Abstract
In recent years, molecular targeted therapy has attracted more attention from researchers due to its high efficiency and fewer side effects. Researchers are attempting to find more specific ways to treat diseases. It has been found that there are different targets for the treatment of diseases such as cancer, obesity, and metabolic syndrome. It is important to find a potential target in order to lessen the side effects of current treatments. G Protein-coupled receptors (GPCRs) are a large family of transmembrane proteins that are expressed in many organs, leading to the activation of internal signal transduction cascades through the binding of different ligands, including neurotransmitters, peptides, and lipids. Due to the critical role of GPCRs in cells, it could be a potential target. G protein-coupled receptor 75 (GPR75) is a novel member of the GPCR family that has an important role in many diseases, such as obesity, cancer, and metabolic syndrome. Until now, three ligands have been detected for GPR75, including 20-HETE, CCL5, and RANTES. Recent studies suggest that 20-HETE, through GPR75, triggers signaling pathways including PI3K/Akt and RAS/MAPK, leading to a more aggressive phenotype in prostate cancer cells. Additionally, the PI3K/Akt and RAS/MAPK signaling pathways activate NF-κB, which is significant in various pathways of cancer development such as proliferation, migration, and apoptosis. The findings indicate that inhibiting GPR75 in humans leads to an increase in insulin sensitivity and glucose tolerance, as well as a reduction in body fat storage. According to these discoveries, GPR75 could be a potential target for drug treatment of diseases such as obesity, metabolic syndrome, and cancer. In this review, we aimed to discuss the therapeutic impact of GPR75 in cancer, metabolic syndrome, and obesity and underscore the possible pathways.
Key Words: G protein, coupled receptors 75, molecular targeted therapy, cancer, obesity, metabolic syndrome
Introduction
Molecular targeted therapy is a new, highly efficient strategy that has recently attracted more attention from researchers. The term “molecular targeted therapy” refers to drugs or other substances that target specific molecules (Ehrlich, 1906). A successful molecular targeted therapy requires the identification of ideal targets with the aim of reducing the burden of common diseases, such as cancer, obesity, and metabolic syndrome. Cancer, the second leading cause of death, is a multifactorial disease and current treatments such as chemotherapy, radiotherapy, and surgery have shown many side effects due to their non-specific approach. Another global health threat is obesity; according to the World Health Organization, the total number of overweight adults 18 years and older was more than 1.9 billion in 2016. More than 650 million of these people were obese (Jafari-Gharabaghlou et al., 2023; West et al., 2018). Obesity, high blood pressure, high blood sugar, and abnormal cholesterol levels are known collectively as metabolic syndrome (Cho et al., 2010). Lifestyle changes are the major way to reduce metabolic risk factors; treatment with medicinal products is also an important therapeutic measure for MetS. The implementation of lifestyle modifications is often challenging for patients, and the sustainability of weight loss outcomes is typically limited, leading to a gradual decline in the efficacy of this clinical approach over time. It has been observed that lifestyle modifications may not be sufficient to rectify pre-existing medical conditions in a considerable portion of the population. Additionally, with advancing age, the intensity of potential risk factors tends to increase, necessitating a heightened reliance on pharmacological interventions. On the other hand, no current drugs lower all metabolic risk factors long-term, so treatment may focus on correcting each risk factor individually with various medications. Also, as the disease worsens, one drug is insufficient, and multiple drugs are needed. This becomes complex when multiple risk factors need to be managed with different medications (Fahed et al., 2022; HanLean, 2015; RAZHABOVA et al., 2020). It has shown different targets for diseases such as cancer, obesity, and metabolic syndrome. It is important to understand how environmental factors interact with specific molecular pathways in order to prevent these diseases (Obici, 2009). Tumor immunology is becoming better understood, resulting in the progression of effective targeted therapies (González-Cao et al., 2015). Targeted therapy aims to deliver drugs directly to certain genes or molecules that have a crucial role in tumor growth. Small-molecule inhibitors and antibody-conjugated nanoparticles are examples of targeted therapy (Ghasemali et al., 2013; Joo et al., 2013; Mohammadian et al., 2016; Nejati et al., 2022; Salmani Javan et al., 2022). Due to the increased specificity and decreased toxicity of targeted therapies, researchers are focusing on new molecular targets (Eatemadi et al., 2016; Nejati et al., 2021). The largest family of membrane receptor proteins in mammals are G-protein coupled receptors (GPCRs), which are involved in a wide variety of physiological and pathological functions. A large variety of extracellular ligands, such as hormones, chemokines, neurotransmitters, autacoids, and enzymes, bind to GPCRs and lead to many cellular physiological functions through interaction with heterotrimeric G proteins (Kawasawa et al., 2003).Seven hydrophobic transmembrane (™) segments, with an extracellular amino-terminal and intracellular carboxyl-terminal, are a typical structure of the GPCR superfamily. The TM section of GPCRs shows numerous homologies to each other. The main diversity of the GPCRs structure is due to the amino-terminal, carboxyl-terminal, and loop of TM5 and TM6. The extreme variety is observed in the amino-terminal region (Kobilka, 2007). A majority of the membrane receptors in humans are GPCRs; approximately 800 members of this family have been identified so far, and more than half of them are olfactory receptors. Based on the similarity of the 7TM sequence, GPCRs can be divided into five families: the rhodopsin family, the adhesion family, the frizzled/taste family, the glutamate family, and the secretin family (Fredriksson et al., 2003). Studies have discovered new models of GPCR activation, including “biased activation,” “intracellular activation,” “dimerization