Abstract
Objective:
The aim is to study the trends of lung cancer (LC) incidence in the regional context in Kazakhstan.
Methods:
The retrospective study was done using descriptive and analytical methods of oncoepidemiology. The extensive, crude and age-specific incidence rates are determined according to the generally accepted methodology used in sanitary statistics. The data were used to calculate the average percentage change (APС) using the Joinpoint regression analysis to determine the trend over the study period.
Results:
Over the 10 years under study, 36,916 new cases of LC were registered in the country (80.5% – in men and 19.5% – in women). During the studied years the average age of patients was 64.2±0.1 years (95%CI=63.9-64.4). The highest incidence rates per 100,000 in the entire population were found in the age groups 65-69 years (147.6±2.7), 70-74 years (159.3±2.5), and 75-79 years (147.1±3.2). The incidence of LC tended to increase only at the age of 80-84 years (APC=+1.26) and the most pronounced average annual decline rates were observed in the age groups of 45-49 years (APC=−4.09), 50-54 years (APC=−4.20) and 85+ years (APC=−4.07). The average annual standardized incidence rate was 22.2 per 100,000, and in dynamics tended to decrease (APC=−2.04). There is a decrease in incidence in almost all regions, with the exception of the Mangystau region (APC=+1.65). During the compilation of cartograms, incidence rates were determined on the basis of standardized indicators: low – up to 20.6, average – from 20.6 to 25.6, high – above 25.6 per 100,000 for the entire population.
Conclusion:
The incidence of lung cancer in Kazakhstan is decreasing. The incidence among the male population is six times higher than among the female, while the rate of decline is more pronounced. The incidence tends to decrease in almost all regions. High rates were found in the northern and eastern regions.
Key Words: Lung cancer, incidence, trends, geographical variation, Kazakhstan
Introduction
Lung cancer is an epidemic that depends on socio-economic, historical and cultural characteristics, as a result, the incidence has variability in different countries (Jemal et al., 2011). Recently, thanks to the global policy against smoking and the improvement of our knowledge about the genetic component of the occurrence of this pathology, there has been a noticeable improvement in the epidemiological indicators of lung cancer (Bade and Dela Cruz, 2020). Despite this, the number of cases of lung cancer and deaths from it is growing worldwide. According to WHO, in 2020, lung cancer ranked second in morbidity (2.2 million new cases) and first in mortality (1.8 million deaths) among oncological diseases in the world (Ferlay et al., 2022).
In Kazakhstan, lung cancer also occupies the first place in the structure of oncopathology, repeating the global picture, while the incidence rate among the male population is seven times higher than the female one (Ferlay et al., 2022). In general, there are about 3.2 million smokers in Kazakhstan today. 24.4% (3.2 million) of the adult population aged 15 years and older (42.2% of men and 6.6% of women) in Kazakhstan reported current tobacco use in any form (WHO global report on trends in prevalence of tobacco use 2000-2025, 2019). Kazakhstan ratified the WHO Framework Convention on Tobacco Control in 2006 and pledged to implement intersectoral measures to protect people from tobacco smoke through law and other measures (Global Adult Tobacco Survey). Legislation on tobacco control in Kazakhstan for the period from 2014 to 2019 included the following measures: a partial ban on smoking in certain closed public places; the establishment of a minimum age for the sale of tobacco products (18 years); requirements for packaging and labeling of tobacco products with health warnings covering 50% of the front and back sides tobacco packs; partial ban on tobacco advertising, promotion and sponsorship; fines for individuals and legal entities that violate the provisions of tobacco control legislation; as well as an annual increase in the minimum price for a pack of cigarettes and excise tax on tobacco products (The Law of the Republic of Kazakhstan on Ratification of the WHO Framework Convention on Tobacco Control).
The incidence of lung cancer is more related to socio-economic status due to smoking habits. About 80% of current smokers live in low- and middle-income countries, and more than half of lung cancer deaths occur in less developed countries (Bray et al., 2018). The incidence of lung cancer is expected to decrease in countries that are currently successfully conducting smoking cessation campaigns.
