Abstract
Objective:
This study aimed to investigate the level of PD-L1 protein expression in patients with BCs who were of Asian descent.
Methods:
Three databases were conducted on this article up to August 10th, 2022. The reference lists of the publications were examined for further studies, and in cases of duplicates, a study with a larger sample size was added. In survival analysis, the hazard ratio (HR) was applied to the circumstances characterized by the frequency of occurrences, and for the clinicopathological characteristic, the best-adjusted odds ratio (OR) with a 95% confidence interval (CI) was employed. The Newcastle-Ottawa Scale (NOS) was utilized to evaluate selection criteria, comparison, and exposure to establish the quality of the technique in the under-consideration studies. The Z test determined the association analysis of OS, DFS, and clinicopathological characteristics with PD-L1 expression.
Result:
All eight trials for OS and six for DFS were considered, with 4.111 and 3.071 participants, respectively. Overexpression of PD-L1 was linked to a reduced OS compared to individuals with undetectable expression (HR= 1.58, 95% CI 1.04–2.40; P=0.03). We analyzed clinicopathological features, and it elevated in individuals with histological grade III (OR=2.39, 95% CI 1.26-4.54; P=0.008) and positive node (OR=0.68, 95% CI 0.48-0.97; P<0.05).
Conclusion:
Overexpression of PD-L1 was associated with a shorter OS in BCs patients. High PDL1 was higher in persons with nodal positivity and histological grade III.
Key Words: Breast cancer, PD-L1, prognostic, biomarker
Introduction
Breast cancer (BCs) is the leading invasive tumor in women commonly. In 2020, it was anticipated that approximately 2.3 million women would receive their first BCs diagnosis. Asia accounted for over half (45.4%!) of all BCs diagnoses (Sung et al., 2021; Lim et al., 2022). The peak of BCs in Asian populations is also relatively experienced at a younger age (40-50 years) compared to non-Asian populations (60-70 years) (Green and Raina, 2008; Leong et al., 2010). Additionally, the M/I ratio of patients with BCs in Asia is far higher than in the rest of the world (0.28). It exceeds the global average and ranks second globally (WHO, 2021). It demonstrates the poor prognosis of BCs patients in the Asian area compared to those in other regions. Several variables contribute to this occurrence, but the immunological link outside of BCs is a topic that is infrequently studied.
One immunological route affecting BCs prognosis is the expression of programmed death-ligand 1 (PD-L1). PD-L1 is an immunological checkpoint in maintaining the normal control of T cell activity and preventing autoimmunity (Hänninen et al., 2021). Nevertheless, the upregulation of PD-L1 and a number of other proteins decreases the efficacy of immune surveillance and raises the survival probability of cancer cells (Cha et al., 2019).
Since antibodies that target PD-1 or PD-L1 are the most promising immunotherapeutic options currently available, it is interesting to explore the influence that PD-L1 has on methods for treating BCs. The significance of PD-L1 as a prognosis marker and its usefulness as a therapy goal for the success of immune checkpoint suppression are two topics that have been the subject of heated debate. PD-L1 expression has been shown to have a substantial association with a positive prognosis in some investigations, and this correlation has been used as a prognostic marker (Ali et al., 2015; Bae et al., 2016; Baptista et al., 2016; Beckers et al., 2016), leading to the hypothesis that PD-L1 expression indicates an efficient immune system against to tumor cells. Nonetheless, a reverse connection has also been discovered in a few investigations. In addition, several additional tumor microenvironment (TME) cells, including macrophages, dendritic cells, and fibroblasts, generate PD-L1, suppressing anti-tumor immunity. It is a significant determinant of cancer occurrence (Schildberg et al., 2016; Zou et al., 2016).
PD-L1 detection can be used to predict how effectively cancer cells would react to immunotherapy targeting PD-L1 and its receptor PD-1, which had been adopted for several other tumors. Additionally, PD-L1 expression can predict clinicopathological BCs features linked with patient prognosis (Javed et al., 2017; Alves, Paredes and Schmitt, 2019).
