Abstract
Serum samples from patients with confirmed human granulocytic ehrlichiosis (HGE) were tested for cytoplasmic, nuclear, and platelet autoantibodies and rheumatoid factor. The indirect fluorescence antinuclear antibody test on Hep-2 cells demonstrated antinuclear titers of ≥40 and ≥160 in 44 and 10%, respectively, of serum samples from HGE patients. Two patients (4%) had anticytoplasmic (mitochondrial and spindle apparatus) antibodies with a titer of 80 and two patients (4%) had anticytoplasmic (mitochondrial) antibodies with a titer of 160 or greater. Flow cytometry was used to demonstrate antiplatelet antibodies in 80% of first serum samples from HGE patients. Rheumatoid factor was not detected. Nuclear and cytoplasmic autoantibodies are a major cause of interference when the indirect fluorescence antibody test is used to detect fluorescence of morulae in Ehrlichia-infected equine neutrophils or HL-60 promyelocytes. Antiplatelet antibodies may contribute to the profound thrombocytopenia which is a characteristic laboratory feature during the acute phase of HGE infection. Whether autoantibodies precede infection or are caused by immune activation of HGE deserves further study.
Laboratory confirmation of suspected human granulocytic ehrlichiosis (HGE) is usually made by testing paired acute-phase and convalescence-phase serum samples by indirect fluorescence assay (IFA) using HGE-infected human HL-60 cells or Ehrlichia equi-infected equine neutrophils (11). PCR detection of the DNA of the 16S ribosomal subunit of HGE (or E. equi) is provided by a small number of specialty reference laboratories (4). Only recently has an immunoblot procedure for the detection of HGE antibodies been offered by specialized research laboratories or become commercially available for research applications (36). A problem frequently encountered with the IFA serologic test is a strong nonspecific fluorescence that interferes with reading the specific staining of ehrlichia morulae (11). Though not frequently mentioned in the medical literature about the human ehrlichioses, this nonspecific fluorescence may lead to inaccurate reading and the reporting of incorrect test results. There are currently no proficiency-testing programs that challenge the ability to accurately detect HGE antibodies.
Hematological abnormalities such as leukopenia, thrombocytopenia, and to a lesser extent, anemia, are encountered during the acute phase of HGE (36). The pathogenesis of this leukopenia associated with the ehrlichioses has not been defined for humans. Antiplatelet antibodies have been induced experimentally in dogs by means of infection with Ehrlichia canis (7, 20, 28, 34). These antiplatelet antibodies are associated with a thrombocytopenia in canine ehrlichiosis, where the E. canis is monocytotropic. Neutrophil and platelet counts in the blood of HGE patients rebound toward normal levels once therapy with doxycline is implemented or, in other cases, once the immune response to the infection is established (1, 3, 13, 18).
We decided to test serum samples from confirmed HGE patients for the presence of autoantibodies, such as cytoplasmic, nuclear, and platelet antibodies, and rheumatoid factor. If such antibodies were detected that would explain the high frequency of nonspecific staining in the IFA and possibly point to a mechanism contributing to the hematological abnormalities identified during the acute phase of HGE infection.
MATERIALS AND METHODS
Serum samples from HGE patients.
Thirty-four single serum samples and 16 sets of first (acute-phase) and follow-up serum samples were analyzed from the serum bank from HGE patients identified by an ehrlichiosis surveillance team and the Wadsworth Center of the New York State Department of Health (33, 35). These sera were dispensed into aliquots and stored at −65°C until the autoantibody testing was performed. A case control study (33) presents the clinical findings on the patients from whom the sera were obtained.
Autoantibody testing by immunofluorescence.
Sera were tested at a screening dilution of 1:40 by a conventional immunofluorescence technique with fixed Hep-2 substrate from Sanofi-Pasteur (Chaska, Minn.). The fluorescein-conjugated anti-human immunoglobulin had a fluorescein-to-protein ratio of 6. An antinuclear antibody (ANA) result was scored positive, according to the criteria of Fritzler and colleagues, if it had fluorescence greater than 1 on a scale of 0 (no fluorescence) to 4 (brightest fluorescence) (15).