While the main etiological factor of lung cancer is tobacco use in 15% of cases of lung cancer in men and up to 53% in women are not associated with smoking. In addition, never smokers account for up to 25% of all lung cancer cases worldwide (Wakelee et al, 2007). Due to the increase in the incidence of lung cancer in non-smokers, there is an increasing need for a better understanding of other etiological factors contributing to the development of lung cancer, in addition to tobacco smoking. The etiological factors of lung cancer include such factors as the burning of biological resources (Kleinerman et al, 2002), air pollution (Li et al, 2017), radioactive substances formed as a result of the decay of uranium (Samet, 2006) and occupational exposure (asbestos) (Fingerhut et al, 2006).
Lung cancer is associated with a huge economic burden and every year more and more attention are paid to prevention, early detection and development of new effective methods of treating this disease. The primary prevention to reduce the incidence and mortality from lung cancer is smoking cessation. Also, the use of lung cancer screening for high-risk patients has a positive effect on the detection of lung cancer at an earlier, more curable stage (Rojewski et al, 2018). Screening with annual low-dose computed tomography reduces lung cancer mortality by 20%, which led to a grade B (moderate-quality evidence) recommendation from the United States (Moyer VA and U.S. Preventive Services Task Force, 2014). In Kazakhstan, a pilot version of lung cancer screening has been launched in some regions (Baltabekov et al, 2013). To date, the results of this program are not yet known.
The purpose of this study is to identify trends in incidence and to evaluate the spatial and temporal features of lung cancer incidence in Kazakhstan, taking into account the administrative-territorial division.
Materials and Methods
Cancer registration and patient recruitment
The cancer registry of the population of Kazakhstan covers considering the administrative-territorial division. New cases of LC were extracted from the reporting forms of the Ministry of Health of the Republic of Kazakhstan (form 7 and form 35) from 2010 to 2019 using the International Disease Code 10, code C34.
Population denominators
Population denominators for calculation of incidence rates were provided by the Bureau of National Statistics. At the same time, data on the number of populations of the republic, taking into account the studied regions, are used, all data are presented on the official website (Bureau of National Statistics, 2022).
Statistical analysis
The main method used in the study of incidence was a retrospective study using descriptive and analytical methods of oncoepidemiology. ASRs were calculated for eighteen different age groups (0-4, 5-9, …, 80-84, and 85+) using the world standard population proposed by WHO (Ahmad et al., 2001) with recommendations from the National Cancer Institute (2013).
The extensive, crude rate (CR) and age-specific incidence rates (ASIR) are determined according to the generally accepted methodology used in sanitary statistics. The annual averages (M, P), mean error (m), Student criterion, 95% confidence interval (95% CI), and average annual upward/downward rates (T%) were calculated. Mean error in statistics usually refers to the average value of the differences between estimates and true values (Glanz, 1999). Student’s criterion, also known as t-test is used to compare the means of two groups, and is based on the difference in means divided by an estimate of the standard error of the difference (Glanz, 1999). We were not smoothing the main calculation formulas in this paper, since they are detailed in the methodological recommendations and textbooks on medical and biological statistics (Merkov and Polyakov, 1974; Glanz, 1999; dos Santos Silva, 1999). The incidence trend was studied for 10 years, while the incidence trend was determined by the least squares method and using the Joinpoint program (https://surveillance.cancer.gov/joinpoint/). The data were used to calculate the average percentage change (APС) using the Joinpoint regression analysis. When compiling cartograms, crude rates and ASRs were used for 10 years (2010-2019). The method of compiling a cartogram proposed in 1974 by S.I. Igissinov (Igissinov, 1974) was used, based on the determination of the standard deviation (σ) from the average (x).
Viewing and processing of the received materials was carried out using the Microsoft 365 software package (Excel, Word, PowerPoint), in addition, online statistical calculators were used (https://medstatistic.ru/calculators/averagestudent.html), where Student criterion was calculated when comparing the average values.
Ethics approval
The study included an analysis of publicly available administrative data and did not involve contacts with individuals. The Local Ethics Commission of the Central Asian Institute for Medical Research approved this study.
Results
During the study period, 36,916 new cases of LC were registered in the country (29,704 (80.5%) – in men, and 7,212 (19.5%) – in women). The distribution of age groups by the number of new cases of LC showed that the groups aged 50 to 79 years were the most numerous – 32,231 (87.3%) and a significant proportion of new cases of LC by age groups (both sexes) characterized by a high proportion of registered cases detected over the age 55 years (namely in the groups of 55-59 years – 16.3%, 60-64 years – 20.7%, 65-69 years – 16.7% and 70-74 years – 14.0%) (Table 1). The proportion of new cases of LC among the male and female population by age group was similar to that among the general population.
