Abstract
Background
Excessive extracellular matrix (ECM) deposition in adipose tissue is a hallmark of fibrosis, leading to disrupted adipose tissue homeostasis and metabolic dysfunction. Hesperetin, a flavonoid compound, has shown promising anti-inflammatory, anti-obesity and anti-diabetic properties. Therefore, we investigated the anti-fibrotic effects of hesperetin, through targeting ECM components and matrix metalloproteinase enzymes.
Methods
3T3-L1 cells were cultured in DMEM, containing 10% FBS and 1% penicillin/streptomycin. Cells were treated with a range of hesperetin concentrations, and the cell viability was determined using MTT assay. Subsequently, the expression of genes encoding collagen VI, osteopontin, matrix metalloproteinase-2 (Mmp-2) and Mmp-9 was analyzed using specific primers and real-time PCR technique. To evaluate protein levels of collagen VI and osteopontin, Western blotting was performed.
Results
Hesperetin affected the viability of 3T3-L1 adipocytes with IC50 of 447.4 µM, 339.2 µM and 258.8 µM (24 h, 48 and 72 h, respectively). Hesperetin significantly reduced the gene and protein expression of both collagen VI and osteopontin in 3T3-L1 pre-adipocytes, in a time- and dose-dependent manner. Hesperetin was also able to cause a remarkable decline in gene expression of Mmp2 and Mmp9.
Conclusion
Hesperetin could potently reduce the production of markers of adipose tissue fibrosis and might be considered a potential anti-fibrotic compound in obesity. Thus, hesperetin has the potency to be used for the treatment of obesity-associated fibrosis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-023-04152-z.
Keywords: Obesity, Adipose tissue fibrosis, Hesperetin, Collagen, Osteopontin, Matrix metalloproteinase
Introduction
Over the past decades, there has been a dramatic increase in global obesity prevalence [1], and according to WHO’s estimate, it will increase by roughly 167 million cases by 2025 (www.who.int). Obesity is associated with increased mortality and numerous complications including type 2 diabetes mellitus (T2DM), non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), cardiovascular disease and a number of cancers [2, 3]. The progression of obesity is closely associated with maladaptive adipose tissue remodeling in response to excess calorie intake. This phenomenon is characterized by adipocyte hypertrophy and/or hyperplasia, which lead to hypoxic conditions and continuous production and deposition of extracellular matrix (ECM) [4]. The matrix itself is capable of promoting differentiation of progenitor cells into adipocytes to accommodate adipose tissue expansion and further fat storage. This excessive deposition of potentially pathological ECM components is defined as adipose tissue fibrosis [5]. Adipose tissue fibrosis has a complex and crucial role in obesity-related metabolic dysfunction. Increased ECM in adipose tissue causes adipocyte death, reduced lipolysis, and disturbed cell-cell interactions [6]. Notably, adipose tissue fibrosis is linked to insulin resistance in subjects with obesity [7, 8]. Inhibition of adipose tissue fibrosis has been shown to be an effective mechanism to improve glucose homeostasis, so targeting adipose tissue fibrosis has been suggested as an efficient tool to alleviate insulin resistance in obesity [9].
Collagen VI is a highly enriched ECM component of adipose tissue and is specific to adipose tissue fibrosis. The removal of collagen VI in knockout mice models of obesity resulted in uninhibited expansion of adipocytes and was accompanied by considerable improvements in lipid clearance, pancreatic hyperplasia, insulin function, and whole-body energy homeostasis, while lower inflammation and necrotic cell death occurred [10]. Moreover, endotrophin which is a component of collagen VI, stimulates fibrosis and inflammation and eventually leads to increased insulin resistance [11].
Osteopontin, a multifunctional ECM-associated protein, is produced by adipose tissue and is significantly elevated in visceral adipose tissue in obesity [12]. Enhancement of osteopontin has been shown to be associated with adipose tissue inflammation and insulin resistance [13]. Deletion of osteopontin was shown to lead to reduced adipose tissue fibrosis and ECM remodeling, reduced MMP2 and MMP9 activity, followed by higher body temperature, improved brown adipose tissue function, reduced body weight and fat mass, and higher insulin sensitivity [14].
Hesperetin, a natural phenolic compound, belonging to flavanone class of flavonoids and an aglycon of hesperidin, is present in citrus fruits such as oranges and grapefruit [15]. It possesses a number of health benefits and exerts anti-hyperlipidemic, anti-hyperglycemic, anti-inflammatory and antioxidant properties [16, 17]. Interestingly, bona fide effects of hesperetin on lipid accumulation and adiposity have been previously demonstrated by various studies [18–21]. However, the role of hesperetin in the adipose tissue fibrosis has yet to be unraveled. Therefore, the objective of this study was to investigate the effect of hesperetin on Collagen VI and osteopontin as the major components of adipose tissue ECM, as well as main matrix metalloproteinases, Mmp2 and Mmp9, in 3T3-L1 pre-adipocytes.
