Abstract
(N)-Methanocarba adenosine derivatives (A3 adenosine receptor (AR) agonists containing bicyclo[3.1.0]hexane replacing furanose) were chain-extended at N6 and C2 positions with terminal alkenes for ring closure. The resulting macrocycles of 17–20 atoms retained affinity, indicating a spatially proximal orientation of these receptor-bound chains, consistent with molecular modeling of 12. C2-Arylethynyl-linked macrocycle 19 was more A3AR-selective than 2-ether-linked macrocycle 12 (both 5′-methylamides, human (h) A3AR affinities (Ki): 22.1 and 25.8 nM, respectively), with lower mouse A3AR affinities. Functional hA3AR comparison of two sets of open/closed analogues in β-arrestin2 and Gi/o protein assays showed certain signaling preferences divergent from reference agonist Cl-IB-MECA 1. The potencies of 1 at all three Gαi isoforms were slightly less than its hA3AR binding affinity (Ki: 1.4 nM), while the Gαi1 and Gαi2 potencies of macrocycle 12 were roughly an order of magnitude higher than its radioligand binding affinity. Gαi2-coupling was enhanced in macrocycle 12 (EC50 2.56 nM, ∼40% greater maximal efficacy than 1). Di-O-allyl precursor 18 cyclized to form 19, increasing the Gαi1 potency by 7.5-fold. The macrocycles 12 and 19 and their open precursors 11 and 18 potently stimulated β-arrestin2 recruitment, with EC50 values (nM) of 5.17, 4.36, 1.30, and 4.35, respectively, and with nearly 50% greater efficacy compared to 1. This example of macrocyclization altering the coupling pathways of small-molecule (nonpeptide) GPCR agonists is the first for potent and selective macrocyclic AR agonists. These initial macrocyclic derivatives can serve as a guide for the future design of macrocyclic AR agonists displaying unanticipated pharmacology.
Keywords: GPCR, adenosine, macrocycles, Gi protein, nucleosides, arrestin
The A3 adenosine receptor (A3AR) is a therapeutic target for inflammatory diseases, ischemia, cancer, neuropathic pain, liver diseases, and other chronic conditions.1−8 Most reported clinical and preclinical candidate molecules acting selectively at this G protein-coupled receptor (GPCR) are nucleoside agonists. We and others have explored the detailed structure activity relationship (SAR) of ribonucleosides and conformationally constrained methanocarba (bicyclo[3.1.0]hexane replacing the furanose) nucleosides (Chart 1) at this receptor.8−12 The methanocarba bicyclic ring substitution is based on fused cyclopropyl and cyclopentyl rings, which depending on the position of fusion can constrain the conformation in either a North (N, 2′-exo) or South (S, 3′-exo) envelope conformation. In the case of the A3AR, the pseudoribose (N) conformer closely approximates the receptor-bound ribose conformation and thereby greatly enhances both affinity and selectivity at this AR subtype and can be combined with a wide range of other substitutions.13 Diverse and sterically large N6 and C2 substitutions on the adenine nucleobase are permitted in A3AR binding, but the ribose (or pseudoribose) 2′, 3′, and 5′ positions are much more sterically and structurally limited.9,10 The ribose moiety in A1, A2A, and A2B experimental structures,14−16 and as modeled for the A3AR, occupies a deep hydrophilic region of the orthosteric binding site. The ribose moiety engages in characteristic-conserved interactions with polar amino acid side chains in transmembrane helices (TMs) 3, 5, 6, and 7. However, the N6 and C2 substituents interact with the receptor external regions, including extracellular loop 2 (EL2).14 By extension of these two latter substituents (and rigidification, in the case of the C2 group) as in compounds 2 and 3,9−11 the A3AR affinity and selectivity may be increased. With respect to the mouse (m) A3AR, it was possible to enhance affinity by introduction of polar OH and OMe groups on a N6-2-phenylethyl substituent, for example, (N)-methanocarba nucleosides 4a and 4b, that reached polar and H-bonding residues on the ELs.9,10
Chart 1. Reference High Affinity A3AR Nucleoside Ligands Containing N6, C2, and 5′ Position Substitutionsa.
a Both ribonucleosides (1 and 2) and conformationally constrained (N)-methanocarba nucleosides (3 and 4) are represented. 4′-Truncated derivative 4a is a low-efficacy partial agonist/antagonist, while 1–3 and 4b are agonists. A3AR binding affinity values provided are published.8−11
Upon examination of the distal portions of the N6 and C2 substituents of 4b that are both pointing outward toward the extracellular medium, we hypothesized that it would be feasible to chemically bridge these two groups to create macrocyclic derivatives. The EL region of Class A GPCRs is known to be more conformationally flexible than deeper helical structures,17 which in the ARs surround the ribose binding site. We hypothesized based on receptor modeling that introduction of a macrocyclic ring on (N)-methanocarba nucleosides might be tolerated in the A3AR EL region. We also questioned whether the presence of a macrocyclic ring, which might affect the overall receptor conformation, may also change the spectrum of G protein interactions of this GPCR. There are already several biased agonists and allosteric modulators identified for the AR family that can favor one signaling pathway over others.18,19 Receptor conformational changes in the EL region could potentially be propagated through internal interaction networks to the cytosolic surface, which is associated with G protein binding.20−22 By analogy, there are known mutations in the ELs that alter functional properties of various GPCRs, including the P2Y1R and P2Y12R, which are activated by ADP.23,24 The A3AR is principally coupled with Gi proteins25 but potentially could interact with multiple G protein-dependent and independent signaling pathways as shown for other GPCRs.26−30
Macrocyclic derivatives of small molecules, often derived from natural products, are used increasingly in medicinal chemistry to improve absorption or to maintain conformational control to enable interaction with a target biomolecule.31−34 Kinase inhibitors have been enhanced by macrocyclization, and synthetic macrocycles are used to inhibit protein–protein interactions.35,36 In some examples, formation of internal H-bonding increases bioavailability, and macrocyclic drugs can adopt multiple conformations to either expose polar groups or shield them to enable crossing lipid membranes, including the blood brain barrier (BBB).34−36 Macrocyclic peptidic and non-peptidic ligands of GPCRs are well explored for peptide receptors, but not commonly for the Class A rhodopsin-like GPCRs that are typically activated by smaller ligands.31,37 One of the few examples of a macrocyclic ligand binding to the A3AR is the antagonist archazolid A, a polyketide macrolide, and various synthetic analogues.38 Now with the availability of hundreds of GPCR structures, including those of the A3AR-related and highly homologous A1AR and A2AAR, we have used a structure-based approach to design potential macrocyclic ligands for this receptor class.
Results
Initial Selection of Linking Structures at the N6 and C2 Positions Based on Reference Compounds
The target adenosine derivatives, as shown in Table 1, were based on the published SAR of A3AR agonists, as represented by known ligands 4–10,8−11 and on molecular modeling of hypothetical macrocycles. 2-Ether derivatives 5–10 were included as model compounds, as they retain high affinity at the A3AR, although the selectivity is variable. In a previous publication, 2-ether derivatives 6 and 9 were shown to be highly efficacious in inducing locomotor depression and reducing in WT (C57BL/6J male) mice following peripheral administration (i.p., Table S1, Supporting Information), principally by activating the A3AR.10,39 2-Isobutyl ether 6 at 3 mg/kg (i.p.) reached a lower core body temperature than reference compounds (full agonists with similar N6-(2-phenylethyl) substituents) containing a 2-alkynyl group. Furthermore, these model C2-ethers contain a free 3-phenolic group on the N6 substituent, which is suitable for further derivatization. Thus, the nucleosides synthesized here, all of which are sterically constrained (N)-methanocarba analogues, contained N6 (aryl-alkyl), C2 (alkyne or ether-linked), and 5′ (methylamide or CH2OH) position substitutions predicted to favor A3AR affinity.
Table 1. AR Affinity of 2-Ethers, Macrocyclic Derivatives and Open-Chain Precursors, Determined Using Reported Radioligand Binding Methods13.

Binding in membranes of HEK293 (hA1, hA2A, hA3, mA1, mA3ARs) cells stably expressing one of the three AR subtypes. The binding affinity for hA1, A2A, and A3ARs was expressed as Ki values using agonists [3H]N6-R-phenylisopropyladenosine 36, [3H]2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarboxamidoadenosine 37, or [125I]N6-(4-amino-3-iodobenzyl)adenosine-5′-N-methyluronamide 38, respectively.9,10,13 A percent in italics refers to inhibition of binding at 10 μM. Nonspecific binding was determined using 5′-N-ethylcarboxamidoadenosine 39 (10 μM). Values are expressed as the mean ± SEM (n = 2–4, unless noted). Ki values were calculated as reported.40
Data from Tosh et al.9
Data from Tosh et al.10 ND, not determined.
Molecular Modeling of Macrocyclic Derivatives
Prior to the synthesis, we turned to molecular modeling to predict the feasibility of closing the macrocyclic ring in a way that would not prevent receptor binding. Spatial considerations based on binding mode predictions of A3AR agonists guided the target macrocycle selection. Since there is no published A3AR X-ray or cryo-EM structure, molecular modeling was carried out by homology to other AR structures (A1AR and A2AAR).14,15
The structural characterization of 2-methoxy derivative 5 through molecular modeling provided a rationale for specific macrocyclic compounds. The hypothetical binding mode of compound 5 was obtained through molecular docking at a hA3AR model (obtained in a previous work9), followed by molecular dynamics (MD) simulations. The proposed docking pose was consistent with what was previously reported for hA3AR agonists, and it resembled the binding mode of adenosine and adenosine-derivatives bound to hA2A and hA1ARs in X-ray crystallographic structures.14,15 Key features are a π–π stacking interaction with F168 (extracellular loop 2, EL2), a bidentate H-bond with N250 (transmembrane helix 6, TM6), a H-bond with T94 (TM3), S271, and H272 (TM7). During MD simulations, the ligand was geometrically stable as exemplified by the RMSD of the ligand heavy atoms along time (Figure S1A, average 1.5 Å over three 30 ns MD simulation replicates) and the pattern of H-bonds, especially with N250 and T94, was maintained (Figure S1D–F).
The N6-(2-phenethyl) moiety of 5 showed a higher RMSD fluctuation as compared to the rest of the molecule (Figure S1B,C), but the RMSD value fluctuated most frequently around 1.5 Å from the initial state. The N6-(2-phenethyl) moiety conformation with respect to the adenine scaffold can be described by three dihedral angles, the one around the amino-methylene bond (C33-N35-C37-C43, using the ligand arbitrary atom names in Figure S2), the one around the ethyl bond (N35-C37-C43-C51), and the one around the methylene-phenyl bond (C37-C43-C51-C52). These dihedral angles appeared to assume limited values during the simulation (Figure S3A–C), with the C33-N35-C37-C43 angle being clustered around approximately 70°, N35-C37-C43-C51 around approximately 180°, and C37-C43-C51-C52 around approximately 70° (with a less populated cluster centered around approximately 250°). The ensemble of compound 5’s MD conformations was clustered based on the conformational variability of the ligand’s N6 substituent (i.e., the RMSD of the 2-phenethyl moiety after superposition of the rigid adenine scaffold). The center of the most populated cluster, collecting around 60% of the MD frames, is reported in Figure 1 to exemplify the preferred compound conformation, which is characterized by 70, 183, and 88° for C33-N35-C37-C43, N35-C37-C43-C51, and C37-C43-C51-C52 dihedral angles, respectively. Given the focused dihedral angle distribution, it was hypothesized that the suggested N6-(2-phenethyl) substituent conformation was preferred for hA3AR binding. For this reason, compound 12 was taken into consideration, as the simpler macrocyclic compound mimicking the predicted conformation of compound 5 within the target binding site. This aimed to chemically constrain compound 5 into a receptor preferred conformation. Compound 12 was designed and docked at the hA3AR orthosteric pocket, and the complex was submitted to MD simulation similarly to compound 5. The dihedral distribution of compound 12 in the MD frames ensemble (Figure S3D–F) was similar to the one observed for compound 5 and even more focused around central values. Phenethyl moiety clustering resulted in a highly populated cluster (80%), whose center is reported in Figure 1B.
Figure 1.

Hypothetical binding mode of compound 5 (green) (A) and 12 (pink) (B) at the hA3AR (grey) orthosteric binding site. The poses were obtained by molecular docking followed by three replicates of 30 ns MD simulations, followed by clustering of the MD frames on the basis of the RMSD of the N6-(2-phenylethyl) substituent (after aligning the trajectories on the ligands’ adenine scaffold). Key interacting residues are shown by grey sticks.
Moreover, to check the variation of the N6 substituent conformation upon binding, compounds 5 and 12 were subjected to MD simulation in the free, unbound state (in a water box). Compound 5’s N6 substituent could assume multiple conformations in the free state, as elucidated by the appearance of multiple dihedral populations describing the phenethyl moiety orientation (Figure S4A–C). Compound 12 explored just two alternative conformations in the free unbound state, one corresponding to the preponderant bound conformation and one with approximately a concerted 180° rotation around the amino-methylene and the methylene-phenyl bond. This suggested that the cyclization would not constrain compound 12 into a unique rigid structure, but it would still limit the extent of the accessible conformational space as compared to compound 5, with a consequent binding advantage.