Table 1.
Number and Age-Specific Incidence Rate of LC in Kazakhstan, 2010-2019
| Age | All | Male | Female | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number (%) | Incidence | Number (%) | Incidence | Number (%) | Incidence | |||||||
| per 100,000 | APC | R2 | per 100,000 | APC | R2 | per 100,000 | APC | R2 | ||||
| <30 | 119 (0.3) | 0.1±0.0 | −2.53 | 0.1005 | 62 (0.2) | 0.1±0.0 | −5.38 | 0.2997 | 57 (0.8) | 0.1±0.0 | −0.37 | 0.0078 |
| 30-34 | 152 (0.4) | 1.1±0.1 | −0.06 | 0.0090 | 87 (0.3) | 1.3±0.2 | +2.54 | 0.0291 | 65 (0.9) | 0.9±0.1 | −4.30 | 0.1460 |
| 35-39 | 269 (0.7) | 2.2±0.1 | −2.40 | 0.1774 | 170 (0.6) | 2.9±0.2 | −3.72 | 0.2958 | 99 (1.4) | 1.6±0.1 | −0.44 | 0.0016 |
| 40-44 | 641 (1.7) | 5.7±0.3 | −1.07 | 0.0589 | 423 (1.4) | 7.8±0.5 | −1.39 | 0.0636 | 218 (3.0) | 3.8±0.2 | −0.76 | 0.0047 |
| 45-49 | 1572 (4.3) | 14.8±0.7 | −4.09 | 0.8039 | 1196 (4.0) | 23.6±1.4 | −5.32 | 0.8433 | 376 (5.2) | 6.8±0.3 | −0.30 | 0.0047 |
| 50-54 | 3514 (9.5) | 34.5±1.5 | −4.20 | 0.9286 | 2872 (9.7) | 60.6±3.5 | −5.52 | 0.9544 | 642 (8.9) | 11.8±0.5 | +1.38 | 0.1043 |
| 55-59 | 6005 (16.3) | 70.2±3.0 | −3.85 | 0.8602 | 5109 (17.2) | 133.4±6.4 | −4.46 | 0.8908 | 896 (12.4) | 18.9±0.7 | −2.14 | 0.3607 |
| 60-64 | 7626 (20.7) | 118.2±3.2 | −2.27 | 0.6491 | 6471 (21.8) | 237.4±8.2 | −3.12 | 0.7876 | 1155 (16.0) | 30.7±1.2 | +1.13 | 0.1055 |
| 65-69 | 6179 (16.7) | 147.6±2.7 | −1.07 | 0.2228 | 5156 (17.4) | 310.8±6.1 | −1.22 | 0.2686 | 1023 (14.2) | 40.1±1.1 | −0.05 | 0.0098 |
| 70-74 | 5163 (14.0) | 159.3±2.5 | −0.81 | 0.3120 | 4100 (13.8) | 344.2±5.9 | −1.19 | 0.5202 | 1063 (14.7) | 52.1±2.4 | +0.49 | 0.0056 |
| 75-79 | 3744 (10.1) | 147.1±3.2 | −1.46 | 0.3655 | 2829 (9.5) | 332.3±7.2 | −1.04 | 0.2500 | 915 (12.7) | 54.0±2.4 | −1.06 | 0.0113 |
| 80-84 | 1383 (3.7) | 101.0±3.3 | +1.26 | 0.1808 | 919 (3.1) | 225.9±8.7 | +1.02 | 0.0860 | 464 (6.4) | 48.5±2.7 | +0.43 | 0.0262 |
| 85+ | 549 (1.5) | 71.3±4.3 | −4.07 | 0.4140 | 310 (1.0) | 158.7±13.5 | −4.87 | 0.2385 | 239 (3.3) | 41.7±3.5 | −5.40 | 0.4324 |
| CR | 36916 (100.0) | 21.2±0.3 | −0.71 | 0.3473 | 29704 (100.0) | 35.3±0.5 | −1.15 | 0.6481 | 7212 (100.0) | 8.0±0.2 | +0.88 | 0.1887 |
| ASR | − | 22.2±0.5 | −2.04 | 0.7989 | − | 44.7±1.2 | −2.42 | 0.8798 | − | 7.3±0.1 | −0.21 | 0.0034 |
| Average Age | − | 64.2±0.1 | +0.17 | 0.7664 | − | 64.0±0.1 | +0.20 | 0.8202 | − | 64.9±0.1 | +0.01 | 0.0083 |
APC, average percentage change; R2, the value of the approximation confidence; CR, crude rate; ASR, age standardized rate
The average age of patients with LC in dynamics increased slightly from 63.5±0.2 years (95%CI=63.2-63.9) in 2009 to 64.7±0.2 years (95%CI=64.4-65.0). The average age of patients was 64.2±0.1 years (95%CI=63.9-64.4) and APC=+0.17 (Table 1). The highest incidence rates per 100,000 in the entire population were found in the age groups 65-69 years (147.6±2.7), 70-74 years (159.3±2.5), and 75-79 years (147.1±3.2) (Figure 1).
