Summary
Mucosal tissues are constantly exposed to the outside environment. They receive signals from the commensal microbiome and tissue specific triggers including alimentary and airborne elements and are tasked to maintain balance in the absence of inflammation and infection. Here, we present neutrophils as sentinel cells in mucosal immunity. We discuss the roles of neutrophils in mucosal homeostasis and overview clinical susceptibilities in patients with neutrophil defects. Finally, we present concepts related to specification of neutrophil responses within specific mucosal tissue microenvironments.
Keywords: Mucosal Immunity, Neutrophils, Neutropenia, Lung, Oral, Gastrointestinal
Introduction; The neutrophil, its development and trafficking into tissues
The neutrophil, a polymorphonuclear leukocyte, is the most abundant immune cell in our circulation and is considered classically as the cornerstone of the innate arm of immunity1. Approximately, 5 × 1010–1011 neutrophils are produced daily in the bone marrow, in order to maintain a steady supply of cells to the circulation and tissues2,3. This constant supply is necessary due to the short life span of neutrophils (6–12 h in mice in the circulation and 5.4 days in humans and within some tissues) and occurs through a well-coordinated developmental process of granulopoiesis, which can adapt quickly and become exaggerated, when needed, to account for increased needs during infection and inflammation.
Granulopoiesis or neutrophil development is initiated from hematopoietic stem cells which differentiate into multipotent progenitor (MPP) cells that do not have further potential for self-renewal. MPPs give rise to lymphoid-primed multipotent progenitors (LMPPs) and subsequently to granulocyte–monocyte progenitors (GMPs). GMPs, with the support of the granulocyte colony-stimulating factor (G-CSF) commit to neutrophil differentiation by turning into myeloblasts and subsequently transitioning through the stages of promyelocyte, myelocyte, metamyelocyte, band cell, and finally into mature neutrophils4. During the differentiation /maturation process, cells will have evident changes in nuclear morphology, granule content, granular protein expression, proliferative capacity, transcriptional activity5,6 and will express distinct cell surface markers. Specifically, primary (azurophil) granules are found at the myeloblast to promyelocyte stage. Secondary (specific) granules are detected at myelocyte and metamyelocyte stages. Tertiary (gelatinase) granules are found at the band cell stage and secretory vesicles are detected only in mature neutrophils. These granules store an arsenal of serine proteases, including elastase, myeloperoxidase, cathelicidins, defensins, and matrix metalloproteinases7. During maturation, the neutrophil nucleus will also mature from a round shape into a banded and then a lobulated morphology. With maturation, transcriptional and proliferative capacity of neutrophils will also diminish, giving rise to what is considered a terminally differentiated cell state8.
Mature neutrophils are retained in the bone marrow through the expression of two chemokine receptors, CXCR2 and CXCR4. Bone marrow stromal cells express CXCL12 and retain CXCR4-expressing neutrophils in the bone marrow. G-CSF stimulates neutrophil exit from the bone marrow by changing the CXCR4-CXCL12 expression profiles9. G-CSF induces upregulation of CXCR2 ligands on megakaryocytes10 and downregulation of both CXCL12 by bone marrow stroma cells11,12, and CXCR4 on neutrophils themselves13. Under homeostatic conditions, only the mature segmented neutrophils exit the bone marrow. However, during inflammatory conditions, immature neutrophils have been observed in the circulation. This is commonly known as a “left shift” and is used as an indicator of inflammation14. These immature neutrophils have been reported in various inflammatory conditions, ranging from cancer, stress, pregnancy, cardiovascular diseases and notably, viral infections. Once in the circulation, neutrophils will migrate into tissues based on a chemokine gradient and will transmigrate through the vasculature into tissues through a well characterized series of events, namely: tethering, rolling, adhesion, crawling, and finally transmigration. This process is initiated by changes on the endothelial surfaces because of stimulation by inflammatory mediators, such as histamine, cytokines and cysteinyl-leukotrienes secreted from tissue-resident leukocytes upon contact with pathogens or directly by pattern-recognition receptor-mediated detection of pathogens15–17.
The current review will further focus on the functional roles of neutrophils particularly within the mucosal tissue microenvironment.
Neutrophil functions are essential for mucosal homeostasis
Mucosal tissues are constantly exposed to the outside environment, including complex communities of the commensal microbiota, airborne particles and alimentary antigens and elements among a plethora of other environmental stimuli. These surfaces are tasked to perform their organ-specific functions while maintaining barrier integrity, avoiding infection, and regulating inflammatory responses to various stimuli and ongoing injury. Given the key role of neutrophils in anti-microbial defense, tissue repair and resolution of inflammation, they become sentinel cells in the regulation of mucosal immunity and tissue homeostasis. Below we expand on neutrophil functionalities which we consider to be essential for mucosal immunity.
Antimicrobial Defense
Neutrophils are potentially most recognized for their properties in innate antimicrobial defense. They typically arrive rapidly at a site of infection or microbial stimulation and can recognize microbes through an array of innate receptors ranging from pattern recognition receptors (such as TLRs and Dectin-1 molecules) which will directly bind microbes or microbial products to receptors for opsonization products such as complement and Fc receptors18. The nature of the microbial recognition as well as the size of the organism encountered18 will then initiate a cascade of signaling which will dictate the flavor of the antimicrobial response19,20.
Neutrophils mediate their anti-microbial activity through three major mechanisms: phagocytosis, degranulation, and neutrophil extracellular trap (NET) release. Phagocytosis, a term coined initially by Eli Metchnikoff in 1880s, describes the uptake of microbes or other particles larger than 0.5μm. Uptake involves inclusion into a cytoplasmic plasma membrane-bound compartment termed the phagosome21. Following phagocytosis, ingested microbes can be eliminated through oxygen-dependent (“respiratory or oxidative burst”) or oxygen-independent (lytic and proteolytic enzymes-dependent) mechanisms. The oxidative killing mechanism involves the NADPH oxidase complex which is present in the phagosome and facilitates the release of reactive oxygen intermediates (ROIs) that can kill internalized microbes. Oxygen-independent mechanisms depend on antimicrobial peptides and enzymes to facilitate the killing and degradation of ingested microbes. In this scenario, neutrophil granules fuse with the phagosomal membrane, delivering microbiocidal peptides and enzymes.
During neutrophil degranulation22 granule-derived soluble proteins are released into the extracellular space. Neutrophil proteins with well-established anti-microbial potential include contents of the primary-azurophilic granules such as elastase, myeloperoxidase (MPO) and anti-microbial peptides (cathelicidins and defensins), the specific granules’ iron-binding protein lactoferrin and matrix metalloproteinases derived from tertiary granules.
Finally, neutrophils release NETs as an anti-microbial defense strategy, particularly aimed at entrapment and inactivation of larger organisms that cannot be phagocytosed23. NETs are strand-like webs of decondensed chromatin, which are rich in histones and decorated by select granule proteins, many of which have anti-microbial potential. Several microbes are effective in triggering NETosis, such as, Staphylococcus aureus bacteria and hyphae from fungi. NETs are thought to bind viruses, bacteria, fungi, and parasites, conceivably to prevent their spread within tissues and translocation into the circulation. Yet, while NETs are thought to originally have anti-microbial protective roles, inability to remove NETs has been linked to various forms of immunopathology ranging from autoimmunity to atherosclerosis and thromboembolism24.
Generally, anti-microbial neutrophil defenses of neutrophils are well characterized for their importance in the containment of bacterial and fungal infection, but not as well established in anti-viral defense.
Tissue repair; the pro-resolving, wound healing neutrophil
At mucosal surfaces there is a constant need for tissue repair and regulation of inflammation. Mucosal tissues are subject to continuous microinjury due constant environmental exposure. Furthermore, homeostatic inflammation in these tissues necessitate continuous tissue repair. In such settings neutrophils can play essential roles related to wound healing including, antimicrobial defense, clearance of the wound bed, repair, and inflammatory resolution where neutrophils will participate (Figure 1). The oral mucosa may be a prototypic site where the function of neutrophils in promoting tissue repair and inflammatory resolution become most evident. The oral mucosa is subject to constant injury and mechanical triggering, even at steady state25. Yet this is an area known for seamless, rapid wound healing without scarring. Studies in humans comparing oral with cutaneous wound healing in the same (young and healthy) individuals have demonstrated that wound healing in the oral mucosa is characterized by rapid recruitment and then resolution of neutrophil responses, without engagement of chronic inflammatory adaptive immune pathways26. This timely innate response which is acute and resolves rapidly appears to promote optimal wound healing in the oral environment. Even in the absence of overt injury, neutrophil-mediated responses become critical for homeostatic repair and maintenance of barrier integrity and tissue functionality27.
Figure 1. Neutrophil functions are essential for mucosal immunity.
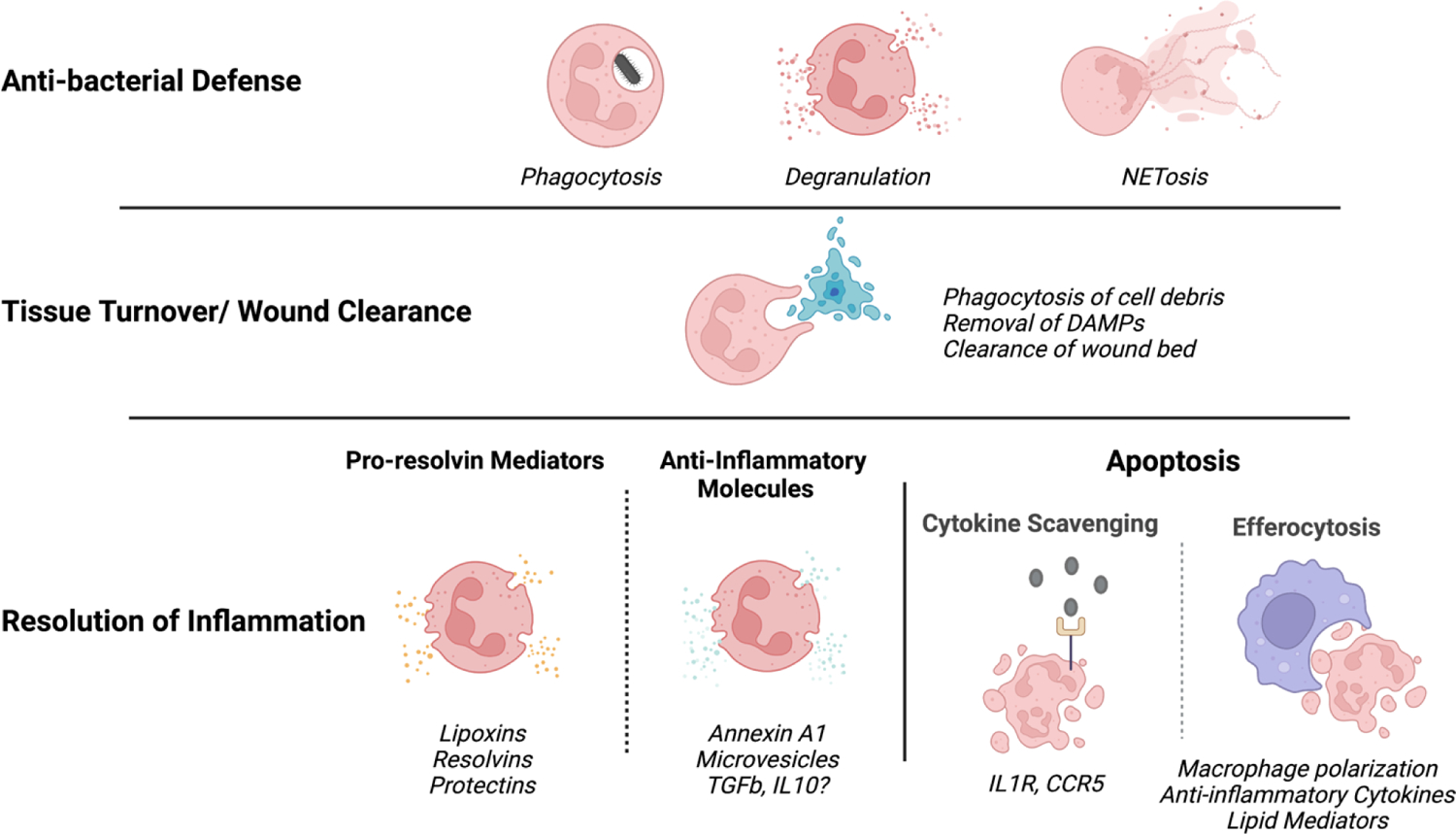
Neutrophil functions are critical for mucosal barrier defense. Anti-microbial functions of neutrophils are essential during infection. Wound clearance through phagocytosis is key during injury but also for homeostatic tissue repair. Resolution of inflammation is necessary to promote healing and repair and for constant regulation of inflammatory reactions at the barrier.
