Introduction
Infectious disease is the second leading cause of death among patients on hemodialysis (HD), and the standardized mortality ratio for all infectious diseases was 7.5 in patients on HD compared to the general population in Japan in 2008 and 2009.1 Uremia in end-stage renal diseases impairs humoral as well as cell-mediated immunity by depleting B cells and dendritic cells, which results in higher morbidity of infectious diseases and mortality in patients on HD.2 Indeed, the mortality of COVID-19 among patients on HD exceeded 20%.3 Therefore, it is important to elucidate whether the infection-preventive effect and reduction of severe disease in clinical trials of SARS-CoV-2 vaccines4 apply to patients on HD.
Recently, several reports have been published on the humoral response of patients on HD to the SARS-CoV-2 vaccine. However, almost all of these reports are based on spike protein-specific antibody assays,5,6 and only 1 study has used the virus neutralization test.7 Furthermore, none of the study results have been discussed based on comparison with healthy subjects using virus neutralization tests including Delta and Omicron variants.
Therefore, we conducted this study to compare and evaluate the humoral response to the SARS-CoV-2 mRNA vaccine in patients on HD with chronic renal failure with that in healthy controls using the plaque reduction neutralization test (PRNT), and to obtain an optimal cut-off value of spike protein-specific antibody titer for virus neutralization in patients on HD.
Results
Characteristics of Participants
The backgrounds of the patients and controls who participated in this study are listed in Supplementary Tables S1 and S2. The significant differences between the 2 groups, the patients on HD and the healthy control, were observed in sex, age, and incidence of diabetes mellitus.
Spike Protein-Specific Antibody Test and PRNT
The results of the spike protein-specific antibody immunoassay test (Elecsys; Roche Diagnostics International Ltd., Rotkreuz, Switzerland) and PRNT are shown in Figure 1. A total of 3 patients on HD had SARS-CoV-2 infection episodes confirmed by PCR test after the second vaccination. These 3 patients were excluded from the analysis. Regarding antinucleocapsid protein antibodies measured at each point, no objective showed positive results except for known infection episodes. The spike antibody test was not performed on a patient on HD at 6 weeks.
Figure 1.
(a) Anti-spike protein-specific antibody transitions after BNT162b2 vaccinations. Anti-spike protein-specific antibodies were measured using the Elecsys Anti-SARS-CoV-2 immunoassay S (Roche Diagnostics International Ltd., Switzerland). Although anti-nucleocapsid protein antibodies were also measured at each point, no objective showed positive results except for known infection episodes. The spike antibody test was not performed in a patient on hemodialysis (HD) at 6 weeks. A total of 3 patients on HD who had SARS-CoV-2 infection after the second vaccination, and 2 patients on HD who deviated from the protocol after the second vaccination were excluded. X axis shows the weeks after the first vaccination. The Anti-S antibody levels were significantly lower in patients on HD than in volunteers at 3, 6, 12, and 30 weeks after the first vaccination. Upward arrows show the timing of vaccinations. ∗P < 0.05 (b) Neutralizing antibody titer transitions after BNT162b2 vaccinations. Cut-off value for this assay is ×10. Plaque reduction neutralization test (PRNT) results showed that the neutralizing activities for SARS-CoV-2 were significantly lower (Wuhan and Delta variant strains) in patients on HD 3 weeks after the first vaccination than in the volunteers’ controls. However, after 12 weeks (corresponding to 9 weeks after the second vaccination), there was no difference in the neutralizing activities between the serum antibodies of the 2 groups. Neutralizing antibodies against the Omicron BA.1 variant were detected in both groups only before and after the third immunization; however, there was no significant difference between the 2 groups. Upward arrows show the timing of vaccinations. ∗P < 0.05. HD, hemodialysis; PRNT, plaque reduction neutralization test.
