Abstract
Introduction
Vitamin K deficiency among patients on hemodialysis (HD) affects the function of matrix GLA protein (MGP), a potent vitamin K-dependent inhibitor of vascular calcification (VC).
Methods
We conducted a single-center randomized controlled trial (RCT) on maintenance HD patients to examine if vitamin K2 supplementation can reduce progression of coronary artery calcification (CAC) over an 18-month study period. Patients were randomized to vitamin K2 group receiving menaquinone-7360 μg 3 times/wk or control group. The primary outcome was CAC scores at the end of the study period. The secondary outcomes were aortic valve calcification (AVC), carotid-femoral pulse wave velocity (cfPWV), aortic augmentation index (AIx), dephosphorylated undercarboxylated MGP (dp-ucMGP) levels, major adverse cardiac events (MACE), and vascular access events.
Results
Of the 178 patients randomized, follow-up was completed for 138 patients. The CAC scores between the 2 groups were not statistically different at the end of 18 months (relative mean difference [RMD] 0.85, 95% CI 0.55–1.31). The secondary outcomes did not differ significantly in AVC (RMD 0.82, 95% CI 0.34–1.98), cfPWV (absolute mean difference [AMD] 0.55, 95% CI −0.50 to 1.60), and AIx (AMD 0.13, 95% CI −3.55 to 3.80). Supplementation with vitamin K2 did reduce dp-ucMGP levels (AMD −86, 95% CI −854 to −117). The composite outcome of MACE and mortality was not statistically different between the 2 groups (Hazard ratio = 0.98, 95% CI 0.50–1.94).
Conclusion
Our study did not demonstrate a beneficial effect of vitamin K2 in reducing progression of VC in this population at the studied dose and duration.
Keywords: aortic augmentation index, carotid-femoral pulse wave velocity, dephosphorylated undercarboxylated MGP, hemodialysis, vascular calcification, vitamin K
Graphical abstract
See Commentary on Page 1711
Cardiovascular disease mortality remains high among patients treated with maintenance dialysis. CAC, a form of VC, is more prevalent and progresses more rapidly in patients on HD compared to age-matched populations without chronic kidney disease (CKD).1,2 A positive dose relationship between CAC score and cardiovascular disease mortality has been reported.3
Established risk factors for VC, including older age, diabetes, hypertension, dyslipidemia, and smoking, are common across both CKD and non-CKD populations.4,5 In recent years, factors unique to the CKD population have been reported, presenting new opportunities to potentially alter the development of VC in the HD population. Hyperphosphatemia, elevated calcium-phosphate product, hyperparathyroidism, and use of calcium-containing phosphate binders may increase the risk of VC in the HD population and therapeutic approaches to mitigate these risk factors have been reported with some degree of efficacy.6,7 Calcitriol, commonly prescribed to suppress elevated parathyroid hormone in patients on HD, has also been implicated to aggravate VC.8 However, a major RCT using cinacalcet, a calcimimetic agent, to control secondary hyperparathyroidism did not result in lower CV mortality, even though it could attenuate progression of VC and cardiac valvular calcification.9,10
Animal and laboratory studies have demonstrated that vitamin K can influence the development and progression of VC by activating vitamin K-dependent MGP.11, 12, 13 MGP, synthesized by vascular smooth muscle cells functions as a natural local inhibitor of VC; however, it only becomes functional after vitamin K-dependent carboxylation.14,15 Patients on maintenance HD may have vitamin K deficiency of varying degrees because of dietary modifications needed to restrict dietary potassium and phosphorus intake.16,17 Vitamin K deficiency can be estimated by measuring dp-ucMGP,18 and studies have demonstrated that oral vitamin K supplementation increases carboxylation of MGP and lowers plasma dp-ucMGP.19 We hypothesized that oral menaquinone-7 (MK-7) will enhance carboxylation of MGP, lower levels of dp-ucMGP and therefore reduce CAC in the HD population. To evaluate the effect of vitamin K2 (MK-7) supplementation on the progression of CAC in patients on maintenance HD, we enrolled patients into the Treatment to Reduce Vascular Calcification in Hemodialysis Patients Using Vitamin K (Trevasc-HDK) study.
Methods
Trevasc-HDK, was a single-center, prospective, open-label interventional clinical trial on patients on HD at the National University Hospital Singapore, randomized to vitamin K2 supplementation or standard care. The study was conducted between July 28, 2016, and February 20, 2019. Clinical data was collected prospectively and reviewed according to schedule by the study team to ensure accuracy, completeness, and to perform data and safety monitoring. The final trial data set was stored in the National University Health System Investigational Medicine Unit with password protection in accordance with the Personal Data Protection Act. Trevasc-HDK was approved by Domain Specific Review Board of National Healthcare Group (2015/01000) and Health Science Authority Singapore and registered with ClinicalTrials.gov (NCT02870829). The latest protocol was revised to include changes in the designated laboratory processing the biomarkers, additional tests done, and change in the inclusion criteria to include patients with a minimum dialysis vintage of 12 months. The protocol was published.20 All patients provided valid informed written consent in keeping with the Helsinki accord.
Participants
Prevalent adults treated by HD for more than 12 months, with an expected life expectancy of more than 18 months and able to provide informed consent were enrolled in the study (Figure 1). Inclusion and exclusion criteria are provided in the Table S1. Patients on warfarin were excluded given that warfarin is a vitamin K antagonist and will affect vitamin K-dependent carboxylation process. Only those with a CAC score (Agatston score) of ≥30 were eligible to be randomized.
Figure 1.
Flow diagram. (1–4) Coronary artery calcification (CAC) score, Aortic valve calcification (AVC) score, ≥ 10% reduction in CAC score and dp-ucMGP: 19 patients in the control group and 18 patients in the vitamin K2 group withdrew, were lost to follow-up, or died. (5) Carotid-femoral pulse wave velocity (cfPWV): 19 patients withdrew, were lost to follow-up, or died, and 3 patients could not be measured at 18 months in the control group because of technical reasons. The numbers were 18 and 2 in the vitamin K2 group. (6) Aortic augmentation index (AIx): 19 patients withdrew, lost to follow-up, or died, and 2 patients could not be measured at 18 months in the control group because of technical reasons. 18 patients withdrew, lost to follow-up, or died, 1 patient at baseline and 2 patients at 18 months could not be measured in the vitamin K2 group. In addition to the above, there were 3 patients in the control group who completed the study but declined to undergo end of study measurements.