Compound 12 was predicted to bind to the hA3AR similarly to compound 5 and other agonists, with the ribose ring pointing deep into the binding pocket and the adenine scaffold enclosed in the TM bundle. The typical key interaction fingerprint of ARs agonists (Figure 1B) was observed,1,14 including the π–π stacking interaction between the aromatic scaffold and F168, the bidentate H-bond between N250 and the exocyclic amino group and the endocyclic N7, the H-bond between OH at the pseudoribose 2′ and 3′ positions and S271 and H272, and between the amide group at position 5′ and T94, with most of these interactions maintained during the MD simulations (Figure S5D–F and Video 1). The N6 and C2 bridging structure comprising the macrocycle faced the extracellular milieu, with the 4-methyoxy and 3-alkyloxy substituents of the phenethyl moiety transiently interacting with residues on EL2 and EL3, such as Q167 and Q261.
Synthesis of Macrocyclic Derivatives
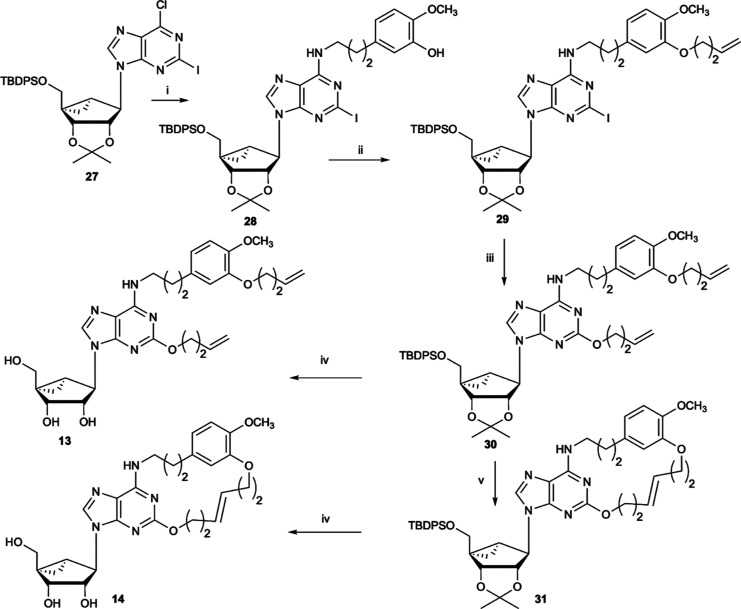
Based on the molecular modeling above, we initially synthesized compound 12 with a 17-membered ring that was predicted to fit the orthosteric binding site (Scheme 1). In compound 12, an adenine C2-ether was designed to be linked by a ring closure metathesis (RCM) reaction to a N6-2-(3-hydroxy-4-methoxyphenyl)-ethyl substituent. The chain attachment to the N6 terminal phenyl ring was also through an ether linkage, specifically to its 3 position. To further explore the SAR, additional macrocyclic derivatives 14, 16, 19, and 21 (Schemes 2–4) were planned based on the possible fit of those slightly enlarged rings (18- to 20-membered) as well. The C2 attachment structure consisted of an alkyl-ether (compounds 12 and 14), an alkyl-alkyne (compounds 16 and 21), or a 3-alkoxy-phenyl-alkyne (compound 19). As the macrocyclic N6 attachment point, we used a N6-(2-phenylethyl) (compounds 12 and 19) or N6-(3-phenylpropyl) (compound 14) substituent containing 3-hydroxy-4-methoxy-phenyl substitution, identified in our previous study as leading to particularly potent A3AR agonists and partial agonists.8,10 However, in the case of the N6-benzyl derivatives 16 and 21, the phenyl substitution consisted of a 3-alkyne. As we have noted,10 the 5′ substitution can also have a major effect on affinity and maximal efficacy of adenosine derivatives, with 5′-methylamides typically being the most efficacious as full agonists, followed by 5′-esters and native ribose-like CH2OH derivatives as partial agonists. We included all three variations in the following macrocyclic derivatives: 4′-CONHCH3 (12, 16, 19), 4′-CO2CH2CH3 (21), and 4′-CH2OH (14). We aimed to compare the pharmacological profiles of these (N)-methanocarba adenosine derivatives, by various binding and functional criteria, between the cyclic and corresponding open rings, and in comparison to a reference agonist 1.
Scheme 1. Synthesis of Ether-Linked Macrocycle (2 Position, 12) Containing a 5′-N-Methyluronamide and Related Non-cyclized Derivative 11.
Reagents and conditions: (i) 4-bromobutene, K2CO3, DMF, 90 °C; (ii) but-3-en-1-ol, NaH, 90 °C; (iii) Grubb’s 2nd gen. catalyst, CH2Cl2, rt; (iv) 10% TFA (aqueous): MeOH (1:1), 70 °C. Compound 23 was synthesized as reported by Tosh et al.10
Scheme 2. Synthesis of an Ether-Linked Macrocycle (2 Position, 14) Containing a 5′-CH2OH Group and Related Non-cyclized Derivative 13.
Reagents and conditions: (i) 5-(3-aminopropyl)-2-methoxyphenol, DIPEA, 2-propanol, rt; (ii) 4-bromobutene, K2CO3, DMF, 80 °C; (iii) but-3-en-1-ol, NaH, 90 °C; (iv) 10% TFA (aqueous)/MeOH (1:1), 70 °C; (v) Grubb’s 2nd gen. catalyst, CH2Cl2, rt.
Scheme 4. Synthesis of Arylalkyne-Linked Macrocycles (2 Position) Containing a 5′-N-Methyluronamide (19) and Related Non-cyclized Derivatives 17 and 18.
Reagents and conditions: (i) 3-ethynylphenol, PdCl2(Ph3P)2, CuI, Et3N, DMF, 60 °C, 2 h; (ii) allyl bromide, K2CO3, DMF, 70 °C; (iii) Grubb’s 2nd gen. catalyst, CH2Cl2, 60 °C; (iv) 10% TFA (aqueous)/MeOH (1:1), 70 °C. Compound 23 (structure shown in Scheme 1) was synthesized as reported by Tosh et al.10
The final step of the macrocyclic synthesis was performed using RCM reactions, as shown in Schemes 1–4. The corresponding uncyclized and acid-deprotected compounds, that is, 11, 13, 15, 18, and 20, all contained two terminal alkenes on separate chains. These alkenes were intended for parallel RCM reactions of the hydroxyl-protected precursors to form the large macrocycles, followed by acid deprotection. The hydroxyl-protected, diallyl precursors, that is, 25 (Scheme 1), 30 (Scheme 2), 34 (Scheme 3), and 37 (Scheme 4), were prepared by routes similar to our previously reported methods for multiply substituted (N)-methanocarba adenosine derivatives.9,10 At the adenine 2 position, an ether was introduced in 29 and 30 by nucleophilic displacement of a 2-iodo group (Schemes 1 and 2). Alternatively, an alkyl-alkyne in 34 (Scheme 3) or a phenylethynyl group in 36 (Scheme 4) was introduced through Sonogashira reactions on a precursor 2-iodo group. A 2-iodo intermediate, 23 (Scheme 1), with an appropriate N6 group already installed was synthesized as reported by Tosh et al.10 For the introduction of 5′-N-methyluronamide groups, ethyl ester precursors (20, 21) were treated with methylamine to yield 15 and 16 (Scheme 3).
Scheme 3. Synthesis of Alkyne-Linked Macrocycles (2 Position) Containing a 5′-Ethyl Ester (16) or a 5′-N-Methyluronamide (21) and Related Non-cyclized Derivatives 15 and 20.
Reagents and conditions: (i) 3-iodobenzylamine, DIPEA, 2-propanol, rt; (ii) hex-1-en-5-yne, PdCl2(Ph3P)2, CuI, Et3N, DMF, rt; (iii) Grubb’s 2nd gen. catalyst, CH2Cl2, rt; (iv) 10% TFA (aqueous)/MeOH (1:1), 70 °C; (v) 40% MeNH2, MeOH, rt.
Pharmacological Evaluation
Radioligand binding inhibition9 at three ARs indicated generally high affinity at the hA3AR (Table 1). Data for the mARs were also included. A comparison of binding affinity with known compounds 4–10 provided a reference for judging the effects of macrocyclization. These known compounds consisted of 2-aryl-ethynyl 4 and 2-ether 5–10 derivatives containing (N)-methanocarba rings. In addition to high hA3AR affinity, three of these derivatives, 6, 8, and 9, displayed moderate affinity (200–600 nM) at the mA1AR and somewhat weaker hA2AAR affinity. We were interested in whether the subsequent macrocyclic cyclization would enhance hA3AR selectivity.
We compared the AR affinity of the open precursor and closed macrocyclic forms: (11 vs 12 [17-membered ring], 13 vs 14 [18-membered], 15 vs 16 [18-membered], 17 and 18 vs 19 [20-membered], and 20 vs 21 [18-membered 5′ esters]). In general, A3AR affinity was well-maintained (either reduced no more than 3-fold or in one instance improved 12-fold (13 [open] vs 14 [closed])), following macrocyclization of the open-chain precursor compounds. Among the macrocycles, hA3AR binding affinity was highest for the 5′-methylamide series, with the C2-ether derivative 12 (17-membered ring) and the C2-phenylethynyl derivative 19 (20-membered ring) displaying respective Ki values of 25.8 and 22.1 nM. Impressively, the C2-arylethynyl macrocycle 19 maintained high selectivity for the hA3AR as compared to the hA1 and hA2A receptors. Interestingly, binding affinity of the 5′-methylamide C2-ether 11 (open) for the hA2AAR increased 10-fold following cyclization (12, closed), while binding affinity for the hA3AR, but not hA1AR, was reduced slightly. Of those tested with the mA3AR, all the macrocycles displayed lower binding affinity as compared to the hA3AR. However, as with the hA3AR, binding affinity was maintained following cyclization within a range of 3-fold. We tested compounds 12 and 19 in a functional hA2BAR cAMP assay in stably transfected CHO cells (Figure S6, Supporting Information), performed as described.13 Both macrocycles showed negligible hA2BAR activation at 1 μM, and the percent activation (relative to NECA as 100%) was 42% and 19%, respectively.
A computation analysis of pharmacokinetic properties using the StarDrop software41 predicted reduced flexibility (4 rotatable bonds versus 6) and hERG liability and slightly higher solubility at pH 7.4 and propensity to cross the BBB for macrocycles 12 and 19 in comparison to reference agonist 1 (Table S2, Supporting Information). Nevertheless, all three molecules were not predicted to readily enter the CNS.
The off-target affinity of most of the analogues at 45 different receptors, channels, and transporters was determined by the NIMH Psychoactive Drug Screening Program (PDSP).42 Only a few weak off-target activities (typical of the methanocarba series6,9,10,13) were observed for both open and corresponding closed rings (Tables 2 and S3, Supporting Information), indicating that the macrocycles are not promiscuous binders. For example, compound 11 bound to the histamine H1 receptor (4.4 ± 0.5 μM), while its closed form 12 bound to σ1 (4.1 ± 1.4 μM) and σ2 (0.60 ± 0.05 μM) receptors. Di-O-allyl open form 18, but not the corresponding closed form 19, bound to the μ- (1.4 μM) and κ- (1.1 μM) opioid receptors. 2-Ether model compounds 5 and 9 had two interactions each, while 8 had no off-target activities.
Table 2. Off-Target Activity as Measured by PDSPa.
| compound | selected structural featuresb | off-target protein, Ki value (μM) |
|---|---|---|
| 5 | 2-O-methyl (open) | BZP 5.1 ± 0.3; 5HT2B 1.9 ± 0.1 |
| 8 | 2-O-cyclopropylmethyl (open) | None |
| 9 | 2-O-isopentyl (open) | BZP 4.1 ± 1.1; TSPO 3.0 ± 0.6 |
| 11 | open precursor of 12 | H1 4.4 ± 0.5 |
| 12 | closed, 17-membered ring | σ1 4.1 ± 1.4; σ2 0.60 ± 0.05; DAT 7.5 ± 0.2 |
| 17 | open, without allyl moieties | σ1 1.0 ± 0.5; α1D 6.4 |
| 18 | open precursor of 19 | σ2 1.4 ± 0.7; MOR 1.4, KOR 1.1 ± 0.3; TSPO 0.46 ± 0.05; 5HT1D 4.0 |
| 19 | closed, 20-membered ring | σ1 2.7 ± 0.9; σ2 1.0 ± 0.3; BZP 3.6 ± 0.8; 5HT2B 2.0 ± 0.3; TSPO 2.1 ± 0.1 |
Data generated by PDSP.42 A total of 45 receptors, channels, and transporters (Supporting Information) were assayed. If not listed here, there was <50% inhibition at 10 μM.
