Summary
Background
Monovalent type 2 oral poliovirus vaccine (mOPV2) and inactivated poliovirus vaccine (IPV) are used to respond to type 2 poliovirus outbreaks. We aimed to assess the effect of two mOPV2 doses on the type 2 immune response by varying the time interval between mOPV2 doses and IPV co-administration with mOPV2.
Methods
We did a randomised, controlled, parallel, open-label, non-inferiority, inequality trial at two study clinics in Dhaka, Bangladesh. Healthy infants aged 6 weeks (42–48 days) at enrolment were randomly assigned (1:1:1:1) to receive two mOPV2 doses (each dose consisting of two drops [0·1 mL in total] of about 105 50% cell culture infectious dose of type 2 Sabin strain) at intervals of 1 week, 2 weeks, 4 weeks (standard or control group), or 4 weeks with IPV (0·5 mL of type 1 [Mahoney, 40 D-antigen units], type 2 [MEF-1, 8 D-antigen units], and type 3 [Saukett, 32 D-antigen units]) administered intramuscularly with the first mOPV2 dose. We used block randomisation, randomly selecting blocks of sizes four, eight, 12, or 16 stratified by study sites. We concealed randomisation assignment from staff managing participants in opaque, sequentially numbered, sealed envelopes. Parents and clinic staff were unmasked to assignment after the randomisation envelope was opened. Laboratory staff analysing sera were masked to assignment, but investigators analysing data and assessing outcomes were not. The primary outcome was type 2 immune response measured 4 weeks after mOPV2 administration. The primary modified intention-to-treat analysis included participants with testable serum samples before and after vaccination. A non-inferiority margin of 10% and p=0·05 (one-tailed) was used.
Findings
Between Dec 7, 2015, and Jan 5, 2016, we randomly assigned 760 infants to receive two mOPV2 doses at intervals of 1 week (n=191), 2 weeks (n=191), 4 weeks (n=188), or 4 weeks plus IPV (n=190). Immune responses after two mOPV2 doses were observed in 161 (93%) of 173 infants with testable serum samples in the 1 week group, 169 (96%) of 177 in the 2 week group, and 176 (97%) of 181 in the 4 week group. 1 week and 2 week intervals between two mOPV2 doses were non-inferior to 4 week intervals because the lower bound of the absolute differences in the percentage of immune responses were greater than −10% (−4·2% [90% CI −7·9 to −0·4] in the 1 week group and −1·8% [−5·0 to 1·5] in the 2 week group vs the 4 week group). The immune response elicited by two mOPV2 doses 4 weeks apart was not different when IPV was added to the first dose (176 [97%] of 182 infants with IPV vs 176 [97%] of 181 without IPV; p=1·0). During the trial, two serious adverse events (pneumonia; one [1%] of 186 patients in the 1 week group and one [1%] of 182 in the 4 week group) and no deaths were reported; the adverse events were not attributed to the vaccines.
Interpretation
Administration of mOPV2 at short intervals does not interfere with its immunogenicity. The addition of IPV to the first mOPV2 dose did not improve poliovirus type 2 immune response.
Funding
US Centers for Disease Control and Prevention.
Introduction
Oral poliovirus vaccine (OPV) is the vaccine of choice to achieve global polio eradication because it provides intestinal and humoral immunity, and is easier to administer and less expensive than inactivated polio vaccine (IPV).1,2 Combining serotypes 1, 2, and 3 into a trivalent OPV (tOPV) reduced the vaccine immuno genicity against each serotype compared with the respective monovalent vaccines; however, this reduction was compensated for by the administration of multiple doses and by separation of the doses by 4–6 weeks to reduce interference among serotypes by intestinal replication.2–5 In undervaccinated populations, OPV strains, especially type 2, can circulate for a long time and reacquire neurovirulence similar to wild polioviruses.6–8 Although rare, type 2 circulating vaccine-derived polioviruses have caused over 600 paralytic cases during 2001–16.9 Because wild polio virus type 2 was declared eradicated in 2015, and to reduce the disease burden of type 2 circulating vaccine-derived polioviruses, all 155 countries using OPV in routine immunisation schedules or campaigns switched to bivalent OPV (bOPV; types 1 and 3) in April, 2016.10
To interrupt potential transmission of type 2 circulating vaccine-derived polioviruses after tOPV withdrawal, the Global Polio Eradication Initiative developed an outbreak response protocol using monovalent type 2 OPV (mOPV2) and IPV.11 On the basis of experience from previous responses to poliovirus outbreaks, a minimum of four campaigns (and up to five or six campaigns) with mOPV2 were estimated to be necessary to stop transmission. Shortening of the interval between mOPV2 campaigns and use of IPV in some campaigns were strategies proposed for areas with a high number of susceptible children12,13 although no immunological data were available to support these strategies.
We did a clinical trial that compared the immunogenicity of two mOPV2 doses given at short intervals of 1 week or 2 weeks with the standard 4 week interval to assess whether shortening of the intervals would interfere with mOPV2 immunogenicity. We also assessed whether administration of IPV with mOPV2 would affect the immune response of mOPV2.
Methods
Study design and participants
We did a randomised, controlled, parallel, open-label, non-inferiority, inequality trial in two study clinics (Mirpur thana and Mohakahli) in Dhaka, Bangladesh. The study was approved by the institutional review board of the International Centre for Diarrhoeal Disease Research, Bangladesh. Fieldworkers recorded new births in the community, and study information was shared with these parents; if interested, they were invited to participate. Participants in the study were healthy infants aged 6 weeks (42–48 days) at enrolment, whose parents provided written consent for participation and could understand and comply with planned study procedures, including not moving outside the study area during the study period. Exclusion criteria were evidence or suspicion of a chronic or acute medical condition that would contraindicate venepuncture or polio vaccine administration, receipt of any polio vaccine (OPV or IPV) before enrolment, or infants from multiple births or born prematurely (<37 weeks’ gestation). Infants were withdrawn from the trial if they had received any polio vaccine outside the study, if parents withdrew consent, or if a contraindication for polio vaccination was identified.
