Abstract
Objective:
The purpose of this study was to assess the immune response and malondialdehyde levels in irradiated rats supplemented with Curcuma xanthorriza Roxb extract as a candidate for mitigating radiation exposure.
Methods:
Twenty-four male Wistar rats were grouped into eight treatment groups, then Curcuma xanthorrhiza Roxb extract was administered orally and irradiated at 6 Gy. Measurement of rats IL-6 and INF-γ was performed using a sandwich ELISA Kit, while the MDA concentration was quantified according to the method of Wills (1971). The statistical test is determined by one way ANOVA test. P-value <0.05 was considered statistically significant.
Result:
The concentration of IL-6 in all groups showed no statistically significant difference (P=0.18). There was an increase in the concentration of IL-6 in the group of rats irradiated with 6 Gy for 7 days and 14 days. Meanwhile, the INF-γ concentration also showed no significant results in all treatment groups (P=0.28). The average of MDA concentration showed a significant difference in the liver and spleen of irradiated rats at 6 Gy for 14 days compared to the control (0.044 nmol/mg vs 0.008 nmol/mg, P=0.03 and 0.032 nmol/mg vs 0.014 nmol/mg, P=0.05, respectively).
Conclusion:
The administration of Curcuma xanthorriza Xorb extract was able to reduce MDA concentrations in the liver and spleen although not statistically significant. In addition, exposure to ionizing radiation at a dose of 6 Gy significantly increased lipid peroxidation in the liver and spleen by 5.5 times and 2.3 times, respectively.
Key Words: Curcuma xanthorriza Roxb extract, Interferon-gamma, Interleukin-6, Malondialdehyde levels
Introduction
Radiation exposure has long been known to contribute to oxidative stress by direct excitation or by ionizing individual atoms. Free radicals formed through molecular ionization will then induce oxidative stress and cause damage to exposed cells (Nuszkiewicz et al., 2020). Exposure to damaging ionizing radiation is closely related to increased oxidative stress at the site of the irradiated cell. This is due to the nature of ionizing radiation which can penetrate the cell until it enters the cell nucleus and then ionizes organic molecules in the nucleus (Belli and Indovina, 2020). Free radicals can cause oxidative stress thereby creating a process of lipid peroxidation in living organisms. In addition, the presence of free radicals can also trigger cell damage due to disruption of the balance between antioxidants and oxidants in the tissue (Alkadi, 2020; Martemucci et al., 2022).
Malondialdehyde (MDA) is one of the end products of the lipid peroxidation process in the blood and organs such as the liver and spleen (Ayala et al., 2014). The increasing accumulation of free radicals causes the production of MDA to also increase. Thus, MDA is a marker of oxidative stress and cellular defects caused by accumulation of free radicals (Hasanuzzaman et al., 2020). During the process of oxidative stress, in addition to releasing MDA, the body will also secrete several pro-inflammatory cytokines, such as Interleukin-6 (IL-6), IL-10, TNF alpha, and Interferon gamma (IFN-γ) (Boarescu et al., 2022). Innate immune cells that play a role in the release of these cytokines are macrophages. According to the way of activation, there are two types of macrophages, namely prototypic macrophages, and anti-inflammatory macrophages. Prototypic macrophages are responsible for producing proinflammatory and bactericidal cytokines, while anti-inflammatory macrophages are responsible for repairing damaged tissue (Fernando et al., 2014).
The cytokines targeted in this study were IL-6 and IFN-γ. IL-6 is a pro-inflammatory cytokine that plays a role in regulating immune and inflammatory responses and inhibits apoptosis during the inflammatory process. IFN-γ, on the other hand, is a pleiotropic molecule associated with anti-proliferative, pro-apoptotic, and anti-tumor mechanisms (Matsuoka et al., 2016; Castro et al., 2018). IFN-γ encodes a soluble cytokine that is a member of the type II interferon class. The encoded protein is secreted by innate and adaptive immune system cells. The active protein is a homodimer that binds to interferon-gamma receptors that trigger cellular responses to viral and microbial infections (Kopitar-Jerala, 2017; Mojic et al., 2018). The IL-6 gene encodes a cytokine that functions in inflammation and B-cell maturation. In addition, the encoded protein has has been shown to be an endogenous pyrogen capable of causing fever in people with autoimmune or infectious diseases. It The protein is mainly produced at sites of acute and chronic inflammation (Kaneko et al., 2019).
