Abstract
Background:
Atezolizumab plus bevacizumab (Ate/Bev) demonstrated promising efficacy and safety in patients with advanced hepatocellular carcinoma (HCC) in the phase III IMbrave150 trial. However, patients with Child–Pugh B HCC were excluded in the abovementioned prospective trial. Therefore, we aimed to investigate the efficacy and safety of Ate/Bev in patients with Child–Pugh B HCC.
Methods:
This multicenter retrospective study included 36 patients with Child–Pugh B advanced HCC who received Ate/Bev at four cancer referral centers between May 2020 and August 2021. Comparative analyses were performed with an independent cohort of patients with Child–Pugh A HCC from the same registry (n = 133).
Results:
All patients received Ate/Bev as first-line systemic treatment for advanced HCC. The objective response and disease control rates of patients in the Child–Pugh groups B and A were 11.1% and 58.3% and 34.6% and 76.7%, respectively. The median progression-free survival (PFS) and overall survival (OS) were 3.0 months [95% confidence interval (CI), 1.7–4.3) and 7.7 months (95% CI, 4.8–10.6) in the Child–Pugh B group, whereas the median PFS and OS were 9.6 months (95% CI, 5.1–14.2) and not reached (95% CI, not available) in the Child–Pugh A group, respectively. Compared to the Child–Pugh A group, grade 3–4 adverse events (AEs) were more common in the Child–Pugh B group (44.4% versus 15.8, p < 0.001), with the most frequent grade 3–4 AEs being gastrointestinal bleeding (n = 6, 16.7%), neutropenia (n = 5, 13.9%), and thrombocytopenia (n = 4, 11.1%).
Conclusions:
In the Child–Pugh B subgroup of patients with advanced HCC, Ate/Bev treatment showed modest clinical activity. However, due to the increased frequency of serious AEs, careful evaluation of treatment response and AE management is required in this subgroup of patients.
Keywords: atezolizumab, bevacizumab, child–Pugh B, hepatocellular carcinoma, systemic treatment
Introduction
Liver cancer is the sixth most prevalent cancer worldwide and the third leading cause of cancer-related deaths globally. 1 Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer. HCC is an inflammation-associated cancer, with ~90% of the HCC occurring in the setting of chronic liver disease, including hepatitis B and hepatitis C and alcoholic and non-alcoholic fatty liver diseases. 2 The vast majority of HCC cases occur in the setting of liver cirrhosis. Therefore, treatment options should be decided in consideration of underlying liver function.
Despite recent advancements, many patients have unresectable HCC at the time of diagnosis, and recurrence or progression after initial treatment is common. 3 Systemic treatment is the mainstay of treatment in this population. However, most approved systemic treatments for unresectable HCC have only been prospectively studied in patients with Child–Pugh A HCC, and patients with impaired liver function (i.e. Child–Pugh B) were excluded from these trials because of the competing risk for liver decompensation. Therefore, only limited systemic treatment options are available for patients with Child–Pugh B HCC, implying large unmet need to guide treatment in patients with more severe hepatic impairment. Based on large observational studies, sorafenib is a recommended first-line treatment option for patients with Child–Pugh B7 HCC in the United States.4,5 In Europe, sorafenib was recommended as an option in the first-line systemic treatment setting for patients with Child–Pugh A HCC only, although these observational studies have not revealed new safety signals in patients with Child–Pugh B HCC.4,6 Nivolumab is recommended for patients with Child–Pugh A or B HCC as first-line systemic treatment in certain circumstances and as subsequent therapy in the United States.5,7
In the phase III IMbrave150 trial, the combination of atezolizumab and bevacizumab (Ate/Bev) has demonstrated significant improvement in survival outcomes in patient with unresectable HCC in Child–Pugh A liver function compared with sorafenib. 8 Based on the phase III results, current international guidelines recommend Ate/Bev as first-line systemic treatment for patients with unresectable HCC, and this combination has been considered standard first-line treatment.5,6,9 However, the safety and efficacy of this combination have not been established in patients with Child–Pugh B liver cirrhosis. Real-world data regarding safety and clinical outcomes of systemic treatment in this population are important to guide the use of systemic treatment. Therefore, we investigated the clinical outcome and safety of Ate/Bev for patients with Child–Pugh B HCC in the real-world setting.
Materials and methods
Patients
This was a retrospective, multicenter, non-comparative, observational study conducted at four cancer referral centers in South Korea, including CHA Bundang Medical Center, Yonsei Cancer Center, Ulsan University Hospital, and Haeundae Paik Hospital. From electronic medical records, we identified patients with unresectable HCC who were treated with Ate/Bev from May 2020 to August 2021. Eligible patients presented with HCC confirmed by a pathologic or noninvasive assessment according to the American Association for the Study of Liver Diseases criteria for patients with cirrhosis, Barcelona Clinic Liver Cancer stage B or C categorization, and Child–Pugh class B liver cirrhosis (i.e. Child–Pugh scores, 7–9). The eligible patients had not previously received systemic therapy for unresectable HCC and had measurable disease that was not amenable to curative or locoregional therapies or that had progressed thereafter. Patients who were followed up at the clinic at least once after the administration of Ate/Bev were included in this analysis. An independent cohort of patients with Child–Pugh class A (n = 133) with the same inclusion criteria apart from Child–Pugh class was included for the comparison of the clinical outcomes.