Previous research has shown that elevated levels of PD-L1 are linked to a poorer prognosis for BCs patient outcomes (Cirqueira et al., 2021). This poor prognosis is consistent with poor clinicopathological characteristics such as the presence of negative estrogen, progesterone receptor status, lymph node metastases, and high histological grade (Alves, Paredes and Schmitt, 2019). However, to date, there has been no study that summarizes the findings of PD-L1 expression in Asian populations, considering that the BCs population in Asia is one with the worst outcomes. As a result, this study aimed to explore the expression of the PD-L1 protein in patients with Asian BCs.
Materials and Methods
Study design and eligibility criteria
A systematic review conducted by the PRISMA (Moher et al., 2009) was completed on three different databases, including PubMed, ScienceDirect, and the Cochrane Library, up until August 10th, 2022. The included papers should: (1) clinical research published in peer-reviewed publications that investigated PD-L1; (2) research given information on PD-L1 as well as clinical and pathological status; and (3) studies with computation data for the calculation of HRs and 95% CIs for OS and DFS; and (4) full-text article. This review’s protocol was filed with the International Prospective Register of Systematic Reviews (PROSPERO), with the registration number CRD42023391913, and the paper was produced in accordance with PRISMA principles.
Search strategy and data extraction
We used the following keywords, and a search was conducted on all English-language publications: (“PD-L1” OR “B7H1” OR “CD-274”) AND (“breast cancer” OR “breast tumor” OR “ca mammae” OR “breast neoplasms”). The reference lists of the published works were looked at for potential new lines of inquiry, and in cases of duplicates, a study with a larger sample size was added. Each research yielded the following information: (1) initial name and year of the author publication; (2) the nation and number of patients; (3) age, median (range) of the sample; (4) IHC method; (5) PD-L1 antibody; (6) PD-L1 positive sample; (7) Follow-up, median and range. Three reviewers performed independently the study selection, quality rating, and data extraction. By reaching a consensus, the fourth and fifth reviewers resolved the dispute between the first three.
We separated survival variables into OS and DFS, as well as clinicopathological characteristics into age, grading histology, tumor size, lymph node, ER status, PR status, HER2 status, Ki67 Index, staging, chemotherapy, and radiation.
Quality assessment
The Newcastle-Ottawa Scale, commonly termed NOS, was applied to assess sample selection, comparison, and exposure to establish the quality of the technique in the under consideration studies (Stang, 2010). Using the findings of the NOS calculation, we could divide the articles’ overall quality into two categories: moderate (4-6) and high (7-9). No letters to the editor, comments, case reports, case series, or reviews were included in this publication.
Statistical analysis
In survival analysis, the hazard ratio (HR) was used to conditions characterized by the probability of occurrences, whereas the best-adjusted odds ratio (OR) with a 95% confidence interval (CI) was employed for clinicopathological characteristics. The Egger test analyzed publication bias, and a p<0.05 indicated the probability bias of publication. The Q test was used to investigate whether or not there was any heterogeneity across the studies, and if it was discovered, the random effect model was applied (p<0.10). Using data from trials for which no specific result was provided, we pooled analyses in Figure 4 to reduce misclassification of exposure and to control for publication bias. Pooled of the survival was calculated using random-effects models and clinicopathological characteristics are both random and fixed models (Figure 4). The Z testing was used for the analysis of PD-L1 expression and clinical and pathological status for OS and DFS. A summary of the statistical study was provided in the form of a forest plot. We utilized Review Manager 5.3 from Revman Cochrane in London, United Kingdom, during the investigation.
Figure 4A.
Age Forest Plot
Results
Study eligibility results
The databases yielded a total of 1.013 publications, of which 952 were eliminated due to irrelevant studies. Article eligibility was determined for 23 articles, and 9 papers were eliminated because they did not match the eligibility requirements (Figure 1). Six research from China, four from South Korea, two from Japan, one from Hongkong, and one from Saudi Arabia comprised the final 14 publications included in this manuscript (Table 1).