Rheumatoid factor screening by latex agglutination.
The Rheumatex latex agglutination test was used as per the kit insert from the manufacturer (Wampole Laboratories, Cranbury, N.J.).
Staining procedure for antiplatelet antibodies.
The method of Breen and coworkers was used for antiplatelet antibody staining and flow cytometric analysis (6). Thirty-two of the first serum samples from confirmed HGE patients, 11 serum samples from apparently healthy blood bank donors (negative controls), and 2 plasma samples from patients with idiopathic thrombocytopenic purpura (ITP) undergoing plasmapheresis (positive controls) were assessed for antiplatelet antibodies. In addition, 12 serum samples from clinically diagnosed and laboratory-confirmed (screening test and Western blot positive) cases of Lyme disease (tick-borne disease controls) were also assessed. All specimens and controls were run simultaneously and in two separate assays using type O blood from two different donors. This assay has previously demonstrated no significant difference between type O donors when known positive and negative specimens were tested (6). The type O blood was drawn fresh from a donor the day of the assay and processed and fixed as described below within 2 to 4 h. Previous studies by Breen and coworkers demonstrated no significant differences in results between donor blood processed at 2 to 4 h and blood processed at 8 to 12 h after collection when blood was collected and stored in 3.8% sodium citrate (6). An antibody to platelet glycoprotein Ib (CD42b) conjugated with fluorescein isothiocyanate (FITC) in conjunction with light scatter was used to specifically identify the platelet population. No other specific platelet markers were used.
Type O blood from a healthy donor was collected in a blue-top Vacutainer tube containing 3.8% sodium citrate. A volume of 5 μl of this type O blood was placed in 2 ml of 0.4% formalin and fixed for 1 h. (Two aliquots were set up for each serum sample assessed.) Following fixation, all aliquots were centrifuged for 7 min at 700 × g, the supernatant was aspirated, and the remaining cells were washed once in 1 ml of Tyrode’s buffer (pH 7.1) and resuspended in 50 μl of Tyrode’s buffer (6, 10). A total of 10 μl of each serum sample was added to two tubes of cells and incubated for 15 min at room temperature. All tubes were washed twice in 1 ml of Tyrode’s buffer by centrifugation for 7 min at 700 × g and resuspended in 50 μl of Tyrode’s buffer. A total of 10 μl of anti-CD42b-FITC, specific for platelet glycoprotein Ib (Coulter-Immunotech, Miami, Fla.), and 10 μl of goat anti-human immunoglobulin G-phycoerythrin (IgG-PE) (Coulter-Immunotech) were added to one tube and 10 μl of isotype controls, mouse IgG1-FITC (Becton-Dickinson, San Jose, Calif.), and goat IgG-PE (Coulter-Immunotech) were added to the second tube for each specimen. After a 15-min incubation at room temperature in the dark, 700 μl of phosphate-buffered saline was added to each tube for analysis.
Flow cytometric fluorescence analysis.
