Abstract

A new era in tumor classification, diagnosis, and prognostic evaluation has begun as a consequence of recent developments in the molecular and genetic characterization of central nervous system tumors. In this newly emerging era, molecular imaging modalities are essential for preoperative diagnosis, surgical planning, targeted treatment, and post-therapy evaluation of gliomas. The radiotracers are able to identify brain tumors, distinguish between low- and high-grade lesions, confirm a patient’s eligibility for theranostics, and assess post-radiation alterations. We previously synthesized and reported the novel l-type amino acid transporter 1 (LAT-1)-targeted amino acid derivative in light of the use of amino acid derivatives in imaging technologies. Further, we have developed a single vial ready to label Tc-lyophilized kit preparations of diethylenetriaminepentaacetic acid-bis-methionine [DTPA-bis(Met)], also referred to as methionine-diethylenetriaminepentaacetic acid-methionine (MDM) and evaluated its imaging potential in numerous clinical studies. This review summarizes our previous publications on 99mTc-DTPA-bis(Met) in different clinical studies such as detection of breast cancer, as a prognostic marker, in detection of recurrent/residual gliomas, for differentiation of recurrent/residual gliomas from radiation necrosis, and for comparison of 99mTc-DTPA-bis(Met) with 11C-L-methionine (11C-MET), with relevant literature on imaging modalities in glioma management.
Keywords: Glioma, Radiotracer, Prognostic marker, Recurrent glioma, Radiation necrosis, Methionine
Proliferation is necessary for tumor growth, and the tumor microenvironment contains a variety of factors that might affect cellular metabolism and produce heterogeneous metabolic activity.1 In order to meet the requirements of growth and proliferation, malignant cells rewire metabolic pathways. These altered metabolic patterns are now recognized as cancer-defining characteristics. According to studies, in order to maintain their growth, tumor cells change particular pathways like glycolysis, fatty acid synthesis, and amino acid synthesis.2 The onset of illness is frequently preceded by changes in tissue function and morphology. In malignant transformation, amino acid transport is frequently enhanced, and this increase in amino acid transport could be linked to alterations in the cell surface of transformed cells. A significant emphasis is currently placed on the synthesis of and research on radiolabeled amino-acid-derived biomolecules with a selective distribution and binding to target structures in living cells and tissues, such as transporters, enzymes, and peptide receptors.3 The ease of synthesis, biodistribution, and production of radiolabeled metabolites in vivo differ across these radiolabeled amino acids.4 Most of the amino acids have been radiolabeled to examine the potential imaging features, usually for positron emission tomography (PET), since the replacement of a carbon atom by 11C does not chemically modify the molecule.4
The incidence of both malignant and non-malignant brain and other central nervous system (CNS) tumors varies greatly by age, sex, and race/ethnicity, and such tumors have a significant negative impact on morbidity and mortality in the United States. Between 2008 and 2017, the overall incidence rate of malignant brain tumors decreased by 0.8% per year, although among children and adolescents it grew by 0.5% to 0.7% per year.5 Gliomas are recognized as extremely heterogeneous tumors with varied cellular interactions and compositions.2 Gliomas are infiltrative tumors that impact the surrounding brain tissue in a diffuse manner. Gliomas affect people of all ages; however, they are more common in adults, with males being more susceptible than females.6,7 Circumscribed gliomas and diffuse gliomas are the two types of gliomas, with the former being benign and curable with complete surgical resection and the latter being more malignant and unable to be cured by surgical resection alone.7 The World Health Organization’s (WHO) classification of tumors of the CNS underwent significant revisions in 2016 as a result of developments in our knowledge of the molecular pathogenesis of gliomas.8 Previously, only microscopic characteristics were used as classification criteria. Entities with the inclusion of genetic information in specific tumors are reclassified according to the new criteria.8,9 Further, the addition of CNS tumor classification by the WHO was published in 2021. This new WHO classification includes even more molecular changes in the diagnosis of numerous tumors. It further reorganizes the gliomas into pediatric-type diffuse low-grade and high-grade gliomas, adult-type diffuse gliomas, ependymal tumors, and circumscribed astrocytic gliomas.10 Because of the increased intracranial pressure and destruction of the surrounding brain tissue, clinical manifestations of nearly all brain tumors are connected to the “mass effect”. Consequently, these pathological conditions have a wide range of clinical manifestations, including personality changes, headaches, focal neurological symptoms, and seizures.11 These consequences may vary depending on the tumor location, anticancer medication, patient’s age, hereditary variables, and the treatment technique used, such as surgery, radiation, and chemotherapy. A glioma can have a negative impact on neuronal functional connections as well as concentrations of brain metabolites. The latter may lead to the destruction of higher-order neurocognitive functions like global cognitive functions, executive functioning, memory, attention, speed of information processing, and graphomotor speed.12 In the case of gliomas, surgery is still the most effective treatment option. The major goal of surgery in advanced local disease is to minimize tumor mass and brain decompression. Glioblastoma has a mostly infiltrative pattern of growth, and surgery is frequently inadequate; therefore, radiotherapy with or without concurrent chemotherapy has emerged as a viable treatment option for such patients.11,13 Regardless of recent improvements in glioblastoma multimodality therapy, which combines surgery, radiotherapy, systemic therapy, and supportive care, the overall prognosis is still poor and long-term survival is scarce,6,14 which highights a clear need for better therapeutic strategies based on imaging and molecular profiling of the tumors.15,16 Some studies have demonstrated that markers based on histopathology as well as radiologic data, such as those obtained from magnetic resonance imaging (MRI) scans, can predict overall survival in glioma patients.17−22
Imaging Techniques in Gliomas
A number of mutations and changes in important molecular pathways that have been connected to the pathogenesis and/or prognosis of glioblastoma are the focus of target-based diagnostics and therapies.23,24 Utilizing molecular traits has the benefit of allowing for the selective detection and subsequent treatment of glioblastoma cells without causing harm to the surrounding healthy brain tissue.25 Accurate non-invasive diagnosis of glioma grade/type, survival, and therapeutic response remains challenging in the age of contemporary imaging. Despite being invasive and expensive, stereotactic brain biopsy continues to be the gold standard for histological and genetic categorization; nonetheless, in 7–15% of individuals, the pathological diagnosis may still be inconclusive.26 The majority of treatment for high-grade gliomas is now maximal safe resection, followed by radiotherapy and concurrent and adjuvant chemotherapy. Histological confirmation by biopsy or surgical resection is the gold standard approach for distinguishing between radiation necrosis and tumor progression, which is often difficult, especially in deep-seated brain tumors. Therefore, non-invasive imaging approaches are required for reliably distinguishing tumor necrosis from recurrence.13,27 Radiation necrosis and recurrent/residual brain gliomas have comparable clinical presentations and MR image appearances, such as perilesional edema, rim-like enhancement, and central low intensity on T2-weighted imaging.28 Hence, non-invasive separation of tumor recurrence from necrosis poses a diagnostic difficulty for imaging modalities. Because brain tumors are newly developed morphological abnormalities at the time of clinical presentation, a radiological, computed tomography (CT) and MRI, workup is required to establish the differential diagnosis. Contrast-enhanced CT (ceCT) can detect a damaged blood–brain barrier (BBB), although it lacks the sensitivity of contrast-enhanced MRI (ceMRI). Differential diagnosis using traditional MRI techniques cannot reliably distinguish between mild and high-grade gliomas.29,30 Despite the fact that a few studies have shown that standard CT and MRI are competent and reliable for the detection and characterization of primary tumors, they still fail to distinguish recurring tumors from radiation necrosis.27,31,32 Glioblastomas are known for disrupting the BBB. Although ceCT and ceMRI can usually detect brain tumors and BBB disruption, the existence of residual/recurrent tumors can provide a diagnostic challenge for anatomical imaging.11,31 Perfusion and diffusion magnetic resonance spectroscopy (MRS) as well as dynamic susceptibility contrast-enhanced (DSCE) imaging have all been demonstrated to be therapeutically beneficial in detecting recurrent/residual malignancy. Santra et al.33 compared the sensitivity, specificity, and accuracy of glucoheptonate (GHA) single photon emission computed tomography (SPECT) and ceMRI for the detection of recurrent gliomas, finding that GHA SPECT had 86.5, 96.5, and 89.4% sensitivity, specificity, and accuracy, respectively, whereas MRI had 94.6, 24.1, and 70.5%.33
The levels of various metabolites in biological tissues can be measured by using MRS. It is a renowned technique in clinical practice for the diagnosis and monitoring of various brain lesions.34,35 The MR signal generates a spectrum of resonances that correspond to the “stimulated” molecular configurations of the isotope. This signature is utilized to diagnose metabolic diseases, particularly those that impact the brain, as well as to provide information on tumor metabolism.36,37 Unlike traditional MRI, proton (1H) MRS can identify biochemical patterns associated with normal brain, tumor histology, and changes in tissue function, providing indicators for disease categorization, diagnosis, and progression in the brain.38−40 MRS can detect a variety of metabolites in vivo, including choline (Cho) and N-acetylaspartate (NAA), both of which have been linked to primary brain tumors and can provide new insights into the metabolism of gliomas.40,41 The NAA, Cho, creatine (Cr), myo-inositol (MI), glutamate, and glutamine (Glu-n) molecules are metabolites that can be detected using standard brain 1H MRS.40−42 NAA, one of the most common compounds in neurons, frequently produces a strong signal in MRS measurements in the brain. In a variety of neuropathological diseases, NAA levels are lowered. Creatine is the second highest peak and acts as a marker for energy-dependent systems in brain cells. A Cho peak indicates higher cellular membrane turnover, which is seen in all processes that lead to hypercellularity. Tumors have a higher Glu-n peak than normal tissues.41,43−46 Differentiating low-grade gliomas from high-grade gliomas is of high prognostic importance for patient care. Peaks of Cho and lipids are strong prognostic indicators of disease progression/regression. The diagnostic accuracy of MRS has been found to be between 85 and 95%.47 Similarly, an increase in Cho has been shown to have good diagnostic utility, as it has been reported to correlate with NAA, and higher Cho levels are linked to lower NAA levels.48−51 Cho, lactate, and lipid levels are generally higher in brain tumors than in non-neoplastic tissue. On the other hand, tumor cells have lower amounts of creatine or tumor NAA than their non-tumor counterparts. A high Cho peak is characteristic of rapidly developing tumors rather than unique for gliomas. An increase in MI peak indicates a zone of active glial activation due to reaction toward infiltrating tumor cells.52 Parra et al. employed volumetric three-dimensional magnetic resonance spectroscopic imaging (MRSI) to map these metabolites throughout a large brain region.53 They evaluated the metabolite-based treatment targets and showed that whole-brain MRSI could be effective for planning radiation therapy for glioblastoma. They also discovered that typical radiation treatment volumes do not adequately characterize areas of metabolically active tumors. In a cohort of 74 patients with newly diagnosed gliomas, Server et al. examined the diagnostic accuracy of conventional MRI, diffusion-weighted imaging (DWI), and MRSI and found that MRSI outperforms MRI and DWI in terms of sensitivity, specificity, positive predictive values (PPV), negative predictive values (NPV), and glioma grading.54 The use of advanced MRI techniques results in improved diagnostic accuracy when compared to utilizing solely conventional MRI for follow-up of treatment response, resulting in greater sensitivity and specificity, according to a meta-analysis encompassing 1174 patients treated for glioblastoma.55 In gliomas, distinguishing between radiation necrosis and tumor recurrence is an important part of post-radiation follow-up. Radiation necrosis can occur 3–12 months after radiation therapy and is associated with excessive gliosis.56 Rabinov et al. analyzed MRS peaks quantitatively to distinguish between glioma recurrence and radiation-induced effects such as delayed necrosis.57 They discovered that a significant drop in Cho, Cr, and NAA metabolite peaks suggested delayed necrosis, but a moderate to high increase in Cho and clearly visible Cr metabolite peaks indicated tumor progression/recurrence. Unlike other body organs, the BBB restricts the substances that are allowed to cross to the brain by diffusion or facilitated transport.31 As a result, many radiotracers that would easily localize in other body cancers would have difficulty reaching the brain and would pass only if the BBB was disrupted.