Treatment and assessment
The standard dosing of Ate/Bev used in the IMbrave150 trial (1200 mg of atezolizumab plus 15 mg/kg of bevacizumab intravenously every 3 weeks) was recommended. Dose interruptions or reductions were made at attending physicians’ discretion. Ate/Bev treatment continued until patients experienced intolerable toxicity or disease progression. Tumors were assessed by computed tomography or magnetic resonance imaging at baseline and every 6–8 weeks according to local institutional guidelines. Tumor responses were graded according to the Response Evaluation Criteria in Solid Tumors version 1.1. The treatment-related adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0.
Statistical analyses
Categorical variables are analyzed as frequency and percentages and were compared using Fisher’s exact test or the chi-squared test, as appropriate. Continuous variables are expressed as median and interquartile range or mean and standard deviation and were compared using unpaired two-tailed t-tests. Progression-free survival (PFS) was defined as the length of time from the start of Ate/Bev to the date of disease progression or death from any cause. Overall survival (OS) was defined as the time from treatment initiation to the date of death from any cause. Survival curves were plotted after Kaplan–Meier analysis and were compared using the log-rank test. The safety and effectiveness outcomes of the Child–Pugh B cohort were compared with those of the Child–Pugh A cohort. For all analyses, p values < 0.05 was considered statistically significant. The Statistical Package for the Social Sciences version 21.0 (IBM, Armonk, NY, USA) was used for all statistical analyses.
Results
Patients characteristics
Baseline patient characteristics are summarized in Table 1. Between May 2020 and August 2021, 36 patients with unresectable HCC in Child–Pugh B were enrolled and included in the analysis. The median age was 61 (range, 42–85 years), and 30 (83.3%) of the patients were male. Hepatitis B virus was the most common etiology of HCC (n = 21, 58.3%). Most patients had a Child–Pugh score of B7 (n = 24, 66.7%), whereas 9 (25.0%) and 3 (8.3%) patients had B8 and B9, respectively. In all, 25 (69.4%) patients had extrahepatic metastasis, with the most common metastatic site being the lung (n = 15, 41.7%), followed by lymph node (n = 10, 27.8%), and bone (n = 4, 11.1%). Macrovascular invasion was noted in 21 (58.3%) patients, and baseline serum alpha-fetoprotein (AFP) level exceeded 400 ng/ml in 21 (58.3%) patients. Among all patients, 27 underwent esophagogastroduodenoscopy (EGD) before treatment, and esophageal varices were found in 18 (n = 18/27, 66.6%) patients, graded as 1 (n = 10), 2 (n = 5), and 3 (n = 3), respectively. Varices were managed according to local practice, three (n = 3/18, 16.7%) patients underwent endoscopic ligation, two (n = 2/18, 11.1%) were on beta-blockers, and two (n = 2/18, 11.1%) received both endoscopic and pharmacological treatment.
Table 1.
Baseline characteristics.
| Characteristics | Child–Pugh B (n = 36) (%) |
Child–Pugh A (n = 133) (%) |
p-value |
|---|---|---|---|
| Age, years, median (range) | 61.0 (42.0–85.0) | 62.0 (34.0–90.0) | 0.643 |
| Sex, male | 30 (83.3) | 109 (82.0) | 1.000 |
| ECOG performance status | 0.005 | ||
| 0/1 | 31 (86.1) | 131 (98.5) | |
| 2 | 5 (13.9) | 2 (1.5) | |
| Etiology | |||
| Hepatitis B | 21 (58.3) | 92 (69.2) | 0.236 |
| Hepatitis C | 5 (13.9) | 6 (4.5) | 0.058 |
| Alcohol | 6 (16.7) | 19 (14.3) | 0.792 |
| Unknown | 4 (11.1) | 16 (12.0) | 1.000 |
| BCLC stage | 0.358 | ||
| B | 10 (27.8) | 26 (19.5) | |
| C | 26 (72.2) | 107 (80.5) | |
| ALBI grade | <0.001 | ||
| 1 | 2 (5.6) | 91 (68.4) | |
| 2 | 30 (83.3) | 42 (31.6) | |
| 3 | 4 (2.4) | 0 (0.0) | |
| AFP ⩾400 ng/ml | 21 (58.3) | 44 (33.1) | 0.007 |
| Varices, present at baseline | 18 (50.0) | 23 (17.3) | 0.001 |
| Ascites, present at baseline | 34 (94.4) | 13 (9.8) | <0.001 |
| Macrovascular invasion | 21 (58.3) | 42 (31.6) | 0.003 |
| Main portal vein invasion | 14 (38.9) | 15 (11.3) | <0.001 |
| Extrahepatic spread | 25 (69.4) | 88 (66.2) | 0.842 |
| Lung | 15 (41.7) | 41 (30.8) | |
| Lymph node | 10 (27.8) | 32 (24.1) | |
| Bone | 4 (11.1) | 21 (15.8) | |
| Peritoneum | 2 (5.6) | 9 (6.8) | |
| Prior treatment | 20 (55.6) | 101 (76.5) | 0.020 |
| Surgery | 6 (16.7) | 44 (33.1) | |
| Radiotherapy | 8 (22.9) | 28 (21.1) | |
| TACE | 13 (36.1) | 85 (64.4) | |
| RFA | 3 (8.3) | 16 (12.0) |
AFP, alpha-fetoprotein; ALBI, albumin–bilirubin; BCLC, Barcelona clinic liver cancer; ECOG, eastern cooperative oncology group; RFA, radiofrequency ablation; TACE, transcatheter arterial chemoembolization.