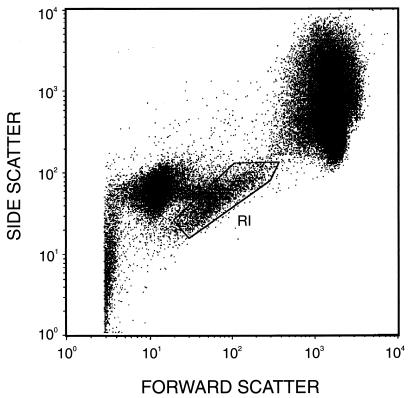
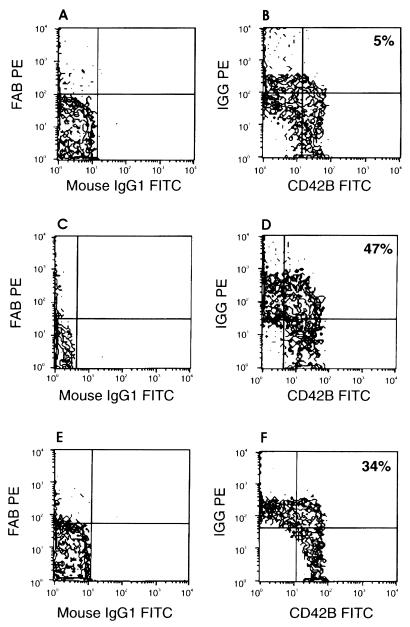
Specimens were analyzed on a FACScan (Becton-Dickinson, Sunnyvale, Calif.), and data were acquired with log amplification for forward scatter (FSC), side scatter (SSC), and fluorescence (FL1 and FL2). Flow cytometric instrument settings and fluorescence compensation were standardized by using FITC- and PE-labeled calibration beads (Calibrite Beads; Becton-Dickinson). Platelets were gated on FSC versus SSC dot plots (Fig. 1) from which FL1 (CD42b-FITC) versus FL2 (IgG-PE) contour plots were derived. Fluorescence quadrants were set with the isotype negative-control tube (Fig. 2A, C, and E). Results are expressed as percentages of the upper-right-quadrant positive-gated events that coexpress human IgG and the platelet specific marker CD42b detected with the murine monoclonal antibody (Fig. 2B, D, and F).
FIG. 1.
Bitmap-gated platelets (R1), gated on FSC versus SSC, represent the CD42B FITC-positive population upon which the platelet antibody binding was measured.
FIG. 2.
Negative-isotype-control tubes (A, C, and E) were used to set quadrant limits for a negative control (B), a positive control from an ITP patient (D), and a representative serum sample from an HGE patient (F).
Laboratory confirmation of Lyme disease sera.
An in-house enzyme immunoassay (EIA) was used to detect Borrelia burgdorferi antibodies (17). Serum samples from clinically diagnosed Lyme disease patients were confirmed by means of this EIA and immunoblotting. This two-tiered testing followed the recommendations of the Centers for Disease Control and Prevention and the Association of State and Territorial Public Health Laboratory Directors (2).
RESULTS
Nuclear and cytoplasmic antibodies detected.
We measured autoantibody titers on single serum samples from 34 HGE patients and on 16 paired serum sets from a further 16 HGE patients. Overall, 26 of 50 HGE patients had nuclear or cytoplasmic antibodies detectable at a titer of 40 or greater. The patterns of autoantibody reactivity and numbers of serum samples showing these patterns are given in Table 1. When paired sera were examined, positive results were found in both the first serum sample and the follow-up serum sample from the seven patients with positive results.
TABLE 1.
Positive results of ANA testing of 66 serum samples from 50 HGE patients by pattern and titera
| Autoantibody fluorescence pattern on Hep-2 cells | No. (%) of patients by antibody titer whose sera were positive for ANA
|
||
|---|---|---|---|
| 40 | 80 | ≥160 | |
| Homogeneous | 1 (2) | 1 (2) | 3 (6) |
| Matrix (fine speckled) | 6 (12) | 8 (16) | 1 (2) |
| Nucleolar | 0 (0) | 0 (0) | 1 (2) |
| Cytoplasmic-mitochondrial | 0 (0) | 1 (2) | 2 (4) |
| Cytoplasmic-spindle apparatus | 0 (0) | 1 (2) | 0 (0) |
| Coarse speckle (CREST)a | 1 (2) | 0 (0) | 0 (0) |
| Total | 8 (16) | 11 (22) | 7 (14) |
CREST, syndrome associated with calcinosis, Raynaud’s phenomenon, esophageal hypomotility, sclerodactyly, and telangiecstasia.
Rheumatoid factor absence.
Serum samples from 50 HGE patients tested negative when tested at the recommended screening dilution (1:20) of the kit. These results equate to less than 3 IU of rheumatoid factor per milliliter of serum (Wampole Laboratories).