Study Design
Eligible patients were assigned to vitamin K2 supplementation or control group based on a 1:1 allocation using random permuted blocks of size 4 and 6, stratified by diabetic status. The random allocation sequence was generated by the ralloc command of Stata software. Randomization and concealed allocation were performed by the clinical trial unit coordinator without involvement of the investigators.
Study Intervention
Patients in the intervention group received oral vitamin K2 (isoform MK-7) at a fixed dose of 360 μg 3 times weekly with their HD sessions for a total duration of 18 months in addition to standard clinical care, whereas the control group received standard clinical care alone. All patients received high-flux HD or hemodiafiltration, anticoagulated with heparin unless contraindicated and used a dialysate calcium of 1.25 mmol/l with magnesium of 0.37 to 0.5 mmol/l, with the majority of patients using a dialysate magnesium of 0.37 mmol/l. During the 18-month treatment phase, the primary physician reviewed patients at intervals of 4 to 6 months as per standard care. Adherence to study medication was checked by pill counts, calculated as the percentage of the number of prescribed pills corrected for the number of returned pills divided by the period (in days) multiplied by 100%. A pill count of more than 85% was deemed as compliance to intervention. Both groups received the usual standard of care management following best practice clinical guidelines.21 The study workflow can be referenced from the published study protocol.20
Measurements
All enrolled patients underwent measurements listed below at baseline and at the end of the study period.
Cardiac-gated multislice, noncontrast computed tomography scan of the coronary arteries and aortic valve were performed using a 256-slice multidetector computed tomography scanner (Brilliance iCT, Philips Healthcare, Cleveland, OH), which has a detector collimation of 128 × 0.625 mm with double z-sampling. Calculation of CAC and AVC scores were performed using a Philips Intellispace Portal 5 workstation. These scores were independently reported by 2 experienced radiologists, blinded to treatment assignment.
Arterial stiffness was assessed using applanation tonometry (SphygmoCorVx, AtCor Medical, West Ryde, NSW, Australia) by trained technicians to determine cfPWV and AIx.22, 23, 24 The technicians and cardiologist reporting these measurements were blinded to treatment assignment.
Dp-ucMGP level was measured at baseline and at the end of the trial. Blood samples were collected in an ethylenediaminetetraacetic acid tube, processed, and stored at −80 °C as plasma before analysis at Coagulation Profile BV, Maastricht University, the Netherlands. Plasma dp-ucMGP levels were determined in a single run using the commercially available IVD CE-marked chemiluminescent InaKtif MGP assay on the IDS-iSYS system (IDS, Boldon, UK).
Outcomes
The primary end point was the absolute difference in log-transformed CAC score between the control and intervention group at the conclusion of the study period. Secondary end points were absolute difference in log-transformed AVC score, percentage of patients with regression of CAC ≥10%, absolute difference in cfPWV, augmentation index, plasma dp-ucMGP levels, mortality from any cause, MACE, and vascular access events.
Safety end points included adverse events, hospitalization, unanticipated problems involving risks to subjects, or others and were reported to the Institutional Review Board.
Statistical Analysis
Sample size was estimated based on the anticipated difference in primary outcome, which is absolute difference in log-transformed CAC scores between groups after 18 months. CAC scores of 1005 and 786 respectively for the control and intervention groups as reported by Block et al.7 were used as a guide. Following natural log-transformation to account for skewness, this yielded corresponding mean logCAC of 6.91 and 6.67. Further assuming a common SD of 0.55 and to achieve a power of 80%, 2-sided level of significance of 5%, a minimum sample size of 170 in total, or 85 per group was required. To account for an attrition rate of 15%, we estimated a total recruitment of 200 patients.
Baseline characteristics were summarized by mean (SD), median (interquartile range) and frequency (percentage) where appropriate. Mean scores of logCAC, logAVC, dp-ucMGP, cfPWV, and aortic AIx between controls and vitamin K2 groups were compared by independent 2-samplet test. The mean difference in logCAC and logAVC between groups was interpreted in terms of ratio of geometric means, also termed RMD, after back transformation to the original scale. If log transformation successfully removes the skewness of data, the geometric mean approximates the median. For AVC with a score of 0, the log-transformed value would be undefined. Therefore, sensitivity analysis was further conducted to compare median difference in AVC score using median regression. The effect of treatment on dp-ucMGP, cfPWV, and aortic AIx, was quantified in terms of AMD. Each end point was subsequently adjusted for baseline values and diabetic status using the analysis of covariance. Patients with regression of CAC ≥10% in both groups were compared by χ2 test, and logistic regression was implemented to adjust for baseline CAC score and diabetic status. Kaplan-Meier survival curves and the log-rank test were used to compare overall survival probabilities and MACE (including all-cause mortality) between the 2 groups. In addition, Cox proportional hazards regression stratified by diabetic status was performed to evaluate the treatment effect on the survival and MACE outcomes. Vascular access events and safety end points, including hospitalizations were summarized by counts. Differences in total number of patients with vascular access events or hospitalization between the 2 groups were compared by χ2 test. The average number of events between the 2 groups were evaluated by negative binomial regression. All statistical analyses were conducted using Stata/SE 17.0 (StataCorp LLC, College Station, TX), assuming 2-sided tests with a 5% significance level based on modified intention-to-treat analysis.
Results
We screened a total of 224 patients at National University Hospital between July 28, 2016, and July 20, 2017. A total of 46 patients were excluded, including with 31 patients having a CAC score <30 and 15 patients declining to consent to participate. A total of 178 patients were recruited and randomized. Of these, 67 patients in the control group and 71 patients in the intervention group were able to fulfill the trial requirements throughout the 18-month duration (Figure 1). Patients who withdrew or were lost to follow-up were included in the survival analysis for the duration of observation.
Baseline Characteristics
The baseline demographic and clinical characteristics of the patients by treatment group are shown in Table 1. At baseline, the median CAC and AVC scores were 790.42 (interquartile range: 265.01–2165.52) and 0.00 (interquartile range: 0.00–31.59), respectively. Baseline demographics, clinical characteristics, and standard biochemistry, and in particular, use of active vitamin D analogs, calcimimetic, and calcium-based phosphate binders were well balanced between the groups. Mean parathyroid hormone level in the vitamin K2 group was slightly lower but not statistically different to that of the control group.