activation,” “transactivation,” and “biphasic activation” (Wang et al., 2018). In biased activation, the ligand can either cause G-protein or β-arrestin activation (Rakesh et al., 2010). β-Arrestin has the ability to regulate the signaling of many GPCRs (Lefkowitz, 2013). Most GPCRs are located and activated on the cell surface, but recent studies have shown that some GPCRs are activated intracellularly and trigger their signaling pathways (Luttrell et al., 1999; Schiaffino et al., 1999). There are two pathways for this approach: First, GPCRs continue to signal after entering the cell with their agonists; second, intracellular GPCRs are located in different organelles and start their signaling pathways from inside the cell (Wang et al., 2018). Some GPCRs are activated individually, but some of them interact with each other and modulate their function through dimerization, referred to as “dimerization activation” (Gomes et al., 2001). In the transactivation model, GPCR ligands can transactivate and activate other receptors, such as the tyrosine kinase receptor (Daub et al., 1996). GPCRs have two stages of activation: an early phase and a late phase. Each of these stages has its own effects and regulates a specific downstream protein, which is referred to as biphasic activation (Schorb et al., 1995). So far, the roles of various GPRs have been identified, such as GPR120, GPR40, GPR35, etc (Table 1). In recent years, GPR75, a unique member of the GPCR family, has attracted a great deal of attention from researchers. GPR75, first identified by Tarttelin et al. in 1999, is a 540-amino-acid protein with only two exons located on human chromosome 2p16. The first exon of GPR75 contains an untranslated sequence, while the second and final exon of GPR75 contains GPR75’s entire translated region, which is not similar to any other gene or transcript (Akbari et al., 2021). The highest expression level of the GPR75 gene is in the brain; however, several recent studies have indicated that the GPR75 receptor is expressed throughout most human tissues, including the brain, heart, kidney, and prostate (Figure 1). A study by Garcia et al. (2017a) demonstrated that 20-HETE binds with high affinity in human endothelial cells and activates the GPR75. Other studies have shown that the GPR75 (Gq) and its ligand 20-HETE activate pro-inflammatory and hypertensive signaling pathways, contributing to diabetes, obesity, endothelial dysfunction, cell proliferation, hypertension, and cardiovascular disease (Pascale et al., 2021). In addition, reports show the important role of 20-HETE in cell growth and development of cancer (Cárdenas et al., 2020). An overview of GPR75 is provided in this review, which will discuss its biological role, mode of action, and role in metabolic syndrome, obesity, and cancer development.
Table 1.
Various GPRs' and Their Functions
| Type | Role | Ref. |
|---|---|---|
| GPR120 | The free fatty acid receptors/Potential Target for Obesity Treatment | (65, 66) |
| GPR55 | Important targets in pain and cancer, and additional diseases as well | (67) |
| GPR35 | Risk gene for inflammatory bowel diseases (IBD) / important targets in pain and cancer, and additional diseases as well | (68) |
| GPR40 | The free fatty acid receptors/Agonists for the Treatment of Type 2 Diabetes Mellitus | (65) |
| GPR41 | Cloned and demonstrated to be receptors for SCFAs/Short chain fatty acids (SCFAs) | (69) |
| GPR43 | Cloned and demonstrated to be receptors for SCFAs/Short chain fatty acids (SCFAs) | (69) |
| GPR119 | GPR119 agonists: prevention and/or treatment of diabetes, obesity, dyslipidemia, or related disorders. | (70) |
| GPR75 | New target in metabolic syndrome and cancer | (20) |
Figure 1.
GPR75 Tissue Expression based on RPKM (reads per kilo-base per million reads placed)
GPR75 and Metabolic Syndrome
Several risk factors, including abnormal cholesterol levels, high blood sugar levels, high blood pressure, and abdominal obesity, are associated with metabolic syndrome (Met S) (Cho et al., 2010). According to lifestyle, region, cultural factors, and degree of urbanization, the prevalence of Met S varies, which is difficult to measure. However, since Met S is about three times more common than diabetes, the global incidence can be estimated to be about one-quarter of the world population (Saklayen, 2018). It has been shown that Met S is closely related to cardiovascular disease and type 2 diabetes, but the connection between Met S and visceral obesity is even more significant, as it has also been linked to other chronic diseases such as brain cancer and some types of cancer (Avgerinos et al., 2019; Yates et al., 2012). 20-HETE, an arachidonic acid metabolite and a vasoactive lipid whose formation is catalyzed by enzymes of the cytochrome P450 (CYP) 4A/F family, plays a critical role in the progression of insulin resistance, obesity, and metabolic syndrome (Issan et al., 2013; Peterson et al., 2016; Tsai et al., 2009; Ward et al., 2006). Furthermore, animal studies have shown that obesity and Met S are linked by 20-HETE levels (Gilani et al., 2018; Joseph et al., 2017; Soler et al., 2018; Theken et al., 2012). Elevated ranges of 20-HETE are associated with excessive blood pressure in lots of experimental models (Sacerdoti et al., 1989; Wang et al., 2006; Wang et al., 1999; Wang et al., 2001).
There is additional evidence that 20-HETE is a very important regulator of sodium homeostasis in animal models (Hoagland et al., 2003; Roman et al., 2006) and humans (Laffer et al., 2003). Regulation of vascular tone (Alonso-Galicia et al., 1997; Garcia et al., 2015; Kauser et al., 1991) and renal function (Escalante et al., 1994; Sánchez-Mendoza et al., 2000; Zou et al., 1994) is also affected by the 20-HETE level. On the other hand, there is an association between 20-HETE and increased triglycerides and decreased HDL in Met S patients (Fava et al., 2012).
20-HETE phosphorylates the epidermal growth factor receptor (EGFR), leading to the release of endothelial nitric oxide synthase (NO), producing inflammatory cytokines, and promoting the activity and expression of angiotensin-converting enzyme (ACE). To evaluate G1T1 and GPR75 in the phosphorylation of EGFR by 20HETE, small interfering RNAs (SiRNA) against GPR75 and G1T1 were used, and it was found that the effects induced by 20HETE were completely inhibited in this case (Cheng et al., 2014; Cheng et al., 2012; Cheng et al., 2008; Garcia et al., 2016; Khodadadi et al., 2022).