When compared to the Child–Pugh A cohort in the same registry, the proportions of patients with Eastern Cooperative Oncology Group performance status of 2 (13.9% versus 1.5%), albumin–bilirubin (ALBI) grade of 3 (2.4% versus 0.0%) esophageal or gastric varices on baseline EGD (50.0% versus 17.0%), and ascites (94.4% versus 9.8%) were significantly higher in the Child–Pugh B cohort (p = 0.005, p = 0.002, p = 0.001, and p < 0.001, respectively). Moreover, the Child–Pugh B cohort had more poor prognostic characteristics at baseline than the Child–Pugh A cohort, including higher baseline serum AFP level (proportion of AFP exceed 400 ng/ml, 58.3% versus 33.1%, p = 0.007) and presence of macrovascular invasion (58.3% versus 31.6%, p = 0.003). The patients in the Child–Pugh A cohort received more prior treatment than those in the Child–Pugh B cohort (76.5% versus 55.6%, p = 0.020).
Treatment outcomes
Of the 36 patients with Child–Pugh class B disease, partial response was achieved in 4 (11.1%) patients with no confirmed complete response. Stable and progressive diseases were the best responses, which were observed in 17 (47.2%) and 15 (41.7%) patients, respectively (Table 2). The objective response rate (ORR) and disease control rate (DCR) were significantly lower in the Child–Pugh B group than in the Child–Pugh A group (ORR, 11.1% versus 34.1%, p = 0.007; DCR, 58.3% versus 76.7%, p = 0.036). When patients were stratified by Child–Pugh score, the ORR and DCR were 12.5% and 58.3% in the Child–Pugh B7 group and 8.3% and 58.3% in the Child–Pugh B8/B9 group, respectively. According to the ALBI grade, the ORR and DCR were 12.5% and 59.4% in patients with ALBI grade 1–2, whereas there was no responder in patients with ALBI grade 3 (ORR, 0.0%; DCR, 50%).
Table 2.
Treatment response.
| Child–Pugh B (n = 36) (%) |
Child–Pugh B7 (n = 24) (%) |
Child–Pugh B8/B9 (n = 12) (%) |
Child–Pugh A (n = 133) (%) |
|
|---|---|---|---|---|
| Best response | ||||
| Complete response | 0 | 0 | 0 | 0 |
| Partial response | 4 (11.1) | 3 (12.5) | 1 (8.3) | 46 (34.6) |
| Stable disease | 17 (47.2) | 11 (45.8) | 6 (50.0) | 56 (42.1) |
| Progressive disease | 15 (41.7) | 10 (41.7) | 5 (41.7) | 31 (23.3) |
| Objective response rate | 11.1 | 12.5 | 8.3 | 34.1 |
| Disease control rate | 58.3 | 58.3 | 58.3 | 76.7 |
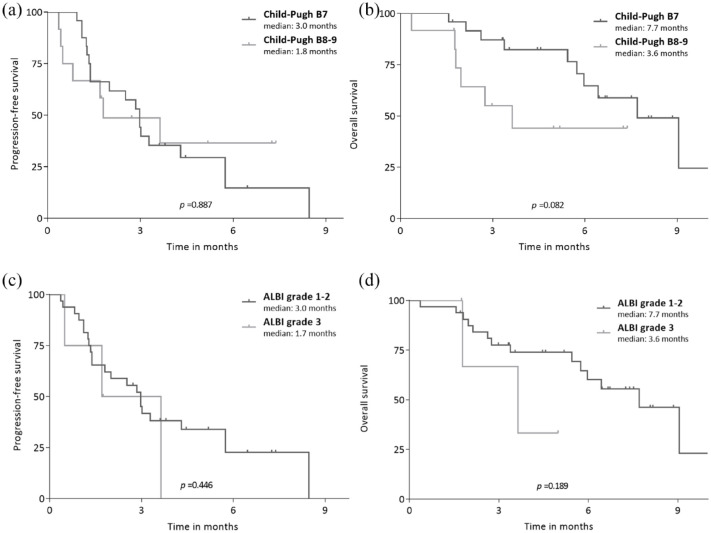
The median follow-up duration was 5.2 [95% confidence interval (CI), 1.9–8.4] months. The median PFS and OS were 3.0 (95% CI, 1.7–4.3) and 7.7 (95% CI, 4.8–10.6) months, respectively (Figure 1). When stratified by Child–Pugh score, patients with Child–Pugh score B8/B9 cirrhosis showed numerically shorter PFS and OS compared to patients with Child–Pugh B7 liver function (median PFS, 1.8 versus 3.0 months, p = 0.887; median OS, 3.6 versus 7.7 months, p = 0.082) (Figure 2(a) and (b)). When stratified by ALBI grade, patients with ALBI grade of 3 showed numerically shorter PFS (1.7 versus 3.0 months, p = 0.446) and OS (3.6 versus 7.7 months, p = 0.189) compared with those with ALBI grade of 1/2 (Figure 2 (c) and (d)).