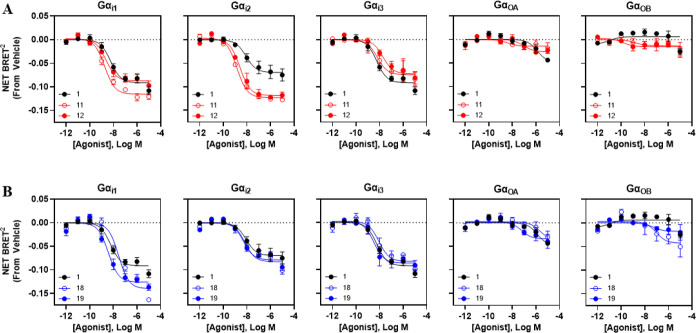
Functional activity of the macrocyclic compounds 12 and 19 having the highest hA3AR binding affinity was assessed in comparison to their respective open-chain precursors (11 and 18) and the reference agonist 1 in bioluminescence resonance energy transfer (BRET)-based G protein coupling and β-arrestin2 translocation assays (Figure 2, Table 3). This was important to address because it was not known a priori whether they would retain agonist activity. The G protein coupling assays measured reduction in net BRET2 ratios for increasing ligand concentration in HEK293T cells expressing various Gα-Rluc8 fusion proteins and a Gβ1-GFP fusion protein, along with Gγ2 and the hA3AR.43 β-Arrestin2 recruitment was assessed by expressing both the hA3AR-Rluc8 and β-arrestin2-YFP fusion proteins in HEK293T cells and measurement of attainment of a BRET1 signal. We confirmed that macrocycles 12 (Figure 2A) and 19 (Figure 2B) were both able to stimulate receptor activation with G protein coupling profiles similar to 1 (Figure 2), but with some prominent differences. All the tested newly synthesized compounds and 1 coupled with high potency (nM range) to each of the three Gαi isoforms and only weakly coupled with GαoA and GαoB proteins. The EC50 values of 1 were 5.8–8.4 nM for the three Gαi isoforms (compared to its hA3AR binding affinity of 1.4 nM), and some potencies of the macrocycles and their open precursors appeared outside that range. The potencies of macrocycle 12 at all three Gαi isoforms were similar to 1 (within a factor of ∼2–3) despite the weaker radioligand binding hA3AR affinity of 12 (∼10-fold with respect to Gαi1 and Gαi2). Interestingly, the 17-membered C-2 ether macrocycle 12 and its precursor 11 exhibited increased coupling with Gαi2 based on ∼40% greater maximal efficacy compared to 1, with EC50 values of 2.56 and 1.41 nM, respectively. However, the precursor/20-membered macrocycle pair 18 and 19 did not show increased efficacy to activate Gαi2. Strikingly, the cyclization of 18 to form 19 increased the potency at Gαi1 (7.5-fold) and Gαi3 (2.7-fold). All the tested compounds, 11, 12, 18, and 19, also potently stimulated β-arrestin2 recruitment, with EC50 values (nM) 1.30, 5.17, 4.35, and 4.36, respectively, and with nearly 50% greater efficacy compared to 1 (Figure 3), suggesting that, with respect to G protein-dependent vs G protein-independent signaling, the macrocyclic compounds and their precursors function more as balanced agonists than 1.
Figure 2.
Pharmacological results comparing activation of various G proteins. Data are presented as the change in net BRET2 values compared to vehicle. Emax and EC50 values are provided in Table 3.
Table 3. Pharmacological Parameters for the Curves Shown in Figures 2 and 3a.
Red (decrease) and green (increased effect) indicate calculated statistical differences in potency or efficacy compared to compound 1 by one-way ANOVA with Bonferroni correction post hoc t-tests. Statistical analysis could not be performed on effects at GαOA (due to a large potency spread) or at GαOB (not feasible to calculate a curve for the reference compound). Methods were according to Fisher et al.25
Figure 3.

Pharmacological results comparing activation of β-arrestin2. Data are presented as the change in net BRET1 compared to vehicle. Emax and EC50 values are provided in Table 3.
Discussion
Initially, the hA3AR interaction of the proposed macrocycle 12 was explored through molecular modeling (Figure 4). The positions of the extended N6 and C2 substituents of known A3AR agonists such as 4b, when receptor-bound, were predicted to be akin to one another, and the proximity was sufficient to propose a covalent bridge between these substituents. Thus, based on the modeling, we predicted that the closure of large macrocyclic (17- to 20-membered) rings would not have a detrimental effect on receptor recognition. In fact, closing the macrocyclic rings might offer an energetic advantage by reducing the entropic cost of the highly flexible N6 and C2 chains during receptor binding.
Figure 4.

Structures of the two most potent macrocyclic derivatives, 12 (MRS7735) and 19 (MRS8033). Predicted interactions between 12 and TMs 3, 6, and 7 and ELs 2 and 3 of the hA3AR are shown.
While we have extensively explored the effects of small bridging ring constraints on the ribose moiety,7−9,13 we now progress to macrocyclic derivatives of AR agonists. Hydrophobic substituents at the N6 and C2 positions of adenosine derivatives tend to favor AR binding affinity.1 2-Phenylethylamine moieties, including at the N6 position, are a common motif in AR ligands, and numerous such AR agonists also bearing a C2-ether or C2-alkyne have been reported.9−11,13 A hypothetical spatial proximity of these two groups in the receptor-bound conformation of AR agonists, that is, an overlapping nonpolar binding region, has been proposed long before structural information was available for the ARs.44−46 The interaction of both of these substituents with extracellular loops (ELs) in A1, A2A, and A3ARs, particularly EL2, was discovered initially using site-directed mutagenesis46−48 and later confirmed with the agonist-bound A2AAR structure.14,49 Currently, the state of structure-based drug discovery for ARs is excellent,1,50,51 due to a high conservation of essential amino acid side chains in the well-characterized binding site. We have confirmed the predicted spatial proximity of receptor-bound N6 and C2 substituents by bridging them covalently in novel macrocyclic A3AR agonists.
Macrocycles, in general, were described by Driggers et al. in 2008 as “underexploited” in medicinal chemistry.52 However, they are now finding application in the development of anticancer drugs, antidiabetic agents, CNS drugs, and so forth.53−56 For example, a macrocyclic anaplastic lymphoma kinase (ALK) inhibitor that has a constrained and compact conformation was shown to penetrate the BBB despite a molecular weight of 450 g/mol.53 Macrocyclization of biologically active peptides using a hydrophobic thiol-reactive cross-linker enhanced their ability to penetrate the BBB.56 Nucleosides including AR agonists typically have limited ability to cross the BBB.57 Although we do not know the tissue distribution of the current macrocycles, there are several potential applications of A3AR ligands that are able to access the CNS including pain, stroke, and traumatic brain injury.2,57
Different chain components and attachment chemistries to form macrocycles were compared. We initially determined the binding affinity at both human and mouse ARs because of the noted species dependence of affinity of A3AR nucleoside agonists, as well as antagonists.10 Macrocyclic derivatives 12 and 19 containing a 5′-methylamide and ether linkage to the N6-(2-phenylethyl) substituent displayed high hA3AR affinity (Ki 25.8 and 22.1 nM, respectively) and moderate affinity at the mA3AR (356 and 648 nM), despite their high molecular weights, 551 and 623 g/mol, respectively, and the steric constraint of the terminal ends of N6 and C2 chains. Upon ring closure, compared to the diallyl precursors, 11 and 18, the hA3AR affinity of 12 was reduced by only 2.4-fold and the affinity of 19 by 2.6-fold. Curiously, compound 16, which is related to A3AR-selective 2-alkylethynyl reference agonists such as MRS5151 (Table S1, Supporting Information),58 was balanced in affinity at the A2AAR and A3AR. There could be a therapeutic application of mixed A2AAR and A3AR agonists, both of which have anti-inflammatory effects.
The closure of the macrocyclic rings converts relatively flexible nucleoside precursors, particularly 11, 13, and 15, to compact and less variable conformations. This conformational consolidation might alter their pharmacokinetic properties (not studied here), as well as pharmacodynamic activity. The cLogP values of 12 and 19 are −0.17 and 1.69, respectively, in comparison to the corresponding open-chain di-allyl analogues 11 and 18 with 0.63 and 2.21, respectively. Additional predicted physicochemical parameters (Table S2, Supporting Information) indicated reduced flexibility and Log D for 12 and 19 compared to 1.
We have shown that the two macrocyclic derivatives 12 and 19 maintain agonist activity and that the signaling profile of each of the derivatives differs from that of the reference agonist 1. The 2-aryl-ethynyl group of 19 resulted in higher hA3AR selectivity (>500-fold) compared to 2-ether-linked 12, similar to the favorable selectivity of uncyclized 2-aryl-ethynyl reference compound 4b and its congeners.8,10,13 In terms of Gαi2 coupling and β-arrestin2 translocation, derivative 12’s signaling efficacy for these two pathways exceeds that of reference agonist 1. On the other hand, derivative 19 exhibits relatively increased signaling efficacy for Gαi1 and β-arrestin2 in comparison to 1. The ring closure directly affects conformational limitations of the outer regions of the A3AR but not the cytosolic side where G proteins and β-arrestin2 interact. Thus, the differences in functional coupling between the various A3AR agonists must be through conformational changes that are propagated from the extracellular regions of the receptor toward the cytosolic interface in a characteristic manner for each analogue.
The macrocycle 14 containing a 5′-CH2OH displayed significantly lower A3AR affinity than the potent 5′-methylamide derivatives, suggesting that the multiply H-bonding methylamide serves as a more effective anchor in the hydrophilic subpocket of the orthosteric binding site. The ester-containing macrocycle 20, which is only H-bond accepting, had even lower A3AR affinity. The macrocycles of highest affinity contained an N6-(2-phenylethyl) moiety, but it was not possible to precisely compare congeners with different numbers of methylene groups in the N6 substituent.
There are other examples in the GPCR literature of ELs and upper parts of the TMs affecting biased signaling.59 Conformational cross-talk between the drug binding site that is in contact with the ELs and the G protein interface is essential for receptor activation. Thus, any conformational perturbation in the EL-pointing regions of the receptor can potentially affect signaling preference. Recently, a structural change in the outward-pointing N6 group of an A1AR agonist induced selectivity for GoB activation among six Gαi/o subtypes and lack of β-arrestin recruitment.60
A3AR agonists are being developed for cancer and various inflammatory and ischemic conditions, including chronic pain, stroke, and traumatic brain injury.2,6,57 The apparent preference of the macrocyclic derivative 12 and its precursor 11 for Gαi2 suggests that there might be a therapeutic advantage, potentially for cancer therapeutics. The corresponding gene GNAI2 promotes IL-1β release and an M1 phenotype in bone marrow-derived macrophages, which makes it highly relevant to inflammation and allergy.61 Both ADORA3 and GNAI2 are highly expressed in microglial cells, which are activated in brain injury.62
In conclusion, by forming macrocyclic rings linking the N6 and C2 positions of adenine, we have altered the physicochemical and pharmacological properties of (N)-methanocarba derivatives as A3AR agonists. A 2-ether macrocycle 12 and a more A3AR-selective C2-arylethynyl derivative 19 were compared in binding and functional assays. There are indications of biased signaling by these derivatives when multiple G protein and β-arrestin pathways are compared, either differences between the open and closed chain forms or between those derivatives and the reference agonist 1. Varying degrees of improvement in signaling efficacy toward Gαi1, Gαi2, and β-arrestin2 occurred. The relationship of the pharmacological profile to conformational factors and to disease-related pathways, as well as using macrocyclization to alter BBB permeability, improves absorption, increases bioavailability, and will be the subject of future studies. These initial macrocyclic derivatives can serve as a guide for the future design of macrocyclic AR agonists that display unanticipated pharmacological parameters.
Materials and Methods
Molecular Modeling
Protein Preparation
The structure of hA3AR was retrieved from a previous work,9 where it was obtained by homology modeling using as templates the X-ray structures of an agonist-bound intermediate state A2AAR for most of the receptor (3QAK14 and 4UHR(49)) and of an antagonist-bound A1AR (5UEN15) for the tip of TM2. The structure was prepared using the Protein Preparation Wizard63 tool of the Schrödinger64 (New York, NY, USA) suite, assigning the following tautomeric states to histidine (residue numbers): HSD 272 and HSE 79, 95, 124, 158 (according to CHARMM nomenclature).
Molecular Docking
Compounds 5 and 12 were docked at the hA3AR putative orthosteric binding site using Glide65,66-XP,67 using F168 and N250 to define the center of the grid, whose inner and outer dimensions were 10 and 30 Å, respectively. A maximum of 20 poses per compound was generated, and the macrocycles sampling through Prime was included in the case of compound 12. One pose per compound was selected by visual inspection.
Molecular Dynamics
The selected complexes between hA3AR and compounds 5 and 12 were prepared for molecular dynamics simulations. After adjusting the orientation according to “Positioning of Proteins in Membrane (PPM)” Web server,68 the complexes were inserted into a 90 × 90 Å 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) lipid bilayer generated with the VMD membrane plugin.69 ACE and CT3 cappings were inserted at the N-/C-termini. The simulation boxes were solvated with TIP3P water molecules, and Na+/Cl– ions were added to neutralize the system and to bring the concentration to 0.154 M. The force field CHARMM3670,71 was used for proteins, lipids, water, ions, and CGenFF72,73 for ligands. Missing ligand parameters were assigned using the ParamChem Web service,74 with few modifications on the (N)-methanocarba which was manually adjusted by analogy to the CGenFF carbocyclic parameters. HTMD75 was used to prepare the systems and ACEMD376 to run the simulations.
The prepared systems were subjected to 500 steps of minimization and 40 ns equilibration in the NPT environment (310 K (Langevin thermostat, a damping constant of 1 ps–1) and 1 atm (Monte Carlo barostat)). During the first 20 ns of the equilibration phase, restraints were placed on protein and ligand atoms (1 kcal mol–1 Å–2 to protein Cα and ligand atoms, 0.5 kcal mol–1 Å–2 to other protein atoms) and linearly reduced.