As innate lymphocytes, neutrophils are rapidly recruited to sites of injury following cell injury and microbial exposure. Indeed, damage associated molecular patterns (DAMPs) released by injured cells as well as pattern associated molecular patterns (PAMPS), stimulate resident cells to recruit neutrophils acutely to sites of damage28. Adenosine triphosphate (ATP) released from necrotic cells can also generate signals that will mediate neutrophil recruitment29. In addition, N-formyl peptides such as fMet-Leu-Phe (fMLP), a key chemoattractant that can be bacteria-derived, can also be released from damaged mitochondria during tissue necrosis and directly recruit neutrophils28.
At the site of injury neutrophils play multiple roles related to clearance of the site of injury/wound bed, promotion of angiogenesis and resolution of inflammation.
Phagocytosis/wound clearance:
As efficient phagocytes, which typically are the first to arrive at sites of injury, neutrophils can clear the site from invading microbes and cellular debris in order to allow for repair to begin. In the wound setting, neutrophils will perform classic anti-microbial defenses (see above) to combat infection. Furthermore, as a typical cleaning crew, these cells will remove cellular debris, cell fragments and DAMPs that can be proinflammatory and inhibit healing. This clearing of the wound bed will create an environment conducive for healing and repair.
Angiogenesis:
Angiogenesis is a key step for wound healing. Neutrophils are increasingly recognized for their pro-angiogenic potential not only during healing but also in carcinogenesis27. Indeed, a subset of angiogenic neutrophils defined by CXCR4hiVEGFR+CD49d+ expression has been identified in humans and mice30. Pro-angiogenic functions of neutrophils include secretion of MMP-931, which can activate the vascular endothelial growth factor (VEGF) to promote neo-angiogenesis31. Additionally, neutrophils have been shown to express VEGF themselves in certain settings and to promote recruitment of other angiogenic cell types27.
Resolution of Inflammation:
Resolution of inflammation is a key step in wound healing. After the acute responses needed for antimicrobial defense and wound clearance have occurred, inflammation has to be resolved to allow for repair. The fact that resolution of inflammation is an active process has been recognized in recent years and neutrophils are well appreciated to be key cellular mediators in inflammatory resolution through a number of processes.
a. Active secretion of pro-resolving and anti-inflammatory mediators
During inflammation, neutrophils get exposed to prostaglandin E2 (PGE2) which induces a lipid class switch in neutrophils from 5-lipoxygenase (LO) to 15-LO expressing pathways32. Increased transcription of 15-LO results in the switching of arachidonic acid products from the classical prostaglandins and leukotrienes to pro-resolving lipoxins (LX). Particularly, LXA4 inhibits further recruitment of granulocytes into the site of inflammation and promotes the efferocytosis of apoptotic neutrophils by macrophages32,33. Neutrophils additionally promote the biosynthesis of pro-resolution lipid mediators such as resolvins and protectins, further promoting the shift from a proinflammatory to a pro-resolution milieu32.
Another key anti-inflammatory/pro-resolving molecule expressed/secreted by neutrophils is the glucocorticoid-inducible protein, annexin A1 (AnxA1), which promotes resolution by recruiting monocytes without inducing their full activation and by facilitating efferocytosis, among other mechanisms34. Neutrophils, typically store AnxA1 in their tertiary granules or cytosol, and traffic it to the cytosolic leaflet of the plasma membrane in the presence of high intracellular Ca2+. Ultimately, AnxA1 is typically released from neutrophils on the surface or within macrovesicles35.
Finally, in the murine setting, neutrophils have been shown to secrete the anti-inflammatory cytokine IL-10 and the mediator secretory leukocyte protease inhibitor (SLPI), an anti-microbial peptide with immunomodulatory activity which promotes tissue healing36.
b. Scavenging of pro-inflammatory mediators
Another mechanism by which neutrophils control the inflammatory environment, is through scavenging pro-inflammatory mediators and not allowing them to signal. Indeed, apoptotic neutrophils express various cytokine/chemokine receptors and sequester corresponding mediators without allowing them to mediate activity. As such, neutrophils constitutively express the type II IL-1 decoy receptor and can upregulate IL1R to sequester IL1 cytokines. Similarly, expression of the chemokine CCR5 on early apoptotic cells will capture and entrap the CCL3 and CCL5 chemokines. It has also been reported that NETs can affect the inflammatory environment by entrapping inflammatory mediators and proteolytically cleaving them through the proteases expressed on their surface37.
c. Efferocytosis
Potentially, the most important mechanism through which neutrophils control inflammation and trigger resolution, is efferocytosis. Efferocytosis is the process of uptake of apoptotic neutrophils by macrophages which triggers a shift in the polarization of macrophage responses from pro-to anti-inflammatory38. After neutrophils have performed their anti-microbial and wound clearing activities, they will revert to an apoptotic cell death. As opposed to necrosis which releases cytosolic DAMPs and triggers inflammation, neutrophils undergoing apoptosis will not trigger inflammatory cascades, but rather signal for efferocytosis and inflammatory resolution27,39. Apoptotic cells, release “find me” signals for professional phagocytes, particularly macrophages, to approach. A key signal for efferocytosis is the externalization of phosphatidylserine from apoptotic cells. Apoptotic cells further express molecules on their surface for binding and internalization. These molecules either directly interact with phagocyte receptors or engage bridging molecules that allow interaction with phagocytes27,39. Efferocytosis of apoptotic cells by macrophages initiates a phenotypic shift in the macrophages from a proinflammatory phenotype to one which is more anti-inflammatory resulting in the promotion of wound healing40. Internalization and phagocytosis of apoptotic cells activates nuclear sterol receptors, such as peroxisome proliferator-activated receptor-γ (PPARγ), PPARδ and liver X receptor-α (LXRα), which stimulate production of the anti-inflammatory cytokines IL-10 and TGFβ and suppress gene expression for pro-inflammatory cytokines. Additionally, efferocytosis triggers the production of proresolving mediators by phagocytes to actively induce resolution of inflammation33,40.
Congenital neutropenia and defects of neutrophil function in humans reveal essential roles of neutrophils in mucosal immunity
Genetic defects in humans affecting the immune system, often referred to as genetic immunodeficiencies, can become particularly enlightening towards our understanding of the specific role of a gene, pathway and or cell population in human health and disease. In this regard, multiple genetic defects associated with neutrophil development and function have been identified in humans41 (Table 1). Most neutrophil defects are well characterized clinically and reveal insights into the distinct neutrophil functionality as regulators of immunity, particularly in the context of different tissue microenvironments.
Table 1. Genetic neutrophil defects.
Here we summarize the genetic defects that affect neutrophil availability and functions and their effect on neutrophil/other cell functions.
| Disease | Gene | OMIM | Affected Cell/Function |
|---|---|---|---|
| Congenital Neutropenia | |||
| Elastase Deficiency | ELANE | 600871 | Neutrophil/Development |
| GFI 1 deficiency | GFII | 600871 | Neutrophil/Development |
| HAX1 deficiency (Kostman) | HAX1 | 605998 | Neutrophil/Development |
| G6PC3 deficiency | G6PC3 | 611045 | Neutrophil Development, chemotaxis, O2 production |
| VPS45 deficiency | VPS45 | 610035 | Neutrophil differentiation, migration |
| Glycogen storage disease type 1b | G6PT1 | 602671 | Myeloid differentiation, chemotaxis, O2− production |
| X-linked neutropenia/myelodysplasia | WAS (gof) | 300299 | Differentiation, mitosis. |
| P14/LAMTOR2 deficiency | LAMTOR2 | 610389 | Endosomal biogenesis |
| Barth Syndrome | TAZ | 300394 | Mitochondrial function |
| Cohen syndrome | VPS13B | 607817 | Neutrophil/Development |
| Clericuzio syndrome | USB1 | 613276 | Neutrophil/Development |
| JAGN1 deficiency | JAGN1 | 616012 | Neutrophil/Development |
| 3-Methylglutaconic aciduria | CLPB | 616254 | Mitochondrial protein |
| G-CSF receptor deficiency | CSF3R | 138971 | Stress granulopoiesis disturbed |
| Shwachman-Diamond Syndrome | SBDS | 607444 | Neutrophil maturation, chemotaxis, ribosomal biogenesis |
| DNAJC21 | |||
| EFL1 | |||
| HYOU1 deficiency | HYOU1 | 601746 | Unfolded protein response |
| SRP54 deficiency | SRP54 | 604857 | Protein translocation to ER, |
| Defects of motility | |||
| Leukocyte adhesion deficiency type 1 (LAD1) | ITGB2 | 600065 | Neutrophil transmigration into tissues |
| Leukocyte adhesion deficiency type 2 (LAD2) | SLC35C1 | 605881 | Rolling, chemotaxis |
| Leukocyte adhesion deficiency type 3 (LAD3) | FERMT | 607901 | Adherence, chemotaxis |
| Rac2 deficiency | RAC2 | 608203 | Adherence, chemotaxis O2− production |
| β actin deficiency | ACTB | 102630 | Motility |
| Localized juvenile periodontitis | FPR1 | 136537 | Formylpeptide induced chemotaxis |
| WDR1 deficiency | WDR1 | 604734 | Spreading, survival, chemotaxis |
| Cystic fibrosis | CFTR | 602421 | Chemotaxis |
| Neutropenia with combined immune deficiency due to MKL1 deficiency | MKL1 | 606078 | Impaired expression of cytoskeletal genes |
| Defects of respiratory burst | |||
| X-linked chronic granulomatous disease (CGD), gp91phox | CYBB | 306400 | Killing (faulty O2− production) |
| Autosomal recessive CGD | CYBA | 608508 | |
| CYBC1 | 618334 | ||
| NCF1 | 608512 | ||
| NCF2 | 608515 | ||
| NCF4 | 613960 | ||
| G6PD deficiency class I | G6PD | 305900 | Reduced O2− production |
| Defects of neutrophil proteases | |||
| Papillon-Lefèvre syndrome | CTSC | 602365 | Cathepsin C deficiency |
| Secondary granule assembly | |||
| neutrophil protease packaging |
Broadly, neutrophil defects have been classified in 3 major categories: Congenital neutropenia(s), defects in neutrophil trafficking and transmigration and defects in neutrophil function. Congenital neutropenia arises most commonly from genetic defects in granulopoiesis, the process of development/differentiation of neutrophils within the bone marrow (see above) and are linked to drastically reduced numbers of neutrophils in the circulation and within tissues. Clinically, patients with congenital neutropenia broadly present with infection susceptibility. Common infections are mucosal and most often involve infections of the respiratory tract (most often bacterial, sinopulmonary infections and pneumonias) which can become systemic42. Oral manifestations are also a hallmark of congenital neutropenia. Severe periodontitis, inflammation in oral tissues that surround the dentition and associated bone destruction at an early age, as well as oral mucosal ulcers are some of the most common manifestations of neutropenic patients. Infections and inflammatory susceptibility in the gastrointestinal tract can also occur.
Defects in neutrophil recruitment, trafficking, and transmigration are characterized by drastically reduced presence of neutrophils within tissues. In most of these diseases, neutrophils develop, egress from the bone marrow and are present in systemic circulation, but have impaired chemotaxis or diapedesis leading to tissue-specific neutropenia. In this regard, such diseases become a window into understanding the role of neutrophils within tissue microenvironments. Clinical susceptibilities in such patients largely resemble the manifestations of neutropenia, including infection susceptibility, impaired wound healing and severe oral inflammatory disease43. Mucosal infections are most often respiratory. Gastrointestinal disease most often presents in the perirectal area and is associated with gram negative bacterial infections44. Periodontitis is also a hallmark of neutrophil motility defects43. In fact, Leukocyte Deficiency I, the prototypic genetic defect of neutrophil trafficking is linked to severe oral disease at an early age, with patients often losing their entire dentition in teenage years45. Furthermore, a specific defect in neutrophil motility linked to a genetic defect in the formyl peptide receptor 1 on neutrophils, leads to a single clinical susceptibility, that of periodontal disease.
