Abstract
Introduction
IgA nephropathy (IgAN) is a progressive autoimmune kidney disease and a leading cause of glomerular disease that can result in kidney failure (KF). The median age at diagnosis is 35 to 37 years and approximately 50% of patients will progress to KF within 20 years. We aimed to enhance the understanding of renal histology and chronic kidney disease (CKD) stage at the time of IgAN diagnosis using a large real-world biopsy cohort.
Methods
This retrospective cohort study evaluated biopsy data and clinical characteristics from adult patients within the US who were diagnosed with IgAN between January 1, 2016 to May 31, 2020. Descriptive statistics were summarized and relationship(s) between each Oxford Classification (MEST-C) component score with 24-hour proteinuria or CKD stage were examined using regression analysis.
Results
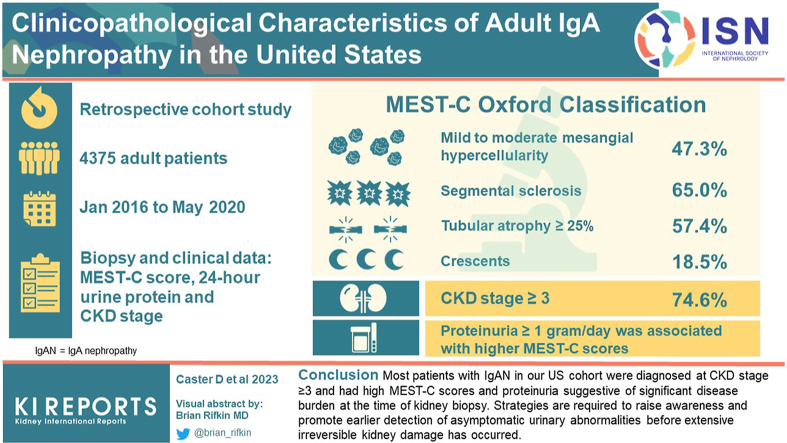
A total of 4375 patients (mean age 47.7 years, 62.7% male) met eligibility criteria. Mild to moderate mesangial hypercellularity (47.3%), segmental sclerosis (65.0%), tubular atrophy ≥25% (57.4%), and crescents (18.5%) were identified; and 74.6% of patients were at CKD stage ≥3. Proteinuria ≥1 g/d was associated with higher MEST-C scores, and the odds of mesangial hypercellularity, segmental sclerosis, tubular atrophy, and crescents increased with CKD stage.
Conclusion
Most patients with IgAN in our US cohort were diagnosed at CKD stage ≥3 and had high MEST-C scores and proteinuria that are suggestive of significant disease burden at the time of kidney biopsy. Strategies are required to raise awareness and promote earlier detection of asymptomatic urinary abnormalities before extensive irreversible kidney damage has occurred.
Keywords: autoimmune, end-stage kidney disease, IgAN, IgA nephropathy, kidney failure, MEST-C
Graphical abstract
IgAN, a progressive autoimmune kidney disease, is the most common primary glomerular disease worldwide and a leading cause of KF.1,2 Estimated incidence is between 1.29 per 100,000 (all ages)3 and 2.5 per 100,000 (adults)4 per year in the US population. Globally, prevalence rates tend to be lower in Africa, moderate in Northern Europe, and higher in East and Pacific Asian regions; however, the extent to which these variations in reported rates may reflect differences in biopsy practices is unclear.4, 5, 6
IgAN is characterized by IgA deposition in the glomerular mesangium, which in turn triggers the release of inflammatory cytokines and complement activation.1,6 Progressive glomerular injury and tubulointerstitial fibrosis are potential long-term consequences. Glomerular changes are diverse, ranging from no changes by light microscopy to crescentic glomerulonephritis.7 The extent of interstitial fibrosis and tubular atrophy in the biopsy sample has been demonstrated to be the strongest histological predictor of disease progression and renal survival.7
IgAN frequently progresses slowly without symptoms and is usually diagnosed when concern is raised due to gross or more commonly microscopic, hematuria and/or proteinuria with or without abnormal renal function.4 By the time of IgAN diagnosis, 50% of adult patients may already have CKD stage 3 or greater.2,8 This delay in diagnosis may expose the kidneys to sustained periods of elevated proteinuria, which has been shown to be the strongest clinical predictor of progression to KF.9,10 Diagnosis, which requires a renal biopsy, often occurs between 25 and 40 years of age, and up to 50% of patients will progress to KF within approximately 20 years from diagnosis.3,11, 12, 13, 14 Patients with IgAN may therefore require kidney replacement therapy at a relatively young age.
Considering that the clinical course of IgAN is so variable, potential parameters that may be predictive of outcome have been studied. Histological findings of renal biopsy samples graded using the Oxford Classification (MEST-C) have been shown to predict decline in renal function.6,15, 16, 17 Predictive power similar to that achieved through clinical monitoring over a 2-year period can be achieved through a combination of a patient’s MEST-C score with clinical data such as glomerular filtration rate, presence of arterial hypertension, and the degree of proteinuria at the time of biopsy.16,18 More recently, the international IgAN risk-prediction tool has been developed by Barbour et al.19 to predict the risk of a 50% decline in estimated glomerular filtration rate (eGFR) or KF within 5-years.
