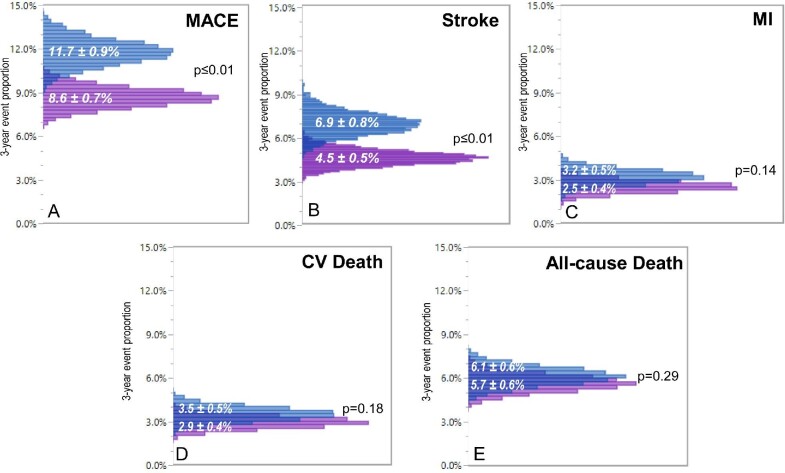
Mean cohort changes in office systolic blood pressure ranged from −11.0 – −21.8 mmHg systolic in the studied high-risk groups. Stroke (4.5%) and all-cause death (5.7%) were the most frequently reported outcome events in the full cohort and event rates varied by subgroup (Table2). The projected relative risk point estimates ranged from 0.57 for stroke in the resistant hypertension cohort to 0.92 for death in the type II diabetes mellitus cohort (Figure1). Probabilistic analysis demonstrated significant absolute reductions in major adverse cardiovascular events over 3 years (8.6 ± 0.7% actual vs. 11.7 ± 0.9% projected control; P < 0.01) primarily due to reduced stroke incidence (4.5 ± 0.5% actual vs. 6.9 ± 0.8% projected control; P < 0.01; Figure2). Over the three-year horizon, the calculated number of patients needed to treat to avoid one major adverse cardiovascular event (number needed to treat) ranged from 19–30 in the studied high-risk cohorts (Figure3 and supplementary materials online, Tables S.3.1–S.3.5).
Table 2.
36-month actual event rates observed
| All patients | rHTN | Type 2 DM | CKD | High ASCVD risk | |
|---|---|---|---|---|---|
| N (full cohort, baseline) | 2651 | 1821 | 1007 | 630 | 737 |
| N (with event data at 36 months) | 1749 | 1208 | 675 | 408 | 510 |
| Baseline oSBP (mmHg) | 166 ± 25 | 175 ± 20 | 165 ± 23 | 164 ± 26 | 168 ± 24 |
| Mean change in oSBP (mmHg) | −14.8 ± 0.4 | −21.5 ± 0.4 | −14.7 ± 0.5 | −11.0 ± 0.7 | −15.6 ± 0.6 |
| Stroke rate, 36 months (%) | 4.5 | 4.8 | 4.0 | 6.1 | 4.7 |
| MI rate, 36 M (%) | 2.5 | 2.3 | 4.0 | 3.4 | 2.2 |
| CV death rate, 36 M (%) | 2.9 | 2.8 | 4.0 | 5.1 | 4.5 |
| MACE rate, 36 M (%) | 8.6 | 8.7 | 10.4 | 13.0 | 10.2 |
| All-cause death rate, 36 M (%) | 5.7 | 5.7 | 7.1 | 10.0 | 8.4 |
Blood pressure values are mean ± SD. Groups and abbreviations as in Table1; MI, myocardial infarction; CV, cardiovascular; MACE, 3-point major cardiovascular Events; ESRD, end stage renal disease.
Figure 1.