Figure 1.
Age-Specific Incidence Rates of LC in Kazakhstan, 2010-2019
The incidence of LC had an upward trend only in one of the studied age groups: 80-84 years (APC=+1.26). In other age groups, the leveled LC incidence was decreasing, with the most pronounced annual average downward rates in the age groups of 45-49 years (APC=−4.09), 50-54 years (APC=−4.20), 55-59 years (APC=−3.85), and 85+ years (APC=−4.07) (Table 1). In the age groups of 30-34 years and 80-84 years, a trend of increasing incidence was revealed in men. On the contrary, in women, the growth trend was found in the age groups of 50-54 years, 60-64 years, 70-74 years and 80-84 years (Table 1).
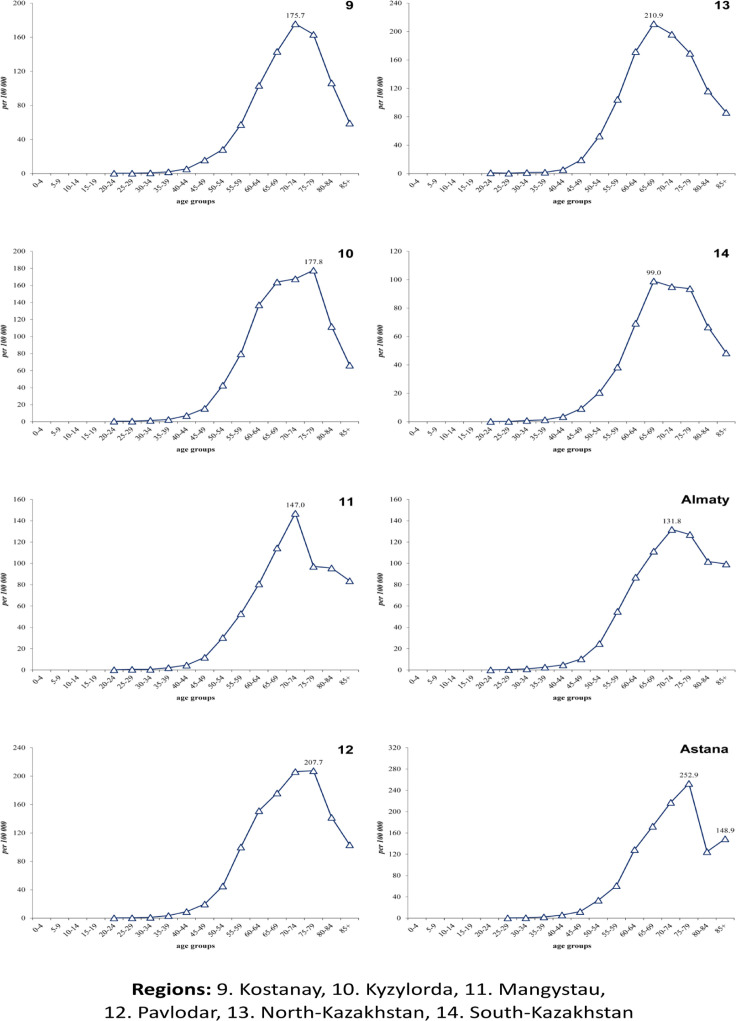
Age-specific incidence rates per 100,000 from lung cancer had regional peculiarities: almost in all regions there is a unimodal increase in incidence, except for the Akmola region (respectively 200.1, 186.6) and the city of Astana (respectively 252.9, 148.9) (Figure 2A and 2B). Unimodal growth was observed with peaks in the groups of 65-69 years, 70-74 years and also in 75-79 years (Figure 2A and 2B). Crude rates of LC incidence tended to decrease from 21.7±0.4 (95%CI=20.9-22.4) (2010) to 20.2±0.3 (95%CI=19.6-20.9) in 2019 per 100,000, the average was 21.2±0.3 per 100,000 (95%CI=20.7-21.7) (APC=−0.71). The standardized incidence rate for the study period in the male population was 44.7±1.2 per 100,000 (APC=−2.42) (Figure 3A), while this indicator in the female population was almost 6 times less and amounted to 7.3±0.1 per 100,000 (APC=−0.21) (Figure 3B). Age – standardized incidence rate for the country was 22.2±0.5 per 100,000 population (APC=−2.04) (Figure 3C).