Few studies have reported the histological characteristics identified via kidney biopsy in adult patients with IgAN and the impact on disease progression.16,20, 21, 22, 23 Given the frequently asymptomatic presentation of IgAN and the potential negative implications of uncontrolled disease on long-term prognosis, we sought to characterize disease stage and histological patterns in a modern large real-world cohort of US adult patients with IgAN at the time of kidney biopsy. We further examined potential associations between MEST-C scores and 24-hour proteinuria as well as CKD stage at the time of diagnosis.
Methods
Study Design and Data Source
This noninterventional, retrospective cohort study comprises patients diagnosed with IgAN in the US between January 1, 2016 to May 31, 2020 using biopsy samples processed by Arkana Laboratories in Little Rock, AR (Arkana). Arkana provides renal pathology, serology, molecular pathology, and neuropathology services from US healthcare institutions across 43 states (Supplementary Table S1). The associated data set contains deidentified demographic and clinical information, which are collected at the time of biopsy assessment in addition to histological findings generated from adult patients with biopsy-confirmed IgAN.
To be included in this analysis, patients must have been at least 18 years of age at the time of kidney biopsy, have received a diagnosis of IgAN based upon biopsy results, and have no history of prior kidney transplant.
Exemption from the requirement for informed consent was given by Solutions Institutional Review Board.
Study Measures
Clinical characteristics in the deidentified data set included the presence or absence of hypertension, presence or absence of hematuria, serum creatinine, and level of proteinuria-based on urinary protein-to-creatinine ratio or 24-hour urinary protein excretion rate. To support proteinuria-based analyses, all proteinuria data are presented as 24-hour urinary protein excretion rate (g/d) with urinary protein-to-creatinine ratio values in g/g converted to g/d. eGFR without race or ethnicity modifier was calculated by the research team according to the CKD-EPI Creatinine Equation 202124 using the serum creatinine value closest to the biopsy date. CKD stage (1–5) was assigned using eGFR values: stage 1 (eGFR ≥90); stage 2 (eGFR 60–89); stage 3A (eGFR 45–59); stage 3B (eGFR 30–44); stage 4 (eGFR (15–29); stage 5 (eGFR <15).
MEST-C scores were assigned by Arkana for the following histological characteristics: mesangial hypercellularity (M0, no significant; M1, mild to moderate); endocapillary hypercellularity (E0, no proliferation; E1, minimal proliferation); segmental sclerosis (S0, absence of sclerosis or adhesions; S1, presence of sclerosis or adhesions); tubular atrophy (T0, ≤25%; T1, 26–50%; T2, > 50%); crescents (C0, no crescents; C1, present in at least 1 glomerulus; C2, present in >25% of glomeruli). IgA, IgG, IgM, Cq1, C3, κ and λ, and fibrinogen levels were assessed using immunofluorescence microscopy.
Sample Processing
Arkana reports use of standard renal biopsy processing techniques to include light, immunofluorescence, and electron microscopy.25 Light microscopy samples were fixed in formalin, embedded in paraffin, serially cut at 3 μm, and stained with hematoxylin and eosin, Jones methenamine silver, Masson trichrome, or periodic acid-Schiff reagent. Tissue for immunofluorescence was embedded in OCT, snap frozen, and sections were cut at 4 μm in a cryostat and stained with fluorescein-tagged polyclonal rabbit antihuman antibodies to IgG, IgA, IgM, C3, C1q, fibrinogen, and κ and λ -light chains (Dako, Carpenteria, CA) for 1 hour and rinsed; a coverslip was applied using aqueous mounting media.
For electron microscopy, 1 mm cubes were removed from the ends of the biopsy sample, dehydrated with graded alcohols, and embedded in Epon/Araldite resin. Sections were cut at 1 μm with an ultramicrotome, stained with toluidine blue, and examined with a light microscope for glomerular evaluation. Thin sections were cut at 60 nm and examined in a Jeol JEM 1011 electron microscope (Jeol, Tokyo, Japan) and photomicrographs taken at 4000, 12,000 and 20,000× magnification.
Statistical Analyses
Descriptive analyses were conducted; categorical variables were summarized using frequency and percentage, and continuous variables were summarized using mean, standard deviation, median, and interquartile range. Multinomial logistic regression was conducted to examine associations (odds ratio and 95% confidence interval) between each MEST-C component (mesangial hypercellularity, endocapillary hypercellularity, segmental sclerosis, tubular atrophy, or crescents) and CKD stage, adjusting for age, sex, and race in each model. Odds of each MEST-C component being present at CKD stages (2, 3A, 3B, 4, and 5) were assessed as compared to CKD stage 1. Logistic regression was used to assess the association between each MEST-C component and proteinuria (24-h urinary protein excretion rate: ≥1 g/d vs. <1 g/d), controlling for age, sex, and race in each model. Multicollinearity among predictors and covariates was evaluated using a correlation matrix, and variance inflation factor. All statistical tests were 2-sided, with a significance level of P < 0.05. Analyses were performed using SAS Studio 3.81 (SAS Institute, Cary, NC). Missing data was considered as a separate category in the primary objective analysis and described using frequency counts and percentages for both categorical and continuous covariates. Only patients without missing values or ‘unknown’ variables were included in regression models and statistical testing of interest.
