Abstract
Background:
Triple negative breast cancer cells (TNBC) are a small part of cancer-inducing cells in breast cancer, which are characterized by high metastatic and self-renewal. Self-renewal has the ability to renew itself and loses control of proliferation. Curcuma longa extract (CL) and Phyllanthus niruri extract (PN) known to have anti-proliferative effects on cancer cells. However, the effects of combination CL and PN on TNBC proliferation still unclear.
Aims:
This study aimed to evaluate the antiproliferative effects of the combination CL and PN on TNBC MDAMB-231 and attempted to elucidate the underlying molecular mechanisms.
Subjects and Methods:
The dried rhizomes of Curcuma longa and the herbs of Phyllanthus niruri were macerated with ethanol for 72 h.The antiproliferative and synergistic effects of combination CL and PN were investigated using 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay. Combination index values were calculated using CompuSyn (ComboSyn, Inc, Paramus, NJ). The cell cycle and apoptosis assay were determined by propidium iodide (PI) and PI-AnnexinV assay under flow cytometer, respectively. The intracellular ROS levels were evaluated using 2′,7′-Dichlorodihydrofluorescein diacetate (DCFDA) assay. The mRNA expressions of proliferation-related genes in the cells were determined using bioinformatic assay.
Results:
The CL and PN single treatment caused a potent and dose-dependent decrease in the percentage of viable cells with IC50 value of 13 μg/mL and 45 μg/mL for 24 h, respectively. The combination index values of the different combinations ranged from 0.08 – 0.90, indicating slightly strong to very strong synergistic effects. The combination of CL and PN also remarkably induced the S- and G2/M-phases cell cycle arrest that leading to apoptosis induction. Furthermore, the combination of CL and PN treatment induced the intracellular reactive oxygen species (ROS) levels. Mechanistically, the AKT1, EP300, STAT3 and EGFR signaling as potential targets of combination CL and PN in antiproliferation and antimetastatic of TNBC.
Conclusions:
The combination of CL and PN exerted promising antiproliferative effects in TNBC. Therefore, CL and PN may be considered a potential source for the development of potent anticancer drugs for breast cancer treatment.
Key Words: Curcuma longa, Phyllanthus niruri, antiproliferation, apoptosis, antimetastatic
Introduction
Breast cancer is one of the most devastating diseases globally and one of the leading causes of death in women worldwide (Kunnumakkara et al., 2008). Approximately 16% of all breast cancer patient are diagnosed with triple negative breast cancer (TNBC) (Zawawy and Khedr, 2022). TNBC is typically associated with a highly metastasis, invasive, and could avoid cell death through apoptosis resistance mechanism (Hanifa et al., 2022). Due to is heterogenicity and lack of defined molecular target, treatment of TNBC remains challenging (Bianchini et al., 2016; Yang et al., 2022). Epidermal growth factor receptor (EGFR), proto-oncogene that enhances cell proliferation and survival (Wee and Wang, 2017). Previous study reported the invasion, metastasis, and proliferation of TNBC cells induced by modulating EGFR (Song et al., 2020a). The mutant EGFR could activate signal transducer and activator of transcription -3 (STAT3) pathway via interleukin-6 upregulation in primary human lung cancer leading to cancer progression (Gao et al., 2007; Wang et al., 2013). On the other hand, activated STAT3 signaling that contributes to the pathogenesis of breast cancer through the prevention of apoptosis (Qin et al., 2019). The recent evidence from preclinical and clinical research have showed that STAT3 plays a critical role in TNBC and STAT3 regulator have shown efficacy in inhibiting TNBC tumor growth and metastasis (Abdelhamed et al., 2016). Furthermore, E1A-associated protein p300 (EP300) has been implicated in TNBC to promoting tumor growth, metastatic potential, and cancer stem cells phenotype leading to apoptosis resistance (Ring et al., 2020; Li et al., 2022). Previous study also reported that mutated EP300 has been promoted cell proliferation and escape the immune checkpoint through programmed death ligand-1 (PD-L1) regulation and miRNA MIR17 (Yeh et al., 2021a). In TNBC cells, the serine/threonine kinase-1 (AKT1) also involved in proliferation and suppressing apoptosis (Martorana et al., 2021). Upregulated STAT3 induced AKT1 pathway through transcription factor EB (TFEB) expression promote cells migration and invasion by upregulation of matrix mettaloproteinase-9 (MMP-9) and -2 (MMP-2) protein expression (Ma et al., 2020). The emerging data suggest that targeting these molecules may be a potential molecular target and biomarker for TNBC.
Indonesian medicinal herbs shows a promising anti-cancer properties, including Curcuma longa and Phyllanthus niruri due to their capability to induce cancer apoptosis (Kunwar et al., 2008; Tang et al., 2010; de Araújo Júnior et al., 2012; Tomeh et al., 2019). Curcuma longa extract is rich in secondary metabolites including flavonoid, terpenoid, alkaloids, and steroid saponins that exhibit anticancer activity on several cancer cells (Shafabakhsh et al., 2019; Hermansyah et al., 2021; Paramita et al., 2022). Previous studies reported that Curcuma longa extract (CL) may increase the regulation of multiple signaling pathways to involve metastasis inhibition in cancer cells including STAT3, JAK, MAPK, and EGFR pathway (Sun et al., 2019; Farghadani and Naidu, 2021). In addition, CL rhizome extract was also reported inhibit metastasis by NF-kB/c-JUN/MMP pathway (Saeed et al., 2022) and targets EGFR in cancer leading to tumor cell killing (Sun et al., 2012; Tajuddin et al., 2019) Curcumin, a major compound derived from CL rhizome in combination with mitomycin significantly induces apoptosis by regulating Bcl-2 family in breast cancer cells (Zhou et al., 2015). Moreover, curcumin overcomes multidrug resistance in various cancer (Roy and Mukherjee, 2014; Gou et al., 2015). We previously showed that Phyllanthus niruri extract (PN) improved the antimetastatic effect of CL extract on MDAMB-231 (Hermansyah et al., 2021). PN possess anticancer activity due to the secondary metabolite’s compounds including flavonoid, alkaloid, terpenoid, and saponin (Jantan et al., 2019). Furthermore, the PN extract performed antitumor activity by regulating regulation of NF-κB, P13K/AKT, and ERK/JNK/P38/MAPKs signaling pathways associated with cell growth, proliferation, metastasis, and apoptotic cell death (Huang et al., 2003; Lee et al., 2011; Tseng et al., 2012; Zheng et al., 2016; Saahene et al., 2021). However, the role of combination therapy CL and PN in TNBC remain largely undefined. The anticancer effect of combination CL-PN and the molecular mechanism to promote apoptosis and inhibit metastasis is required. Therefore, current research trends are focusing on finding potentially therapeutically manageable combinations of herbal compounds, which could significantly help to decrease the risk of metastasis development and disease recurrence. This study focuses on elucidating the mechanism of CL extract when combined with PN extract in TNBC cells under in vitro study and bioinformatic approach. Our findings from this study can be fundamental for the development of combination CL and PN extract as an alternative agent for the inhibition of TNBC metastasis on breast cancer cells.