Antiplatelet antibodies in first serum samples.
Thirty of the first serum samples from confirmed HGE patients were assessed for the presence of antiplatelet antibodies by flow cytometry. In addition serum samples from 11 apparently healthy individuals and 2 known ITP patients with antiplatelet antibodies were also assessed by this same method. Twelve serum samples from clinically diagnosed and laboratory-confirmed cases of Lyme disease were also tested for antiplatelet antibodies. Four of these Lyme disease serum samples were low positive on the Lyme disease EIA screening test (optical density [OD], 0.200 to 0.240), four were mid-positive (OD 0.350 to 0.600), and four were high positive (OD, 1.30 to 1.90). The antiplatelet antibody negative-control serum samples from healthy donors gave less than 15% positive-gated events in the upper-right quadrants of the histograms, as shown in Fig. 2B (ranging from 5 to 15%). The positive-control serum samples gave a maximum of 47% positive-gated events (Fig. 2D). Previous analysis of these positive-control specimens yielded positive-gated events in the range of 41 to 52%. On this basis serum samples providing less than 15% positive-gated events were considered negative whereas those yielding results >15% were considered positive for antiplatelet antibodies. The 12 serum samples from the Lyme disease patients were all negative (<15%) for antiplatelet antibodies. Twenty-five of the 30 HGE patients had positive antiplatelet antibodies (>15% positive-gated events) by our criteria. The results ranged from 15 to 42% upper-right-quadrant positive-gated events, with a median of 27% and a mean of 28%. A representative histogram of platelet antibodies from serum samples of HGE patients is shown in Fig. 2F. Remarkably, 12 of the 30 serum samples were strongly positive compared with the positive-control samples which came from two different patients with ITP.
Correlation of antinuclear and anticytoplasmic antibodies with antiplatelet antibodies.
Nineteen of the 30 serum samples which had been tested for antiplatelet antibodies produced an antibody titer of 40 or greater in the test for antinuclear and anticytoplasmic antibodies. Fourteen of these 19 serum samples were reactive in both the assay for antiplatelet antibodies and the assay for ANAs. None of these sera had detectable levels of rheumatoid factor.
DISCUSSION
Thrombocytopenia and leukopenia are defining laboratory parameters during the acute phase of both HGE and human monocytotropic ehrlichiosis (HME) (1, 3, 12, 13, 23, 30). These hematologic abnormalities are included in the case definitions of both these ehrlichial infections (8). Anemia, though not a prevalent laboratory finding, has been observed in some human subjects and in some of the animal ehrlichioses (5, 7, 9, 14, 22, 28). This anemia in some instances is associated with rouleaux formations and a positive result for a direct antiglobulin test (Coomb’s test) (34a). Nevertheless, an etiology for the rapid decrease in the leukocytes and platelets has not been described. The median age of the HGE patients in the present study was 48 years (33). There is little reason to suspect that the vector, Ixodes scapularis, preferentially feeds on, or more frequently encounters, older subjects, since there is ample evidence for an abundance of Lyme disease cases in children (1, 16, 21, 27, 29). For these reasons, we hypothesize that either patients with preexisting autoantibodies may exhibit greater morbidity when infected with the HGE agent than do children or the HGE infection may somehow induce these cytoplasmic, nuclear, and platelet antibodies in older subjects more readily than in children. Such immune dysregulation may be associated with the severity of HGE infection, which resulted in a 50% hospitalization rate of the HGE patients identified in New York state (36). One of the few hospitalized HGE pediatric patients identified by PCR at the New York state laboratory had concurrent dysregulation of the immune system by Epstein-Barr virus infection (34a). Immune dysfunction contributes to the secondary infections which have been identified in fatal HGE cases (32).