As a result of blocking GPR75 in humans, energy intake and body fat storage are reduced. In a study, two groups of mice were used: first, Cyp4a12 transgenic mice whose blood 20HETE was increased by doxycycline, and second, mice whose GPR75 was inhibited by a bolus of GPR75-targeted shRNA. Cyp4a12 transgenic mice treated with doxycycline resulted in their blood pressure, ACE expression, and activity being increased, while the other group, which was given a bolus of GPR75-targeted shRNA at the same time, did not show any increase in blood pressure and ACE expression had not changed (Garcia et al., 2017). 20HETE activates GPCR-EGFR by a c-Src kinase that starts Raf / MEK / ERK signal pathway cascade (Alonso-Galicia et al., 1999; Cheng et al., 2012). 20-HETE binding to GPR75 triggers signaling pathways in endothelial and vascular smooth muscle cells, which cause vascular ACE expression, hypertension, endothelial dysfunction, remodeling, and contractility (Garcia et al., 2017). 20-HETE binding to GPR75 results in cleavage of Gαq/11 from GPR75 and associated GPCR-kinase interacting protein1 (G1T1) to GPR75, eventually causing phosphorylation of the large conductance voltage and calcium-activated potassium subunit β subunit of the Ca2+-activated K+ channels (Bkca), which is ultimately responsible for inactivating it and causing vasoconstriction (Alioua et al., 2002).
GPR75 and Cancer
Until now, three ligands have been dedicated to GPR75, including RANTES, CCL5, and 20HETE; each of them triggers a variety of signaling pathways (Cárdenas et al., 2020). As a result of 20HETE bonding with GPR75, PKC/PLC, C-src, MAPK, NF-κB, and AKT signaling pathways are activated, leading to pro-inflammation, diabetes, vascular dysfunction remodeling, hypertension, and malignant cellular transformation (Cárdenas et al., 2020; Pascale et al., 2021). 20HETE increases the invasive nature of non-small lung cancer cells and the metastasis potential in prostate cancer cells. It has been shown that blocking the 20-HETE pathway can reduce the risk of brain, breast, and kidney cancer, as well as the migration and invasiveness of triple-negative breast cancer cells. By incubating Pc-3 cells with 20HETE, the PKC& signaling pathway switched from the cytoplasm to the plasma membrane. In addition, E-cadherin expression slowed down, but the expression of mesenchymal, vimentin markers, and MMP-2 activity increased. Generally, 20HETE via GPR75 may enhance the metastatic properties of cancer cells. Furthermore, 20HETE regulates the polymerization of actin by increasing the expression of stress fibers and focal adhesion kinase, which in turn affects cell adhesion and polarization, thus affecting cell migration and invasion (Cárdenas et al., 2020).
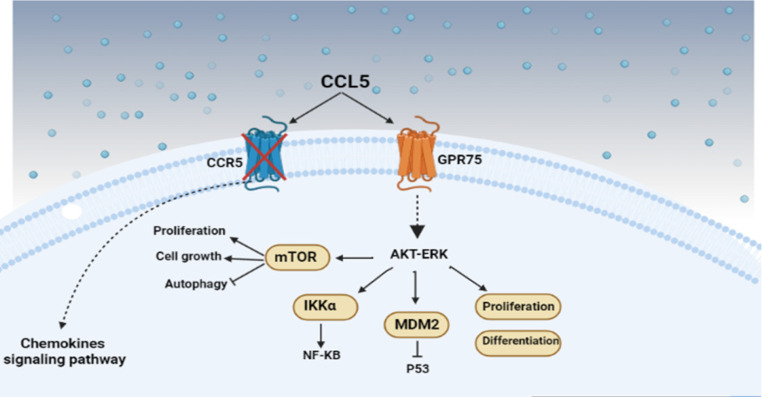
Another ligand of GPR75 is CCL5, which causes phosphorylation and activation of AKT, glycogen synthase kinase B, and extracellular signal-regulated kinase (ERK1/2), without changing the amount of these proteins. It has been reported that, by knocking down GPR75 via Crisper-Cas9, CCL5’s potential to activate ERK has been blocked. (Dedoni et al., 2018) (Figure2).
Figure 2.
Binding 20HETE to GPR75 Leads to Dissociation of Gαq11 from GPR75. Gαq11 activates PLC, convertor of PIP2 to IP3 and DAG. IP3 increases cytoplasm Ca2+ through the releasing ERK Ca2+. DAG cooperates with Ca2+ to activate PKC and eventually PI3K/AKT is activated. Another pathway activates NF-KB via MAPK and initiate downstream cascade
ERK is a member of the mitogen-activated protein kinase (MAPK) family that regulates proliferation and differentiation processes. ERK transmits extracellular signals to intracellular targets. It has been determined that dysregulation of the ERK pathway is one of the causes of cancer spread. In addition, high expression of MAPK/ERK could promote epithelial-mesenchymal transition (EMT) and the expression of matrix metalloproteinases (MMPs) (Jadid et al., 2023; Maik-Rachline et al., 2019; Song et al., 2018). 20HETE competes CCL5 for binding to the GPR75. 20HETE through GPR75 increases ica2+, the inositol phosphate (IP-1), and B-arrestin, while CCL5, via GPR75, prevents 20HETE binding to this receptor so the mentioned pathways will be inhibited (Pascale et al., 2021). B-arrestin is a family of proteins that regulate the signal pathway of various GPCR. β-arrestin is a family of proteins that regulate the signal pathway of various GPCRs. β-arrestin adjusts cell proliferation, migration, invasion, anti-apoptotic, and drug resistance pathways (Song et al., 2018). Binding 20HETE to GPR75 in human endothelial cells leads to the dissociation of subunit Gαq11 and C-src from G1T1, which is bound to GPR75. Then, Gαq11 activates PLC, which is a converter of PIP 2 to IP3 and diacylglycerol. These events cause PKC to be phosphorylated and activated, resulting in the activation of nuclear factor K-B (NF-κB) (FanRoman, 2017) (Figure3).