Figure 1.
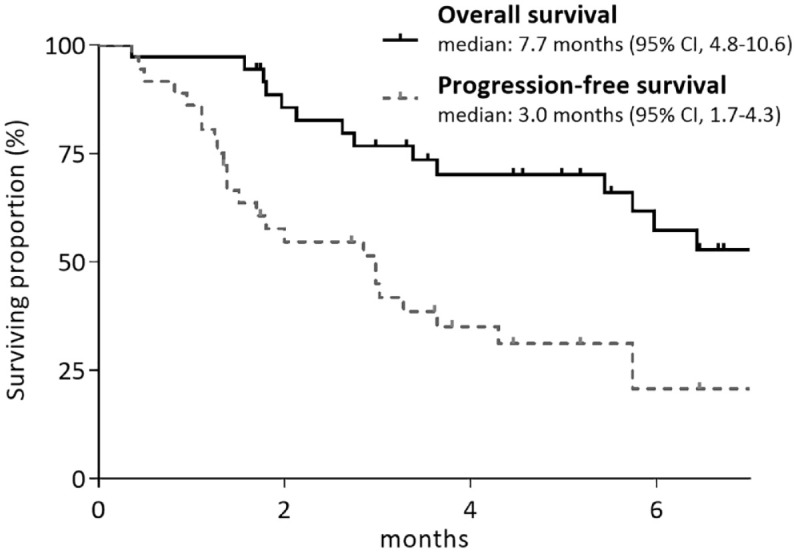
Survival outcomes in patients with Child–Pugh B (n = 36).
Figure 2.
PFS and OS according to ALBI grade and Child–Pugh scores. PFS and OS between subgroups determined by Child–Pugh score (a, b) and ALBI grade (c, d).
ALBI, albumin–bilirubin; OS, overall survival; PFS, progression-free survival.
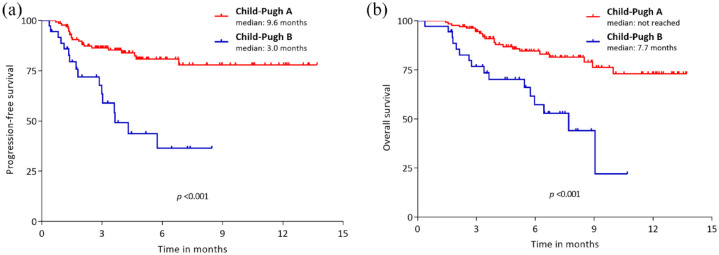
Compared to the Child–Pugh A group, the Child–Pugh B group showed significantly poorer PFS (median, 3.0 versus 9.6 months; p < 0.001) and OS (median, 7.7 months versus not reached; p < 0.001) (Figure 3(a) and (b)). In the multivariate analyses of survival outcomes, Child–Pugh B liver function [PFS: hazard ratio (HR), 2.14; 95% CI, 1.29–3.58; p = 0.004; OS: HR, 2.24; 95% CI, 1.15–4.36; p = 0.018], neutrophil-to-lymphocyte ratio (NLR) >5, and AFP exceeding 400 ng/ml were associated with worse PFS and OS outcomes (Table 3). This finding was reproduced even when Child–Pugh was replaced with ALBI grade. ALBI grade 3 (PFS: HR, 3.51; 95% CI, 1.09–11.34; p = 0.037; OS: HR, 7.55; 95% CI, 1.61–35.36; p = 0.010), NLR > 5, and AFP exceeding 400 ng/ml were associated with worse PFS and OS outcomes (Supplemental Table 1).
Figure 3.
PFS (a) and OS (b) between patients with Child–Pugh classes A and B.
OS, overall survival; PFS, progression-free survival.
Table 3.
Multivariate analysis for survival outcomes in all patients (n = 169).
| PFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | |
| Male sex | 0.73 (0.43–1.25) | 0.251 | 0.76 (0.44–1.30) | 0.316 | 0.63 (0.32–1.26) | 0.189 | 0.62 (0.30–1.27) | 0.188 |
| Age ⩾65 years | 1.01 (0.64–1.57) | 0.984 | 0.93 (0.58–1.50) | 0.768 | 0.89 (0.47–1.67) | 0.708 | 0.75 (0.38–1.50) | 0.419 |
| HBV etiology | 0.85 (0.53–1.34) | 0.479 | – | – | 0.89 (0.47–1.70) | 0.730 | – | – |
| ECOG PS 2 | 1.80 (0.73–4.49) | 0.204 | – | – | 3.27 (1.16–9.24) | 0.025 | 1.18 (0.37–3.82) | 0.779 |
| BCLC stage C | 1.02 (0.61–1.70) | 0.943 | – | – | 1.51 (0.66–3.41) | 0.327 | – | – |
| Macrovascular invasion | 1.88 (1.21–2.91) | 0.005 | 2.97 (1.59–5.54) | 0.001 | 1.95 (0.99–3.84) | 0.052 | ||
| Extrahepatic spread | 1.15 (0.72–1.82) | 0.566 | 1.39 (0.71–2.73) | 0.342 | – | – | ||
| AFP > 400 ng/ml | 2.11 (1.35–3.29) | 0.001 | 1.78 (1.11–2.86) | 0.017 | 3.26 (1.72–6.18) | <0.001 | 2.56 (1.29–5.08) | 0.007 |
| NLR > 5 | 1.96 (1.12–3.41) | 0.018 | 1.85 (1.05–3.24) | 0.033 | 2.81 (1.40–5.63) | 0.004 | 2.73 (1.32–5.62) | 0.007 |
| Child–Pugh class B | 2.77 (1.72–4.47) | <0.001 | 2.14 (1.29–3.58) | 0.004 | 5.19 (1.22–22.04) | 0.025 | 2.24 (1.15–4.36) | 0.018 |
AFP, alpha-fetoprotein; BCLC, Barcelona clinic liver cancer; CI, confidence interval; ECOG, eastern cooperative oncology group; HBV, hepatitis B; NLR, neutrophil-to-lymphocyte ratio; PS, performance status.
Safety
The AE profiles are presented in Table 4. The most common AEs of any grade were hyperbilirubinemia (n = 23, 63.9%), aspartate aminotransferase (AST) elevation (n = 20, 55.6%), fatigue (n = 15, 41.7%), and thrombocytopenia (n = 14, 38.9%). In all, 16 (44.4%) patients had grade 3–4 AEs, and the most frequent grade 3–4 AEs were gastrointestinal (GI) bleeding (n = 6, 16.7%), neutropenia (n = 5, 13.9%), and thrombocytopenia (n = 4, 11.1%). Patients who experienced grade 3 GI bleeding in the Child–Pugh B group (n = 6) had esophageal and/or gastric varices on baseline EGD. Of the six patients, two had prior grade 3 varices, one had grade 2, and three patients reported grade 1 esophageal varices. Varices were treated according to local guidelines prior to treatment initiation (endoscopic ligation, n = 1; both endoscopic ligation and beta-blocker treatment, n = 1). Ate/Bev was discontinued in 5 (14.3%) patients because of AEs. AEs that led to treatment discontinuation included GI bleeding in 4 (11.1%) patients and GI perforation in 1 (2.8%) patient.
Table 4.
Adverse events.
| Any grades | ⩾ Grades 3 | |||||
|---|---|---|---|---|---|---|
| Child–Pugh B (%) | Child–Pugh A (%) | p-value | Child–Pugh B (%) | Child–Pugh A (%) | p-value | |
| Total | 35 (97.2) | 118 (88.7) | 0.198 | 16 (44.4) | 21 (15.8) | <0.001 |
| Fatigue | 15 (41.7) | 55 (41.4) | 0.973 | – | – | – |
| Pruritus | 5 (13.9) | 18 (13.5) | 0.956 | 0 (0.0) | 2 (1.5) | 0.459 |
| Rash | 4 (11.1) | 19 (14.3) | 0.787 | 0 (0.0) | 2 (1.5) | 0.459 |
| Anorexia | 10 (27.8) | 29 (21.8) | 0.505 | 1 (2.8) | 2 (1.5) | 0.515 |
| Nausea | 10 (27.8) | 26 (19.5) | 0.358 | 0 (0.0) | 1 (0.8) | 0.602 |
| Vomiting | 2 (5.6) | 8 (6.0) | 0.917 | 1 (2.8) | 1 (0.8) | 0.319 |
| Diarrhea | 1 (2.8) | 7 (5.3) | 0.690 | – | – | – |
| Hypertension | 6 (16.7) | 72 (54.1) | <0.001 | 1 (2.8) | 6 (4.5) | 0.643 |
| Proteinuria | 11 (30.6) | 48 (36.1) | 0.537 | 0 (0.0) | 2 (1.5) | 0.459 |
| Pulmonary embolism | 0 (0.0) | 1 (0.8) | 0.602 | 0 (0.0) | 1 (0.8) | 0.602 |
| GI perforation | 1 (2.8) | 2 (1.5) | 0.608 | 1 (2.8)* | 2 (1.5) | 0.608 |
| GI hemorrhage | 6 (16.7) | 4 (3.0) | 0.002 | 6 (16.7) | 1 (0.8) | <0.001 |
| Hypothyroidism | 2 (5.6) | 6 (4.6) | 0.808 | – | – | – |
| Neutropenia | 9 (25.0) | 27 (20.3) | 0.541 | 5 (13.9) | 0 (0.0) | <0.001 |
| Anemia | 12 (33.3) | 37 (27.8) | 0.518 | 1 (2.8) | 0 (0.0) | 0.054 |
| Thrombocytopenia | 14 (38.9) | 54 (41.2) | 0.801 | 4 (11.1) | 1 (0.8) | 0.001 |
| AST elevation | 20 (55.6) | 58 (43.6) | 0.202 | 3 (8.3) | 7 (5.3) | 0.489 |
| ALT elevation | 13 (36.1) | 35 (26.3) | 0.248 | 2 (5.6) | 1 (0.8) | 0.053 |
| Hyperbilirubinemia | 23 (63.9) | 23 (17.3) | <0.001 | 2 (5.6) | 0 (0.0) | 0.006 |
| Dose reduction or interruption | 0 (0.0) | 4 (3.1) | 0.295 | – | – | – |
| Discontinuation | 5 (14.3) | 12 (9.2) | 0.374 | – | – | – |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GI, gastrointestinal.