The HD patient group had significantly lower spike protein-specific antibodies than the volunteer nondialysis group at weeks 3, 6, 12, and 30. However, there was no significant difference between the 2 groups at week 33 (Figure 1a).
PRNT results showed that the neutralizing activity against the Wuhan strain and Delta variant was significantly lower in patients on HD at week 3. However, after week 12, there was no difference in the neutralizing activity between the 2 groups (Figure 1b). The early humoral response was lower in patients on HD. Therefore, we categorized the patients on HD into antibody-positive and antibody-negative groups according to the PRNT result at week 3 and compared the factors potentially related to the lower immune response in the 2 groups (Supplementary Table S3). However, significant associations were not observed between a lower humoral response and the previously reported factors5,6 in the present study.
Correlation Between Spike Protein-Specific Antibodies and PRNT Titers
The correlations between spike protein-specific antibody titers assessed by the immunoassay and PRNT titers for all 3 COVID-19 variants were evaluated in the volunteer group and HD group (Supplementary Figure S1). These correlations were very high in both the control and HD patient groups but slightly lower for the Delta and Omicron variants.
Accuracy of Spike Protein-Specific Antibody (Elecsys) for Positive Neutralizing Activity Against Each Viral Strain
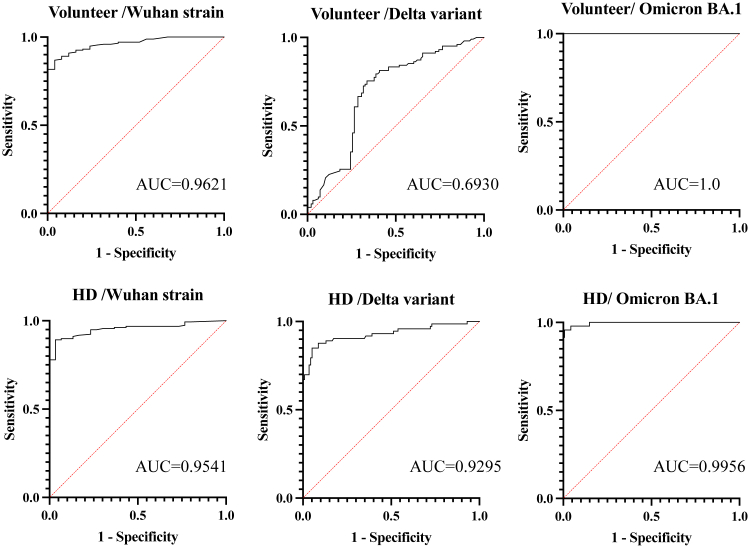
The predictive accuracy of the spike protein-specific antibody assessed by immunoassay (Elecsys) for neutralizing activity (PRNT50 ≥10) against each SARS-CoV-2 strain was evaluated in both groups using receiver operating curves (ROC) (Figure 2). The optimal cut-off values calculated by the Youden index were as follows: in the volunteer group, 148 U/ml (sensitivity: 86.8%, specificity: 96%) against the Wuhan strain, 457 U/ml (sensitivity: 75.4%, specificity: 66.3%) against the Delta variant, and 5410 U/ml (sensitivity: 100%, specificity: 100%) against the Omicron BA.1 variant; in the HD group, 28.8 U/ml against the Wuhan strain (sensitivity: 89.2%, specificity: 96.6%), 388 U/ml against the Delta variant (sensitivity: 84.9%, specificity: 94.7%); and 3300 U/ml for the Omicron BA.1 variant (sensitivity: 95.7%, specificity: 99.2%). The cut-off values in HD group were approximately 10 times higher for the Delta variant than for the Wuhan strain, and even 10 times higher for the Omicron variant BA.1. As for the differences in the accuracy between the control group and the patients on HD, they were not obvious in our samples of the Wuhan and the Omicron BA. 1 strains (the AUCs in the control/patients on HD were 0.9621/0.9541 and 1.0000/0.9956, respectively). In contrast, the difference was not trivial in the Delta variant (the AUCs in the control/patients on HD were 0.6930/0.9295).