Defects in neutrophil functionality also present with diverse mucosal manifestations. However, it is important to consider that defects in neutrophil functionality result from defects in genes that are expressed exclusively in the neutrophil compartment and thus affect additional cell sources. Most recognized is the group of chronic granulomatous disease (CGD) defects, which are linked to a deficiency in superoxide production due to defects in the NADPH oxidase machinery44. These patients are also susceptible to infections and inflammatory disease at mucosal barriers, and present with a distinct infection susceptibility that includes specific bacteria and fungi as well as a more distinct susceptibility to inflammatory colitis (Table 2). Finally, a select defect in a neutrophil protease, cathepsin C, which affects the processing of neutrophil serine proteases and their packaging in secondary granules, presents with a single mucosal susceptibility, that of severe periodontal disease, potentially highlighting unique functionality of neutrophils in the oral barrier.
Table. 2. Mucosal Clinical Manifestations in Patients with Neutrophil Defects.
Here we summarize various defects in neutrophil functionality and their effect on distinct infection susceptibility of the host.
| Common manifestations in patients with Congenital Neutropenia: |
| Bacterial Infections; commonly respiratory |
| Staphylococcus aureus |
| Streptococci, enterococci, pneumococci |
| Pseudomonas aeruginosa |
| Gram negative bacilli |
| Oral manifestations (inflammatory): Periodontitis, Oral Ulcers |
| Diffuse gastrointestinal disease- sometimes associated with enteric infection |
| Common manifestations in patients with Neutrophil motility defects: |
| Mucosal bacterial infections; Respiratory, GI, Perirectal area |
| Infections often become necrotic |
| Staphylococcus aureus and Gram-negative enteric organisms |
| Oral manifestations (inflammatory): Periodontitis, Oral Ulcers |
| Common mucosal manifestations in patients with Neutrophil Function Defects: |
| Defective O2 production |
| Respiratory infections, Gastrointestinal disease |
| Bacterial infections, granulomas; Staphylococcus aureus, Burkholderia cepacia |
| Nocardia species, gram negative enteric bacilli. |
| Increased risk for infection with mycobacterial species |
| Invasive fungal infections with molds and yeast (Aspergillus) |
| Cathepsin C defect |
| Oral disease; severe periodontitis |
Collectively, patients with severe neutrophil defects present with susceptibility to bacterial infections in mucosal barriers, primarily in the respiratory tract and secondarily in the GI tract. They also present with defective wound healing and aberrant hyperinflammatory responses to microbiota which manifest both as defective cutaneous wound healing as well as hyperinflammatory /destructive responses at the oral mucosa, an area constantly exposed to commensal microbiota and subject to ongoing injury during mastication25.
This concept of hyper-inflammatory responses being a key feature in many immune-deficiencies, has been recently increasingly recognized46,47 and appears to largely represent a misguided compensatory inflammatory response when the appropriate arm of the immune system is unable to perform its intended function. In this regard, in the setting of neutropenia or defective neutrophil trafficking, neutrophil recruitment pathways, such as the IL17 response become exaggerated in an effort to compensate for the low abundance of neutrophils. This mechanism, coined the “neutrostat” is set in place to regulate granulopoiesis and neutrophil recruitment into tissues48. However, in the absence of tissue neutrophils this axis becomes dysregulated and leads to immunopathology and defective wound healing.
Tissue Specification of Neutrophil Responses
The concept of tissue specification for immune cell populations has been coined to express the adaptations undertaken by immune cell subsets in response to tissue- specific stimuli. Typically, such adaptations serve to “program” immune cells to serve the functional needs and environmental exposures unique to different tissue microenvironments. Within the myeloid compartment, macrophage populations are well recognized for their plasticity toward adaptations that serve the functionality of the specific tissue they inhabit49.
Neutrophils, however, when they exit the bone marrow in their mature state, have been long thought of as terminally differentiated cells with a short life span, minimal capacity for proliferation and low transcriptional activity and therefore have not been considered as a cell with functional plasticity and potential for further differentiation within tissues50. However, it has been increasingly recognized that neutrophils expressing distinct cell surface markers and with specialized immune functionalities exist both in the circulation and within tissues. Whether these represent “bonafide subpopulations” or cell states is not clear51, but it’s well appreciated that neutrophils display adaptability within the tissue microenvironment and in response to states of inflammation, infection, carcinogenesis etc.
Recent studies, utilizing emerging technologies based on single cell sequencing and phenotyping approaches have demonstrated tissue specification of neutrophils within distinct tissue microenvironments52. In fact, through this work, it has become evident that neutrophils extend their lifespan within tissues which allows for tissue-specific adaptations. Within the tissue microenvironment neutrophils appear (even at steady state) to acquire unique transcriptional and chromatin adaptations and to perform distinctive, including non-canonical functions (such as promotion of angiogenesis). Interestingly, such adaptations occur within the tissue and in some cases (i.e. in the lung) within particular microenvironments of the specific milieu52.
Neutrophil phenotype and functionality in tissues appears to relate to a number of factors including developmental stage, ageing and response to particular stimuli.
Development state is a key factor in determining neutrophil adaptability. Immature neutrophils can exit the bone marrow into the circulation during inflammation, infection and disease states53 and are typically characterized by a higher proliferative and differentiation capacity5,6. Additionally, neutrophils in varying stages of development can exit the bone marrow, particularly during states of emergency granuolopoiesis54 leading to a developmentally heterogeneous population of neutrophils that can potentially display various unique effector functions in response to the inflammatory stimulus. The spectrum of developmental stages of the neutrophil which can be encountered from the BM to the circulation and across tissues has been recently termed ‘neutrotime” to represent neutrophil across the spectrum of developmental time55.
Neutrophil aging, a neutrophil- intrinsic transcriptional program regulated by diurnal changes in gene expression, has also been shown to be a key factor affecting phenotype and functionality56. Aged neutrophils have a much higher phagocytic potential as compared with the non-aged neutrophils57, respond faster to inflammatory signals and have a greater survival capacity when responding to infectious agents. However, aged neutrophils have also been linked to severe disease pathology in inflammatory models, further supporting divergent functionality with aging56.
Finally, neutrophil adaptation depends on the tissue-specific triggers encountered in each environment in health but also in the setting of injury, infection, cancer, autoimmunity, and give rise to different effector responses depending on the specific situation. Therefore, it is not surprising that neutrophils with pro-inflammatory versus immunomodulatory and reparative programs have been identified51. As an example, neutrophil- myeloid suppressor cells have been identified in certain settings, and N1/N2 neutrophils have been designated to characterize neutrophils with anti- and pro-tumor functionality51.
A key signal that regulates neutrophil ageing and functionality, which is particularly relevant for the mucosal barriers, is the microbiome58. Neutrophil ageing has been shown in vivo to be driven by microbiota through TLR and MyD88 (myeloid differentiation factor 88-mediated signaling) pathways. In fact, depletion of microbiota is connected to a reduced number of ageing and proinflammatory neutrophils and with protection from neutrophil-mediated immunopathology. Another tissue trigger linked to neutrophil activation in mucosal tissues is fibrin. Fibrin, a classic molecule linked to hemostasis, becomes deposited in the extravascular space of tissues not only with injury but also during inflammation. In the mucosal tissues, constant homeostatic inflammation is linked to extravascular fibrin deposition and fibrin mediated inflammation, particularly when fibrin is inadequately cleared59. Recent work has demonstrated that fibrin deposited in the oral mucosa engages tissue neutrophils through its CD11b binding site (the αMβ2 integrin receptor) and triggers neutrophil effector functions including ROS production and NETosis to drive immunopathology59.
While the effects of tissue cues on neutrophil functionality have not yet been fully understood, it appears that some tissue microenvironments may promote accumulation of neutrophils at steady state serving as reservoirs for neutrophils, either displaying marginated neutrophils in their vasculature (such as seen in the lung or spleen), serving as bonafide reservoirs (as proposed for lung)51 or displaying a constant stream of transmigration even in health (such as observed in the oral mucosa)60,61. Interestingly, the respiratory tract and the oral mucosa, which are natural habitats for neutrophil transmigration even in health, are also the tissue microenvironments most susceptible to clinical manifestations in patients with neutropenia.
Neutrophil functionality and heterogeneity in mucosal tissues
Neutrophil heterogeneity in the respiratory tract mucosa in health and disease
The respiratory tract mucosa is exposed to an enormous quantity of diverse airborne elements and infectious agents. However, respiratory pathology is an exception rather than a rule reflecting a strong natural defense system operating in the respiratory mucosa. Neutrophils appear to be a key component in respiratory surveillance and homeostasis as neutrophil deficiencies lead to clear phenotypes of respiratory infection both in the upper and lower respiratory tract. Particularly bacterial infections with gram negative bacilli are common in patients with genetic defects in neutrophil development or motility (Table. 2).
Indeed, neutrophils are encountered even at steady state in the respiratory tract. Neutrophils are present constitutively in the nasal mucosa. The lung, in particular, is considered a reservoir for neutrophils. Neutrophils can be found as a marginated, intravascular pool, adhering to the endothelium of capillaries and postcapillary venules in the lung. In humans, they acquire higher expression levels of CD11b, but lower expression of CD62L and have been shown to be activated once in the lung and are present regardless of the inflammatory condition62. Additionally, a lung-specific neutrophil signature has been recently described in mice with specialized functionality relevant to vascular growth and repair52.
Neutrophils have been associated both with protection towards infection as well as pathology at the respiratory tract and distinct neutrophil subsets have been associated with health and disease at this interface. Indeed, neutrophil infiltration is a common characteristic in both viral and bacterial respiratory diseases. In both mice and humans, viral respiratory diseases (VRDs) caused by Influenza A virus (IAV), respiratory syncytial virus (RSV), rhinovirus (RV), metapneumovirus, adenovirus, and SARS-CoV2, have all been associated with neutrophil recruitment and activation63–68. Increased number of neutrophils in the lung is a hallmark for RSV disease severity in both humans and mice69,70. Severe RSV-infected infants’ bronchoalveolar lavage is dominated by neutrophils69. Severe IAV and SARS-CoV-2 infections also show an increase in lung neutrophil markers, and genes of neutrophil function and activation71–76. Additionally, neutrophil infiltration is also noted in upper respiratory tract (URT) infections. RV and adenovirus are typical causes of URT infections, such as the common cold. Neutrophils have been shown to infiltrate the nasal mucosa and secretions in the early stages of cold from symptomatic RV-infected patients77. High levels of neutrophils, and neutrophil-associated antimicrobial peptides (HNP-1, −3, and −4) have been observed in the upper respiratory tracts of Adenovirus-infected children78.
Bacterial infections in the respiratory tract have been associated both with lack of and overabundance of neutrophils. Neutropenic individuals, or those with impaired neutrophil antimicrobial activity, are shown to be at increased risk for pneumonia79–81. Similarly, depletion of neutrophils in animal models prior to several bacterial82–84 or viral68,85 pulmonary infections resulted in increased pathogen burdens and heightened mortality. These findings emphasize the fact that the neutrophils are important early on in controlling pathogen burdens. However, an exacerbated neutrophilic response contributes significantly to respiratory tract mucosal immunopathology via increased activation of neutrophils79,86. In fact, defective neutrophil resolution in the lungs leads to poorer outcomes, and depletion of these cells after the onset of disease enhances host survival in several animal models of bacterial and viral pulmonary infections82,85. Therefore, successful control of pulmonary infections requires a balance of efficient clearance of invading pathogens by neutrophils with the subsequent resolution of these immune cells to prevent tissue damage. These observations emphasize the importance of characterizing the role and heterogeneity of neutrophils in respiratory diseases (Figure 2).
Figure 2. Neutrophil heterogeneity in respiratory tract.