Radiotracers and Tomographic Modalities in Nuclear Medicine
Radiolabeled amino acids targeted at tumor-specific receptors, transporters, and enzymes in cancer cells are promising and extensively studied molecular tools for the diagnosis and treatment of cancer.3 A variety of radiolabeled amino acids have been produced for tumor imaging. The transport and metabolic characteristics of specific radiolabeled amino acids must be matched with the relevant tumor biology and clinical concern in order for this class of tracers to be applied for oncologic imaging in the best possible way. When imaging brain tumors, 11C-l-methionine (11C-MET) and 18F-fluoro-ethyl-l-tyrosine (18F-FET) offer more accurate estimates of the gross tumor volumes and margins, a clearer view of non-enhancing gliomas, and a more precise assessment of the therapeutic response compared to MRI alone.58 Tumor cells have a high expression of amino acid transporter genes, which allow them to take up a huge number of amino acids. At least 17 different classes of transporters have been identified. Three systems are thought to transport neutral amino acids: system L, system A, and alanine-serine-cysteine (ASC).59 The amino acid transport system L transports 11C-L-MET into cells.59,60 The increased protein synthesis and trans-methylation metabolic processes of tumors allow 11C-L-MET to become incorporated into tumor molecules, resulting in 11C-L-MET accumulation in malignancies.60
Development of amino acid-based tracers has been primarily focused on system L amino acid transport for PET imaging. At the molecular level, numerous transporters, including l-type amino acid transporter 1 (LAT-1), LAT-2, LAT-3, and LAT-4, have been identified, and LAT-1 is constantly found to be highly expressed in cancer cells among these identified transporters.61−63 LAT-1 functions as a transporter for long branched-chain and aromatic neutral amino acids, working independently of sodium (Na+) and pH levels with a 1:1 stoichiometry.64,65 The LAT-1 transporter consists of two subunits that are connected through a covalent linkage formed by a disulfide bond. The functional subunit responsible for amino acid exchange in the transporter is the light-chain subunit (LAT-1, also known as SLC7A5). However, the heavy-chain subunit (CD98 or 4F2hc, also known as SLC3A2) is a glycoprotein that is coupled with the light-chain subunit and serves as a molecular chaperone to help localize LAT-1 at the plasma membrane.61,64,66,67 A schematic diagram of the LAT-1 transporter is shown in Figure 1.
Figure 1.
(A) Structure of LAT1-4F2hc, adapted from PDB 7DSN (https://www.rcsb.org/structure/7DSN). (B) Representative docking pose of 99mTc-DTPA-bis(Met) with LAT-1 (chain B) (Autodock Vina 1.5.2) and docking site visualization in PyMOL.
In the recent past, a study revealed that 4F2hc played a critical role in the transport function of the complex.68 The binding affinity of DTPA-bis(Met) to the LAT-1 transporter was studied on tumor cell lines such as U-87MG, BMG, and human embryonic kidney (HEK) by binding assay using 99mTc-DTPA-bis(Met) as the labeled ligand. We observed a significant binding of DTPA-bis(Met) to LAT-1 transporters on tumor cell lines. The Kd values were found to be 0.067, 0.077, and 0.10 nM in U-87MG, BMG, and HEK cell lines, respectively. We found a Km value of 12.95 ± 3.8 nM and a maximal transport rate velocity (Vmax) of 80.35 ± 0.42 pmol μg protein–1 min–1 of 99mTc-DTPA-bis(Met) in U-87MG cells by enzyme kinetic study. This result was further strengthened by trans-stimulation of 35S-l-methionine efflux in U-87MG cells.63
There is an increased expression of LAT-1 in various human tumors, including malignant glioma, multiple myeloma, and cholangiocarcinoma, as well as cancers affecting the lung, bone, thyroid, pancreas, prostate, bladder, uterine cervix, breast, and other malignancies compared to benign tissue used as a control.64,69 The association between increased LAT-1 expression and significantly reduced survival rates in several cancer types suggests that LAT-1 could be utilized as a prognostic biomarker to predict the outcomes in diverse types of cancer.70,71 The grade of the glioma and the poor prognosis of glioma patients are related to LAT-1 overexpression.72 Haining et al.72 studied the expression of LAT-1 and 4F2hc in 62 patients of human brain glioma using immunohistochemistry and reported increased expression of LAT-1 and 4F2hc in all examined specimens. In numerous cancers, there is also an increased amino acid transport, which has been successfully targeted using radiolabeled amino acid substrate for PET and SPECT imaging.73 Most of the efforts in developing amino acid-based tracers have been primarily focused on system L amino acid transport. Radiolabeled l-substrates, including 11C-MET, 6-[18F]fluoro-l-3,4-dihydroxy-phenylalanine (18F-FDOPA), 3-[18F]fluoro-α-methyl-l-tyrosine (FAMT), O-(2-[18F]fluoroethyl)-l-tyrosine (FET), and 3-[123I]iodo-α-methyl-l-tyrosine (IMT), have been widely used for oncologic imaging in patients and have shown particular efficacy in imaging brain tumors. This is due to the upregulation of system L transport in glioma cells and is active at the intact BBB.74−78 [18F]Fluoroboronotyrosine (FBY) is a boron-derived tyrosine with therapeutic and diagnostic potential that is dependent on LAT-1 for its transportation.79 Most malignant brain tumors exhibit increased FBY activity, and recurrent gliomas, primary GBM and metastatic brain tumors, all showed high tumor-to-background ratios, which may aid in the stratification of malignancy and future boron neutron capture therapy.80 FBY shows promise in assessing brain tumors, and its metabolism has been found to be significantly increased in most neoplasms, whereas it remained low in non-cancerous lesions.79,80 In comparison to other amino acid PET tracers, like FET and 4-borono-2-18F-fluorophenylalanine (FBPA), FBY exhibited reduced background activity, with mean and maximum SUV values of 0.037 and 0.116, respectively, allowing a clearer visualization of tumors, and may help in identifying small lesions and defining treatment targets.80
Preclinical and clinical studies show that the energy-independent LAT-1 system preferentially takes up 99mTc-methionine, referring to 99mTc-DTPA-bis(Met) in tumor cells. In a preliminary study, it localizes selectively in recurrent/residual gliomas and has excellent target-to-non-target (T/NT) image contrast.81,82 In a meta-analysis, Zhao et al. found that fluorine-18-fluorodeoxyglucose (18F-FDG) PET has limited diagnostic performance in brain tumor differentiation; however, this varies depending on the stage of the tumor.83 In contrast, 11C-MET PET has a good diagnostic accuracy in brain tumor differentiation. Tripathi et al. revealed in a recent comparative study that 11C-MET should be the radiotracer of choice in the evaluation of recurrence of primary brain tumors, since 11C-MET has a better sensitivity for detection and delineation of the possible recurrent tumor, as well as secondary deposits.84 The advantage of amino acid PET tracers over FDG is that they do not build up excessively in the healthy brain.85 PET imaging with 11C-MET could not become a routine investigation due to the very short half-life of 11C (20 min), and it is limited to those centers with an in-house cyclotron and the requisite synthesis equipment. Another promising PET-based tracer that can be synthesized with a high yield is 18F-FET; the long half-life of 18F (110 min) avoids the drawbacks of the short half-life of 11C. 18F-FET is a promising new amino acid tracer that can be manufactured in large quantities for therapeutic use and can be used in PET scans.86 In contrast to 18F-FDG and 11C-MET, 18F-FET has been demonstrated in animal studies to have no absorption in inflammatory cells or lymph nodes, which promises a higher specificity for the detection of tumor cells.87−89
The two primary tomographic modalities used in nuclear medicine are SPECT and PET, which both give functional data on various tissues and disorders. The assessment of brain tumors uses various nuclear medicine radiotracers. Although a majority of cutting-edge molecular imaging radiotracers are compatible with PET imaging, SPECT remains a more affordable and widely accessible alternative modality. PET and SPECT imaging techniques used in nuclear medicine have been used to identify recurring tumors from treatment-induced necrosis. When compared to PET, SPECT has a lower cost and is more widely available, but its lesser resolution of around 0.8 mm compared to that of PET (0.4 mm) may be a limitation.90 Various SPECT tracers have been used to detect recurrent tumors, with thallium-201 being one of the first to be extensively investigated.91 Due to the 140 keV γ-ray energy and high photon flux, 99mTc-labeled compounds have proven to be superior to those labeled with 201Tl, resulting in improved spatial resolution and a lower radiation burden on the patient.9299mTc-DTPA-bis(Met) has the same biological characteristics as 11C-labeled l-methionine; therefore, 99mTc-DTPA-bis(Met) will allow metabolic imaging with a gamma camera and also expose the patient to a lesser radiation burden, apart from being inexpensive. Based on radiology assessment of neuro-oncology (RANO) criteria, hybrid 11C-MET PET/MRI demonstrated accuracy significantly higher than that of MRI (96% vs 82%) and higher than that of 11C-MET PET (96% vs 88%) to distinguish treatment-related alterations from true progression in recurrent glioblastoma.93
Considering the application of amino acid derivatives in imaging technologies, we have earlier synthesized and reported DTPA-bis(Met), an amino acid derivative based on DTPA. This compound was synthesized by covalently conjugating two molecules of methionine to DTPA, and it was labeled in high radiochemical purity and specific activity (166–296 MBq/mol) with 99mTc.6399mTc-DTPA-bis(Met) showed excellent tumor targeting and has promising utility as a SPECT radiopharmaceutical for imaging methionine-dependent human tumors. Breast, head, and neck cancers of varying histology were successfully imaged with 99mTc-DTPA-bis(Met). The timeline from the conceptualization of this SPECT radiopharmaceutical to the clinical trial is shown in Figure 2.
Figure 2.
Timeline diagram depicting the events of 99mTc-DTPA-bis(Met) from conceptualization to clinical trials.
To reach from preclinical to clinical research, a comprehensive evaluation was carried out, which led to the development of this molecule as a SPECT tracer for human application. The preclinical and clinical studies of this compound are summarized in Figure 3.
Figure 3.
Highlights of preclinical and clinical studies of 99mTc-DTPA-bis(Met).
99mTc-DTPA-bis(Met) showed substantial promise for tumor scintigraphy, as significant accumulation was observed in athymic mice bearing a subcutaneous BMG cell line (malignant glioma).63 The U-87MG, BMG, and EAT tumor-bearing mice showed considerable tumor uptake and good contrast in the biodistribution study.63 A representative whole-body γ image of a female athymic mouse with a subcutaneous BMG tumor is shown in Figure 4A. DTPA-bis(Met) was also labeled with M(III) metal ions (68Ga, Gd, and Eu). Further, multimodal scans in athymic mice bearing a xenograft of the tumor U-87MG are shown in Figure 4B–D.82
Figure 4.
(A) Whole-body γ image of a female athymic mouse with a subcutaneous BMG tumor. (B) Reconstructed PET-CT imaging of a U-87MG xenograft in the right thigh. (C) 3D T1-weighted MR images followed by intravenous administration of Gd complex; the tumor shows a positive signal at 7 T. (D) U-87MG xenograft optical imaging of the left thigh. Panel (A) reproduced with permission from ref (63). Copyright 2010 American Chemical Society. Panels (B)–(D) reproduced with permission from ref (82). Copyright 2014 Bentham Science Publishers Ltd.