Curcuma xanthorrhiza Roxb (Indonesian people call “Temulawak”) is a plant that is often used as medicine which belongs to the Zingiberacea genus and is found in many forests in tropical areas, especially in Indonesia. This genus has around 93-100 species, although there is still controversy about the exact number. Curcuma contains many secondary metabolites that are beneficial to health (Akarchariya et al., 2017; Syamsudin et al., 2019). Sesquiterpenoids and monoterpenoids are the main components of this plant. The oil extracted from Curcuma has many pharmacological functions, including antidiarrheal, carminative, antilarvatic, antiviral, anti-inflammatory, antioxidant, and anticancer properties (Angel et al., 2014; Sikha et al., 2015; Herath et al., 2017; Dosoky and Setzer, 2018). Information and investigation related to the role of Curcuma as a radiation mitigator has not been widely carried out. Moreover, the use and development of traditional medicines, especially in the Asian region, is very rapid. In addition, studies of the benefits of Curcuma on immune response and MDA concentrations in rats after being exposed to radiation has not been widely studied. This is the novelty that this paper wants to achieve. The purpose of this study was to assess the immune response and malondialdehyde levels in irradiated rats supplemented with Curcuma xanthorriza Roxb extract as a mitigator candidate from radiation exposure.
Materials and Methods
Ethical approval
The ethical approval certificate for this present study was issued by Institutional Animal Care and Use Committee, Indonesia, on November 15, 2021 with certificate number 011/KEPPHP-BATAN/XII/2021.
Preparation of Curcuma xanthorriza Xorb extract
Roots of Curcuma xanthorriza were obtained from the Indonesian Medicinal and Aromatic Crops Research Institute (IMACRI), while the identification process was carried out at the Indonesian Institute of Sciences, National Research and Innovation Agency, Bogor, West Java, Indonesia. Dosage is expressed as milligrams of dry extract per kilograms of rat body weight. The resulting extract was then redissolved in its solvent before each individual experiment. Captopril was used as standard drug in this study.
Animals
The experimental animals used were male Wistar rats, aged 8-12 weeks, and weighing 180-220 grams. These animals were obtained from iRATco Veterinary Laboratory Service, Bogor, Indonesia. All test animals were housed and acclimatized under optimal conditions of temperature (21-24oC) and lighting (12 hours dark/light cycle) at the Integrated Animal Laboratory, National Research and Innovation Agency (BRIN), Indonesia. Before use, the animals were given a pelleted rodent diet (iRATco) and weighed regularly once a week.
Samples collection
Twenty-four male Wistar rats were grouped into eight groups (n=3 each): group K (control), group A (6 Gy irradiation, 2 hours), group B (6 Gy irradiation, 7 days), group C (6 Gy irradiation, Curcuma, 7 days), group D (6 Gy irradiation, Captopril, 7 days), group E (6 Gy irradiation, 14 days), group F (6 Gy irradiation, Curcuma, 14 days) and group G (6 Gy irradiation, Captopril, 14 days). The concentration of curcuma extract was 100 milligrams per kilogram of body weight. The number of animals tested in this study was calculated using the Frederer formula, (n-1) (t-1) ≥15, with “t” means the number of treatment groups and “n’ means the minimum number of animals used in each group. Based on this formula, eight groups were formed and three animals were in each group.
Radiation exposure was given at a dose of 6 Gy and a dose rate of 1 Gy/min using a cobalt-60 IRPASENA gamma ray device at the Research Center for Radiation Process Technology, BRIN, Indonesia. The distance from the Co-60 source to the sample was 108 cm. Each rat was fixed in a perforated acrylic container. The irradiated rats were placed on a rotating platform to ensure the same dose to all tissues. Rats were anesthetized with ketamine-xylazine (75-100 mg per kg body weight) intraperitoneally. Surgery was performed by opening the thoracic cage, and blood was immediately removed using a 10 mL syringe and was placed into a tube containing heparin for plasma isolation. Whole blood was centrifuged at 3,000 rpm within 30 minutes. Supernatant (plasma) was taken and was stored at -20°C.