Grade 5 toxicity.
Bold statistically significant (p<0.05).
We also compared the frequency of AEs with that of the Child–Pugh A group in the same registry. Although the overall incidence of any AEs was comparable between the Child–Pugh B and A groups (97.2% versus 88.7%, p = 0.198), grade 3–4 AEs were more frequent in the Child– TAM1148023Pugh B group than in the Child–Pugh A group (44.4% versus 15.8%, p < 0.001).
At the time of the analysis, 16 deaths (44.4%) had occurred in the Child–Pugh B group. The cause of death was disease progression (n = 11, 68.8%), deterioration in liver function without disease progression (n = 4, 25%), and serious adverse events (n = 1, GI perforation).
Discussion
In this study, we evaluated the safety and effectiveness of Ate/Bev in patients with unresectable Child–Pugh B HCC from four tertiary hospitals in Korea. The patients with Child–Pugh B HCC receiving first-line Ate/Bev showed an ORR of 11.1%, a median PFS of 3.0 months, and a median OS of 7.7 months. These results are worse compared to the Child–Pugh A group from the same registry, which demonstrated an ORR of 34.1%, a median PFS of 9.6 months, and a median OS that was not reached. Therefore, our study results indicate that the therapeutic benefit of Ate/Bev is significantly reduced in Child–Pugh B HCC patients, whereas the clinical benefit of this combination in the Child–Pugh A group is comparable with the results from the IMbrave150 study.
Management of patients with HCC is challenging in those with impaired liver function. 10 The median survival of untreated patients with Child-Pugh B HCC was 2–5 months in previous observational studies.11,12 There is currently limited evidence for systemic therapy in patients with Child–Pugh B HCC. Sorafenib was the most widely used agent in patients with Child–Pugh B HCC. In previous retrospective and prospective studies, sorafenib demonstrated an OS of only 2.5–5.2 months in patients with Child–Pugh B, implying an unmet need for novel and effective therapy options for this population.4,13–15 Recently, the efficacy of immune checkpoint inhibitor monotherapy has been evaluated in patients with Child–Pugh B HCC. In real-world data, the median OS of patients with Child–Pugh B treated with anti-programmed cell death protein 1 monotherapy ranged from 3.8 to 8.6 months.16–18 In phase I/II CheckMate 040 trial, nivolumab demonstrated favorable efficacy in patients with impaired liver function (Child–Pugh B7–B8), with median OS of 9.8 and 7.3 months in sorafenib-naive (n = 25) and sorafenib-experienced (n = 24) patients, respectively. 7 In recent analyses, the Ate/Bev combination in Child–Pugh B group showed modest efficacy (ORR: 21–25%, median: PFS 3.4–6.0 months, and median OS: 6.4–6.7 months) in real-world practice.19,20 Taken together, the role of Ate/Bev in the treatment of patients with Child–Pugh B remains controversial. Although Ate/Bev combination therapy yields superior results in patients with Child–Pugh A, further studies are required to determine whether this is reproduced in patients with Child–Pugh B (ClinicalTrials.gov identifier: NCT04829383).
Considering the vulnerability of patients with impaired liver function, it is necessary to identify the patient subset that has higher benefit–risk ratio by Ate/Bev treatment. We found that there were differences even within the Child–Pugh B subset; patients with Child–Pugh B7 showed a trend of better ORR, median PFS, and OS results compared with the Child–Pugh B8–B9 group. This finding is in line with recently published data from Japan, in which patients with Child–Pugh B8-9 showed poorer survival outcomes (median PFS; 3.0 months and OS: 4.3 months) than the Child–Pugh 7 patients (PFS: 6.3 months and OS: 7.3 months). 19 This trend was also shown in the analysis by baseline ALBI grade, wherein patients who had ALBI grade 1–2 cirrhosis at baseline demonstrated numerically improved ORR and survival outcomes, although the difference was not statistically significant due to small sample size. Careful patient selection is crucial to define the subset of patients who will receive meaningful benefits from Ate/Bev combination treatment in Child–Pugh B population, which encompass the varying degrees of hepatic impairment. Child–Pugh score and ALBI grading may be considered useful parameters to guide Ate/Bev treatment in patients with Child–Pugh B HCC.