Results
Baseline demographics and clinical characteristics
A total of 4375 patients met the full eligibility criteria for this study (Table 1). The mean age was 47.7 years, and most patients were male (62.7%). Among persons with known race (n = 2998 [68.5% of sample]), 72.4% were Caucasian, 7.5% were African American, 7.7% were Hispanic and 8.2% were Asian. Clinical indication of hypertension (presence/absence) was available for 3230 of 4375 patients (73.8% of sample) with 88.3% (2852/3230) exhibiting elevated blood pressure. Hematuria was found to be present in 93.3% (2761/2958) of patients with available data. Mean (SD) serum creatinine and eGFR were 3.0 (3.0) mg/dl and 44.2 (32.8) ml/min per 1.73 m2, respectively, among the 3828 patients with available creatinine values (histograms shown in Supplementary Figure S1). Among this subset, 74.6% presented at CKD stage 3 or higher. Just over half (52.1%) of the cohort had a proteinuria value, measured at mean (SD) of 4.2 (4.0) g/d. Proteinuria values ≥1 g/d were observed in 85.0% (1937/2280) of patients with available data.
Table 1.
Demographics and clinical characteristics
| Category | Frequency (N) | Percentage (%) |
|---|---|---|
| Overall | 4 375 | 100.0% |
| Age | ||
| Mean, yrs (SD) | 47.7 (16.6) | |
| Median, yrs (Q1, Q3) | 46 (34, 60) | |
| Sex | ||
| Male | 2742 | 62.7% |
| Race/Ethnicity | 2998 | 68.5% |
| Caucasian | 2172 | 72.4% (known race) |
| African American | 225 | 7.5% (known race) |
| Hispanic | 230 | 7.7% (known race) |
| Asian | 247 | 8.2% (known race) |
| Other | 124 | 4.1% (known race) |
| Unknown | 1377 | 31.5% |
| Hypertension | 3230 | 73.8% |
| Yes | 2852 | 88.3% |
| Hematuria | 2958 | 67.6% |
| Present | 2761 | 93.3% |
| Creatinine | 3828 | 87.5% |
| Mean, mg/dl (SD) | 3.0 (3.0) | |
| Median, mg/dl (Q1, Q3) | 2.0 (1.3, 3.4) | |
| eGFRa (ml/min/1.73 m2) | 3828 | 87.5% |
| Mean (SD) | 44.2 (32.8) | |
| Median (Q1, Q3) | 35.3 (18.9, 60.6) | |
| CKD stage | 3828 | 87.5% |
| Stage 1 | 491 | 12.8% |
| Stage 2 | 481 | 12.6% |
| Stage 3 | 1250 | 32.7% |
| Stage 3A | 481 | 12.6% |
| Stage 3B | 769 | 20.1% |
| Stage 4 | 865 | 22.6% |
| Stage 5 | 741 | 19.4% |
| Proteinuria | 2280 | 52.1% |
| Mean, g/d (SD) | 4.2 (4.0) | |
| Median, g/d (Q1, Q3) | 3.0 (1.5, 5.8) | |
| ≥1 g/d | 1937 | 85.0% |
| ≥3.5 g/d | 1018 | 44.6% |
eGFR, estimated glomerular filtration rate.
Calculated for patients with available creatinine data using 2021 CKD-EPI creatinine calculation.
Histological characteristics for the overall cohort determined using immunofluorescence microscopy (Supplementary Table S2) along with a breakdown of demographic and clinical characteristics stratified by age and race (Supplementary Table S3), and presence or absence of proteinuria ≥1 g/d (Supplementary Table S4) are available in online supplemental content. Patient counts by CKD stage or MEST-C score (Supplementary Table S5) and proteinuria value or MEST-C score (Supplementary Table S6) are also available.