Figure 1.
A PRISMA Flowchart for Selected Articles
Table 1 displays the features of the studies that have been listed. These studies’ sample sizes ranged from 44 to 1.091 patients. There were a total of 4.929 participants participating in the trials. Five retrospective studies were determined to be suitable for the analysis. In the above investigations, PD-L1 positive rates varied from 13.7% to 57.0%. The HRs and 95% confidence intervals were derived directly from the source papers. All studies evaluated PD-L1 expression using IHC. According to the NOS quality evaluation, the investigations were of moderate to high quality.
Analysis of OS, DFS, and PD-L1 expression
We studied the link between OS, DFS, and PDL1 expression in BCs. All eight trials for OS and six for DFS were considered, with 4.111 and 3.071 participants, respectively. PD-L1 overexpression was linked with the poorer OS than the absence of PD-L1 in individuals diagnosed with BCs (HR = 1.58, 95% CI 1.04–2.40; P=0.03) (Figure 2). Overexpression of PD-L1 was not linked with DFS (HR = 1.35, 95% CI 0.92–1.98; P =0.13) (Figure 3). There was found to be a significant amount of heterogeneity in OS (I2=65%, P<0.01) and DFS (I2=79%, P<0.01). Therefore, a model with random effects was utilized for the investigation.
Figure 2.
A Forest Plot Shows BCs Patients' Overall Survival (OS) Rate and PD-L1 Expression
Figure 3.
Disease Free Survival (DFS) Rate and PD-L1 Expression in BCs Patients are Shown in a Forest Plot
Analysis of clinico and pathological characteristics
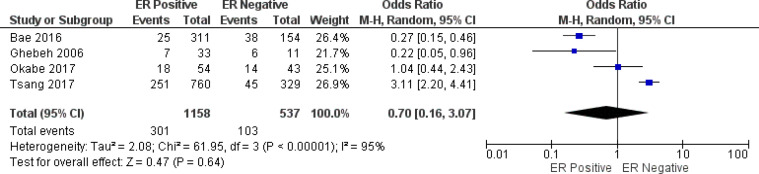
In this work, we analyzed clinicopathological features and PD-L1 expression. PD-L1 was elevated in histological grade III (OR =2.39, 95% CI 1.26 - 4.54; P =0.008), and positive node (OR =0.68, 95% CI 0.48-0.97; P<0.05). PD-L1 overexpression was not linked with age (OR=1.06, 95% CI 0.62-1.82, P=0.83), size of tumor (OR=0.97, 95% CI 0.55-4.07, P=1.70), ER (OR =0.70, 95% CI 0.16-3.07; P=0.64), PR (OR=0.63, 95% CI 0.12-3.15; P=0.57), HER2 (OR=1.45, 95% CI 0.69-3.07; P = 0.33), Ki67 index (OR=3.02, 95% CI 0.11-83.45; P=0.51), staging tumor (OR=1.18, 95% CI 0.89-1.57; P = 0.24), chemotherapy status (OR=0.66, 95% CI 0.39-1.11; P = 0.12), and radiotherapy status (OR =3.86, 95% CI 0.35-42.31; P = 0.27) (Figure 4). During the course of the investigation of the node variable (P = 0.36; I2=6%), staging (P=0.10, I2 =42%), and chemotherapy (P = 0.61; I2= 0 %), heterogeneity was not identified. Thus, a model with a fixed effect was adopted. The remainder of the analyses were carried out using the random effects model.
Heterogeneity and bias potential across studied
Data evaluating OS, DFS, PD-L1, and selected clinicopathological parameters revealed heterogeneity. This study adopted the random effect model, whereas the fixed effect model was applied to investigate the PD-L1 and positive node status, staging, also treatment status. According to Egger’s tests, publication bias did not impact the HR for OS and DFS in the included studies. These studies yielded respective P values of 0.74 and 0.39.