Figure 3.
CCL5 has Two Receptors Including CCR5 and GPR75. Binding of CCL5 to GPR75 initiate cascade signal pathway which AKT-ERK act as upstream regulator. AKT-ERK triggers MDM2, IKKα, and mTOR resulting in proliferation and differentiation
It has been shown that tumors that continuously activate NF-κB result in resistance to chemotherapy. On the other hand, some cancers that are treated with chemotherapy or radiotherapy increase the activity of NF-κB, PI3K/AKT, and RAS/MAPK signaling pathways, which activate NF-κB and are substantial in various pathways of cancer development such as proliferation, migration, and apoptosis (BonizziKarin, 2004). Another activity of NF-κB has been detected in several malignancies. NF-κB regulates apoptosis by activating several genes which suppress cell death via both mitochondrial pathways (intrinsic) and death receptors (extrinsic). It also has a role in the expression of several members of the anti-apoptosis family, such as BCL2. NF-κB also regulates several cell cycle genes, such as cyclins D1, D2, D3, cyclin E, C-myc, and C-mycb, which contribute to the proliferation and invasion of cancer cells (Dolcet et al., 2005; Sahabi et al., 2022). RANTES is another ligand of GPR75 that activates MAPK through the PLC/PI3K/AKT signal pathway. Phosphorylation and activation of AKT and MAPK result in proliferation and cell survival (Ignatov et al., 2006). The PI3K/AKT/mTOR signaling pathway plays a critical role in maintaining cellular function. PI3K initiates this pathway by activating AKT, which subsequently regulates many downstream proteins, playing an important role in cell survival, proliferation, migration, metabolism, angiogenesis, and inhibiting apoptosis. Furthermore, increasing Ica2+ can cause the formation of a Ca2+/calmodulin complex and activate calcium/calmodulin-dependent kinase-kinase (CAMKK). CAMKK causes phosphorylation and activation of AKT. One of the pathways that AKT inhibits apoptosis is phosphorylation of Bad in serine 112 and phosphorylation of caspase 9. On the other hand, Bad can be phosphorylated and inhibited by other kinases such as PKA, Ca/CAMKII, and Ca2+/CAMKIV in serine 136. Generally, the PI3K/AKT/mTOR signaling pathway and MAPK-ERK pathway have been observed in many cancer types, including neuroendocrine neoplasms (RevathideviMunirajan, 2019).
GPR75 and Obesity
Obesity is a multi-factorial disease that is a serious public health problem, associated with a large proportion of disorders, including type 2 diabetes, hypertension, cardiovascular disease, and some particular cancers (LoosYeo, 2022).
Recent studies have shown that GPR75 is associated with obesity. In a study, the genomes of 645,626 people from different countries were sequenced and a significant relationship between the GPR75 number and body mass index (BMI) was observed. The lower the expression of GPR75, the more balanced the weight. The case with the protein-truncating variant in GPR75 led to a lower BMI, more appropriate weight, and reduced risk of obesity. It has been shown that knocking out GPR75 in mice causes resistance to weight gain. In an experiment, three groups of mice with GPR75-/- and GPR75 +/- genotypes and a wild type were fed a high-fat diet (HFD), and after 14 weeks it was observed that the GPR 75 -/- had about 44% and the GPR 75 +/- had about 25% less weight gain than the wild type group and also had more suitable blood sugar, insulin sensitivity, and a lower ratio of leptin to adiponectin (Akbari et al., 2021; Alagheband et al., 2022; Powell et al., 2022). Mice whose GPR75 had been knocked out were fed less than wild-type (WT) mice, and their oxygen consumption and energy expenditure varied from that of the WT group. This evidence suggests that the energy consumption of mice whose GPR75 was knocked out was higher than the energy that they received. These mice were also thinner, more sensitive to insulin, had lower blood insulin levels, and improved glucose tolerance. GPR75 could be a potential target for drug-targeting treatment, the inhibition of which could result in a reduction of body fat as well as improvements in other metabolic processes (Powell et al., 2022). 20-HETE is one of the GPR75 ligands that triggers a variety of signaling pathways. According to numerous studies, 20-HETE is linked to the progression of obesity, insulin resistance, and Met S. Clinical observations have shown that a value of 20-HETE in plasma and urine may be associated with BMI. In vitro studies have demonstrated that 20-HETE stimulates adipogenesis and also inhibits insulin activity. Bonding of 20-HETE with GPR75 stimulates the IP3/DAG signaling pathway and results in increased intracellular Ca2+ and activation of PKCα. Afterwards, PKCα activates phosphatase, which is responsible for dephosphorylating the insulin receptor, so the insulin receptor is not activated (Gilani et al., 2021). A study observed that 20HETE expression is at a high level in cyp4a14-/- mice. In this study, two groups of mice were inducted. one group was fed by normal diet and the other group was fed by HFD. HFD-fed mice gained much more weight than mice fed a normal diet. In the HFD group, applied 20-SLA, as a 20HETE antagonist, was given to mice. While HFD developed hyperglycemia and hyperinsulinemia, 20-SLA decreases the effect of HFD on gain weight and normalized blood sugar and insulin levels. This information suggests that 20HETE is associated with, obesity due to HFD and insulin resistance, and impaired insulin signaling pathway.
A study revealed that 20-HETE expression is at a high level in cyp4a14-/- mice. In this study, two groups of mice were induced; one group was fed a normal diet and the other group was fed a HFD. HFD-fed mice gained much more weight than mice fed a normal diet. In the HFD group, the 20-SLA, as a 20-HETE antagonist, was given to mice. While HFD developed hyperglycemia and hyperinsulinemia, 20-SLA decreased the effect of HFD on weight gain and normalized blood sugar and insulin levels. This information suggests that 20-HETE is associated with obesity due to HFD and insulin resistance, and impaired insulin signaling pathway (Gilani et al., 2018).