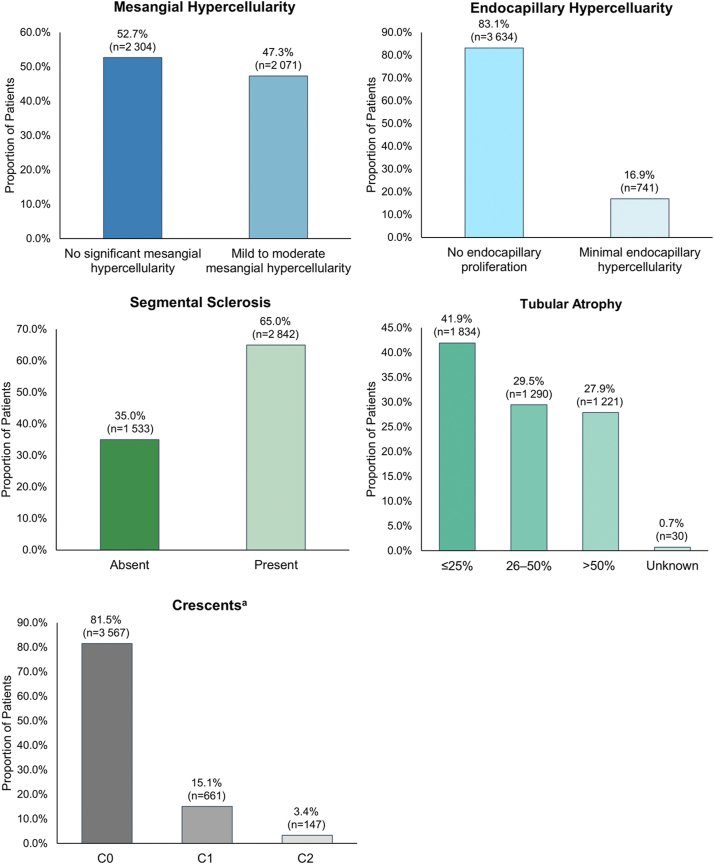
Among the 4375 patient biopsy samples evaluated, almost half (47.3%) showed mild to moderate mesangial hypercellularity and 16.9% exhibited minimal endocapillary hypercellularity (Figure 1). Segmental sclerosis and/or adhesion of tuft to Bowman capsule was present in almost two-thirds of biopsies (65.0%). Tubular atrophy of 26% to 50% of kidney tissue was observed in 29.5% of patients, whereas 27.9% exhibited tubular atrophy in >50% of kidney tissue. Crescents were present in at least 1 glomerulus in 15.1% of patients, and 3.4% had crescents in >25% of glomeruli.
Figure 1.
Distribution of histological characteristics in patients with IgAN at time of kidney biopsy. aC0, no crescents; C1, crescent present in at least one glomerulus; C2, present in >25% of the glomeruli.
Associations Between MEST-C and CKD Stage
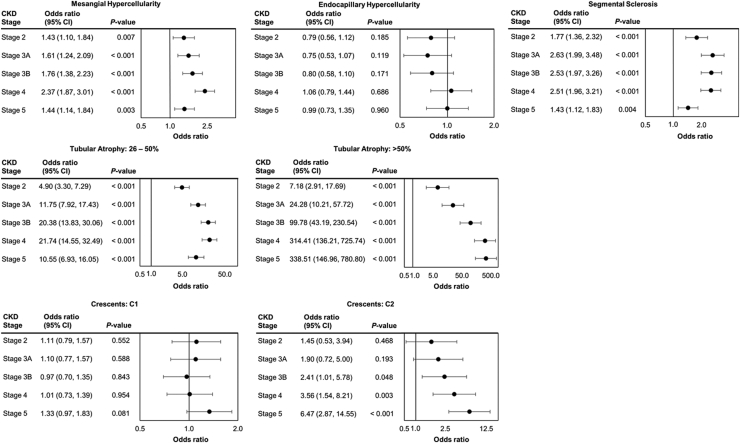
Mesangial hypercellularity was associated with increased odds of being in a more severe CKD stage than stage 1, with the adjusted odds ratios (95% confidence interval) being 1.43 (1.10, 1.84), 1.61 (1.24, 2.09), 1.76 (1.38, 2.23), 2.37 (1.87, 3.01), and 1.44 (1.14, 1.84) for stages 2, 3A, 3B, 4, and 5, respectively (Figure 2). Mesangial hypercellularity had the highest odds ratio for stage 4 versus stage 1, which indicates that most of patients with mesangial hypercellularity were in stage 4 when their biopsy was taken. Similarly, CKD stages 3A, 3B, and 4 were each associated with greater odds of segmental sclerosis compared with CKD stages 1 and 2. Odds of tubular atrophy >50% of kidney tissue increased significantly with higher CKD stages to a maximum of 338.51 (146.76, 780.80; P < 0.001) among persons diagnosed at CKD stage 5. The odds of observing crescents in >25% of glomeruli increased with higher CKD stage, reaching statistical significance for stage 3B (2.41 [1.01, 5.78]; P = 0.048), beyond stage 4 (3.56 [1.54, 8.21]; P = 0.003), and stage 5 (6.47 [2.87, 14.55]; P < 0.001). Although the presence of endocapillary hypercellularity showed a similar directional pattern with greatest odds (1.06 [0.79, 1.44]; P = 0.686) identified in patients with CKD stage 4, this finding was not statistically significant.
Figure 2.
Associations between histological characteristics and CKD stage. C1, crescent present in 1%–24% of glomeruli; C2, present in >25% of glomeruli. Reference groups: CKD stage: stage 1; Mesangial Hypercellularity (Ref: No significant mesangial hypercellularity); Endocapillary Hypercellularity (Ref: No endocapillary proliferation); Segmental Sclerosis (Ref: Absence); Tubular Atrophy (Ref: ≤25%); Crescents (Ref: C0 (no crescents)). CKD, chronic kidney disease.