Table 1.
Baseline characteristics and clinical data
| Characteristics | Controls (n = 89) | Vitamin K2 (n = 89) |
|---|---|---|
| Median age (yrs), (range) | 61 (24–78) | 62 (29–80) |
| Gender, n (%) | ||
| Male | 47 (52.8) | 54 (60.7) |
| Female | 42 (47.2) | 35 (39.3) |
| Mean body mass index (kg/m2) (SD) | 27.6 (7.3) | 27.5 (6.3) |
| Median Dialysis vintage (yrs), (IQR) | 4 (2–7) | 3 (1–7) |
| Mean systolic blood pressure (mm Hg), (SD) | 156 (19) | 154 (23) |
| Smoking history, n (%) | ||
| Never smoker | 64 (71.9) | 60 (67.4) |
| Previous smoker | 13 (14.6) | 17 (19.1) |
| Current smoker | 12 (13.5) | 12 (13.5) |
| Medical history, n (%) | ||
| Diabetes mellitus | 56 (62.9) | 57 (64.0) |
| Hypertension | 75 (84.3) | 72 (80.9) |
| Ischemic heart disease | 20 (22.5) | 24 (27.0) |
| Peripheral vascular disease | 11 (12.4) | 11 (12.4) |
| History of cerebrovascular events | 6 (6.7) | 11 (12.4) |
| Mean left ventricular ejection fraction (%), (SD) | 60 (8) | 59 (10) |
| Mean dialysis duration (minutes) (SD) | 246 (16) | 247 (16) |
| Mean single-pool Kt/Vurea (SD) | 1.57 (0.24) | 1.61 (0.27) |
| History of medication, n (%) | ||
| Activated vitamin D | 69 (77.5) | 68 (76.4) |
| Calcimimetics | 7 (7.9) | 7 (7.9) |
| Phosphate-binder | 86 (96.6) | 80 (90.0) |
| Non-calcium phosphate binder only | 10 (11.2) | 9 (10.1) |
| Calcium phosphate-binder only | 67 (75.3) | 61 (68.5) |
| Calcium and non-calcium phosphate binders | 6 (6.7) | 13 (14.6) |
| Statin | 67 (75.3) | 74 (83.2) |
| Antiplatelet | 58 (65.2) | 61 (68.5) |
| Meana laboratory parameters (SD) | ||
| Albumin (g/l) | 40 (3) | 39 (4) |
| PTH (pmol/l) | 56.4 (34.8) | 43.2 (26.9) |
| Corrected serum calcium (mmol/l) | 2.38 (0.17) | 2.33 (0.16) |
| Serum phosphate (mmol/l) | 1.60 (0.39) | 1.57 (0.47) |
| dp-ucMGP (pmol/l) | 2940 (1085) | 3087 (1579) |
| Radiological parameters | ||
| Median CAC score (IQR) | 972.00 (295.90–2604.18) | 687.80 (252.91–1733.5) |
| Median AVC score (IQR) | 0.23 (0.00–25.61) | 0.00 (0.00–33.77) |
| Mean cfPWV (m/s) | 11.40 (3.74) | 11.61 (3.60) |
| Mean Aortic AIx | 29 (12) | 29 (9) |
AI, augmentation index; AVC, aortic vascular calcification; CAC, coronary artery calcification; cfPWV, carotid-femoral pulse wave velocity; dp-ucMGP, dephosphorylated undercarboxylated MGP; HD, hemodialysis; HDF, hemodiafiltration; IQR, interquartile range; MGP, matrix GLA protein; PTH, parathyroid hormone; yrs, years.
Denoted using mean unless otherwise indicated.
Primary and Secondary End Points of VC and Arterial Stiffness
A total of 138 patients completed the 18-month follow-up and had a repeat CAC measurement. At 18 months, the geometric mean CAC score in the vitamin K group was slightly lower, 882.05 (SD 3.51), as compared to the control group, 1043.54 (SD 3.77) (Table 2). The unadjusted RMD was 0.85 (95% CI 0.55–1.31) and the 2 groups did not differ significantly.
Table 2.
Effects of treatment on study outcomes at 18 months
|
Outcomes |
Controls (n = 89) | Vitamin K2 (n = 89) | Unadjusted Effect Measurea (95% CI) |
P-Value | Adjusted Effect Measurea (95% CI) |
P-Value |
|---|---|---|---|---|---|---|
| Primary outcome | ||||||
| Geometric mean CAC score (IQR) | 1043.54 (438.06–2975.90) | 882.05 (298.89–2194.58) | 0.85 (0.55–1.31) | 0.45 | 0.93 (0.71–1.21) | 0.58 |
| Secondary outcomes | ||||||
| Geometric mean AVC score (IQR)b | 31.39 (12.29–145.72) | 25.77 (10.37–155.88) | 0.82 (0.34–1.98) | 0.66 | 0.82 (0.40–1.69) | 0.60 |
| Number of patients with regression of CAC ≥ 10% (%) | 1 (1.5) | 3 (4.2) | 2.91 (0.23–155.11) | 0.62 | 2.95 (0.30–29.25) | 0.36 |
| Mean dp-ucMGP (SD) pmol/l | 2986 (1113) | 2500 (1075) | −486 (−854 to −117) | 0.01 | −525 (−823 to −226) | 0.001 |
| Mean cfPWV (SD) m/s | 10.72 (2.91) | 11.27 (3.19) | 0.55 (−0.50 to 1.60) | 0.30 | 0.24 (−0.53 to 1.01) | 0.9 |
| Mean Aortic AIx (SD) | 29 (11) | 29 (10) | 0.13 (−3.55 to 3.80) | 0.90 | 0.01 (−3.10 to 3.12) | 0.9 |
AIx, augmentation index; AVC, aortic valve calcification; CAC, coronary artery calcification; cfPWV, carotid-femoral pulse wave velocity; CI, confidence interval; dp-ucMGP, dephosphorylated undercarboxylated MGP; IQR, interquartile range; MGP, matrix GLA protein; SD, standard deviation.