The initial screening of this compound was carried out in osteosarcoma, breast, thyroid, head, and neck cancers. The first study in human breast cancer was carried out in 47 female patients having palpable breast masses and published in 2009.94 Semiquantitative analysis from regions of interest (ROI) showed the maximum radiotracer uptake at the tumor site compared to that of symmetric counterparts, with a maximum ROI of 5.31 for osteosarcoma and ROIs for breast cancer, brain cancer, and regional lymph node cancer of 3.7, 4.72, and 3.47, respectively.94 Further, we have developed a single vial ready to label with 99mTc lyophilized kit preparations of DTPA-bis(Met) and evaluated the imaging potential of this kit in numerous studies.63,95−98 The timeline of 99mTc-DTPA-bis(Met) is shown in Figure 2. This Review aims to summarize our research work on 99mTc-DTPA-bis(Met) in preclinical and clinical studies, which provides a way to research more potential amino acid-based radiotracers for clinical applications. Mainly, this review highlights the diagnostic potential of 99mTc-DTPA-bis(Met) in numerous clinical studies including breast cancer detection, as a prognostic marker, detection of recurrent/residual glioma, detection and differentiation of recurrent/residual glioma from radiation necrosis, and comparison of 99mTc-DTPA-bis(Met) with 11C-MET.
Breast Cancer Detection by 99mTc-DTPA-Bis(Met)
We have evaluated the diagnostic utility of 99mTc-DTPA-bis(Met) for the detection of breast cancer.94,95 In 2009, Sharma et al. performed scintimammography in 47 female patients (median age 44 years) with palpable breast masses using 99mTc-DTPA-bis(Met) following an intravenous radiotracer injection of 555 MBq.9499mTc-DTPA-bis(Met) planar chest scintigraphy was carried out in the anterior and lateral positions. The radiotracer was injected into an upper limb vein on the side opposite the known breast anomaly, and imaging was done at 1 and 3 h. On the basis of histological findings, 33 cases were malignant, and 14 cases were benign. Out of 33 individuals with breast cancer, 29 patients had true-positive results from scintimammography, and 13 out of 14 patients with benign breast lesions had true-negative results. In this study, we found the sensitivity, specificity, and PPV to be 87.8, 92.8, and 96.6%, respectively. In another study by Sharma et al., 30 female patients with primary breast carcinoma were exposed to 99mTc-DTPA-bis(Met) scintigraphy.95 Whole-body imaging was performed on all the patients at different time points, 5 and 10 min and 1, 2, and 4 h after an intravenous administration of 555–740 MBq radioactivity of 99mTc-DTPA-bis(Met). Moreover, scintimammography at 1, 2, and 4 h was also carried out on these patients. The radiotracer is eliminated predominantly through the kidneys and concentrates in breast cancer lesions with high T/NT ratios. For the identification of primary breast cancer, 99mTc-DTPA-bis(Met) scintimammography has excellent sensitivity and PPV of 96.0% each. A semiquantitative analysis of the scintimammography data was performed to determine the lesion-to-background ratio (LBR) of the radiotracer as a function of imaging time intervals. A semiquantitative analysis of the positive breast lesions (n = 29) showed a mean LBR of 3.6 ± 0.48, 3.10 ± 0.24, and 2.5 ± 0.4 based on scintimammography images taken at 1, 2, and 4 h, respectively. However, the LBR for the negative lesions (n = 3) was significantly lower, measuring 1.7 ± 0.07, 1.6 ± 0.40, and 1.4 ± 0.25 at 1, 2, and 4 h, respectively. We observed that our single-vial kit formulations of 99mTc-DTPA-bis(Met) can also be used for the scintigraphic detection of breast cancer and other methionine-dependent tumors expressing LAT.
99mTc-DTPA-Bis(Met) as a Prognostic Marker
Prognostic markers are clinical tools used in oncology to assess a patient’s risk of a specific outcome, such as the recurrence of the illness following primary treatment. They are essential in clinical practice as they classify patients into various risk groups, which inform treatment plans and facilitate patient counseling.99 It can be challenging to predict the prognosis of nervous system tumors, and it is nevertheless generally poor, especially for patients with metastatic lesions.100 PET employing radiolabeled amino acids has significant potential in the identification of glioma recurrence/residual/necrosis because malignant cells exhibit a higher rate of amino acid uptake and metabolism. Particularly, O-(2-18F-fluoroethyl)-l-tyrosine (18F-FET) PET and 11C-L-MET PET provide significant data on glioma metabolism and have shown promise as glioma management tools. As a result, amino acid-based PET imaging, when used in conjunction with traditional imaging modalities, can offer complementary diagnostic and therapeutic information and can serve as a guide for the sampling or excision of certain target tissues.101,102 The commonly used MET-PET is constrained by the 20-min short half-life of 11C and needs inside cyclotron equipment. Therefore, there is a clinical need to create a more useful imaging technique that can serve as a more affordable alternative to glioma PET imaging. Considering this, we have developed a single-vial lyophilized kit, DTPA-bis(Met), that is ready to label with 99mTc, and the clinical application of this kit has been evaluated in SPECT imaging.63,103 The tumor uptake of 99mTc-DTPA-bis(Met) is comparable to that of 11C-MET (transport through LAT-1). LAT-1 is more frequently expressed in cancer from low-grade to high-grade neuroendocrine tumors and hence directly affects their malignant potential and prognostication.82,95,104Figure 5 shows a recurrent tumor in a 37-year-old female patient with astrocytoma grade II. An increased 99mTc-DTPA-bis(Met) tracer uptake can be clearly seen in Figure 5B. Figure 6 shows the tumor recurrence in a 37-year-old female patient with oligodendroglioma grade III at 6 months after surgery/radiotherapy. An increased concentration of the 99mTc-DTPA-bis(Met) tracer is observed in the left frontotemporal/parietal areas (Figure 6A).
Figure 5.
Representative image of a female patient (37 years old) with a grade II astrocytoma and recurrent tumor in the left frontal lobe of the corpus callosum. (A) T1-weighted contrast-enhanced magnetic resonance image. (B) Improved 99mTc-DTPA-bis(Met) tracer uptake and T/NT ratio = 5.77. (C) Enhanced perfusion (nCBV = 5.1) in the same area. (D) Cho metabolite map from MR, exhibiting stronger Cho signals in the central area. (E) Multivoxel spectra from the region with the strongest signal for Cho elevation and (F) representing recurrent tumor. Reproduced with permission from ref (98). Copyright 2021 Mary Ann Liebert, Inc.
Figure 6.
An oligodendroglioma (grade III) in a 37-year-old female patient 6 months following surgery and radiotherapy. Several areas of elevated tracer concentration in the left frontotemporal and parietal areas can be seen on a transaxial image taken with 99mTc-DTPA-bis(Met) SPECT (A). The MR picture (transaxial, T2-weighted, contrast-enhanced) reveals heterogeneous hyperintense enhancement in the corresponding area (B). Co-registered transaxial slices of 99mTc-DTPA-bis(Met) SPECT and MR-T2 (C). Perfusion and bloodflow maps (D, E) are seen in the tumor on DSCE-MRI, which also exhibits a distinctive T2 signal intensity perfusion curve (F). Both quantitative 99mTc-DTPA-bis(Met) SPECT (T/NT ratio = 5.1) and MR perfusion (nCBV = 9.1) data supported tumor recurrence. Reproduced with permission from ref (97). Copyright 2018 Wolters Kluwer Health, Inc.
Clinical applications for targeted biopsies or radiation therapy planning require the identification of metabolically active primary, residual, or recurrent tumors and the precise delineation of tumor boundaries. 99mTc-DTPA-bis(Met) can be used as a prognostic marker for the management of glioma for high-dose radiotherapy boost and post-treatment assessment. Moreover, it is a cost-effective substitute for amino acid-based PET imaging. The utility of 99mTc-DTPA-bis(Met) uptake as a non-invasive and early indicator of disease progression in glioma patients had evaluated and its diagnostic performance compared with those of DSCE-MRI and MRS for identifying post-treatment response assessment and survival.97 In this study, 114 glioma patients were followed post-operatively by sequential 99mTc-DTPA-bis(Met) SPECT, DSCE-MRI, and MRS at baseline 6, 12, and 22.5 months after chemo/radiotherapy. A significantly lower T/NT ratio was observed in responders compared to non-responders. The sensitivity and specificity of 99mTc-DTPA-bis(Met) SPECT for identifying tumor recurrence from radiation necrosis at a cutoff ratio of 1.90 were estimated at 97.9 and 92%, respectively. 99mTc-DTPA-bis(Met) and DSCE-MRI provided comparable results for predicting response assessment versus chemo/radiotherapy (Figure 7).
Figure 7.
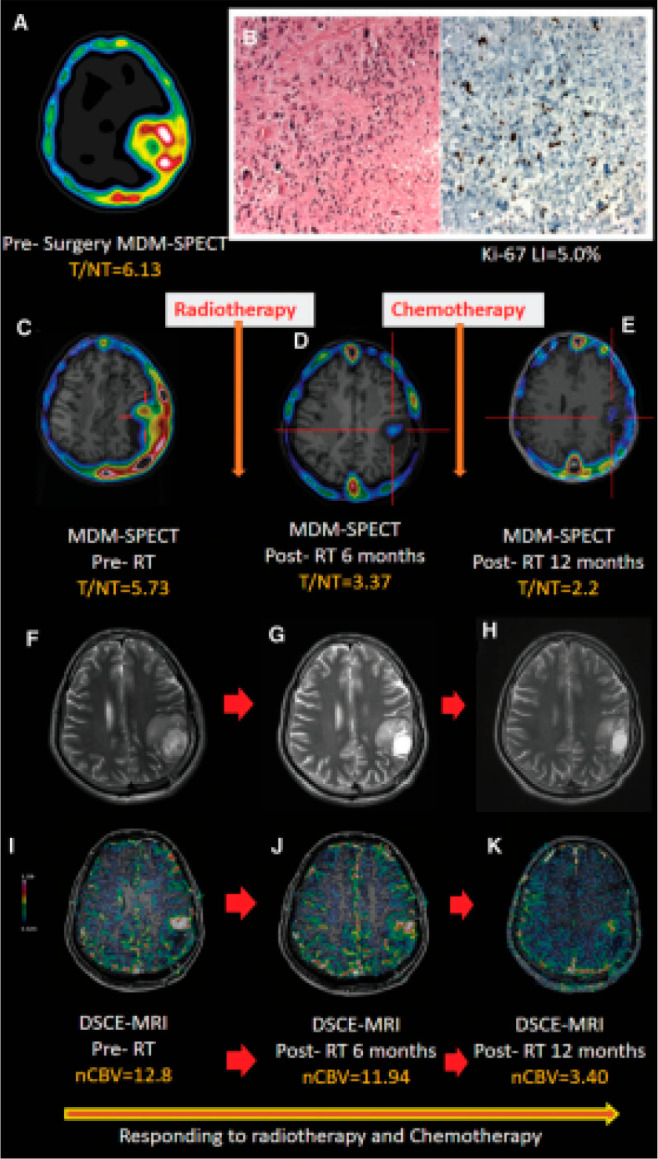
(A) Presurgery imaging of [99mTc]MDM SPECT in a 28-year-old male patient with GBM-IV showing an enlarged tracer uptake (T/NT = 6.13) in the left parietal lobe. (B) Histology (H&E staining, 40×) images show (left panel) several mitoses with regions of palisading necrosis, moderate cellular and nuclear pleomorphism, and lower proliferation index of Ki-67 LI = 5.0% (right panel). In the follow-up scans, serial axial T2-weighted MR images (F, G, and H) did not show any noticeable enhancement on the tumor bed. A significant treatment response (Responder) was shown by follow-up SPECT/MRI, which showed that the baseline pre-radiotherapy (C) tracer uptake (5.73) and (I) nCBV (12.8) decreased (D, J) after 6 months (T/NT = 3.36, nCBV = 11.94) and (E, K) at 12 months (T/NT = 2.3, nCBV = 3.40). Abbreviations: GBM, glioblastoma multiforme; nCBV, normalized cerebral blood volume; SPECT, single-photon emission computed tomography; T/NT, target to non-target; [99mTc]MDM, 99mTc-DTPA-bis(Met). Reproduced with permission from ref (98). Copyright 2021 Mary Ann Liebert, Inc.