Measurement of immune responses
The rats IL-6 and INF-γ measurement were carried out using ELISA Kit from EliKineTM. Specific antibodies for IL-6 and IFN-γ were pre-coated onto microplates. The standard and sample are pipetted into the well and any IL-6 and IFN-γ present are bound by the immobilized antibody. After removing unbound substances, a biotin-conjugated antibody specific for IL-6 and IFN-γ was added to the wells. After washing, a proprietary EliKineTM Streptavidin-HRP conjugate is added to the well. After washing, TMB Substrate was added, TMB turned blue under HRP catalysis and turned yellow after the solution addition stopped. Measure the optical density (OD) value with a microplate reader at a wavelength of 450 nm. IL-6 and IFN-γ concentrations were comparable to OD450 nm standard values.
Quantification of MDA levels
MDA levels were quantified according to the Wills (1971) method with some modifications (Wills, 1971). MDA standard curves were prepared using tetraethoxypropane (TEP) at five-point concentrations from 0 to 5 nmol/mL. The standard MDA solution was put into a 1.5 mL microtube containing 400 µl of distilled water. 200 µl of 20% trichloroacetic acid (TCA) and 400 µl of 0.67% thiobarbituric acid (TBA) were added then incubated at 96-100oC for 10 minutes. The mixture was kept at room temperature until there were no bubbles in the tube. The optical density value is read at a wavelength of 530 nm. The procedure for measuring MDA in liver and spleen tissue is the same as for determining the standard curve. After adding 20% TCA, the samples (200 µl each) were homogenized briefly and then centrifuged at 5,000 rpm for 10 minutes. The MDA concentration was calculated by plotting the absorbance value into the linear regression formula of the standard curve.
Statistical analysis
In all experiments, the data obtained are presented as the mean ± SEM. Statistical analysis was performed using SPSS software version 25. The data normality test was performed using the Shapiro-Wilk test. The level of significance between IL-6, IFN-γ, and MDA levels was determined by one-way analysis of variance (ANOVA) test and continued with Tukey’s post-hoc assessment if significant differences were found. An independent sample T-test was performed to determine the concentration of IL-6, INF-γ, and MDA between irradiated groups with and without Curcuma supplementation. P-value <0.05 was considered statistically significant.