Randomisation and masking
We randomly assigned (1:1:1:1) eligible infants to one of four study groups: two mOPV2 doses at intervals of 1 week, 2 weeks, 4 weeks (standard or control group), or 4 weeks with IPV added to the first mOPV2 dose. We used block randomisation, stratified by study sites, randomly selecting blocks of sizes four, eight, 12, or 16, to decrease the ability of study staff to predict the start and end of blocks. The study staff who prepared the randomisation sequence had no engagement with trial participants. The staff managing participants were masked to group assignment because it was concealed in opaque, sequentially numbered, sealed envelopes. Parents and clinic staff were unmasked to assignment after the randomisation envelope was opened. Laboratory staff analysing sera were masked to assignment; investigators analysing data and assessing outcomes were not masked to assignment.
Procedures
Infants received the first dose of vaccine (mOPV2 or mOPV2 plus IPV) at the first (baseline) visit at age 6 weeks. Study staff also recorded participants’ vaccination history, breastfeeding patterns, weight, and length, and obtained an initial blood sample. Weight was measured with electronic scales precise to 100 g and length was measured with measuring boards precise to 1 mm. The mean of two consecutive measures of length and weight were used to establish whether stunting (reduced length for age) or wasting (reduced weight for length) were present by use of child-growth standard curves from the WHO Multicenter Growth Reference Study.14 Stunting or wasting were defined as at least 2 SDs less than the mean of the reference population at baseline. The second study visit was scheduled at 7, 8, or 10 weeks of age, depending on the assigned study group. During this second visit, staff asked parents about presence of diarrhoea, clinical events, and vaccinations received since the last visit, and provided the second dose of study vaccine. Blood samples were also obtained during this visit for infants in the 4 week group and 4 week plus IPV group before vaccine administration. During the third study visit, which was scheduled 4 weeks following the second mOPV2 dose (at 11, 12, or 14 weeks of age), study staff again asked parents about presence of diarrhoea, clinical events, and vaccinations received since the last visit, and obtained a blood sample from participants in all study arms.
SanofiPasteur (Lyon, France) manufactured the mOPV2 and IPV used in this trial. One mOPV2 dose consisted of two drops (0·1 mL in total) containing about 105 50% cell culture infectious dose of type 2 Sabin strain. IPV contained type 1 (Mahoney, 40 D-antigen units), type 2 (MEF-1, eight D-antigen units), and type 3 (Saukett, 32 D-antigen units), and was administered as a 0·5 mL intramuscular injection in the upper thigh of infants on the side opposite to administration of the pentavalent vaccine (containing diphtheria, tetanus, pertussis, hepatitis B, and Haemophilus influenzae serotype b vaccines). All vaccines were stored in the manufacturer-recommended cold-chain conditions.
Blood samples (1 mL) were collected by venepuncture at the study clinic and transported to the International Centre for Diarrhoeal Disease Research, Bangladesh laboratory at 2–8°C. Within 24 h of collection, the serum was separated by centrifugation and stored at −20°C until study completion. At the end of the study, sera were shipped to the Centers for Disease Control and Prevention laboratory in Atlanta, GA, USA. Sera were tested in triplicate and antibody titres against serotypes 1, 2, and 3 were measured by use of a microneutralisation test. A reciprocal titre of 8 or more was considered seropositive.
Outcomes
The primary objectives of the trial were to assess the effect of two mOPV2 doses on the type 2 immune response by varying the time interval between mOPV2 doses, and to assess the effect of co-administering IPV with mOPV2. To address varying time intervals between mOPV2 doses, we compared type 2 immune responses following two doses of mOPV2 at 6 weeks and 7 weeks of age with that of mOPV2 administered at 6 weeks and 10 weeks of age (1 week group vs 4 week group; non-inferiority comparison). We also compared type 2 immune responses following two doses of mOPV2 at 6 weeks and 8 weeks of age with that of mOPV2 administered at 6 weeks and 10 weeks of age (2 week group vs 4 week group; non-inferiority com parison). To assess IPV co-administration with mOPV2, we compared type 2 immune responses following two doses of mOPV2 at 6 weeks and 10 weeks of age with that of mOPV2 plus IPV administered at 6 weeks of age and mOPV2 alone at 10 weeks of age (4 week group vs 4 week plus IPV group; inequality comparison). A secondary objective further assessed the effect of IPV co-administration by comparing the type 2 immune response following one dose of mOPV2 at 6 weeks of age with that of mOPV2 plus IPV administered at 6 weeks of age. The primary outcome of the trial was type 2 immune response measured 4 weeks after mOPV2 administration. Immune response was defined as a change from seronegative at baseline to seropositive (seroconversion) after vaccination or an increase in the antibody titres by at least four times between two specimens (boosting), assuming exponential decay of maternal antibody titres with a half-life of 28 days.15 The secondary outcome was median antibody titre, also measured 4 weeks after administration of a mOPV2 dose.
Participants were monitored for adverse events at the study clinics for 30 min following each administration of study vaccine, and parents were asked to seek medical care immediately and notify study staff should illness or an adverse event occur. All adverse events were reviewed by the principal investigator and all serious adverse events were reported within 24 h to the regulatory agencies, ethical review committees, and data safety monitoring board. Participants were offered other childhood vaccines recommended by the Expanded Programme on Immunization of Bangladesh Ministry of Health and Family Welfare, as appropriate for age. At the completion of study activities, participants received three tOPV doses 4 weeks apart and one IPV dose, in accordance with Bangladesh’s routine immunisation schedule.
Statistical analysis
The target sample size was 700 infants, with an enrolment target of 888. We assumed 90% of infants would achieve a type 2 immune response with two doses of mOPV2 in the standard 4 week group.16 We estimated a sample size of 175 participants per arm to have 90% power to detect non-inferiority in the type 2 immune response induced by two mOPV2 doses given at 1 week or 2 week intervals versus the standard 4 week interval with a continuity corrected Z test with pooled variance. This assumes a non-inferiority margin of 10% and p=0·05 (one-tailed). The enrolment target was increased to 222, assuming 10% of enrolled infants would have baseline titres too high to detect an immune response (seroconversion or boosting) and 10% attrition. The type 2 immune response in the 1 week (11 weeks of age) or 2 week groups (12 weeks of age) was deemed non-inferior to that in the standard 4 week study group (14 weeks of age) if the lower bound of the 90% CI of the differences in immune responses, calculated with a two-sided Wald test, was greater than −10%. We chose a priori to set the type 1 error at 5%; therefore, we used a 90% two-sided CI. We selected non-inferiority to compare short and standard interval schedules because the potential benefit of a faster increase in population immunity achieved with a short-interval schedule would offset a reduced, but not programmatically meaningful decline in type 2 immune response. The margin of non-inferiority was based on a probable and acceptable public health decline in immune response in lieu of quicker programmatic action. Distributions of antibody titres were compared by use of the Kruskal-Wallis test. Antibody titres were plotted as reverse cumulative distribution curves, which were constructed by representing on the vertical axis the proportion of participants with antibody titres equal to or greater than that represented on the horizontal axis.