Materials and Methods
Plant materials
The rhizomes of Curcuma longa and the herbs of Phyllanthus niruri were collected from Tawangmangu, Karanganyar, Central Java, Indonesia (latitude 7°40’39.3” north; longitude 111°08’09.4” E) in November 2019 and February 2020, respectively. These plants were identified and verified by biologists from the Indonesian Medicinal Plants and Traditional Medicine Research and Development Center (B2P2TOOT). For the bioassay, the rhizomes of Curcuma longa and herbs of Phyllanthus niruri were dried and circulated at 40°C, and renewed in an air oven until completely dehydrated.
Extraction methods
Curcuma longa (500g) and Phyllanthus niruri (500g) were washed and dried, and the successively macerated with ethanol 96% (Merck, Darmstadt, Germany) for 72 hours in room temperature 25°C ± 2°C. The macerated was filtered through Whatman No.1 filter paper and evaporated under reduced pressure rotary vacuum evaporator (IKA HB 10 basic) at 40°C and 80 rpm. Curcuma longa extract (CL) 5.11% yield and Phyllanthus niruri (PN) 2.37% yield were stored at -20°C and protected from light.
Phytochemical screening of Curcuma longa and Phyllanthus niruri extract
The CL and PN were tested for the presence of flavonoids, alkaloids, tannins, steroids, terpenoids, and saponins. The qualitative results are expressed as (+) for the presence and (−) for the absence of phytochemicals. The flavonoids were test using Wilstater’s test according to (Fernanda et al., 2019). Briefly, 2 mg of each CL and PN was mixed with HCl (Merck, Darmstadt, Germany) 500µL and 0.02 mg magnesium (Merck, Darmstadt, Germany). The presence of flavonoids is characterized by the occurrence of discoloration. The presence of alkaloids indicated with a brown colored precipitate that determined under Wagner’s test, 15 mg of APE was stirred with 1% HCl (6 mL) on water bath for 5 minutes and filtered. The filtrate was added with few drops of Wagner solution (2 gram of Potassium iodide (Merck, Darmstadt, Germany) and 1,27 g of Iodine (Merck, Darmstadt, Germany) in 95 mL of distilled water) (Y et al., 2016). Furthermore, Tannins content was analysis the CLE and PNE with 1% ferric chloride (Merck, Darmstadt, Germany), the black or blue coloration was taken a positive result of tannins (Sri Sulasmi et al., 2019). Liebermann-Burchard test was used to determine the presence of steroids and terpenoids, briefly 100 mg of CL and PN was shaken with chloroform and added the few drops of acetic anhydride was added to the test tube and boiled in a water bath and rapidly cooled in iced water. Concentrated H2SO4 (Merck, Darmstadt, Germany) (2 mL) was added alongside of the test tube. Formation of a brown ring at the junction of two layers and turning the upper layer to green shows the presence of steroids while formation of deep red color indicates the presence of triterpenoids (Adu et al., 2019). The saponin presence was analysis under Forth’s test, 500 mg of CL and PN was shaken with 10 mL of distilled water. The formation of frothing, which persists on warming in a water bath for 5 min, shows the presence of saponins (Adu et al., 2019).
Cell culture
MDA-MB-231 human breast cancer cell line was obtained from the American Type Culture Collection (#HTB26 ATCC, Manassas, VA, USA). MDAMB-231 is cultured in high glucose Dulbecco’s modified Eagle’s Medium (DMEM) (Gibco, USA) enriched with 10% fetal bovine serum (Gibco, USA), 12.5μg/ml amphotericin B (Gibco, USA), 150μg/ml streptomycin, and 150 IU/ml penicillin (Gibco, USA). The cells were cultured at 37°C and 5% CO2.
Cell viability assay
The effect of CL and PN on MDAMB-231 breast cancer cell proliferation was determined using MTT assay (Ikawati et al., 2020). Briefly, 5x103 cells/well were cultured in 96-well plate for 24 h. Then, the treatment with 0-200 μg/mL of CL or PN was applied for 24, 48, and 72 h. Untreated cells are considered as negative controls (DMEM containing DMSO 0.01%). After the treatment, the medium containing the extracts was replaced by fresh complete medium and 0.5 mg/mL MTT (Sigma-Aldrich) was added for 4h. Then, DMSO was added to dilute formazan crystals, and the OD of the supernatant was measured at λ595 nm under ELISA reader (Biorad iMarkTM Microplate Reader). The IC50 value was calculated through linier regression equation (Y= bX + a). The data for this study was conducted through three replication experiments (Amalina et al., 2021a).
Combination activity
We evaluated the combination effect of CL, PN, and their combination on MDAMB-231 cells under MTT assay (Hermansyah et al., 2021). The cells were treated with one IC50, one-half IC50, one-fourth IC50, and one-eighth IC50 of CL, PN, and combination thereof for 24 h. After 24 h of treatment, the percentage of viable cells was determined using the above in vitro cytotoxicity assay method (Ikawati et al., 2020; Hermansyah et al., 2021). Furthermore, the drug interactions between CL and PN were determined using isobologram analysis and denoted with the combination index under CompuSyn software (ComboSyn Inc, Paramus, New Jersey).
Cell cycle analysis
MDAMB-231 breast cancer cells were cultured in the 6-well plates in the presences of CL and PN in the single and combination treatment for 24 h. After treatment, the cells were incubated with BD Cycletest (BD Biosciences, USA) according to manufacture instructions. Finally, the percentage of cell distribution were determined by flow cytometry (DB Accury C6 plus, BD Biosciences, USA) (Suzery et al., 2020).
Apoptosis analysis
The cells were treated with CL, PN, and the combination in several concentration for 24 h. The quantification of apoptosis cells was measured by Annexin V-PI assay (BD Biosciences, USA). Briefly, after incubation the cells were harvested and incubated with 5μL Annexin V-FITC and 5μL PI (50μg/mL) for 30 min at 4°C in the dark. Finally, the cells were analyzed by flow cytometry (DB Accury C6 plus, BD Biosciences, USA) (Jenie et al., 2019).
ROS levels assay
For all ROS experiments, MDAMB-231 cells were collected by centrifugation, washed with PBS and incubated with 2.5 μM DCFDA (Sigma-Aldrich, USA) in supplemented buffer (10% FBS in PBS) for 30 min in the dark at 37°C. Each cell was treated with CL, PN, and combination thereof then incubated for 4 h in 37°C CO2 5%. Intracellular ROS was determined by flow cytometry (DB Accury C6 plus, BD Biosciences, USA) (Mursiti et al., 2021).