Deterministic projections for the full, resistant hypertension, diabetes mellitus, chronic kidney disease, and high atherosclerotic cardiovascular disease risk cohorts for: (A) relative risks; (B) events avoided over 3 years per 1000 treated patients; and (C) numbers needed to treat for major adverse cardiac events, stroke, myocardial infarction, cardiovascular death events. MACE, major adverse cardiac events; MI, myocardial infarction; CV, cardiovascular; rHTN, resistant hypertension; DM, type-2 diabetes mellitus; CKD, chronic kidney disease; ASCVD, atherosclerotic cardiovascular disease.
Figure 2.
Distribution of mean rates of (A) major adverse cardiac events, (B) stroke, (C) myocardial infarction, (D) cardiovascular death, and (E) all-cause death for the actual treated group (purple) vs. calculated control (blue) for the full registry cohort based on simulation results considering parameter uncertainty. MACE, major adverse cardiac events; MI, myocardial infarction.
Figure 3.
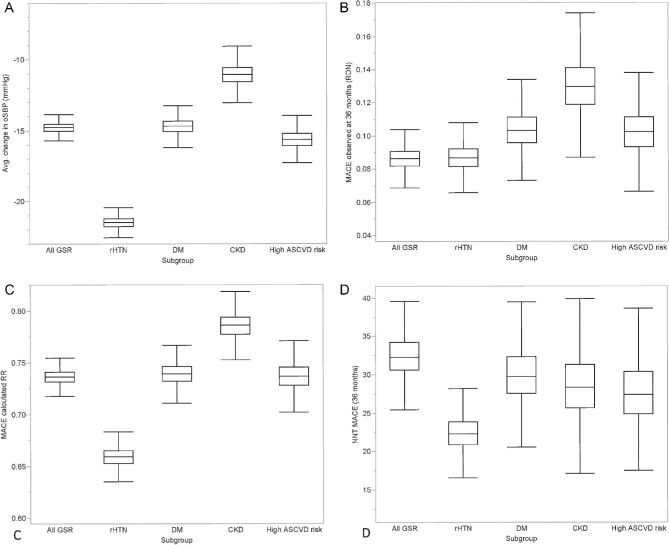
Sensitivity analysis accounting for sample uncertainty in subgroups analyses is shown. Panels illustrate: (A) distribution of actual office systolic blood pressure reductions over 3 years following radiofrequency renal denervation in the full Global SYMPLICITY Registry cohort and high-risk subgroups chronic kidney disease, high basal atherosclerotic cardiovascular disease risk, resistant hypertension, and diabetes mellitus cohorts; (B) frequency of actual major adverse cardiac events event occurrence in each group; (C) calculated risk reduction distribution for actual BP drops based on meta regression analysis; and (D) calculated distribution of numbers needed to treat in each group to avoid one major adverse cardiac events event within the 3-year follow up. oSBP,office systolic blood pressure; RDN, renal denervation; CKD, chronic kidney disease; ASCVD, atherosclerotic cardiovascular disease; rHTN, resistant hypertension; DM, type-2 diabetes mellitus; MACE, major adverse cardiac events; BP, blood pressure; NNT, numbers needed to treat.
In sensitivity analysis, the assumed reduction of effect size to 10 mmHg led to a limited reduction in control event rates (projected MACE events 10.9% instead of 11.7% for all GSR and 10.9% instead of 13.2% for resistant hypertension cohorts; see supplementary materials online, Tables S.4.1–S 4.5 for detailed results of all cohorts). Applying the BPLTTC-derived risk functions led to a projected MACE control event rate for the full cohort of 10.8% instead of 11.7% (vs. 8.6% actual events; P < 0.01—see supplementary material online, Table S.5.1). The MACE NNTs for the full cohort increased from 33 in the main analysis to 44 for the 10 mmHg effect size scenario and 46 for the BPLTTC risk function scenario.