Associations Between MEST-C and Proteinuria
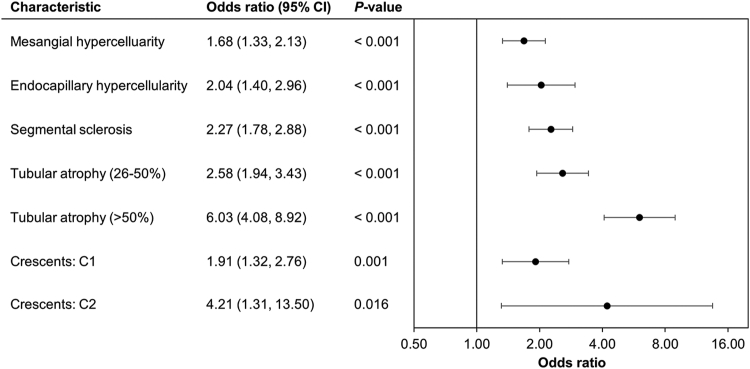
Proteinuria ≥1 g/d had a statistically significant association with the presence of all MEST-C histological characteristics assessed in this study (Figure 3). Patients with crescents present in at least 25% of their glomeruli had greater odds of having proteinuria ≥ 1 g/d at biopsy (4.21 [1.31,13.50]; P = 0.016), which increased further in patients with >50% tubular atrophy (6.03 [4.08, 8.92]; P < 0.001). Patients were also at least twice as likely to have proteinuria ≥1 g/d if they demonstrated endocapillary hypercellularity (2.04 [1.40, 2.96]; P < 0.001), segmental sclerosis (2.27 [1.78, 2.88]; P < 0.001) and tubular atrophy of 26%–50% (2.58 [1.94, 3.43]; P < 0.001), and 1.68 times as likely if they presented with mesangial hypercellularity (1.68 [1.33, 2.13]; P < 0.001). A similar pattern was evidenced among patients with proteinuria values ≥3.5 g/d compared with <3.5 g/d (Supplementary Figure S2).
Figure 3.
Associations between histological characteristics and proteinuria (≥1 vs. <1 g/d). C1, crescent present in 1%–25% of glomeruli; C2, present in >25% of glomeruli. Reference groups: Proteinuria (Ref: <1 g/d); Mesangial Hypercellularity (Ref: No significant mesangial hypercellularity); Endocapillary Hypercellularity (Ref: No endocapillary proliferation); Segmental Sclerosis (Ref: Absence); Tubular Atrophy (Ref: ≤25%); Crescents (Ref: C0 [no crescents]).
Discussion
This study included 4375 IgAN-positive biopsy samples; 3828 from patients with an available serum creatinine level for estimation of CKD stage at time of diagnosis, and 2280 with available proteinuria data.
Among the 3828 US adults with known serum creatinine values, three-quarters (75%) received their diagnosis at CKD stage 3 or later, whereas 42% had advanced to CKD stage 4 or 5 by the time of diagnosis. Our US-based real-world cohort findings of advanced CKD stage at time of diagnosis (42% CKD stage 4 or 5) are remarkably higher than those reported by Hastings et al.,8 who found that 29% of US-based patients diagnosed with IgAN had progressed to either CKD stage 4 or 5 at the time of kidney biopsy. In contrast, an assessment of kidney biopsy samples from 771 Japanese patients (87% adult and 13% pediatric) with primary IgAN demonstrated that less than a third had advanced to CKD stage ≥3 and approximately 5% to stage 4 or 5 at the time of IgAN diagnosis.26 Diagnosis of IgAN at an earlier stage of disease in Japan may stem from the presence of mandatory kidney screening programs across Japan, first implemented in the 1970s with later programmatic updates.27 IgAN is often asymptomatic with only urinary abnormalities in its early stages, which means that in countries without mandatory screening, it is likely that most of the patients reaching the biopsy stage are already displaying symptoms and are therefore at a later stage in their disease.6,28
Another key objective of this study was to characterize the histological patterns of disease at IgAN diagnosis. Our results demonstrated that a high proportion of patients in our US cohort had substantial kidney damage, with M1, E1, S1, T1/2, and C1/2 scores being assigned to high proportions of biopsies. We show that 47% of patients presented with mesangial hypercellularity, 17% with endocapillary hypercellularity, 65% with segmental sclerosis, 57% with >25% tubular atrophy, and 19% with crescents. This high proportion of patient biopsies with segmental sclerosis and tubular atrophy is consistent with an advanced stage of CKD at the time of diagnosis in our cohort. Similar frequencies of both S1 and T1/2 scores have been reported in studies conducted in Brazil23 and India,29 whereas several other studies have shown at least 50% of patients present with segmental sclerosis, but lesser amounts of tubular atrophy.