We assumed 98% of infants would achieve a type 2 immune response with two doses of mOPV2 and one IPV dose (4 week plus IPV group). No previous studies have assessed type 2 immunogenicity of simultaneous administration of mOPV2 and IPV. A sample size of 175 infants in the 4 week plus IPV group (enrolment target of 222) would provide 80% power through use of a two-sided test with a significance of p=0·05 to compare with the 4 week group with continuity corrected Z test with pooled variance. We used Fisher’s exact test to compare the proportion of participants showing type 2 immune response in the 4 week group and 4 week plus IPV group after receiving two doses (14 weeks of age) or one dose (10 weeks of age) of mOPV2. We also used the Kruskal-Wallis test to compare distributions of antibody titre.
Several post-hoc analyses were done to further investigate initial findings. We assessed the benefit of an additional dose of mOPV2 by comparing type 2 immune response and titres 4 weeks after the first and second dose of mOPV2 (10 weeks and 14 weeks of age, respectively) within participants in the 4 week group. McNemar’s test was used for within-participant comparisons of proportions and the signed rank test for antibody titre distributions. We also assessed whether IPV co-administration affected type 2 immune response in a subset of participants who were seronegative at baseline by comparing immune response and titres 4 weeks after one and two doses of mOPV2 (10 weeks and 14 weeks of age, respectively) in the 4 week group and 4 week plus IPV group. Fisher’s exact test was used for comparisons of proportions of patients between study groups and the Kruskal-Wallis test for comparison of distributions of antibody titres. Results of post-hoc analyses were assessed by use of a Bonferroni corrected significance level of less than 0·01; not all post-hoc analyses are presented. We did not apply Bonferroni correction for a-priori hypotheses.
Baseline analyses included infants who completed all study visits and provided samples (intention to treat). Participants with adjusted baseline titres too high to detect a four times increase in a poliovirus serotype were excluded from further analysis for that serotype (modified intention to treat). Except for baseline findings, results are presented for infants who completed the study per modified intention to treat; results from per-protocol analyses were similar (not reported here). All analyses were done with SAS version 9.317 and graphics were created with R version 3.3.3.
This trial is registered at ClinicalTrials.gov, number NCT02643368, and is closed to accrual.
Role of the funding source
The sponsor of the study participated in study design, data analysis, data interpretation, and writing of the in the report. The sponsor did not participate in data collection. The corresponding author had full access to all the data study, except personally identified information, and had final responsibility for the decision to submit for publication.
Results
Between Dec 7, 2015, and Jan 5, 2016, 760 infants were randomly assigned to four study groups (figure 1). 19 participants (three in the 1 week group, five in the 2 week group, four in the 4 week group, and seven in the 4 week plus IPV group) withdrew consent and four (two in the 1 week and 2 week groups) were lost to follow-up. Thus 737 infants were included in the intention-to-treat analysis. At baseline (6 weeks of age), the study groups were similar for age, sex, mothers’ education, presence of wasting or stunting, exclusive breastfeeding, or antibody seroprevalence to poliovirus types 1, 2, or 3 (table 1).
Figure 1: Trial profile.
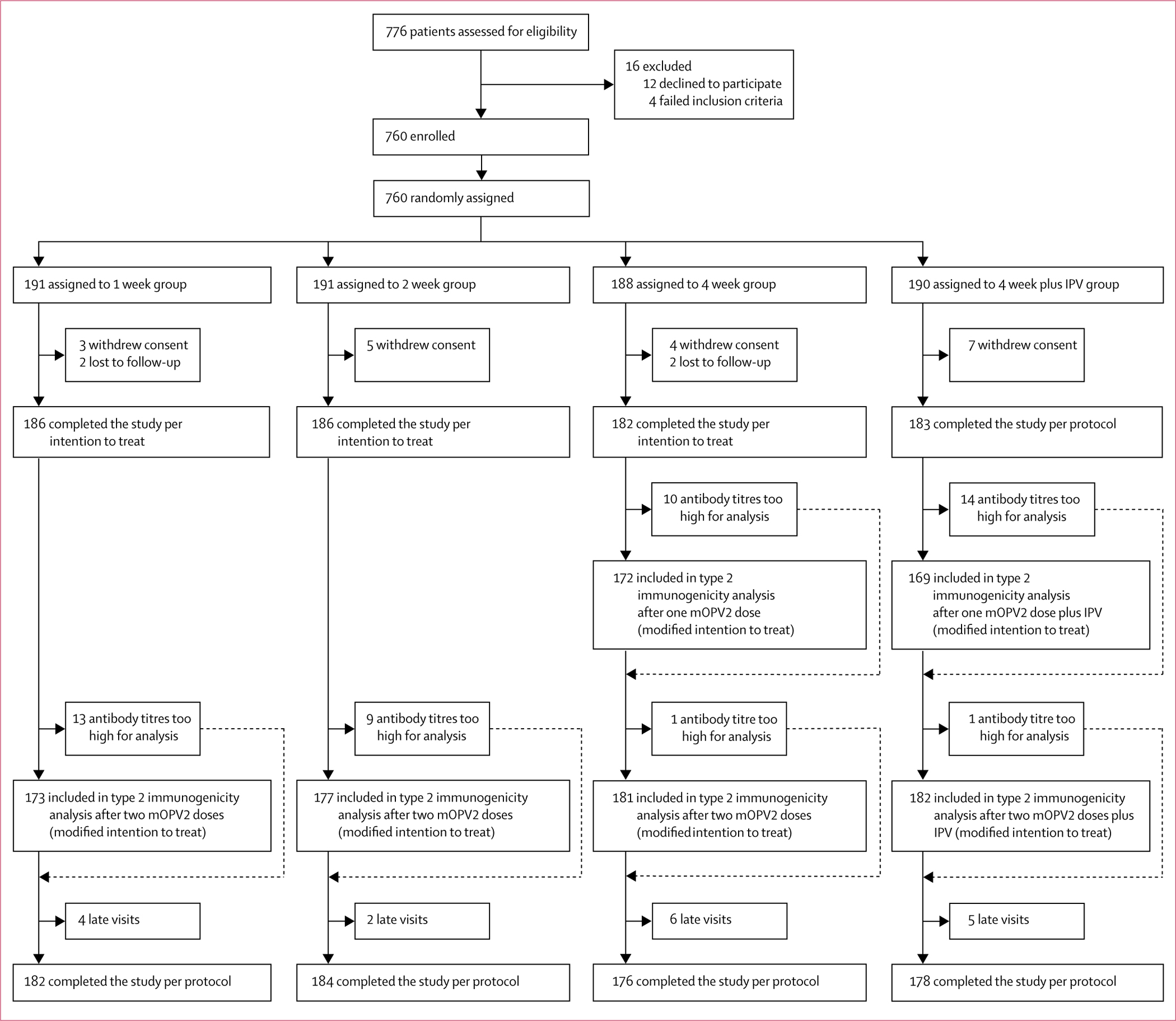
mOPV2=monovalent oral poliovirus vaccine type 2. IPV=inactivated poliovirus vaccine.