Autoantibody prevalence in apparently healthy individuals increases with age (15). Whether the antibodies are benign or pathogenic is associated with the target specificity, isotype, ability to fix complement, and electric charge. There are well-established associations of certain ANAs with connective tissue disorders and systemic rheumatic diseases and associations of cytoplasmic antibodies with autoimmune endocrine disorders and liver inflammation (15). Systemic lupus erythematosus (SLE) is one disorder where antibodies to double-stranded DNA or the Sm (Smith) antigen are relatively specific (19). These antibodies help confirm the clinical diagnosis and may be useful in predicting disease severity or outcome. Platelet-associated immune globulin, also found in SLE patients, is frequently associated with mild thrombocytopenia because of more rapid platelet clearance. Clinical features of SLE, a chronic condition, overlap with clinical manifestation of infection with the HGE agent. In both conditions fatigue, malaise, and fever are prominent (>95% of patients) as are hematologic abnormalities (19, 33). Neurologic, cardiopulmonary, and gastrointestinal problems occur in subsets of both patient groups (19, 33).
Diagnostic laboratories frequently observe significant changes in test-ordering practices associated with dissemination of disease information in professional publications and lay publications such as news magazines and newspapers. Public attention to a newly described tick-borne infection (HGE) may have diverted some health care vigilance from concerns about multisystemic rheumatologic conditions. Our findings in this study suggest that further observation of these patients with multiple autoantibody specificities is warranted, whether the antibodies are benign or pathogenic. Some patients in areas in which HGE is endemic and who have symptoms consistent with HGE may be experiencing a flare-up of a systemic autoimmune disease where immunomodulatory agents but not antibiotics would be appropriate therapy.
Analysis of anticytoplasmic and antinuclear antibody frequencies in the HGE patient serum samples showed about twice the levels described in a survey of these antibody frequencies in healthy female blood donors (15). Moreover, an analysis by Merkel and coworkers of cytoplasmic antinuclear cytoplasmic antibody (ANCA) and perinuclear ANCA in serum samples from 200 randomly selected blood donors found zero specimens to be positive (24). Demonstration of elevated autoantibody titers in serum samples of HGE patients is significant not only because it may be associated with the pathogenesis of the infection and opportunistic secondary infections but also because these antibodies cause nonspecific fluorescence that interferes with accurate reading of the ehrlichia-infected equine neutrophil or HL-60 human promyelocyte cells in the IFA serological test. Casual mention of nonspecific fluorescence interference is given in some of the reviews of serological diagnostic tests for ehrlichioses, but very few published studies have compared alternative diagnostic tests (PCR or immunoblot) with the IFA (25). Our initial observation of a large number of nonspecific reactions in the IFA prompted these investigations to find a cause of the interference. Antinuclear and anticytoplasmic antibodies can easily explain the interfering fluorescence signal seen on the equine neutrophils and the HL-60 human promyelocytes. The antiplatelet antibodies measured in vitro may be platelet-associated immunoglobulin in vivo and may explain the trapping of antibody-coated platelets in the spleen and other lymphoid tissue, allowing depletion of circulating platelets during the acute febrile phase of HGE and possibly HME. Nevertheless, the etiology of autoantibodies in HGE patients is uncertain. One possible mechanism of autoantibody production may be immune activation by bacterial DNA, as presented by Pisetsky (26). Induction of autoantibodies, B-cell activation, and increased cytokine levels follow immunostimulation by quadriplex bacterial DNA (26). Genetically susceptible individuals may produce cross-reactive antibodies when their immune systems are stimulated by bacterial DNA. Support is given to this hypothesis by the fact that bacterial DNA sequences have been found in immune complexes from SLE sera (31).