Excluding endocapillary hypercellularity, the remaining MEST-C scores showed a significant association with CKD stage; higher CKD stage, particularly stage 4, resulted in significantly higher odds for the presence of positive MEST-C scores. CKD stage 5 was observed to have a lower odds ratio than stage 4 for mesangial hypercellularity, segmental sclerosis, and mild to moderate tubular atrophy (T1). The lower odds of segmental sclerosis observed at CKD stage 5 may be the result of the greater levels of global glomerulosclerosis expected in patients with advanced disease. A greater proportion of patients in CKD stage 5 were found to exhibit tubular atrophy in >50% of tubules, correlating with the expected increase in odds, and a lower proportion exhibited tubular atrophy in 26% to 50% of tubules (Supplementary Table S5). This trend in higher T scores with progressive levels of renal deterioration is expected, and in alignment with the 2016 Oxford Classification working group update.17 It is important to highlight that the MEST-C classification system30 was designed as a prognostic algorithm. To ensure that assessment of the prognostic value included patients whose disease had not progressed too far by the time of diagnosis, the initial validation analyses were limited to patients with an eGFR of at least 30 ml/min per 1.73 m2. Our study provides evidence from a large cohort of patients that the association of MEST-C scores with clinical characteristics at diagnosis is evident for patients with IgAN who were first diagnosed at CKD stages 4 and 5 as well as CKD stages 1 to 3. Diagnosis when a patient has reached an advanced CKD stage, with severe histological disease and high levels of proteinuria may lead to worse outcomes for patients than if diagnosis and treatment were initiated earlier. Data from biopsies collected in the STOP-IgAN trial demonstrated that M1, T1/2, and C1/2 scores were associated with poorer outcomes.16 M1 score was associated with a higher rate of eGFR loss over the 3-year trial period, T1/T2 score was associated with lower eGFR at randomization and with progression to KF among patients receiving immunosuppression, whereas patients with C1/C2 scores were also more likely to progress to KF.
Most patients with available data in our cohort had proteinuria ≥1 g/d, with a mean value of 4.2 g/d. Analysis of the association between proteinuria and MEST-C in our cohort revealed that the presence of each MEST-C component came with higher odds of having proteinuria ≥1 g/d, particularly segmental sclerosis and tubular atrophy. Both tubular atrophy and segmental sclerosis are late findings of injury in proteinuric kidney diseases, and the toxic tubular injury related to proteinuria is well established in the literature.31, 32, 33 Our findings suggest that patients within this US cohort are experiencing substantial delays before diagnosis. This delay may be leading to sustained periods of elevated proteinuria, contributing to the high levels of kidney damage observed in biopsy samples at the time of diagnosis. Our results are consistent with a recent study by Gowrishankar et al.,34 which demonstrated in an Indian cohort that presence of each MEST-C component was associated with numerically higher proteinuria, but the difference was significant only for T score.
The data in this study show that patients had extensive tissue damage at the time of biopsy, and the degree of proteinuria and loss of eGFR was strikingly high. It is therefore clear that diagnosis is currently taking place at too late of a disease stage. With the emergence of novel, efficient, and safe treatments for IgAN, it is now vital that the diagnosis of IgAN be made earlier in the course of the disease. The improvement of recommendations regarding these practices is already ongoing, with the US Preventive Services Task Force making their draft research plan for CKD screening publicly available in January 2023.35 Diagnosis of IgAN before extensive segmental sclerosis and tubular atrophy occur would optimize the ability to apply present and potential future therapeutics to arrest or slow down IgAN disease progression earlier in the disease course.
Limitations
When using secondary data for comparative analyses, several limitations exist. Although this study represents a modern large sample of US adult patients with IgAN, not all US states are included in the database and those that are included may not be equally represented; thereby possibly limiting our ability to generalize results to the overall US IgAN patient population.
Patient demographic and clinical information was provided to Arkana on a secondary level by the clinicians who ordered the biopsy. In some cases, the forms accompanying the biopsy tissue were not completed with all requested information such as serum creatinine or urinary protein values. For example, in this study only 52% of patients had available proteinuria data, whereas 88% had available serum creatinine values. Whether these data points are absent due to a systematic patient-related cause or simply missing at random is beyond the ability of this study to determine.
Finally, MEST-C scores were not validated for patients with eGFR <30 ml/min per 1.73 m2 in the original Oxford classification analysis.30 Our results in patients with CKD stages 4 and 5 should therefore be interpreted with more caution than results for CKD stages 1–3 when considering potential implications of MEST-C scores at diagnosis in relation to long-term renal outcomes.
Conclusion
Most US patients with IgAN were diagnosed at CKD stage ≥3 and had elevated MEST-C scores for mesangial hypercellularity, segmental sclerosis, and tubular atrophy, which suggests significant disease duration at the time of kidney biopsy. Proteinuria ≥1 g/d and higher CKD stage at diagnosis are both associated with higher MEST-C scores in diagnostic biopsies and may therefore be early indicators of the need for diagnostic biopsy.
Strategies to improve awareness and facilitate earlier detection of IgAN are needed to enable administration of current and future therapies before extensive and irreversible kidney damage has occurred, thereby maximizing the potential to slow progression to KF.