Materials and methods
Chemicals and reagents
Hesperetin was obtained from Sigma-Aldrich (W431300, Germany). Cell culture reagents, including culture medium (L0093) and antibiotics (L0022) were obtained from Biowest (France). Fetal bovine serum (FBS) was purchased from Biosera (FB-1001/100, France). Hybrid-R RNA extraction kit (305 − 101, GeneAll Biotechnology, Korea) was used for the isolation of total cellular RNA and the corresponding cDNA was synthesized with HyperScript™ RT Master Mix (601–710, GeneAll Biotechnology, Korea). RealQ Plus 2x SYBR Green Master Mix high ROX™ (A325402, Ampliqon, Denmark) was used for real-time PCR. BCA Protein Assay Kit (23,225) was purchased from Thermo Fisher Scientific, UK). RIPA cell lysis buffer (PL008-5X) was attained from Biobasic (Canada). All the chemicals, including MTT (M5655), dimethyl sulfoxide (DMSO) (67-68-5), and general laboratory reagents were obtained from Sigma-Aldrich (Germany).
Cell culture
3T3-L1 cells were purchased from Cell Bank of the Iranian Biological Resource Center (Tehran, Iran) and maintained in DMEM (Dulbecco’s modified Eagle’s medium), containing 10% FBS and 1% penicillin/streptomycin at 37 °C with 5% CO2. Cells were treated with different concentrations of hesperetin, after solubilization in DMSO, considering the final concentration of DMSO to be less than 0.1%. The highest concentration of DMSO in the treatments was also added as the negative control.
Cell viability test
The viability of 3T3-L1 cells was evaluated using MTT assay. Cells were seeded in a 96-well plate at a density of 4 × 103 cells/well. Then the cells were treated with various concentrations of hesperetin, including 25, 50, 100, 200, 300, 400, 500, 600, 700 and 800 µM for 24, 48 and 72 h. After incubation, the media was replaced with media containing 10 µl of MTT (stock concentration-5 mg/mL in PBS), followed by 3 h of incubation at 37℃ until formazan crystals were formed. The reaction was stopped by adding 100 µl DMSO to each well. After incubating in a dark place, at room temperature for 10 min, the absorbance was measured at 570 nm wavelength using a plate-reader. Cell viability was then calculated relative to control untreated cells.
RNA isolation and real-time PCR
Total RNA was extracted from 3T3-L1 cells and the purity of RNA was measured with NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, USA). Reverse transcription was performed using 1 µg total RNA and real-time PCR was done in triplicate with SYBR green method, using an initial denaturation step (15 min at 95℃) and the subsequent 40 cycles of 30 s at 95℃ and 1 min at 61 °C. Beta-actin was used as the housekeeping gene and the relative gene expression was calculated by the 2−ΔΔCt formula. The primer sequences are presented in Table 1.
Table 1.
The information of primers used for real-time PCR.
| Gene name | Gene acronym | Accession No. | Sequence | Product length |
|---|---|---|---|---|
| collagen type VI | Col6a3 | XM_030245451.2 |
5’- AACCCTCCACATACTGCTAATTC-3’ 5’- TCGTTGTCACTGGCTTCATT-3’ |
70 |
| Osteopontin (secreted phosphoprotein 1) | Opn (Spp1) | NM_001204203.1 |
5’- GCCTGTTTGGCATTGCCTCCTC-3’ 5’- CACAGCATTCTGTGGCGCAAGG-3’ |
158 |
| matrix metallopeptidase 2 | Mmp2 | XM_006530751.4 |
5’- CAGGGAATGAGTACTGGGTCTATT-3’ 5’- ACTCCAGTTAAAGGCAGCATCTAC-3’ |
119 |
| matrix metallopeptidase 9 | Mmp9 | NM_013599.5 |
5’- AATCTCTTCTAGAGACTGGGAAGGAG-3’ 5’- AGCTGATTGACTAAAGTAGCTGGA-3’ |
128 |
| Beta-actin | Actb | NM_007393.5 |
5’- GTCCTCCTGGCATACCATAGA-3’ 5’- AGCTCAGTAACAGTCCGCCTAGA-3’ |
101 |
The species of all the primers are Mus musculus
Western blotting
Total protein extraction and immunoblotting was performed as described previously [22]. In brief, 3T3-L1 cells were washed with ice-cold PBS, homogenized and lysed in lysis buffer containing protease and phosphatase inhibitor cocktail (Sigma-Aldrich, Germany). Protein concentrations were measured by BCA method, using bovine serum albumin as the reference standard. Total proteins (40 µg per well) were separated by 8% sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes (03010040001, Roche Applied Sciences, Germany). The membranes were incubated for 4 h at room temperature with 0.1% Tween 20 in tris-buffered saline (TBST) containing 5% skim milk for osteopontin and and 5% BSA for collagen VI determination, respectively. Subsequently, blots were washed and incubated overnight at 4 °C with buffer containing primary antibodies; either 1:1000 dilution of antibody against mouse collagen VI (Col6A1) (B-4, sc-377,143), 1:500 dilution of antibody against mouse osteopontin (AKm2A1, sc-21,742) (Santa Cruze Biotechnology, Inc., USA), or antibody against mouse GAPDH (6C5, ab-8245, Abcam, USA). Membranes were rinsed three times with TBST before and after the incubation with the secondary antibody for 1.5 h, at room temperature. Horseradish peroxidase-conjugated anti-mouse IgG Fc binding protein (sc-525,409, Santa Cruze Biotechnology, Inc., USA) was used as the secondary antibody. Immunodetection was performed using enhanced chemiluminescent (ECL) detection reagent (Amersham biosciences, UK) and the bands were detected through exposing blots to X-ray films. Quantification of the visualized bands was carried out using Image J software (NIH, Bethesda, USA).