Experimental infection of dogs with E. canis by other investigators has shown the induction of antiplatelet antibodies in this canine infection, which is analogous to HME (20, 34). Earlier studies documented rapid clearance of platelets in dogs with canine ehrlichiosis (28). The rate of platelet destruction was associated with the degree of thrombocytopenia. Rapid treatment of dogs in the acute phase of disease resulted in normalization of platelet counts. The splenic sequestration resembled what occurs in ITP, but it occurred too rapidly for antibodies to be considered a part of the process. In the HGE patient sera studied here the antibodies with an affinity for platelets were present during acute illness and may thus be considered a part of the pathogenic mechanism that may also involve apoptosis and cytokines. Certainly the analysis of experimental animal model systems for HGE such as horses may be necessary to document whether the antibodies preexist the infection or are induced by modulation of immune responses during the HGE infection (9, 22). This apparent high prevalence of autoantibodies suggests that it may be prudent for clinicians to question HGE patients about coexisting autoimmune diseases or previous positive tests for autoantibodies. If patients with clinically apparent HGE have a prior history of autoantibodies or rheumatological disease, it may be fruitful to investigate the morbidity of the HGE infection in such patients compared with that of HGE patients lacking autoantibodies. In geographic regions within the range of I. scapularis a portion of patients with ITP may be found to be a subset of the HGE patients. Patients with an ehrlichial infection and apparent clinically recognized disease may be individuals with preexisting dysregulation of their immune system, by either autoimmunity or by infection with other agents. The individual patient factors associated with resistance or susceptibility to clinical disease during infection with the agent of HGE remain to be determined. The relative potency of the immune system in children may be related to the small numbers of pediatric patients with clinical HGE or the apparent resistance of children to what is recognized as HGE.
ACKNOWLEDGMENTS
We thank Christopher Pullis and Jeremy Raccio for assistance with sample preparation and analysis.
This work was supported in part by a grant from the Centers for Disease Control and Prevention (815-3478A) of the Public Health Service.
REFERENCES
- 1.Aguero-Rosenfeld M, Horowitz H W, Wormser G P, McKenna D F, Nowakowski J, Munoz J, Dumler J S. Human granulocytic ehrlichiosis: a case series from a medical center in New York state. Ann Intern Med. 1996;125:904–908. doi: 10.7326/0003-4819-125-11-199612010-00006. [DOI] [PubMed] [Google Scholar]
- 2.Association of State and Territorial Public Health Laboratory Directors and U.S. Centers for Disease Control and Prevention. Proceedings of the Second National Conference on Serologic Diagnosis of Lyme Disease. Washington, D.C: ASTPHLD; 1994. Recommendation 1.4 WB criteria; pp. 1–2. [Google Scholar]
- 3.Bakken J S, Krueth J, Wilson-Nordskog C, Tilden R L, Asanovich K, Dumler J S. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1996;275:199–205. [PubMed] [Google Scholar]
- 4.Bakken J S, Dumler J S, Chen S-M, Eckman M R, Van Etta L L, Walker D H. Human granulocytic ehrlichiosis in the upper midwest—a new species emerging? JAMA. 1994;272:212–218. [PubMed] [Google Scholar]
- 5.Berrington A, Moats R, Lester S. A case of Ehrlichia equi in an adult horse in British Columbia. Can Vet J. 1996;37:174–175. [PMC free article] [PubMed] [Google Scholar]