According to the Kaplan–Meier study, patients with a T/NT ratio of 1.9 or less had a longer median survival time of 53.8 months than those with a T/NT ratio of 1.9 or more, with a survival time of 37.2 months, as shown in Figure 8.
Figure 8.
Bar graphs showing that the T/NT ratios (A) and nCBV (B) are comparatively higher in non-responders than responders, and that non-responders consistently have higher values for all of these parameters. Kaplan–Meier curves showing overall survival (months) at the end of follow-up in all grades of glioma patients with cutoff T/NT ratios of <1.9 (green curve) versus >1.9 (blue curve) presenting higher survival in the former (C). Kaplan–Meier curves showing proportional survival (months) at the end of follow-up in patients with T/NT ratio >1.9 (low-grade glioma, green; high-grade glioma, purple) and T/NT ratio <1.9 (low-grade glioma, red; HGG, high-grade glioma) (D). Reproduced with permission from ref (98). Copyright 2021 Mary Ann Liebert, Inc.
In another study, we also used 99mTc-DTPA-bis(Met) to characterize glioma preoperatively and compared the results with MR perfusion/spectroscopy and histopathological/Ki-67 scoring. We found that quantitative 99mTc-DTPA-bis(Met) had a high degree of specificity and sensitivity for differentiating between high-grade and low-grade gliomas, and it also suggested a possible use for glial and non-glial tumor differential diagnosis.105
Detection of Recurrent/Residual Glioma Using 99mTc-DTPA-bis(Met)
In our study, we used a 99mTc-DTPA-bis(Met) kit for detection of recurrent/remnant glioma and compared the results with ceMRI and 18F-FLT PET.103 Out of 32 patients, the results were concordant in 28 patients and discordant in 5 patients with 99mTc-DTPA-bis(Met) SPECT and ceMRI. Further, the 99mTc-DTPA-bis(Met) SPECT, 18F-FLT PET, and ceMRI results were positive in 9 of 16 patients and negative in 5 of 16 patients. However, the remaining 2 patients were negative in ceMRI and positive in 99mTc-DTPA-bis(Met) SPECT and 18F-FLT PET. In the case of 99mTc-DTPA-bis(Met) SPECT, the sensitivity, specificity, PPV, NPV, and diagnostic accuracy (DA) were 88.24, 81.25, 83.3, 86.7, and 84.8%, respectively. In the present study, we observed the comparable diagnostic accuracy of 99mTc-DTPA-bis(Met) SPECT with 18F-FLT PET and ceMRI. Therefore, it can be used in the management of glioma patients. Figure 9 shows the uptake of 99mTc-DTPA-bis(Met) in a 52-year-old patient with histopathologically proven primary glioblastoma and comparable results from 18F-FLT PET and ceMRI. In our separate study, we used 99mTc-DTPA-bis(Met) as a tracer for recurrent glioma detection and compared with 18F-FDG PET-CT and ceMRI.96 In this study, 39 histologically proven patients underwent 99mTc-DTPA-bis(Met) SPECT-CT, 18F-FDG PET-CT, and ceMRI. The PPV for 99mTc-DTPA-bis(Met) SPECT-CT, 18F-FDG PET-CT, and ceMRI were 95.6, 56.2, and 92.3%, respectively. The negative predictive values for 99mTc-DTPA-bis(Met) SPECT-CT, 18F-FDG PET-CT, and ceMRI were 61.5, 79.4, and 42.9%, respectively. Moreover, the sensitivity and specificity for these three modalities were 75.9 and 90%, 82.8 and 80%, and 87.1 and 30%, respectively. 99mTc-DTPA-bis(Met) SPECT-CT showed significantly higher specificity compared to ceMRI but not compared to 18F-FDG PET-CT. However, the sensitivity and accuracy of all three modalities were non-significant.
Figure 9.
A mildly enhanced lesion was detected via ceMRI in the right frontal lobe (A), in concurrence with focal uptake of 99mTc-MDM (B) and 18F-FLT (C). A 52-year-old male patient with histopathologically confirmed primary GBM underwent follow-up ceMRI (D) at 3 months with substantial enhancement and increased 99mTc-MDM uptake (E) in the corresponding area. 99mTc-MDM = 99mTc-DTPA-bis(Met). Reproduced with permission from ref (103). Copyright 2015 Wolters Kluwer Health, Inc.
It has always been difficult to distinguish between radiation necrosis and recurrent tumors in gliomas. Early and accurate diagnosis of tumor recurrence and its delineation from therapy-related alterations are essential for effective management of glioma. Compared to a single imaging examination, combined multiparametric imaging may improve the diagnosis accuracy. We have used 99mTc-DTPA-bis(Met) SPECT for the detection and differentiation of recurrent/residual glioma from radiation necrosis, and the results were compared with those from DSCE-MRI.97 In this study, 28 histologically proven glioma patients were recruited prospectively. At 6 months following surgery and radio/chemotherapy, these patients had 99mTc-DTPA-bis(Met) SPECT-CT and DSCE-MRI imaging. Nine of the 28 patients additionally underwent SPECT imaging at 1–2 weeks following surgery. When compared to radiation necrosis, 99mTc-DTPA-bis(Met) SPECT-CT analysis revealed a considerably greater T/NT ratio of the radiotracer in tumor recurrence. The diagnosis of recurrent/residual glioma or radiation necrosis was determined in 18 and 10 patients using the results of 99mTc-DTPA-bis(Met) SPECT-CT, DSCE-MRI, and clinical observations. The sensitivity and specificity of 99mTc-DTPA-bis(Met) SPECT-CT were 92.0 and 78.6%, respectively, comparable to the sensitivity and specificity of DSCE-MRI, 92.0 and 71.4%, respectively. Therefore, combining 99mTc-DTPA-bis(Met) SPECT and DSCE-MRI in glioma patients may provide an accurate method for differentiating radiation-induced necrosis from tumor recurrence.
Comparison of 99mTc-DTPA-Bis(Met) with 11C-Methionine in Glioma Patients
In our recent study, Kumar et al.106 did a head-to-head comparison of 99mTc-DTPA-bis(Met) with 11C-Met in glioma patients. Twenty-one patients were enrolled for the study, out of which 10 were post-operated patients (8 male and 2 female) and the other group had 11 primary glioma patients (3 male and 8 female). All of the patients underwent 99mTc-DTPA-bis(Met) SPECT-CT followed by 11C-Met PET-MR within 2.3 ± 0.5 days. The studies showed that, for Post-Op gliomas, all the indices significantly discriminated tumor recurrence from radiation necrosis (P = 0.002 for T/NT mean, p = 0.044 for T/NT max, p = 0.044 for L/N mean, p = 0.022 for SUV max, P = 0.044 for L/N max) except for SUV mean (p = 0.089). Likewise, with respect to primary gliomas, all the indices significantly discriminated low-grade gliomas from high-grade gliomas (p = 0.024 for T/NT mean, p = 0.012 for T/NT max, p = 0.006 for SUV mean, p = 0.006 for SUV max, p = 0.012 for L/N max) except for L/N mean (p = 0.067). 11C-Met has sensitivity and specificity of 87% and 100%, respectively, in post-op cases to differentiate recurrence from necrosis, as compared to 72% and 60% in primary cases. A significant and strong positive correlation (Spearman’s coefficient (rs) = 0.611, P < 0.004) was observed between volume of distribution-positron emission tomography (Vt) PET and volume of distribution (Vt) MR. Likewise, a significant and strong positive correlation (Spearman’s coefficient (rs) = 0.610, P < 0.004) was found between Vt-SPECT and Vt-MR.106 The dice coefficient was calculated and showed the concordance between the SPECT and PET findings, especially for high-grade tumors. 99mTc-DTPA-bis(Met) can be an economical amino acid SPECT tracer for differentiating recurrence from necrosis. A comparative study between 99mTc-DTPA-bis(Met) and 11C-Met in patients is shown in Table 1.
Table 1. Comparative Study Using 99mTc-DTPA-Bis(Met) (MDM) and 11C-Methionine in Patients (n = 21)a.
|
Final
diagnosis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| S.N. | Sex | Age (years) | Patient type | Primary tumor type/grade | Location of tumor | 99mTc-MDMT/NT max | 11C-methionineL/N max | SPECT | PET |
| 1 | M | 9 | Post OP | GBM IV | Parietal (L) | 1.28 | 2.46 | Necrosis | Necrosis |
| 2 | M | 47 | Post OP | GBM IV | Temporal (L) | 6.95 | 3.78 | Recurrence | Recurrence |
| 3 | M | 60 | Post OP | GBM IV | Temporal (R) | 2.42 | 3.97 | Recurrence | Recurrence |
| 4 | F | 34 | Post OP | AODG III | Frontal (R) | 1.05 | 1.42 | Necrosis | Necrosis |
| 5 | M | 37 | Post OP | Astro IV | Frontal (L) | 2.1 | 6.55 | Recurrence | Recurrence |
| 6 | M | 44 | Post OP | Astro III | Frontal (R) | 2 | 2.19 | Recurrence | Recurrence |
| 7 | M | 37 | Post OP | Astro II | Frontal (L) | 3.36 | 4.86 | Recurrence | Recurrence |
| 8 | M | 51 | Post OP | ODG II | Temporal (R) | 2.23 | 6 | Recurrence | Recurrence |
| 9 | M | 60 | Post OP | AODG IV | Parietal (L) | 2.2 | 8.63 | Recurrence | Recurrence |
| 10 | F | 34 | Post OP | AODG III | Frontal (R) | 2.65 | 4.31 | Recurrence | Recurrence |
| 11 | F | 27 | Pre OP | Diffuse Astro II | Temporal (R) | 1.13 | 2.7 | LGG | LGG |
| 12 | M | 42 | Pre OP | Anaplastic Astro II | Frontal (R) | 1.07 | 1.04 | LGG | LGG |
| 13 | M | 42 | Pre OP | AODG III | Frontal (L) | 1.94 | 3.47 | HGG | HGG |
| 14 | F | 52 | Pre OP | Anaplastic Astro III | Frontal (R) | 2.34 | 3.77 | HGG | HGG |
| 15 | F | 47 | Pre OP | AODG III | Frontal (R) | 2.3 | 3.02 | HGG | HGG |
| 16 | F | 31 | Pre OP | Anaplastic Astro III | Frontal (L) | 3.1 | 5.59 | HGG | HGG |
| 17 | F | 10 | Pre OP | AODG III | Midbrain + Pons | 2.46 | 2.1 | HGG | HGG |
| 18 | F | 30 | Pre OP | Anaplastic Astro III | Frontal (L) | 1.74 | 5.04 | HGG | HGG |
| 19 | M | 57 | Pre OP | Anaplastic Astro III | Temporal (L) | 2.22 | 4.54 | HGG | HGG |
| 20 | F | 27 | Pre OP | ODG II | Frontal (L) | 1.82 | 1.58 | LGG | LGG |
| 21 | M | 50 | Pre OP | ODG III | Temporal (R) | 8.6 | 4.09 | HGG | HGG |
Abbreviations: Post OP, post-operated; Pre OP, pre-operated; Astro, astrocytoma; AWD, alive with disease; GBM, glioblastoma; L, left; ODG, oligodendroglioma; AODG, anaplastic oligodendroglioma; PFS, progression-free survival; R, right; M, male; F, female; LGG, Low-grade glioma; HGG, high-grade glioma.