Figure 2A.
Age-Specific Incidence Rate of Lung Cancer in Kazakhstan by region, 2010-2019
Figure 2B.
Age-Specific Incidence Rate of Lung Cancer in Kazakhstan by region, 2010-2019
Figure 3A.
Dynamics of LC Incidence (ASR) in Kazakhstan among the male population, 2010-2019
Figure 3B.
Dynamics of LC Incidence (ASR) in Kazakhstan among the Female Population, 2010-2019
Figure 3C.
Dynamics of LC Incidence (ASR) in Kazakhstan among Both Sexes, 2010-2019
Based on the calculated average annual CR and ASR LC indicators, the cartograms were compiled. The levels of LC CR per 100,000 based on the following criteria were determined: low – up to 18.4, average – from 18.4 to 28.4, high – above 28.4. As a result, the following groups of regions were revealed (Figure 4A):
Figure 4.
Cartogram of Lung Cancer Incidence in Kazakhstan, 2010-2019
1. Regions with the lowest indicators (up to 18.4 per 100,000): South Kazakhstan (8.9), Mangystau (11.4), Almaty (14.9), Kyzylorda (15.5), Zhambyl (15.6), Astana city (17.0), Almaty city (17.9).
2. Regions with average indicators (from 18.4 to 28.4 per 100,000): Atyrau (19.0). Aktobe (21.0), West Kazakhstan (26.4), Karaganda (28.1).
3. Regions with high indicators (28.4 and above per 100,000): Kostanay (32.5), Akmola (34.0), East Kazakhstan (34.3), Pavlodar (35.9), North Kazakhstan (42.5).
The levels of LC ASR per 100,000 population based on the following criteria were determined: low – up to 20.6, average – from 20.6 to 25.6, high – above 25.6. As a result, the following groups of regions were determined (Figure 4B):
1. Regions with the lowest indicators (up to 20.6 per 100,000): South Kazakhstan (13.6), Almaty (16.6), Mangystau (17.5), Almaty city (17.6), Zhambyl (18.6).
2. Regions with average indicators (from 20.6 to 25.6 per 100,000): Kyzylorda (21.2), Karaganda (23.7), Aktobe (23.8), West Kazakhstan (25.0), Atyrau (25.1), Kostanay (25.2).
3. Regions with high indicators (25.6 per 100,000 and above): Astana city (26.2), East Kazakhstan (26.9), Akmola (28.6), Pavlodar (29.3), North Kazakhstan (30.2).
According to the cartogram, the highest incidence rates of lung cancer belong to the northern and eastern regions of the country. The southern regions belong to the regions with low indicators, while the western and central regions mainly belong to the regions with average indicators.
Analyzing the average percentage change of standardized indicators (Figure 5A and 5B), it was found that there was a downward trend in almost all regions (the minimum indicator was in Zhambyl (APC=−0.38) and the maximum in Kyzylorda (APC=−5.0), with the exception of the Mangystau region (APC=+1.65) (Figure 5A).
Figure 5A.
Trends of Age-Standardized Incidence Rates of Lung Cancer in Kazakhstan, 2010-2019
Figure 5B.