Statistical analysis
Statistical analysis was carried out with the aid of GraphPad Prism 5 software (San Diego, USA). All obtained data were expressed as mean ± SEM and analyzed by one-way analysis of variance (ANOVA), with Dunnett’s post-hoc test. The cutoff for significance was considered p < 0.05.
Results
The effect of hesperetin on the viability of 3T3-L1 pre-adipocytes
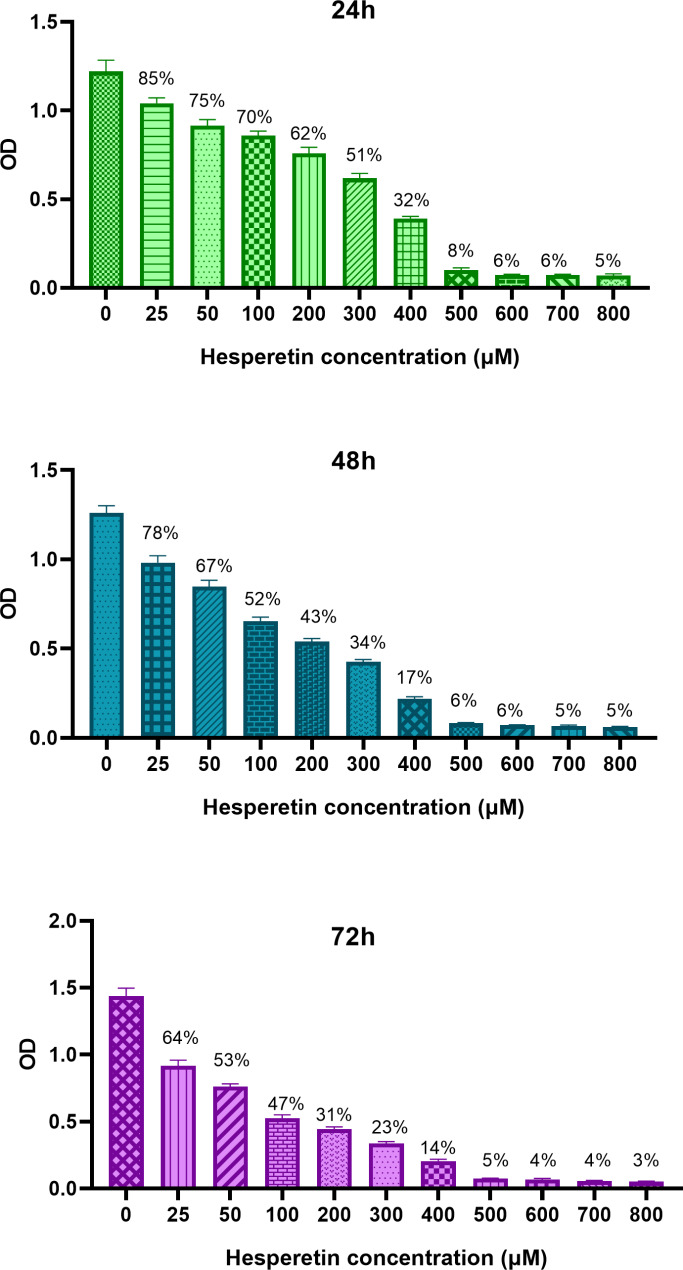
To investigate the cytotoxic effect of hesperetin on 3T3-L1 cells, first we examined cell viability in response to various concentrations of hesperetin. As shown in Fig. 1, the IC50 was found to be 447.4 µM, 339.2 µM and 258.8 µM for 24 h, 48 h and 72 h of treatment, respectively. Considering these data, we selected lower concentrations of hesperetin (25, 100 and 150 µM) for further experiments.
Fig. 1.