Figure 4B.
Grade Forest Plot
Figure 4C.
Tumor Forest Plot
Figure 4D.
Node Forest Plot
Figure 4E.
ER Forest Plot
Figure 4E.
ER Forest Plot
Figure 4G.
HER2 Forest Plot
Figure 4H.
Ki67 Forest Plot
Figure 4I.
Stadium Forest Plot
Figure 4J.
Chemotherapy Forest Plot
Figure 4K.
Radiotherapy Forest Plot
Table 1.
Characteristics of the Articles Considered in Our Baseline Research
| Author | Year | Country | Number of Patients | Age, median (range) | IHC method | Antibody | PD-L1 positive | Follow-up Median (range) |
Quality Assessment (score) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Company | Source | Type | Clone | Cut-off | |||||||||
| Li (Li et al., 2016) | 2016 | China | 501 | 53 (29-83) | H-score | Abcam, UK | Rabbit | PAB | ab58810 | >100 | 231/501 (46.1) | 64 (1-80) | 7 |
| Park (Park et al., 2016) | 2016 | South Korea | 333 | 47 (28-78) | H-score | Abcam, UK | Rabbit | PAB | NA | >3 | 163/316 (51.6) | 118 (5-154) | 6 |
| Qin (Qin et al., 2015) | 2015 | China | 870 | 47 (21-84) | Percentage | Beverly, USA | Rabbit | MAB | NA | >5% | 189/870 (21.7) | 98 (17-265) | 6 |
| AiErken (AiErken et al., 2017) | 2017 | China | 215 | 49 (27-78) | Percentage | Beverly, USA | Rabbit | PAB | NA | >50% | 70/215 (32.6) | 68 (7-159) | 8 |
| Bae (Bae et al., 2016) | 2016 | South Korea | 465 | mean. 52.3 (24-81) | H-score | Beverly, USA | Rabbit | MAB | E1L3N | >100 | 63/465 (13.5) | 41 (1-158) | 7 |
| Choi (Choi et al., 2018) | 2018 | South Korea | 539 | 50 (24-79) | Percentage | Abcam, UK | Rabbit | PAB | NA | >5% | 117/539 (21.7) | 53 (4-135) | 6 |
| Ghebeh (Ghebeh et al., 2006) | 2006 | Saudi Arabia | 44 | 45 | Percentage | Dako Corp.. | Rabbit | PAB | MIH1 | >5% | 13/44 (29.5) | NA | 6 |
| Guo (Guo et al., 2016) | 2016 | China | 183 | 50 | Percentage | Tucson, AZ | Rabbit | MAB | SP142 | >10% | 25/183 (13.7) | 76.4 | 7 |
| Kim (Kim et al., 2017) | 2017 | South Korea | 167 | (23-85) | Allred score | Canvers, USA | Rabbit | MAB | E1L3N | the sum of the 2 scores | 81/167 (48.5) | 1157 (43-1748) | 8 |
| Li F (Li, Ren and Wang, 2018) | 2018 | China | 112 | 60 | Percentage | Abcam, UK | Rabbit | PAB | NA | >50% | 22/112 (19.6) | NA | 8 |
| Lou (Lou et al., 2017) | 2017 | China | 64 | 55 | Percentage | NA | NA | NA | NA | >50% | 24/64 (37.5) | NA | 7 |
| Mori (Mori et al., 2017) | 2017 | Japan | 248 | mean. 57.4 (32-84) | Percentage | Beverly, USA | Rabbit | MAB | E1L3N | >50% | 103/248 (41.5) | 68 (2-150) | 7 |
| Okabe (Okabe et al., 2017) | 2017 | Japan | 97 | 58 (27-84) | H-score | Abcam, USA | Rabbit | MAB | EPR1161(2) | >100 | 32/97 (57) | 88.9 | 6 |
| Tsang (Tsang et al., 2017) | 2017 | Hongkong | 1091 | mean. 54.5 (22-94) | Percentage | NA | NA | NA | NA | >5% | 295/1091 (27) | 63 (1-120) | 7 |
Note: IHC, immunohistochemistry; PD-L1, programmed death ligand 1; UK, United Kingdom; USA, United States of America; AZ, Arizona; NA, not available; PAB, polyclonal antibody; MAB, monoclonal antibody
Table 2.