Bioinformatic analysis
We collected the active compounds of CL and PN with anti-breast cancer activity using Dr. Duke’s phytochemical and ethnobotanical databases (https://phytochem.nal.usda.gov/). We obtained curcumin from CL, and limonene and rutin from PN which have anti-cancer effect to be used for further analysis. We used several web tools to acquire the potential target genes for curcumin, limonene, and rutin including Similarity Ensemble Approach (SEA) (https://sea.bkslab.org/), SwissTargetPrediction (http://www.swisstargetprediction.ch/), and STITCH chemical association networks (http://stitch.embl.de/). Gene associated with TNBC proliferation were obtained from PubMed using the key words “Triple negative breast cancer proliferation”. Furthermore, we used Venny 2.1 (https://bioinfogp.cnb.csic.es/tools/venny/) to obtain the overlapping genes between CL target genes, PN target genes, and TNBC proliferation (OG). The protein-protein interaction (PPI) was constructed the network using functional protein association networks (STRING-DB) (https://string-db.org/) with high confidence score than 0.7. Then the network analysis was generated through the latest Cytoscape. According to degree, we ranked the selected genes and chose the top 10 genes analyzed in CytoHubba (Cahyono et al., 2021; Mursiti et al., 2021). We also curated the overall survival (OS) analysis for 10 genes under Kaplan–Meier Plotter database (http://www.kmplot.com/) and selected TCGA based on subtype TNBC. The plot consists of separate patients divided into the high- and low-expression subgroups based on the gene transcriptional expression level of a given gene, the hazard ratio (HR) with the 95% confidence interval, and the log-rank P value were calculated and displayed on the chart. Furthermore, we conducted pathway analysis using the Kyoto Encyclopedia of Genes and Genomes database (KEGG) (www.genome.jp/kegg). Functional enrichment and pathway enrichment analyses were analyzed under the Database for Annotation, Visualization and Integrated Discovery (DAVID) (http://davidd.ncifcrf.gov) (Amalina et al., 2021b). Pvalue was adjusted by the method of BenjaminiHochberg to control the false discovery rate. Enriched GO terms and KEGG pathways were identified as significant with P<0.05 (Tjipta et al., 2022).
Statistical analysis
Statistical analyses were accomplished with software SPSS 26.0 (SPSS Inc., Chicago, IL, USA). All quantitative variables are expressed as mean and standard deviation. The data obtained were collected, compiled, and tested for normality with the Shapiro-Wilk test and homogeneity test with the Lavene test. Data analysis used one-way ANOVA and a least significant difference [LSD] comparison post hoc test p value <0.05 indicated statistical significance.
Results
Phytochemical screening of Curcuma longa extract and Phyllanthus niruri extract
The phytochemical screening of crude ethanolic extract of Curcuma longa extract and Phyllanthus niruri extract revealed the presence of some secondary metabolites such as alkaloids, tannins, flavonoids, terpenoids, saponins, and steroids (Table 1). However, CL does not contain saponins.
Table 1.
Phytochemical Screening of Secondary Metabolites from Curcuma Longa Extract and Phyllanthus Niruri Extract
| Chemical componenet |
Name of the test | CL | PN |
|---|---|---|---|
| Alkaloids | Wagner test | + | + |
| Flavonoids | Wilstater test | + | + |
| Tannins | Braemer’s test | + | + |
| Saponins | Forth test | - | + |
| Steroids | Lieberman Burchardt test | + | + |
| Terpenoids | Lieberman Burchardt test | + | + |
Anti-proliferative effects of single treatment Curcuma longa extract and Phyllanthus niruri extract on MDAMB-231 cells
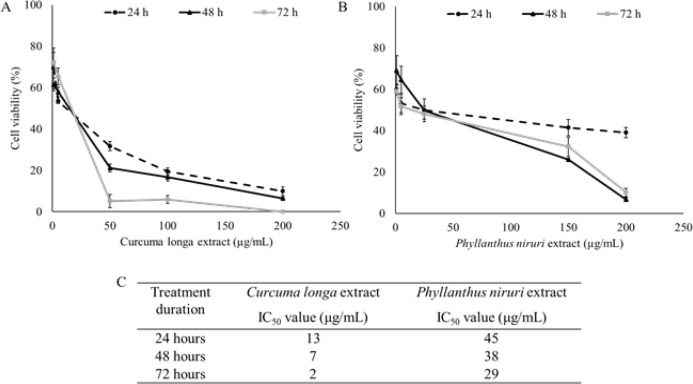
The anti-proliferative activities of CL and PN in the TNBC MDAMB-231 cells were used MTT assay. CL caused a significant dose-and time-dependent reduction in the number of colonies compared to that of the untreated cells (Figure 1A). A similar pattern was found in the PNE antiproliferative effect (Figure 1B). After 24, 48, and 72 h incubations, IC50 value were calculated respectively, for CL on MDAMB-231 cells to be 13, 7, and 2 μg/mL, for PNE to be 45, 38, and 29 μg/mL (Figure 1C). The cytotoxic effect of CL was found higher than PNE in TNBC cells.
Figure 1.
Percentage of MDAMB-231 Cells Survival after 24, 48, and 72 h after Treatment with (A) CL and (B) PN with Several Concentration 0-200 μg/mL. (C) IC50 value of CL and PN in several treatment duration. The results are presented as the mean ± standard deviation for n = 3, p < 0.05.
Synergistic effect of CL and PN on MDAMB-231 cells
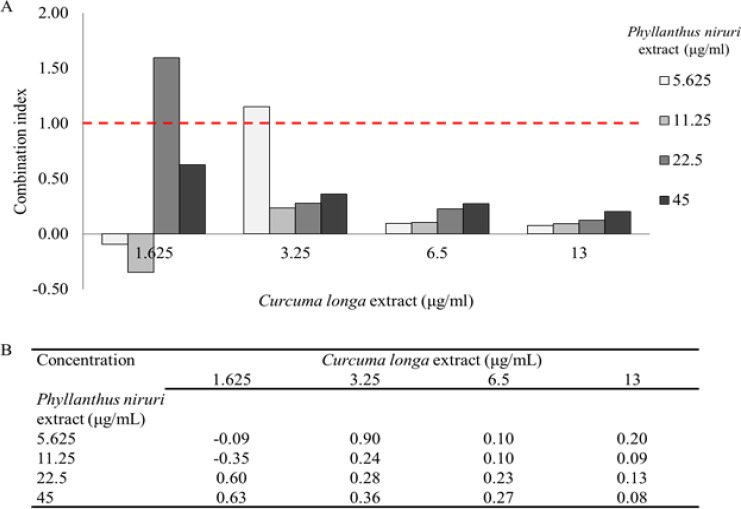
MDAMB-231 cells were treated with several concentration of CL and PN in single and combination for 24 h to investigate the combined effect. The IC50 value obtained after cytotoxic single treatment were used to evaluate their concentration on CL and PN combinations. The concentration used were calculated as one, one-half, one-fourth, one-eighth IC50. The combining CL and PN enhanced their anticancer effect against MDAMB-231. In comparison with CL and PN in single treatment, this combination resulted in greater efficacy in inducing antiproliferative of MDAMB-231 cells (Figure 2A). Furthermore, the combination index value of CL and PN under CompuSyn calculation showed slightly strong to very strong synergistic effects with combination index values ranged from 0.08 – 0.90 (Figure 2B).
Figure 2.