Results
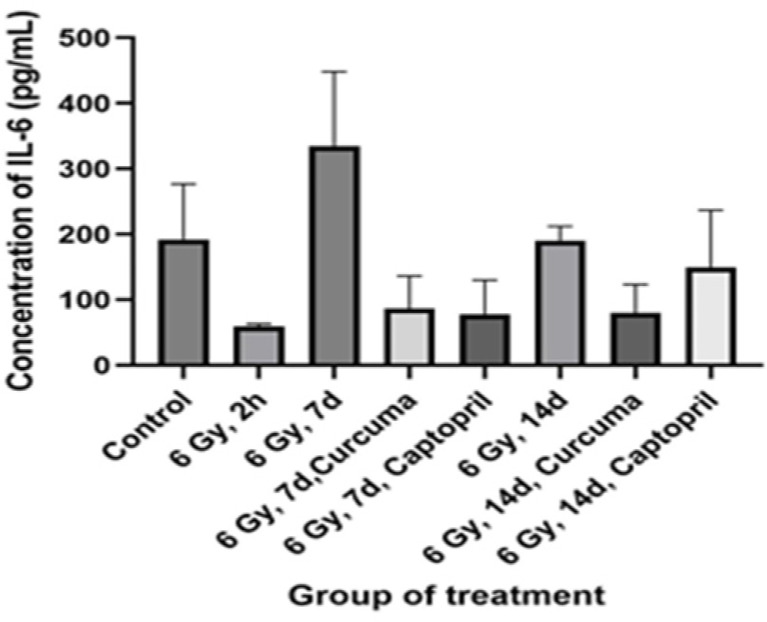
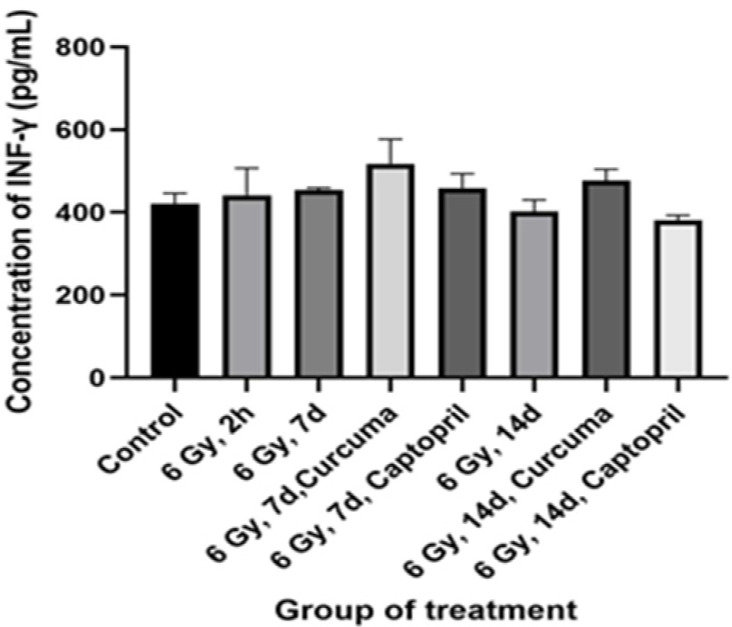
Effects of different doses of the ethyl alcohol extract of Curcuma xanthorriza Xorb (Temulawak) on IL-6 and INF-γ levels are shown in Table 1, Figures 1 and 2, respectively. In this study, as shown in Figure 1, the mean plasma concentrations of the cytokine IL-6 in rat plasma in all groups showed no statistically significant difference (P=0.18). There was an increase in the concentration of IL-6 in the group of rats irradiated with 6 Gy for 7 days and 14 days. Meanwhile, in Figure 2, the average INF-γ concentration also showed a not significant results in all treatment groups (P=0.28). Table 2 showed the concentration of IL-6, IFN-ϒ, and MDA between irradiated groups with and without Curcuma supplementation. There were no significant differences between all the groups tested.
Table 1.
The Concentration of IL-6, INF-γ, and MDA Concentration in Rats Treated with 6 Gy Irradiation
| Concentration of | ||||
|---|---|---|---|---|
| Groups | IL-6 (pg/mL) | INF-gamma (pg/mL) | MDA in liver (nmol/mg) | MDA in spleen (nmol/mg) |
| Control | 192.48±17.34 | 420.81±44.53 | 0.0076±0.005 | 0.0138±0.002 |
| 2h, 6 Gy | 58.95±5.78 | 441.99±92.02 | 0.0211±0.011 | 0.0239±0.008 |
| 7d, 6 Gy | 334.5±161.55 | 454.49±7.77 | 0.02±0.009 | 0.0259±0.005 |
| Curcuma, 7d, 6 Gy | 86.4±6.47 | 517.71±102.13 | 0.0118±0.001 | 0.0164±0.001 |
| Captopril, 7d, 6 Gy | 77.5±6.57 | 459.08±60.14 | 0.0055±0.005 | 0.0169±0.001 |
| 14d, 6 Gy | 190.68±30.51 | 401.85±40.62 | 0.0438±0.009* | 0.0316±0.001* |
| Curcuma, 14d, 6 Gy | 79.91±18.37 | 476.67±48.07 | 0.0278±0.008 | 0.0256±0.01 |
| Captopril, 14d, 6 Gy | 149.32±124.04 | 380.03±18.74 | 0.0204±0.001 | 0.0268±0.003 |
| P-value | 0.18 | 0.28 | 0.03 | 0.05 |
Note: One-way ANOVA, Tukey’s post hoc test, two sided P-value<0.05
Figure 1.
Effects of Curcuma xanthorriza Xorb Extract (100 mg/kg body weight) and 6 Gy Irradiation Dose on the Value of IL-6. Comparisons were made using ANOVA followed by post-hoc Tukey's multiple comparison test (P-value=0.18). Note: P-value<0.05 was considered statistically significant
Figure 2.