Table 1:
Baseline characteristics of the intention-to-treat population
| 1 week group (n=186) | 2 week group (n=186) | 4 week group (n=182) | 4 week plus IPV group (n=183) | |
|---|---|---|---|---|
|
| ||||
| Age (days) | 43 (42–48) | 43 (42–48) | 43 (42–48) | 43 (42–48) |
| Boys | 88 (47%) | 88 (47%) | 93 (51%) | 95 (52%) |
| Mother's education | ||||
| No formal school | 44 (24%) | 54 (29%) | 33 (18%) | 44 (24%) |
| Some school | 133 (72%) | 128 (69%) | 142 (78%) | 135 (74%) |
| Graduate | 9 (5%) | 4 (2%) | 7 (4%) | 4 (2%) |
| Stunting present* | 34 (18%) | 28 (15%) | 26 (14%) | 33 (18%) |
| Wasting present* | 7 (4%) | 12 (6%) | 14 (8%) | 12 (7%) |
| Exclusive breastfeeding | 104 (56%) | 89 (48%) | 94 (52%) | 88 (48%) |
| Type 1 poliovirus | ||||
| Seropositive | 96 (52%) | 90 (48%) | 86 (47%) | 94 (51%) |
| Reciprocal titres | 9 (6–57) | 7 (6–23) | 7 (6–28) | 9 (6–45) |
| Type 2 poliovirus | ||||
| Seropositive | 107 (57%) | 100 (54%) | 94 (52%) | 111 (61%) |
| Reciprocal titres | 11 (6–57) | 11 (6–36) | 9 (6–36) | 11 (6–57) |
| Type 3 poliovirus | ||||
| Seropositive | 44 (24%) | 43 (23%) | 37 (20%) | 44 (24%) |
| Reciprocal titres | 6 (6–7) | 6 (6–7) | 6 (6–7) | 6 (6–7) |
Data are n (%) or median (IQR).
Length for age and weight for length were compared with the SD of an international reference population recommended by WHO.14
713 (94%) of 760 infants had testable serum samples before and after vaccination and were included in the modified intention-to-treat analysis for type 2 immune response (figure 1). After two mOPV2 doses, type 2 immune responses were non-inferior with the short-interval schedule compared with the standard-interval schedule, with the lower bound of the absolute difference in immune response greater than −10% in both comparisons (table 2). Type 2 immune response was observed in 161 (93%) of 173 infants in the 1 week group, 169 (96%) of 177 in the 2 week group, and 176 (97%) of 181 in the 4 week group (table 2). Median titres were 1448 in all study groups (table 2), with no significant differences in titre distributions (figure 2).
Table 2:
Poliovirus type 2 response after vaccination with different study vaccines and schedules in modified intention-to-treat population
| 4 weeks after two mOPV2 doses |
4 weeks after two mOPV2 doses with or without IPV |
4 weeks after one mOPV2 dose with or without IPV |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 week group | 2 week group | 4 week group | 1 week vs 4 week group (difference [90% CI] or p value) | 2 week vs 4 week group (difference [90% CI] or p value) | 4 week plus IPV group | 4 week group | p value† | 4 week plus IPV group | 4 week group | p value† | |
|
| |||||||||||
| Immune response | 161/173 (93⋅1%; 88⋅3–96⋅0) | 169/177 (95 5%; 91⋅3-97⋅7) | 176/181 (97⋅2%; 93⋅7-98⋅8) | −4⋅2% (−7⋅9 to −0⋅4)* | −1⋅8% (−5⋅0 to 1⋅5)* | 176/182 (96⋅7%; 93⋅0-98⋅5) | 176/181 (97⋅2%; 93⋅7-98⋅8) | 1⋅0 | 154/169 (91⋅1%; 85⋅9-94⋅6) | 157/172 (91⋅3%; 86⋅1-94⋅6) | 1⋅0 |
| Antibody titres | 1448 (1152⋅1448) | 1448 (910⋅1448) | 1448 (1152⋅1448) | p=0⋅243† | p=0⋅059† | 1448 (1152⋅1448) | 1448 (1152⋅1448) | 0⋅758 | 1448 (724⋅1448) | 1152 (724⋅1448) | 0⋅691 |
Outcomes for poliovirus type 2 response include the proportion of infants with immune response expressed as n/N (%; 95% CI), and reciprocal antibody titres expressed as median (IQR). mOPV2=monovalent oral poliovirus vaccine type 2. IPV=inactivated poliovirus vaccine.
Non-inferiority test results are presented using the difference (experimental-standard) in the proportion of infants with immune response with 90% CIs.