- 6.Breen C, Parker R, Golightly M. A stable low volume whole blood flow cytometric assay for detection of platelet surface associated antibodies and platelet antibodies in serum. Lake Placid, N.Y: International Society of Analytical Cytology Meeting; 1994. [Google Scholar]
- 7.Buhles W C, Jr, Huxsoll D L, Ristic M. Tropical canine pancytopenia: clinical, hematologic and serologic response of dogs to Ehrlichia canis infection, tetracycline therapy, and challenge inoculation. J Infect Dis. 1974;130:357–367. doi: 10.1093/infdis/130.4.357. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Case definitions for infectious conditions under public health surveillance. Morbid Mortal Weekly Rep. 1997;46:RR–10. [PubMed] [Google Scholar]
- 9.Chang, Y.-F., V. Novosel, E. Dubovi, S. J. Wong, F. Chu, C.-F. Chang, F. Del Piero, S. Shin, and D. H. Lein. Experimental infection of the human granulocytic ehrlichiosis agent in horses. Vet. Parasitol, in press. [DOI] [PubMed]
- 10.Davis B. Platelet-associated immunoglobulin assay (PAIg) In: Robinson P, editor. Handbook of flow cytometry methods. New York, N.Y: Wiley-Liss; 1993. pp. 132–134. [Google Scholar]
- 11.Dumler J S. Laboratory diagnosis of human rickettsial and ehrlichial infections. Clin Microbiol Newsl. 1996;18:57–61. [Google Scholar]
- 12.Dumler J S, Bakken J S. Ehrlichial diseases of humans: emerging tick-borne infections. Clin Infect Dis. 1995;20:1102–1110. doi: 10.1093/clinids/20.5.1102. [DOI] [PubMed] [Google Scholar]
- 13.Dumler J S, Bakken J S. Human granulocytic ehrlichiosis in Wisconsin and Minnesota: a frequent infection with the potential for persistence. J Infect Dis. 1996;173:1027–1030. doi: 10.1093/infdis/173.4.1027. [DOI] [PubMed] [Google Scholar]
- 14.Dumler, J. S. 1997. Is human granulocytic ehrlichiosis a new Lyme disease? Review and comparison of clinical, laboratory, epidemiological, and some biological features. Clin. Infect. Dis. 25(Suppl. 1):543–547. [DOI] [PubMed]
- 15.Fritzler M J, Pauls J D, Kinsella T D, Bowen T J. Antinuclear, anticytoplasmic, and anti-Sjogren’s syndrome antigen A (SS-A/Ro) antibodies in female blood donors. Clin Immunol Immunopathol. 1985;36:120–128. doi: 10.1016/0090-1229(85)90045-5. [DOI] [PubMed] [Google Scholar]
- 16.Gerber M A, Shapiro E D, Burke G S, Parcells V J, Bell G L. Lyme disease in children in southeastern Connecticut. New Engl J Med. 1996;335:1270–1274. doi: 10.1056/NEJM199610243351703. [DOI] [PubMed] [Google Scholar]
- 17.Golightly M G, Thomas J A. Enzyme immunoassay for antibodies to Borrelia burgdorferi. In: Isenberg H D, editor. Clinical microbiology procedures handbook. Washington, D.C: American Society for Microbiology; 1992. pp. 9.8.1–9.8.8. [Google Scholar]
- 18.Hardalo C J, Quagliarello V, Dumler J S. Human granulocytic ehrlichiosis in Connecticut: report of a fatal case. Clin Infect Dis. 1995;21:910–914. doi: 10.1093/clinids/21.4.910. [DOI] [PubMed] [Google Scholar]
- 19.Hahn B H. Systemic lupus erythematosus. In: Braunwald E, et al., editors. Harrison’s principles of internal medicine. 11th ed. New York, N.Y: McGraw-Hill; 1987. pp. 1418–1423. [Google Scholar]
- 20.Harrus S, Warner T, Weiss D J, Keysary A, Bark H. Kinetics of serum antiplatelet antibodies in experimental acute canine ehrlichiosis. Vet Immunol Immunopathol. 1996;51:13–20. doi: 10.1016/0165-2427(95)05516-9. [DOI] [PubMed] [Google Scholar]
- 21.Johnson B J B, Robbins K E, Bailey R E, Cao B-L, Sviat S L, Craven R B, Mayer L M, Dennis D T. Serodiagnosis of Lyme disease: accuracy of a two-step approach using a flagella-based ELISA and immunoblotting. J Infect Dis. 1996;174:346–353. doi: 10.1093/infdis/174.2.346. [DOI] [PubMed] [Google Scholar]