Baseline values and diabetes mellitus were adjusted for in primary and secondary outcomes analyses.
The effect measures refers to relative mean difference (ratio of geometric means) for CAC score and AVC score, odds ratio for regression of CAC≥10%, and absolute mean difference for dp-ucMGP, cfPWV and aortic AIx.
56 patients had an AVC score of zero after 18 months.
The geometric mean AVC was lower in the vitamin K group than in the control group, but the RMD was not statistically significant (RMD 0.82, 95% CI 0.34–1.98). Only 3 patients in the vitamin K2 group and 1 patient in the control group had a regression of CAC ≥10%. There was no difference between the 2 groups (Odds ratio 2.91, 95% CI 0.23–155.11). The other secondary end points, cfPWV and AIx were also not significantly different between interventions (AMD 0.55 [95% CI −0.50 to 1.60] and AMD 0.13 [95% CI −3.55 to 3.80], respectively).
Dp-ucMGP
Dp-ucMGP level was significantly lower (2500 pmol/l ± 1075) in the vitamin K2 group than that in the control group (2986 pmol/l ± 1113) (AMD −486, 95% CI −854 to −117) (Table 2). Notably, the control group also saw a marginal rise in dp-ucMGP levels from 2940 pmol/l (SD ± 1085) to 2986 pmol/l (SD ± 1113). Two outliers were observed. However, the result of the sensitivity analysis was similar after removing them.
The conclusions for the primary and secondary outcome analyses remained unaltered even after adjustment for the respective baseline covariates and comorbidities, including diabetes mellitus.
MACEs and Mortality
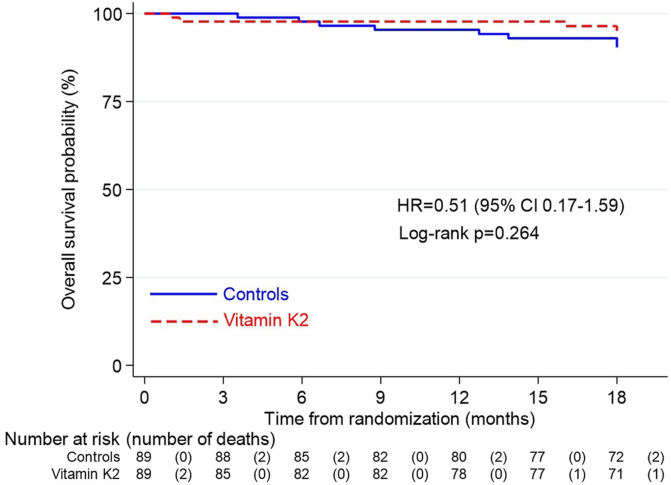
MACE were comparable in the vitamin K2 group and control group (13 vs. 9 events). There were 8 deaths in total in the control group compared to 4 deaths in the Vitamin K2 treated patients (Hazard ratio = 0.51, 95% CI 0.17–1.59) (Figure 2). The composite outcome of MACE and all-cause mortality was not different between the 2 groups. (Hazard ratio = 0.98, 95% CI 0.50–1.94) (Figure 3). For both these outcomes, the results remained unaltered even after performing Cox regression analysis stratified by diabetes mellitus.
Figure 2.
Kaplan-Meier estimate of overall survival probability. CI, confidence interval; HR, hazard ratio.
Figure 3.
Kaplan-Meier estimate of MACE (including all-cause mortality)-free survival probability. MACE, major adverse cardiac events.
Vascular Access Events
Vascular access events were comparable between the 2 groups, both in terms of the total number of patients with vascular events (Relative risk 1.03, 95% CI 0.73–1.44) and the average number of vascular events per patients (Incidence rate ratio 1.01, 95% CI 0.61–1.67) (Table 3).
Table 3.
Summary of vascular access events
| Vascular access data | Controls (n = 89) | Vitamin K2 (n = 89) | Effect measurea (95% CI) | P-value |
|---|---|---|---|---|
| Total patients with vascular access events | 38 | 39 | 1.03 (0.73–1.44) | 0.88 |
| Average vascular access events per patient | 1 | 1 | 1.01 (0.61–1.67) | 0.90 |
| Total vascular access events | 97 | 98 | ||
| Vascular access type | ||||
| Arterio-venous fistula | 71 | 72 | ||
| Arterio-venous graft | 23 | 19 | ||
| Dialysis catheter | 3 | 7 |
The effect measure refers to relative risk for total patients with vascular access events, and incidence rate ratio for average vascular access events per patient.
Adverse Events
No patient in the Vitamin K2 group reported adverse events related to medication use. There were more hospitalizations in the Vitamin K2 group, but the 2 groups were not significantly different (Table 4).
Table 4.
Summary of hospitalization
| Hospitalization data | Controls (n = 89) | Vitamin K2 (n = 89) | Effect measurea (95% CI) | P-value |
|---|---|---|---|---|
| Total patients with hospitalization | 60 | 54 | 0.90 (0.72–1.12) | 0.35 |
| Average hospitalizations per patient | 1 | 2 | 1.27 (0.90–1.80) | 0.18 |
| Total hospitalizations | 133 | 169 | ||
| Categories of hospitalization | ||||
| Infection | 55 | 79 | ||
| Fluid overload | 11 | 13 | ||
| Gastrointestinal-related | 20 | 14 | ||
| Cardiac-relatedb | 4 | 4 | ||
| Others | 43 | 59 |
The effect measure refers to relative risk for total patients with hospitalization, and incidence rate ratio for average hospitalizations per patient.
Refers to non-MACE cardiac-related events.
Discussion
Trevasc-HDK is the largest randomized clinical trial to date reporting the use of vitamin K2 supplementation to mitigate the progression of VC in patients established on maintenance HD, and the first such study in an Asian HD population. In our study, vitamin K2 supplementation did not significantly reduce progression of VC as measured by the degree of CAC, despite a substantial reduction in dp-ucMGP. Our results are consistent with some of the recently published smaller RCTs that also failed to demonstrate a beneficial effect of vitamin K2 to mitigate VC in this population.25,26 Secondary outcome measures in our study also did not support a significant beneficial impact of vitamin K2 supplementation. Interestingly, there were twice the number of deaths in the control group when compared to the intervention group though again this was not statistically significant. Notably, there was no increase in adverse events with vitamin K2 and in particular no increase in vascular access clotting.