Inequality hypothesis tests were done with Fisher's exact test for proportions and with Kruskal-Wallis test for antibody titre distributions.
Figure 2: Reverse cumulative distribution and boxplot of poliovirus type 2 titres following varying schedules of mOPV2 with or without IPV.
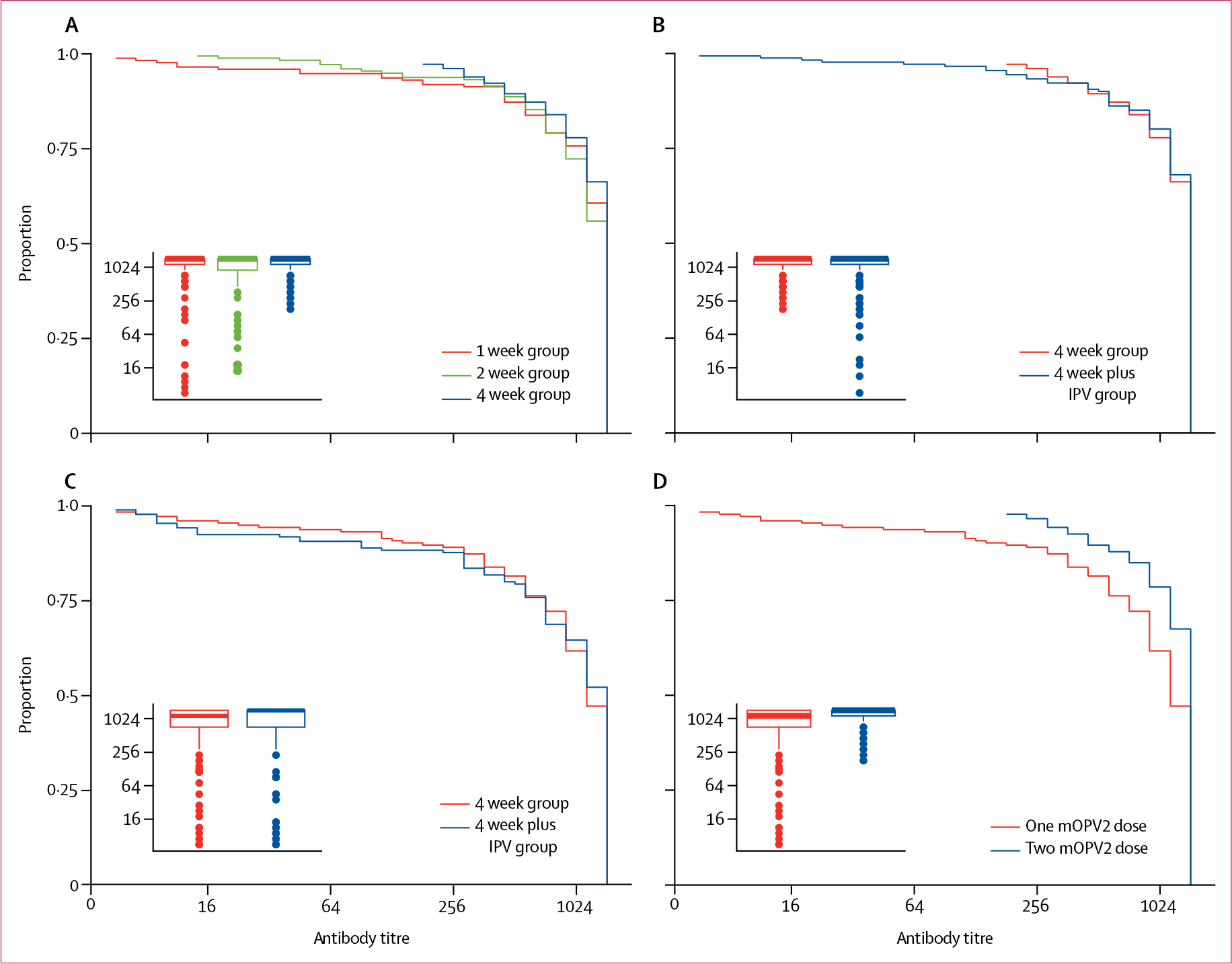
(A) Titres following two doses of mOPV2 given at a 1 week or 2 week interval versus a standard 4 week interval. Inset of boxplot compares median and IQR of type 2 titres. (B) Titres following two doses of mOPV2 (4 week group) versus two doses of mOPV2 with one IPV dose (4 week plus IPV group). Inset of boxplot compares median and IQR of type 2 titres. (C) Titres following one dose of mOPV2 (4 week group) versus one dose of mOPV2 with one IPV dose (4 week plus IPV group). Inset of boxplot compares median and IQR of type 2 titres. (D) Titres following one dose of mOPV2 versus following two doses of mOPV2 administered at a 4 week interval in the same participants (4 week group). Inset of boxplot compares median and IQR of type 2 titres. y axes show proportion of participants with antibody titres equal to or greater than that represented on the x axis. mOPV2=monovalent oral poliovirus vaccine type 2.
IPV administration did not significantly change the type 2 immune response induced by mOPV2. Immune response was observed in 157 (91%) of 172 infants after one dose of mOPV2 alone (4 week group), and in 154 (91%) of 169 infants after one dose of mOPV2 with IPV (4 week plus IPV group; p=1·00; table 2). Following two doses of mOPV2, 176 (97%) of 181 infants had an immune response in the 4 week group compared with 176 (97%) of 182 infants in the 4 week plus IPV group (p=1·00; table 2). Titre distributions were also not different between study arms (p=0·69 for one-dose and p=0·758 for two-dose comparisons between these study arms; figure 2).
Of 172 participants in the 4 week group, immune response was observed in 157 (91%) infants after the first mOPV2 dose compared with 170 (99%) infants after the second mOPV2 dose (p=0·002). Type 2 antibody titres increased with the second dose (p<0·0001; figure 2).
Among children who were antibody seronegative at baseline, immune response after one mOPV2 dose was observed in 84 (95·5%; 95% CI 88·9–98·2) of 88 infants who did not receive IPV, and in 71 (98·6%; 95% CI 92·5–99·8) of 72 infants who received IPV (p=0·38). Following two mOPV2 doses with a 4 week interval, immune response was observed in all infants (72 [100%] of 72 infants with IPV; 88 [100%] of 88 without IPV).