Effects of Curcuma xanthorriza Xorb Extract (100 mg/kg body weight) and 6 Gy Irradiation Dose on the Value of INF-γ (P-value=0.28)
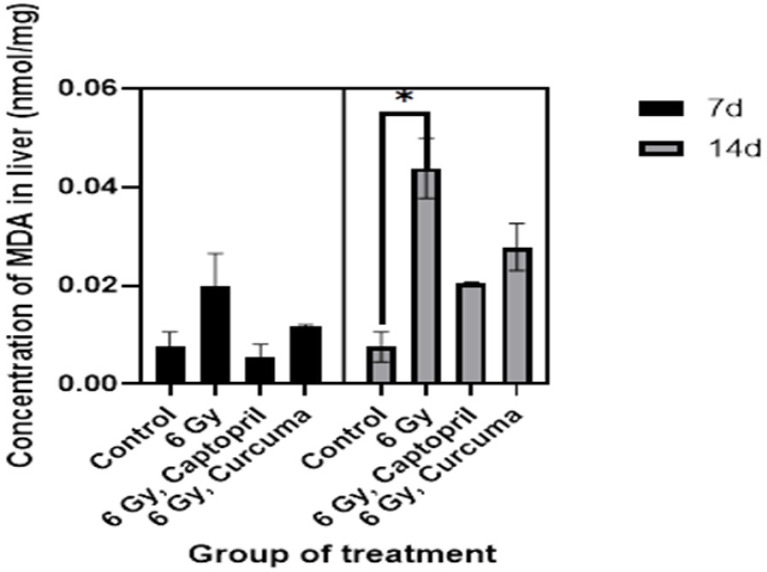
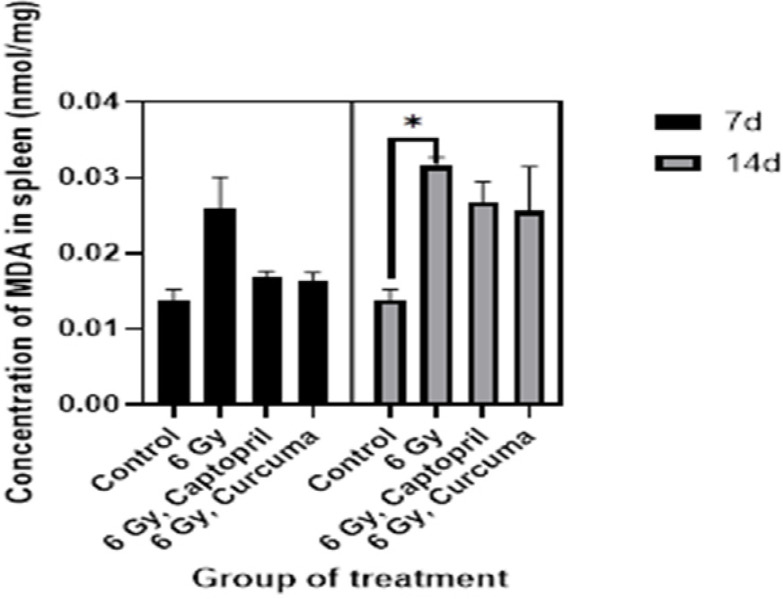
Effects of different doses of the ethyl alcohol extract of Curcuma xanthorriza Xorb (Temulawak) on MDA levels in liver and spleen are shown in Figures 3 and 4, respectively. In Figure 3, the average MDA concentration showed a significant difference in the liver of irradiated rats at 6 Gy for 14 day compared to the control (0.044 nmol/mg vs 0.008 nmol/mg, P=0.03). Meanwhile, in Figure 4 we can see that the average of MDA concentration also showed a significant increase in the spleen of irradiated rats at 6 Gy for 14 days compared to the control (0.032 nmol/mg vs 0.014 nmol/mg, P=0.05).
Figure 3.
Effects of Curcuma xanthorriza Xorb Extract (100 mg/kg body weight) and 6 Gy Irradiation Dose on the Value of MDA in Liver (P=0.03). Note: *P-value<0.05 was considered statistically significant
Figure 4.
Effects of Curcuma xanthorriza Xorb Extract (100 mg/kg body weight) and 6 Gy Irradiation Dose on the Value of MDA in Spleen (P-value=0.05). Note: *P-value<0.05 was considered statistically significant
Table 2.
The Concentration of IL-6, INF-ϒ, and MDA Engagement between Irradiated Groups with and without Curcuma Supplementation
| Group | IL-6 (pg/mL) | INF-gamma (pg/mL) | MDA in liver (nmol/mg) | MDA in spleen (nmol/mg) |
|---|---|---|---|---|
| 7d, 6 Gy | 334.5±161.55 | 454.49±7.77 | 0.02±0.009 | 0.0259±0.005 |
| Curcuma, 7d, 6 Gy | 86.4±6.47 | 517.71±102.13 | 0.0118±0.001 | 0.0164±0.001 |
| P-value | 0.11 | 0.47 | 0.43 | 0.24 |
| 14d, 6 Gy | 190.68±30.51 | 401.85±40.62 | 0.0438±0.009 | 0.0316±0.001 |
| Curcuma, 14d, 6 Gy | 79.91±18.37 | 476.67±48.07 | 0.0278±0.008 | 0.0256±0.01 |
| P-value | 0.16 | 0.17 | 0.13 | 0.49 |
Note: Independent sample T-test, significant if P-value<0.05
Discussion
The results of this study in rats showed a strong correlation between exposure to ionizing radiation and the level of lipid peroxidation which is characterized by the formation of MDA. Malondialdehyde (MDA) is a dialdehyde compound which is the end product of lipid peroxidation in the body, through enzymatic or non-enzymatic processes (Jove et al., 2020). High concentrations of MDA indicate an oxidation process in the cell membrane. In order to protect against ROS attacks, the human body has an organized antioxidant system, both enzymatic antioxidants and non-enzymatic antioxidants, which work synergistically (Kurutas, 2016; Aranda-Rivera et al., 2020). Antioxidants protect body cells against oxidative damage and can prevent the production of oxidative products. Imbalance between oxidants and antioxidants, i.e. if ROS production exceeds antioxidant capacity, it has the potential to cause damage, which is called oxidative stress (Pizzino et al., 2017).