Regarding safety outcomes, although the overall incidence of any AEs was comparable between the Child–Pugh A and B groups in this study, grade 3–4 AEs were more frequently observed in patients with Child–Pugh B than in patients with Child–Pugh A. These findings are in line with the result of a previous study, wherein patients who did not meet the inclusion criteria of the IMbrave150 study more likely experienced decompensation of liver function with the occurrence of large-volume ascites and/or high-grade hepatic encephalopathy and were at higher risk of treatment discontinuation due to its association with deteriorated liver function than patients who met the inclusion criteria. 21 In our study, the most frequent grade 3–4 AEs were GI bleeding (16.7%), and the reasons for permanent discontinuation of treatment other than progression of disease were bevacizumab-related AEs, such as GI bleeding or perforation in most patients. This finding is different from the previous nivolumab monotherapy, which reported a comparable safety profile in patients with Child–Pugh A and B HCCs. 7 This suggests that it may be necessary to define patients who can benefit from Ate/Bev combination in patients with Child–Pugh B, even when the increased risk of bevacizumab-related AEs is taken into account.
Our study has some limitations. Since our study utilized a retrospective study design, this study was subject to unintentional bias. A lack of independent radiological review limits the quality of radiology assessment. Although our study was based on multiple institutions, the number of analyzed patients was relatively small, and the results may not reflect the general population with advanced HCC in Child–Pugh B. However, our data are clinically meaningful, as our results offer real-world analysis of Ate/Bev in patients with Child–Pugh B HCC who were excluded in prospective studies. Given the limited reports on Ate/Bev in the Child–Pugh B group, our data may provide evidences to guide clinical decisions.
The results presented in this study suggest the modest efficacy of Ate/Bev in patients with Child–Pugh B HCC. However, Ate/Bev increased the risk of severe AEs, including GI bleeding in patients with Child–Pugh B, different from patients with Child–Pugh A. Further prospective studies involving Ate/Bev are warranted in patients with HCC with Child–Pugh B liver function.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359221148541 for Atezolizumab plus bevacizumab in patients with child–Pugh B advanced hepatocellular carcinoma by Jaekyung Cheon, Hyeyeong Kim, Han Sang Kim, Chang Gon Kim, Ilhwan Kim, Beodeul Kang, Chan Kim, Sanghoon Jung, Yeonjung Ha and Hong Jae Chon in Therapeutic Advances in Medical Oncology
Acknowledgments
Not applicable.
Footnotes
ORCID iDs: Han Sang Kim  https://orcid.org/0000-0002-6504-9927
https://orcid.org/0000-0002-6504-9927
Hong Jae Chon  https://orcid.org/0000-0002-6979-5812
https://orcid.org/0000-0002-6979-5812
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jaekyung Cheon, Department of Medical Oncology, CHA Bundang Medical Center, CHA University, Seongnam, South Korea.
Hyeyeong Kim, Department of Hematology-Oncology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, South Korea.
Han Sang Kim, Division of Medical Oncology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea.
Chang Gon Kim, Division of Medical Oncology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea.
Ilhwan Kim, Department of Oncology, Haeundae Paik Hospital, Inje University College of Medicine, Busan, South Korea.
Beodeul Kang, Department of Medical Oncology, CHA Bundang Medical Center, CHA University, Seongnam, South Korea.
Chan Kim, Department of Medical Oncology, CHA Bundang Medical Center, CHA University, Seongnam, South Korea.
Sanghoon Jung, Department Radiology, CHA Bundang Medical Center, CHA University, Seongnam, South Korea.
Yeonjung Ha, Department of Gastroenterology, CHA Bundang Medical Center, CHA University, 59 Yatap-ro, Bundang-gu, Seongnam 13496, South Korea.
Hong Jae Chon, Department of Medical Oncology, CHA Bundang Medical Center, CHA University, 59 Yatap-ro, Bundang-gu, Seongnam 13496, South Korea.
Declarations
Ethics approval and consent to participate: This study was approved by the institutional review board of each participating center (CHA Bundang Medical Center, 2021-12-041; Yonsei Cancer Center, 4-2021-1292; Ulsan University Hospital, 2021-12-038; Haeundae Paik Hospital, 2021-12-024-003) and was performed in accordance with the ethical standards of the institutional research committee and the recent Declaration of Helsinki. The informed consent of enrolled patients was waived owing to the retrospective nature of the study.
Consent for publication: Not applicable
Author contribution(s): Jaekyung Cheon: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Validation; Visualization; Writing – original draft; Writing – review & editing.
Hyeyeong Kim: Conceptualization; Data curation; Formal analysis; Investigation; Resources; Writing – review & editing.
Han Sang Kim: Data curation; Formal analysis; Investigation; Resources; Writing – review & editing.
Chang Gon Kim: Formal analysis; Investigation; Resources; Writing – review & editing.
Ilhwan Kim: Formal analysis; Investigation; Resources; Writing – review & editing.
Beodeul Kang: Formal analysis; Investigation; Resources; Writing – review & editing.
Chan Kim: Formal analysis; Investigation; Resources; Writing – review & editing.
Sanghoon Jung: Formal analysis; Investigation; Resources; Writing – review & editing.