28 adverse events were reported during the study, with two classified as serious (pneumonia that required admission to hospital; one [1%] of 186 patients in the 1 week group and one [1%] of 182 in the 4 week group; table 3). No deaths were reported and all adverse events resolved with treatment. No adverse events were attributed to study vaccines.
Table 3:
Distribution of adverse events per study arm among all enrolled study participants
| 1 week group | 2 week group | 4 week group | 4 week plusIPV group | Total | |
|---|---|---|---|---|---|
|
| |||||
| Mild to moderate adverse events | |||||
| Burn injury | 0 | 0 | 0 | 1 | 1 |
| Conjunctivitis | 0 | 0 | 2 | 1 | 3 |
| Diarrhoea | 2 | 0 | 0 | 2 | 4 |
| Vaccine-related fever | 1 | 0 | 0 | 2 | 3 |
| Respiratory infection | 1 | 1 | 4 | 1 | 7 |
| Oral candidiasis | 0 | 1 | 0 | 1 | 2 |
| Skin infection | 1 | 0 | 2 | 1 | 4 |
| Measles | 0 | 1 | 0 | 0 | 1 |
| Varicella | 0 | 1 | 0 | 0 | 1 |
| Serious adverse events | |||||
| Pneumonia | 1 | 0 | 1 | 0 | 2 |
| Total | 6 | 4 | 9 | 9 | 28 |
Data are n.
Discussion
Immunogenicity of two mOPV2 doses at 1 week or 2 week intervals was non-inferior to immunogenicity of two mOPV2 doses at a 4 week interval. Administration of IPV with the first mOPV2 dose did not improve type 2 immune response compared with mOPV2 alone although it was associated with higher antibody titres.
The higher immunogenicity observed in our study than in a study16 from India that used the same mOPV2 formulation, in which 35 (21%) of 170 infants responded to the first dose given at birth and 114 (84%) of 135 infants after the second dose given 1 month later, is consistent with the expected reduction in immunogenicity of OPV when administered at birth.18 On the basis of the observed immunogenicity of mOPV2,16,18 high population immunity is expected to be achieved with only two or three vaccination campaigns as long as the campaigns achieve high vaccination coverage (ie, >90%) among the population at risk. Reduction of the minimum number of campaigns required in response to a type 2 poliovirus outbreak might enable the programme to focus on improving the quality of the campaigns with strategies that reach children chronically missed and reduce the likelihood of vaccination campaigns causing a new type 2 vaccine-derived poliovirus outbreak. Additionally, a reduction of the number of mOPV2 campaigns would decrease the demand for mOPV2 doses and aid more effective management of the mOPV2 stockpile.
Similar to that observed with mOPV1 and bOPV, provision of several doses of mOPV2 at short intervals of 1 week or 2 weeks does not interfere with the immunological response.19,20 These data support the use of campaigns done at short intervals to interrupt poliovirus transmission in conflict areas where the susceptible population can only be reached at particular times and in areas where the baseline population immunity is low.21 Short-interval rounds can achieve high population immunity faster, thus reducing the duration and extent of the outbreak if the short interval does not affect campaign coverage.
Our study showed that the administration of IPV with the initial dose of mOPV2 did not improve the immunogenicity of one or two mOPV2 doses. The absence of an additive effect from IPV was also observed in infants who were seronegative at baseline, which suggests that it cannot be attributed to the interference of maternal antibodies. This finding is similar to those observed in other studies that showed that IPV does not improve immune response after an immunisation schedule with three or four doses of tOPV, although it might increase antibody titres.22,23 Whereas, IPV combined with OPV has been shown to be more effective than OPV alone when administered to children who have been previously vaccinated or live in areas where high prevalence of diarrheal diseases and poor sanitary conditions decrease the efficacy of oral vaccines, or both.24–26 Considering the increase in the operational cost and complexity associated with the use of IPV in campaigns,12 and the potential negative effect of IPV use on mOPV2 coverage and the existing global IPV supply constraints,27 the target population and timing of IPV use in campaigns with mOPV2 needs to be carefully considered on the basis of epidemiology (eg, co-circulation of serotypes) and population immunity.
Our study has several limitations. Response to OPVs varies by multiple factors and is lower in developing countries than in developed countries.28,29 Maternal antibodies, younger age at vaccine administration, and presence of diarrhoea are known to reduce OPV response. Therefore, the immune response of mOPV2 in outbreak response, when administered to children older than those enrolled in this trial who have lost their maternal antibodies, is likely to be higher. Community exposure to type 2 poliovirus was possible because the study was implemented before the global switch from tOPV to bOPV, and it might have increased the proportion of participants showing an immune response to type 2. However, because the trial was randomised, any bias resulting from increased immune response should have been non-differential and should not have affected the interpretation of the results. Finally, maternal antibodies could have affected our assessment of antibody titres because laboratory assays are unable to distinguish maternal antibodies from vaccine response antibodies.
Using the information provided in this study, the Strategic Advisory Group of Experts on Immunization recommended that WHO revise the global type 2 poliovirus outbreak response protocol,30 decreasing the number of mOPV2 vaccination campaigns from four to six to two to three, and emphasised the need to focus on the achievement of high coverage during each campaign to maximise the advantage of the high mOPV2 immunogenicity. Additionally, the Strategic Advisory Group of Experts on Immunization recommended carefully targeting IPV use in light of global IPV supply limitations and the absence of improvement in mOPV2 immunogenicity with IPV co-administration.
The findings from this clinical trial, in conjunction with the recommendations of the Strategic Advisory Group of Experts on Immunization, will strengthen the response to type 2 poliovirus outbreaks by focusing polio programme resources on improving vaccination campaign coverage, thus reaching the unvaccinated, and reducing the number of mOPV2 campaigns and the need to add IPV to these campaigns for type 2 outbreak responses.
Research in context.