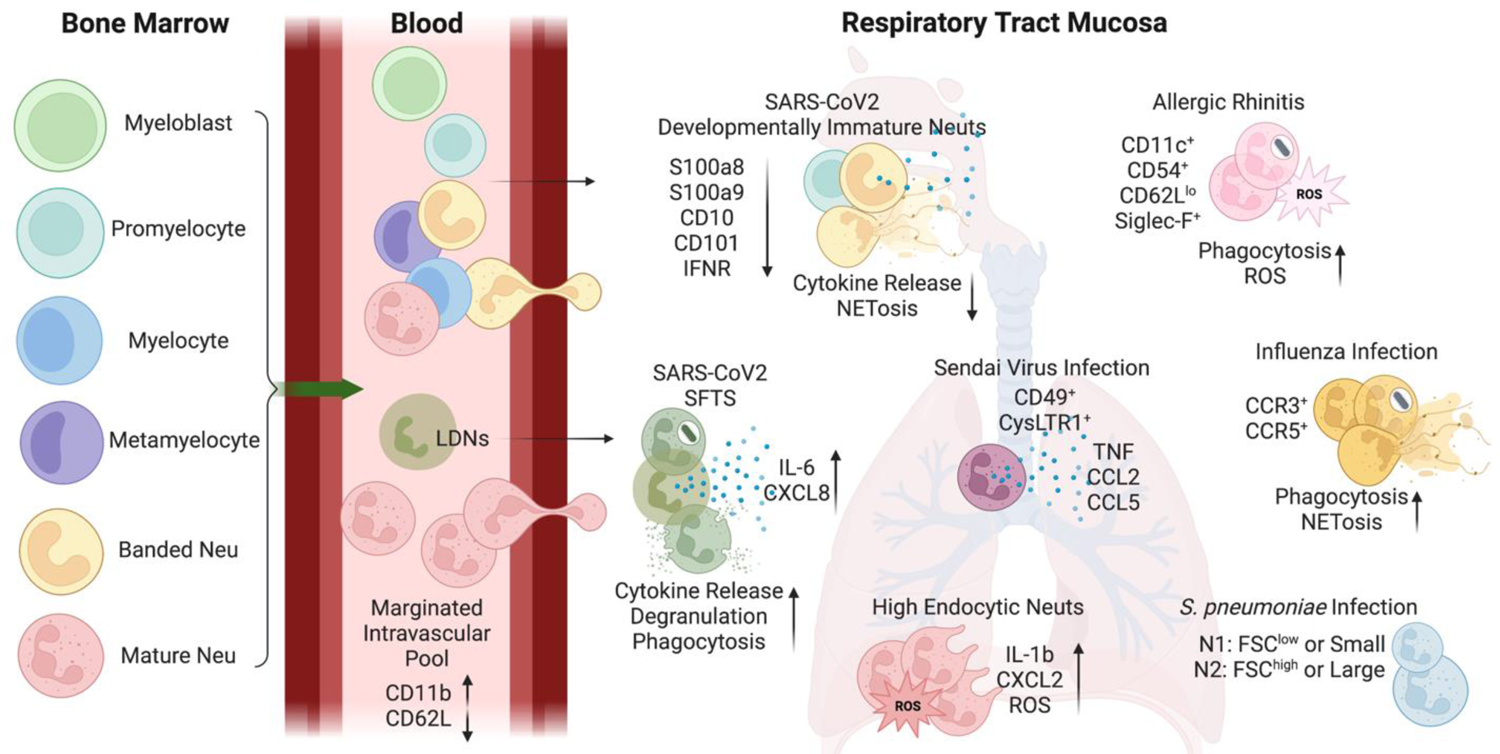
Developmentally divergent neutrophils (Neu) have been detected in the circulation and respiratory tract mucosa during inflammatory conditions. Lungs contain a marginated intravascular pool of neutrophils with tissue-specific marker expression. Additionally, immature neutrophils and low-density neutrophils (LDNs) have been identified in the respiratory mucosa during disease states. Furthermore, select neutrophil subtypes have been identified in distinct infections. These subsets may exert various effector functions with different capabilities.
C-C motif chemokine ligand (CCL); C-C motif chemokine receptor (CCR); cysteinyl leukotriene receptor 1 (CysLTR1); C-X-C chemokine receptors (CXCR); forward scatter (FSC); interferon receptor (IFNR); interleukin (IL); neutrophil extracellular trap formation (NETosis); reactive oxygen species (ROS); S100 calcium binding protein A (S100A); severe fever with thrombocytopenia syndrome (SFTS); tumor necrosis factor (TNF)
Neutrophil subsets in the respiratory tract have been associated with distinct developmental and activation states, resulting from different environmental exposures5,51,87,88. Neutrophils developmental state appears to contribute to a first level of heterogeneity. During inflammation, immature neutrophils have been observed in inflamed tissues, marked as a “left shift” of precursor neutrophils14. Recent evidence with single-cell sequencing technologies has identified developmentally immature neutrophils in the blood and lungs of SARS-CoV2-infected patients, displaying lower expression levels of S100a8, S100a9, CD10, and CD10189. These neutrophil populations consisted of pro-neutrophils, pre-neutrophils, and immature neutrophils that associate highly with severe disease90. In fact, presence of immature neutrophils corelated with COVID-19 disease severity and has been proposed as a negative prognostic marker91. Immature neutrophils have been shown to respond weakly to interferon (IFNα or IFNγ), as they lack type-1 IFN signaling receptors and associated genes92, and thus have an impaired ability to respond to viral stimuli, leading to a lower propensity for NETosis and cytokine release. Single-cell analysis of SARS-CoV2-infected patient leukocytes confirms this, showing proNeus and preNeus in the blood have much lower expression of IFN signaling genes90. Moreover, both proNeus and preNeus have low or no expression of CD16 required for antibody-dependent cellular phagocytosis. This further differentiates the antiviral potential between mature and immature neutrophil subsets. Retrospective studies of infants with various viral respiratory diseases have observed high frequencies of immature neutrophils, which were not influenced by bacterial coinfections93. During viral infections in infants, three major neutrophil populations have been identified, namely immature CD16loCD62Lhi neutrophils, mature CD16hiCD62Lhi neutrophils, and a suppressive CD16hiCD62Llo subset94,95. The functional role of these immature neutrophils in the tissue or the rationality for their recruitment during viral respiratory disease is still unclear. It is proposed that they are recruited because of the high inflammation that stimulates the rushed mobilization of immature neutrophils from the bone marrow to the circulation and sites of inflammation. These immature neutrophils are perhaps less efficient in providing required assistance in viral clearance and forming NETs and may cause a feedback loop that triggers the recruitment of more immature granulocytes. Indeed, studies on immature neutrophils during inflammatory conditions suggest that immature neutrophils can perform conventional inflammatory responses, such as bacterial phagocytosis and killing via the production of reactive oxygen species, although less efficiently than mature neutrophils96. However, some groups have disagreed with this view, demonstrating enhanced activation of immature neutrophils. One group has noted that immature neutrophils produced increased level of extracellular reactive oxygen species in vitro97 and a separate in vitro human study has demonstrated increased immature neutrophil migration through CXCL8 signaling, with a higher predisposition towards NETosis correlating with severe COVID-19 disease98. Thus, the exact functional role of these immature neutrophils is yet to be clarified.
A second layer of neutrophil heterogeneity is associated with disease status. In the human nasal mucosa, three neutrophil subsets have been detected in chronic rhinosinusitis patients using single-cell level analysis, a 3 C-C motif chemokine ligand+ (CCL3+), a guanylate binding protein 5+ (GBP5+) and a statherin+ (STATH+) population99. However, their functional differences were not further explored. Also in the nasal mucosa, neutrophil subsets characterized by cell surface expression of CD11c+ CD54+ CD62Llo Siglec-F+ molecules have been observed to accumulate during nasal inflammation in a mouse allergic rhinitis model. Compared to conventional neutrophils, Siglec-F+ neutrophils exhibited increased phagocytotic functions and a tendency toward increased ROS generation100. A separate population of SiglecF-expressing cells were detected in the mice lungs upon exposure to diesel exhaust particles. This new subset of neutrophils is noted to express high levels of cysteinyl leukotrienes and neutrophil extracellular traps101. Furthermore, flow cytometric analysis of neutrophils from mice models of influenza infection noted increased expression of a chemokine receptor repertoire. This included expression of chemokine receptors CCR3 and CCR5, typical eosinophilic CCRs102. Stimulation of these CCRs with corresponding ligands increased neutrophil migratory capacity, phagocytic activity, and NETosis. Perhaps this enhanced expression of chemokine receptor repertoire represents a mechanism to refine neutrophil effector functions in specific infiltrating tissues. Similarly, infiltrated neutrophils from human chronic inflammatory lung diseases have been shown to highly express CCR3 and CCR5103. In response to influenza A viral infection, infiltrating murine neutrophils were detected to express a typical eosinophilic marker IL-5 receptor alpha (IL-5Rα), which lead to IL-5-mediated suppression of neutrophil respiratory burst104,105. Mice infected with Sendai virus (SeV), the murine parainfluenza virus type 1, recruited a CD49d+ subset of neutrophils (CD49d+ PMN) that induced the expression of the high-affinity receptor for IgE (FcεRI) on lung conventional dendritic cells (cDC)106. CD49d+ neutrophils have also been documented in humans during acute respiratory illness107. As seen in mice, human CD49d+ neutrophils isolated from nasal lavage during a respiratory viral infection expressed CysLTR1. In mice, CD49d+ CysLTR1+ neutrophils represented a “pro-atopic” neutrophil subset that produced TNF, CCL2, and CCL5, where inhibition of CysLTR1 signaling was sufficient to reduce accumulation of CD49d+ neutrophils in the lungs and development of post-viral atopic airway disease106. Similar to viral infections, bacterial infections may also trigger the presence of specific neutrophil subsets. In agreement with this idea, two previously defined neutrophil subsets were identified in lungs during health and experimental S. pneumoniae infection in mice108. These two subsets were identified based on their difference in size (N1: FSClow or small and N2: FSChigh or large). Moreover, FSClow cells showed low granularity, a low number of vacuoles and a nucleus mainly constituted by heterochromatin. On the other hand, FSChigh neutrophils are larger cells, have low presence -or absence- of granules and have presence of multiple vacuoles. Gonzalez et al noted that these neutrophils are the source of IL10 that is required for subduing the inflammatory damage in murine lung. They further observed that FSChigh neutrophils produce significantly higher levels of IL-10 compared to FSClow neutrophils109. Morphologically, FSClow and FSChigh subtypes display similar characteristics to the murine neutrophil subtypes reported in a systemic inflammatory syndrome in response to intravenous Staphylococcus aureus infection110. In addition, infection of IL-5 deficient mice with Actinobacillus pleuropneumoniae significantly increased PMN-II (N2) cells in the lung111.
The endocytosis capacity of neutrophils has recently been suggested as a factor for neutrophil heterogeneity. Two subsets of neutrophils have been characterized in murine lungs, bone marrow, peripheral blood, and spleen both under steady state and after inflammatory challenge based on endocytosis capacity112. These two subsets were characterized by their capability to endocytose albumin nanoparticles (ANP), one that readily endocytosed ANP (ANPhigh neutrophils) and another that failed to endocytose ANP (ANPlow neutrophils). ANPhigh neutrophils showed transcriptomic heterogeneity, produced increased amounts of reactive oxygen species (ROS) and inflammatory chemokines and cytokines compared with ANPlow neutrophils in the lung. In a lung inflammation model, ANPhigh neutrophils have been shown to be capable of exacerbating lung inflammation by increasing the inflammatory cytokine Il-1β and chemokine CXCL2 expression112.
In humans, low-density neutrophils (LDNs) have been described (particularly in the systemic circulation) and associated with various inflammatory and autoimmune diseases113. More recently LDN have been associated with viral respiratory disease in the context of SARS- CoV2 infection. The activation status of neutrophils also reported to create a level of heterogeneity. Low-density neutrophils (LDNs) detected in COVID-19 patients are suggested to be a degranulated form of neutrophils capable of immunosuppression114,115. This group of neutrophils consisted of a mixture of immature and mature phenotypes114–116. Specifically, the CD16int LDN population displayed enhanced cytokine production upon stimulation, increased phagocytosis and degranulation compared with CD16hi population114. However, the increased functionality of LDN in SARS- CoV2 infection is not observed in all studies117. LDN have also been identified in the viral disease, severe fever with thrombocytopenia syndrome (SFTS) and shown to be increased dramatically in patients compared with the healthy donors118. Interestingly, in this context as well LDN appeared to have heightened activation and secreted higher amounts of pro-inflammatory cytokines, such as IL-8, IL-6, and IL-17 and demonstrated enhanced potential to induce endothelial cell injury118.