The final diagnosis of low-grade and high-grade tumors was confirmed by following up with the patient’s tumor histopathology report on the day of surgery. In post-op gliomas, a final diagnosis of tumor recurrence and radiation necrosis was confirmed through histopathologic examination of the tumor resected out during the surgery.
Conclusion and Future Perspective
Despite the prevalence of sophisticated imaging, it is not easy to accurately assess the response of glioblastoma to treatment with numerous entities that can mimic tumor progression or recurrence on structural MRI, specifically radiation necrosis and pseudoprogression.107−109 Non-invasive functional MRI techniques such as MR perfusion and MRS advance the diagnostic accuracy, but these techniques may not be available everywhere and are frequently challenging to replicate.110−112 Because tumor recurrence and post-radiation alterations can seem identical on MRI, distinguishing between recurring brain metastasis and radiation necrosis remains a clinical issue in the follow-up of patients with brain metastasis.113 Therefore, in order to plan the treatment for glioma management, a differentiation between true tumor progression and radiation necrosis must be determined. Since the LAT system transporters are overexpressed in the majority of gliomas, amino acid PET tracers have a relatively high sensitivity for gliomas. Nuclear medicine tomographic techniques with amino acid tracers are gaining popularity, and amino acid-based radiotracers are still being researched to have better imaging capabilities than 18F-FDG-PET.114,115 The ability of 18F-FDG PET to distinguish between radiation necrosis and tumor recurrence has been reported to be inadequate due to its physiological brain uptake. This issue is solved by using an amino acid-based tracer.113 Different SPECT and PET radiotracers can be used to image various molecular processes in gliomas with improved specificity. Even though the majority of cutting-edge molecular imaging radiotracers are well-suited for PET imaging, SPECT remains a more affordable and widely accessible alternative modality. Our single-vial ready-to-label 99mTc-DTPA-bis(Met) (MDM) kit is found to be effective in numerous clinical studies for various applications such as the detection of breast cancer, as a prognostic marker, for detection of recurrent/residual glioma, and for detection and differentiation of recurrent/residual glioma from radiation necrosis. Therefore, it may be a potential tracer for preoperative diagnosis, surgical planning, targeted treatment, and post-therapy evaluation of glioma. The molecular characterization of CNS tumors will keep developing. Imaging biomarkers for non-invasive molecular tumor profiling will also advance and become a component of integrated diagnosis, utilized to guide treatment planning for clinical studies and to improve patient care. The primary aim in the near future will be the development of reliable imaging end points that can accurately differentiate between glioma response, progression, and therapeutic effects.
Acknowledgments
The authors are thankful to Director, INMAS, for his constant guidance and encouragement. We are grateful to the Head of the Indian National Institutes of PGIMER (Chandigarh), AIIMS (Delhi), AIIMS (Rishikesh), and NIMHANS (Bangalore) for their persistent support in clinical studies.
Glossary
Abbreviations
- 11C-MET
carbon-11-labeled l-methionine
- 18F-FDG
fluorine-18-labeled fluorodeoxyglucose
- 18F-FET
fluorine-18-labeled fluoroethyl-l-tyrosine
- 18F-FLT
fluorine-18-labeled 3′-fluoro-3′-deoxythymidine
- 1H
proton
- ASC
alanine-serine-cysteine
- BBB
blood–brain barrier
- ceCT
contrast-enhanced computed tomography
- ceMRI
contrast-enhanced magnetic resonance imaging
- Cho
choline
- CNS
central nervous system
- Cr
creatine
- CT
computed tomography
- DSCE
dynamic susceptibility contrast-enhanced
- DSCE-MRI
dynamic susceptibility contrast-enhanced magnetic resonance imaging
- DTPA
diethylenetriaminepentaacetic acid
- GHA
glucoheptonate
- Glu-n
glutamine
- L/N
lesion/normal
- LAT-1
l-type amino acid transporter
- LBR
lesion-to-background ratio
- DTPA-bis(Met)
diethylenetriaminepentaacetic acid-bis-methionine
- MDM
methionine-DTPA-methionine
- MI
myo-inositol
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- MRSI
magnetic resonance spectroscopic imaging
- NAA
N-acetylaspartate
- PET
positron emission tomography
- RANO
radiology assessment of neuro-oncology
- SPECT
single-photon emission computed tomography
- SUV
standard uptake value
- T/NT
target-to-non-target ratio
- WHO
World Health Organization
Author Contributions
P.P.H.: conceptualization, data curation, writing - original draft preparation. S.K.Y.: data curation, writing - original draft preparation. P.K.: resources, supporting. V.D.: resources, supporting. N.R.: resources, supporting. R.K.: resources, supporting. B.S.: resources, supporting. A.K.M.: supervision, reviewing and editing.
The authors declare no competing financial interest.
Special Issue
Published as part of the ACS Pharmacology & Translational Science virtual special issue “Diagnostic and Therapeutic Radiopharmaceuticals”.
References
- DeBerardinis R. J.; Lum J. J.; Hatzivassiliou G.; Thompson C. B. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008, 7, 11–20. 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Berens M. E.; Sood A.; Barnholtz-Sloan J. S.; Graf J. F.; Cho S.; Kim S.; Kiefer J.; Byron S. A.; Halperin R. F.; Nasser S.; Adkins J.; et al. Multiscale, multimodal analysis of tumor heterogeneity in IDH1 mutant vs wild-type diffuse gliomas. PLoS One 2019, 14 (12), e0219724 10.1371/journal.pone.0219724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulová M. B.; Mikuš P. Advances in development of radiometal labeled amino acid-based compounds for cancer imaging and diagnostics. Pharmaceuticals 2021, 14, 167. 10.3390/ph14020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager P. L.; Vaalburg W.; Pruim J.; de Vries E. G.; Langen K. J.; Piers D. A. Radiolabeled amino acids: basic aspects and clinical applications in oncology. J. Nucl. Med. 2001, 42 (3), 432–445. [PubMed] [Google Scholar]
- Miller K. D.; Ostrom Q. T.; Kruchko C.; Patil N.; Tihan T.; Cioffi G.; Fuchs H. E.; Waite K. A.; Jemal A.; Siegel R. L.; Barnholtz-Sloan J. S. Brain and other central nervous system tumor statistics 2021. CA: Cancer J. Clin. 2021, 71 (5), 381–406. 10.3322/caac.21693. [DOI] [PubMed] [Google Scholar]
- Tan A. C.; Ashley D. M.; López G. Y.; Malinzak M.; Friedman H. S.; Khasraw M. Management of glioblastoma: State of the art and future directions. CA: Cancer J. Clin. 2020, 70 (4), 299–312. 10.3322/caac.21613. [DOI] [PubMed] [Google Scholar]
- Yang K.; Wu Z.; Zhang H.; Zhang N.; Wu W.; Wang Z.; Dai Z.; Zhang X.; Zhang L.; Peng Y.; Ye W.; et al. Glioma targeted therapy: insight into future of molecular approaches. Mol. Cancer 2022, 21 (1), 39. 10.1186/s12943-022-01513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis D. N.; Perry A.; Reifenberger G.; Von Deimling A.; Figarella-Branger D.; Cavenee W. K.; Ohgaki H.; Wiestler O. D.; Kleihues P.; Ellison D. W. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016, 131, 803–820. 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- Johnson D. R.; Guerin J. B.; Giannini C.; Morris J. M.; Eckel L. J.; Kaufmann T. J. 2016 updates to the WHO brain tumor classification system: what the radiologist needs to know. Radiographics 2017, 37 (7), 2164–2180. 10.1148/rg.2017170037. [DOI] [PubMed] [Google Scholar]
- Horbinski C.; Berger T.; Packer R. J.; Wen P. Y. Clinical implications of the 2021 edition of the WHO classification of central nervous system tumours. Nat. Rev. Neurol. 2022, 18 (9), 515–529. 10.1038/s41582-022-00679-w. [DOI] [PubMed] [Google Scholar]
- Schillaci O.; Filippi L.; Manni C.; Santoni R. Single-photon emission computed tomography/computed tomography in brain tumors. Semin. Nucl. Med. 2007, 37 (1), 34–47. 10.1053/j.semnuclmed.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Satoer D.; Visch-Brink E.; Smits M.; Kloet A.; Looman C.; Dirven C.; Vincent A. Long-term evaluation of cognition after glioma surgery in eloquent areas. J. Neuro-oncol. 2014, 116, 153–160. 10.1007/s11060-013-1275-3. [DOI] [PubMed] [Google Scholar]
- Shah A. H.; Snelling B.; Bregy A.; Patel P. R.; Tememe D.; Bhatia R.; Sklar E.; Komotar R. J. Discriminating radiation necrosis from tumor progression in gliomas: a systematic review what is the best imaging modality?. J. Neuro-oncol. 2013, 112, 141–152. 10.1007/s11060-013-1059-9. [DOI] [PubMed] [Google Scholar]
- Stupp R.; Taillibert S.; Kanner A.; Read W.; Steinberg D. M.; Lhermitte B.; Toms S.; Idbaih A.; Ahluwalia M. S.; Fink K.; Di Meco F.; et al. Effect of tumor-treating fields plus maintenance Temozolomide vs maintenance Temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 2017, 318 (23), 2306–2316. 10.1001/jama.2017.18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan C. W.; Verhaak R. G.; McKenna A.; Campos B.; Noushmehr H.; Salama S. R.; Zheng S.; Chakravarty D.; Sanborn J. Z.; Berman S. H.; Beroukhim R.; et al. The somatic genomic landscape of glioblastoma. Cell 2013, 155, 462–477. 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]; Erratum:; Cell 2014, 157, 753. 10.1016/j.cell.2014.04.004
- Sottoriva A.; Spiteri I.; Piccirillo S. G.; Touloumis A.; Collins V. P.; Marioni J. C.; Curtis C.; Watts C.; Tavaré S. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 4009–4014. 10.1073/pnas.1219747110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kickingereder P.; Burth S.; Wick A.; Götz M.; Eidel O.; Schlemmer H. P.; Maier-Hein K. H.; Wick W.; Bendszus M.; Radbruch A.; Bonekamp D. Radiomic profiling of glioblastoma: identifying an imaging predictor of patient survival with improved performance over established clinical and radiologic risk models. Radiology 2016, 280 (3), 880–889. 10.1148/radiol.2016160845. [DOI] [PubMed] [Google Scholar]