- 22.Madigan J E, Richter P J, Jr, Kimsey R B, Barlough J E, Bakken J S, Dumler J S. Transmission and passage in horses of the agent of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1141–1144. doi: 10.1093/infdis/172.4.1141. [DOI] [PubMed] [Google Scholar]
- 23.Magnarelli L A, Dumler J S. Ehrlichioses: emerging infectious diseases in tick-infested areas. Clin Microbiol Newsl. 1996;18:81–88. [Google Scholar]
- 24.Merkel P A, Polisson R P, Chang Y-C, Skates S J, Niles J L. Prevalence of antineutrophilic cytoplasmic antibodies in a large inception cohort of patients with connective tissue disease. Ann Intern Med. 1997;126:866–873. doi: 10.7326/0003-4819-126-11-199706010-00003. [DOI] [PubMed] [Google Scholar]
- 25.Mott J, Rikihisa Y, Zhang Y, Reed S M, Yu C Y. Comparison of PCR and culture to the indirect fluorescent-antibody test for diagnosis of Potomac horse fever. J Clin Microbiol. 1997;35:2215–2219. doi: 10.1128/jcm.35.9.2215-2219.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pisetsky D S. Immune activation by bacterial DNA: a new genetic code. Immunity. 1996;5:303–310. doi: 10.1016/s1074-7613(00)80256-3. [DOI] [PubMed] [Google Scholar]
- 27.Shrestha M, Grodzicki R, Steere A C. Diagnosing early Lyme disease. Amer J Med. 1985;78:235–240. doi: 10.1016/0002-9343(85)90432-2. [DOI] [PubMed] [Google Scholar]
- 28.Smith R D, Ristic M, Huxsoll D L, Baylor R A. Platelet kinetics in canine ehrlichiosis: evidence for increased platelet destruction as the cause of thrombocytopenia. Infect Immun. 1975;11:1216–1221. doi: 10.1128/iai.11.6.1216-1221.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steere A C. Diagnosis and treatment of Lyme arthritis. Adv Rheumatol. 1997;81:179–194. doi: 10.1016/s0025-7125(05)70510-1. [DOI] [PubMed] [Google Scholar]
- 30.Telford S R, III, Lepore T J, Shaw P, Warner C K, Dawson J E. Human granulocytic ehrlichiosis in Massachusetts. Ann Intern Med. 1995;123:277–279. doi: 10.7326/0003-4819-123-4-199508150-00006. [DOI] [PubMed] [Google Scholar]
- 31.Terada K, Okuhara E, Kawarada Y. Antigen DNA isolated from immune complexes in plasma of patients with systemic lupus erythematosus hybridizes with Escherichia coli lacZ gene. Clin Exp Immunol. 1991;85:66–69. doi: 10.1111/j.1365-2249.1991.tb05683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker D H, Dumler J S. Human monocytic and granulocytic ehrlichioses—discovery and diagnosis of emerging tick-borne infections and the critical role of the pathologist. Arch Pathol Lab Med. 1997;121:785–791. [PubMed] [Google Scholar]
- 33.Wallace B J, Brady G, Ackman D M, Wong S J, Jacquette G, Lloyd E E, Birkhead G S. Human granulocytic ehrlichiosis in New York. Arch Intern Med. 1998;158:769–773. doi: 10.1001/archinte.158.7.769. [DOI] [PubMed] [Google Scholar]
- 34.Waner T, Harrus S, Weiss D J, Bark H, Keysary A. Demonstration of serum antiplatelet antibodies in experimental acute canine ehrlichiosis. Vet Immunol Immunopathol. 1995;48:177–182. doi: 10.1016/0165-2427(95)05420-b. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 34a.Wong, S. J. Unpublished data.
- 35.Wong S J, Grady L J. Ehrlichia infection as a cause of severe respiratory distress. New Engl J Med. 1996;334:273. doi: 10.1056/NEJM199601253340418. [DOI] [PubMed] [Google Scholar]
- 36.Wong S J, Brady G S, Dumler J S. Serological responses to Ehrlichia equi, Ehrlichia chaffeensis, and Borrelia burgdorferi in patients from New York state. J Clin Microbiol. 1997;35:2198–2205. doi: 10.1128/jcm.35.9.2198-2205.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]