Discussion
The results of the current study indicate that radiofrequency renal denervation may lower the occurrence of major adverse cardiovascular events by almost one third within 3-years. This finding is based on a projection of the blood pressure lowering effects observed in the largest real-world registry. This benefit was apparent in a patient population with uncontrolled hypertension despite baseline treatment with greater than four medications on average. The mean calculated number needed to treat to avoid one major adverse cardiovascular event over 3 years was 32 in the full cohort and was lower in the high-risk subgroups with calculated numbers needed to treat of 21, 30, 28, and 27 for resistant hypertension, type II diabetes mellitus, chronic kidney disease, and overall high atherosclerotic cardiovascular disease risk, respectively (Figure1). Therefore, higher risk patients with more comorbidities or higher baseline blood pressure may stand to benefit most from radiofrequency renal denervation treatment, consistent with recommendations promulgated in recent US and European consensus statements.10,23 The study approach is novel in that relative risks from meta-regression data were not applied to estimate potential events in patients treated with the intervention, but rather were applied to project events in a simulated control group that maintained baseline office systolic blood pressure throughout the follow up period. We believe this approach is innovative in that it can be applied whenever observed event rates in treated patients are available along with observed blood pressure reductions. Modeling projected events in a simulated control group may further enable cost-effectiveness and budget impact evaluations at the observed timeframe and beyond.
Recent randomized, sham-controlled trials included relatively smaller proportions of higher cardiovascular risk patients.6–8 Prospective evaluation of the effect of the renal denervation procedure on clinical outcomes appears to be impractical because of ethical considerations for potentially under-treated control group patients.17 Likewise, existing—and imminent—regulatory approvals of renal denervation in many geographies would render randomization of a large-scale population necessary for a prospective outcome trial difficult. Notably, the US Food and Drug Administration have acknowledged that blood pressure reduction is an acceptable surrogate trial endpoint because of the strongly established relationship between blood pressure reduction and improved cardiovascular outcome.24 Such a relationship has not only been observed in epidemiological studies but also in several meta-analyses reporting a nearly linear relationship between blood pressure lowering and reduction of cardiovascular events, irrespective of the baseline blood pressure.14–16 Interestingly, a recent retrospective analysis of a large single center cohort (N = 296) of resistant hypertensive renal denervation-treated patients with a median of 4 years follow up demonstrated significantly fewer major adverse cardiovascular events in patients classified as treatment ‘responders’.25 These results suggest that renal denervation-induced blood pressure reductions are indeed associated with major adverse cardiovascular event reduction as assumed in the present analysis. Our projections, based on previously published meta-analysis of cardiovascular risk reduction and blood pressure reduction, may guide estimates of the potential events avoided and the range of numbers needed to treat in the short-term. Such analysis may complement previous26 and future analysis models extrapolating longer-term clinical effectiveness and associated costs avoided following renal denervation treatment.
Previous investigations besides the Global SYMPLICITY Registry have also demonstrated the safety and efficacy of renal denervation in high risk cohorts of uncontrolled hypertension, including resistant hypertension,27–29 elderly patients,30 insulin resistance,31,32 chronic kidney disease,33–36 obstructive sleep apnea37,38 and isolated systolic hypertension.39 The present analysis focused on high-risk populations associated with hypertension including chronic kidney disease and type II diabetes mellitus. We also examined patients with overall high composite risk. Our modelled projections confirm and extend these previous findings by showing sustained blood pressure reduction vs. baseline in all cohorts and estimating a potential one-third reduction in specific major adverse cardiovascular events associated with observed blood pressure lowering.