Combination Efect of CL and PN on MDAMB-231 Cells. (A) The combination index plot in several concentration and (B) combination index value of CL and PN calculated using CompuSyn. The results are presented as the mean ± standard deviation for n = 3, p < 0.05
Effect of CL and PN on cell cycle distribution
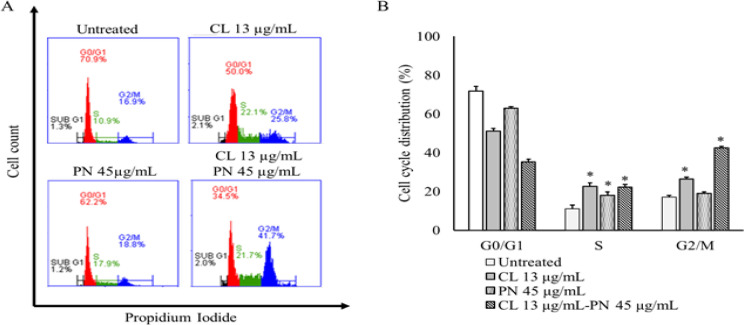
To investigate the factors contributing to the growth inhibition of MDAMB-231 cells, we analyzed the effect of CL and PN on cell cycle distribution under single and combination treatment in several concentration for 24 h. The flow cytometry results showed the single treatment CL 13 μg/mL and PN 45 μg/mL induce S- and G2/M-phases cell cycle arrest (Figure 3A). The average proportion of S phase and G2/M phases after CL 13 μg/mL treatment was 22.57 ± 1.80% and 26.35 ± 1.10%, respectively. In addition, the percentage of S phase and G2/M phases induced by PN lower than CL was 18.09 ± 1.65% and 19.00 ± 0.86%, respectively. Moreover, the percentage of cells in the G2/M phase significantly increase in a combination treatment of CL 13 μg/mL and PN 45 μg/mL up to 42.59 ± 0.74% (Figure 3B). The results suggest that the combination treatment of CL and PN blocks MDAMB-231 cells in G0/G1 phase and increases the percentage of cells in the G2/M phase.
Figure 3.
CL and PN Induced Cell Cycle Arrest on MDAMB-231 Cells. (A) Flow cytometry analysis of percentage cell in each phase of cell cycle for the indicated treatment. (B) Percentage of cell cycle distribution of CL or PN in single and combination treatment for 24 h. The results are presented as the mean ± standard deviation for n = 3, p < 0.05
Effect of CL and PN on apoptosis induction
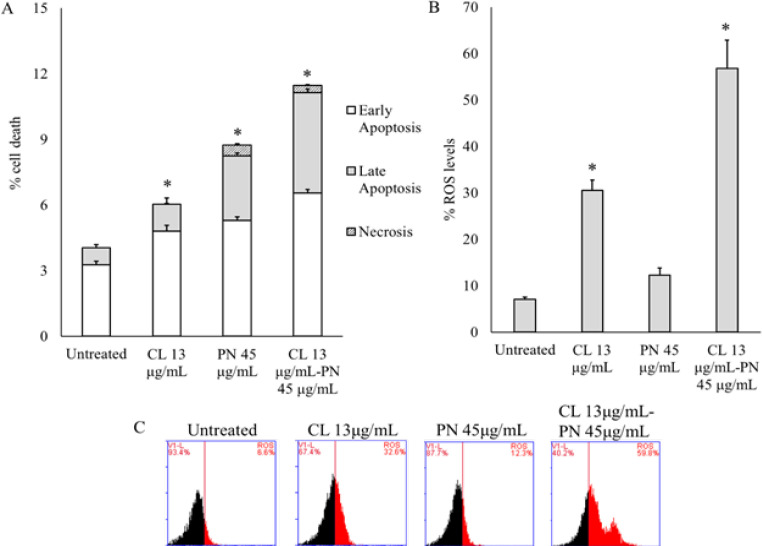
To determine the effect of CL or PN alone and combination on apoptosis induction was onbserved via Annexin V-PI flow cytometry assay. The presence of CL and PN was significantly induced apoptosis up to 6.03 ± 0.26% and 8.73 ± 0.15%, respectively for 24 h. Interstingly, the combination treatment was shown to induce apoptosis at a higher rate than alone treatment up to 11.47 ± 0.18% (Figure 4A). To clarify the molecular mechanisms underlying combination CL and PN-induced apoptosis, we analysed the intracellular ROS levels.
Figure 4.
CL and PN Induce Cell Apoptosis through Increasing Intracellular ROS Level. (A) Percentage of apoptosis cells were detected with Annexin V/PI staining by flow cytometry analysis. (B) Percentage of intracellular ROS levels were (C) analysed with DCFDA flow cytometry assay. The results are presented as the mean ± standard deviation for n = 3, p < 0.05
CL and PN induce the intracellular ROS levels in MDAMB-231 cells
The ROS is essential for most cellular activities and survival (Weinberg et al., 2019). The role of induction ROS levels in CL and PN-induces TNBC cancer cell apoptosis was further investigated. We found that CL significantly increased the ROS levels, but PN doesn’t (Figure 4B). However, the combination therapy strongly increased intracellular ROS levels up to 56.80 ± 6.08% (Figure 4C).
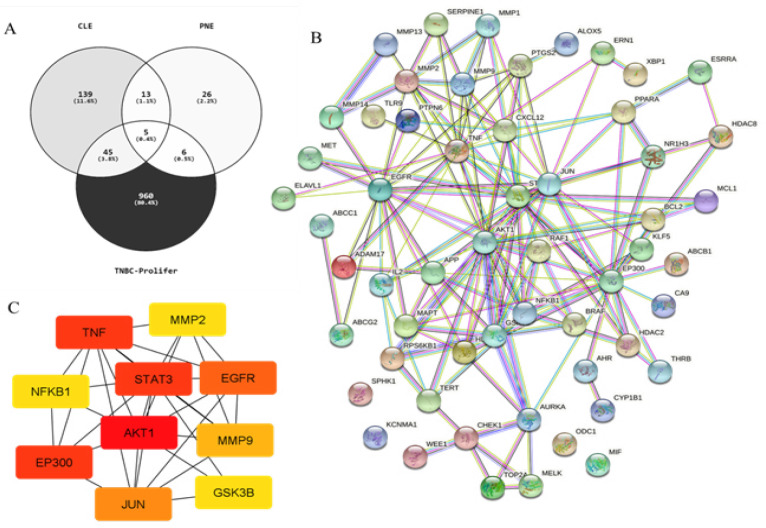
Bioinformatic analysis
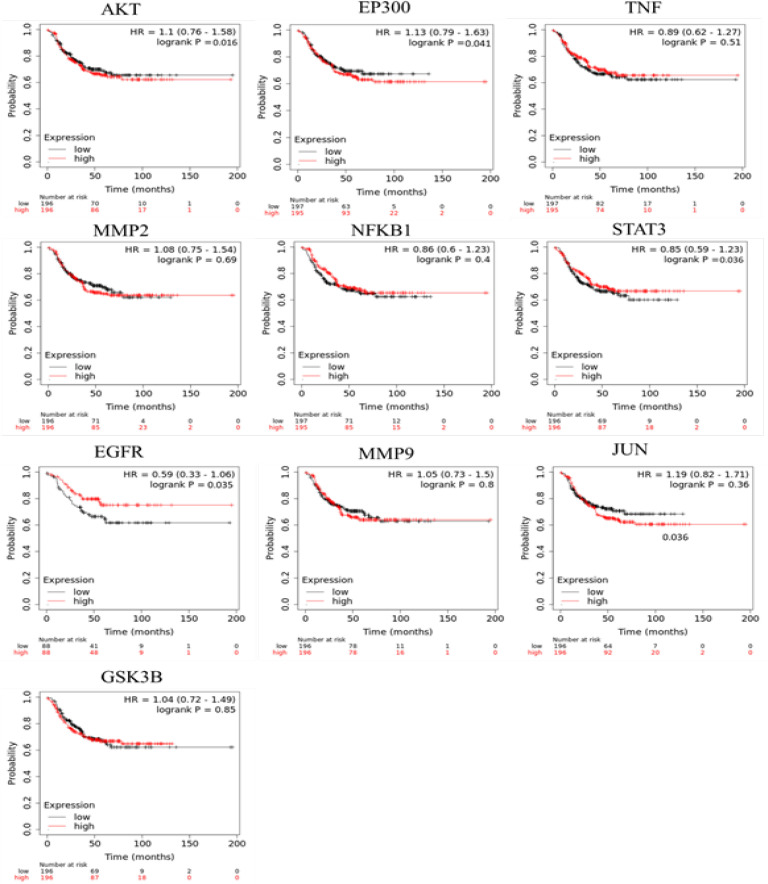
We screened out a total of 206 genes and 50 genes were encoded as a direct protein target of CL (curcumin) and PN (limonene and rutin) (DPT), respectively (Supplementary Data 1). A PubMed generated 1016 genes associated with triple negative breast cancer proliferation (TNBC-Proliferation) (Supplementary Data 2). Furthermore, under Venny 2.1 we analysis the overlapping genes and the gene list from PubMed generated 56 genes that were regulated by DPT and were related with TNBC proliferation (Figure 5A) (Supplementary Data 3). Protein-protein interaction (PPI) network investigated the systemic interaction between the overlapping gene we got above. Fifty-six the overlapping genes (DEG) in total ere mapped to the DEG PPI network with 56 nodes and 149 edges (Figure 5B). And the Cytoscape CytoHubba was applied for further analysis of DEG in PPI network, and we got a result of 10 particular nodes being identified which were all up-regulated DEG (Figure 5C). To Investigate the survival data of 10 genes were identified, K-M plotter indicated that four (AKT, EP300, STAT3 and EGFR) of them had a significant survival rate while other 6 genes had not (P<0.05) (Figure 6). For estrogen receptor (ER) negative, progesterone receptor (PR) negative, and HER negative subtypes 2168, 1989, and 6262 samples are used respectively. The results also show of the survival analysis with the smallest log-rank p-value of gene for each subtype. The p-values of AKT, EP300, STAT3, and EGFR are less than 0.05. This indicates that these genes can be used as potential prognostic markers of TNBC subtypes.