Evidence before this study
Removal of live polioviruses, including those in the oral poliovirus vaccine (OPV), is essential to achieve and sustain polio eradication. Following the recommendations of the Strategic Advisory Group of Experts on Immunization, the Global Polio Eradication Initiative started a phased OPV cessation in April, 2016, starting with type 2 poliovirus, by globally replacing trivalent oral poliovirus vaccine (tOPV), which contains polioviruses types 1, 2, and 3, with bivalent OPV (bOPV), which contains polioviruses types 1 and 3.
To interrupt type 2 poliovirus outbreaks after withdrawal of tOPV, the Global Polio Eradication Initiative developed a new outbreak response protocol that recommended the use of monovalent type 2 OPV (mOPV2) and inactivated poliovirus vaccine (IPV). In the outbreak response protocol, the recommended number and schedule of mOPV2 campaigns and the use of IPV in conjunction with mOPV2 was based on previous experience with tOPV and mOPV type 1.
We searched PubMed for papers published between Jan 1, 2000, and Nov 1, 2017, using the terms, “oral polio vaccine”, “monovalent oral polio vaccine”, and “inactivated polio vaccine” for studies comparing immunogenicity of poliovirus vaccines. We limited the search to studies in English published after 2000 because the mOPVs used in the 1960s had variable dosing and formulations. Those mOPVs were replaced by tOPV worldwide in 1963 with only few countries continuing to use locally manufactured mOPVs. In 2004, the technical oversight committee for the Global Polio Eradication Initiative recommended the development and licensure of mOPVs. We only selected clinical trials that had used mOPV2 for primary polio vaccination—ie, without previous receipt of any type 2 polio vaccine.
A trial in India reported on type 2 immunogenicity of two doses of mOPV2 with a 4 week interval between doses but did not assess impact of shortening the interval between mOPV2 doses or the impact of IPV co-administration on mOPV2 immunogenicity. A multi-country trial in Latin America vaccinated infants with a single dose of mOPV2 after three doses of bOPV. Type 2 immunogenicity was assessed 1 week after mOPV2. This trial did not assess the impact of IPV co-administration on mOPV2 immunogenicity.
Added value of this study
This is the first study to report results of an open-label, randomised controlled trial that assessed the immunogenicity of two mOPV2 doses including shortening of the interval between mOPV2 doses and the co-administration of IPV. After one dose of mOPV2, immune response was observed in 157 (91%) of 172 participants. After two mOPV2 doses, immune responses were non-inferior with the 1 week or 2 week schedules compared with the standard 4 week schedule. Titre distributions were also not significantly different among the study arms. IPV administration did not modify the immune response induced by the first dose of mOPV2.
Implications of all the available evidence
Using findings from this trial, the Strategic Advisory Group of Experts on Immunization recommended that WHO revise the global type 2 poliovirus outbreak response protocol. On the basis of the high immunogenicity of mOPV2 observed in this study, the Strategic Advisory Group of Experts on Immunization recommended reducing the number of mOPV2 campaigns from four to six to two to three, and they recommended a more restricted use of IPV because of global IPV supply constraints and the absence of improvement in mOPV2 immunogenicity with IPV co-administration. These revisions in the outbreak response protocol will allow the Global Polio Eradication Initiative to focus polio programme resources on improving mOPV2 vaccination campaign coverage and reducing the need for additional mOPV2 vaccination campaigns or the need to add IPV to mOPV2 vaccination campaigns.
Acknowledgments
The International Centre for Diarrhoeal Disease Research, Bangladesh acknowledge with gratitude the commitment of Centers for Disease Control and Prevention to its research efforts and is grateful to the Governments of Bangladesh, Canada, Sweden, and the UK for providing core and unrestricted support. We thank the study staff at the Mirpur and Mohakahli study clinics in Dhaka, Bangladesh; Kimberly Porter at Centers for Disease Control and Prevention; Deborah Moore, Yiting Zhang, Sharla McDonald, Larin McDuffie, William Hendley, and Mario Nicolas at the Polio and Picornavirus Laboratory Branch in the Centers for Disease Control and Prevention; all parents and infants who participated in this study; and SanofiPasteur for donating vaccines used in this study. The findings and conclusions in this study are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Footnotes
Declaration of interests
MSO reports grants from Bill & Melinda Gates Foundation and royalties to institution for licensing of enterovirus and parechovirus strains for redistribution as reagents or controls from Zeptometrix outside the submitted work. All other authors declare no competing interests.
Contributor Information
Khalequ Zaman, International Centre for Diarrhoeal Disease Research, Bangladesh, Dhaka, Bangladesh.
Concepción F Estívariz, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Michelle Morales, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Mohammad Yunus, International Centre for Diarrhoeal Disease Research, Bangladesh, Dhaka, Bangladesh.
Cynthia J Snider, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Howard E Gary, Jr, Centers for Disease Control and Prevention, Atlanta, GA, USA.
William C Weldon, Centers for Disease Control and Prevention, Atlanta, GA, USA.
M Steven Oberste, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Steven G Wassilak, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Mark A Pallansch, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Abhijeet Anand, Centers for Disease Control and Prevention, Atlanta, GA, USA.
References
- 1.Dowdle WR, Cochi SL. Global eradication of poliovirus: history and rationale. In: Semler BL, Wimmer E, eds. Molecular biology of picornaviruses. Washington, DC: ASM Press, 2002: 473–80. [Google Scholar]
- 2.Sutter RW, Kew OM, Cochi SL, Aylward RB. Poliovirus vaccine—live. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines 6th edn. China: Elsevier, 2013: 598–645. [Google Scholar]
- 3.Caceres VM, Sutter RW. Sabin monovalent oral polio vaccines: review of past experiences and their potential use after polio eradication. Clin Infect Dis 2001; 33: 531–41. [DOI] [PubMed] [Google Scholar]
- 4.Birmingham ME, Aylward RB, Cochi SL, Hull HF. National immunization days: state of the art. J Infect Dis 1997; 175 (suppl 1): S183–88. [DOI] [PubMed] [Google Scholar]
- 5.Cochi SL, Linkins RW. The final phase of polio eradication: new vaccines and complex choices. J Infect Dis 2012; 205: 169–71. [DOI] [PubMed] [Google Scholar]
- 6.Kew OM, Wright PF, Agol VI, et al. Circulating vaccine-derived polioviruses: current state of knowledge. Bull World Health Organ 2004; 82: 16–23. [PMC free article] [PubMed] [Google Scholar]
- 7.Wassilak S, Pate MA, Wannemuehler K, et al. Outbreak of type 2 vaccine-derived poliovirus in Nigeria: emergence and widespread circulation in an underimmunized population. J Infect Dis 2011; 203: 898–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estivariz CF, Watkins MA, Handoko D, et al. A large vaccine-derived poliovirus outbreak on Madura Island—Indonesia, 2005. J Infect Dis 2008; 197: 347–54. [DOI] [PubMed] [Google Scholar]
- 9.Global Polio Eradication Initiative. Global circulating vaccine-derived poliovirus cases, 2000–2017. http://polioeradication.org/polio-today/polio-now/this-week/circulating-vaccine-derived-poliovirus (accessed March 20, 2016).