Thirty nanoseconds MD simulations were run in the NVT ensemble, fixing the temperature to 310 K (a damping constant of 0.1 ps–1). A timestep of 4 fs was employed. Simulations were run in triplicates.
A 9 Å cutoff was employed for nonbonded interactions, a switching distance of 7.5 Å for van der Waals interactions, and the particle-mesh Ewald (PME) was employed for long-range electrostatic interactions. The NIH HPC Biowulf cluster,79 with NVIDIA Tesla V100 GPUs, was used to run the simulations.
Trajectory Analysis
Protein alignments and RMS computations were performed using Wordom.77 H-bonds between the ligand and the surrounding residues (within 4 Å) were computed using the Hbonds plugin of VMD 1.9.3 (using a distance of 3.5 Å and an angle of 30°). Clustering was performed using the QT-like algorithm of Wordom (1 Å as cutoff value). The ligand adenine scaffold was aligned, and the trajectory clustered on the basis of the RMSD of the phenethyl coordinates. The center of the most populated clusters was selected for the manuscript. Dihedral angles were measured using the module ProDy.78 Plots were generated with gnuplot, matplotlib, and seaborn.
Chemical Synthesis
Materials and Instrumentation
All reagents and solvents were purchased from Sigma-Aldrich (St. Louis, MO). 1H NMR spectra were obtained with a Bruker 400 spectrometer using CDCl3, CD3OD, and DMSO as solvents. Chemical shifts are expressed in δ values (ppm) with tetramethylsilane (δ 0.00) for CDCl3 and DMSO, and water (δ 3.30) for CD3OD. NMR spectra were collected with a Bruker AV spectrometer equipped with a z-gradient [1H, 13C, 15N]-cryoprobe. TLC analysis was carried out on glass sheets precoated with silica gel F254 (0.2 mm) from Aldrich. The purity of final nucleoside derivatives was checked using a Hewlett–Packard 1100 HPLC equipped with a Zorbax SB-Aq 5 μm analytical column (50 × 4.6 mm; Agilent Technologies Inc., Palo Alto, CA). Mobile phase: linear gradient solvent system, 5 mM TBAP (tetrabutylammonium dihydrogenphosphate) −CH3CN from 80:20 to 0:100 in 13 min; the flow rate was 0.5 mL/min. Peaks were detected by UV absorption with a diode array detector at 230, 254, and 280 nm. All derivatives tested for biological activity showed >95% purity by HPLC analysis (detection at 254 nm). Low-resolution mass spectrometry was performed with a JEOL SX102 spectrometer with 6 kV Xe atoms following desorption from a glycerol matrix or on an Agilent LC/MS 1100 MSD, with a Waters (Milford, MA) Atlantis C18 column. High-resolution mass spectroscopic (HRMS) measurements were performed on a proteomics optimized Q-TOF-2 (Micromass-Waters) using external calibration with polyalanine, unless noted. Observed mass accuracies are those expected based on the known performance of the instrument as well as trends in masses of standard compounds observed at intervals during the series of measurements. Reported masses are observed masses uncorrected for this time-dependent drift in mass accuracy. All of the monosubstituted alkyne intermediates were purchased from Sigma-Aldrich (St. Louis, MO), Small Molecules, Inc. (Hoboken, NJ), Anichem (North Brunswick, NJ), PharmaBlock, Inc. (Sunnyvale, CA), Frontier Scientific (Logan, UT), and Tractus (Perrineville, NJ). Grubbs second generation catalyst was purchased from Sigma-Aldrich (M204). cLog P values were calculated using ChemDraw (v. 21.0 for MAC, PerkinElmer, Waltham, MA, USA). Prediction of Log D, TPSA, and BBB log([brain]/[blood]), and additional parameters was calculated using the StarDrop software (v. 7.3.2).41
Intermediates 22 and 32 were synthesized as reported (Tosh et al., 2009).58 Intermediate 27 was prepared as reported (Tosh et al., 2015).8 Refer to Scheme 1 for compounds 11, 12, 24a, 24b, 25, and 26. Refer to Scheme 2 for compounds 13, 14, and 28–31. Refer to Scheme 3 for compounds 15, 16, 20, 21, and 33–35. Refer to Scheme 4 for compounds 17–19 and 36–38.
General Method for Deprotection of an Isopropylidene Group
(1S,2R,3S,4R,5S)-4-(2-(But-3-en-1-yloxy)-6-((3-(but-3-en-1-yloxy)-4-methoxy phenethyl)amino)-9H-purin-9-yl)-2,3-dihydroxy-N-methylbicyclo[3.1.0]hexane-1-carboxamide (11)
A solution of compound 25 (15 mg, 0.02 mmol) in methanol (1 mL) and 10% aq. TFA (1 mL) was heated at 70 °C overnight. Solvent was evaporated under vacuum, and the residue was purified on flash silica gel column chromatography (CH2Cl2/MeOH = 20:1) to provide the compound 11 (12 mg, 85%) as colorless syrup. 1H NMR (CD3OD, 400 MHz): δ 7.85 (s, 1H), 6.88–6.80 (m, 3H), 6.01–5.86 (m, 2H), 5.21–5.02 (m, 4H), 4.72 (s, 1H), 4.42–4.34 (m, 2H), 4.02 (d, J = 6.8 Hz, 1H), 3.96 (t, J = 6.8 Hz, 2H), 3.79 (s, 3H), 2.91 (t, J = 7.2 Hz, 2H), 2.85 (s, 3H), 2.58–2.53 (m, 2H), 2.51–2.46 (m, 2H), 2.09–2.06 (m, 1H), 1.80 (t, J = 5.2 Hz, 1H), 1.36–1.30 (m, 1H). HRMS calculated for C30H39N6O6 (M + H)+, 579.2931; found, 579.2931.
(1S,2R,3S,4R,5S)-2,3-Dihydroxy-4-((E)-54-methoxy-19H-6,13-dioxa-2-aza-1(6,2)-purina-5(1,3)-benzenacyclotridecaphan-9-en-19-yl)-N-methylbicyclo[3.1.0]hexane-1-carboxamide (12)
Grubbs second generation catalyst (34 mg, 0.016 mmol) was added to a solution of compound 25 (25 mg, 0.04 mmol) in dry dichloromethane (180 mL), and the mixture was stirred at room temperature for 24 h. Solvent was evaporated under vacuum, and the residue was roughly purified on flash silica gel column chromatography (CH2Cl2/MeOH = 35:1) to give the compound 26 (20 mg),which was dissolved with MeOH (2.0 mL) and 10% aq. TFA (2.0 mL) and the resulting mixture was heated at 70 °C for 4 h. Solvent was evaporated under vacuum, and the residue was purified on flash silica gel column chromatography (CH2Cl2/MeOH = 20:1) to afford the 17-membered macrocyclic derivative 12 (9.5 mg, 54%) as a colorless syrup. 1H NMR (CD3OD, 400 MHz): δ 7.85 (s, 1H), 6.84–6.71 (m, 3H), 5.78–5.68 (m, 2H), 5.03 (d, J = 6.4 Hz, 1H), 4.71 (s, 1H), 4.54 (t, J = 7.6 Hz, 2H), 4.04–3.98 (m, 3H), 3.80 (s, 3H), 2.86–2.83 (m, 4H), 2.57–2.55 (m, 2H), 2.41–2.30 (m, 2H), 2.08–2.05 (m, 1H), 1.80 (t, J = 4.8 Hz, 1H), 1.37–1.30 (m, 1H). HRMS calculated for C28H35N6O6 (M + H)+, 551.2618; found, 551.2625.
(1R,2R,3S,4R,5S)-4-(2-(But-3-en-1-yloxy)-6-((3-(3-(but-3-en-1-yloxy)-4-methoxyphenyl) propyl)amino)-9H-purin-9-yl)-1-(hydroxymethyl)bicyclo[3.1.0]hexane-2,3-diol (13)
Compound 13 (90%) was prepared from compound 30 following the same method as for compound 11. 1H NMR (CD3OD, 400 MHz): δ 8.28 (s, 1H), 6.86–6.75 (m, 3H), 5.98–5.88 (m, 2H), 5.17–5.06 (m, 4H), 4.77 (d, J = 6.8 Hz, 1H), 4.73 (s, 1H), 4.34 (t J = 6.8 Hz, 2H), 4.28 (d, J = 11.6 Hz, 1H), 3.98 (d, J = 6.8 Hz, 2H), 3.87 (d, J = 6.4 Hz, 1H), 3.80 (s, 3H), 3.58 (br s, 2H), 2.68 (t, J = 7.2 Hz, 2H), 2.55–2.48 (m, 4H), 2.02–1.95 (m, 2H), 1.63–1.60 (m, 1H), 1.53 (t, J = 5.2 Hz, 1H), 0.75–0.72 (m, 1H). HRMS calculated for C30H40N5O6 (M + H)+, 566.2979; found, 566.2980.
(1R,2R,3S,4R,5S)-1-(Hydroxymethyl)-4-((E)-64-methoxy-19H-7,14-dioxa-2-aza-1(6,2)-purina-6(1,3)-benzenacyclotetradecaphan-10-en-19 yl)bicyclo[3.1.0]hexane-2,3-diol (14)
Compound 14 (92%) was prepared from compound 31 following the same method as for compound 11. 1H NMR (CD3OD, 400 MHz): δ 8.40 (s, 1H), 6.86–6.74 (m, 3H), 5.68–5.66 (m, 2H), 4.81 (s, 1H), 4.78 (d, J = 6.4 Hz, 1H), 4.48–4.47 (m, 2H), 4.28 (d, J = 11.6 Hz, 1H), 3.92–3.90 (m, 3H), 3.78 (s, 3H), 3.49–3.48 (m, 2H), 2.72–2.69 (m, 2H), 2.49–2.47 (m, 2H), 2.05–2.03 (m, 2H), 1.63–1.60 (m, 1H), 1.54 (t, J = 4.8 Hz, 1H), 0.78–0.75 (m, 1H). HRMS calculated for C28H36N5O6 (M + H)+, 538.2666; found, 538.2668.
General Method for Conversion of Esters to N-Methylamides
(1S,2R,3S,4R,5S)-4-(2-(Hex-5-en-1-yn-1-yl)-6-((3-(hex-5-en-1-yn-1-yl)benzyl)amino)-9H-purin-9-yl)-2,3-dihydroxy-N-methylbicyclo[3.1.0]hexane-1-carboxamide (15)
The MeNH2 solution (1.0 mL, 40%) was added to a solution of ester derivative 20 (6.5 mg, 0.011 mmol) in MeOH (1.0 mL), and the mixture was stirred at room temperature overnight. The solvent was evaporated under vacuum, and the residue was purified on flash silica gel column chromatography (CH2Cl2/MeOH = 40:1) to yield the N-methylamide derivative 15 (4 mg, 68%) as a colorless syrup. 1H NMR (CD3OD, 400 MHz): δ 8.08 (s, 1H), 7.40 (s, 1H), 7.33–7.25 (m, 3H), 6.03–5.88 (m, 2H), 5.19–5.01 (m, 5H), 4.85 (s, 1H), 4.78 (br s, 2H), 4.00 (d, J = 7.2 Hz, 1H), 2.87 (s, 3H), 2.55 (t, J = 7.2 Hz, 2H), 2.47 (t, J = 7.2 Hz, 2H), 2.43–2.38 (m, 2H), 2.35–2.30 (m, 2H), 2.11–2.08 (m, 1H), 1.87 (t, J = 4.8, Hz, 1H), 1.40–1.37 (m, 1H). HRMS calculated for C32H35N6O3 (M + H)+, 551.2771; found, 551.2772.
Macrocyclic-5′-Methyl Amide Derivative (16)
Compound 16 (73%) was prepared from compound 21 following the same method as for compound 15. 1H NMR (CD3OD, 400 MHz): δ 8.07 (s, 1H), 8.02 (s, 1H), 7.31–7.18 (m, 3H), 5.88–5.84 (m, 2H), 5.03 (d, J = 7.6 Hz, 1H), 4.80 (s, 1H), 3.97 (d, J = 6.8 Hz, 1H), 2.87 (s, 3H), 2.65–2.62 (m, 2H), 2.50–2.41 (m, 4H), 2.31–2.27 (m, 2H), 2.07–2.04 (m, 1H), 1.83 (t, J = 4.8 Hz, 1H), 1.38–1.34 (m, 1H). HRMS calculated for C30H31N6O3 (M + H)+, 523.2458; found, 523.2461.
(1S,2R,3S,4R,5S)-2,3-Dihydroxy-4-(6-((3-hydroxy-4-methoxyphenethyl)amino)-2-((3-hydroxyphenyl)ethynyl)-9H-purin-9-yl)-N-methylbicyclo[3.1.0]hexane-1-carboxamide (17)
Compound 17 (91%) was prepared from compound 36 following the same method as for compound 11. 1H NMR (CD3OD, 400 MHz): δ 8.14 (s, 1H), 7.26 (t, J = 8.0 Hz, 1H), 7.14 (d, J = 7.6 Hz, 1H), 7.07 (s, 1H), 6.91 (d, J = 8.0 Hz, 1H), 6.84 (d, J = 8.0 Hz, 1H), 6.77 (s, 1H), 6.74 (d, J = 8.0 Hz, 1H), 5.06 (d, J = 6.4 Hz, 1H), 4.91 (s, 1H), 4.03 (d, J = 6.4 Hz, 1H), 3.88–3.82 (m, 5H), 2.89 (t, J = 7.2 Hz, 2H), 2.85 (s, 3H), 2.13–2.10 (m, 1H), 1.88 (t, J = 4.8 Hz, 1H), 1.41–1.38 (m, 1H). HRMS calculated for C30H31N6O6 (M + H)+, 571.2305; found, 571.2302.