- Mobadersany P.; Yousefi S.; Amgad M.; Gutman D. A.; Barnholtz-Sloan J. S.; Velázquez Vega J. E.; Brat D. J.; Cooper L. A. Predicting cancer outcomes from histology and genomics using convolutional networks. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, e2970-e2979 10.1073/pnas.1717139115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae S.; Choi Y. S.; Ahn S. S.; Chang J. H.; Kang S. G.; Kim E. H.; Kim S. H.; Lee S. K. Radiomic MRI phenotyping of glioblastoma: improving survival prediction. Radiology 2018, 289 (3), 797–806. 10.1148/radiol.2018180200. [DOI] [PubMed] [Google Scholar]
- Rathore S.; Akbari H.; Rozycki M.; Abdullah K. G.; Nasrallah M. P.; Binder Z. A.; Davuluri R. V.; Lustig R. A.; Dahmane N.; Bilello M.; O’Rourke D. M.; Davatzikos C. Radiomic MRI signature reveals three distinct subtypes of glioblastoma with different clinical and molecular characteristics, offering prognostic value beyond IDH1. Sci. Rep. 2018, 8 (1), 5087. 10.1038/s41598-018-22739-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macyszyn L.; Akbari H.; Pisapia J. M.; Da X.; Attiah M.; Pigrish V.; Bi Y.; Pal S.; Davuluri R. V.; Roccograndi L.; Dahmane N.; et al. Imaging patterns predict patient survival and molecular subtype in glioblastoma via machine learning techniques. Neuro Oncol. 2016, 18 (3), 417–425. 10.1093/neuonc/nov127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iftikhar M.; Rathore S.; Nasrallah M. Analysis of microscopic images via deep neural networks can predict outcome and IDH and 1p/19q codeletion status in gliomas. J. Neuropathol. Exp. Neurol. 2019, 78 (6), 553. [Google Scholar]
- Wang H.; Xu T.; Jiang Y.; Xu H.; Yan Y.; Fu D.; Chen J. The challenges and the promise of molecular targeted therapy in malignant gliomas. Neoplasia 2015, 17 (3), 239–255. 10.1016/j.neo.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J. R. D.; Regad T. Targeting cellular pathways in glioblastoma multiforme. Signal Transduct. Target Ther. 2017, 2, 1–11. 10.1038/sigtrans.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolcaen J.; Kleynhans J.; Nair S.; Verhoeven J.; Goethals I.; Sathekge M.; Vandevoorde C.; Ebenhan T. A perspective on the radiopharmaceutical requirements for imaging and therapy of glioblastoma. Theranostics 2021, 11 (6), 7911. 10.7150/thno.56639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulsen S. Achieving higher diagnostic results in stereotactic brain biopsy by simple and novel technique. Open Access Maced. J. Med. Sci. 2015, 3, 99–104. 10.3889/oamjms.2015.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S. M.; Zhang B.; Wu Y. W.; Zhang W.; Chen Y. Y. Detection of glioma recurrence by 11C-methionine positron emission tomography and dynamic susceptibility contrast-enhanced magnetic resonance imaging: a meta-analysis. Nucl. Med. Commun. 2013, 34 (8), 758–766. 10.1097/MNM.0b013e328361f598. [DOI] [PubMed] [Google Scholar]
- Lizarraga K. J.; Allen-Auerbach M.; Czernin J.; DeSalles A. A.; Yong W. H.; Phelps M. E.; Chen W. 18F-FDOPA PET for differentiating recurrent or progressive brain metastatic tumors from late or delayed radiation injury after radiation treatment. J. Nucl. Med. 2014, 55, 30–36. 10.2967/jnumed.113.121418. [DOI] [PubMed] [Google Scholar]
- Ginsberg L. E.; Fuller G. N.; Hashmi M.; Leeds N. E.; Schomer D. F. The significance of lack of MR contrast enhancement of supratentorial brain tumors in adults: histopathological evaluation of a series. Surg. Neurol. 1998, 49 (4), 436–440. 10.1016/S0090-3019(97)00360-1. [DOI] [PubMed] [Google Scholar]
- Scott J. N.; Brasher P. M. A.; Sevick R. J.; Rewcastle N. B.; Forsyth P. A. How often are nonenhancing supratentorial gliomas malignant? A population study. Neurology 2002, 59, 947–949. 10.1212/WNL.59.6.947. [DOI] [PubMed] [Google Scholar]
- Herholz K.; Langen K. J.; Schiepers C.; et al. Brain tumors. Semin. Nucl. Med. 2012, 42, 356–370. 10.1053/j.semnuclmed.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins M. E.; Barest G. D.; Schaefer P. W.; Hochberg F. H.; Gonzalez R. G.; Lev M. H. Radiation necrosis versus glioma recurrence: conventional MR imaging clues to diagnosis. Am. J. Neuroradiol. 2005, 26 (8), 1967–1972. [PMC free article] [PubMed] [Google Scholar]
- Santra A.; Sharma P.; Kumar R.; Bal C.; Kumar A.; Julka P. K.; Malhotra A. Comparison of glucoheptonate single photon emission computed tomography and contrast-enhanced MRI in detection of recurrent glioma. Nucl. Med. Commun. 2011, 32 (3), 206–211. 10.1097/MNM.0b013e328341c3e9. [DOI] [PubMed] [Google Scholar]
- Durmo F.; Rydelius A.; Baena S. C.; Askaner K.; Lätt J.; Bengzon J.; Englund E.; Chenevert T. L.; Björkman-Burtscher I. M.; Sundgren P. C. Multivoxel 1H-MR Spectroscopy Biometrics for Preoprerative Differentiation Between Brain Tumors. Tomography 2018, 4 (4), 172–181. 10.18383/j.tom.2018.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weybright P.; Sundgren P. C.; Maly P.; Hassan D. G.; Nan B.; Rohrer S.; Junck L. Differentiation between brain tumor recurrence and radiation injury using MR spectroscopy. AJR Am. J. Roentgenol. 2005, 185 (6), 1471–1476. 10.2214/AJR.04.0933. [DOI] [PubMed] [Google Scholar]
- Golder W. Magnetic resonance spectroscopy in clinical oncology. Oncol. Res. Treat. 2004, 27, 304–309. 10.1159/000077983. [DOI] [PubMed] [Google Scholar]
- Rosen Y.; Lenkinski R. E. Recent advances in magnetic resonance neurospectroscopy. Neurotherapeutics 2007, 4, 330–345. 10.1016/j.nurt.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling C.; Bollen A. W.; Noworolski S. M.; McDermott M. W.; Barbaro N. M.; Day M. R.; Henry R. G.; Chang S. M.; Dillon W. P.; Nelson S. J.; Vigneron D. B. Preoperative proton MR spectroscopic imaging of brain tumors: Correlation with histopathologic analysis of resection specimens. Am. J. Neuroradiol. 2001, 22 (4), 604–612. [PMC free article] [PubMed] [Google Scholar]
- McKnight T. R.; von dem Bussche M. H.; Vigneron D. B.; Lu Y.; Berger M. S.; McDermott M. W.; Dillon W. P.; Graves E. E.; Pirzkall A.; Nelson S. J. Histopathological validation of a three-dimensional magnetic resonancespectroscopy index as a predictor of tumor presence. J. Neurosurg. 2002, 97 (4), 794–802. 10.3171/jns.2002.97.4.0794. [DOI] [PubMed] [Google Scholar]
- García-Gómez J. M.; Luts J.; Julià-Sapé M.; Krooshof P.; Tortajada S.; Robledo J. V.; Melssen W.; Fuster-García E.; Olier I.; Postma G.; Monleón D.; et al. Multiproject-multicenterevaluation of automatic brain tumor classification by magnetic resonance spectroscopy. Magn. Reson. Materials Phys. Biol. Med. 2009, 22, 5–18. 10.1007/s10334-008-0146-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galijasevic M.; Steiger R.; Mangesius S.; Mangesius J.; Kerschbaumer J.; Freyschlag C. F.; Gruber N.; Janjic T.; Gizewski E. R.; Grams A. E. Magnetic Resonance Spectroscopy in Diagnosis and Follow-Up of Gliomas: State-of-the-Art. Cancers 2022, 14, 3197. 10.3390/cancers14133197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatchian E.; Ehsani S.; Montazerabadi A. Evaluation of Two Post-Processing Analysis Methods of Proton Magnetic Resonance Spectroscopy in Glioma Tumors. J. Biomed. Phys. Eng. 2023, 13 (1), 39–44. 10.31661/jbpe.v0i0.2001-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. J.; Jin H.; Lu Y.; Oh J.; Chang S.; Nelson S. J. Identifycation of MRI and 1H MRSI parameters that may predict survival for patients with malignant gliomas. NMR Biomed. 2004, 17 (1), 10–20. 10.1002/nbm.858. [DOI] [PubMed] [Google Scholar]
- Oshiro S.; Tsugu H.; Komatsu F.; Abe H.; Onishi H.; Ohmura T.; Iwaasa M.; Sakamoto S.; Fukushima T. Quantitative assessment of gliomas by proton magnetic resonance spectroscopy. Anticancer Res. 2007, 27 (6A), 3757–3763. [PubMed] [Google Scholar]
- Harting I.; Hartmann M.; Jost G.; Sommer C.; Ahmadi R.; Heiland S.; Sartor K. Differentiating primary central nervous system lymphoma from glioma in humans using localized proton magnetic resonance spectroscopy. Neurosci. Lett. 2003, 342 (3), 163–166. 10.1016/S0304-3940(03)00272-6. [DOI] [PubMed] [Google Scholar]
- Tabatabaei P.; Bergström P.; Henriksson R.; Bergenheim A. T. Glucose metabolites, glutamate and glycerol in malignant glioma tumours during radiotherapy. J. Neurooncol. 2008, 90, 35–39. 10.1007/s11060-008-9625-2. [DOI] [PubMed] [Google Scholar]
- Herminghaus S.; Dierks T.; Pilatus U.; Möller-Hartmann W.; Wittsack J.; Marquardt G.; Labisch C.; Lanfermann H.; Schlote W.; Zanella F. E. Determination of histopathological tumor grade in neuroepithelial brain tumors by using spectral pattern analysis of in vivo spectroscopic data. J. Neurosurg. 2003, 98 (1), 74–81. 10.3171/jns.2003.98.1.0074. [DOI] [PubMed] [Google Scholar]
- Law M.; Yang S.; Wang H.; Babb J. S.; Johnson G.; Cha S.; Knopp E. A.; Zagzag D. Glioma Grading: Sensitivity, Specificity, and Predictive Values of Perfusion MR Imaging and Proton MR Spectroscopic Imaging Compared with Conventional MR Imaging. Am. J. Neuroradiol. 2003, 24 (10), 1989–1998. [PMC free article] [PubMed] [Google Scholar]
- Tedeschi G.; Lundbom N.; Raman R.; Bonavita S.; Duyn J. H.; Alger J. R.; Di Chiro G. Increased choline signal coinciding with malignant degeneration of cerebral gliomas: a serial proton magnetic resonance spectroscopy imaging study. J. Neurosurg. 1997, 87 (4), 516–524. 10.3171/jns.1997.87.4.0516. [DOI] [PubMed] [Google Scholar]
- Demaerel P. In vivo localized single-voxel proton magnetic resonance spectroscopy of intracranial tumors. Int. J. Neuroradiol. 1997, 3, 94–110. [Google Scholar]
- Li X.; Lu Y.; Pirzkall A.; McKnight T.; Nelson S. J. Analysis of the spatial characteristics of metabolic abnormalities in newly diagnosed glioma patients. J. Magn. Reson. Imaging 2002, 16 (3), 229–237. 10.1002/jmri.10147. [DOI] [PubMed] [Google Scholar]