VC is common, and the CAC score is an independent predictor of mortality, in patients on maintenance HD.27 MGP acts as a local inhibitor of VC,14,15 and requires carboxylation by vitamin K2 to function.11,12 Dp-ucMGP is a widely accepted surrogate biomarker of vitamin K adequacy, with increased dp-ucMGP levels accepted as a biomarker of vitamin K deficiency.28,29 Several studies have reported that dp-ucMGP levels are significantly higher in patients with CKD and on maintenance HD compared to healthy subjects.28,30,31 This has led to the hypothesis that vitamin K2 supplementation will lower dp-ucMGP levels, restore MGP function, and limit progression of or even regress VC.19
The mean baseline level of dp-ucMGP in our patients was, as expected, significantly higher than that of the general population, with 1 study reporting a reference range of <300 to 532 pmol/l in healthy volunteers.30 The levels in our study population were comparable to or higher than those in other published series.28,31, 32, 33 In our patients, oral supplementation with vitamin K2 significantly reduced dp-ucMGP levels but the levels were still higher than those reported in healthy persons. There is uncertainty as to how low dp-ucMGP levels need to be reduced to reverse severe chronic VC in patients on HD. The failure to demonstrate an advantage of vitamin K2 supplementation may have been a reflection of the 3 times weekly dosing schedule, which we had chosen to ensure compliance.34 Data from the UK National Dietary and Nutrition Survey suggests that an adequate dietary intake for vitamin K2 is 54 μg/d for men and 36 μg/d for women.35 In addition to often low intake of vitamin K2 among patients on HD, other factors, such as uremic inhibition of the vitamin K cycle and possibly interference of absorption by phosphate binders, may further exacerbate vitamin K2 deficiency.32,36, 37, 38 We selected a vitamin K2 dose of 360 μg 3 times/wk (approximately 154 μg/d), because there were very few published studies on the safety of higher doses of vitamin K2 supplementation in patients on HD at the time we were designing this study. Two very short term studies of 6 to 8 weeks’ duration suggested that higher doses could be tolerated and effect a greater reduction in dp-ucMGP.19,39 There are reports that the dietary intake of vitamin K2 in Asians is higher than that in a Caucasian population and therefore a lower dose of vitamin K2 may be sufficient.40,41 However, this was not supported by the baseline dp-ucMGP levels in our study, which were comparable to studies in Caucasian populations and in those consuming a Mediterranean diet.19,31
Compared to other studies investigating vitamin K2 supplementation, the 20% reduction in dp-ucMGP in this trial was similar to that reported in a short 8 week study giving the same dose36 and lower than the 27% reduction in a 6-week German study, which administered 135 μg/d.19 The Valkyrie study documented an even greater 46.6% reduction in dp-ucMGP in 132 patients on HD who were requiring anticoagulation given 2000 μg of vitamin K2, 3 times weekly for 18 months. Despite this greater reduction in dp-ucMGP, levels remained about twice those of healthy persons, and no significant improvement in CAC, AVC, and cfPWV were observed.25 The RenaKvit study, a double blind, placebo-controlled randomized trial of 48 patients, reported that the initial reduction in dp-ucMGP in the treatment group given vitamin K2 supplementation was not sustained over a 2-year follow-up period, raising the question of efficacy over time.42 The primary outcome of arterial calcification was not altered by vitamin K2 supplementation. Similarly, CAC and abdominal aortic calcification scores were also not statistically different between the treatment and placebo groups.
Vitamin K isoforms being lipophilic are transported to target tissue via lipoproteins. A recent study comparing pharmacokinetics of vitamin K in patients on dialysis and healthy individuals was also quite revealing of the differences in vitamin K uptake and distribution into circulating lipoproteins after subjects were given oral vitamin K.43 Even among menaquinone types, there are differences in the distribution of triacylglycerol-rich lipoprotein fraction, low-density lipoprotein, and even high-density lipoprotein.44 Kessler et al. reported that high-density lipoprotein particles obtained from uremic patients in vitro had a lower uptake of MK-7 and did not increase carboxylation of MGP.43 This in vitro finding supports the possibility that there may be diminished biological activity of MK-7 at the site of the vascular smooth muscle cells in patients on dialysis and therefore may potentially explain the lack of in vivo efficacy of vitamin K2 supplementation in this population.43 What is not known is whether the in vitro experimental model mirrors the real-life clinical scenario because the specific underlying pathobiological mechanisms remain to be determined; and if higher doses of MK-7 can overcome this apparent lack of activity. Although clinical human trials including ours have consistently demonstrated a reduction in dp-ucMGP with MK-7 supplementation, the reduction in dp-ucMGP does not consistently correlate with VC between studies. We only recruited patients with a CAC score >30, with the majority having scores in the moderate and severe disease categories,10,45 which was higher than some other studies.46 Therefore, the degree of pre-existing VC could have limited any potential benefit of vitamin K2 supplementation. In the ADVANCE study, a >15% increase in CAC score over 1 year occurred most often in patients with the lowest baseline score, regardless of group assignment.10 In contrast, the ViKTORIES study conducted in transplant patients with much lower CAC scores showed only minimal increase in CAC over the same period.47 Therefore, baseline CAC score may not fully define likelihood of CAC progression and its influence on the success or failure of VC mitigation strategies. A review of major RCTs that included CAC as an end point reported a universal increase in annualized CAC across the trials in both non-CKD and CKD populations, such that CAC scores may not be a good or meaningful primary end point.48,49
We found no significant effect of vitamin K2 supplementation on progression of AVC, in keeping with 2 earlier smaller trials,25,35 though 1 non-CKD trial did report a positive effect.50 Over the 12-month study period involving subjects with estimated glomerular filtration rate of more than 60 ml/min per 1.73 m2, the AVC volume score progressed by 10.0% in patients in the vitamin K group compared with 22.0% in the placebo group.50 Of note, the mean dp-ucMGP at baseline for both groups were below 500 pmol/l and further decreased to approximately 200 pmol/l with vitamin K1 supplementation. Arterial stiffening increases in patients on HD, and cfPWV, a robust marker of stiffening, is a strong independent predictor of mortality.51 The absence of significant improvement in cfPWV after 18 months of vitamin K2 supplementation in our study, accords with a similar study in patients with CKD.52
Our study population had no adverse events related directly to vitamin K2 supplementation. The use of vitamin K has raised concerns of possible increased risk of thrombosis. However, the vitamin K–dependent clotting factors have a limited number of glutamine residues available for gamma-carboxylation, beyond which there can be no further carboxylation, and therefore supplementation is not associated with increased risk of thrombosis.14,39,53,54 There were no excess vascular access complications (arteriovenous fistula, arteriovenous graft, or catheter) in our patients, which is consistent with other RCTs of vitamin K supplementation in patients on HD. There were 8 deaths in the control group, which is consistent with the annual mortality of our local HD population.55 Although there were only half the number of deaths in the vitamin K2 supplementation group, this was not statistically significant, and the study was not sufficiently powered to detect a difference in mortality.