Neutrophil function and heterogeneity in the gastrointestinal tract mucosa in health and disease
Upper gastrointestinal tract/oral mucosa
Neutrophils are present within several niches of the oral cavity. Under steady-state conditions, oral PMNs are found in saliva119, within oral/mucosal tissues120 and in the periodontal pocket121, the area between teeth and oral mucosal (gingiva). In fact, PMNs continuously transmigrate into the gingival area of the oral mucosa, even in health, pass through the epithelial connection of the tooth with the mucosa (the junctional epithelium) and reach the periodontal pocket122. Indeed, even in the absence of overt inflammation, 95% of cells present in the periodontal pocket are neutrophils122. This continuous transmigration of neutrophils to the oral/periodontal tissues, suggests important homeostatic functions of tissue neutrophils at the oral barrier (Figure 3). Neutrophils are traditionally thought to arrive to the periodontal pocket to perform classic antimicrobial defenses against the rich and diverse microbial biofilm which forms a complex biofilm on the tooth-root surface123. However, neutrophil recruitment is only partially regulated by the commensal microbiota at the gingival interphase. Germ free mice have been shown to have continuous neutrophil transmigration to the periodontal pocket, albeit with reduced numbers compared to WT counterparts124. Another tissue-specific cue that appears to regulate neutrophil recruitment at the oral barrier is ongoing damage from mastication which triggers Th17 immunity and downstream neutrophil recruitment25. Given the nature of constant triggers at the oral/gingival barrier it can be theorized that neutrophils have important homeostatic functions at this interface that relate to both anti-microbial defense and wound healing/ inflammatory resolution. Consistent with this notion, patients with neutropenia and/or neutrophil migration defects present with a severe periodontal phenotype, that of destructive gingival/mucosal inflammation that mediates severe bone destruction at an early age43. Interestingly, absence of tissue neutrophils does not predispose patients to an overt oral infection, but rather to an exaggerated inflammatory response mediated by the IL23/IL17 pathway45,47,48, reflecting critical roles of neutrophils in the regulation of inflammation at this barrier in mice and humans. Similar to lack of tissue neutrophils, exaggerated neutrophil infiltration and activation also is associated with oral/periodontal inflammation60,125, suggesting that a balanced neutrophil response is necessary for periodontal tissue homeostasis. Beyond tissue repair and immune regulation, neutrophil surveillance of the commensal microbiome is thought to be a key function at this barrier126. However, this has been more complicated to demonstrate in vivo, as neutrophil deficiency is always associated with hyperinflammation. In humans, the genetic neutrophil defect linked with impaired neutrophil anti-microbial responses, Papillon Lefevre, may be the best example of a setting in which defects in neutrophil anti-microbial function can predispose to disease127. Yet in this setting as well, neutrophil regulatory functions have also been implicated128. Importantly, while neutrophils and neutrophil-associated anti-microbials do have the capacity to kill/inactivate a variety of oral microbiota in vitro129, a large body of literature supports the notion that many periodontal pathobionts have evolved evasion strategies to escape neutrophil-mediated immune surveillance ranging from specific leukotoxins to sophisticated enzymes126,130. Yet neutrophils from patients with periodontitis, and in particular oral PMN have been shown to have a hyperactive phenotype with increased activation of anti-microbial defenses including ROS production and NETosis131–133. Whether these functions are protective or detrimental in the setting of the oral disease periodontitis may depend on the extent of neutrophil recruitment and activation.
Figure 3. Neutrophil function and heterogeneity in oral mucosa.
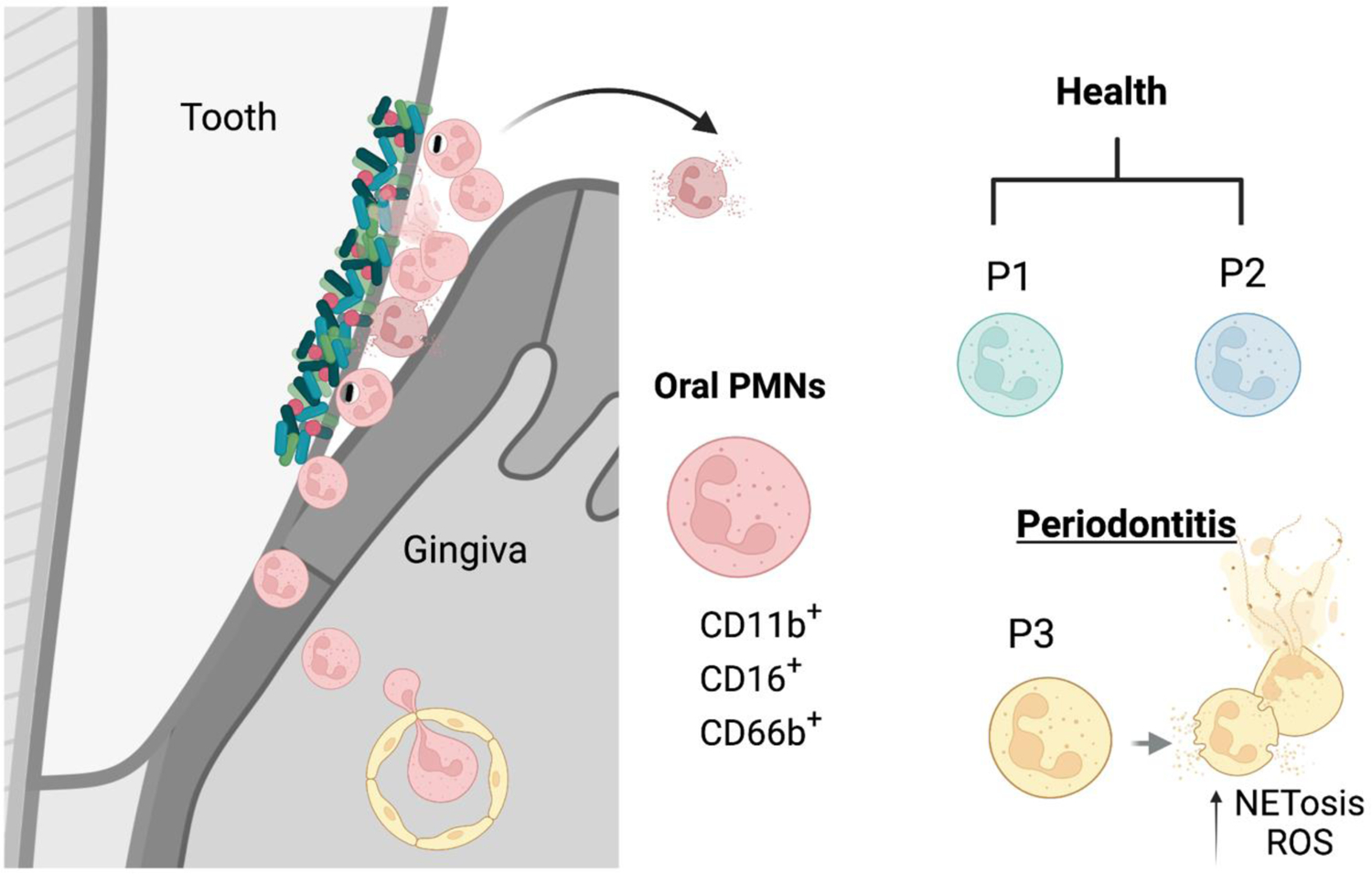
The gingival crevice, the epithelium of the periodontal pocket, is a site of constant transmigration of neutrophils even in health. Neutrophils constantly transmigrate from the vasculature to the gingival tissue and through the bottom of the pocket into the oral cavity. This tissue has a great extent of homeostatic inflammation that is primarily characterized by neutrophil responses. Neutrophils transmigrating into the oral cavity have been subclassified into two populations of health-associated para-inflammatory neutrophils (P1 and P2) and a proinflammatory subtype (P3) in periodontitis. Oral neutrophils express CD11b, CD16, CD66 and varying levels of CD10, CD18, CD55, CD63, CD64, and CD170. P3 neutrophils in particularly are characterized by increased expression of activation markers and increased propensity towards ROS production and NETosis.
The functionality of distinct neutrophil subsets and/ or cell states has been recently explored at the oral barrier134. While human oral tissue neutrophils have been only characterized by expression of CD15/CD16 surface molecules120, more comprehensive characterization has been performed in neutrophils exiting the oral mucosa and found within the oral cavity134. Neutrophils in the oral cavity, termed oral PMNs (oPMN) have been characterized by constitutive expression of markers CD11b, CD16 and CD66b (consistent with a classical neutrophil phenotype)135. They have been further subclassified by the same group of investigators into three oral neutrophil subsets or cell states: two types of parainflammatory neutrophils found in the healthy oral cavity, and proinflammatory neutrophils found in the oral cavity during chronic periodontal disease136 (Figure 3). Expression of various neutrophil activation markers was evident at varying degrees within oral neutrophils. Indeed CD10, CD11b, CD18, CD55, CD63, CD64, CD66, CD16 and CD170, were all detected on oral neutrophils. Pro-inflammatory neutrophils presented with significantly higher expression of CD10, CD11b, CD18, CD55, CD63, CD64, and CD66 compared to both para-inflammatory subsets. CD16 and CD170 was elevated on proinflammatory neutrophils only compared to the P2 parainflammatory subset. Proinflammatory oral neutrophil populations have increased potency for NETosis and increased ROS production as compared with parainflammatory neutrophils. Finally, the two healthy oral neutrophil populations could be distinguished based on four markers; CD55 and CD63 was elevated in P2, while expression of CD16 and CD170 was reduced on the P2 population versus the P1 population136. How these subpopulations may differentially contribute to oral mucosal immunity in health and disease is not yet well understood.
Lower gastrointestinal mucosal barrier
Unlike the upper gastrointestinal tract (the oral cavity) and the respiratory tract, neutrophils are not a prevalent cell population of the lower gastrointestinal tract in health. Detailed phenotyping of human and murine gastrointestinal tissues does not reveal abundance of neutrophils along the length of the lower gastrointestinal tract, during development or with ageing, in the absence of overt inflammation137–139. In fact, it is thought that in the lower intestinal tract, at steady state, signals involved in neutrophil recruitment are largely suppressed by the commensal microbiota140 (Figure 4). Indeed, microbial metabolites derived by commensals have been well documented to engage immunoregulatory pathways in the intestine, suppressing neutrophil accumulation during homeostasis140. For example, intestinal commensals metabolize dietary tryptophan into indole derivatives which can serve as ligand for aryl-hydrocarbon receptor (AHR). AHR ligands promote the differentiation of regulatory T cells (Tregs) which can inhibit inflammation and neutrophil recruitment140,141. Gut microbiota, among other functions, regulate bile acids, which will also contribute to local immunomodulation ultimately indirectly suppressing neutrophil recruitment and inflammatory responses against the commensal microbiota140,142. Furthermore, select microbial commensals have been shown to inhibit the ability of pathogens to trigger neutrophil recruitment and/or activation143.
Figure 4. Cross regulation of the microbiome with neutrophils at the gastrointestinal mucosa.