The viability of 3T3-L1 cells after 24 h, 48 h and 72 h treatment with various concentrations of hesperetin (25–800 µM), evaluated by MTT assay. The obtained results are compared with untreated control and presented as mean ± SD of at least three replicates
Hesperetin reduced gene expression of Col6a3 and OPN in 3T3-L1 pre-adipocytes
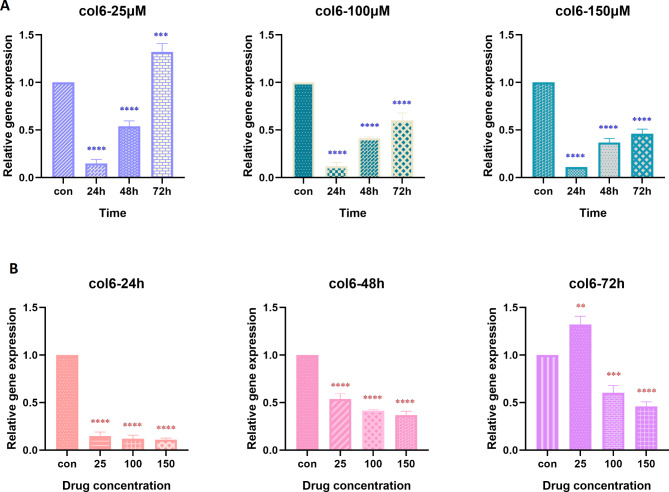
The expression of genes that encode coallagen VI (Col6a3) and osteopontin (Opn) was analyzed by real-time PCR technique. Figure 2 shows the effect of hesperetin on Col6a3 gene expression in 3T3-L1 pre-adipocytes. Hesperetin could reduce Col6a3 gene expression in a time- and dose-dependent manner. Moreover, we observed that the most significant effects of hesperetin on Col6a3 gene expression was evident at 48 h and 72 h of treatment with all selected hesperetin concentrations (p value < 0.0001).
Fig. 2.
The time-dependent (A) and dose-dependent (B) effect of hesperetin on the expression of Col6 gene, encoding Collagen VI, in 3T3-L1 pre-adipocytes. The controls in panel A are mean of the controls in 24 h, 48 h, and 72 h. The obtained results are presented as mean ± SD of at least three replicates. * p < 0.05, ** p < 0.01, *** p < 0.001, ****p < 0.0001
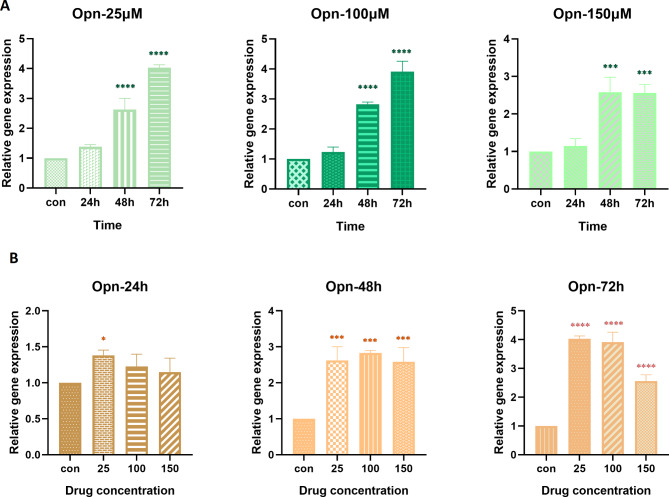
The effect of selected concentrations of hesperetin on Opn gene expression is illustrated in Fig. 3. From the presented results it is apparent that hesperetin led to a significant time- and dose-dependent decrease in the expression of Opn gene (p value < 0.0001).
Fig. 3.
The time-dependent (A) and dose-dependent (B) effect of hesperetin on the expression of Opn gene, encoding osteopontin, in 3T3-L1 pre-adipocytes. The controls in panel A are mean of the controls in 24 h, 48 h, and 72 h. The obtained results are presented as mean ± SD of at least three replicates. *** p < 0.001, ****p < 0.0001
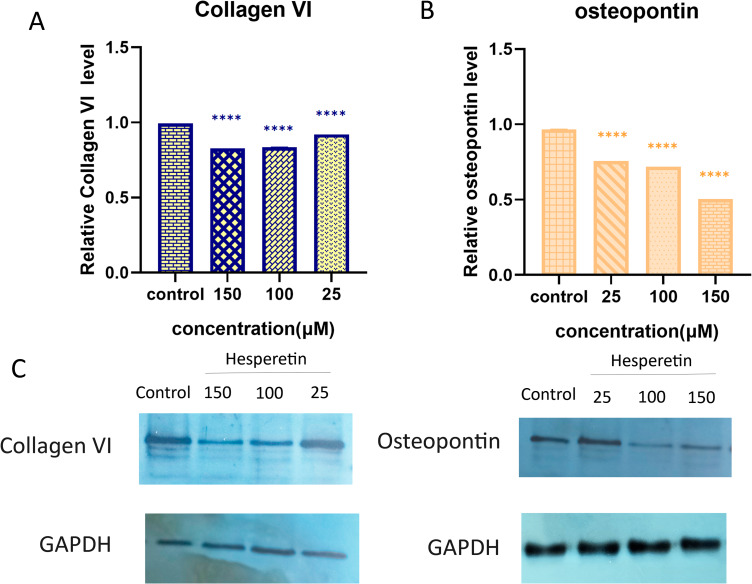
Hesperetin decreases protein level of collagen VI and osteopontin in 3T3-L1 pre-adipocytes
In order to further confirm the suppressive impact of hesperetin on collagen VI and osteopontin protein expression in pre-adipocytes, we conducted western blotting. From the data in Fig. 4, it can be seen that there was a remarkable decrease in the levels of both collagen VI and osteopontin proteins (p value < 0.0001) in response to treatment with hesperetin. Nevertheless, low dose of hesperetin (25 µM) did not cause a significant change in osteopontin protein level.
Fig. 4.