The strength of our study is that this is the largest randomized trial using vitamin K2 supplementation, and the first in an Asian HD population. Although we recruited 178 patients, just above the minimum of 170 based on our sample size calculations, only 138 patients completed the entire 18-month study period and underwent end-of-study CAC and laboratory measurements. The number of patients recruited was limited by the allowable funding extension period. Past landmark studies on intervention to reduce progression of CAC in an HD population, including 2 recent publications reported much lower number of patients at the end-of-study period.25,56 The Valkyrie study group randomized 132 patients into 3 treatment arms but had a total of 47 deaths during study period and 8 more patients who did not want to continue.25 Isaka et al.56 reported on the outcome of different levels of phosphate control on CAC progression and successfully randomized 156 patients (of a planned 200 patients) but ended the study with a total of 97 patients in their defined per protocol set. We had assumed a 15% attrition rate, and 25 participants dropped out (excluding deaths). The 14% drop-out rate is much lower than those reported in earlier reports of vitamin K2 supplementation in HD patients. At the time when this study was conceptualized, measurement of vitamin K was not easily accessible. We elected instead to evaluate functional sufficiency by measuring vitamin K–dependent carboxylation of MGP. In our study, we measured compliance to vitamin K2 intake by self-reporting and pill count. The reduction in level of dp-ucMGP among those supplemented with vitamin K2 does imply certain degree of adherence.
In conclusion, Trevasc-HDK does not suggest a beneficial effect of oral vitamin K2 supplementation in attenuating VC or arterial stiffness at a dose of 360 μg 3 times/wk despite a significant reduction in dp-ucMGP. Our findings are consistent with 3 earlier smaller RCTs on vitamin K supplementation, which reported progression of VC in HD patients. Our population is unique in it being a multiethnic Asian population with low mortality rate despite a significant degree of VC. Although we cannot exclude a quantifiable benefit of vitamin K2 supplementation at higher doses or earlier in the natural history of VC, we must consider the many other factors that contribute to the initiation and progression of VC via non-vitamin K–dependent mechanisms.57,58 The answer may be that it will take a multifaceted approach to overcome VC.
Disclosure
LS reports consultancy fee from Immunodiagnostic systems (IDS), outside the submitted work and grants from NattoPharma, Boehringer Ingelheim, and Bayer outside the submitted work. LJS is a shareholder in Coagulation Profile. All the other authors declare no conflict of interest.
Acknowledgments
We are grateful to the National University Health System’s Investigational Medicine Unit, Department of Diagnostic Imaging and Cardiovascular Imaging Core Laboratory for their role in the execution of the trial. We thank Nattopharma ASA for providing vitamin K2 for the trial.
Funding
This work was supported by the National Medical Research Council Singapore. [NMRC/CNIG/1138/2015]. The funder had no role in the study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Data Availability Statement
Anonymized individual patient data can be requested with data sharing agreement.
Footnotes
Table S1. Inclusion and exclusion criteria for Trevasc-HDK.
CONSORT checklist and Inclusion and exclusion criteria for Trevasc-HDK.
Supplementary Material
Table S1. Inclusion and exclusion criteria for Trevasc-HDK.
CONSORT checklist and Inclusion and exclusion criteria for Trevasc-HDK.
References
- 1.Goodman W.G., Goldin J., Kuizon B.D., et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/nejm200005183422003. [DOI] [PubMed] [Google Scholar]
- 2.Raggi P., Boulay A., Chasan-Taber S., et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39:695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 3.Matsuoka M., Iseki K., Tamashiro M., et al. Impact of high coronary artery calcification score (CACS) on survival in patients on chronic hemodialysis. Clin Exp Nephrol. 2004;8:54–58. doi: 10.1007/s10157-003-0260-0. [DOI] [PubMed] [Google Scholar]
- 4.Kronmal R.A., McClelland R.L., Detrano R., et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 5.Tomiyama C., Higa A., Dalboni M.A., et al. The impact of traditional and non-traditional risk factors on coronary calcification in pre-dialysis patients. Nephrol Dial Transplant. 2006;21:2464–2471. doi: 10.1093/ndt/gfl291. [DOI] [PubMed] [Google Scholar]
- 6.Block G.A., Port F.K. Re-evaluation of risks associated with hyperphosphatemia and hyperparathyroidism in dialysis patients: recommendations for a change in management. Am J Kidney Dis. 2000;35:1226–1237. doi: 10.1016/s0272-6386(00)70064-3. [DOI] [PubMed] [Google Scholar]
- 7.Block G.A., Spiegel D.M., Ehrlich J., et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68:1815–1824. doi: 10.1111/j.1523-1755.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 8.McCabe K.M., Zelt J.G., Kaufmann M., et al. Calcitriol accelerates vascular calcification irrespective of vitamin K status in a rat model of chronic kidney disease with hyperphosphatemia and secondary hyperparathyroidism. J Pharmacol Exp Ther. 2018;366:433–445. doi: 10.1124/jpet.117.247270-. [DOI] [PubMed] [Google Scholar]
- 9.Investigators E.T., Chertow G.M., Block G.A., et al. Effect of Cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482–2494. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- 10.Raggi P., Chertow G.M., Torres P.U., et al. The ADVANCE study: a randomized study to evaluate the effects of Cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant. 2011;26:1327–1339. doi: 10.1093/ndt/gfq725. [DOI] [PubMed] [Google Scholar]