Figure 5.
Common DEG PPI Network of Overlapping Genes Constructed by STRING Online Database and Cytoscape Analysis. (A) Overlapping genes analysis by venny 2.1. (B) Node meant protein; the edge meant the interaction of protein. (C) Degree score analysis via Cytoscape CytoHubba tool
Figure 6.
Survival Analysis (Kaplan-Meier plots) of up-Regulated DEG. biomarkers high values is shown in red and low values are shown in black
KEGG pathway enrichment analysis was performed by using DAVID. There were 56 pathways obtained and 36 of them were statistically significant (P<0.05). The top nine important pathways involved in EGFR tyrosine kinase inhibitor resistance, PD-L1 expression and PD-1 checkpoint pathway cancer, ErbB signaling pathway, Notch signaling pathway, NF-kappa B signaling pathway, cell cycle, P53 signaling pathway, focal adhesion, and regulation of actin cytoskeleton (Table 2).
Table 2.
KEGG Analysis of DEG
| Pathway ID | Name | Count | p-value | Genes |
|---|---|---|---|---|
| 1521 | EGFR tyrosine kinase inhibitor resistance | 9 | 3.10E-10 | MET, EGFR, RPS6KB1, GSK3B, AKT1, STAT3, RAF1, BRAF, BCL2 |
| 5235 | PD-L1 expression and PD-1 checkpoint pathway cancer | 9 | 6.34E-10 | AKT1, STAT3, PTPN6, JUN, EGFR, RAF1, TRL9, NFKB1, RPS6KB1 |
| 4012 | ErbB signaling pathway | 7 | 8.41E-08 | EGFR, AKT1, RPS6KB1, GSK3B, BRAF, RAF1, JUN |
| 4330 | Notch signaling pathway | 3 | 0.0018 | ADAM17, EP300, HDAC2 |
| 4064 | NF-kappa B signaling pathway | 5 | 6.08E-05 | PTGS2, TNF, CXCL2, NFKB1, BCL2 |
| 4110 | Cell cycle | 5 | 0.00012 | EP300, HDAC2, GSK3B, CHEK1, WEE1 |
| 4115 | P53 signaling pathway | 3 | 0.004 | CHEK1, BCL2, SERPINE1 |
| 4510 | Focal adhesion | 8 | 9.50E-07 | MET, EGFR, AKT1, GSK3B, RAF1, JUN, BRAF, BCL2 |
| 4810 | Regulation of actin cytoskeleton | 4 | 0.009 | BRAF, RAF1, EGFR, CXCL2, |
Discussion
Natural products especially the herbal products induce the cancer cell death by induction of apoptosis, alteration of cell cycle and by modifying the various signaling pathways. The major problem with the currently available chemotherapeutic agents is the side-effects, which kill both cancerous and normal cells since they are not selective. In light of this, the use of plant to kill cancer cells is a more promising area of cancer research thereby reduces the adverse effects of cancer treatment. The previous reports showed 38% viable cells when breast cancer stem cells (BCSCs) treated with Curcuma longa (CL) (Hermansyah et al., 2021). Phyllanthus niruri (PN) has previously shown to be an effective in MCF-7 and A549 cells (Lee et al., 2011; Parvathaneni et al., 2014). Previous study also reported that the combination therapy more effective which indicated by a higher number of cells undergoing apoptosis (Amalina et al., 2021b). In order to identify more effective antiproliferative biomarker in TNBC under CL and PN treatment, we used invitro and bioinformatic approach.
Cytotoxic assay with MTT showed that CL and PN exhibited cytotoxic activity in MDAMB-231 cells at doses- and time-dependent manner. A natural compound for combination therapy should be potent, but also less toxic toward normal cells (Yan et al., 2017). Previous acute and chronic toxicity studies showed that CL and PN are not toxic in animals (de Queiroz et al., 2013; Abdel-Shafy et al., 2020). Further, the study was continued using CL-PN combination to investigate the synergistic effect mechanism of combination thereof. Combination of CL and PN showed that PN augmented the inhibitory effect of CL on the growth of MDAMB-231 cells in vitro. The antiproliferative effect of combination treatment with CL and PN depends on their individual concentrations, suggesting a synergistic effect. The combination index value of CL and PN under CompuSyn calculation showed slightly strong to very strong synergistic effects with combination index values ranged from 0.08 – 0.90. The previous paper revealed that CL it has potent cytotoxic effect on leukemic cancer and breast cancer (Larasati et al., 2018; Hermansyah et al., 2021). Thus, those findings support the use of CL and PN as a combination agent in TNBC treatment.