The effect of hesperetin on (A) collagen VI and (B) osteopontin protein levels in 3T3-L1 cells, compared to untreated control. (C) a representative Western blotting result is shown. Data are presented as mean ± SD of at least three replicates, ** P < 0.01, ****p < 0.0001
Hesperetin reduced the gene expression of Mmp2 and Mmp9 in 3T3-L1 pre-adipocytes
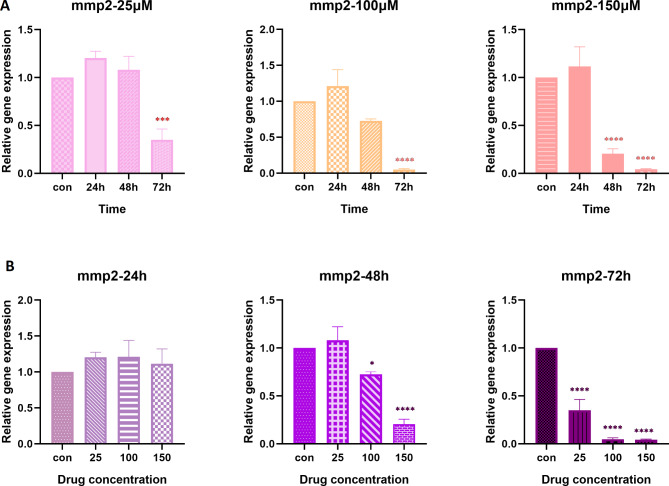
Amongst the matrix metalloproteinase enzymes, Mmp-2 and Mmp-9 are the main contributors to adipose tissue fibrosis, the levels of which are noticeably surged in response to the accumulated ECM components [23, 24]. With regard to the inhibitory effect of hesperetin on collagen VI and osteopontin as major ECM proteins, we were keen to investigate the expression level of Mmp2 and Mmp9 after hesperetin exposure. As it is shown in Fig. 5 there is a clear trend of decreasing Mmp2 gene expression in a time- and dose-dependent manner as compared to untreated control cells. Data showed that Mmp2 gene expression was significantly decreased following treatment with almost all concentrations of hesperetin and different time spans (p value < 0.0001). However, treatment with 25 µM hesperetin resulted in no statistically significant change in Mmp2 gene expression.
Fig. 5.
The effect of hesperetin on the gene expression of Mmp2 in different times (A) and concentrations (B). The controls in panel A are the mean of the control in 24 h, 48 h, and 72 h. The obtained results are presented as mean ± SD of at least three replicates. ** p < 0.01, *** p < 0.001, ****p < 0.0001
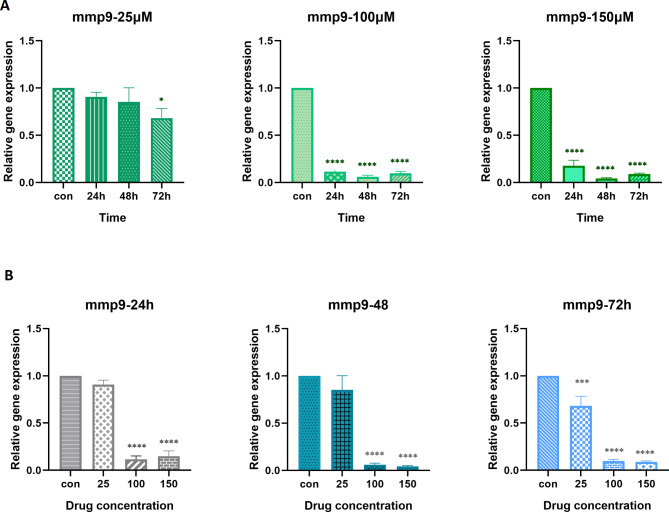
Hesperetin could also effectively decrease Mmp9 gene expression in almost all doses and time spans in comparison to control cells (Fig. 6). Although 25 µM hesperetin caused no significant change on the Mmp-9 gene expression after 24 and 48 h of treatment. Furthermore, it was found that there was no notable difference between 100 µM and 150 µM concentrations of hesperetin at different time intervals.
Fig. 6.
The effect of hesperetin on the gene expression of Mmp9 in different times (A) and concentrations (B). The controls in panel A are the mean of the controls in 24 h, 48 h, and 72 h. The obtained results are presented as mean ± SD of at least three replicates. * p < 0.05, *** p < 0.001, ****p < 0.0001
Discussion
Many studies have examined the effect of hesperetin on obesity and its complications [25–28]. Citrus flavonoids, including hesperetin were shown to suppress gene expression of stearoyl-CoA desaturase, an enzyme whose inhibition reduces hyperlipidemia and adiposity [29]. Additionally lipolytic actions of tumor necrosis factor α (TNF-α), which promotes insulin resistance, can be attenuated by hesperetin in 3T3-L1 cells [28]. Intriguingly, hesperetin and hesperidin have the ability to stimulate the release of one of the appetite-regulating hormones, named cholecystokinin (CCK), suggesting that hesperetin might serve as a candidate biomolecule for the suppression of appetite and weight gain [30].