In conclusion, targeted therapy has attracted a great deal of attention from researchers in recent years due to its specificity in the treatment of diseases such as obesity, metabolic syndrome, and cancer. Therefore, there are many attempts to find effective targets. GPR75 is a new member of the GPCR family with an important role in obesity, metabolic syndrome, and cancer. According to recent studies, knocking down GPR75 leads to a reduced range of energy intake and body fat stores. In addition, GPR75, through signaling pathways including PI3K/Akt, RAS/MAPK, and PKC/PLC, has an effect on pro-inflammation, proliferation, and malignant transformation. Since 20-HETE is one of the GPR75 ligands and it stimulates adipogenesis and also inhibits insulin activity, it can be said that GPR75 plays a critical role in this process. Based on these findings, GPR75 could be a potential molecular target that needs more research for the development of existing treatments.
Non-standard Abbreviations and Acronyms
20-HETE: 20-hydroxy-5,8,11,14-eicosatetraenoic acid
ACE: angiotensin-converting enzyme
AKT: protein kinas B
BCL2: B-cell lymphoma 2
Camkk: calcium/calmodulin dependent protein kinase kinase
C-src: Cellular proto-oncogene tyrosine-protein kinase
CYP: Cytochrome P450
DAG: Diacylglycerol
EGFR: epidermal growth factor receptor
ERK: extra cellular signal-regulated kinase
G1T1: G-protein-coupled receptor kinase interactor-1
GPCR: G-protein Coupled Receptor
GPR75: G protein coupled receptor75
iCa2+: Intracellular Ca2+
IP-1: Inositol Phosphate
IP-3: Inositol 3 phosphate
MAPK: Mitogen-activated protein kinase
MDR: Multi-Drug Resistance
Met S: Metabolic Syndrome
NO: nitric oxide synthase
PI3K: phosphoinositol 3kinase
PKC: Protein kinas C
PLC: phospholipase C
shRNA: Short hairpin RNA
SiRNA: small interfering RNAs
TM: transmembrane
Author Contribution Statement
Writing - original draft preparation: Mohammad Reza Dashti and Fatemeh Ghorbanzadeh; Editing: Davoud Jafari-gharabaghlou and Mahdi Farhoudi Sefidan Jadid; Conceptualization, Supervision: Nosratollah Zarghami .
Acknowledgements
Not applicable.
Competing Interests
No potential competing interest was reported by the authors.
References
- A Tanagho P, S Shohdy K. GPR 120: the potential target for obesity treatment. Endocr Metab Immune Disord Drug Targets. 2016;16:8–11. doi: 10.2174/1871530316666151123115611. [DOI] [PubMed] [Google Scholar]
- Abdel-Magid AF. Treatment of diabetes, obesity, dyslipidemia, and related disorders with GPR119 agonists. ACS Med Chem Lett. 2018;10:14–5. doi: 10.1021/acsmedchemlett.8b00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari P, Gilani A, Sosina O, et al. Sequencing of 640,000 exomes identifies GPR75 variants associated with protection from obesity. Science. 2021;373:eabf8683. doi: 10.1126/science.abf8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagheband Y, Jafari-gharabaghlou D, Imani M, et al. Design and fabrication of a dual-drug loaded nano-platform for synergistic anticancer and cytotoxicity effects on the expression of leptin in lung cancer treatment. J Drug Deliv Sci Technol. 2022;73:103389. [Google Scholar]
- Alioua A, Mahajan A, Nishimaru K, et al. Coupling of c-Src to large conductance voltage-and Ca2+-activated K+ channels as a new mechanism of agonist-induced vasoconstriction. Proc Natl Acad Sci U S A. 2002;99:14560–5. doi: 10.1073/pnas.222348099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Galicia M, Drummond HA, Reddy KK, Falck JR, Roman RJ. Inhibition of 20-HETE production contributes to the vascular responses to nitric oxide. Hypertension. 1997;29:320–5. doi: 10.1161/01.hyp.29.1.320. [DOI] [PubMed] [Google Scholar]
- Alonso-Galicia M, Falck JR, Reddy KM, Roman RJ. 20-HETE agonists and antagonists in the renal circulation. Am J Physiolo Renal Physiol. 1999;277:790–6. doi: 10.1152/ajprenal.1999.277.5.F790. [DOI] [PubMed] [Google Scholar]
- Ang Z, Ding JL. GPR41 and GPR43 in obesity and inflammation–protective or causative? Front Immunol. 2016;7:28. doi: 10.3389/fimmu.2016.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–35. doi: 10.1016/j.metabol.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Bonizzi G, Karin M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–8. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Cárdenas S, Colombero C, Panelo L, et al. GPR75 receptor mediates 20-HETE-signaling and metastatic features of androgen-insensitive prostate cancer cells. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158573. doi: 10.1016/j.bbalip.2019.158573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Edin ML, Hoopes SL, et al. Vascular characterization of mice with endothelial expression of cytochrome P450 4F2. FASEB J. 2014;28:2915–31. doi: 10.1096/fj.13-241927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Garcia V, Ding Y, et al. Induction of Angiotensin-Converting Enzyme and Activation of the Renin–Angiotensin System Contribute to 20-Hydroxyeicosatetraenoic Acid–Mediated Endothelial Dysfunction. Arterioscler Thromb Vasc Biol. 2012;32:1917–24. doi: 10.1161/ATVBAHA.112.248344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Ou JS, Singh H, et al. 20-hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. Am J Physiol Heart Circ Physiol. 2008;294:1018–26. doi: 10.1152/ajpheart.01172.2007. [DOI] [PubMed] [Google Scholar]