(1S,2R,3S,4R,5S)-4-(6-((3-(Allyloxy)-4-methoxyphenethyl)amino)-2-((3-(allyloxy)phenyl) ethynyl)-9H-purin-9-yl)-2,3-dihydroxy-N-methylbicyclo[3.1.0]hexane-1-carboxamide (18)
Compound 18 (90%) was prepared from compound 37 following the same method as for compound 11. 1H NMR (CD3OD, 400 MHz): δ 8.12 (s, 1H), 7.35 (d, J = 8.0 Hz, 1H), 7.24–7.22 (m, 2H), 7.06 (d, J = 8.0 Hz, 1H), 6.91 (s, 1H), 6.88–6.83 (m, 2H), 6.14–5.99 (m, 2H), 5.46–5.17 (m, 4H), 5.06 (d, J = 6.4 Hz, 1H), 4.91 (s, 1H), 4.62 (d, J = 5.2 Hz, 2H), 4.52 (d, J = 5.2 Hz, 2H), 4.02 (d, J = 6.4 Hz, 1H), 3.87 (br s, 2H), 3.80 (s, 3H), 2.93 (t, J = 7.2 Hz, 2H), 2.85 (s, 3H), 2.12–2.09 (m, 1H), 1.88 (t, J = 4.8 Hz, 1H), 1.41–1.38 (m, 1H). HRMS calculated for C36H39N6O6 (M + H)+, 651.2931; found, 651.2942.
Macrocyclic-5′-Methyl Amide Derivative (19)
Grubbs second generation catalyst (8 mg, 0.009 mmol) was added to a solution of compound 37 (16 mg, 0.023 mmol) in dry dichloromethane (116 mL) and heated at 60 °C overnight. The solvent was evaporated under vacuum, and the residue was roughly purified on flash silica gel column chromatography (CH2Cl2/MeOH = 35:1) to give the compound 38 (10 mg),which was dissolved with MeOH (1.0 mL) and 10% aq. TFA (1.0 mL), and the resulting mixture was heated at 70 °C for 4 h. The solvent was evaporated under vacuum, and the residue was purified on flash silica gel column chromatography (CH2Cl2/MeOH = 20:1) to afford the 20-membered macrocyclic derivative 19 (6 mg, 43%) as a colorless syrup. 1H NMR (CD3OD, 400 MHz): δ 8.12 (s, 1H), 7.41 (s, 1H), 7.31 (t, J = 8.0 Hz, 1H), 7.18–7.17 (m, 1H), 7.01 (d, J = 8.0 Hz, 1H), 6.92 (d, J = 8.0 Hz, 1H), 6.86 (d, J = 7.6 Hz, 1H), 6.77–6.76 (m, 1H), 5.99–5.90 (m, 2H), 5.08 (d, J = 6.8 Hz, 1H), 4.91–4.81 (m, 3H), 4.79 (d, J = 4.4 Hz, 2H), 4.05 (d, J = 7.2 Hz, 1H), 3.83 (s, 3H), 3.75–3.71 (m, 2H), 2.99–2.89 (m, 5H), 2.12–2.10 (m, 1H), 1.89 (t, J = 4.8 Hz, 1H), 1.40–1.37 (m, 1H). HRMS calculated for C34H35N6O6 (M + H)+, 623.2618; found, 623.2615.
Ethyl (1S,2R,3S,4R,5S)-4-(2-(hex-5-en-1-yn-1-yl)-6-((3-(hex-5-en-1-yn-1-yl)benzyl)amino)-9H-purin-9-yl)-2,3-dihydroxybicyclo[3.1.0]hexane-1-carboxylate (20)
Compound 20 (91%) was prepared from compound 34 following the same method as for compound 11. 1H NMR (CD3OD, 400 MHz): δ 7.98 (s, 1H), 7.39 (s, 1H), 7.32–7.24 (m, 3H), 6.03–5.87, 5.18–5.02 (m, 5H), 4.82 (s, 1H), 4.78 (br s, 2H), 4.27–4.21 (m, 2H), 4.09 (d, J = 6.8 Hz, 1H), 2.55 (t, J = 7.2 Hz, 2H), 2.49 (t, J = 7.2 Hz, 2H), 2.45–2.38 (m, 2H), 2.35–2.30 (m, 2H), 2.22–2.20. (m, 1H), 1.93 (t, J = 5.2 Hz, 1H), 1.66–1.63 (m, 1H), 1.31 (t, J = 7.2 Hz, 3H). HRMS calculated for C33H36N5O4 (M + H)+, 566.2767; found, 566.2765.
Macrocyclic-5′-Ethyl Ester Derivative (21)
Compound 21 (92%) was prepared from compound 35 following the same method as for compound 11. 1H NMR (CD3OD, 400 MHz): δ 8.07 (s, 1H), 7.94 (s, 1H), 7.31–7.18 (m, 3H), 5.87–5.83 (m, 2H), 5.15 (d, J = 6.8 Hz, 1H), 4.78 (s, 1H), 4.63 (d, J = 6.0 Hz, 1H), 4.27–4.22 (m, 2H), 4.06 (d, J = 6.8 Hz, 1H), 2.63–2.60 (m, 2H), 2.50–2.42 (m, 4H), 2.30–2.27 (m, 2H), 2.20–2.17 (m, 1H), 1.91 (t, J = 5.2 Hz, 1H), 1.64–1.60 (m, 1H), 1.30 (t, J = 7.2 Hz, 3H). HRMS calculated for C31H32N5O4 (M + H)+, 538.2454; found, 538.2451.
General Method for Alkylation of 3-Hydroxyphenyl Group
(3aR,3bS,4aS,5R,5aS)-5-(6-((3-(But-3-en-1-yloxy)-4-methoxyphenethyl)amino)-2-iodo-9H-purin-9-yl)-N,2,2-trimethyltetrahydrocyclopropa[3,4]cyclopenta[1,2-d][1,3]dioxole-3b(3aH)-carboxamide (24a)
K2CO3 (162 mg, 1.17 mmol) and 4-bromobutene (71 μL, 0.66 mmol) were added to a solution of compound 23 (73 mg, 0.11 mmol) in dry DMF (2.0 mL) and heated at 90 °C overnight. The reaction mixture was quenched by water, and an aqueous layer was extracted with ethyl acetate (3 times). Combined organic layer was dried over Na2SO4, filtered, and evaporated under vacuum, and the residue was purified on flash silica gel column chromatography (CH2Cl2/MeOH = 40:1) to give the O-alkylated desired product 24a (42 mg, 52%) as a colorless syrup. Further elution of the column with (CH2Cl2/MeOH = 25:1) provided an isopropylidene-deprotected O-alkylated derivative 24b (14 mg, 19%) as a syrup. Data for 24a: 1H NMR (CD3OD, 400 MHz): δ 7.94 (s, 1H), 6.87–6.84 (m, 2H), 6.81–6.79 (m, 1H), 5.97–5.86 (m, 1H), 5.72 (d, J = 6.8 Hz, 1H), 5.16–5.06 (m, 2H), 4.91 (s, 1H), 4.83 (d, J = 6.4 Hz, 1H), 3.99–3.95 (m, 2H), 3.80–3.75 (m, 4H), 2.91–2.86 (m, 4H), 2.52–2.47 (m, 2H), 2.14–2.10 (m, 1H), 1.54–1.49 (m, 4H), 1.38 (t, J = 5.2 Hz, 1H), 1.30 (s, 3H). HRMS calculated for C29H36N6O5I (M + H)+, 675.1792; found, 675.1796. Data for isopropylidene-deprotected O-alkylated derivative [(1S,2R,3S,4R,5S)-4-(6-((3-(but-3-en-1-yloxy)-4-methoxyphenethyl)amino)-2-iodo-9H-purin-9-yl)-2,3-dihydroxy-N-methylbicyclo [3.1.0]hexane-1-carboxamide] 24b: 1H NMR (CD3OD, 400 MHz): δ 7.94 (s, 1H), 6.86–6.80 (m, 3H), 5.97–5.87 (m, 1H), 5.16–5.06 (m, 3H), 4.78 (s, 1H), 4.01–3.98 (m, 3H), 3.80 (s, 3H), 2.90–2.86 (m, 2H), 2.52–2.47 (m, 2H), 2.04–2.01 (m, 1H), 1.81 (t, J = 4.8 Hz, 1H), 1.39–1.34 (m, 1H). HRMS calculated for C26H32N6O5I (M + H)+, 635.1479; found, 635.1487.
General Method for the Conversion of 2-Iodo to an Ether Group
(3aR,3bS,4aS,5R,5aS)-5-(2-(But-3-en-1-yloxy)-6-((3-(but-3-en-1-yloxy)-4-methoxy phenethyl)amino)-9H-purin-9-yl)-N,2,2-trimethyltetrahydrocyclopropa[3,4]cyclopenta[1,2-d][1,3]dioxole-3b(3aH)-carboxamide (25)
A solution of compound 24a (28 mg, 0.041 mmol) in but-3-en-1-ol (1 mL) in the presence of NaH (10 mg, 0.41) was heated at 90 °C for >6 h. The reaction mixture was quenched with water, and the aqueous layer was extracted with ethyl acetate (3 times), dried (Na2SO4) filtered and evaporated. The crude reaction mixture was purified on flash silica gel column chromatography (CH2Cl2/MeOH = 40:1) to provide the compound 25 (25 mg, 97%) as a colorless syrup. 1H NMR (CD3OD, 400 MHz): δ 8.14 (s, 1H), 6.87–6.80 (m, 3H), 6.02–5.8 (m, 2H), 5.69 (d, J = 7.2 Hz, 1H), 5.22–5.02 (m, 4H), 4.90 (s, 1H), 4.84 (d, J = 6.8 Hz, 1H), 4.45–4.29 (m, 2H), 3.95 (t, J = 6.8 Hz, 2H), 3.79 (s, 3H), 2.90 (t, J = 6.8 Hz, 2H), 2.81 (s, 3H), 2.60–2.55 (m, 2H), 2.50–2.45 (m, 2H), 2.22–2.15 (m, 1H), 1.54 (s, 3H), 1.51–1.47 (m, 1H), 1.37 (t, J = 5.2 Hz, 1H), 1.29 (s, 3H). HRMS calculated for C33H43N6O6 (M + H)+, 619.3244; found, 619.3254.
5-(3-((9-((3aR,3bR,4aS,5R,5aS)-3b-(((tert-Butyldiphenylsilyl)oxy)methyl)-2,2-dimethylhexahydrocyclopropa[3,4]cyclopenta[1,2-d][1,3]dioxol-5-yl)-2-iodo-9H-purin-6-yl)amino)propyl)-2-methoxyphenol (28)
5-(3-Aminopropyl)-2-methoxyphenol hydrochloride (318 mg, 1.46 mmol) and DIPEA (0.51 mL, 2.93 mmol) were added to a solution of compound 27 (206 mg, 0.29 mmol) in isopropanol (5 mL) stirred at room temperature overnight. The solvent was evaporated under vacuum, and the residue was purified on flash silica gel column chromatography (hexane/ethyl acetate = 1:1) to give the compound 28 (205 mg, 84%) as colorless syrup. 1H NMR (CD3OD, 400 MHz): δ 8.1 (s, 1H), 7.67–7.62 (m, 4H), 7.43–7.27 (m, 6H), 6.84–6.65 (m, 3H), 5.31 (d, J = 7.2 Hz, 1H), 4.91 (s, 1H), 4.72 (d, J = 6.4 Hz, 1H), 4.22 (d, J = 10.4 Hz, 1H), 3.92 (d, J = 10.4 Hz, 1H), 3.82 (s, 3H), 3.57 (br s, 2H), 2.66 (t, J = 7.6 Hz, 2H), 1.99–1.95 (m, 2H), 1.57–1.53 (m, 4H), 1.27 (s, 3H), 1.12–1.07 (m, 10H), 1.03–0.99 (m, 1H). HRMS calculated for C41H49N5O5SiI (M + H)+, 846.2548; found, 846.2539.