The results also compare favorably with prior reports of number needed to treat for various device and drug therapies. For example, the SPRINT trial of intensive vs. standard blood pressure control in patients with uncontrolled blood pressure and increased cardiovascular risk reported an actual risk reduction of 25% for major adverse cardiovascular events, which was associated with a number needed to treat of 61 after an average follow up of slightly over 3 years, although a slightly different definition of major adverse cardiovascular events was applied in that study.40
Study limitations
The present analysis has limitations. First, our findings used blood pressure data from a single-arm registry, with assumptions that blood pressure measurements were not influenced by reporting bias and that control subjects maintained their baseline blood pressure over the study period. However, this assumption appears reasonable since Global SYMPLICITY Registry patients had uncontrolled blood pressure for an average of 16 years prior to receiving the renal denervation procedure. Also, the registry-observed changes in office systolic blood pressure are directionally higher than office systolic blood pressure changes demonstrated in the recent sham-controlled trials. However, these were performed in cohorts with different characteristics and risk profile compared with the real-world population in the Global SYMPLICITY registry. Nevertheless, the conducted sensitivity analysis using an effect size of 10 mmHg derived from recent sham-controlled trial suggest event reductions and NNTs would remain favorable and directionally comparable. Second, we relied on relative risk estimates derived from a published meta-regression that consider change in blood pressure as the only factor for estimation of risk reduction. This report is the largest meta-regression published to-date and specifically focusing on hypertension interventions, as opposed to associations between point-in-time blood pressure measures and clinical events reported in meta-analyses of broader populations.41 However, we also performed an additional sensitivity analysis based on data provided by the BPLTTC.14,16 Again, the calculated risk reductions and event rates, while somewhat smaller, did not materially change the analysis findings and therefore support the robustness of the results. Finally, to complement deterministic analyses, we applied probabilistic simulations to account for uncertainty in the observed clinical event rates, blood pressure changes, and relative risks from the published meta-regression.
Conclusions
In summary, the meta regression analysis-based projections of clinical events avoided resulted in significant major adverse cardiovascular event reduction and relatively low numbers needed to treat through 3 years follow-up in a real-world uncontrolled hypertension population treated with radiofrequency renal denervation, with consistent results in high-risk sub-cohorts. The relatively even distribution of predicted risk reduction across the full cohort and comorbid groups suggests that renal denervation can benefit all patients with uncontrolled hypertension, including higher risk uncontrolled hypertensive patients. These shorter-term data might provide useful orientation for clinicians and policymakers interested in extrapolating the potential clinical and economic implications of renal denervation treatment over longer-term horizons.
Supplementary Material
Acknowledgements
The authors thank Professor Kazem Rahimi FRCP DM MSc FESC (Oxford University) and the Blood Pressure Lowering Treatment Trialists’ Collaboration for providing the relative risk functions for the sensitivity analysis. The authors also thank Beth Ferri, PhD, CMPP and Benjamin Woods, PhD for editorial support under the direction of the first author.
Contributor Information
Roland E Schmieder, Department of Nephrology and Hypertension, University Hospital Erlangen, Erlangen, Bavaria 91054, Germany.
Felix Mahfoud, Internal Medicine and Cardiology, Saarland University Hospital, Homburg/Saar, Saarland 66421, Germany.
Giuseppe Mancia, Department of Medicine, University of Milano-Bicocca, Monza, Lombardia 20126, Italy.
Krzysztof Narkiewicz, Hypertension and Diabetology, Medical University of Gdansk, Gdansk 80-210, Poland.
Luis Ruilope, Cardiorenal Investigation, Institute of Research, Hospital Universitario 12 de Octubre and CIBERCV and School of Doctoral Studies and Research, Universidad Europea de Madrid, Madrid 28041, Spain.
David W Hutton, School of Public Health, University of Michigan, Ann Arbor, MI 48109, USA.
Khoa N Cao, Wing Tech Inc., Menlo Park, CA 94025, USA.
Douglas A Hettrick, Coronary and Renal Denervation, Medtronic, Santa Rosa, CA 95403, USA.
Martin Fahy, Coronary and Renal Denervation, Medtronic, Santa Rosa, CA 95403, USA.
Markus P Schlaich, Dobney Hypertension Centre, School of Medicine—Royal Perth Hospital Unit, The University of Western Australia, Perth, WA 6009, Australia.
Michael Böhm, Internal Medicine and Cardiology, Saarland University Hospital, Homburg/Saar, Saarland 66421, Germany.