Trends of Age-Standardized Incidence Rates of Lung Cancer in Kazakhstan, 2010-2019
Discussion
In Kazakhstan, the incidence of lung cancer is decreasing. Over 10 years, the incidence has decreased by 6.7%. But despite this, unfortunately, our republic belongs to regions with a high incidence rate of lung cancer.
The incidence among the male population tends to decrease with a high level of approximation, as in many countries of Europe, North America and in some Asian countries. Most likely, the decrease in incidence is due to the anti-tobacco policy that these countries adhere to (Torre et al, 2016). The trends of decreasing incidence among the male population are similar to the trends of incidence of both sexes in all regions. However, the trends in the occurrence of lung cancer among women differ from those among men due to the different progression of addiction to smoking. The incidence of lung cancer among women is increasing in countries where the tobacco epidemic began later (especially in Western and Southern Europe and most countries of Eastern Europe and South America) (Torre et al., 2014). In six regions of Kazakhstan (Kostanay – APC=+0.78, Almaty – APC=+1.47, West Kazakhstan – APC=+1.80, Zhambyl – APC=+2.83, North Kazakhstan – APC=+3.21, Mangystau – APC=+7.67) and in the city of Almaty (APC=+0.20), the incidence among the female population tends to increase. Perhaps this is due to an increase in the proportion of women who smoke. During the period 2014-2019, there was a significant decrease in smoking among men from 43.4% to 38.3% (a relative decrease of 11.8%) and a significant increase in smoking among women from 4.5% to 6.4% (a relative increase of 42.3%) (Global Adult Tobacco Survey, 2019). The increase in the number of women who smoke is commensurate with the increase in the incidence of lung cancer in women. In addition, women have an increased risk of lung cancer than men due to hormonal influences, a reduced ability to repair DNA damage and an increased amount of DNA adduct (Rivera, 2011). Also, the incidence rate increases slightly in women in certain age groups (50-54 years, 60-74 years, 80-84 years), but the overall incidence rate in women is still lower than in men. As we can see, the epidemiology of lung cancer differs between the sexes.
During the study period, about 87% of new cases belong to the age group of 50-79 years. Lung cancer develops decades after the start of smoking and, thus, is rare before the age of 30 and reaches a maximum in the elderly. The average age of patients in Kazakhstan was 64 years. The incidence of lung cancer tends to decrease after about 80 years, probably due to competing deaths from other causes or reduced classification accuracy (Spitz et al., 2006). According to the cartogram of lung cancer incidence in Kazakhstan, it was revealed that in the northern and eastern regions the indicator was the highest. The World Health Organization has identified chronic exposure to radon and its decay products in residential premises as the second cause of lung cancer after tobacco use and as the main risk factor in people who have never smoked (WHO Handbook on Indoor Radon, 2009.). There are no regional data on smoking in our republic. But according to a study by Bersimbaev and Bulgakova, high levels of radon are observed in the northern and eastern regions of Kazakhstan due to natural sources of radiation and long-term and large-scale uranium mining (Bersimbaev and Bulgakova, 2015). Radon can enhance the effects of other factors, such as cigarette smoke, dust and exhaust gases. Tobacco smoke increases the oncogenic effect of radon by 2-10 times, and radon significantly reduces the latent period of lung cancer (Méndez et al., 2011).
Lung cancer remains an incredibly lethal disease. Raising public awareness, tightening tobacco control strategies, and introducing screening for people at high risk can have a beneficial effect on reducing the prevalence of lung cancer. Studying trends in lung cancer incidence is important for planning health services and providing baseline data for assessing the impact of public health interventions.
The main advantage of the study is the identification of new trends in incidence both in the republic as a whole and at the regional level for 10 years. Limitations of the study are the lack of data on smoking history and morphological data of tumors.
Author Contribution Statement
DY, DT, DK, ZT, KA – Collection and preparation of data, primary processing of the material and their verification; TZ, KI, AO, GO, FD – Statistical processing and analysis of the material, writing the text of the article (material and methods, results); ZK, AI, US, SU, SK, KR – Writing the text of the article (introduction, discussion); NI, IK, GI, ZB, DT – Concept, design and control of the research, approval of the final version of the article. All authors approved the final version of the manuscript.