N-(3-(3-(But-3-en-1-yloxy)-4-methoxyphenyl)propyl)-9-((3aR,3bR,4aS,5R,5aS)-3b-(((tert-butyldiphenylsilyl)oxy)methyl)-2,2-dimethylhexahydrocyclopropa[3,4]cyclopenta[1,2-d][1,3]dioxol-5-yl)-2-iodo-9H-purin-6-amine (29)
Compound 29 (73%) was prepared from compound 28 following the same method as for compound 24a. 1H NMR (CD3OD, 400 MHz): δ 8.09 (s, 1H), 7.67–7.62 (m, 4H), 7.42–7.27 (m, 6H), 6.84–6.77 (m, 3H), 5.97–5.87 (m, 1H), 5.30 (d, J = 6.8 Hz, 1H), 5.16–5.04 (m, 2H), 4.90 (s, 1H), 4.73 (d, J = 6.8 Hz, 1H), 4.21 (d, J = 10.8 Hz, 1H), 3.98 (d, J = 6.4 Hz, 2H), 3.89 (d, J = 10.8 Hz, 1H), 3.78 (s, 3H), 3.59–3.56 (m, 2H), 2.68 (d, J = 7.2 Hz, 2H), 2.53–2.48 (m, 2H), 2.04–1.97 (m, 2H), 1.59–1.52 (m, 4H), 1.27 (s, 3H), 1.10–1.07 (m, 10H), 1.00–0.97 (m, 1H). HRMS calculated for C45H55N5O5SiI (M + H)+, 900.3017; found, 900.3008.
2-(But-3-en-1-yloxy)-N-(3-(3-(but-3-en-1-yloxy)-4-methoxyphenyl)propyl)-9-((3aR,3bR,4aS,5R,5aS)-3b-(((tert-butyldiphenylsilyl)oxy)methyl)-2,2-dimethylhexahydro cyclopropa[3,4]cyclopenta[1,2-d][1,3]dioxol-5-yl)-9H-purin-6-amine (30)
Compound 30 (69%) was prepared from compound 29 following the same method as for compound 25. 1H NMR (CD3OD, 400 MHz): δ 8.12 (s, 1H), 7.66–7.64 (m, 4H), 7.45–7.27 (m, 6H), 6.85–6.76 (m, 3H), 5.96–5.81 (m, 2H), 5.38 (d, J = 7.2 Hz, 1H), 5.16–5.02 (m, 4H), 4.93 (s, 1H), 4.75 (d, J = 6.8 Hz, 1H), 4.33–4.18 (m, 3H), 3.96 (t, J = 7.6 Hz, 2H), 3.78 (s, 3H), 3.63–3.58 (m, 3H), 2.71–2.68 (t, J = 7.2 Hz, 2H), 2.52–2.44 (m, 4H), 2.04–1.97 (m, 2H), 1.64–1.62 (m, 1H), 1.52 (s, 3H), 1.27 (s, 3H), 1.32 (t, J = 5.2 Hz, 1H), 1.07 (s, 9H), 0.94–0.91 (m, 1H). HRMS calculated for C49H62N5O6Si (M + H)+, 844.4469; found, 844.4459.
(E)-19-((3aR,3bR,4aS,5R,5aS)-3b-(((tert-Butyldiphenylsilyl)oxy)methyl)-2,2-dimethyl hexahydrocyclopropa[3,4]cyclopenta[1,2-d][1,3]dioxol-5-yl)-64-methoxy-19H-7,14-dioxa-2-aza-1(6,2)-purina-6(1,3)-benzenacyclotetradecaphan-10-ene (31)
Grubbs second generation catalyst (10 mg, 0.012 mmol) was added to a solution of compound 30 (25.6 mg, 0.03 mmol) in dry dichloromethane (136 mL), and the mixture was stirred at room temperature for 5 h. The solvent was evaporated under vacuum, and the residue was roughly purified on flash silica gel column chromatography (hexane/ethyl acetate = 1:1) to afford the compound 31 (11 mg, 45%), as a colorless syrup. 1H NMR (CD3OD, 400 MHz): δ 8.10 (s, 1H), 7.68–7.65 (m, 4H), 7.46–7.32 (m, 6H), 6.84–6.72 (m, 3H), 5.67–5.53 (m, 2H), 5.38 (d, J = 7.2 Hz, 1H), 4.94 (s, 1H), 4.75 (d, J = 6.8 Hz, 1H), 4.33 (t, J = 7.2 Hz, 2H), 4.19 (d, J = 10.8 Hz, 1H), 3.93–3.87 (m, 2H), 3.78 (s, 3H), 3.68 (d, J = 10.8 Hz, 1H), 3.58–3.49 (m, 2H), 2.71–2.69 (m, 2H), 2.50–2.48 (m, 4H), 2.05–2.02 (m, 2H), 1.68–1.65 (m, 1H), 1.53 (s, 3H), 1.29 (s, 3H), 1.15 (t, J = 4.8 Hz, 1H), 1.10 (s, 9H), 0.97–0.91 (m, 1H). HRMS calculated for C47H58N5O6Si (M + H)+, 816.4136; found, 816.4152.
Ethyl (3aR,3bS,4aS,5R,5aS)-5-(2-Iodo-6-((3-iodobenzyl)amino)-9H-purin-9-yl)-2,2-dimethyltetrahydrocyclopropa[3,4]cyclopenta[1,2-d][1,3]dioxole-3b(3aH)-carboxylate (33)
Compound 33 (80%) was prepared from compound 32 following the same method as for compound 28. 1H NMR (CD3OD, 400 MHz): δ 7.93 (s, 1H), 7.77 (s, 1H), 7.58 (d, J = 8.0 Hz, 1H), 7.39 (d, J = 8.0 Hz, 1H), 7.06 (t, J = 8.0 Hz, 1H), 5.82 (d, J = 6.8 Hz, 1H), 4.81 (d, J = 6.8 Hz, 1H), 4.65 (br s, 2H), 4.32–4.23 (m, 2H), 2.26–2.22 (m, 1H), 1.63–1.59 (m, 1H), 1.52–1.47 (m, 4H), 1.33 (t, J = 7.2 Hz, 3H), 1.26 (s, 3H). HRMS calculated for C24H26N5O4I2 (M + H)+, 702.0074; found, 702.0076.
Ethyl (3aR,3bS,4aS,5R,5aS)-5-(2-(Hex-5-en-1-yn-1-yl)-6-((3-(hex-5-en-1-yn-1-yl)benzyl) amino)-9H-purin-9-yl)-2,2-dimethyltetrahydrocyclopropa[3,4]cyclopenta[1,2-d][1,3]dioxole-3b(3aH)-carboxylate (34)
PdCl2(PPh3)2 (19 mg, 0.027 mmol), CuI (2.5 mg, 0.013 mmol), hex-1-en-5-yne (65 mg, 0.81 mmol), and triethylamine (0.18 mL, 1.35 mmol) were added to a solution of compound 33 (95 mg, 0.13 mmol) in anhydrous DMF (2.0 mL), and the mixture stirred at room temperature overnight. The solvent was evaporated under vacuum, and the residue was purified on flash silica gel column chromatography (hexane/ethyl acetate = 1:2) to give the compound 34 (59 mg, 72%) as a syrup. 1H NMR (CD3OD, 400 MHz): δ 8.05 (s, 1H), 7.38 (s, 1H), 7.30–7.22 (m, 3H), 6.04–5.88 (m, 3H), 5.08–5.01 (m, 4H), 4.99 (s, 1H), 4.83 (d, J = 7.2 Hz, 1H), 4.71 (br s, 2H), 4.29–4.15 (m, 2H), 2.55–2.52 (m, 2H), 2.47–2.39 (m, 4H), 2.33 (t, J = 7.2 Hz, 2H), 2.27–2.24 (m, 1H), 1.68–1.64 (m, 1H), 1.54–1.52 (m, 4H), 1.31–1.26 (m, 6H). HRMS calculated for C36H40N5O4 (M + H)+, 606.3080; found, 606.3081.
Isopropylidine-Protected Macrocyclic-5′-Ester Derivative (35)
Compound 35 (54%) was prepared from compound 34 following the same method as for compound 31. 1H NMR (CD3OD, 400 MHz): δ 8.08 (s, 1H), 8.01 (s, 1H), 7.30–7.17 (m, 3H), 5.89–5.83 (m, 2H), 4.97 (s, 1H), 4.79 (d, J = 7.2 Hz, 1H), 4.62 (br s, 2H), 4.32–4.19 (m, 2H), 2.64–2.61 (m, 2H), 2.50–2.44 (m, 4H), 2.30–2.24 (m, 3H), 1.67–1.63 (m, 1H), 1.56–1.52 (m, 4H), 1.33 (t, J = 7.2 Hz, 3H), 1.27 (s, 3H). HRMS calculated for C34H36N5O4 (M + H)+, 578.2767; found, 578.2763.
(3aR,3bS,4aS,5R,5aS)-5-(6-((3-Hydroxy-4-methoxyphenethyl)amino)-2-((3-hydroxyphenyl) ethynyl)-9H-purin-9-yl)-N,2,2-trimethyltetrahydrocyclopropa[3,4]cyclopenta[1,2-d][1,3]dioxole-3b(3aH)-carboxamide (36)
Compound 36 (70%) was prepared from compound 23 following the same method as for compound 34. 1H NMR (CD3OD, 400 MHz): δ 8.10 (s, 1H), 7.25 (t, J = 7.6 Hz, 1H), 7.18 (d, J = 7.6 Hz, 1H), 7.11 (s, 1H), 6.90 (d, J = 6.4 Hz, 1H), 6.87 (d, J = 8.0 Hz, 1H), 6.77 (s, 1H), 6.73 (d, J = 8.0 Hz, 1H), 5.79 (d, J = 7.2 Hz, 1H), 5.01 (s, 1H), 3.85–3.81 (m, 5H), 2.88 (t, J = 7.2 Hz, 2H), 2.79 (s, 3H), 2.17–2.13 (m, 1H), 1.54–1.53 (m, 4H), 1.41 (t, J = 5.2 Hz, 1H), 1.32 (s, 3H). HRMS calculated for C33H35N6O6 (M + H)+, 611.2618; found, 611.2625.
(3aR,3bS,4aS,5R,5aS)-5-(6-((3-(Allyloxy)-4-methoxyphenethyl)amino)-2-((3-(allyloxy) phenyl)ethynyl)-9H-purin-9-yl)-N,2,2-trimethyltetrahydrocyclopropa[3,4]cyclopenta[1,2-d][1,3]dioxole-3b(3aH)-carboxamide (37)
K2CO3 (138 mg, 1.0 mmol) and allyl bromide (50 μL, 0.6 mmol) were added to a solution of compound 36 (61 mg, 0.1 mmol) in dry DMF (2 mL) and heated at 70 °C overnight. The reaction mixture was quenched by water, and the aqueous layer was extracted with ethyl acetate (3 times). Combined organic layer was dried over Na2SO4, filtered, and evaporated under vacuum, and the residue was purified on flash silica gel column chromatography (CH2Cl2/MeOH = 35:1) to give the diallylated derivative 37 (36 mg, 53%) as a colorless syrup. 1H NMR (CD3OD, 400 MHz): δ 8.12 (s, 1H), 7.36 (d, J = 8.0 Hz, 1H), 7.29–7.27 (m, 2H), 7.06–7.04 (m, 1H), 6.90 (s, 1H), 6.87–6.82 (m, 2H), 6.14–5.97 (m, 2H), 5.80 (d, J = 7.2 Hz, 1H), 5.46–5.17 (m, 4H), 5.01 (s, 1H), 4.62 (d, J = 4.8 Hz, 2H), 4.50 (d, J = 4.8 Hz, 2H), 3.86 (br s, 2H), 3.79 (s, 3H), 2.92 (t, J = 7.2 Hz, 2H), 2.79 (d, J = 4.4 Hz, 3H), 2.16–2.12 (m, 1H), 1.59–1.54 (m, 4H), 1.41 (t, J = 5.2 Hz, 1H), 1.30 (s, 3H). HRMS calculated for C39H43N6O6 (M + H)+, 691.3244; found, 691.3246.
Pharmacology
Competitive Radioligand Binding Assays
The radioligands used for standard binding inhibition assays in membranes from HEK293T cells expressing an AR were (abbreviation, concentration used (nM), KD value (nM), specific activity): [3H]N6-R-phenylisopropyladenosine ([3H]R-PIA, 1.0, 1.5, and 14.8 Ci/mmol), [3H]-2-[p-(2-carboxyethyl)phenylethylamino]-5′-N-ethylcarboxamido-adenosine ([3H]CGS21680, 10, 16.2, and 30.5 Ci/mmol), and [125I]N6-(4-amino-3-iodobenzyl)adenosine-5′-N-methyluronamide ([125I]I-AB MECA, 0.2, 1.22, and 2170 Ci/mmol) for A1, A2A, and A3 receptors, respectively. These radioligands were purchased from PerkinElmer (Waltham, MA, USA). Nonspecific binding was determined using adenosine 5′-N-ethyluronamide (10 μM).
G Protein Coupling and β-arrestin2 Translocation Assays
Procedures for the BRET-based G protein coupling and β-arrestin2 translocation assays are as reported.25,43,55,60 BRET22 fluorescence indicates receptor-mediated activation of each specific G protein isoform.
Data Analysis and Statistics
The formula used for calculating Emax and EC50 values from data obtained from agonist concentration–response curves was: E = (Emax × x) / (EC50 + x), with x being the agonist concentration. Statistical comparisons were made using a one-way ANOVA followed by Bonferroni-adjusted t-tests for post hoc comparison or a two-tailed Student’s t-test, as indicated..
Acknowledgments
We acknowledge the support from the National Institutes of Health Intramural Research Program, NIDDK (ZIADK031117) and NHLBI R01 (HL133589). We thank John Lloyd (NIDDK) for mass spectral determinations and Robert O’Connor (NIDDK) for NMR spectra. We thank Dr. Bryan L. Roth (Univ. North Carolina at Chapel Hill) and National Institute of Mental Health’s Psychoactive Drug Screening Program (Contract # HHSN-271-2008-00025 C) for screening data.