Jan B Pietzsch, Wing Tech Inc., Menlo Park, CA 94025, USA.
Funding
Medtronic.
Conflicts of interest:
R.E.S. reports grants and personal fees from Medtronic, Recor, and Ablative Solutions.
F.M. is supported by Deutsche Gesellschaft für Kardiologie; and has received scientific support and speaker honoraria from Bayer, Boehringer Ingelheim, Medtronic, and ReCor Medical.
G.M. has received speaker fees from Boehringer Ingelheim, Ferrer, Gedeon Richter, Medtronic Vascular, Menarini, Merck Healthcare KGaA, Neopharmed-Gentili,
Novartis, Recordati, Sanofi, and Servier.
K.N. has received speaker honoraria from Adamed, Berlin Chemie/Menarini, Egis, Gedeon Richter, Krka, Polpharma, Sandoz, and Servier; and has received honoraria or consultation fees from Medtronic, Servier, Krka, Berlin-Chemie/Menarini, Egis, Sandoz, Idorsia, Polpharma, and Gedeon Richter.
L.R. has served as an advisor/speaker for Medtronic.
D.H. reports consulting fees from Medtronic (through Wing Tech Inc.).
K.C. reports consulting fees from Medtronic (through Wing Tech Inc.).
D.A.H. is an employee of Medtronic.
M.F. is an employee of Medtronic.
M.P.S. is supported by an NHMRC Senior Research Fellowship; and has received consulting fees and/or travel and research support from Medtronic, Abbott, Novartis, Servier, Pfizer, and Boehringer Ingelheim.
J.B.P. reports consulting fees from Medtronic and Aktiia SA (through Wing Tech Inc.).
Data availability
The data, analytical methods, and study materials are owned by the funder and therefore will not be made available to other researchers for the purposes of reproducing the results or replicating the procedure.
References
- 1. Collaborators GBDRF . Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet North Am Ed. 2020;396:1223–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mills KT, Stefanescu A, He J.. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collaboration NCDRF . Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hamdidouche I, Jullien V, Boutouyrie P, Billaud E, Azizi M, Laurent S.. Drug adherence in hypertension: from methodological issues to cardiovascular outcomes. J Hypertens. 2017;35:1133–1144. [DOI] [PubMed] [Google Scholar]
- 5. Gupta P, Patel P, Strauch B, Lai FY, Akbarov A, Maresova Vet al. Risk factors for nonadherence to antihypertensive treatment. Hypertension. 2017;69:1113–1120. [DOI] [PubMed] [Google Scholar]
- 6. Bohm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder REet al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet North Am Ed. 2020;395:1444–1451. [DOI] [PubMed] [Google Scholar]
- 7. Kandzari DE, Bohm M, Mahfoud F, Townsend RR, Weber MA, Pocock Set al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet North Am Ed. 2018;391:2346–2355. [DOI] [PubMed] [Google Scholar]
- 8. Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies Jet al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet North Am Ed. 2018;391:2335–2345. [DOI] [PubMed] [Google Scholar]
- 9. Azizi M, Sanghvi K, Saxena M, Gosse P, Reilly JP, Levy Tet al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet North Am Ed. 2021;397:2476–2486. [DOI] [PubMed] [Google Scholar]
- 10. Schmieder RE, Mahfoud F, Mancia G, Azizi M, Bohm M, Dimitriadis Ket al. European Society of Hypertension position paper on renal denervation 2021. J Hypertens. 2021;39:1733–1741. [DOI] [PubMed] [Google Scholar]
- 11. Townsend R, Walton A, Hettrick DA, Hickey GL, Weil J, Sharp ASPet al. Incidence of renal artery damage following percutaneous renal denervation with radio frequency renal artery ablation systems: Review and Meta-Analysis of published reports. EuroIntervention. 2020;16:89–962020, doi: 10.4244/EIJ-D-19-00902. [DOI] [PubMed] [Google Scholar]