- 11.Proudfoot D., Shanahan C.M. Molecular mechanisms mediating vascular calcification: role of matrix gla protein (Review Article) Nephrol (Carlton) 2006;11:455–461. doi: 10.1111/j.1440-1797.2006.00660.x. [DOI] [PubMed] [Google Scholar]
- 12.Schurgers L.J., Cranenburg E.C., Vermeer C. Matrix Gla-protein: the calcification inhibitor in need of vitamin K. Thromb Haemost. 2008;100:593–603. [PubMed] [Google Scholar]
- 13.Zaragatski E., Grommes J., Schurgers L.J., et al. Vitamin K antagonism aggravates chronic kidney disease-induced neointimal hyperplasia and calcification in arterialized veins: role of vitamin K treatment? Kidney Int. 2016;89:601–611. doi: 10.1038/ki.2015.298. [DOI] [PubMed] [Google Scholar]
- 14.Theuwissen E., Smit E., Vermeer C. The role of vitamin K in soft-tissue calcification. Adv Nutr. 2012;3:166–173. doi: 10.3945/an.111.001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murshed M., Schinke T., McKee M.D., Karsenty G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J Cell Biol. 2004;165:625–630. doi: 10.1083/jcb.200402046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaesler N., Schurgers L.J., Floege J. Vitamin K and cardiovascular complications in chronic kidney disease patients. Kidney Int. 2021;100:1023–1036. doi: 10.1016/j.kint.2021.06.037. [DOI] [PubMed] [Google Scholar]
- 17.Regulska-Ilow B., Rozanska D., Zatonska K., Szuba A. Estimation of vitamin K content and its sources in the diet of the polish participants of the PURE study. Nutrients. 2022;14:1917. doi: 10.3390/nu14091917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schurgers L.J., Barreto D.V., Barreto F.C., et al. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: a preliminary report. Clin J Am Soc Nephrol. 2010;5:568–575. doi: 10.2215/CJN.07081009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westenfeld R., Krueger T., Schlieper G., et al. Effect of vitamin K2 supplementation on functional vitamin K deficiency in hemodialysis patients: a randomized trial. Am J Kidney Dis. 2012;59:186–195. doi: 10.1053/j.ajkd.2011.10.041. [DOI] [PubMed] [Google Scholar]
- 20.Haroon S.W., Tai B.C., Ling L.H., et al. Treatment to reduce vascular calcification in hemodialysis patients using vitamin K (Trevasc-HDK): a study protocol for a randomized controlled trial. Med (Baltim) 2020;99 doi: 10.1097/MD.0000000000021906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl (2011) 2017;7:1–59. doi: 10.1016/j.kisu.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chirinos J.A. Arterial stiffness: basic concepts and measurement techniques. J Cardiovasc Transl Res. 2012;5:243–255. doi: 10.1007/s12265-012-9359-6. [DOI] [PubMed] [Google Scholar]
- 23.Chen C.H., Nevo E., Fetics B., et al. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95:1827–1836. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 24.Pauca A.L., O’Rourke M.F., Kon N.D. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–937. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- 25.De Vriese A.S., Caluwe R., Pyfferoen L., et al. Multicenter randomized controlled trial of vitamin K antagonist replacement by Rivaroxaban with or without vitamin K2 in hemodialysis patients with atrial fibrillation: the Valkyrie study. J Am Soc Nephrol. 2020;31:186–196. doi: 10.1681/ASN.2019060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oikonomaki T., Papasotiriou M., Ntrinias T., et al. The effect of vitamin K2 supplementation on vascular calcification in haemodialysis patients: a 1-year follow-up randomized trial. Int Urol Nephrol. 2019;51:2037–2044. doi: 10.1007/s11255-019-02275-2. [DOI] [PubMed] [Google Scholar]
- 27.Shantouf R.S., Budoff M.J., Ahmadi N., et al. Total and individual coronary artery calcium scores as independent predictors of mortality in hemodialysis patients. Am J Nephrol. 2010;31:419–425. doi: 10.1159/000294405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cranenburg E.C., Koos R., Schurgers L.J., et al. Characterisation and potential diagnostic value of circulating matrix gla protein (MGP) species. Thromb Haemost. 2010;104:811–822. doi: 10.1160/TH09-11-0786. [DOI] [PubMed] [Google Scholar]
- 29.Dalmeijer G.W., van der Schouw Y.T., Magdeleyns E., Ahmed N., Vermeer C., Beulens J.W. The effect of menaquinone-7 supplementation on circulating species of matrix gla protein. Atherosclerosis. 2012;225:397–402. doi: 10.1016/j.atherosclerosis.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 30.Griffin T.P., Islam M.N., Wall D., et al. Plasma dephosphorylated-uncarboxylated Matrix Gla-Protein (dp-ucMGP): reference intervals in Caucasian adults and diabetic kidney disease biomarker potential. Sci Rep. 2019;9 doi: 10.1038/s41598-019-54762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aoun M., Makki M., Azar H., Matta H., Chelala D.N. High Dephosphorylated-Uncarboxylated MGP in hemodialysis patients: risk factors and response to vitamin K2, A pre-post intervention clinical trial. BMC Nephrol. 2017;18:191. doi: 10.1186/s12882-017-0609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cranenburg E.C., Schurgers L.J., Uiterwijk H.H., et al. Vitamin K intake and status are low in hemodialysis patients. Kidney Int. 2012;82:605–610. doi: 10.1038/ki.2012.191. [DOI] [PubMed] [Google Scholar]
- 33.Schlieper G., Westenfeld R., Kruger T., et al. Circulating nonphosphorylated carboxylated matrix gla protein predicts survival in ESRD. J Am Soc Nephrol. 2011;22:387–395. doi: 10.1681/ASN.2010040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marles R.J., Roe A.L., Oketch-Rabah H.A. US Pharmacopeial Convention safety evaluation of menaquinone-7, a form of vitamin K. Nutr Rev. 2017;75:553–578. doi: 10.1093/nutrit/nux022. [DOI] [PubMed] [Google Scholar]