Summary of Breast Cancer Patient Characteristics
| Characteristic | Variable | Number of Studies | Model | Breast Cancer Total Sample |
Value (%) | OR | 95% CI | p |
|---|---|---|---|---|---|---|---|---|
| Age | 5 | 1.06 | 0.62 - 1.82 | 0.83 | ||||
| >50 | Random | 724 | 212 (29.2%) | |||||
| <=50 | Random | 639 | 176 (27.5%) | |||||
| Grade | 10 | 2.39 | 1.26 - 4.54 | 0.008 | ||||
| III | Random | 1725 | 529 (30.6%) | |||||
| I-II | Random | 1977 | 512 (25.8%) | |||||
| Tumor Size | 8 | 0.97 | 0.55 - 1.70 | 0.91 | ||||
| >2 cm | Random | 911 | 355 (38.9%) | |||||
| <= 2 cm | Random | 873 | 320 (36.6%) | |||||
| Nodal Status | 4 | 0.68 | 0.48 - 0.97 | 0.03 | ||||
| Node Positive | Fixed | 338 | 67 (19.8%) | |||||
| Node Negative | Fixed | 502 | 122 (24.3%) | |||||
| ER Status | 4 | 0.70 | 0.16 - 3.07 | 0.64 | ||||
| ER Positive | Random | 1158 | 301 (25.9%) | |||||
| ER Negative | Random | 537 | 103 (19.1%) | |||||
| PR Status | 3 | 0.63 | 0.12 - 3.15 | 0.57 | ||||
| PR Positive | Random | 793 | 223 (28.1%) | |||||
| PR Negative | Random | 798 | 148 (18.5%) | |||||
| HER-2 Status | 4 | 1.45 | 0.69 - 3.07 | 0.33 | ||||
| HER-2 Positive | Random | 311 | 79 (25.4%) | |||||
| HER-2 Negative | Random | 1382 | 325 (23.5%) | |||||
| Ki67 Index | 2 | 3.02 | 0.11 - 83.45 | 0.51 | ||||
| >14% | Random | 649 | 148 (22.8%) | |||||
| <=14% | Random | 379 | 43 (11.3%) | |||||
| Stage | 8 | 1.18 | 0.89 - 1.57 | 0.24 | ||||
| Advanced | Fixed | 281 | 94 (33.4%) | |||||
| Early | Fixed | 2180 | 590 (27.0%) | |||||
| Chemotherapy | 2 | 0.66 | 0.39 - 1.11 | 0.12 | ||||
| No | Fixed | 109 | 19 (17.4) | |||||
| Yes | Fixed | 976 | 240 (24.5%) | |||||
| Radiotherapy | 2 | 3.86 | 0.35 - 42.31 | 0.27 | ||||
| No | Random | 198 | 85 (32.8%) | |||||
| Yes | Random | 585 | 29 (4.9%) | |||||
OR, Odd ratio; CI, Confidence interval; ER, Estrogen receptor; PR, Progesteron receptor; HER-2, Human epidermal growth factor-2
Discussion
There is evidence that both PD-1 and both of its ligands, PD-L1 and PD-L2, are present in the tumor microenvironment, maintaining a balance between activation, immunopathology, and T-cell tolerance in prolonged periods of antigen exposure. Under certain conditions, cancer cells can avoid monitoring the immune system mediated by increased PD-L1 expression. On tumor cells, PD-L1 reaches out and attaches to its receptor tumor-specific T lymphocytes, which results in PD-1/PD-L1 interactions. Due of this interaction, the proliferation and migration of T cells are slowed down, and cytotoxic mediators release. Additionally, this interaction stimulates the death of tumor-specific T cells and the development of CD4+ T cells into FOXP3+ CD4+ regulatory T cells. This cascade of events disrupts the function of T cells, as a direct consequence, a decreased anti-tumor immune response (Wang et al., 2017; Carlsson et al., 2020).