- 12. Sanders MF, Reitsma JB, Morpey M, Gremmels H, Bots ML, Pisano Aet al. Renal safety of catheter-based renal denervation: systematic review and meta-analysis. Nephrology Dialysis Transplantation. 2017;32:1440–1447. [DOI] [PubMed] [Google Scholar]
- 13. Mahfoud F, Bohm M, Schmieder R, Narkiewicz K, Ewen S, Ruilope Let al. Effects of renal denervation on kidney function and long-term outcomes: 3-year follow-up from the Global SYMPLICITY Registry. Eur Heart J. 2019;40:3474–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson Jet al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet North Am Ed. 2016;387:957–967. [DOI] [PubMed] [Google Scholar]
- 15. Thomopoulos C, Parati G, Zanchetti A.. Effects of blood pressure lowering on outcome incidence in hypertension. 1. Overview, meta-analyses, and meta-regression analyses of randomized trials. J Hypertens. 2014;32:2285–2295. [DOI] [PubMed] [Google Scholar]
- 16. Collaboration BPLTT . Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet North Am Ed. 2021;397:1625–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kandzari DE, Mahfoud F, Bhatt DL, Bohm M, Weber MA, Townsend RRet al. Confounding factors in renal denervation trials: revisiting old and identifying new challenges in trial design of device therapies for hypertension. Hypertension. 2020;76:1410–1417. [DOI] [PubMed] [Google Scholar]
- 18. Mahfoud F, Mancia G, Schmieder R, Narkiewicz K, Ruilope L, Schlaich Met al. Renal denervation in high-risk patients with hypertension. J Am Coll Cardiol. 2020;75:2879–2888. [DOI] [PubMed] [Google Scholar]
- 19. Bohm M, Mahfoud F, Ukena C, Hoppe UC, Narkiewicz K, Negoita Met al. First report of the Global SYMPLICITY Registry on the effect of renal artery denervation in patients with uncontrolled hypertension. Hypertension. 2015;65:766–774. [DOI] [PubMed] [Google Scholar]
- 20. Bohm M, Mahfoud F, Ukena C, Bauer A, Fleck E, Hoppe UCet al. Rationale and design of a large registry on renal denervation: the Global SYMPLICITY registry. EuroIntervention. 2013;9:484–492. [DOI] [PubMed] [Google Scholar]
- 21. Whelton PK, Carey RM, Aronow WS, Casey DE, Jr., Collins KJ, Dennison Himmelfarb Cet al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:2199–2269.29146533 [Google Scholar]
- 22. Mahfoud F, Kandzari DE, Kario K, Townsend RR, Weber MA, Schmieder REet al. Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): a randomised, sham-controlled trial. Lancet North Am Ed. 2022;399:1401–1410. [DOI] [PubMed] [Google Scholar]
- 23. Kandzari DE, Townsend RR, Bakris G, Basile J, Bloch MJ, Cohen DLet al. Renal denervation in hypertension patients: proceedings from an expert consensus roundtable cosponsored by SCAI and NKF. Catheter Cardiovasc Interv.98, 416–426, 2021. [DOI] [PubMed] [Google Scholar]
- 24. (CDER) USDoHaHSFaDACfDEaR . Guidance for industry hypertension indication: drug labeling for cardiovascular outcome claims https://www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugs2020. [Google Scholar]
- 25. Fengler K, Reimann P, Rommel KP, Kresoja KP, Blazek S, Unterhuber Met al. Comparison of long-term outcomes for responders versus non-responders following renal denervation in resistant hypertension. J Am Heart Assoc. 2021;10:e022429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geisler BP, Egan BM, Cohen JT, Garner AM, Akehurst RL, Esler MDet al. Cost-effectiveness and clinical effectiveness of catheter-based renal denervation for resistant hypertension. J Am Coll Cardiol. 2012;60:1271–1277. [DOI] [PubMed] [Google Scholar]