- Cho AS, Jeon S-M, Kim MJ, et al. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem Toxicol. 2010;48:937–43. doi: 10.1016/j.fct.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Daub H, Ulrich Weiss F, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–60. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- Dedoni S, Campbell LA, Harvey BK, Avdoshina V, Mocchetti I. The orphan G-protein-coupled receptor 75 signaling is activated by the chemokine CCL 5. J Neurochem. 2018;146:526–39. doi: 10.1111/jnc.14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446:475–82. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- Eatemadi A, Daraee H, Aiyelabegan HT, et al. Synthesis and characterization of chrysin-loaded PCL-PEG-PCL nanoparticle and its effect on breast cancer cell line. Biome Pharmacother. 2016;84:1915–22. doi: 10.1016/j.biopha.2016.10.095. [DOI] [PubMed] [Google Scholar]
- Escalante B, Erlij D, Falck JR, McGIFF JC. Cytochrome P-450 arachidonate metabolites affect ion fluxes in rabbit medullary thick ascending limb. Am J Physiol Cell Physiol. 1994;266:1775–82. doi: 10.1152/ajpcell.1994.266.6.C1775. [DOI] [PubMed] [Google Scholar]
- Fahed G, Aoun L, Bou Zerdan M, et al. Metabolic syndrome: Updates on pathophysiology and management in 2021. Int J Mol Sci. 2022;23:786. doi: 10.3390/ijms23020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Roman RJ. GPR75 identified as the first 20-HETE receptor: a chemokine receptor adopted by a new family. Cric Res. 2017;120:1696–8. doi: 10.1161/CIRCRESAHA.117.311022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava C, Montagnana M, Danese E, et al. The functional variant V433M of the CYP4F2 and the metabolic syndrome in Swedes. Prostaglandins Other Lipid Mediat. 2012;98:31–6. doi: 10.1016/j.prostaglandins.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Lagerström MC, Lundin L-G, Schiöth HB. The G-protein-coupled receptors in the human genome form five main families Phylogenetic analysis paralogon groups and fingerprints. Mol Pharmacol. 2003;63:1256–72. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- Garcia V, Gilani A, Shkolnik B, et al. 20-HETE Signals Through G-Protein-Coupled Receptor GPR75 (G(q)) to Affect Vascular Function and Trigger Hypertension. Circ Res. 2017;120:1776–88. doi: 10.1161/CIRCRESAHA.116.310525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia V, Joseph G, Shkolnik B, et al. Angiotensin II receptor blockade or deletion of vascular endothelial ACE does not prevent vascular dysfunction and remodeling in 20-HETE-dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2015;309:71–8. doi: 10.1152/ajpregu.00039.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia V, Shkolnik B, Milhau L, Falck JR, Schwartzman ML. 20-HETE activates the transcription of Angiotensin-Converting enzyme via nuclear Factor-κB translocation and promoter binding. J Pharmacol Exp Ther. 2016;356:525–33. doi: 10.1124/jpet.115.229377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemali S, Nejati-Koshki K, Akbarzadeh A, et al. Inhibitory effects of β-cyclodextrin-helenalin complexes on H-TERT gene expression in the T47D breast cancer cell line-results of real time quantitative PCR. Asian Pac J Cancer Prev. 2013;14:6949–53. doi: 10.7314/apjcp.2013.14.11.6949. [DOI] [PubMed] [Google Scholar]
- Gilani A, Agostinucci K, Hossain S, et al. 20-HETE interferes with insulin signaling and contributes to obesity-driven insulin resistance. Prostaglandins Other Lipid Mediat. 2021;152:106485. doi: 10.1016/j.prostaglandins.2020.106485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilani A, Pandey V, Garcia V, et al. High-fat diet-induced obesity and insulin resistance in CYP4a14−/− mice is mediated by 20-HETE. Am J Physiol Regul Integr Comp Physiol. 2018;315:934–44. doi: 10.1152/ajpregu.00125.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Jordan BA, Gupta A, et al. G protein coupled receptor dimerization: implications in modulating receptor function. J Mol Med. 2001;79:226–42. doi: 10.1007/s001090100219. [DOI] [PubMed] [Google Scholar]
- González-Cao M, Karachaliou N, Viteri S, et al. Targeting PD-1/PD-L1 in lung cancer: current perspectives. Lung Cancer. 2015;6:55. doi: 10.2147/LCTT.S55176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han TS, Lean ME. Metabolic syndrome. Medicine. 2015;43:80–7. [Google Scholar]
- Hoagland KM, Flasch AK, Roman RJ. Inhibitors of 20-HETE formation promote salt-sensitive hypertension in rats. Hypertension. 2003;42:669–73. doi: 10.1161/01.HYP.0000084634.97353.1A. [DOI] [PubMed] [Google Scholar]
- Ignatov A, Robert J, Gregory Evans C, Schaller H. RANTES stimulates Ca2+ mobilization and inositol trisphosphate (IP3) formation in cells transfected with G protein coupled receptor 75. Br J Pharmacol. 2006;149:490–7. doi: 10.1038/sj.bjp.0706909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issan Y, Hochhauser E, Guo A, et al. Elevated level of pro-inflammatory eicosanoids and EPC dysfunction in diabetic patients with cardiac ischemia. Prostaglandins Other Lipid Mediat. 2013;100:15–21. doi: 10.1016/j.prostaglandins.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadid MFS, Jafari-Gharabaghlou D, Bahrami MK, Bonabi E, Zarghami N. Enhanced anti-cancer effect of curcumin loaded-niosomal nanoparticles in combination with heat-killed Saccharomyces cerevisiae against human colon cancer cells. J Drug Deliv Sci Technol. 2023:104167. [Google Scholar]