Herein, we focused on the anti-fibrotic properties of hesperetin and thus, aimed to find its impact on the major ECM constituents (collagen VI and osteopontin). Adipose tissue ECM is a complex and rich meshwork of dynamically changing interconnected macromolecules, of which collagen VI and osteopontin are among the main constituents [31]. It has been previously shown that in human body, expression of collagen VI and osteopontin increases during obesity [32, 33]. Khan et al., demonstrated an inverse correlation between collagen VI deposition in adipocyte-surrounding ECM and insulin sensitivity as well as inflammatory phenotype of obesity [10]. Osteopontin has also been shown to be expressed in adipose tissue and the associated macrophages, and promotes inflammation [34]. A myriad of studies have proposed osteopontin as an important adipokine, playing a key role in linking obesity to insulin resistance through adipose tissue macrophage recruitment [13, 33]. We had also previously shown that visfatin, an inflammatory adipocytokines, can promote fibrosis through modulation of the expression of ECM components [22]. However, attenuation of adipose tissue fibrosis by natural products is a topic which has not been scrutinized well.
Our results in the present study showed for the first time that hesperetin could remarkably reduce collagen VI and osteopontin at protein and mRNA levels in 3T3-L1 pre-adipocytes. A major hallmark of white adipose tissue fibrosis is the accumulation of ECM proteins, which increases the risk of insulin resistance [35, 36]. Hence, hesperetin-mediated downregulation of collagen VI and osteopontin might be considered as an anti-fibrotic property. The effect of hesperetin on the ECM components has not been previously investigated in pre-adipocytes; however, consistent with our findings, it has shown to be effective in the amelioration of liver fibrosis in different hepatocyte injury models. For example, hesperetin has been reported to exert protective effects against CCl4-induced liver fibrosis in animal models [37, 38], as well as high-fat diet-induced non-alcoholic fatty liver disease [39]. Furthermore, the anti-fibrotic influence of hesperetins has been demonstrated in experimental hepatocytes [39, 40]. These studies have mainly focused on anti-oxidative and anti-inflammatory properties of hesperetin; however, down-regulation of Col1α1, Col3α1 and TIMP-1 in hepatocytes has been reported [38, 40]. The exact molecular mechanism behind the protective effect of hesperetin in fibrosis is not well-defined, but the activation of AMPK/SIRT3, suppression of Glioma associated oncogene-1 (Gli-1), increased expression of Nrf2, and attenuation of the aberrant expression of patched1 in the Hedgehog pathway have been proposed as underlying mechanisms in the amelioration of liver fibrosis [37, 38, 40, 41].
Not many herbal derivatives have been investigated in relation to the markers of ECM accumulation in adipocytes; nevertheless, the scarce available research shows the ability of these compounds to combat adipose tissue fibrosis. Saponins derived from Panax japonicus plant was shown to decrease collagen deposition and suppress the expression of genes involved in fibrosis in epididymal white adipose tissue of obese mice [42]. In another study by Wang et al., barberine was shown capable of attenuating adipose tissue fibrosis in mice model of obesity [43]. Isoliquiritigenin, a flavonoid derived from Glycyrrhiza uralensis plant, improves fibrosis in adipose tissue though inhibition of TLR4- and Mincle‐induced expression of fibrosis‐related genes in adipocytes and macrophages [44].
The matrix metalloproteinases are fundamental enzymes in ECM homeostasis, playing a major role in ECM degradation [45]. Increased levels of MMP-2 and MMP-9 in subjects with obesity and type 2 diabetes, as well as obesity-related insulin resistance have been demonstrated in several studies [22, 45, 46]. Here, we showed that Mmp2 and Mmp9 mRNA levels were decreased in 3T3-L1 cells treated with hesperetin. These findings are consistent with previous studies performed in tumor cells, which reported that hesperetin is capable of suppressing both MMP-2 and MMP-9 [47, 48]. These data further support that hesperetin not only reduces ECM proteins but also suppresses the expression of MMP enzymes.
The limitation of this study was that we did not include animal model of obesity and therefore could not evaluate adipose tissue fibrosis at the tissue levels. However, given the potency of the hesperetin in the modulation of crucial markers of fibrosis, animal studies to confirm these beneficial effects, especially long-term influences on insulin resistance, would be plausible to consider for future studies. Since repression of adipose tissue fibrosis improves systemic glucose homeostasis independent of body-weight loss [9], remedies to alleviate this condition would be highly beneficial in the reduction of obesity-associated complications.
Conclusion
In conclusion, the evidence from this study reveals the benefit of hesperetin against the pathophysiology of adipose tissue fibrosis, through downregulation of ECM proteins and major MMPs. The efficacy of hesperetin in reducing the markers of fibrosis introduces this compound as a probable anti-fibrotic therapeutic option in obesity and therefore, hesperetin might be considered as a solution to improve obesity-associated metabolic disorders.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank all the staff and faculty of the Department of Biochemistry, Iran University of Medical Sciences, for their help and support during this research.