Yeonjung Ha: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Validation; Writing – review & editing.
Hong Jae Chon: Conceptualization; Formal analysis; Funding acquisition; Investigation; Resources; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Research Foundation of Korea grant funded by the Korea government [MSIT] [NRF-2020R1C1C1010722 to HJC].
HJC has a consulting or advisory role at Eisai, Roche, Bayer, ONO, MSD, BMS, Celgene, Sanofi, Servier, AstraZeneca, Sillajen, Menarini, GreenCross Cell and has received research grants from Roche, Dong-A ST, Boryung Pharmaceuticals. JC received research grants from Bayer; consulting or advisory role at Roche and Eisai. The other authors have no conflicts of interest to declare.
Availability of Data and Materials: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2. Yang YM, Kim SY, Seki E. Inflammation and liver cancer: molecular mechanisms and therapeutic targets. Semin Liver Dis 2019; 39: 26–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sherman M. Recurrence of hepatocellular carcinoma. N Engl J Med 2008; 359: 2045–2047. [DOI] [PubMed] [Google Scholar]
- 4. Marrero JA, Kudo M, Venook AP, et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: the GIDEON study. J Hepatol 2016; 65: 1140–1147. [DOI] [PubMed] [Google Scholar]
- 5. Benson AB, D’Angelica MI, Abbott DE, et al. Hepatobiliary cancers, Version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2021; 19: 541–565. [DOI] [PubMed] [Google Scholar]
- 6. Vogel A, Martinelli E. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO clinical practice guidelines. Annals of Oncology 2021; 32: 801–805. [DOI] [PubMed] [Google Scholar]
- 7. Kudo M, Matilla A, Santoro A, et al. CheckMate 040 cohort 5: A phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J Hepatol 2021; 75: 600–609. [DOI] [PubMed] [Google Scholar]
- 8. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020; 382: 1894–1905. [DOI] [PubMed] [Google Scholar]
- 9. Kudo M, Kawamura Y, Hasegawa K, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer 2021; 10: 181–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int 2009; 29: 502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giannini EG, Farinati F, Ciccarese F, et al. Prognosis of untreated hepatocellular carcinoma. Hepatology 2015; 61: 184–190. [DOI] [PubMed] [Google Scholar]
- 12. Jeon D, Song GW, Lee HC, et al. Treatment patterns for hepatocellular carcinoma in patients with Child-Pugh class B and their impact on survival: a Korean nationwide registry study. Liver Int 2022; 42: 2830–2842. [DOI] [PubMed] [Google Scholar]
- 13. McNamara MG, Slagter AE, Nuttall C, et al. Sorafenib as first-line therapy in patients with advanced Child-Pugh B hepatocellular carcinoma-a meta-analysis. Eur J Cancer 2018; 105: 1–9. [DOI] [PubMed] [Google Scholar]
- 14. Pressiani T, Boni C, Rimassa L, et al. Sorafenib in patients with Child-Pugh class A and B advanced hepatocellular carcinoma: a prospective feasibility analysis. Ann Oncol 2013; 24: 406–411. [DOI] [PubMed] [Google Scholar]
- 15. Chiu J, Tang YF, Yao TJ, et al. The use of single-agent sorafenib in the treatment of advanced hepatocellular carcinoma patients with underlying Child-Pugh B liver cirrhosis: a retrospective analysis of efficacy, safety, and survival benefits. Cancer 2012; 118: 5293–5301. [DOI] [PubMed] [Google Scholar]
- 16. Lee PC, Chao Y, Chen MH, et al. Predictors of response and survival in immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. Cancers 2020; 12: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scheiner B, Kirstein MM, Hucke F, et al. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentre real-world cohort. Aliment Pharmacol Ther 2019; 49: 1323–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim HS, Hong JY, Cheon J, et al. Different organ-specific response to nivolumab to determine the survival outcome of patients with advanced hepatocellular carcinoma (aHCC). J Clin Oncol 2020; 38: 4584–4584. [Google Scholar]
- 19. Tanaka T, Hiraoka A, Tada T, et al. Therapeutic efficacy of atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma in patients with Child-Pugh class A or B liver function in real-world clinical practice. Hepatol Res 2022; 52: 773–783. [DOI] [PubMed] [Google Scholar]
- 20. D’Alessio A, Fulgenzi CAM, Nishida N, et al. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh A and B cirrhosis: a real-world study. Hepatology 2022; 76: 1000–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Castro T, Jochheim LS, Bathon M, et al. Atezolizumab and bevacizumab in patients with advanced hepatocellular carcinoma with impaired liver function and prior systemic therapy: a real-world experience. Ther Adv Med Oncol 2022; 14: 17588359221080298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359221148541 for Atezolizumab plus bevacizumab in patients with child–Pugh B advanced hepatocellular carcinoma by Jaekyung Cheon, Hyeyeong Kim, Han Sang Kim, Chang Gon Kim, Ilhwan Kim, Beodeul Kang, Chan Kim, Sanghoon Jung, Yeonjung Ha and Hong Jae Chon in Therapeutic Advances in Medical Oncology