It is assumed that increased PD-L1 expression in BCs results either from the dynamic production of IFN during the anti-tumor immune response or from the activation of PD-L1 gene expression of many related oncogenes, but the precise mechanism for this is not yet known. In the solid tumor, PD-L1 overexpression has been related to multiple genetic changes, including the amplification of 9p24.1, the site of PD-L1. Due to the rarity of PD-L1 copy number alterations, this does not appear to be the reason for elevated PD-L1 expression in BCs. In addition, it is known that disturbances in signaling systems, such as hyperactivation of the PI3K pathway, can also increase PD-L1 expression. According to an earlier study, AKT phosphorylation and PI3K pathway activation have been linked with high PD-L1 due to PTEN loss. High PD-L1 is particularly found in the luminal subtype, which can also be connected to PIK3CA mutations (Tsang et al., 2017).
Numerous studies have been conducted to establish if high PD-L1 is linked with the worst prognosis or histological characteristics. However, there has been a lack of consistency in the outcomes. A comprehensive study of BCs patients was performed for the meta-analysis. High PD-L1 was linked with lowering OS. Nonetheless, this PD-L1 overexpression did not affect the DFS value of BCs patients. It is correlated with reduced TIL activity in patients with BCs overexpress PD-L1. PD-L1 promotes tumor development by decreasing the amount of PD-1 TIL by aggressively blocking the immune system’s response to tumor antigens (Tsang et al., 2017). Similar patterns are observed in ovarian, lung, stomach, and kidney (renal cell carcinoma) cancers (Bae et al., 2016).
PD-L1 expression was elevated in individuals with high histological grades, according to the findings of this investigation, as well as positive lymph node status. There was no connection between PD-L1 overexpression and age, tumor size, estrogen receptors, progesterone receptors, HER2 expression, Ki67 index, tumor stage, chemotherapy, or radiation in this investigation (Rizka et al., 2021; Wihandani et al., 2021). A substantial correlation between high PD-L1, high histological grade and the presence of lymph node metastases was consistent with decreased patient survival. It is because high-grade BCs patients typically exhibit rapid proliferation and poor differentiation (Ghebeh et al., 2006; Kartini et al., 2023)
This research has many drawbacks. It will alter the variety of research subjects represented by the data comprised in the meta-analysis. Second, this meta-analysis did not assess the expression depending on BCs subtypes, so the influence remains unclear. In conclusion, overexpression of PD-L1 was associated with a lowered OS in BCs patients. PDL1 expression was higher in persons with nodal positivity and histological grade III. However, additional it is necessary to conduct prospective research to provide further evidence and corroborate the findings of the current study conclusions.
Author Contribution Statement
Conceptual: PATA, IWS, DMW. Design: SW. Control/supervision: PATA, IGPS. Data collection/processing: SW, IPGSS. Extraction/analysis/interpretation: SW, PATA, IGPS. Literature review: IWS, DMW, IGPS. Writing the article: SW, IPGSS. Critical review: PATA, IWS, DMW, SW, IPGSS, IGPS. Everyone who contributed to the project has reviewed the final manuscript.
Acknowledgements
General
We would like to express our gratitude to our warm family of the RISEarch Oncology group for their steadfast support and encouragement throughout the authoring of this article.
Ethical Declaration
This research does not require ethical approval as a meta-analysis.
Data Availability
This work incorporates data previously published by other authors, with all data included in the findings section.
Study Registration
This review’s protocol was filed with the International Prospective Register of Systematic Reviews (PROSPERO), with the registration number CRD42023391913, and the paper was produced in accordance with PRISMA principles.
Conflict of Interest
There was no disclosure of any potential conflicts of interest that existed.