Antioxidant compounds derived from natural materials, such as Curcuma (Temulawak), are considered a promising alternative to synthetic radiomitigators because they can eliminate free radicals generated by the interaction of radiation with water molecules in cells (Parcheta et al., 2021). In addition, some cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-17 (IL-17), and interferon-γ (INF-γ) also play important roles during the stages of the inflammatory cascade in cells (Megha et al., 2021). The results of this study indicated that there was no effect of giving Curcuma xanthorriza Xorb extract on decreasing the concentration of cytokines IL-6 and INF-γ, although the measurement results showed a decreased trend compared to the control and 6 Gy radiation treatment. This seems to be different from several previous studies which reported that the secondary metabolites contained in curcumin were able to inhibit the inflammatory process (Zhang et al., 2015; Dai et al., 2018; Zhang et al., 2019). Nevertheless, Curcumin has good potential as a radiomitigator agent because of its flavonoid content to reduce free radical levels caused by lipid peroxidation (Hall et al., 2016).
This study also examined the effects of radiation and administration of Curcumin on the level of lipid peroxidation, through the formation of MDA. The results showed that irradiation at a dose of 6 Gy increased MDA concentrations significantly by 5.5 times in the liver and 2.3 times in the spleen compared to controls. A previous study by Okassova et al (2022) reported that gamma radiation could significantly increase MDA levels in the liver by 1.76 times and spleen by 1.4 times in animals exposed to gamma radiation. Concentration of MDA in plasma is an important biomarker in radiation exposure to assess oxidative stress and its impact on lipid peroxidation levels (Maurya et al., 2021). In other study, it was found that the DNA damage response was strongly associated with age and time of exposure with a decrease of 0.6 percent (P-value: 0.008) and 0.58 percent (P-value<0.05), respectively. Whereas gender, smoking habit, and equivalent dose were not correlated with DNA damage (Surniyantoro et al., 2022). In addition, radiation exposure also had a significant effect on decreasing red blood cells (RBCs) levels by 0.541 × 106/µL per 1 mSv increase in radiation dose (Surniyantoro et al., 2019). The equivalent dose correlated significantly with an increase in micronuclei frequency (MN) of 16.3 per 1 mSv equivalent dose (P=0.001). In addition, the frequency of MN is closely related to age, equivalent dose, and time of exposure (Surniyantoro et al., 2018).
The study we have conducted reveal that exposure to ionizing radiation can increase MDA concentrations and as a result is a condition of oxidative stress that appears as a form of imbalance between free radicals and antioxidants in the body. The administration of Curcuma xanthorriza Xorb extract did not show a significant effect on reducing the MDA concentrations, although the value of MDA concentrations showed a decrease in the group that was given the extract compared to the group of mice that were not given Curcuma extract. However, when compared with Captopril as a standard drug, which also did not show a significant effect, the Curcuma extract was predicted to have the better ability to reduce lipid peroxidation levels. This is supported by the lower MDA concentration after administration of the Curcuma extract compared to Captopril.
In conclusion, we reported that administration of Curcuma xanthorriza Xorb extract was able to reduce MDA concentrations in both liver and spleen although not statistically significant. In addition, we also found that exposure to ionizing radiation at a dose of 6 Gy significantly increased lipid peroxidation in the liver and spleen by 5.5 times and 2.3 times, respectively.