The potential reason for the perceived cytotoxicity of the compounds was indorsed to the formation of reactive oxygen species (ROS), which subsequently induces cell death (Mursiti et al., 2021). The results of this study proved that combination treatment of CL and PN blocks MDAMB-231 cells in G0/G1 phase, increases the percentage of cells in the G2/M phase, and significantly induced apoptosis through ROS level elevation. Cell cycle checkpoint dysfunction might lead to abnormal cell proliferation and cancer development. Furthermore, the G2/M checkpoint prevented damaged DNA from entering mitosis and was critical in DNA damage-induced apoptosis (Khazaei et al., 2017). Most anticancer drugs induced cancerous DNA damage, blocked mitosis and arrested cells at the G2/M phase (Amalina et al., 2017; Jenie et al., 2019). According to the findings of this investigation, the combination was more effective than single treatment in causing G2/M cell cycle arrest. The results also indicated that combination of CL and PN-induced cell death is mediated by induction in ROS in breast cancer MDAMB-231 cells. Increased ROS activated phosphatidylinositide 3-kinase/ protein kinase B (PI3K/Akt) signaling pathway and lead to the secretion of cytokines chemokine ligand 7, colony-stimulating factor-1 (CSF-1), and interlukin-8 leading to macrophag M2 polarization, thus inhibiting to cell growth. In addition, the high levels of ROS inhibit tumor invasion and metastasis by inhibiting matrix mettaloprotease (MMP)-dependent extracellular matrix (ECM) formation (Wang et al., 2021). Furthermore, ROS-activated PI3K/Akt signaling pathway can inhibit glycogen synthase kinase 3β (GSK-3β) that promote the nuclear-translocation of β-catenin, finally inhibiting epithelial mesenchymal transtition (EMT) leading to metastasis inhibition (Huang et al., 2021). Therefore, Combination treatment is more effective in promoting cancer cell death resulting in enhanced ROS levels. However, the limitation of this study we didn’t evaluate the effect of combination treatment in the normal cells to evaluating the selectivity of these agents.
To determine the molecular mechanism of combination CL and PN on TNBC cell proliferation, we conduct bioinformatic analysis using several public data-based and tools analyses. A total of 256 genes as a direct protein target of CL (curcumin) and PN (limonene and rutin) (DPT) were selected by three data sets including SEA, SwissTargetProtein, and STITCH. Under Venn software analysis, 56 overlapping genes were extracted that associated with TNBC proliferation. The KEGG pathway analysis were significantly enriched in various cell pathways. Furthermore, in this study we observed the top four genes as a potential of antiproliferation biomarker in TNBC, including AKT1, EP300, STAT3, and EGFR. Research reported that downregulation of AKT1 gene inhibit cellular growth and survival by phosphorylating and regulating many targets, including the mammalian target of rapamycin (mTOR) (Cohen, 2013; Martini et al., 2014). The inhibition of AKT1 inhibits metastasis of TNBC cancer subtype (Johnson et al., 2021). In addition, suppressing AKT1 expression dramatically decreased metastatic colonization of lungs by inducing apoptosis or inhibiting invasion (Johnson et al., 2021)
On the other hand, signaling transduction protein EP300 in TNBC could silence miRNA MIR17 leading to PD-L1 regulation (Yeh et al., 2021a). Furthermore, the ligand PD-L1 is speculated to play a major role in suppressing the adaptive arm of immune system in many diseases (Mooradian and Sullivan, 2017). It is shown that the upregulation of PD-L1 may allow cancers to evade the host immune system. EP300 also activated miRNA MIR17 to negatively regulate target genes BECN1 and CD28 to promote cell proliferation and escape the immune checkpoint (Gayther et al., 2000). In this study also revealed that STAT3 as a specific biomarker for TNBC (Xu et al., 2018). STAT3 plays a crucial role of mediating tumor-induced immune suppression in various microenvironment conditions (Lee et al., 2010). The overexpression of STAT3 modified by acetylation regulates target genes BECN1 and CD28 to trigger autophagy and the inhibition of the immune response (Yeh et al., 2021b). Here, in the core signaling pathway of TNBC, autophagy has the ability to regulate T-cell functions, which inhibit the immune response, and cell proliferation to reduce the consumption and accumulate abundant energy for angiogenesis. Another study revealed that EGFR silencing inhibits TNBC cell proliferation and increase apoptosis through the suppression of STAT3 (Song et al., 2020b). Nude mouse tumor formation assays verified that EGFR silencing could suppress tumor cell growth (Song et al., 2020b; Deng et al., 2022).
In conclussion, Through this study, we summarized that combination of CL and PN exhibited a synergistic effect on MDAMB-231 highly metastasis breast cancer by inducing the S- and G2/M-phases cell cycle arrest and ROS levels leading to apoptosis induction. More importantly, the AKT1, EP300, STAT3 and EGFR signaling as potential targets of combination CL and PN in antiproliferation and antimetastatic of TNBC.
Author Contribution Statement
DH and NDA: Writing – review; DAP and NDA: Formal analysis, Project administration, Resources, Supervision, Writing - review & editing; DAP: Project administration, Resources, & Software. DH: Supervision..
Acknowledgements
General
The authors would like to express their gratitude to stem cell and cancer research Indonesia for sharing the laboratory facility.
Funding Statement
The authors express their great appreciation for the financial support received for this work from Ministry of Education and Culture Republic of Indonesia (PDUPT Grant) 2021.
Ethical Declaration
Not applicable because this study does not involve experiments on animals or human subject.
Data Availability
The study data is available with authors.
Conflict of Interest
authors have no conflicts of interests to disclose.