References
- AiErken NJ, Shi HJ, Zho Y, et al. High PD-L1 expression is closely associated with tumor-infiltrating lymphocytes and leads to good clinical outcomes in Chinese triple negative breast cancer patients. Int J Biol Sci. 2017;13:1172–9. doi: 10.7150/ijbs.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali HR, Glont SE, Blows FM, et al. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol. 2015;26:1488–93. doi: 10.1093/annonc/mdv192. [DOI] [PubMed] [Google Scholar]
- Alves AM, Paredes J, Schmitt F. Expression of PD-L1 in primary breast carcinoma and lymph node metastases. Surg Exp Pathol. 2019;2:1–6. [Google Scholar]
- Bae SB, Cho HD, Oh MH, et al. Expression of programmed death receptor ligand 1 with high tumor-infiltrating lymphocytes is associated with better prognosis in breast cancer. J Breast Canc. 2016;19:242–51. doi: 10.4048/jbc.2016.19.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista MZ, Sarian LO, Derchain SFM, et al. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol. 2016;47:78–84. doi: 10.1016/j.humpath.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Beckers RK, Selinger CL, Vilain R, et al. Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology. 2016;69:25–34. doi: 10.1111/his.12904. [DOI] [PubMed] [Google Scholar]
- Carlsson J, Sundqvist P, Kosuta V, et al. PD-L1 expression is associated with poor prognosis in renal cell carcinoma. Appl Immunohistochem Mol Morphol. 2020;28:213–20. doi: 10.1097/PAI.0000000000000766. [DOI] [PubMed] [Google Scholar]
- Cha JH, Chan LC, Li CW, et al. Mechanisms Controlling PD-L1 Expression in Cancer. Mol Cell. 2019;76:359–70. doi: 10.1016/j.molcel.2019.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Chang JS, Koo JS, et al. Differential prognostic impact of strong PD-L1 Expression and 18 F-FDG uptake in triple-negative breast cancer. Am J Clin Oncol-Canc. 2018;41:1049–57. doi: 10.1097/COC.0000000000000426. [DOI] [PubMed] [Google Scholar]
- Cirqueira MB, Mendonca CR, Noll M, et al. Prognostic role of pd-l1 expression in invasive breast cancer: A systematic review and meta-analysis. Cancers. 2021;13:1–20. doi: 10.3390/cancers13236090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghebeh H, Mohammed S, Al-Omair A, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: Correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–8. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M, Raina V. Epidemiology, screening and diagnosis of breast cancer in the Asia-Pacific region: Current perspectives and important considerations. Asia-Pac J Clin Onco. 2008;4:5–13. [Google Scholar]
- Guo L, Li W, Zhu X, et al. PD-L1 expression and CD274 gene alteration in triple-negative breast cancer: implication for prognostic biomarker. SpringerPlus. 2016;5:1–8. doi: 10.1186/s40064-016-2513-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst Rev. 2022;18:e1230. doi: 10.1002/cl2.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubair WK, Hendrickson JD, Severs EL, et al. Modulation of Inflammatory Arthritis in Mice by Gut Microbiota Through Mucosal Inflammation and Autoantibody Generation. Arthritis Rheumatol. 2018;70:1220–33. doi: 10.1002/art.40490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed A, Arguello D, Johnston C, et al. PD-L1 expression in tumor metastasis is different between uveal melanoma and cutaneous melanoma. Immunotherapy. 2017;9:1323–30. doi: 10.2217/imt-2017-0066. [DOI] [PubMed] [Google Scholar]
- Kartini D, Panigoro S, Alam IA, et al. Fibroblast growth factor 2 expression on lymph node metastasis in early-stage breast cancer. Bali Med J. 2023;12:621–5. [Google Scholar]