Author Contribution Statement
H.N.E.S. drafted the manuscript, collected the samples, performed the experiments (IL-6 and INF-γ), analyzed the experimental data, performed the statistical analysis, designed the tables and figures, and revised the manuscript, T.K. team leader, administered the ethical clearance, collected the samples, performed the experiment (MDA), and revised the manuscript, D.T. collected the samples and performed the experiment (MDA), D.Y., Y.L. collected the samples, I.K.H.B. aided in interpreting the results.
Acknowledgements
The authors are also grateful to our co-workers in Molecular Radiobiology Laboratory, PRTKMMN, Jakarta. This study was supported by Research Organization for Nuclear Energy (ORTN), National Research and Innovation Agency (BRIN), Indonesia.
Funding
This study was supported by Research Organization for Nuclear Energy (ORTN), National Research and Innovation Agency (BRIN), Indonesia, via Project Research Fund (RP-HITN): B-125/III/TN/3/2022.
Ethical Approval
The ethical approval certificate for this present study was issued by Institutional Animal Care and Use Committee, Indonesia, on November 15, 2021 with certificate number 011/KEPPHP-BATAN/XII/2021.
Conflict of Interest
All of authors state that this study was conducted without any financial relationship construed as a potential conflict of interest.
References
- Akarchariya N, Sirilun S, Julsrigival J, Chansakaowa S. Chemical profiling and antimicrobial activity of essential oil from Curcuma aeruginosa Roxb Curcuma glans K Larsen & J Mood and Curcuma cf xanthorrhiza Roxb collected in Thailand. Asian Pac J Trop Biomed. 2017;7:881–5. [Google Scholar]
- Alkadi H. A Review on free radicals and antioxidants. Infect Disord Drug Targets. 2020;20:16–26. doi: 10.2174/1871526518666180628124323. [DOI] [PubMed] [Google Scholar]
- Angel GR, Menon N, Vimala B, Nambisan B. Essential oil composition of eight starchy Curcuma species. Ind Crops Prod. 2014;60:233–8. [Google Scholar]
- Aranda-Rivera AK, Cruz-Gregorio A, Arancibia-Hernandez YL, Hernandez-Cruz EY, Pedraza-Chaverri J. RONS and oxidative stress: An overview of basic concepts. Oxygen. 2020;2:437–78. [Google Scholar]
- Ayala A, Munoz MF, Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:1–31. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belli M, Indovina L. The response of living organisms to low radiation environment and its implications in radiation protection. Front Public Health. 2020;8:1–15. doi: 10.3389/fpubh.2020.601711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boarescu PM, Boarescu I, Pop RM, et al. Evaluation of oxidative stress biomarkers, pro-inflammatory cytokines, and histological changes in experimental hypertension, dyslipidemia, and type 1 diabetes mellitus. Int J Mol Sci. 2022;23:1–17. doi: 10.3390/ijms23031438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro F, Cardoso AP, Goncalves RM, Serre K, Oliveira MJ. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front Immunol. 2018;9:1–19. doi: 10.3389/fimmu.2018.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Gu L, Su Y, et al. Inhibition of curcumin on influenza A virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF-κB pathways. Int Immunopharmacol. 2018;54:177–87. doi: 10.1016/j.intimp.2017.11.009. [DOI] [PubMed] [Google Scholar]
- Dosoky NS, Setzer WN. Chemical composition and biological activities of essential oils of Curcuma species. Nutrients. 2018;10:1–42. doi: 10.3390/nu10091196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando MR, Reyes JL, Iannuzzi J, Leung G, McKay DM. The pro-inflammatory cytokine, interleukin-6, enhances the polarization of alternatively activated macrophages. PLoS One. 2014;9:1–12. doi: 10.1371/journal.pone.0094188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S, Rudrawar S, Zunk M, et al. Protection against radiotherapy-induced toxicity. Antioxidants. 2016;5:1–18. doi: 10.3390/antiox5030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M, Bhuyan MHMB, Zulfiqar F, et al. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants (Basel) 2020;9:1–52. doi: 10.3390/antiox9080681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herath HMIC, Wiyasiriwardene TDCMK, Premakumara GAS. Comparative GC-MS analysis of all Curcuma species grown in Sri Lanka by multivariate test. Ruhunu J Sci. 2017;8:1–9. [Google Scholar]