Abbreviations
- ECM
extracellular matrix
- Mmp
matrix metalloproteinase
- T2DM
type 2 diabetes mellitus
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- DMSO
dimethyl sulfoxide
- SDS
sodium dodecyl sulfate
- PVDF
polyvinylidene difluoride
- TBST
Tween 20 in tris-buffered saline
- ECL
enhanced chemiluminescent
- ANOVA
one-way analysis of variance
- CCK
cholecystokinin
- TNF-α
tumor necrosis factorα
Authors’ contributions
AT performed the experiments; SEM prepared the manuscript draft; PG contributed in the conduction of experiments; MN and MTY contributed in the conceptualization and analysis of the research; SY contributed in the conduction of experiments; MN performed the conceptualization and analysis and prepared the final manuscript. All authors reviewed the manuscript.
Funding
This study was financially supported by Iran University of Medical Sciences, grant number: 1400-1-4-19488.
Data Availability
Data will be made available from corresponding author (Mitra Nourbakhsh, nourbakhsh.m@iums.ac.ir) upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by Ethics Committee of Iran University of Medical Sciences, ethics code: IR.IUMS.FMD.REC.1400.490. This study was performed on cell line and did not include human subjects.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Collaboration NRF. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19· 2 million participants. The Lancet. 2016;387(10026):1377–96. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemieux I, Després J-P. Metabolic syndrome: past, present and future. MDPI; 2020. p. 3501. [DOI] [PMC free article] [PubMed]
- 3.Aleksandrova K, Egea Rodrigues C, Floegel A, Ahrens W. Omics biomarkers in obesity: novel etiological insights and targets for precision prevention. Curr Obes Rep. 2020;9(3):219–30. doi: 10.1007/s13679-020-00393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buechler C, Krautbauer S, Eisinger K. Adipose tissue fibrosis. World J Diabetes. 2015;6(4):548–53. doi: 10.4239/wjd.v6.i4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Datta R, Podolsky MJ, Atabai K. Fat fibrosis: friend or foe? JCI Insight. 2018;3(19). [DOI] [PMC free article] [PubMed]
- 6.DeBari MK, Abbott RD. Adipose tissue fibrosis: mechanisms, models, and Importance. Int J Mol Sci [Internet]. 2020; 21(17). [DOI] [PMC free article] [PubMed]
- 7.DeBari MK, Abbott RD. Adipose tissue fibrosis: mechanisms, models, and Importance. Int J Mol Sci. 2020;21(17). [DOI] [PMC free article] [PubMed]
- 8.Smith GI, Mittendorfer B, Klein S. Metabolically healthy obesity: facts and fantasies. J Clin Investig. 2019;129(10):3978–89. doi: 10.1172/JCI129186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasegawa Y, Ikeda K, Chen Y, Alba DL, Stifler D, Shinoda K, et al. Repression of adipose tissue fibrosis through a PRDM16-GTF2IRD1 Complex improves systemic glucose homeostasis. Cell Metabol. 2018;27(1):180–94e6. doi: 10.1016/j.cmet.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29(6):1575–91. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun K, Park J, Gupta OT, Holland WL, Auerbach P, Zhang N, et al. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat Commun. 2014;5:3485. doi: 10.1038/ncomms4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gómez-Ambrosi J, Catalan V, Ramírez B, Rodriguez A, Colina I, Silva C, et al. Plasma osteopontin levels and expression in adipose tissue are increased in obesity. J Clin Endocrinol Metabolism. 2007;92(9):3719–27. doi: 10.1210/jc.2007-0349. [DOI] [PubMed] [Google Scholar]
- 13.Nomiyama T, Perez-Tilve D, Ogawa D, Gizard F, Zhao Y, Heywood EB, et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J Clin Investig. 2007;117(10):2877–88. doi: 10.1172/JCI31986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lancha A, Rodríguez A, Catalán V, Becerril S, Sáinz N, Ramírez B, et al. Osteopontin deletion prevents the development of obesity and hepatic steatosis via impaired adipose tissue matrix remodeling and reduced inflammation and fibrosis in adipose tissue and liver in mice. PLoS ONE. 2014;9(5):e98398. doi: 10.1371/journal.pone.0098398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HK, Jeong TS, Lee MK, Park YB, Choi MS. Lipid-lowering efficacy of hesperetin metabolites in high-cholesterol fed rats. Clin Chim Acta. 2003;327(1–2):129–37. doi: 10.1016/S0009-8981(02)00344-3. [DOI] [PubMed] [Google Scholar]