Figure 2.
Receiver operating curves (ROC) of anti-spike protein-specific antibodies for positive neutralizing antibodies. The predictive accuracy of the spike protein-specific antibodies (Elecsys) for neutralizing activity (PRNT ≥10) against each SARS-CoV-2 strain in both groups was evaluated using ROC. Except for the Delta strain in the volunteer group, the other ROCs showed a very high AUC of >0.9. The optimal cut-off values calculated by the Youden index were as follows: in the volunteer group, 148 U/ml (sensitivity: 86.8%, specificity: 96%) against the Wuhan strain; 457 U/ml (sensitivity: 75.4%, specificity: 66.3%) against the Delta variant; 5410 U/ml (sensitivity: 100%, specificity: 100%) against the Omicron BA.1 variant; HD group, 28.8 U/ml against the Wuhan strain (sensitivity: 89.2%, specificity: 96.6%); 388 U/ml against the Delta variant (sensitivity: 84.9%, specificity: 94.7%); and 3300 U/ml for the Omicron BA.1 variant (sensitivity: 95.7%, specificity: 99.2%). There were no remarkable differences between the 2 groups. As expected, the cut-off value was approximately 10 times higher against the Delta strain than against the Wuhan strain and even 10 times higher against the Omicron BA.1 than in the Delta variant.
Discussion
The present study showed that the humoral response after the first dose of the SARS-CoV-2 mRNA vaccine was significantly reduced in patients on HD than in the control group. However, the neutralizing activities were similar between the HD and control groups 9 weeks after the second vaccination, and before or after the third vaccination. Commercial spike protein-specific antibody titers were significantly higher in the healthy volunteers up to 9 weeks after the second vaccination, although the difference was small, as previously reported.5,6
This trend was similar to that observed in patients with autoimmune diseases taking immunosuppressive drugs such as steroids and JAK inhibitors, except for tumor necrosis factor-α inhibitors and CD20 antibodies.8,9
Furthermore, a very high correlation was observed between spike protein-specific antibody titers and neutralizing antibody titers against the Wuhan strain in both control and dialysis patient groups because the spike-specific antibody was developed for the wild-type Wuhan strain. These correlations were very high in both the control and HD patient groups but slightly lower for the Delta and Omicron variants. The prediction accuracy of the spike protein-specific antibodies (Elecsys) for neutralizing activity (PRNT50 ≥10) against each SARS-CoV-2 strain was very high, with an AUC of 0.9 or higher, except for the Delta variant in the volunteer group. It was not clear why the correlation for the Delta variant in the volunteer group was relative lower than other groups.
We also analyzed the correlation between PRNT and commercially available spike protein-specific antibody kit results. Therefore, we set optimal cut-off values for each SARS-CoV-2 strain and compared the cut-off values of HD with those of the control group. The cut-off values in the HD and control groups were similar for each viral strain; the cut-off value of 28.8 U/ml for the Wuhan strain in the HD group was lower than the previously reported value of 196 U/ml.7
The limitations of our study are as follows. First, the number of participants was relatively small; so, the predictive accuracy and the cut-off values can vary in other samples. Second, the patients on HD were significantly older than those in the control group, although the ages of the 2 groups were not as far apart as in previous reports.5,6 Third, cellular immunity was not evaluated.
The strengths of this study are that almost none of the patients on HD were taking steroids or immunosuppressive drugs and were unaffected by these medications. In addition, we evaluated the neutralizing activity of antibodies in patients on HD over time using several strains of live SARS CoV-2 viruses; and were able to correlate the results with spike protein-specific antibody titers determined using commercially available kits and calculate optimal cut-off values for each virus strain.