- 27. Azizi M, Sapoval M, Gosse P, Monge M, Bobrie G, Delsart Pet al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet North Am Ed. 2015;385:1957–1965. [DOI] [PubMed] [Google Scholar]
- 28. Volz S, Spaak J, Elf J, Jagren C, Lundin C, Stenborg Aet al. Renal sympathetic denervation in Sweden: a report from the Swedish registry for renal denervation. J Hypertens. 2018;36:151–158. [DOI] [PubMed] [Google Scholar]
- 29. Naduvathumuriyil T, Held U, Steigmiller K, Denegri A, Cantatore S, Obeid Set al. Clinical benefits and safety of renal denervation in severe arterial hypertension: a long-term follow-up study. The Journal of Clinical Hypertension. 2020;22:1854–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ziegler AK, Bertog S, Kaltenbach B, Id D, Franke J, Hofmann Iet al. Efficacy and safety of renal denervation in elderly patients with resistant hypertension. Catheter Cardiovasc Interv. 2015;86:299–303. [DOI] [PubMed] [Google Scholar]
- 31. Miroslawska AK, Gjessing PF, Solbu MD, Fuskevag OM, Jenssen TG, Steigen TK.. Renal denervation for resistant hypertension fails to improve insulin resistance as assessed by hyperinsulinemic-euglycemic step clamp. Diabetes. 2016;65:2164–2168. [DOI] [PubMed] [Google Scholar]
- 32. Mahfoud F, Schlaich M, Kindermann I, Ukena C, Cremers B, Brandt MCet al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation. 2011;123:1940–1946. [DOI] [PubMed] [Google Scholar]
- 33. Hering D, Marusic P, Duval J, Sata Y, Head GA, Denton KMet al. Effect of renal denervation on kidney function in patients with chronic kidney disease. Int J Cardiol. 2017;232:93–97. [DOI] [PubMed] [Google Scholar]
- 34. Hering D, Mahfoud F, Walton AS, Krum H, Lambert GW, Lambert EAet al. Renal denervation in moderate to severe CKD. J Am Soc Nephrol. 2012;23:1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kiuchi MG, Graciano ML, Chen S, Carreira MA, Kiuchi T, Andrea BRet al. Improvement of the renal function after renal sympathetic denervation in refractory hypertensive patients with chronic kidney disease: possible predictors. Int J Cardiol. 2015;199:10–12. [DOI] [PubMed] [Google Scholar]
- 36. Marin F, Fezzi S, Gambaro A, Ederle F, Castaldi G, Widmann Met al. Insights on safety and efficacy of renal artery denervation for uncontrolled-resistant hypertension in a high risk population with chronic kidney disease: first Italian real-world experience. J Nephrol. 2021;34:1445–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Warchol-Celinska E, Prejbisz A, Kadziela J, Florczak E, Januszewicz M, Michalowska Iet al. Renal denervation in resistant hypertension and obstructive sleep apnea: randomized proof-of-concept phase II trial. Hypertension 2018;72:381–390. [DOI] [PubMed] [Google Scholar]
- 38. Witkowski A, Kadziela J.. Obstructive sleep apnoea, resistant hypertension and renal denervation. EuroIntervention.R105–R109, 2013;9Suppl R:R105-9. [DOI] [PubMed] [Google Scholar]
- 39. Fengler K, Rommel KP, Lapusca R, Blazek S, Besler C, Hartung Pet al. Renal denervation in isolated systolic hypertension using different catheter techniques and technologies. Hypertension 2019;74:341–348. [DOI] [PubMed] [Google Scholar]
- 40. Group SR, Wright JT, Jr., Williamson JD, Whelton PK, Snyder JK, Sink KMet al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang WY, Melgarejo JD, Thijs L, Zhang ZY, Boggia J, Wei FFet al. Association of office and ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA. 2019;322:409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data, analytical methods, and study materials are owned by the funder and therefore will not be made available to other researchers for the purposes of reproducing the results or replicating the procedure.