Glossary
Abbreviations
- AR
adenosine receptor
- ACN
acetonitrile
- BRET
bioluminescence resonance energy transfer
- DIPEA
diisopropylethylamine
- DMEM
Dulbecco’s modified Eagle’s medium
- EL
extracellular loop
- FBS
fetal bovine serum
- IL
interleukin
- MD
molecular dynamics
- PDSP
NIMH Psychoactive Drug Screening Program
- RMSF
root-mean-square fluctuation
- TEA
triethylamine
- TEAC
triethylammonium hydrogen carbonate
- TM
transmembrane domain.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.3c00126.
The authors declare no competing financial interest.
Special Issue
Published as part of the ACS Pharmacology & Translational Science virtual special issue “GPCR Signaling”.
Supplementary Material
References
- Jacobson K. A.; Merighi S.; Varani K.; Borea P. A.; Baraldi S.; Aghazadeh Tabrizi M.; Romagnoli R.; Baraldi P. G.; Ciancetta A.; Tosh D. K.; Gao Z. G.; Gessi S. A3 adenosine receptors as modulators of inflammation: from medicinal chemistry to therapy. Med. Res. Rev. 2018, 38, 1031–1072. 10.1002/med.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luongo L.; Guida F.; Maione S.; Jacobson K. A.; Salvemini D. Adenosine metabotropic receptors in chronic pain management. Front. Pharmacol. 2021, 12, 651038. 10.3389/fphar.2021.651038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmer S. M.; Manojlovic N. S.; Marinca M. V.; Petrov P.; Cherciu N.; Ganea D.; Ciuleanu T. E.; Pusca I. A.; Beg M. S.; Purcell W. T.; Croitoru A. E.; Ilieva R. N.; Natošević S.; Nita A. L.; Kalev D. N.; Harpaz Z.; Farbstein M.; Silverman M. H.; Bristol D.; Itzhak I.; Fishman P. Namodenoson in advanced hepatocellular carcinoma and Child–Pugh B cirrhosis: Randomized placebo-controlled clinical trial. Cancers 2021, 13, 187. 10.3390/cancers13020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.-W.; Han C.-T.; Sakaguchi Y.; Lee J.; Youn H.-Y. Safety evaluation of FM101, an A3 adenosine receptor modulator, in rat, for developing as therapeutics of glaucoma and hepatitis. EXCLI J. 2020, 19, 187–200. 10.17179/excli2019-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi I.-Y.; Lee J.-C.; Ju C.; Hwang S.; Cho G.-S.; Lee H. W.; Choi W. J.; Jeong L. S.; Kim W.-K. A3 adenosine receptor agonist reduces brain ischemic injury and inhibits inflammatory cell migration in rats. Am. J. Pathol. 2011, 179, 2042–2052. 10.1016/j.ajpath.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle T. M.; Largent-Milnes T. M.; Chen Z.; Staikopoulos V.; Esposito E.; Dalgarno R.; Fan C.; Tosh D. K.; Cuzzocrea S.; Jacobson K. A.; Trang T.; Hutchinson M. R.; Bennett G. J.; Vanderah T. W.; Salvemini D. Chronic morphine-induced changes in signaling at the A3 adenosine receptor contribute to morphine-induced hyperalgesia, tolerance and withdrawal. J. Pharmacol. Exp. Ther. 2020, 374, 331–341. 10.1124/jpet.120.000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta C.; Rotondo J. C.; Lanzillotti C.; Campione G.; Martini F.; Tognon M. Cancer biology and molecular genetics of A3 adenosine receptor. Oncogene 2022, 41, 301–308. 10.1038/s41388-021-02090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosh D. K.; Padia J.; Salvemini D.; Jacobson K. A. Efficient, large-scale synthesis and preclinical studies of MRS5698, a highly selective A3 adenosine receptor agonist that protects against chronic neuropathic pain. Purinergic Signalling 2015, 11, 371–387. 10.1007/s11302-015-9459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosh D. K.; Salmaso V.; Rao H.; Bitant A.; Fisher C. L.; Lieberman D. I.; Vorbrüggen H.; Reitman M. L.; Gavrilova O.; Gao Z. G.; Auchampach J. A.; Jacobson K. A. Truncated (N)-methanocarba nucleosides as partial agonists at mouse and human A3 adenosine receptors: Affinity enhancement by N6-(2-phenylethyl) substitution. J. Med. Chem. 2020, 63, 4334–4348. 10.1021/acs.jmedchem.0c00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosh D. K.; Salmaso V.; Campbell R. G.; Rao H.; Bitant A.; Pottie E.; Stove C. P.; Liu N.; Gavrilova O.; Gao Z. G.; Auchampach J. A.; Jacobson K. A. A3 adenosine receptor agonists containing dopamine moieties for enhanced interspecies affinity. Eur. J. Med. Chem. 2022, 228, 113983. 10.1016/j.ejmech.2021.113983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonioli L.; Lucarini E.; Lambertucci C.; Fornai M.; Pellegrini C.; Benvenuti L.; Di Cesare Mannelli L.; Spinaci A.; Marucci G.; Blandizzi C.; Ghelardini C.; Volpini R.; Dal Ben D. The anti-inflammatory and pain-relieving effects of AR170, an adenosine A3 receptor agonist, in a rat model of colitis. Cells 2020, 9, 1509. 10.3390/cells9061509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzein E.; Palle V.; Wu Y.; Maa T.; Zeng D.; Zablocki J. 2-Pyrazolyl-N6-substituted adenosine derivatives as high affinity and selective adenosine A3 receptor agonists. J. Med. Chem. 2004, 47, 4766–4773. 10.1021/jm049682h. [DOI] [PubMed] [Google Scholar]
- Tosh D. K.; Salmaso V.; Rao H.; Campbell R.; Bitant A.; Gao Z. G.; Auchampach J. A.; Jacobson K. A. Direct comparison of (N)-methanocarba and ribose-containing 2-arylalkynyladenosine derivatives as A3 receptor agonists. ACS Med. Chem. Lett. 2020, 11, 1935–1941. 10.1021/acsmedchemlett.9b00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F.; Wu H.; Katritch V.; Han G. W.; Jacobson K. A.; Gao Z. G.; Cherezov V.; Stevens R. C. Structure of an agonist-bound human A2A adenosine receptor. Science 2011, 332, 322–327. 10.1126/science.1202793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glukhova A.; Thal D. M.; Nguyen A. T.; Vecchio E. A.; Jörg M.; Scammells P. J.; May L. T.; Sexton P. M.; Christopoulos A. Structure of the adenosine A1 receptor reveals the basis for subtype selectivity. Cell 2017, 168, 867–877.e13. 10.1016/j.cell.2017.01.042. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Zhang J.; Weng Y.; Xu Y.; Lu W.; Liu W.; Liu M.; Hua T.; Song G. Cryo-EM structure of the human adenosine A2B receptor–Gssignaling complex. Sci. Adv. 2022, 8, eadd3709 10.1126/sciadv.add3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemister-Buffington J.; Wolf A. J.; Raschka S.; Kuhn L. A. Machine learning to identify flexibility signatures of class a GPCR inhibition. Biomolecules 2020, 10, 454. 10.3390/biom10030454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill S. M.; Baltos J.-A.; White P. J.; May L. T. Biased agonism at adenosine receptors. Cell. Signalling 2021, 82, 109954. 10.1016/j.cellsig.2021.109954. [DOI] [PubMed] [Google Scholar]
- Gao Z. G.; Verzijl D.; Zweemer A.; Ye K.; Göblyös A.; Ijzerman A. P.; Jacobson K. A. Functionally biased modulation of A3 adenosine receptor agonist efficacy and potency by imidazoquinolinamine allosteric enhancers. Biochem. Pharmacol. 2011, 82, 658–668. 10.1016/j.bcp.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrens-Fontanals M.; Stepniewski T. M.; Gloriam D. E.; Selent J. Structural dynamics bridge the gap between the genetic and functional levels of GPCRs. Curr. Opin. Struct. Biol. 2021, 69, 150–159. 10.1016/j.sbi.2021.04.005. [DOI] [PubMed] [Google Scholar]
- Fleetwood O.; Carlsson J.; Delemotte L. Identification of ligand-specific G protein-coupled receptor states and prediction of downstream efficacy via data-driven modeling. Elife 2021, 10, e60715 10.7554/eLife.60715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egyed A.; Domány-Kovács K.; Koványi B.; Horti F.; Kurkó D.; Kiss D. J.; Pándy-Szekeres G.; Greiner I.; Keserű G. M. Controlling receptor function from the extracellular vestibule of G-protein coupled receptors. Chem. Commun. 2020, 56, 14167–14170. 10.1039/d0cc05532h. [DOI] [PubMed] [Google Scholar]
- Cattaneo M.; Zighetti M. L.; Lombardi R.; Martinez C.; Lecchi A.; Conley P. B.; Ware J.; Ruggeri Z. M. Molecular bases of defective signal transduction in the platelet P2Y12 receptor of a patient with congenital bleeding. Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 1978–1983. 10.1073/pnas.0437879100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro S.; Hoffmann C.; Jacobson K. A. Role of the extracellular loops of G protein-coupled receptors in ligand recognition: A molecular modeling study of the human P2Y1 receptor. Biochemistry 1999, 38, 3498–3507. 10.1021/bi982369v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher C. L.; Fallot L. B.; Wan T. C.; Keyes R. F.; Suresh R. R.; Rothwell A. C.; Gao Z. G.; McCorvy J. D.; Smith B. C.; Jacobson K. A.; Auchampach J. A. Structure activity relationship of dual action purine nucleoside allosteric modulators of the A3 adenosine receptor. ACS Pharmacol. Transl. Sci. 2022, 5, 625–641. 10.1021/acsptsci.2c00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomivuori C. M.; Latorraca N. R.; Wingler L. M.; Eismann S.; King M. C.; Kleinhenz A. L. W.; Skiba M. A.; Staus D. P.; Kruse A. C.; Lefkowitz R. J.; Dror R. O. Molecular mechanism of biased signaling in a prototypical G protein-coupled receptor. Science 2020, 367, 881–887. 10.1126/science.aaz0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock A.; Bermudez M. Allosteric coupling and biased agonism in G protein-coupled receptors. FEBS J. 2021, 288, 2513–2528. 10.1111/febs.15783. [DOI] [PubMed] [Google Scholar]
- Seyedabadi M.; Gharghabi M.; Gurevich E. V.; Gurevich V. V. Receptor-arrestin interactions: the GPCR perspective. Biomolecules 2021, 11, 218. 10.3390/biom11020218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Soto M.; Verma R. K.; Willette B. K. A.; Gonye E. C.; Moore A. M.; Moritz A. E.; Boateng C. A.; Yano H.; Free R. B.; Shi L.; Sibley D. R. A structural basis for how ligand binding site changes can allosterically regulate GPCR signaling and engender functional selectivity. Sci. Signaling 2020, 13, eaaw5885 10.1126/scisignal.aaw5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker D.; Wang C.; Katritch V.; Han G. W.; Huang X. P.; Vardy E.; McCorvy J. D.; Jiang Y.; Chu M.; Siu F. Y.; Liu W.; Xu H. E.; Cherezov V.; Roth B. L.; Stevens R. C. Structural features for functional selectivity at serotonin receptors. Science 2013, 340, 615–619. 10.1126/science.1232808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Marín J.; Reyes-Resina I.; Martínez-Pinilla E.; Navarro G.; Franco R. Natural compounds as guides for the discovery of drugs targeting G-protein-coupled receptors. Molecules 2020, 25, 5060. 10.3390/molecules25215060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeau C.; Ries B.; Stadelmann T.; Tremblay J.; Poulet S.; Fröhlich U.; Côté J.; Boudreault P. L.; Derbali R. M.; Sarret P.; Grandbois M.; Leclair G.; Riniker S.; Marsault É. Modulation of the passive permeability of semipeptidic macrocycles: N- and C-methylations fine-tune conformation and properties. J. Med. Chem. 2021, 64, 5365–5383. 10.1021/acs.jmedchem.0c02036. [DOI] [PubMed] [Google Scholar]
- Sindhikara D.; Wagner M.; Gkeka P.; Güssregen S.; Tiwari G.; Hessler G.; Yapici E.; Li Z.; Evers A. Automated design of macrocycles for therapeutic applications: From small molecules to peptides and proteins. J. Med. Chem. 2020, 63, 12100–12115. 10.1021/acs.jmedchem.0c01500. [DOI] [PubMed] [Google Scholar]
- Garcia Jimenez D.; Poongavanam V.; Kihlberg J. Macrocycles in drug discovery–Learning from the past for the future. J. Med. Chem. 2023, 66, 5377–5396. 10.1021/acs.jmedchem.3c00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrhein J. A.; Knapp S.; Hanke T. Synthetic opportunities and challenges for macrocyclic kinase inhibitors. J. Med. Chem. 2021, 64, 7991–8009. 10.1021/acs.jmedchem.1c00217. [DOI] [PubMed] [Google Scholar]