References
- Abdelhamed S, Ogura K, Yokoyama S, Saiki I, Hayakawa Y. AKT-STAT3 pathway as a downstream target of egfr signaling to regulate PD-l1 expression on NSCLC cells. J Cancer. 2016;7:1579–86. doi: 10.7150/jca.14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Shafy S, Alanazi AD, Gabr HSM, et al. Efficacy and safety of ethanolic Curcuma longa extract as a treatment for sand tampan ticks in a rabbit model. Vet World. 2020;13:812–20. doi: 10.14202/vetworld.2020.812-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu JK, Amengor CDK, Kabiri N, et al. Validation of a Simple and Robust Liebermann-Burchard Colorimetric Method for the Assay of Cholesterol in Selected Milk Products in Ghana. Int J Food Sci. 2019 doi: 10.1155/2019/9045938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalina N, Nurhayati IP, Meiyanto E. Doxorubicin Induces Lamellipodia Formation and Cell Migration. Indones J Cancer Chemoprevention. 2017;8:61. [Google Scholar]
- Amalina ND, Wahyuni S, Harjito Cytotoxic effects of the synthesized Citrus aurantium peels extract nanoparticles against MDA-MB-231 breast cancer cells. J Phys Conf Ser. 2021a;19:18. [Google Scholar]
- Amalina ND, Wahyuni S, Harjito Cytotoxic effects of the synthesized Citrus aurantium peels extract nanoparticles against MDA-MB-231 breast cancer cells. J Phys Conf Ser. 2021b;1918:032006. [Google Scholar]
- Araújo Júnior RF de, Souza TP de, Pires JGL, et al. A dry extract of Phyllanthus niruri protects normal cells and induces apoptosis in human liver carcinoma cells. Exp Biol Med. 2012;237:1281–8. doi: 10.1258/ebm.2012.012130. [DOI] [PubMed] [Google Scholar]
- Bianchini G, Balko JM, Mayer IA, Sanders ME, Giann L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13:674–90. doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahyono B, Amalina ND, Suzery M, Bima DN. Exploring the Capability of Indonesia Natural Medicine Secondary Metabolite as Potential Inhibitors of SARS-CoV-2 Proteins to Prevent Virulence of COVID-19. silico and Bioinformatic Approach. 2021;9: 336–42. [Google Scholar]
- Cohen MM. The AKT genes and their roles in various disorders. Am J Med Genet A. 2013;161:2931–7. doi: 10.1002/ajmg.a.36101. [DOI] [PubMed] [Google Scholar]
- Deng YM, Zhao C, Wu L, Qu Z, Wang XY. Cannabinoid Receptor-1 suppresses M2 macrophage polarization in colorectal cancer by downregulating EGFR. Cell Death Discov. 2022;8:273. doi: 10.1038/s41420-022-01064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farghadani R, Naidu R. Curcumin: Modulator of key molecular signaling pathways in hormone-independent breast cancer. Cancers (Basel) 2021:13. doi: 10.3390/cancers13143427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernanda MAHF, Andriani RD, Estulenggani Z, Kusumo GG. Identification and Determination of Total Flavonoids in Ethanol Extract of Old and Young Angsana Leaves (Pterocarpus indicus Willd ) Using Visible Spectrophotometry. (Scitepress. 2019: 541–4. [Google Scholar]
- Gao SP, Mark KG, Leslie K, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117:3846–56. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayther SA, Batley SJ, Linger L, et al. Mutations truncating the EP300 acetylase in human cancers. Nat Genet. 2000;24:300–3. doi: 10.1038/73536. [DOI] [PubMed] [Google Scholar]
- Gou Q, Liu L, Wang C, et al. Polymeric nanoassemblies entrapping curcumin overcome multidrug resistance in ovarian cancer. Colloids Surf B Biointerfaces. 2015;126:26–34. doi: 10.1016/j.colsurfb.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Hanifa M, Wulandari R, Zulfin UM, et al. Different Cytotoxic Effects of Vetiver Oil on Three Types of Cancer Cells, Mainly Targeting CNR2 on TNBC. Asian Pac J Cancer Prev. 2022;23:253–63. doi: 10.31557/APJCP.2022.23.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermansyah D, Putra A, Munir D, et al. Synergistic Effect of Curcuma longa Extract in Combination with Phyllanthus niruri Extract in Regulating Annexin A2 , Epidermal Growth Factor Receptor , Matrix Metalloproteinases , and Pyruvate Kinase M1 / 2 Signaling Pathway on Breast Cancer Stem Cell. Open Access Maced J Med Sci. 2021;9:271–85. [Google Scholar]
- Huang R, Chen H, Liang J, et al. Dual role of reactive oxygen species and their application in cancer therapy. J Cancer. 2021;12:5543–61. doi: 10.7150/jca.54699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ST, Yang RC, Yang LJ, Lee PN, Pang JHS. Phyllanthus urinaria triggers the apoptosis and Bcl-2 down-regulation in Lewis lung carcinoma cells. Life Sci. 2003;72:1705–16. doi: 10.1016/s0024-3205(03)00016-x. [DOI] [PubMed] [Google Scholar]
- Ikawati M, Jenie RI, Utomo RY, et al. Genistein enhances cytotoxic and antimigratory activities of doxorubicin on 4T1 breast cancer cells through cell cycle arrest and ROS generation. J Appl Pharm Sci. 2020;10:95–104. [Google Scholar]
- Jantan I, Haque MA, Ilangkovan M, ArshadL An insight into the modulatory effects and mechanisms of action of phyllanthus species and their bioactive metabolites on the immune system. Front Pharmacol. 2019;10:878. doi: 10.3389/fphar.2019.00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenie RI, Amalina ND, Ilmawati GPN, et al. Cell cycle modulation of CHO-K1 cells under genistein treatment correlates with cells senescence, apoptosis and ROS level but in a dose-dependent manner. Adv Pharm Bull. 2019;9:453–61. doi: 10.15171/apb.2019.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Chow Z, Lee E, et al. Role of AMPK and Akt in triple negative breast cancer lung colonization. Neoplasia (United States) 2021;23:429–38. doi: 10.1016/j.neo.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazaei S, Esa NM, Ramachandran V, et al. In vitro antiproliferative and apoptosis inducing effect of Allium atroviolaceum bulb extract on breast, cervical, and liver cancer cells. Front Pharmacol. 2017;8:1–16. doi: 10.3389/fphar.2017.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Kunwar A, Barik A, Mishra B, et al. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim Biophys Acta Gen Subj. 2008;1780:673–9. doi: 10.1016/j.bbagen.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Larasati YA, Yoneda-Kato N, Nakamae I, et al. Curcumin targets multiple enzymes involved in the ROS metabolic pathway to suppress tumor cell growth. Sci Rep. 2018;8:1–13. doi: 10.1038/s41598-018-20179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Pal SK, Reckamp K, Figlin RA, Yu H. STAT3: A Target to Enhance Antitumor Immune Response. Curr Top Microbiol Immunol. 2010;344:41–59. doi: 10.1007/82_2010_51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Jaganath IB, Wang SM, Sekaran SD. Antimetastatic effects of phyllanthus on human lung (A549) and breast (MCF-7) cancer cell lines. PLoS One. 2011;6:1–14. doi: 10.1371/journal.pone.0020994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CH, Chan MH, Sinica A, et al. The activation of EP300 by F11R leads to EMT and acts as a prognostic factor in triple-negative breast cancers. J Pathol Clin Res. 2022;9:165–81. doi: 10.1002/cjp2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JH, Qin L, Li X. Role of STAT3 signaling pathway in breast cancer. Cell Commun Signal. 2020:18. doi: 10.1186/s12964-020-0527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini M, Santis MC De, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: An updated review. Ann Med. 2014;46:372–83. doi: 10.3109/07853890.2014.912836. [DOI] [PubMed] [Google Scholar]
- Martorana F, Motta G, Pavone G, et al. AKT Inhibitors: New Weapons in the Fight Against Breast Cancer? Front Pharmacol. 2021;12:662232. doi: 10.3389/fphar.2021.662232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooradian MJ, Sullivan RJ. Immunomodulatory effects of current cancer treatment and the consequences for follow-up immunotherapeutics. Future Oncol. 2017;13:1649–63. doi: 10.2217/fon-2017-0117. [DOI] [PubMed] [Google Scholar]