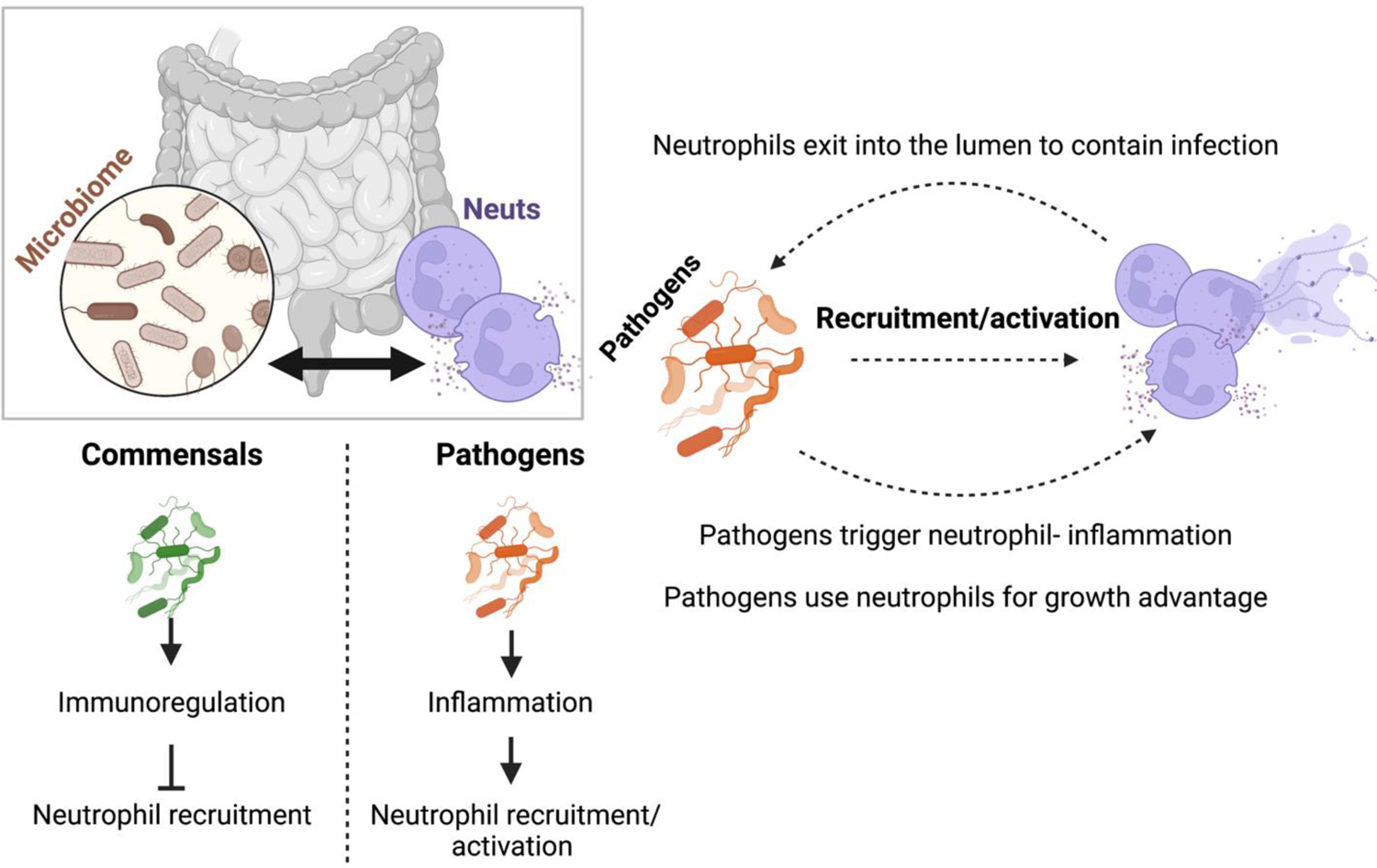
At the GI tract, microbiota play a key role in the regulation of neutrophil responses. Commensal microbiota generally engages immunoregulatory responses which inhibit excessive neutrophil infiltration. Pathogenic microbiota trigger neutrophil recruitment and activation at the GI tract. Neutrophils recruited by pathogens can exit into the lumen to mediate protection to the host. However, many pathogens have found ways to use neutrophil responses for their growth advantage and thus perpetuate neutrophil infiltration and activation for their benefit.
Yet, severe neutropenia does predispose to infection, indicating that neutrophils do have a role in the homeostatic containment of microbes, particularly bacteria in the GI tract. Lower GI infections and inflammation are not as prevalent in patients with congenital neutropenia (compared to respiratory infections and oral inflammatory disease), yet gastrointestinal disease does occur, and is often associated with enteric bacterial infection (Table 2). In experimental models, severe defects in neutrophil recruitment (such as seen in Cxcr2−/− mice) also predispose to significant shifts in gastrointestinal microbial communities and to infection144,145. Neutrophils are thought to contribute to bacterial infection containment through their classic anti-microbial defenses, including phagocytosis, degranulation and NETosis146. In this regard, mice deficient in the cathelin-related antimicrobial peptide (CRAMP), the mouse ortholog of cathelicidin (LL-37) display gut microbiome dysbiosis and inflammation, suggesting a major role for neutrophil anti-microbial peptides in regulating commensal colonization147
However, both gut commensals and pathogens appear to have evolved multiple mechanisms to evade neutrophil killing, including degradation of NETs and inactivation of neutrophil anti-microbial peptides148. Furthermore, neutrophil protective mechanisms are thought of as a double-edged sword, contributing more often to immunopathology rather than protection at the lower gastrointestinal tract. Interestingly, while health-associated commensals are thought to negatively regulate neutrophil recruitment and activation, pathogenic bacteria have been shown to trigger neutrophil activation and at times immunopathology. In some instances, the neutrophil response becomes protective and contains microbial translocation. As such Salmonella and Pseudomonas, can activate neutrophil recruitment and transmigration into the gut lumen where they create organized structures that inhibit microbial growth and translocation into the tissue149. In other instances, pathogens, use neutrophil recruitment to their advantage and drive immunopathology. Several “proinflammatory” microbiota species have been shown to trigger gastrointestinal inflammation in susceptible mice, associated with neutrophil recruitment, including the adherent-invasive E coli, Clostridium difficile, and Bacteroides. These species not only promote inflammation but also suppress the grown of health- associated commensals which promote immunoregulation140,150,151. In another scenario, Salmonella, a diarrheal pathogen, can use neutrophils for its growth advantage. Salmonella, promotes neutrophil recruitment into the intestinal lumen, where they produce reactive oxygen species (ROS). ROS generated by neutrophils will react with sulphur compounds (thiosulphate) in the lumen and form a new respiratory electron acceptor, tetrathionate. Salmonella has the ability to use tetrathionate as an electron acceptor and thus use respiration for growth and compete with other microbes in the gut152. Pathogenic E.coli can also use neutrophil-mediated inflammation for its benefit. During inflammation, inducible nitric oxide synthetase promotes production of nitrate with is used by E.coli for its growth advantage153. Additionally, microbes such as E. coli and C. albicans have been shown to trigger neutrophil activation and NETosis potentially promoting both surveillance and immunopathology154. Thus, in the setting of gastrointestinal infection, neutrophil function may involve both beneficial and detrimental effects for the host.
In inflammatory bowel disease (IBD), immunopathology, is clearly associated with neutrophil infiltration. In fact, levels of neutrophil infiltration correlate with clinical and pathologic severity in IBD. Furthermore, levels of systemic and fecal lactotransferrin and calprotectin (typically associated with neutrophil degranulation and NETosis), correlate with severity of gastrointestinal pathology154. Neutrophil elastase and myeloperoxidase in tissues of colitis patients is also a marker of disease severity. Moreover, neutrophil NETosis has been well documented in patients with ulcerative colitis and suggested to potentially contribute to immunopathology, by promoting both inflammation and coagulation cascades155.
Yet neutrophils have also been linked to immunoregulatory roles even in the setting of colitis. Beyond their anti-microbial activity, neutrophils can have clear anti-inflammatory activities. In fact, a distinct neutrophil subset has been documented in both mice with colitis and patients with ulcerative colitis or Crohn’s disease156, with inferred anti-inflammatory functions. This neutrophil subset expressed the CD marker, CD177, produced decreased levels of inflammatory cytokines but increased levels of IL-22 and transforming growth factor-β (TGF- β). In addition, CD177+ neutrophils showed increased bactericidal potential as they produced higher amounts for ROS, antimicrobial peptides and neutrophil extracellular traps compared with CD177− neutrophils. Importantly these cells were shown to be protective in colitis as CD177−/− mice developed more severe colitis in a DSS colitis model, compared with wild-type counterparts. In the setting of CD177 deficiency, neutrophils secreted reduced levels of IL22 which compromised intestinal barrier and impaired antibacterial immunity. Consistent with these findings another study had previously indicated that IL22 secreting neutrophils are critical for barrier function and antimicrobial immunity as they trigger production of the antimicrobial peptides, RegIIIβ and S100A8 by colonic epithelial cells157.
As in other settings in the context of gastrointestinal inflammation and disease, it becomes evident that neutrophil functionality is context dependent and can serve either as beneficial or detrimental depending on the scenario.
Concluding remarks
At mucosal barriers, neutrophils play essential roles related to anti-microbial defense, wound healing and resolution of inflammation. However, exaggerated and or deregulated neutrophil responses are clearly associated with immunopathology in mucosal tissues both in the setting of infection and inflammatory disease. Furthermore, specification of neutrophils within mucosal tissue microenvironments may further underlie functionality of neutrophil subsets in health and disease within each milieu. Further understanding of neutrophil specification and functionality within different tissues may open avenues towards therapeutic regulation of neutrophil responses, particularly for the setting of mucosal disease.
Acknowledgments
This work was funded by the intramural program of NIDCR/NIH (N.M.M.) and NIDCR 1K99DE030124-01A1 (L.M.S.). The authors thank Teresa Wild for her critical review of the manuscript and Dr. SM Holland for discussions on clinical susceptibilities in patients with neutrophil immunodeficiencies. Figures have been created using Biorender.
Footnotes
All authors declare that they have no competing interests.
References
- 1.Kobayashi SD, Voyich JM, Burlak C, DeLeo FR. Neutrophils in the innate immune response. Arch Immunol Ther Exp (Warsz). 2005;53(6):505–517. [PubMed] [Google Scholar]
- 2.Dancey JT, Deubelbeiss KA, Harker LA, Finch CA. Neutrophil kinetics in man. J Clin Invest. 1976;58(3):705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorgens A, Radtke S, Horn PA, Giebel B. New relationships of human hematopoietic lineages facilitate detection of multipotent hematopoietic stem and progenitor cells. Cell Cycle. 2013;12(22):3478–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Vietinghoff S, Ley K. Homeostatic regulation of blood neutrophil counts. J Immunol. 2008;181(8):5183–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yvan-Charvet L, Ng LG. Granulopoiesis and Neutrophil Homeostasis: A Metabolic, Daily Balancing Act. Trends Immunol. 2019;40(7):598–612. [DOI] [PubMed] [Google Scholar]
- 6.Bjerregaard MD, Jurlander J, Klausen P, Borregaard N, Cowland JB. The in vivo profile of transcription factors during neutrophil differentiation in human bone marrow. Blood. 2003;101(11):4322–4332. [DOI] [PubMed] [Google Scholar]
- 7.Hager M, Cowland JB, Borregaard N. Neutrophil granules in health and disease. J Intern Med. 2010;268(1):25–34. [DOI] [PubMed] [Google Scholar]
- 8.Blanter M, Gouwy M, Struyf S. Studying Neutrophil Function in vitro: Cell Models and Environmental Factors. J Inflamm Res. 2021;14:141–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Summers C, Rankin SM, Condliffe AM, et al. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31(8):318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohler A, De Filippo K, Hasenberg M, et al. G-CSF-mediated thrombopoietin release triggers neutrophil motility and mobilization from bone marrow via induction of Cxcr2 ligands. Blood. 2011;117(16):4349–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3(7):687–694. [DOI] [PubMed] [Google Scholar]
- 12.Semerad CL, Christopher MJ, Liu F, et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106(9):3020–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HK, De La Luz Sierra M, Williams CK, Gulino AV, Tosato G. G-CSF down-regulation of CXCR4 expression identified as a mechanism for mobilization of myeloid cells. Blood. 2006;108(3):812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honda T, Uehara T, Matsumoto G, Arai S, Sugano M. Neutrophil left shift and white blood cell count as markers of bacterial infection. Clin Chim Acta. 2016;457:46–53. [DOI] [PubMed] [Google Scholar]
- 15.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–689. [DOI] [PubMed] [Google Scholar]
- 16.Sadik CD, Kim ND, Luster AD. Neutrophils cascading their way to inflammation. Trends Immunol. 2011;32(10):452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nat Med. 2011;17(11):1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosales C, Uribe-Querol E. Phagocytosis: A Fundamental Process in Immunity. Biomed Res Int. 2017;2017:9042851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bardoel BW, Kenny EF, Sollberger G, Zychlinsky A. The balancing act of neutrophils. Cell Host Microbe. 2014;15(5):526–536. [DOI] [PubMed] [Google Scholar]
- 20.Nemeth T, Sperandio M, Mocsai A. Neutrophils as emerging therapeutic targets. Nat Rev Drug Discov. 2020;19(4):253–275. [DOI] [PubMed] [Google Scholar]
- 21.Nordenfelt P, Tapper H. Phagosome dynamics during phagocytosis by neutrophils. J Leukoc Biol. 2011;90(2):271–284. [DOI] [PubMed] [Google Scholar]
- 22.Lacy P. Mechanisms of degranulation in neutrophils. Allergy Asthma Clin Immunol. 2006;2(3):98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Branzk N, Papayannopoulos V. Molecular mechanisms regulating NETosis in infection and disease. Semin Immunopathol. 2013;35(4):513–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thalin C, Hisada Y, Lundstrom S, Mackman N, Wallen H. Neutrophil Extracellular Traps: Villains and Targets in Arterial, Venous, and Cancer-Associated Thrombosis. Arterioscler Thromb Vasc Biol. 2019;39(9):1724–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dutzan N, Abusleme L, Bridgeman H, et al. On-going Mechanical Damage from Mastication Drives Homeostatic Th17 Cell Responses at the Oral Barrier. Immunity. 2017;46(1):133–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iglesias-Bartolome R, Uchiyama A, Molinolo AA, et al. Transcriptional signature primes human oral mucosa for rapid wound healing. Sci Transl Med. 2018;10(451). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peiseler M, Kubes P. More friend than foe: the emerging role of neutrophils in tissue repair. J Clin Invest. 2019;129(7):2629–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonald B, Pittman K, Menezes GB, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330(6002):362–366. [DOI] [PubMed] [Google Scholar]