- 35.Manna P., Kalita J. Beneficial role of vitamin K supplementation on insulin sensitivity, glucose metabolism, and the reduced risk of type 2 diabetes: a review. Nutrition. 2016;32:732–739. doi: 10.1016/j.nut.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Caluwe R., Vandecasteele S., Van Vlem B., Vermeer C., De Vriese A.S. Vitamin K2 supplementation in haemodialysis patients: a randomized dose-finding study. Nephrol Dial Transplant. 2014;29:1385–1390. doi: 10.1093/ndt/gft464. [DOI] [PubMed] [Google Scholar]
- 37.Kaesler N., Magdeleyns E., Herfs M., et al. Impaired vitamin K recycling in uremia is rescued by vitamin K supplementation. Kidney Int. 2014;86:286–293. doi: 10.1038/ki.2013.530. [DOI] [PubMed] [Google Scholar]
- 38.Neradova A., Schumacher S.P., Hubeek I., Lux P., Schurgers L.J., Vervloet M.G. Phosphate binders affect vitamin K concentration by undesired binding, an in vitro study. BMC Nephrol. 2017;18:149. doi: 10.1186/s12882-017-0560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caluwe R., Verbeke F., De Vriese A.S. Evaluation of vitamin K status and rationale for vitamin K supplementation in dialysis patients. Nephrol Dial Transplant. 2020;35:23–33. doi: 10.1093/ndt/gfy373. [DOI] [PubMed] [Google Scholar]
- 40.Yan L., Zhou B., Greenberg D., et al. Vitamin K status of older individuals in northern China is superior to that of older individuals in the UK. Br J Nutr. 2004;92:939–945. doi: 10.1079/bjn20041261. [DOI] [PubMed] [Google Scholar]
- 41.Kaneki M., Hodges S.J., Hosoi T., et al. Japanese fermented soybean food as the major determinant of the large geographic difference in circulating levels of vitamin K2: possible implications for hip-fracture risk. Nutrition. 2001;17:315–321. doi: 10.1016/s0899-9007(00)00554-2. [DOI] [PubMed] [Google Scholar]
- 42.Levy-Schousboe K., Frimodt-Moller M., Hansen D., et al. Vitamin K supplementation and arterial calcification in dialysis: results of the double-blind, randomized, placebo-controlled RenaKvit trial. Clin Kidney J. 2021;14:2114–2123. doi: 10.1093/ckj/sfab017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaesler N., Schreibing F., Speer T., et al. Altered vitamin K biodistribution and metabolism in experimental and human chronic kidney disease. Kidney Int. 2022;101:338–348. doi: 10.1016/j.kint.2021.10.029. [DOI] [PubMed] [Google Scholar]
- 44.Schurgers L.J., Vermeer C. Differential lipoprotein transport pathways of K-vitamins in healthy subjects. Biochim Biophys Acta. 2002;1570:27–32. doi: 10.1016/s0304-4165(02)00147-2. [DOI] [PubMed] [Google Scholar]
- 45.Kakani E., Elyamny M., Ayach T., El-Husseini A. Pathogenesis and management of vascular calcification in CKD and dialysis patients. Semin Dial. 2019;32:553–561. doi: 10.1111/sdi.12840. [DOI] [PubMed] [Google Scholar]
- 46.Shantouf R., Kovesdy C.P., Kim Y., et al. Association of serum alkaline phosphatase with coronary artery calcification in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:1106–1114. doi: 10.2215/CJN.06091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lees J.S., Rankin A.J., Gillis K.A., et al. The ViKTORIES trial: a randomized, double-blind, placebo-controlled trial of vitamin K supplementation to improve vascular health in kidney transplant recipients. Am J Transplant. 2021;21:3356–3368. doi: 10.1111/ajt.16566. [DOI] [PubMed] [Google Scholar]
- 48.McCullough P.A., Chinnaiyan K.M. Annual progression of coronary calcification in trials of preventive therapies: a systematic review. Arch Intern Med. 2009;169:2064–2070. doi: 10.1001/archinternmed.2009.382. [DOI] [PubMed] [Google Scholar]
- 49.Vlasschaert C., Goss C.J., Pilkey N.G., McKeown S., Holden R.M. Vitamin K supplementation for the prevention of cardiovascular disease: where is the evidence? A systematic review of controlled trials. Nutrients. 2020;12 doi: 10.3390/nu12102909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brandenburg V.M., Reinartz S., Kaesler N., et al. Slower progress of aortic valve calcification with vitamin K supplementation: results from a prospective interventional proof-of-concept study. Circulation. 2017;135:2081–2083. doi: 10.1161/CIRCULATIONAHA.116.027011. [DOI] [PubMed] [Google Scholar]
- 51.Blacher J., Guerin A.P., Pannier B., Marchais S.J., Safar M.E., London G.M. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 52.Witham M.D., Lees J.S., White M., et al. Vitamin K supplementation to improve vascular stiffness in CKD: the K4Kidneys randomized controlled trial. J Am Soc Nephrol. 2020;31:2434–2445. doi: 10.1681/ASN.2020020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inoue T., Fujita T., Kishimoto H., et al. Randomized controlled study on the prevention of osteoporotic fractures (OF study): a phase IV clinical study of 15-mg menatetrenone capsules. J Bone Miner Metab. 2009;27:66–75. doi: 10.1007/s00774-008-0008-8. [DOI] [PubMed] [Google Scholar]
- 54.Vissers L.E., Dalmeijer G.W., Boer J.M., Monique Verschuren W.M., van der Schouw Y.T., Beulens J.W. Intake of dietary phylloquinone and menaquinones and risk of stroke. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Office NROD Singapore Renal Registry Annual Report, 2018. 2018. https://www.nrdo.gov.sg/docs/librariesprovider3/default-document-library/singapore-renal-registry-annual-report-2018.pdf?sfvrsn=de5a657f_0
- 56.Isaka Y., Hamano T., Fujii H., et al. Optimal phosphate control related to coronary artery calcification in dialysis patients. J Am Soc Nephrol. 2021;32:723–735. doi: 10.1681/ASN.2020050598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schlieper G., Schurgers L., Brandenburg V., Reutelingsperger C., Floege J. Vascular calcification in chronic kidney disease: an update. Nephrol Dial Transplant. 2016;31:31–39. doi: 10.1093/ndt/gfv111. [DOI] [PubMed] [Google Scholar]
- 58.Demer L.L., Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–2948. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized individual patient data can be requested with data sharing agreement.