Disclosure
DJC has received consulting fees from Travere Therapeutics, Inc., Aurinia, Calliditas, Chinook, and GSK; payment or honoraria from Aurinia and GSK; served as a member of the Lupus Foundation of America Medical Scientific Advisory Council; and has received grant support from the National Institutes of Health (R01DK126777). PDW is a consultant for Travere Therapeutics, Inc. CWA has no competing interests to declare. KW is a stockholder and former employee of Travere Therapeutics, Inc. ARR and JH are employees of Genesis Research (Hoboken, NJ, USA) which received compensation from Travere Therapeutics, Inc. for the design and conduct of this study. MB is an employee of Travere Therapeutics, Inc.
Acknowledgments
Medical writing support was provided by Nancy Hedlund PhD, MedNavigate and David Cork PhD, Genesis Research, which received compensation from Travere Therapeutics, Inc. This work was funded by Travere Therapeutics, Inc.
Footnotes
Figure S1. Histograms showing the distribution of patient creatinine (top) and eGFR (bottom) measurements at baseline.
Figure S2. Associations between histological characteristics and proteinuria (≥3.5 vs. <3.5 g/d).
Table S1. States with US healthcare institutions receiving services from Arkana Laboratories.
Table S2. Histological characteristics for overall cohort determined using immunofluorescence microscopy.
Table S3. Demographic and clinical characteristics stratified by age and race.
Table S4. Demographic and clinical characteristics stratified by availability of proteinuria data.
Table S5. Patient Count by MEST-C Characteristic/CKD stage.
Table S6. Patient Count by MEST-C Characteristic/Proteinuria level.
Supplementary Material
Figure S1. Histograms showing the distribution of patient creatinine (top) and eGFR (bottom) measurements at baseline.
Figure S2. Associations between histological characteristics and proteinuria (≥3.5 vs. <3.5 g/d).
Table S1. States with US healthcare institutions receiving services from Arkana Laboratories.
Table S2. Histological characteristics for overall cohort determined using immunofluorescence microscopy.
Table S3. Demographic and clinical characteristics stratified by age and race.
Table S4. Demographic and clinical characteristics stratified by availability of proteinuria data.
Table S5. Patient Count by MEST-C Characteristic/CKD stage.
Table S6. Patient Count by MEST-C Characteristic/Proteinuria level.
References
- 1.Scionti K., Molyneux K., Selvaskandan H., Barratt J., Cheung C.K. New insights into the pathogenesis and treatment strategies in IgA nephropathy. Glomerular Dis. 2022;2:15–29. doi: 10.1159/000519973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wyatt R.J., Julian B.A. IgA nephropathy. N Engl J Med. 2013;368:2402–2414. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 3.Kwon C.S., Daniele P., Forsythe A., Ngai C. A systematic literature review of the epidemiology, health-related quality of life impact, and economic burden of immunoglobulin A nephropathy. J Health Econ Outcomes Res. 2021;8:36–45. doi: 10.36469/001c.26129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGrogan A., Franssen C.F., de Vries C.S. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26:414–430. doi: 10.1093/ndt/gfq665. [DOI] [PubMed] [Google Scholar]
- 5.Kiryluk K., Li Y., Sanna-Cherchi S., et al. Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodrigues J.C., Haas M., Reich H.N. IgA nephropathy. Clin J Am Soc Nephrol. 2017;12:677–686. doi: 10.2215/CJN.07420716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts I.S.D. Pathology of IgA nephropathy. Nat Rev Nephrol. 2014;10:445–454. doi: 10.1038/nrneph.2014.92. [DOI] [PubMed] [Google Scholar]
- 8.Hastings M.C., Bursac Z., Julian B.A., et al. Life expectancy for patients from the Southeastern United States with IgA nephropathy. Kidney Int Rep. 2018;3:99–104. doi: 10.1016/j.ekir.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le W., Liang S., Hu Y., et al. Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant. 2012;27:1479–1485. doi: 10.1093/ndt/gfr527. [DOI] [PubMed] [Google Scholar]
- 10.Reich H.N., Troyanov S., Scholey J.W., Cattran D.C., Toronto Glomerulonephritis Registry Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. 2007;18:3177–3183. doi: 10.1681/asn.2007050526. [DOI] [PubMed] [Google Scholar]
- 11.Berthoux F.C., Mohey H., Afiani A. Natural history of primary IgA nephropathy. Semin Nephrol. 2008;28:4–9. doi: 10.1016/j.semnephrol.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Knoop T., Vikse B.E., Mwakimonga A., Leh S., Bjørneklett R. Long-term outcome in 145 patients with assumed benign immunoglobulin A nephropathy. Nephrol Dial Transplant. 2017;32:1841–1850. doi: 10.1093/ndt/gfx242. [DOI] [PubMed] [Google Scholar]