- 30.Massena S, Christoffersson G, Vagesjo E, et al. Identification and characterization of VEGF-A-responsive neutrophils expressing CD49d, VEGFR1, and CXCR4 in mice and humans. Blood. 2015;126(17):2016–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christoffersson G, Vagesjo E, Vandooren J, et al. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood. 2012;120(23):4653–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. 2018;128(7):2657–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones HR, Robb CT, Perretti M, Rossi AG. The role of neutrophils in inflammation resolution. Semin Immunol. 2016;28(2):137–145. [DOI] [PubMed] [Google Scholar]
- 34.Sugimoto MA, Vago JP, Teixeira MM, Sousa LP. Annexin A1 and the Resolution of Inflammation: Modulation of Neutrophil Recruitment, Apoptosis, and Clearance. J Immunol Res. 2016;2016:8239258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leoni G, Neumann PA, Kamaly N, et al. Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Invest. 2015;125(3):1215–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nugteren S, Samsom JN. Secretory Leukocyte Protease Inhibitor (SLPI) in mucosal tissues: Protects against inflammation, but promotes cancer. Cytokine Growth Factor Rev. 2021;59:22–35. [DOI] [PubMed] [Google Scholar]
- 37.Hahn J, Schauer C, Czegley C, et al. Aggregated neutrophil extracellular traps resolve inflammation by proteolysis of cytokines and chemokines and protection from antiproteases. FASEB J. 2019;33(1):1401–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doran AC, Yurdagul A Jr., Tabas I. Efferocytosis in health and disease. Nat Rev Immunol. 2020;20(4):254–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10(6):427–439. [DOI] [PubMed] [Google Scholar]
- 40.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120(15):e60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tangye SG, Al-Herz W, Bousfiha A, et al. Human Inborn Errors of Immunity: 2019 Update on the Classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol. 2020;40(1):24–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donadieu J, Fenneteau O, Beaupain B, Mahlaoui N, Chantelot CB. Congenital neutropenia: diagnosis, molecular bases and patient management. Orphanet J Rare Dis. 2011;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silva LM, Brenchley L, Moutsopoulos NM. Primary immunodeficiencies reveal the essential role of tissue neutrophils in periodontitis. Immunol Rev. 2019;287(1):226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenzweig SD, Holland SM. Phagocyte immunodeficiencies and their infections. J Allergy Clin Immunol. 2004;113(4):620–626. [DOI] [PubMed] [Google Scholar]
- 45.Moutsopoulos NM, Konkel J, Sarmadi M, et al. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss. Sci Transl Med. 2014;6(229):229ra240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fodil N, Langlais D, Gros P. Primary Immunodeficiencies and Inflammatory Disease: A Growing Genetic Intersection. Trends Immunol. 2016;37(2):126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moutsopoulos NM, Zerbe CS, Wild T, et al. Interleukin-12 and Interleukin-23 Blockade in Leukocyte Adhesion Deficiency Type 1. N Engl J Med. 2017;376(12):1141–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stark MA, Huo Y, Burcin TL, et al. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22(3):285–294. [DOI] [PubMed] [Google Scholar]
- 49.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14(10):986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silvestre-Roig C, Fridlender ZG, Glogauer M, Scapini P. Neutrophil Diversity in Health and Disease. Trends Immunol. 2019;40(7):565–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deniset JF, Kubes P. Neutrophil heterogeneity: Bona fide subsets or polarization states? J Leukoc Biol. 2018;103(5):829–838. [DOI] [PubMed] [Google Scholar]
- 52.Ballesteros I, Rubio-Ponce A, Genua M, et al. Co-option of Neutrophil Fates by Tissue Environments. Cell. 2020;183(5):1282–1297 e1218. [DOI] [PubMed] [Google Scholar]
- 53.Chavakis T, Wielockx B, Hajishengallis G. Inflammatory Modulation of Hematopoiesis: Linking Trained Immunity and Clonal Hematopoiesis with Chronic Disorders. Annu Rev Physiol. 2022;84:183–207. [DOI] [PubMed] [Google Scholar]
- 54.Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol. 2014;14(5):302–314. [DOI] [PubMed] [Google Scholar]
- 55.Grieshaber-Bouyer R, Radtke FA, Cunin P, et al. The neutrotime transcriptional signature defines a single continuum of neutrophils across biological compartments. Nat Commun. 2021;12(1):2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adrover JM, Del Fresno C, Crainiciuc G, et al. A Neutrophil Timer Coordinates Immune Defense and Vascular Protection. Immunity. 2019;51(5):966–967. [DOI] [PubMed] [Google Scholar]
- 57.Uhl B, Vadlau Y, Zuchtriegel G, et al. Aged neutrophils contribute to the first line of defense in the acute inflammatory response. Blood. 2016;128(19):2327–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang D, Chen G, Manwani D, et al. Neutrophil ageing is regulated by the microbiome. Nature. 2015;525(7570):528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silva LM, Doyle AD, Greenwell-Wild T, et al. Fibrin is a critical regulator of neutrophil effector function at the oral mucosal barrier. Science. 2021;374(6575):eabl5450. [DOI] [PubMed] [Google Scholar]
- 60.Williams DW, Greenwell-Wild T, Brenchley L, et al. Human oral mucosa cell atlas reveals a stromal-neutrophil axis regulating tissue immunity. Cell. 2021;184(15):4090–4104 e4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moutsopoulos NM, Konkel JE. Tissue-Specific Immunity at the Oral Mucosal Barrier. Trends Immunol. 2018;39(4):276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fortunati E, Kazemier KM, Grutters JC, Koenderman L, Van den Bosch v J. Human neutrophils switch to an activated phenotype after homing to the lung irrespective of inflammatory disease. Clin Exp Immunol. 2009;155(3):559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garg S, Jain S, Dawood FS, et al. Pneumonia among adults hospitalized with laboratory-confirmed seasonal influenza virus infection-United States, 2005–2008. BMC Infect Dis. 2015;15:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kirsebom FCM, Kausar F, Nuriev R, Makris S, Johansson C. Neutrophil recruitment and activation are differentially dependent on MyD88/TRIF and MAVS signaling during RSV infection. Mucosal Immunol. 2019;12(5):1244–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Totura AL, Whitmore A, Agnihothram S, et al. Toll-Like Receptor 3 Signaling via TRIF Contributes to a Protective Innate Immune Response to Severe Acute Respiratory Syndrome Coronavirus Infection. mBio. 2015;6(3):e00638–00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wareing MD, Shea AL, Inglis CA, Dias PB, Sarawar SR. CXCR2 is required for neutrophil recruitment to the lung during influenza virus infection, but is not essential for viral clearance. Viral Immunol. 2007;20(3):369–378. [DOI] [PubMed] [Google Scholar]
- 67.McCarthy MK, Zhu L, Procario MC, Weinberg JB. IL-17 contributes to neutrophil recruitment but not to control of viral replication during acute mouse adenovirus type 1 respiratory infection. Virology. 2014;456–457: 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Camp JV, Jonsson CB. A Role for Neutrophils in Viral Respiratory Disease. Front Immunol. 2017;8:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McNamara PS, Ritson P, Selby A, Hart CA, Smyth RL. Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis. Arch Dis Child. 2003;88(10):922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goritzka M, Makris S, Kausar F, et al. Alveolar macrophage-derived type I interferons orchestrate innate immunity to RSV through recruitment of antiviral monocytes. J Exp Med. 2015;212(5):699–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lucas C, Wong P, Klein J, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584(7821):463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cavallaro EC, Liang KK, Lawrence MD, Forsyth KD, Dixon DL. Neutrophil infiltration and activation in bronchiolitic airways are independent of viral etiology. Pediatr Pulmonol. 2017;52(2):238–246. [DOI] [PubMed] [Google Scholar]
- 73.Tang BM, Shojaei M, Teoh S, et al. Neutrophils-related host factors associated with severe disease and fatality in patients with influenza infection. Nat Commun. 2019;10(1):3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiong Y, Liu Y, Cao L, et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect. 2020;9(1):761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meizlish ML, Pine AB, Bishai JD, et al. A neutrophil activation signature predicts critical illness and mortality in COVID-19. Blood Adv. 2021;5(5):1164–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turner RB. The role of neutrophils in the pathogenesis of rhinovirus infections. Pediatr Infect Dis J. 1990;9(11):832–835. [DOI] [PubMed] [Google Scholar]
- 78.Priyadharshini VS, Ramirez-Jimenez F, Molina-Macip M, et al. Human Neutrophil Defensin-1, −3, and −4 Are Elevated in Nasal Aspirates from Children with Naturally Occurring Adenovirus Infection. Can Respir J. 2018;2018:1038593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pechous RD. With Friends Like These: The Complex Role of Neutrophils in the Progression of Severe Pneumonia. Front Cell Infect Microbiol. 2017;7:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rolston KV. The spectrum of pulmonary infections in cancer patients. Curr Opin Oncol. 2001;13(4):218–223. [DOI] [PubMed] [Google Scholar]
- 81.Grudzinska FS, Brodlie M, Scholefield BR, et al. Neutrophils in community-acquired pneumonia: parallels in dysfunction at the extremes of age. Thorax. 2020;75(2):164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bou Ghanem EN, Clark S, Roggensack SE, et al. Extracellular Adenosine Protects against Streptococcus pneumoniae Lung Infection by Regulating Pulmonary Neutrophil Recruitment. PLoS Pathog. 2015;11(8):e1005126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garvy BA, Harmsen AG. The importance of neutrophils in resistance to pneumococcal pneumonia in adult and neonatal mice. Inflammation. 1996;20(5):499–512. [DOI] [PubMed] [Google Scholar]
- 84.Tateda K, Moore TA, Deng JC, et al. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J Immunol. 2001;166(5):3355–3361. [DOI] [PubMed] [Google Scholar]
- 85.Kulkarni U, Zemans RL, Smith CA, et al. Excessive neutrophil levels in the lung underlie the age-associated increase in influenza mortality. Mucosal Immunol. 2019;12(2):545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bhowmick R, Maung N, Hurley BP, et al. Systemic disease during Streptococcus pneumoniae acute lung infection requires 12-lipoxygenase-dependent inflammation. J Immunol. 2013;191(10):5115–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grieshaber-Bouyer R, Nigrovic PA. Neutrophil Heterogeneity as Therapeutic Opportunity in Immune-Mediated Disease. Front Immunol. 2019;10:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ng LG, Ostuni R, Hidalgo A. Heterogeneity of neutrophils. Nat Rev Immunol. 2019;19(4):255–265. [DOI] [PubMed] [Google Scholar]
- 89.Silvin A, Chapuis N, Dunsmore G, et al. Elevated Calprotectin and Abnormal Myeloid Cell Subsets Discriminate Severe from Mild COVID-19. Cell. 2020;182(6):1401–1418 e1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schulte-Schrepping J, Reusch N, Paclik D, et al. Severe COVID-19 Is Marked by a Dysregulated Myeloid Cell Compartment. Cell. 2020;182(6):1419–1440 e1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carissimo G, Xu W, Kwok I, et al. Whole blood immunophenotyping uncovers immature neutrophil-to-VD2 T-cell ratio as an early marker for severe COVID-19. Nat Commun. 2020;11(1):5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martinelli S, Urosevic M, Daryadel A, et al. Induction of genes mediating interferon-dependent extracellular trap formation during neutrophil differentiation. J Biol Chem. 2004;279(42):44123–44132. [DOI] [PubMed] [Google Scholar]
- 93.Noyola E, Noor A, Sweeney N, et al. Prevalence of Bandemia in Respiratory Viral Infections: A Pediatric Emergency Room Experience. Front Pediatr. 2020;8:576676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cortjens B, Ingelse SA, Calis JC, et al. Neutrophil subset responses in infants with severe viral respiratory infection. Clin Immunol. 2017;176:100–106. [DOI] [PubMed] [Google Scholar]
- 95.Pillay J, Kamp VM, van Hoffen E, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest. 2012;122(1):327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Drifte G, Dunn-Siegrist I, Tissieres P, Pugin J. Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Crit Care Med. 2013;41(3):820–832. [DOI] [PubMed] [Google Scholar]