- Hattingen E.; Raab P.; Franz K.; Zanella F. E.; Lanfermann H.; Pilatus U. Myoinositol: a marker of reactive astrogliosis in glial tumors?. NMR Biomed. 2008, 21 (3), 233–241. 10.1002/nbm.1186. [DOI] [PubMed] [Google Scholar]
- Parra N. A.; Maudsley A. A.; Gupta R. K.; Ishkanian F.; Huang K.; Walker G. R.; Padgett K.; Roy B.; Panoff J.; Markoe A.; Stoyanova R. Volumetric spectroscopic imaging of glioblastoma multiforme radiation treatment volumes. Int. J. Radiation Oncol. Biol. Phys. 2014, 90 (2), 376–384. 10.1016/j.ijrobp.2014.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Server A.; Kulle B.; Gadmar Ø. B.; Josefsen R.; Kumar T.; Nakstad P. H. Measurements of diagnostic examination performance using quantitative apparent diffusion coefficient and proton MR spectroscopic imaging in the preoperative evaluation of tumor grade in cerebral gliomas. Eur. J. Radiol. 2011, 80, 462–470. 10.1016/j.ejrad.2010.07.017. [DOI] [PubMed] [Google Scholar]
- van Dijken B. R.; van Laar P. J.; Holtman G. A.; van der Hoorn A. Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur. Radiol. 2017, 27, 4129–4144. 10.1007/s00330-017-4789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herminghaus S.; Pilatus U.; Möller-Hartmann W.; Raab P.; Lanfermann H.; Schlote W.; Zanella F. E. Increased choline levels coincide with enhanced proliferative activity of human neuroepithelial brain tumors. NMR Biomed. 2002, 15 (6), 385–392. 10.1002/nbm.793. [DOI] [PubMed] [Google Scholar]
- Rabinov J. D.; Lee P. L.; Barker F. G.; Louis D. N.; Harsh G. R.; Cosgrove G. R.; Chiocca E. A.; Thornton A. F.; Loeffler J. S.; Henson J. W.; Gonzalez R. G. In vivo 3-T MR spectroscopy in the distinction of recurrent glioma versus radiation effects: initial experience. Radiology 2002, 225 (3), 871–879. 10.1148/radiol.2253010997. [DOI] [PubMed] [Google Scholar]
- Huang C.; McConathy J. Radiolabeled amino acids for oncologic imaging. J. Nucl. Med. 2013, 54, 1007–1010. 10.2967/jnumed.112.113100. [DOI] [PubMed] [Google Scholar]
- Barai S.; Bandopadhayaya G. P.; Julka P. K.; Kale S. S.; Malhotra A.; Haloi A. K.; Seith A.; Sing N. G. Evaluation of 99mTc-L-methionine brain SPECT for detection of recurrent brain tumor; a pilot study with radiological and pathological correlation. Acta Radiol. 2004, 45 (6), 649–657. 10.1080/02841850410006740. [DOI] [PubMed] [Google Scholar]
- Kobayashi M.; Hashimoto F.; Ohe K.; Nadamura T.; Nishi K.; Shikano N.; Nishii R.; Higashi T.; Okazawa H.; Kawai K. Transport mechanism of 11C-labeled L- and D-methionine in human-derived tumor cells. Nucl. Med. Biol. 2012, 39 (8), 1213–1218. 10.1016/j.nucmedbio.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Verrey F. System L: heteromeric exchangers of large, neutral amino acids involved in directional transport. Pflügers Archiv 2003, 445, 529–533. 10.1007/s00424-002-0973-z. [DOI] [PubMed] [Google Scholar]
- Bodoy S.; Martin L.; Zorzano A.; Palacin M.; Estévez R.; Bertran J. Identification of LAT4, a novel amino acid transporter with system L activity. J. Biol. Chem. 2005, 280, 12002–12011. 10.1074/jbc.M408638200. [DOI] [PubMed] [Google Scholar]
- Hazari P. P.; Shukla G.; Goel V.; Chuttani K.; Kumar N.; Sharma R.; Mishra A. K. Synthesis of specific SPECT-radiopharmaceutical for tumor imaging based on methionine: 99mTc-DTPA-bis (methionine). Bioconjugate Chem. 2010, 21 (2), 229–239. 10.1021/bc900197n. [DOI] [PubMed] [Google Scholar]
- Puris E.; Gynther M.; Auriola S.; Huttunen K. M. L-Type amino acid transporter 1 as a target for drug delivery. Pharm. Res. 2020, 37, 1–17. 10.1007/s11095-020-02826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H. N. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990, 70 (1), 43–77. 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- Kanai Y.; Segawa H.; Miyamoto K.; Uchino H.; Takeda E.; Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J. Biol. Chem. 1998, 273 (37), 23629–32. 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- Yan R.; Li Y.; Müller J.; Zhang Y.; Singer S.; Xia L.; Zhong X.; Gertsch J.; Altmann K. H.; Zhou Q. Mechanism of substrate transport and inhibition of the human LAT1–4F2hc amino acid transporter. Cell discovery 2021, 7 (1), 16. 10.1038/s41421-021-00247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R.; Zhao X.; Lei J.; Zhou Q. Structure of the human LAT1–4F2hc heteromeric amino acid transporter complex. Nature, 2019, 568 (7750), 127–130. 10.1038/s41586-019-1011-z. [DOI] [PubMed] [Google Scholar]
- Ikotun O. F.; Marquez B. V.; Huang C.; Masuko K.; Daiji M.; Masuko T.; McConathy J.; Lapi S. E. Imaging the L-type amino acid transporter-1 (LAT1) with Zr-89 immuno PET. PloS one 2013, 8 (10), e77476 10.1371/journal.pone.0077476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimaiti M.; Sakamoto S.; Yamada Y.; Sugiura M.; Rii J.; Takeuchi N.; et al. Expression of L-type amino acid transporter 1 as a molecular target for prognostic and therapeutic indicators in bladder carcinoma. Sci. Rep. 2020, 10 (1), 1292. 10.1038/s41598-020-58136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa N.; Ichinoe M.; Mikami T.; Nakada N.; Hana K.; Koizumi W.; et al. High expression of L-type amino acid transporter 1 (LAT1) predicts poor prognosis in pancreatic ductal adenocarcinomas. J. Clin. Pathol. 2012, 65 (11), 1019–1023. 10.1136/jclinpath-2012-200826. [DOI] [PubMed] [Google Scholar]
- Haining Z.; Kawai N.; Miyake K.; Okada M.; Okubo S.; Zhang X.; Fei Z.; Tamiya T. Relation of LAT1/4F2hc expression with pathological grade, proliferation and angiogenesis in human gliomas. BMC Clin Pathol. 2012, 12, 4. 10.1186/1472-6890-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa N.; Ichinoe M.; Mikami T.; Nakada N.; Hana K.; Koizumi W.; et al. High expression of L-type amino acid transporter 1 (LAT1) predicts poor prognosis in pancreatic ductal adenocarcinomas. J. Clin. Pathol. 2012, 65 (11), 1019–1023. 10.1136/jclinpath-2012-200826. [DOI] [PubMed] [Google Scholar]
- Chen W.; Silverman D. H.; Delaloye S.; Czernin J.; Kamdar N.; Pope W.; Satyamurthy N.; Schiepers C.; Cloughesy T. 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J. Nucl. Med. 2006, 47 (6), 904–911. [PubMed] [Google Scholar]
- Grosu A. L.; Astner S. T.; Riedel E.; Nieder C.; Wiedenmann N.; Heinemann F.; Schwaiger M.; Molls M.; Wester H. J.; Weber W. A. An interindividual comparison of O-(2-[18F]fluoroethyl)-L-tyrosine (FET)- and L-[methyl-11C]methionine (MET)-PET in patients with brain gliomas and metastases. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81 (4), 1049–1058. 10.1016/j.ijrobp.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Pauleit D.; Floeth F.; Tellmann L.; Hamacher K.; Hautzel H.; Müller H. W.; Coenen H. H.; Langen K. J. Comparison of O-(2–18F-fluoroethyl)-L-tyrosine PET and 3–123I-iodo-alpha-methyl-L-tyrosine SPECT in brain tumors. J. Nucl. Med. 2004, 45 (3), 374–381. [PubMed] [Google Scholar]
- Pauleit D.; Stoffels G.; Bachofner A.; Floeth F. W.; Sabel M.; Herzog H.; Tellmann L.; Jansen P.; Reifenberger G.; Hamacher K.; Coenen H. H.; Langen K. J. Comparison of (18) F-FET and (18) F-FDG PET in brain tumors. Nucl. Med. Biol. 2009, 36 (7), 779–787. 10.1016/j.nucmedbio.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Watanabe H.; Inoue T.; Shinozaki T.; Yanagawa T.; Ahmed A. R.; Tomiyoshi K.; Oriuchi N.; Tokunaga M.; Aoki J.; Endo K.; Takagishi K. PET imaging of musculoskeletal tumours with fluorine-18 alpha-methyltyrosine: comparison with fluorine-18 fluorodeoxyglucose PET. Eur. J. Nucl. Med. 2000, 27 (10), 1509–1517. 10.1007/s002590000344. [DOI] [PubMed] [Google Scholar]
- Kong Z.; Li Z.; Chen J.; Liu S.; Liu D.; Li J.; Li N.; Ma W.; Feng F.; Wang Y.; Yang Z.; Liu Z. Metabolic characteristics of [18F] fluoroboronotyrosine (FBY) PET in malignant brain tumors. Nuclear Medicine and Biology 2022, 106, 80–87. 10.1016/j.nucmedbio.2022.01.002. [DOI] [PubMed] [Google Scholar]
- Kong Z.; Li Z.; Chen J.; Ma W.; Wang Y.; Yang Z.; Liu Z. Larger 18F-fluoroboronotyrosine (FBY) active volume beyond MRI contrast enhancement in diffuse gliomas than in circumscribed brain tumors. EJNMMI Res. 2022, 12 (1), 22. 10.1186/s13550-022-00896-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B.; Prasad V.; Schuchardt C.; et al. Can the Standardized Uptake Value derived from Diagnostic Ga- DOTATATE PET/CT Imaging Predict the radiation dose delivered to the Metastatic Liver NET Lesions on Lu- DOATATATE peptide receptor radionuclide therapy?. JPMER 2013, 47, 7–13. 10.5005/jp-journals-10028-1050. [DOI] [Google Scholar]
- Hazari P.; Prakash S.; Meena V.; Jaswal A.; Khurana H.; Mishra S.; Somu Bhonsle H.; Singh L.; Mishra A. LAT1 targeted delivery of methionine based Imaging probe derived from M (III) metal ions for early diagnosis of proliferating tumours using molecular imaging modalities. Curr. Cancer Drug. Targets. 2015, 14 (9), 817–831. 10.2174/1568009614666141020102337. [DOI] [PubMed] [Google Scholar]
- Zhao C.; Zhang Y.; Wang J. A Meta-Analysis on the diagnostic performance of 18F-FDG and 11C-Methionine PET for differentiating brain tumors. Am. J. Neuroradiol. 2014, 35, 1058–1065. 10.3174/ajnr.A3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi M.; Sharma R.; Varshney R.; Jaimini A.; Jain J.; Souza M. M.; Bal J.; Pandey S.; Kumar N.; Mishra A. K.; Mondal A. Comparison of F-18 FDG and C-11 Methionine PET/CT for the Evaluation of Recurrent Primary Brain Tumors. Clin. Nucl. Med. 2012, 37 (2), 158–163. 10.1097/RLU.0b013e318238f51a. [DOI] [PubMed] [Google Scholar]
- Zhang-Yin J. T.; Girard A.; Bertaux M. What does pet imaging bring to neuro-oncology in 2022? A review. Cancers 2022, 14, 879. 10.3390/cancers14040879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salber D.; Stoffels G.; Pauleit D.; Reifenberger G.; Sabel M.; Shah N. J.; Hamacher K.; Coenen H. H.; Langen K. J. Differential uptake of [18F]FET and [3H]l-methionine in focal cortical ischemia. Nucl. Med. Biol. 2006, 33 (8), 1029–1035. 10.1016/j.nucmedbio.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Kaim A. H.; Weber B.; Kurrer M. O.; Westera G.; Schweitzer A.; Gottschalk J.; von Schulthess G. K.; Buck A. (18) F-FDG and (18) F-FET uptake in experimental soft tissue infection. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 648–654. 10.1007/s00259-002-0780-y. [DOI] [PubMed] [Google Scholar]