- Mursiti S, Amalina ND, Marianti A. Inhibition of breast cancer cell development using Citrus maxima extract through increasing levels of Reactive Oxygen Species (ROS) J Phys Conf Ser. 2021:1918. [Google Scholar]
- Paramita DA, Hermansyah D, Paramita DA, Amalina ND. Regulation of p53 and survivin by Curcuma longa extract to caspase-3 dependent apoptosis in triple negative breast cancer cells. Med Glas. 2022;19:189–96. doi: 10.17392/1453-22. [DOI] [PubMed] [Google Scholar]
- Parvathaneni M, Battu GR, Gray AI, Gummalla P. Investigation of anticancer potential of hypophyllanthin and phyllanthin against breast cancer by in vitro and in vivo methods. Asian Pac J Trop Dis. 2014;4:S71–6. [Google Scholar]
- Qin JJ, Yan L, Zhang J, Zhang WD. STAT3 as a potential therapeutic target in triple negative breast cancer: A systematic review. J Exp Clin Cancer Res. 2019;38:195. doi: 10.1186/s13046-019-1206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiroz FM de, Oliveira Matias KW de, Cunha MMF da, Schwarz A. Evaluation of (anti)genotoxic activities of Phyllanthus niruri L in rat bone marrow using the micronucleus test. Braz J Pharm Sci. 2013;49:137–48. [Google Scholar]
- Ring A, Kaur P, Lang JE. EP300 knockdown reduces cancer stem cell phenotype, tumor growth and metastasis in triple negative breast cancer. BMC Cancer. 2020:20. doi: 10.1186/s12885-020-07573-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Mukherjee S. Reversal of resistance towards cisplatin by curcumin in cervical cancer cells. Asian Pac J Cancer Prev. 2014;15:1403–10. doi: 10.7314/apjcp.2014.15.3.1403. [DOI] [PubMed] [Google Scholar]
- Saahene RO, Agbo E, Barnes P, et al. A Review: Mechanism of Phyllanthus urinaria in Cancers - NF- B, P13K/AKT, and MAPKs Signaling Activation. Evid Based Complement Alternat Med. 2021:2021. doi: 10.1155/2021/4514342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed MEM, Yücer R, Dawood M, et al. In Silico and In Vitro Screening of 50 Curcumin Compounds as EGFR and NF-κB Inhibitors. Int J Mol Sci. 2022;23:3966. doi: 10.3390/ijms23073966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafabakhsh R, Pourhanifeh MH, Mirzaei HR, et al. Targeting regulatory T cells by curcumin: A potential for cancer immunotherapy. Pharmacol Res. 2019;147:104353. doi: 10.1016/j.phrs.2019.104353. [DOI] [PubMed] [Google Scholar]
- Song X, Liu Z, Yu Z. EGFR promotes the development of triple negative breast cancer through JAK/STAT3 signaling. Cancer Manag Res. 2020a;12:703–17. doi: 10.2147/CMAR.S225376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Liu Z, Yu Z. EGFR promotes the development of triple negative breast cancer through JAK/STAT3 signaling. Cancer Manag Res. 2020b;12:703–17. doi: 10.2147/CMAR.S225376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sri Sulasmi E, Saptasari M, Mawaddah K, Ama Zulfia F. Tannin identification of 4 species pterydophyta from baluran national park. Journal of Physics: Conference Series. 2019 [Google Scholar]
- Sun XD, Liu XE, Huang DS. Curcumin induces apoptosis of triple-negative breast cancer cells by inhibition of EGFR expression. Mol Med Rep. 2012;6:1267–70. doi: 10.3892/mmr.2012.1103. [DOI] [PubMed] [Google Scholar]
- Sun Y, Liu L, Wang Y, et al. Curcumin inhibits the proliferation and invasion of MG-63 cells through inactivation of the p-JAK2/ p-STAT3 pathway. Onco Targets Ther. 2019;12:2011–21. doi: 10.2147/OTT.S172909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzery M, Cahyono B, Amalina ND. Antiproliferative and apoptosis effect of hyptolide from Hyptis pectinata (L ) Poit on human breast cancer cells. App Pharm Sci. 2020;10:1–6. [Google Scholar]
- Tajuddin WNBWM, Lajis NH, Abas F, Othman I, Naidu R. Mechanistic understanding of curcumin’s therapeutic effects in lung cancer. Nutrients. 2019:11. doi: 10.3390/nu11122989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YQ, Jaganath IB, Sekaran SD. Phyllanthus spp induces selective growth inhibition of PC-3 and mewo human cancer cells through modulation of cell cycle and induction of apoptosis. PLoS One. 2010;5:1–11. doi: 10.1371/journal.pone.0012644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjipta A, Hermansyah D, Suzery M, Cahyono B, Amalina ND. Application of Bioinformatics Analysis to Identify Important Pathways and Hub Genes in Breast Cancer Affected by HER-2. Int J Cell Biomed Sci. 2022;1:18–27. [Google Scholar]
- Tomeh MA, Hadianamrei R, Zhao X. A review of curcumin and its derivatives as anticancer agents. Int J Mol Sci. 2019:20. doi: 10.3390/ijms20051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng HH, Chen PN, Kuo WH, et al. Antimetastatic potentials of phyllanthus urinaria L on A549 and Lewis lung carcinoma cells via repression of matrix-degrading proteases. Integr Cancer Ther. 2012;11:267–78. doi: 10.1177/1534735411417128. [DOI] [PubMed] [Google Scholar]
- Wang Y, Boxel-Dezaire AHH van, Cheon H, Yang J, Stark GR. STAT3 activation in response to IL-6 is prolonged by the binding of IL-6 receptor to EGF receptor. Proc Natl Acad Sci U S A. 2013;110:16975–980. doi: 10.1073/pnas.1315862110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Qi H, Liu Y, et al. The double-edged roles of ROS in cancer prevention and therapy. Theranostics. 2021;11:4839–57. doi: 10.7150/thno.56747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee P, Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel) 2017:9. doi: 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg F, Ramnath N, Nagrath D. Reactive oxygen species in the tumor. Cancers (Basel) 2019;11:1–20. doi: 10.3390/cancers11081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Zhou Y, Li WEI, Zhang BIN, et al. Tumor derived mesenchymal stem cell secreted IL 6 enhances resistance to cisplatin via the STAT3 pathway in breast cancer. Oncol Lett. 2018;15:9142–50. doi: 10.3892/ol.2018.8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufai Y, Isah Y, Isyaka MS. Comparative Phyto-Constituents Analysis from the Root Bark and Root Core Extractives of Cassia ferruginea (Schrad D C) Plant. Scholars J Agric Vet Sci. 2016;3:275–83. [Google Scholar]
- Yan X, Qi M, Li P, Zhan Y, Shao H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci. 2017;7:1–16. doi: 10.1186/s13578-017-0179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Li Y, Wang H, et al. Therapeutic progress and challenges for triple negative breast cancer: targeted therapy and immunotherapy. Mol Biomed. 2022:3. doi: 10.1186/s43556-022-00071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh SJ, Hsu BJ, Chen BS. Systems medicine design for triple-negative breast cancer and non-triple-negative breast cancer based on systems identification and carcinogenic mechanisms. Int J Mol Sci. 2021a;22:1–24. doi: 10.3390/ijms22063083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh SJ, Hsu BJ, Chen B sen. Systems medicine design for triple-negative breast cancer and non-triple-negative breast cancer based on systems identification and carcinogenic mechanisms. Int J Mol Sci. 2021b;22:1–24. doi: 10.3390/ijms22063083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawawy S, Khedr G. Pattern of Failure and Treatment Results in Triple Negative Breast Cancer Patients. Adv Breast Cancer Res. 2022;11:75–88. [Google Scholar]
- Zheng ZZ, Chen LH, Liu SS, et al. Bioguided Fraction and Isolation of the Antitumor Components from Phyllanthus niruri L. Biomed Res Int. 2016:2016. doi: 10.1155/2016/9729275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Ye M, Lu Y, et al. Curcumin improves the tumoricidal effect of mitomycin C by suppressing ABCG2 expression in stem cell-like breast cancer cells. PLoS One. 2015;10:1–12. doi: 10.1371/journal.pone.0136694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study data is available with authors.