Acknowledgements
The authors greatly appreciate the contribution of the Ministry of Healthcare of the Republic of Kazakhstan to the current research by providing the data.
This study was not funded, it was performed within the framework of the Daulet Yessenbayev’s dissertation, the topic of the dissertation was approved at the University Council of Akhunbaev Kyrgyz State Medical Academy.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Ahmad OE, Boschi-Pinto C, Lopez AD, et al. Age standardization of rates: a new who standard, 2001. GPE Discussion Paper Series: No.31 EIP/GPE/EBD World Health Organization. 2001. [Google Scholar]
- Bade BC, Dela Cruz CS. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin Chest Med. 2020;41:1–24. doi: 10.1016/j.ccm.2019.10.001. [DOI] [PubMed] [Google Scholar]
- Baltabekov NT, Baipeiso DM, Rezni VL, Nasrytdinova NY. Status of early diagnosis of lung cancer in Kazakhstan and ways to improve in areas of high incidence. The pilot project KazSRIO&R. Oncol Radiol Kazakhstan. 2013;30:9–12. [Google Scholar]
- Bersimbaev RI, Bulgakova O. The health effects of radon and uranium on the population of Kazakhstan. Genes Environ. 2015;37:18. doi: 10.1186/s41021-015-0019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries (published correction appears. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- dos Santos Silva I. Cancer epidemiology: principles and methods. Lion, France: IARC; 1999. p. 441. [Google Scholar]
- Fingerhut M, Nelson DI, Driscoll T, et al. The contribution of occupational risks to the global burden of disease: summary and next steps. Med Lav. 2006;97:313–21. [PubMed] [Google Scholar]
- Glanz S. Biomedical statistics. Moscow: Practice; 1998. p. 459. (Russian) [Google Scholar]
- Igissinov SI. Preparation and application method of cartograms in oncology. Healthcare Kazakhstan. 1974;2:69–71. [Google Scholar]
- Jemal A, Bray F, Center MM. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Kleinerman RA, Wang Z, Wang L, et al. Lung cancer and indoor exposure to coal and biomass in rural China. J Occup Environ Med. 2002;44:338–44. doi: 10.1097/00043764-200204000-00014. [DOI] [PubMed] [Google Scholar]
- Li J, Li WX, Bai C, Song Y. Particulate matter-induced epigenetic changes and lung cancer. Clin Respir J. 2017;11:539–46. doi: 10.1111/crj.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez D, Alshanqeety O, Warner KE, Lantz PM, Courant PN. The impact of declining smoking on radon-related lung cancer in the United States. Am J Public Health. 2011;101:310–4. doi: 10.2105/AJPH.2009.189225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkov AM, Polyakov LY. Health Statistics. Leningrad: Medicine; 1974. p. 384. (Russian) [Google Scholar]
- Moyer VA. Screening for lung cancer: U S Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330–8. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- Rojewski AM, Tanner NT, Dai L, et al. Tobacco Dependence Predicts Higher Lung Cancer and Mortality Rates and Lower Rates of Smoking Cessation in the National Lung Screening Trial. Chest J. 2018;154:110–8. doi: 10.1016/j.chest.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JM. Residential radon and lung cancer: end of the story? J Toxicol Environ Health A. 2006;69:527–31. doi: 10.1080/15287390500260879. [DOI] [PubMed] [Google Scholar]
- Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol. 2016;893:1–19. doi: 10.1007/978-3-319-24223-1_1. [DOI] [PubMed] [Google Scholar]
- Torre LA, Siegel RL, Ward EM, Jemal A. International variation in lung cancer mortality rates and trends among women. Cancer Epidemiol Biomarkers Prev. 2014;23:1025–36. doi: 10.1158/1055-9965.EPI-13-1220. [DOI] [PubMed] [Google Scholar]
- WHO global report on trends in prevalence of tobacco use 2000-2025, third edition. World Health Organization; 2019. https://www.who.int/publications/i/item/who-global-report-on-trends-in-prevalence-of-tobacco-use-2000-2025-third-edition . [Google Scholar]
- Zeeb H, Shannon F, editors. WHO Handbook on Indoor Radon: A Public Health Perspective. Geneva: Switzerland: World Health Organization; 2009. [PubMed] [Google Scholar]