- Jafari-Gharabaghlou D, Dadashpour M, Khanghah OJ, Salmani-Javan E, Zarghami N. Potentiation of Folate-Functionalized PLGA-PEG nanoparticles loaded with metformin for the treatment of breast Cancer: possible clinical application. Mol Biol Rep. 2023;2023:1–11. doi: 10.1007/s11033-022-08171-w. [DOI] [PubMed] [Google Scholar]
- Joo WD, Visintin I, Mor G. Targeted cancer therapy–are the days of systemic chemotherapy numbered? Maturitas. 2013;76:308–14. doi: 10.1016/j.maturitas.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph G, Soler A, Hutcheson R, et al. Elevated 20-HETE impairs coronary collateral growth in metabolic syndrome via endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2017;312:528–40. doi: 10.1152/ajpheart.00561.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauser K, Clark JE, Masters BS, et al. Inhibitors of cytochrome P-450 attenuate the myogenic response of dog renal arcuate arteries. Circ Res. 1991;68:1154–63. doi: 10.1161/01.res.68.4.1154. [DOI] [PubMed] [Google Scholar]
- Kawasawa Y, McKenzie LM, Hill DP, Bono H, Yanagisawa M. G protein-coupled receptor genes in the FANTOM2 database. Genome Res. 2003;13:1466–77. doi: 10.1101/gr.1087603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodadadi M, Jafari-Gharabaghlou D, Zarghami N. An update on mode of action of metformin in modulation of meta-inflammation and inflammaging. Pharmacol Rep. 2022;74:310–22. doi: 10.1007/s43440-021-00334-z. [DOI] [PubMed] [Google Scholar]
- Kobilka BK. G protein coupled receptor structure and activation. Biochim Biophys Acta. 2007;1768:794–807. doi: 10.1016/j.bbamem.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffer CL, Laniado-Schwartzman M, Wang M-H, Nasjletti A, Elijovich F. Differential regulation of natriuresis by 20-hydroxyeicosatetraenoic acid in human salt-sensitive versus salt-resistant hypertension. Circulation. 2003;107:574–8. doi: 10.1161/01.cir.0000046269.52392.14. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ. A brief history of G-protein coupled receptors (Nobel Lecture) Angewandte Chemie Int Ed. 2013;52:6366–78. doi: 10.1002/anie.201301924. [DOI] [PubMed] [Google Scholar]
- Loos RJ, Yeo GS. The genetics of obesity: from discovery to biology. Nat Rev Genet. 2022;23:120–33. doi: 10.1038/s41576-021-00414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell L, Ferguson S, Daaka Y, et al. β-Arrestin-dependent formation of β2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–61. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- Maik-Rachline G, Hacohen-Lev-Ran A, Seger R. Nuclear ERK: mechanism of translocation, substrates, and role in cancer. Int J Mol Sci. 2019;20:1194. doi: 10.3390/ijms20051194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G, Alvarez-Curto E, Watterson K, Ulven T, Hudson B. Characterizing pharmacological ligands to study the long-chain fatty acid receptors GPR 40/FFA 1 and GPR 120/FFA 4. Br J Pharmacol. 2015;172:3254–65. doi: 10.1111/bph.12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadian F, Abhari A, Dariushnejad H, et al. Effects of chrysin-PLGA-PEG nanoparticles on proliferation and gene expression of miRNAs in gastric cancer cell line. Iran J Cancer Prev. 2016;9 doi: 10.17795/ijcp-4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejati K, Alivand M, Arabzadeh A. MicroRNA-22 in female malignancies: focusing on breast, cervical, and ovarian cancers. Pathol Res Pract. 2021;223:153452. doi: 10.1016/j.prp.2021.153452. [DOI] [PubMed] [Google Scholar]
- Nejati K, Rastegar M, Fathi F, Dadashpour M, Arabzadeh A. Nanoparticle-based drug delivery systems to overcome gastric cancer drug resistance. J Drug Deliv Sci Technol. 2022:103231. [Google Scholar]
- Obici S. Molecular targets for obesity therapy in the brain. Endocrinology. 2009;150:2512–7. doi: 10.1210/en.2009-0409. [DOI] [PubMed] [Google Scholar]
- Pascale JV, Park EJ, Adebesin AM, et al. Uncovering the signalling, structure and function of the 20-HETE-GPR75 pairing: Identifying the chemokine CCL5 as a negative regulator of GPR75. Br J Pharmacol. 2021;178:3813–28. doi: 10.1111/bph.15525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson SJ, Vanella L, Gotlinger K, et al. Oxidized HDL is a potent inducer of adipogenesis and causes activation of the Ang-II and 20-HETE systems in human obese females. Prostaglandins Other Lipid Mediat. 2016;123:68–77. doi: 10.1016/j.prostaglandins.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Powell DR, Doree DD, DaCosta CM, et al. Mice Lacking Gpr75 are Hypophagic and Thin. Diabetes Metab Syndr Obes. 2022;15 doi: 10.2147/DMSO.S342799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quon T, Lin L-C, Ganguly A, Tobin AB, Milligan G. Therapeutic opportunities and challenges in targeting the orphan G protein-coupled receptor GPR35. ACS Pharmacol Transl Sci. 2020;3:801–12. doi: 10.1021/acsptsci.0c00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakesh K, Yoo B, Kim IM, et al. β-Arrestin–biased agonism of the angiotensin receptor induced by mechanical stress. Sci Signal. 2010;3:ra46–ra. doi: 10.1126/scisignal.2000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razhabova GK, Dzhumaev KS, Odilovna KB, Axmedova GI, Dzhumaev K. Metabolic syndrome: methods of prevention and treatment. Arterial Hypertension. 2020;7 [Google Scholar]