- Kim A, Lee SJ, Kim YK, et al. Programmed death-ligand 1 (PD-L1) expression in tumour cell and tumour infiltrating lymphocytes of HER2-positive breast cancer and its prognostic value. Sci Rep-UK. 2017;7:1–10. doi: 10.1038/s41598-017-11905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong SPL, Shen ZZ, Liu TJ, et al. Is Breast cancer the same disease in Asian and Western countries? World J Surg. 2010;34:2308–24. doi: 10.1007/s00268-010-0683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Ren Y, Wang Z. Programmed death 1 Ligand 1 expression in breast cancer and its association with patients’ clinical parameters. J Canc Res Ther. 2018;14:150–4. doi: 10.4103/jcrt.JCRT_602_17. [DOI] [PubMed] [Google Scholar]
- Li Z, Dong P, Ren M, et al. PD-L1 expression is associated with tumor FOXP3+regulatory T-cell infiltration of breast cancer and poor prognosis of patient. J Cancer. 2016;7:784–93. doi: 10.7150/jca.14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YX, Lim ZL, Ho PJ, et al. Breast Cancer in Asia: Incidence, Mortality, Early Detection, Mammography Programs, and Risk-Based Screening Initiatives. Cancers. 2022;14:4218. doi: 10.3390/cancers14174218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou J, Zhou Y, Huang J, et al. Relationship between PD-L1 expression and clinical characteristics in patients with breast invasive ductal carcinoma. Open Med-Warsaw. 2017;12:288–92. doi: 10.1515/med-2017-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med-Warsaw. 2009;3:e123–30. [PMC free article] [PubMed] [Google Scholar]
- Mori H, Kubo M, Yamaguchi R, et al. The combination of PD-L1 expression and decreased tumorinfiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer. Oncotarget. 2017;8:15584–92. doi: 10.18632/oncotarget.14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe M, Toh U, Iwakuma N, et al. Predictive factors of the tumor immunological microenvironment for long-term follow-up in early stage breast cancer. Cancer Sci. 2017;108:81–90. doi: 10.1111/cas.13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Kong SY, Ro JY, et al. Prognostic Implications of Tumor-Infiltrating Lymphocytes in Association with Programmed Death Ligand 1 Expression in Early-Stage Breast Cancer. Clin Breast Cancer. 2016;16:51–8. doi: 10.1016/j.clbc.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Qin T, Zeng Y, Qin G, et al. High PD-L1 expression was associated with poor prognosis in 870 Chinese patients with breast cancer. Oncotarget. 2015;6:33972–81. doi: 10.18632/oncotarget.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizka A, Purwanto H, Budianto MB, Rohman MS. Role of angiotensin-converting enzyme inhibitors on changes in troponin levels in breast cancer with anthracycline chemotherapy. Bali Med J. 2021;10:728–32. [Google Scholar]
- Schildberg FA, Klein SR, Freeman GJ, et al. Coinhibitory Pathways in the B7-CD28 Ligand-Receptor Family. Immunity. 2016;44:955–72. doi: 10.1016/j.immuni.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Tsang JYS, Au WL, Lo KY, et al. PD-L1 expression and tumor infiltrating PD-1+ lymphocytes associated with outcome in HER2+ breast cancer patients. Breast Cancer Res Tr. 2017;162:19–30. doi: 10.1007/s10549-016-4095-2. [DOI] [PubMed] [Google Scholar]
- Wang J, Yu M, Guan S, et al. Prognostic significance of microRNA-100 in solid tumors: An updated meta-analysis. Oncotargets Ther. 2017;10:493–502. doi: 10.2147/OTT.S122774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Estimated Age-Standardized Incidence Rates (World) in 2020, Breast, Females, All Ages, Asia. GLOBOCAN. 2021: 721–62. [Google Scholar]
- Wihandani DM, Saputra IPGS, Remitha NPSI, et al. The potential effect and delivery of piperine on chemoresistant breast cancer. Bali Med J. 2021;10:608–16. [Google Scholar]
- Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy. Sci Transl Med. 2016;8:1–34. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This work incorporates data previously published by other authors, with all data included in the findings section.