In conclusion, the initial humoral response after SARS-CoV-2 mRNA vaccination was lower in patients on HD than in the controls. This indicates that the booster vaccination is indispensable in patients on HD. Therefore, in a future pandemic caused by a new virus or the emergence of a new variant with extremely reduced efficacy of existing vaccines, patients on HD will likely require multiple doses of a new vaccine. Continued evaluation of the response to future Omicron-compatible vaccines and the neutralizing antibody activity against new mutant strains is also needed.
Disclosure
All the authors declared no competing interests.
Acknowledgment
This research was supported by a grant from the Public Foundation of the Vaccination Research Center of Japan.
Author Contributions
Conceptualization was by TT, MK, NH, and OK. Patients’ recruitment was done by SF, MK, KTorigoe, SA, KMuta, and TN. Methodology was by TT, MMNT, and K. Morita. Data curation and statistical analysis were conducted by TT, NA, and SM. Writing of original draft preparation was by TT. Review and editing were done by YI, KTakeda, NI, SI, MT, and MK. Supervision was done by KY, KI, and HM.
Footnotes
Supplementary Methods.
Supplementary Reference.
Figure S1. Correlation between spike protein-specific antibodies and PRNT titers.
Table S1. Baseline characteristics of volunteers and hemodialysis patients.
Table S2. The factors comparison between HD patients with PRNT negative and positive at 3 weeks after 1st vaccination.
Table 3. The baseline characteristics and lab data comparison between HD patients with PRNT negative and positive at 3 weeks after 1st vaccination.
STROBE Statement.
Supplementary Materials
Supplementary Methods.
Supplementary Reference.
Figure S1. Correlation between spike protein-specific antibodies and PRNT titers.
Table S1. Baseline characteristics of volunteers and hemodialysis patients.
Table S2. The factors comparison between HD patients with PRNT negative and positive at 3 weeks after 1st vaccination.
Table 3. The baseline characteristics and lab data comparison between HD patients with PRNT negative and positive at 3 weeks after 1st vaccination.
STROBE Statement.
References
- 1.Wakasugi M., Kawamura K., Yamamoto S., Kazama J.J., Narita I. High mortality rate of infectious diseases in dialysis patients: a comparison with the general population in Japan. Ther Apher Dial. 2012;16:226–231. doi: 10.1111/j.1744-9987.2012.01062.x. [DOI] [PubMed] [Google Scholar]
- 2.Vaziri N.D., Pahl M.V., Crum A., Norris K. Effect of uremia on structure and function of immune system. J Ren Nutr. 2012;22:149–156. doi: 10.1053/j.jrn.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li P., Guan Y., Zhou S., et al. Mortality and risk factors for COVID-19 in hemodialysis patients: a systematic review and meta-analysis. Sci Prog. 2022;105 doi: 10.1177/00368504221110858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grupper A., Sharon N., Finn T., et al. Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 2021;16:1037–1042. doi: 10.2215/CJN.03500321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanai D., Wakui H., Haze T., et al. Improved immune response to the third COVID-19 mRNA vaccine dose in hemodialysis patients. Kidney Int Rep. 2022;7:2718–2721. doi: 10.1016/j.ekir.2022.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohmer N., Rabenau H.F., Ciesek S., et al. Heterologous immunization with BNT162b2 followed by mRNA-1273 in dialysis patients: seroconversion and presence of neutralizing antibodies. Nephrol Dial Transplant. 2022;37:1132–1139. doi: 10.1093/ndt/gfac018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jena A., Mishra S., Deepak P., et al. Response to SARS-CoV-2 vaccination in immune mediated inflammatory diseases: systematic review and meta-analysis. Autoimmun Rev. 2022;21 doi: 10.1016/j.autrev.2021.102927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benucci M., Damiani A., Gobbi F.L., et al. Role of booster with BNT162b2 mRNA in SARS-CoV-2 vaccination in patients with rheumatoid arthritis. Immunol Res. 2022;70:493–500. doi: 10.1007/s12026-022-09283-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.