- Revathidevi S, Munirajan AK. Akt in cancer: mediator and more. Seminars Cancer Bio. 2019;59:80–91. doi: 10.1016/j.semcancer.2019.06.002. [DOI] [PubMed] [Google Scholar]
- Roman RJ, Hoagland KM, Lopez B, et al. Characterization of blood pressure and renal function in chromosome 5 congenic strains of Dahl S rats. Am J Physiol Renal Physiol. 2006;290:1463–71. doi: 10.1152/ajprenal.00360.2005. [DOI] [PubMed] [Google Scholar]
- Sacerdoti D, Escalante B, Abraham NG, et al. Treatment with tin prevents the development of hypertension in spontaneously hypertensive rats. Science. 1989;243:388–90. doi: 10.1126/science.2492116. [DOI] [PubMed] [Google Scholar]
- Sahabi S, Jafari-Gharabaghlou D, Zarghami N. A new insight into cell biological and biochemical changes through aging. Acta Histochem. 2022;124:151841. doi: 10.1016/j.acthis.2021.151841. [DOI] [PubMed] [Google Scholar]
- Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20:1–8. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmani Javan E, Lotfi F, Jafari-Gharabaghlou D, et al. Development of a magnetic nanostructure for co-delivery of metformin and silibinin on growth of lung cancer cells: Possible action through leptin gene and its receptor regulation. Asian Pac J Cancer Prev. 2022;23:519–27. doi: 10.31557/APJCP.2022.23.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Mendoza A, López-Sánchez P, Vázquez-Cruz B, et al. Angiotensin II modulates ion transport in rat proximal tubules through CYP metabolites. Biochem Biophys Res Commun. 2000;272:423–30. doi: 10.1006/bbrc.2000.2807. [DOI] [PubMed] [Google Scholar]
- Schiaffino MV, d’Addio M, Alloni A, et al. Ocular albinism: evidence for a defect in an intracellular signal transduction system. Nat Genet. 1999;23:108–12. doi: 10.1038/12715. [DOI] [PubMed] [Google Scholar]
- Schorb W, Conrad KM, Singer HA, Dostal DE, Baker KM. Angiotensin II is a potent stimulator of MAP-kinase activity in neonatal rat cardiac fibroblasts. J Mol Cell Cardiol. 1995;27:1151–60. doi: 10.1016/0022-2828(95)90051-9. [DOI] [PubMed] [Google Scholar]
- Soler A, Hunter I, Joseph G, et al. Elevated 20-HETE in metabolic syndrome regulates arterial stiffness and systolic hypertension via MMP12 activation. J Mol Cell Cardiol. 2018;117:88–99. doi: 10.1016/j.yjmcc.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q, Ji Q, Li Q. The role and mechanism of βarrestins in cancer invasion and metastasis. Int J Mol Med. 2018;41:631–9. doi: 10.3892/ijmm.2017.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theken KN, Deng Y, Schuck RN, et al. Enalapril reverses high-fat diet-induced alterations in cytochrome P450-mediated eicosanoid metabolism. Am J Physiol Endocrinol Metab. 2012;302:500–9. doi: 10.1152/ajpendo.00370.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai IJ, Croft KD, Mori TA, et al. 20-HETE and F2-isoprostanes in the metabolic syndrome: the effect of weight reduction. Free Radic Biol Med. 2009;46:263–70. doi: 10.1016/j.freeradbiomed.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Tudurí E, Imbernon M, Hernández-Bautista RJ, et al. GPR55: a new promising target for metabolism? J Mol Endocrinol. 2017;58:191–202. doi: 10.1530/JME-16-0253. [DOI] [PubMed] [Google Scholar]
- Wang JS, Singh H, Zhang F, et al. Endothelial dysfunction and hypertension in rats transduced with CYP4A2 adenovirus. Circ Res. 2006;98:962–9. doi: 10.1161/01.RES.0000217283.98806.a6. [DOI] [PubMed] [Google Scholar]
- Wang MH, Guan H, Nguyen X, et al. Contribution of cytochrome P-450 4A1 and 4A2 to vascular 20-hydroxyeicosatetraenoic acid synthesis in rat kidneys. Am J Physiol Renal Physiol. 1999;276:246–53. doi: 10.1152/ajprenal.1999.276.2.F246. [DOI] [PubMed] [Google Scholar]
- Wang MH, Zhang F, Marji J, et al. CYP4A1 antisense oligonucleotide reduces mesenteric vascular reactivity and blood pressure in SHR. Am J Physiol Regul Integr Comp Physiol. 2001;280:255–61. doi: 10.1152/ajpregu.2001.280.1.R255. [DOI] [PubMed] [Google Scholar]
- Wang W, Qiao Y, Li Z. New insights into modes of GPCR activation. Trends Pharmacol Sci. 2018;39:367–86. doi: 10.1016/j.tips.2018.01.001. [DOI] [PubMed] [Google Scholar]
- Ward NC, Hodgson JM, Puddey IB, Beilin LJ, Croft KD. 20-Hydroxyeicosatetraenoic acid is not associated with circulating insulin in lean to overweight humans. Diabetes Res Clin Pract. 2006;74:197–200. doi: 10.1016/j.diabres.2006.04.001. [DOI] [PubMed] [Google Scholar]
- West SL, Caterini J, Banks L, Wells GD. The epidemic of obesity and poor physical activity participation: will we ever see a change? J Funct Morphol. 2018;3:34. [Google Scholar]
- Yates KF, Sweat V, Yau PL, Turchiano MM, Convit A. Impact of metabolic syndrome on cognition and brain: a selected review of the literature. Arteriosclen Thromb Vasc Biol. 2012;32:2060–7. doi: 10.1161/ATVBAHA.112.252759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou AP, Imig JD, Ortiz de Montellano P, et al. Effect of P-450 omega-hydroxylase metabolites of arachidonic acid on tubuloglomerular feedback. Am J Physiol Renal Physio. 1994;266:934–41. doi: 10.1152/ajprenal.1994.266.6.F934. [DOI] [PubMed] [Google Scholar]