- Dougherty P. G.; Qian Z.; Pei D. Macrocycles as protein-protein interaction inhibitors. Biochem. J. 2017, 474, 1109–1125. 10.1042/BCJ20160619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brice-Tutt A. C.; Wilson L. L.; Eans S. O.; Stacy H. M.; Simons C. A.; Simpson G. G.; Coleman J. S.; Ferracane M. J.; Aldrich J. V.; McLaughlin J. P. Multifunctional opioid receptor agonism and antagonism by a novel macrocyclic tetrapeptide prevents reinstatement of morphine-seeking behaviour. Br. J. Pharmacol. 2020, 177, 4209–4222. 10.1111/bph.15165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeff S.; Rivière S.; Ruiz J.; Abdelrahman A.; Schulz-Fincke A.-C.; Köse M.; Tiburcy F.; Wieczorek H.; Gütschow M.; Müller C. E.; Menche D. Synthesis of novel potent archazolids: Pharmacology of an emerging class of anticancer drugs. J. Med. Chem. 2020, 63, 1684–1698. 10.1021/acs.jmedchem.9b01887. [DOI] [PubMed] [Google Scholar]
- Carlin J. L.; Jain S.; Gizewski E.; Wan T. C.; Tosh D. K.; Xiao C.; Auchampach J. A.; Jacobson K. A.; Gavrilova O.; Reitman M. L. Hypothermia in mouse is caused by adenosine A1 and A3 receptor agonists and AMP via three distinct mechanisms. Neuropharmacology 2017, 114, 101–113. 10.1016/j.neuropharm.2016.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung-Chi C.; Prusoff W. H. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- D Segall M. Multi-parameter optimization: Identifying high quality compounds with a balance of properties. Curr. Pharm. Des. 2012, 18, 1292–1310. 10.2174/138161212799436430. [DOI] [PubMed] [Google Scholar]
- Besnard J.; Ruda G. F.; Setola V.; Abecassis K.; Rodriguiz R. M.; Huang X.-P.; Norval S.; Sassano M. F.; Shin A. I.; Webster L. A.; Simeons F. R. C.; Stojanovski L.; Prat A.; Seidah N. G.; Constam D. B.; Bickerton G. R.; Read K. D.; Wetsel W. C.; Gilbert I. H.; Roth B. L.; Hopkins A. L. Automated design of ligands to polypharmacological profiles. Nature 2012, 492, 215–220. 10.1038/nature11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. H. J.; DiBerto J. F.; English J. G.; Glaudin A. M.; Krumm B. E.; Slocum S. T.; Che T.; Gavin A. C.; McCorvy J. D.; Roth B. L.; Strachan R. T. TRUPATH, an open-source biosensor platform for interrogating the GPCR transducerome. Nat. Chem. Biol. 2020, 16, 841–849. 10.1038/s41589-020-0535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen S. A.; Quinn R. J. Adenosine receptors: new opportunities for future drugs. Bioorg. Med. Chem. 1998, 6, 619–641. 10.1016/s0968-0896(98)00038-8. [DOI] [PubMed] [Google Scholar]
- Dooley M. J.; Quinn R. J. The three binding domain model of adenosine receptors: molecular modeling aspects. J. Med. Chem. 1992, 35, 211–216. 10.1021/jm00080a002. [DOI] [PubMed] [Google Scholar]
- Kim J.; Jiang Q.; Glashofer M.; Yehle S.; Wess J.; Jacobson K. A. Glutamate residues in the second extracellular loop of the human A2a adenosine receptors are required for ligand recognition. Mol. Pharmacol. 1996, 49, 683–691. [PMC free article] [PubMed] [Google Scholar]
- Olah M. E.; Jacobson K. A.; Stiles G. L. Role of the second extracellular loop of adenosine receptors in agonist and antagonist binding: Analysis of chimeric A1/A3 adenosine receptors. J. Biol. Chem. 1994, 269, 24692–24698. 10.1016/s0021-9258(17)31446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong H. T.; Gao Z. G.; Jacobson K. A. Nucleoside modification and concerted mutagenesis of the human A3 adenosine receptor to probe interactions between the 2-position of adenosine analogs and Gln167 in the second extracellular loop. Nucleosides, Nucleotides Nucleic Acids 2005, 24, 1507–1517. 10.1080/15257770500265778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebon G.; Edwards P. C.; Leslie A. G. W.; Tate C. G. Molecular determinants of CGS21680 binding to the human adenosine A2A receptor. Mol. Pharmacol. 2015, 87, 907–915. 10.1124/mol.114.097360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matricon P.; Nguyen A. T.; Vo D. D.; Baltos J. A.; Jaiteh M.; Luttens A.; Kampen S.; Christopoulos A.; Kihlberg J.; May L. T.; Carlsson J.; Carlsson J. Structure-based virtual screening discovers potent and selective adenosine A1 receptor antagonists. Eur. J. Med. Chem. 2023, 257, 115419. 10.1016/j.ejmech.2023.115419. [DOI] [PubMed] [Google Scholar]
- Jespers W.; Oliveira A.; Prieto-Díaz R.; Majellaro M.; Åqvist J.; Sotelo E.; Gutiérrez-de-Terán H. Structure-based design of potent and selective ligands at the four adenosine receptors. Molecules 2017, 22, 1945. 10.3390/molecules22111945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driggers E. M.; Hale S. P.; Lee J.; Terrett N. K. The exploration of macrocycles for drug discovery – an underexploited structural class. Nat. Rev. Drug Discovery 2008, 7, 608–624. 10.1038/nrd2590. [DOI] [PubMed] [Google Scholar]
- Murray B. W.; Zhai D.; Deng W.; Zhang X.; Ung J.; Nguyen V.; Zhang H.; Barrera M.; Parra A.; Cowell J.; Lee D. J.; Aloysius H.; Rogers E. TPX-0131, a Potent CNS-penetrant, next-generation inhibitor of wild-type ALK and ALK-resistant mutations. Mol. Cancer Ther. 2021, 20, 1499–1507. 10.1158/1535-7163.MCT-21-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitty A.; Zhong M.; Viarengo L.; Beglov D.; Hall D. R.; Vajda S. Quantifying the chameleonic properties of macrocycles and other high-molecular-weight drugs. Drug Discovery Today 2016, 21, 712–717. 10.1016/j.drudis.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. J.; Lee S. H.; Park S. O.; Kang H.; Lee J. S.; Lee K. N.; Jung M. E.; Kim J.; Lee J. Novel macrocyclic C-aryl glucoside SGLT2 inhibitors as potential antidiabetic agents. Bioorg. Med. Chem. 2011, 19, 5468–5479. 10.1016/j.bmc.2011.07.045. [DOI] [PubMed] [Google Scholar]
- Fadzen C. M.; Wolfe J. M.; Cho C. F.; Chiocca E. A.; Lawler S. E.; Pentelute B. L. Perfluoroarene-based peptide macrocycles to enhance penetration across the blood-brain barrier. J. Am. Chem. Soc. 2017, 139, 15628–15631. 10.1021/jacs.7b09790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston T. E.; Hinz S.; Müller C. E.; Holstein D. M.; Wendling J.; Melton R. J.; Campbell M.; Korinek W. S.; Suresh R. R.; Sethre-Hofstad D.; Gao Z. G.; Tosh D. K.; Jacobson K. A.; Lechleiter J. D. Nucleotide P2Y1 receptor agonists are In vitro and In vivo prodrugs of A1/A3 adenosine receptor agonists: Implications for roles of P2Y1 and A1/A3 receptors in health and disease. Purinergic Signalling 2020, 16, 543–559. 10.1007/s11302-020-09732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosh D. K.; Chinn M.; Ivanov A. A.; Klutz A. M.; Gao Z. G.; Jacobson K. A. Functionalized congeners of A3 adenosine receptor-selective nucleosides containing a bicyclo[3.1.0]hexane ring system. J. Med. Chem. 2009, 52, 7580–7592. 10.1021/jm900426g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler L. M.; Elgeti M.; Hilger D.; Latorraca N. R.; Lerch M. T.; Staus D. P.; Dror R. O.; Kobilka B. K.; Hubbell W. L.; Lefkowitz R. J. Angiotensin analogs with divergent bias stabilize distinct receptor conformations. Cell 2019, 176, 468–478.e11. 10.1016/j.cell.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall M. J.; Hill E.; Huckstepp R.; Barkan K.; Deganutti G.; Leuenberger M.; Preti B.; Winfield I.; Carvalho S.; Suchankova A.; Wei H.; Safitri D.; Huang X.; Imlach W.; La Mache C.; Dean E.; Hume C.; Hayward S.; Oliver J.; Zhao F. Y.; Spanswick D.; Reynolds C. A.; Lochner M.; Ladds G.; Frenguelli B. G. Selective activation of Gαob by an adenosine A1 receptor agonist elicits analgesia without cardiorespiratory depression. Nat. Commun. 2022, 13, 4150. 10.1038/s41467-022-31652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vural A.; Nabar N. R.; Hwang I.-Y.; Sohn S.; Park C.; Karlsson M. C. I.; Blumer J. B.; Kehrl J. H. Gαi2 signaling regulates inflammasome priming and cytokine production by biasing macrophage phenotype determination. J. Immunol. 2019, 202, 1510–1520. 10.4049/jimmunol.1801145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao C.-C.; Sankowski R.; Prinz M.; Smolders J.; Huitinga I.; Hamann J. GPCRomics of homeostatic and disease-associated human microglia. Front. Immunol. 2021, 12, 674189. 10.3389/fimmu.2021.674189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavi Sastry G.; Adzhigirey M.; Day T.; Annabhimoju R.; Sherman W. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J. Comput.-Aided Mol. Des. 2013, 27, 221–234. 10.1007/s10822-013-9644-8. [DOI] [PubMed] [Google Scholar]
- Schrodinger Release 2021-2. Maestro; Schrödinger, LLC: New York, NY, 2020.
- Friesner R. A.; Banks J. L.; Murphy R. B.; Halgren T. A.; Klicic J. J.; Mainz D. T.; Repasky M. P.; Knoll E. H.; Shelley M.; Perry J. K.; Shaw D. E.; Francis P.; Shenkin P. S. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- Halgren T. A.; Murphy R. B.; Friesner R. A.; Beard H. S.; Frye L. L.; Pollard W. T.; Banks J. L. Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004, 47, 1750–1759. 10.1021/jm030644s. [DOI] [PubMed] [Google Scholar]
- Friesner R. A.; Murphy R. B.; Repasky M. P.; Frye L. L.; Greenwood J. R.; Halgren T. A.; Sanschagrin P. C.; Mainz D. T. Extra precision Glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- Lomize M. A.; Pogozheva I. .D.; Joo H.; Mosberg H. I.; Lomize A. L. OPM database and PPM Web server: resources for positioning of proteins in membranes. Nucleic Acids Res. 2012, 40, D370–D376. 10.1093/nar/gkr703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey W.; Dalke A.; Schulten K. VMD: visual molecular dynamics. J. Mol. Graphics 1996, 14, 33–38. 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Best R. B.; Zhu X.; Shim J.; Lopes P. E. M.; Mittal J.; Feig M.; Mackerell A. D. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone Φ, Ψ and side-chain χ1 and χ2 dihedral angles. J. Chem. Theory Comput. 2012, 8, 3257–3273. 10.1021/ct300400x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauda J. B.; Venable R. M.; Freites J. A.; O’Connor J. W.; Tobias D. J.; MondragonRamirez C.; Vorobyov I.; MacKerell A. D.; Pastor R. W. Update of the CHARMM allatom additive force field for lipids: validation on six lipid types. J. Phys. Chem. B 2010, 114, 7830–7843. 10.1021/jp101759q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanommeslaeghe K.; MacKerell A. D. Automation of the CHARMM general force field (CGenFF) I: bond perception and atom typing. J. Chem. Inf. Model. 2012, 52, 3144–3154. 10.1021/ci300363c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanommeslaeghe K.; Raman E. P.; MacKerell A. D. Automation of the CHARMM general force field (CGenFF) II: assignment of bonded parameters and partial atomic charges. J. Chem. Inf. Model. 2012, 52, 3155–3168. 10.1021/ci3003649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHARMM general force field (CGenFF) program. https://cgenff.umaryland.edu/ (accesed September 15, 2022).
- Doerr S.; Harvey M. J.; Noe F.; De Fabritiis G. HTMD: high-throughput molecular dynamics for molecular discovery. J. Chem. Theory Comput. 2016, 12, 1845–1852. 10.1021/acs.jctc.6b00049. [DOI] [PubMed] [Google Scholar]
- Harvey M. J.; Giupponi G.; Fabritiis G. D. ACEMD: accelerating biomolecular dynamics in the microsecond time scale. J. Chem. Theory Comput. 2009, 5, 1632–1639. 10.1021/ct9000685. [DOI] [PubMed] [Google Scholar]
- Seeber M.; Felline A.; Raimondi F.; Muff S.; Friedman R.; Rao F.; Caflisch A.; Fanelli F. Wordom: a user-friendly program for the analysis of molecular structures, trajectories, and free energy surfaces. J. Comput. Chem. 2011, 32, 1183–1194. 10.1002/jcc.21688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakan A.; Meireles L. M.; Bahar I. ProDy: Protein Dynamics Inferred from Theory and Experiments. Bioinformatics 2011, 27, 1575–1577. 10.1093/bioinformatics/btr168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIOWULF . High Performance Computing at the NIH. http://hpc.nih.gov (accesed September 15, 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.