- 97.Wang L, Ai Z, Khoyratty T, et al. ROS-producing immature neutrophils in giant cell arteritis are linked to vascular pathologies. JCI Insight. 2020;5(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Belchamber KBR, Thein OS, Hazeldine J, et al. Dysregulated Neutrophil Phenotype and Function in Hospitalised Non-ICU COVID-19 Pneumonia. Cells. 2022;11(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang W, Xu Y, Wang L, et al. Single-cell profiling identifies mechanisms of inflammatory heterogeneity in chronic rhinosinusitis. Nat Immunol. 2022. [DOI] [PubMed] [Google Scholar]
- 100.Matsui M, Nagakubo D, Satooka H, Hirata T. A novel Siglec-F(+) neutrophil subset in the mouse nasal mucosa exhibits an activated phenotype and is increased in an allergic rhinitis model. Biochem Biophys Res Commun. 2020;526(3):599–606. [DOI] [PubMed] [Google Scholar]
- 101.Shin JW, Kim J, Ham S, et al. A unique population of neutrophils generated by air pollutant-induced lung damage exacerbates airway inflammation. J Allergy Clin Immunol. 2022;149(4):1253–1269 e1258. [DOI] [PubMed] [Google Scholar]
- 102.Rudd JM, Pulavendran S, Ashar HK, et al. Neutrophils Induce a Novel Chemokine Receptors Repertoire During Influenza Pneumonia. Front Cell Infect Microbiol. 2019;9:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hartl D, Krauss-Etschmann S, Koller B, et al. Infiltrated neutrophils acquire novel chemokine receptor expression and chemokine responsiveness in chronic inflammatory lung diseases. J Immunol. 2008;181(11):8053–8067. [DOI] [PubMed] [Google Scholar]
- 104.Gorski SA, Lawrence MG, Hinkelman A, et al. Expression of IL-5 receptor alpha by murine and human lung neutrophils. PLoS One. 2019;14(8):e0221113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mesnil C, Raulier S, Paulissen G, et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest. 2016;126(9):3279–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cheung DS, Ehlenbach SJ, Kitchens RT, et al. Cutting edge: CD49d+ neutrophils induce FcepsilonRI expression on lung dendritic cells in a mouse model of postviral asthma. J Immunol. 2010;185(9):4983–4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cheung DS, Sigua JA, Simpson PM, et al. Cysteinyl leukotriene receptor 1 expression identifies a subset of neutrophils during the antiviral response that contributes to postviral atopic airway disease. J Allergy Clin Immunol. 2018;142(4):1206–1217 e1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Penaloza HF, Salazar-Echegarai FJ, Bueno SM. Interleukin 10 modulation of neutrophil subsets infiltrating lungs during Streptococcus pneumoniae infection. Biochem Biophys Rep. 2018;13:12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gonzalez LA, Melo-Gonzalez F, Sebastian VP, et al. Characterization of the Anti-Inflammatory Capacity of IL-10-Producing Neutrophils in Response to Streptococcus pneumoniae Infection. Front Immunol. 2021;12:638917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tsuda Y, Takahashi H, Kobayashi M, et al. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity. 2004;21(2):215–226. [DOI] [PubMed] [Google Scholar]
- 111.Chen P, Bao C, Zhu R, et al. IL-5 enhances the resistance of Actinobacillus pleuropneumoniae infection in mice through maintaining appropriate levels of lung M2, PMN-II and highly effective neutrophil extracellular traps. Vet Microbiol. 2022;269:109438. [DOI] [PubMed] [Google Scholar]
- 112.Bachmaier K, Stuart A, Singh A, et al. Albumin Nanoparticle Endocytosing Subset of Neutrophils for Precision Therapeutic Targeting of Inflammatory Tissue Injury. ACS Nano. 2022;16(3):4084–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ley K, Hoffman HM, Kubes P, et al. Neutrophils: New insights and open questions. Sci Immunol. 2018;3(30). [DOI] [PubMed] [Google Scholar]
- 114.Morrissey SM, Geller AE, Hu X, et al. A specific low-density neutrophil population correlates with hypercoagulation and disease severity in hospitalized COVID-19 patients. JCI Insight. 2021;6(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cabrera LE, Pekkarinen PT, Alander M, et al. Characterization of low-density granulocytes in COVID-19. PLoS Pathog. 2021;17(7):e1009721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tay SH, Celhar T, Fairhurst AM. Low-Density Neutrophils in Systemic Lupus Erythematosus. Arthritis Rheumatol. 2020;72(10):1587–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hardisty GR, Llanwarne F, Minns D, et al. High Purity Isolation of Low Density Neutrophils Casts Doubt on Their Exceptionality in Health and Disease. Front Immunol. 2021;12:625922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li Y, Li H, Wang H, et al. The proportion, origin and pro-inflammation roles of low density neutrophils in SFTS disease. BMC Infect Dis. 2019;19(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.al-Essa L, Niwa M, Kohno K, Tsurumi K. A proposal for purification of salivary polymorphonuclear leukocytes by combination of nylon mesh filtration and density-gradient method: a validation by superoxide- and cyclic AMP-generating responses. Life Sci. 1994;55(17):PL333–338. [DOI] [PubMed] [Google Scholar]
- 120.Dutzan N, Konkel JE, Greenwell-Wild T, Moutsopoulos NM. Characterization of the human immune cell network at the gingival barrier. Mucosal Immunol. 2016;9(5):1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scully C, Wilkinson PC. Inflammatory polymorphonuclear neutrophil leukocytes; orientation, chemotactic, locomotor and phagocytic capabilities of neutrophils from the human gingival crevice. J Clin Lab Immunol. 1985;17(2):69–73. [PubMed] [Google Scholar]
- 122.Hajishengallis E, Hajishengallis G. Neutrophil homeostasis and periodontal health in children and adults. J Dent Res. 2014;93(3):231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mark Welch JL, Ramirez-Puebla ST, Borisy GG. Oral Microbiome Geography: Micron-Scale Habitat and Niche. Cell Host Microbe. 2020;28(2):160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zenobia C, Luo XL, Hashim A, et al. Commensal bacteria-dependent select expression of CXCL2 contributes to periodontal tissue homeostasis. Cell Microbiol. 2013;15(8):1419–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hajishengallis G, Moutsopoulos NM, Hajishengallis E, Chavakis T. Immune and regulatory functions of neutrophils in inflammatory bone loss. Semin Immunol. 2016;28(2):146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Uriarte SM, Edmisson JS, Jimenez-Flores E. Human neutrophils and oral microbiota: a constant tug-of-war between a harmonious and a discordant coexistence. Immunol Rev. 2016;273(1):282–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sorensen OE, Clemmensen SN, Dahl SL, et al. Papillon-Lefevre syndrome patient reveals species-dependent requirements for neutrophil defenses. J Clin Invest. 2014;124(10):4539–4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Roberts H, White P, Dias I, et al. Characterization of neutrophil function in Papillon-Lefevre syndrome. J Leukoc Biol. 2016;100(2):433–444. [DOI] [PubMed] [Google Scholar]
- 129.Gorr SU, Abdolhosseini M. Antimicrobial peptides and periodontal disease. J Clin Periodontol. 2011;38 Suppl 11:126–141. [DOI] [PubMed] [Google Scholar]
- 130.Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16(12):745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moonen CGJ, Hirschfeld J, Cheng L, et al. Oral Neutrophils Characterized: Chemotactic, Phagocytic, and Neutrophil Extracellular Trap (NET) Formation Properties. Front Immunol. 2019;10:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Saito I, Komiyama K, Moro I, et al. Ultrastructural and immunocytochemical characterization of polymorphonuclear leukocytes from gingival crevice in man. J Periodontol. 1987;58(7):493–497. [DOI] [PubMed] [Google Scholar]
- 133.Ling MR, Chapple IL, Matthews JB. Peripheral blood neutrophil cytokine hyper-reactivity in chronic periodontitis. Innate Immun. 2015;21(7):714–725. [DOI] [PubMed] [Google Scholar]
- 134.Hirschfeld J. Neutrophil Subsets in Periodontal Health and Disease: A Mini Review. Front Immunol. 2019;10:3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lakschevitz FS, Hassanpour S, Rubin A, et al. Identification of neutrophil surface marker changes in health and inflammation using high-throughput screening flow cytometry. Exp Cell Res. 2016;342(2):200–209. [DOI] [PubMed] [Google Scholar]
- 136.Fine N, Hassanpour S, Borenstein A, et al. Distinct Oral Neutrophil Subsets Define Health and Periodontal Disease States. J Dent Res. 2016;95(8):931–938. [DOI] [PubMed] [Google Scholar]
- 137.Tabula Muris C. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature. 2020;583(7817):590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Elmentaite R, Kumasaka N, Roberts K, et al. Cells of the human intestinal tract mapped across space and time. Nature. 2021;597(7875):250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fawkner-Corbett D, Antanaviciute A, Parikh K, et al. Spatiotemporal analysis of human intestinal development at single-cell resolution. Cell. 2021;184(3):810–826 e823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang D, Frenette PS. Cross talk between neutrophils and the microbiota. Blood. 2019;133(20):2168–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Loughman JA, Hunstad DA. Induction of indoleamine 2,3-dioxygenase by uropathogenic bacteria attenuates innate responses to epithelial infection. J Infect Dis. 2012;205(12):1830–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Guo C, Xie S, Chi Z, et al. Bile Acids Control Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome. Immunity. 2016;45(4):802–816. [DOI] [PubMed] [Google Scholar]
- 143.Vong L, Lorentz RJ, Assa A, Glogauer M, Sherman PM. Probiotic Lactobacillus rhamnosus inhibits the formation of neutrophil extracellular traps. J Immunol. 2014;192(4):1870–1877. [DOI] [PubMed] [Google Scholar]
- 144.Mei J, Liu Y, Dai N, et al. Cxcr2 and Cxcl5 regulate the IL-17/G-CSF axis and neutrophil homeostasis in mice. J Clin Invest. 2012;122(3):974–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Watanabe K, Gilchrist CA, Uddin MJ, et al. Microbiome-mediated neutrophil recruitment via CXCR2 and protection from amebic colitis. PLoS Pathog. 2017;13(8):e1006513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. [DOI] [PubMed] [Google Scholar]
- 147.Yoshimura T, McLean MH, Dzutsev AK, et al. The Antimicrobial Peptide CRAMP Is Essential for Colon Homeostasis by Maintaining Microbiota Balance. J Immunol. 2018;200(6):2174–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cullen TW, Schofield WB, Barry NA, et al. Gut microbiota. Antimicrobial peptide resistance mediates resilience of prominent gut commensals during inflammation. Science. 2015;347(6218):170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Molloy MJ, Grainger JR, Bouladoux N, et al. Intraluminal containment of commensal outgrowth in the gut during infection-induced dysbiosis. Cell Host Microbe. 2013;14(3):318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Palmela C, Chevarin C, Xu Z, et al. Adherent-invasive Escherichia coli in inflammatory bowel disease. Gut. 2018;67(3):574–587. [DOI] [PubMed] [Google Scholar]
- 151.Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104(34):13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Winter SE, Thiennimitr P, Winter MG, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467(7314):426–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Winter SE, Winter MG, Xavier MN, et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science. 2013;339(6120):708–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Honda M, Kubes P. Neutrophils and neutrophil extracellular traps in the liver and gastrointestinal system. Nat Rev Gastroenterol Hepatol. 2018;15(4):206–221. [DOI] [PubMed] [Google Scholar]
- 155.He Z, Si Y, Jiang T, et al. Phosphotidylserine exposure and neutrophil extracellular traps enhance procoagulant activity in patients with inflammatory bowel disease. Thromb Haemost. 2016;115(4):738–751. [DOI] [PubMed] [Google Scholar]
- 156.Zindl CL, Lai JF, Lee YK, et al. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc Natl Acad Sci U S A. 2013;110(31):12768–12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhou G, Yu L, Fang L, et al. CD177(+) neutrophils as functionally activated neutrophils negatively regulate IBD. Gut. 2018;67(6):1052–1063. [DOI] [PubMed] [Google Scholar]