- Jove M, Mota-Martorell N, Pradas I, et al. The advanced lipoxidation end-product malondialdehyde-lysine in aging and longevity. Antioxidants (Basel) 2020;9:1–20. doi: 10.3390/antiox9111132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko N, Kurata M, Yamamoto T, Morikawa S, Masumoto J. The role of interleukin-1 in general pathology. Inflamm Regener. 2019;39:1–16. doi: 10.1186/s41232-019-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopitar-Jerala N. The role of interferons in inflammation and inflammasome activation. Front Immunol. 2017;8:1–8. doi: 10.3389/fimmu.2017.00873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. 2016;15:1–22. doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martemucci G, Costagliola C, Mariano M, et al. Free radical properties, source and targets, antioxidant consumption and health. Oxygen. 2022;2:48–78. [Google Scholar]
- Matsuoka Y, Nakayama H, Yoshida R, et al. IL-6 controls resistance to radiation by suppressing oxidative stress via the Nrf2-antioxidant pathway in oral squamous cell carcinoma. Br J Cancer. 2016;115:1234–44. doi: 10.1038/bjc.2016.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurya RP, Prajapat MK, Singh VP et al. Serum malondialdehyde as a biomarker of oxidative stress in patients with primary ocular carcinoma: Impact on response to chemotherapy. Clin Ophthalmol. 2021;15:871–9. doi: 10.2147/OPTH.S287747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megha KB, Joseph X, Akhil V, Mohanan PV. Cascade of immune mechanism and consequences of inflammatory disorders. Phytomedicine. 2021;91:1–17. doi: 10.1016/j.phymed.2021.153712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojic M, Takeda K, Hayakawa Y. The dark side of IFN-γ: Its role in promoting cancer immunoevasion. Int J Mol Sci. 2018;19:1–13. doi: 10.3390/ijms19010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuszkiewicz J, Wozniak A, Szewczyk-Golec K. Ionizing radiation as a source of oxidative stress-The protective role of melatonin and vitamin D. Int J Mol Sci. 2020;21:1–22. doi: 10.3390/ijms21165804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okassova AK, Britko V, Okassov DB, et al. Study of Lipid peroxidation-antioxidant defense systems in rats under radiation exposure. Maced J Med Sci. 2022;10:236–9. [Google Scholar]
- Parcheta M, Swislocka R, Orzechowska S, et al. Recent developments in effective antioxidants: The structure and antioxidant properties. Materials. 2021;14:1–24. doi: 10.3390/ma14081984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzino G, Irrera N, Cucinotta M, et al. Oxidative stress: Harms and benefits for human health. Oxid Med Cell Longev. 2017;2017:1–13. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikha A, Harini A, Prakash H. Pharmacological activities of wild turmeric (Curcuma aromatica Salisb): A review. J Pharmacogn Phytochem. 2015;3:1–4. [Google Scholar]
- Surniyantoro HNE, Lusiyanti Y, Rahardjo T, Nurhayati S, Tetriana D. Association between XRCC1 exon 10 (Arg399Gln) gene polymorphism and micronucleus as a predictor of DNA damage among radiation workers. Biodiversitas. 2018;19:1676–82. [Google Scholar]
- Surniyantoro HNE, Rahardjo T, Lusiyanti Y, et al. Assessment of Ionizing Radiation Effects on the Hematological Parameters of Radiation-Exposed Workers. Atom Indonesia. 2019;45:123–9. [Google Scholar]
- Surniyantoro HNE, Yusuf D, Rahardjo T, et al. Assessment of hOGG1 Genetic Polymorphism (rs1052133) and DNA Damage in Radiation-Exposed Workers. Asian Pac J Cancer Prev. 2022;23:4005–12. doi: 10.31557/APJCP.2022.23.12.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syamsudin RAMR, Perdana F, Mutiaz FS, et al. Temulawak plant (Curcuma xanthorrhiza Roxb) as a traditional medicine. Farmako Bahari. 2019;10:51–65. [Google Scholar]
- Wills ED. Effects of lipid peroxidation on membrane-bound enzymes of the endoplasmic reticulum. Biochem J. 1971;123:983–91. doi: 10.1042/bj1230983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liang D, Dong L, et al. Anti-inflammatory effects of novel curcumin analogs in experimental acute lung injury. Respir Res. 2015;16:1–13. doi: 10.1186/s12931-015-0199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Swamy S, Balijepalli S, et al. Direct pulmonary delivery of solubilized curcumin reduces severity of lethal pneumonia. FASEB J. 2019;33:13294–309. doi: 10.1096/fj.201901047RR. [DOI] [PMC free article] [PubMed] [Google Scholar]