- Rau F. C.; Weber W. A.; Wester H. J.; Herz M.; Becker I.; Krüger A.; Schwaiger M.; Senekowitsch-Schmidtke R. O-(2-[(18) F]fluoroethyl)-l-tyrosine (18F-FET): a tracer for differentiation of tumour from inflammation in murine lymph nodes. Eur. J. Nucl. Med. Mol. Imaging 2002, 29 (8), 1039–1046. 10.1007/s00259-002-0821-6. [DOI] [PubMed] [Google Scholar]
- Stöber B.; Tanase U.; Herz M.; Seidl C.; Schwaiger M.; Senekowitsch-Schmidtke R. Differentiation of tumour and inflammation: characterisation of [methyl-(3) H]methionine (MET) and O-(2-[(18) F] fluoroethyl)-l-tyrosine (FET) uptake in human tumour and inflammatory cells. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 932–939. 10.1007/s00259-005-0047-5. [DOI] [PubMed] [Google Scholar]
- Fotopoulos A. D.; Alexiou G. A. Is there still a place for SPET in the era of PET brain imaging?. Hell. J. Nucl. Med. 2012, 15, 89–91. 10.1967/s002449910026. [DOI] [PubMed] [Google Scholar]
- Vos M. J.; Tony B. N.; Hoekstra O. S.; Postma T. J.; Heimans J. J.; Hooft L. Systematic review of the diagnostic accuracyof 201Tl single photon emission computed tomography in the detection ofrecurrent glioma. Nucl. Med. Commun. 2007, 28 (6), 431–439. 10.1097/MNM.0b013e328155d131. [DOI] [PubMed] [Google Scholar]
- Soler C.; Beauchesne P.; Maatougui K.; Schmitt T.; Barral F. G.; Michel D.; Dubois F.; Brunon J. Technetium-99m sestamibi brain single-photon emission tomography for detection of recurrent gliomas afterradiation therapy. Eur. J. Nucl. Med. 1998, 25, 1649–1657. 10.1007/s002590050344. [DOI] [PubMed] [Google Scholar]
- Deuschl C.; Kirchner J.; Poeppel T. D.; Schaarschmidt B.; Kebir S.; El Hindy N.; Hense J.; Quick H. H.; Glas M.; Herrmann K.; Umutlu L.; et al. 11C-MET PET/MRI for detection of recurrent glioma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 593–601. 10.1007/s00259-017-3916-9. [DOI] [PubMed] [Google Scholar]
- Sharma R.; Tripathi M.; Panwar P.; Chuttani K.; Jaimini A.; Maitra S.; Chopra M. K.; Sawroop K.; Shukla G.; Mondal A.; Mishra A. 99mTc-methionine scintimammography in the evaluation of breast cancer. Nucl. Med. Commun. 2009, 30 (5), 338–342. 10.1097/MNM.0b013e32832999dc. [DOI] [PubMed] [Google Scholar]
- Sharma S.; Singh B.; Mishra A. K.; Rathod D.; Hazari P. P.; Chuttani K.; Chopra S.; Singh P. M.; Abrar M. L.; Mittal B. R.; Singh G. Lat-1 based primary breast cancer detection by [99m] tc-labeled dtpa-bis-methionine scintimammography: First results using indigenously developed single vial kit preparation. Cancer Biother. Radiopharm. 2014, 29, 283–288. 10.1089/cbr.2014.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora G.; Sharma P.; Sharma A.; Mishra A. K.; Hazari P. P.; Biswas A.; Garg A.; Aheer D.; Kumar R. 99mTc-methionine hybrid SPECT/CT for detection of recurrent glioma: comparison with 18F-FDG PET/CT and contrast-enhanced MRI. Clin. Nucl. Med. 2018, 43 (5), e132–e138. 10.1097/RLU.0000000000002036. [DOI] [PubMed] [Google Scholar]
- Rani N.; Singh B.; Kumar N.; Singh P.; Hazari P. P.; Singh H.; Kumar G.; Radotra B.; Kumar M.; Bhattacharya A.; Sharma R.; et al. Differentiation of recurrent/residual glioma from radiation necrosis using semi quantitative 99mTc MDM (Bis-Methionine-DTPA) brain SPECT/CT and dynamic susceptibility contrast-enhanced MR perfusion: a comparative study. Clin. Nucl. Med. 2018, 43 (3), e74–e81. 10.1097/RLU.0000000000001943. [DOI] [PubMed] [Google Scholar]
- Rani N.; Singh B.; Kumar N.; Singh P.; Hazari P. P.; Vyas S.; Hooda M.; Chitkara A.; Shekhawat A. S.; Gupta S. K.; Radotra B. D.; Mishra A. K. [99mTc]-Bis-Methionine-DTPA Single-Photon Emission Computed Tomography Impacting Glioma Management: A Sensitive Indicator for Postsurgical/Chemoradiotherapy Response Assessment. Cancer Biother Radiopharm. 2021, 36 (7), 568–578. 10.1089/cbr.2020.3696. [DOI] [PubMed] [Google Scholar]
- Riley R. D.; Sauerbrei W.; Altman D. G. Prognostic markers in cancer: the evolution of evidence from single studies to meta-analysis, and beyond. Br. J. Cancer. 2009, 100 (8), 1219–1229. 10.1038/sj.bjc.6604999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shooli H.; Dadgar H.; Wáng Y. X. J.; Vafaee M. S.; Kashuk S. R.; Nemati R.; Jafari E.; Nabipour I.; Gholamrezanezhad A.; Assadi M.; Larvie M. An update on PET-based molecular imaging in neuro-oncology: challenges and implementation for a precision medicine approach in cancer care. Quant. Imaging Med. Surg. 2019, 9 (9), 1597. 10.21037/qims.2019.08.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. Clinical applications of PET in brain tumors. J. Nucl. Med. 2007, 48, 1468–1481. 10.2967/jnumed.106.037689. [DOI] [PubMed] [Google Scholar]
- Weber W. A.; Wester H. J.; Grosu A. L.; Herz M.; Dzewas B.; Feldmann H. J.; Molls M.; Stöcklin G.; Schwaiger M. O-(2-[18F] fluoroethyl)-L-tyrosine and L-[methyl-11C] methionine uptake in brain tumours: initial results of a comparative study. Eur. J. Nucl. Med. Mol. Imaging 2000, 27, 542–549. 10.1007/s002590050541. [DOI] [PubMed] [Google Scholar]
- Singh B.; Kumar N.; Sharma S.; Watts A.; Hazari P. P.; Rani N.; Vyas S.; Anish B.; Mishra A. K. 99mTc-MDM brain SPECT for the detection of recurrent/remnant glioma-comparison with ceMRI and 18F-FLT PET imaging: initial results. Clin. Nucl. Med. 2015, 40 (10), e475–e479. 10.1097/RLU.0000000000000881. [DOI] [PubMed] [Google Scholar]
- Maecke H. R.; André J. I. 68Ga-PET radiopharmacy: a generator-based alternative to 18F-radiopharmacy. PET Chem. 2007, 64, 215–242. 10.1007/978-3-540-49527-7_8. [DOI] [PubMed] [Google Scholar]
- Rani N.; Singh B.; Kumar N.; Singh P.; Hazari P. P.; Jaswal A.; Gupta S. K.; Chhabra R.; Radotra B. D.; Mishra A. K. The diagnostic performance of 99mTc-methionine single-photon emission tomography in grading glioma preoperatively: a comparison with histopathology and Ki-67 indices. Nucl. Med. Commun. 2020, 41 (9), 848–857. 10.1097/MNM.0000000000001230. [DOI] [PubMed] [Google Scholar]
- Kumar P.; Kumar A.; Nagaraj C.; Saini J.; Mangalore S.; Sadashiva N.; Beniwal M.; Panwar P.; Mishra A. K. To evaluate the diagnostic efficacy of [99mTc] methionine: A head-to-head comparison with [11C] methionine in glioma patients. Eur. J. Nucl. Med. Mol. Imaging 2022, 49 (Suppl 1), S454. [Google Scholar]
- Tiwari P.; Prasanna P.; Wolansky L.; Pinho M.; Cohen M.; Nayate A.P.; Gupta A.; Singh G.; Hatanpaa K.J.; Sloan A.; Rogers L.; Madabhushi A. Computer-extracted texture features to distinguish cerebral radionecrosis from recurrent brain tumors on multiparametric MRI: a feasibility study. Am. J. Neuroradiol. 2016, 37 (12), 2231–2236. 10.3174/ajnr.A4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbasi A. W.; Westerlaan H. E.; Holtman G. A.; Aden K. M.; van Laar P. J.; van der Hoorn A. Incidence of tumour progression and pseudoprogression in highgrade gliomas: a systematic review and meta-analysis. Clin. Neuroradiol. 2018, 28, 401–411. 10.1007/s00062-017-0584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larroza A.; Moratal D.; Paredes-Sánchez A.; Soria-Olivas E.; Chust M. L.; Arribas L. A.; Arana E. Support vector machine classification of brain metastasis and radiation necrosis based on texture analysis in MRI. J. Magn. Reson. Imaging 2015, 42 (5), 1362–1368. 10.1002/jmri.24913. [DOI] [PubMed] [Google Scholar]
- Hu L. S.; Eschbacher J. M.; Heiserman J. E.; Dueck A. C.; Shapiro W. R.; Liu S.; Karis J. P.; Smith K. A.; Coons S. W.; Nakaji P.; Spetzler R. F.; et al. Reevaluating the imaging definition of tumor progression: perfusion MRI quantifies recurrent glioblastoma tumor fraction, pseudoprogression, and radiation necrosis to predict survival. Neuro Oncol. 2012, 14 (7), 919–930. 10.1093/neuonc/nos112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalaa I.; Henry R.; Dillon W. P.; Graves E. E.; McKnight T. R.; Lu Y.; Vigneron D. B.; Nelson S. J. Perfusion, diffusion and spectroscopy values in newly diagnosed cerebral gliomas. NMR Biomed. 2006, 19 (4), 463–475. 10.1002/nbm.1059. [DOI] [PubMed] [Google Scholar]
- van Dijken B. R.; van Laar P. J.; Smits M.; Dankbaar J. W.; Enting R. H.; van der Hoorn A. Perfusion MRI in treatment evaluation of glioblastomas: Clinical relevance of current and future techniques. J. Magn. Reson. Imaging 2019, 49 (1), 11–22. 10.1002/jmri.26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papin-Michault C.; Bonnetaud C.; Dufour M.; Almairac F.; Coutts M.; Patouraux S.; Virolle T.; Darcourt J.; Burel-Vandenbos F. Study of LAT1 expression in brain metastases: towards a better understanding of the results of positron emission tomography using amino acid tracers. PloS one 2016, 11 (6), e0157139 10.1371/journal.pone.0157139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W.; McConathy J.; Williams L.; Camp V. M.; Malveaux E. J.; Zhang Z.; Olson J. J.; Goodman M. M. Synthesis, radiolabeling, and biological evaluation of (R)-and (S)-2-amino-3-[18F] fluoro-2-methylpropanoic acid (FAMP) and (R)-and (S)-3-[18F] fluoro-2-methyl-2-N-(methylamino) propanoic acid (N MeFAMP) as potential PET radioligands for imaging brain tumors. J. Med. Chem. 2010, 53 (2), 876–886. 10.1021/jm900556s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W.; McConathy J.; Olson J. J.; Goodman M. M. System A amino acid transport-targeted brain and systemic tumor PET imaging agents 2-amino-3-[18 F] fluoro-2-methylpropanoic acid and 3-[18 F] fluoro-2-methyl-2-(methylamino) propanoic acid. Nucl. Med. Biol. 2015, 42 (1), 8–18. 10.1016/j.nucmedbio.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]