- 10.Hampton LM, Farrell M, Ramirez-Gonzalez A, et al. Cessation of trivalent oral poliovirus vaccine and introduction of inactivated poliovirus vaccine—worldwide, 2016. MMWR Morb Mortal Wkly Rep 2016; 65: 934–38. [DOI] [PubMed] [Google Scholar]
- 11.Global Polio Eradication Initiative. Standard operating procedures: responding to a poliovirus event and outbreak. Part 2: protocol for poliovirus type 2. Geneva: World Health Organization, 2017. http://polioeradication.org/tools-and-library/resources-for-polio-eradicators/gpei-tools-protocols-and-guidelines/ (accessed March 15, 2017). [Google Scholar]
- 12.Sheikh MA, Makokha F, Hussein AM, et al. Combined use of inactivated and oral poliovirus vaccines in refugee camps and surrounding communities—Kenya, December 2013. MMWR Morb Mortal Wkly Rep 2014; 63: 237–41. [PMC free article] [PubMed] [Google Scholar]
- 13.Bahl S, Verma H, Bhatnagar P, et al. Fractional-dose inactivated poliovirus vaccine immunization campaign—Telangana State, India, June 2016. MMWR Morb Mortal Wkly Rep 2016; 65: 859–63. [DOI] [PubMed] [Google Scholar]
- 14.WHO. Multicentre Growth Reference Study Group: WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva: World Health Organization, 2006. [Google Scholar]
- 15.Resik S, Tejeda A, Lago PM, et al. Randomized controlled clinical trial of fractional doses of inactivated poliovirus vaccine administered intradermally by needle-free device in Cuba. J Infect Dis 2010; 201: 1344–52. [DOI] [PubMed] [Google Scholar]
- 16.Sutter RW, John TJ, Jain H, et al. Immunogenicity of bivalent types 1 and 3 oral poliovirus vaccine: a randomised, double-blind, controlled trial. Lancet 2010; 376: 1682–88. [DOI] [PubMed] [Google Scholar]
- 17.SAS Institute. SAS/STAT user’s guide, version 6.4, 4th edn. Cary, NC: SAS Institute, 1989. [Google Scholar]
- 18.Halsey N, Galazka A. The efficacy of DPT and oral poliomyelitis immunization schedules initiated from birth to 12 weeks of age. Bull World Health Organ 1985; 63: 1151–69. [PMC free article] [PubMed] [Google Scholar]
- 19.Estívariz CF, Anand A, Gary HE Jr, et al. Immunogenicity of three doses of bivalent, trivalent, or type 1 monovalent oral poliovirus vaccines with a 2 week interval between doses in Bangladesh: an open-label, non-inferiority, randomised, controlled trial. Lancet Infect Dis 2015; 5: 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mir F, Quadri F, Mach O, et al. Monovalent type-1 oral poliovirus vaccine given at short intervals in Pakistan: a randomised controlled, four-arm, open-label, non-inferiority trial. Lancet Infect Dis 2015; 15: 889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farag NH, Alexander J, Hadler S, et al. Progress toward poliomyelitis eradication—Afghanistan and Pakistan, January 2013–August 2014. MMWR Morb Mortal Wkly Rep 2014; 63: 973–77. [PMC free article] [PubMed] [Google Scholar]
- 22.Faden H, Modlin JF, Thoms ML, McBean AM, Ferdon MB, Ogra PL. Comparative evaluation of immunization with live attenuated and enhanced-potency inactivated trivalent poliovirus vaccines in childhood: systemic and local immune responses. J Infect Dis 1990; 162: 1291–97. [DOI] [PubMed] [Google Scholar]
- 23.Parent du Chatelet I, Merchant AT, Fisher-Hoch S, et al. Serological response and poliovirus excretion following different combined oral and inactivated poliovirus vaccines immunization schedules. Vaccine 2003; 21: 1710–18. [DOI] [PubMed] [Google Scholar]
- 24.Estivariz CF, Jafari H, Sutter RW, et al. Immunogenicity of supplemental doses of poliovirus vaccine for children aged 6–9 months in Moradabad, India: a community-based, randomised controlled trial. Lancet Infect Dis 2012; 12: 128–35. [DOI] [PubMed] [Google Scholar]
- 25.Jafari H, Deshpande JM, Sutter RW, et al. Polio eradication. Efficacy of inactivated poliovirus vaccine in India. Science 2014; 345: 922–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardemil CV, Estivariz C, Shrestha L, et al. The effect of diarrheal disease on bivalent oral polio vaccine (bOPV) immune response in infants in Nepal. Vaccine 2016; 34: 2519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO. Meeting of the Strategic Advisory Group of Experts on immunization, April 2016—conclusions and recommendations. Wkly Epidemiol Rec 2016; 21: 266–84. [PubMed] [Google Scholar]
- 28.Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis 1991; 13: 926–39. [DOI] [PubMed] [Google Scholar]
- 29.WHO Collaborative Study Group. Factors affecting the immunogenicity of oral poliovirus vaccine: a prospective evaluation in Brazil and the Gambia. World Health Organization Collaborative Study Group on Oral Poliovirus Vaccine. J Infect Dis 1995; 171: 1097–106. [DOI] [PubMed] [Google Scholar]
- 30.WHO. Meeting of the Strategic Advisory Group of Experts on immunization, October 2016—conclusions and recommendation. Wkly Epidemiol Rec 2016; 48: 561–84. [PubMed] [Google Scholar]