- 13.Manno C., Strippoli G.F.M., D’Altri C., Torres D., Rossini M., Schena F.P. A novel simpler histological classification for renal survival in IgA nephropathy: a retrospective study. Am J Kidney Dis. 2007;49:763–775. doi: 10.1053/j.ajkd.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Moriyama T., Tanaka K., Iwasaki C., et al. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a Single Center in Japan. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts I.S., Cook H.T., Troyanov S., et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546–556. doi: 10.1038/ki.2009.168. [DOI] [PubMed] [Google Scholar]
- 16.Schimpf J., Klein T., Fitzner C., et al. Renal outcomes of STOP-IgAN trial patients in relation to baseline histology (MEST-C scores) BMC Nephrol. 2018/11//2018;19:328. doi: 10.1186/s12882-018-1128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trimarchi H., Barratt J., Cattran D.C., et al. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91:1014–1021. doi: 10.1016/j.kint.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Barbour S.J., Espino-Hernandez G., Reich H.N., et al. The MEST score provides earlier risk prediction in lgA nephropathy. Kidney Int. 2016;89:167–175. doi: 10.1038/ki.2015.322. [DOI] [PubMed] [Google Scholar]
- 19.Barbour S.J., Coppo R., Zhang H., et al. Evaluating a new international risk-prediction tool in IgA nephropathy. JAMA Intern Med. 2019;179:942–952. doi: 10.1001/jamainternmed.2019.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexander S., Varughese S., Franklin R., et al. Epidemiology, baseline characteristics and risk of progression in the first South-Asian prospective longitudinal observational IgA nephropathy cohort. Kidney Int Rep. 2021;6:414–428. doi: 10.1016/j.ekir.2020.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung C., Lee J., Jang S., et al. Age-adjusted global glomerulosclerosis predicts renal progression more accurately in patients with IgA nephropathy. Sci Rep. 2020;10:6270. doi: 10.1038/s41598-020-63366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konieczny A., Donizy P., Gołębiowski T., et al. Clinical and histopathological factors influencing IgA nephropathy outcome. Diagnostics (Basel) 2021;11 doi: 10.3390/diagnostics11101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mariani G., Freitas L.L.L., Zollner R.L., Ribeiro M. Renal outcome in IgA nephropathy according to Oxford classification and ultrastructural analysis in a Brazilian center. Clin Nephrol. 2018;89:270–276. doi: 10.5414/CN109062. [DOI] [PubMed] [Google Scholar]
- 24.Inker LA, Eneanya ND, Coresh J, SchmidChronic Kidney Disease Epidemiology Collaboration, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker P.D. The renal biopsy. Arch Pathol Lab Med. 2009;133:181–188. doi: 10.1043/1543-2165-133.2.181. [DOI] [PubMed] [Google Scholar]
- 26.Kamano C., Shimizu A., Joh K., et al. A cross-sectional study in patients with IgA nephropathy of correlations between clinical data and pathological findings at the time of renal biopsy: a Japanese prospective cohort study. Clin Exp Nephrol. 2021;25:509–521. doi: 10.1007/s10157-021-02022-x. [DOI] [PubMed] [Google Scholar]
- 27.Imai E., Yamagata K., Iseki K., et al. Kidney Disease Screening Program in Japan: history, outcome, and perspectives. Clin J Am Soc Nephrol. 2007;2:1360–1366. doi: 10.2215/CJN.00980207. [DOI] [PubMed] [Google Scholar]
- 28.Thompson A., Carroll K., Inker A., et al. Proteinuria reduction as a surrogate end point in trials of IgA nephropathy. Clin J Am Soc Nephrol. 2019;14:469–481. doi: 10.2215/CJN.08600718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexander S., Varughese S., Franklin R., et al. Three-year clinical outcomes of the first South Asian prospective longitudinal observational IgA nephropathy cohort. Kidney Int Rep. 2021;7:305–318. doi: 10.1016/j.ekir.2021.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cattran D.C., Coppo R., Cook H.T., et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 31.Cravedi P., Remuzzi G. Pathophysiology of proteinuria and its value as an outcome measure in chronic kidney disease. Br J Clin Pharmacol. 2013;76:516–523. doi: 10.1111/bcp.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baines R.J., Brunskill N.J. Tubular toxicity of proteinuria. Nat Rev Nephrol. 2011;7:177–180. doi: 10.1038/nrneph.2010.174. [DOI] [PubMed] [Google Scholar]
- 33.Sharma S., Smyth B. From proteinuria to fibrosis: an update on pathophysiology and treatment options. Kidney Blood Press Res. 2021;46:411–420. doi: 10.1159/000516911. [DOI] [PubMed] [Google Scholar]
- 34.Gowrishankar S., Gupta Y., Vankalakunti M., et al. Correlation of Oxford MEST-C scores with clinical variables for IgA nephropathy in South India. Kidney Int Rep. 2019;4:1485–1490. doi: 10.1016/j.ekir.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Draft research plan: chronic kidney disease: screening. U.S. Preventive Services Task Force. https://www.uspreventiveservicestaskforce.org/uspstf/document/draft-research-plan/chronic-kidney-disease-screening Updated 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.