- 16.Scoditti E. Neuroinflammation and Neurodegeneration: the promising protective role of the Citrus Flavanone Hesperetin. Nutrients. 2020;12(8):2336. doi: 10.3390/nu12082336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jayaraman R, Subramani S, Sheik Abdullah SH, Udaiyar M. Antihyperglycemic effect of hesperetin, a citrus flavonoid, extenuates hyperglycemia and exploring the potential role in antioxidant and antihyperlipidemic in streptozotocin-induced diabetic rats. Biomed Pharmacother. 2018;97:98–106. doi: 10.1016/j.biopha.2017.10.102. [DOI] [PubMed] [Google Scholar]
- 18.Mosqueda-Solís A, Sánchez J, Portillo MP, Palou A, Picó C. Combination of Capsaicin and Hesperidin reduces the effectiveness of each compound to decrease the adipocyte size and to induce Browning features in adipose tissue of western Diet Fed rats. J Agric Food Chem. 2018;66(37):9679–89. doi: 10.1021/acs.jafc.8b02611. [DOI] [PubMed] [Google Scholar]
- 19.Kim JK, Jeong HW, Kim AY, Hong YD, Lee JH, Choi JK, et al. Green satsuma mandarin orange (Citrus unshiu) extract reduces adiposity and induces uncoupling protein expression in skeletal muscle of obese mice. Food Sci Biotechnol. 2019;28(3):873–9. doi: 10.1007/s10068-018-0503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosqueda-Solís A, Lasa A, Gómez-Zorita S, Eseberri I, Picó C, Portillo MP. Screening of potential anti-adipogenic effects of phenolic compounds showing different chemical structure in 3T3-L1 preadipocytes. Food Funct. 2017;8(10):3576–86. doi: 10.1039/C7FO00679A. [DOI] [PubMed] [Google Scholar]
- 21.Serino A, Salazar G. Protective role of polyphenols against vascular inflammation, Aging and Cardiovascular Disease. Nutrients. 2018;11(1). [DOI] [PMC free article] [PubMed]
- 22.Ezzati-Mobaser S, Malekpour-Dehkordi Z, Nourbakhsh M, Tavakoli-Yaraki M, Ahmadpour F, Golpour P, et al. The up-regulation of markers of adipose tissue fibrosis by visfatin in pre-adipocytes as well as obese children and adolescents. Cytokine. 2020;134:155193. doi: 10.1016/j.cyto.2020.155193. [DOI] [PubMed] [Google Scholar]
- 23.Derosa G, Ferrari I, D’Angelo A, Tinelli C, Salvadeo SA, Ciccarelli L, et al. Matrix metalloproteinase-2 and – 9 levels in obese patients. Endothelium. 2008;15(4):219–24. doi: 10.1080/10623320802228815. [DOI] [PubMed] [Google Scholar]
- 24.Tinahones FJ, Coín-Aragüez L, Mayas MD, Garcia-Fuentes E, Hurtado-Del-Pozo C, Vendrell J, et al. Obesity-associated insulin resistance is correlated to adipose tissue vascular endothelial growth factors and metalloproteinase levels. BMC Physiol. 2012;12:4. doi: 10.1186/1472-6793-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.XUE M, WEICKERT M, RABBANI N. THORNALLEY P. 338-OR: reversal of insulin resistance by Resveratrol and Hesperetin combination in overweight and obese subjects correlates with decrease in expression of Thioredoxin interacting protein. Diabetes. 2021;70(Supplement_1).
- 26.Lee Y-J, Seo M-J, Lee O-H, Kim K-J, Lee B-Y. Hesperetin inhibits lipid accumulation and ROS production during adipocyte differentiation in 3T3-L1 cells. J Food Biochem. 2017;41(3):e12348. doi: 10.1111/jfbc.12348. [DOI] [Google Scholar]
- 27.Rabbani N, Xue M, Weickert MO, Thornalley PJ. Reversal of insulin resistance in overweight and obese subjects by trans-resveratrol and hesperetin combination—link to Dysglycemia, blood pressure, Dyslipidemia, and Low-Grade inflammation. Nutrients. 2021;13(7):2374. doi: 10.3390/nu13072374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida H, Takamura N, Shuto T, Ogata K, Tokunaga J, Kawai K, et al. The citrus flavonoids hesperetin and naringenin block the lipolytic actions of TNF-α in mouse adipocytes. Biochem Biophys Res Commun. 2010;394(3):728–32. doi: 10.1016/j.bbrc.2010.03.060. [DOI] [PubMed] [Google Scholar]
- 29.Nichols LA, Jackson DE, Manthey JA, Shukla SD, Holland LJ. Citrus flavonoids repress the mRNA for stearoyl-CoA desaturase, a key enzyme in lipid synthesis and obesity control, in rat primary hepatocytes. Lipids Health Dis. 2011;10(1):36. doi: 10.1186/1476-511X-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim HY, Park M, Kim K, Lee YM, Rhyu MR. Hesperetin stimulates cholecystokinin secretion in Enteroendocrine STC-1 cells. Biomol Ther (Seoul) 2013;21(2):121–5. doi: 10.4062/biomolther.2012.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruiz-Ojeda FJ, Plaza-Díaz J, Anguita-Ruiz A, Méndez-Gutiérrez A, Aguilera CM. Adipose Extracellular Matrix Remodeling in Obesity and Insulin Resistance. Cellular and Biochemical Mechanisms of Obesity. 2021:215 – 29.
- 32.Williams L, Layton T, Yang N, Feldmann M, Nanchahal J. Collagen VI as a driver and disease biomarker in human fibrosis. FEBS J. 2022;289(13):3603–29. doi: 10.1111/febs.16039. [DOI] [PubMed] [Google Scholar]
- 33.Vianello E, Kalousová M, Dozio E, Tacchini L, Zima T, Corsi Romanelli MM. Osteopontin: the molecular bridge between fat and cardiac–renal disorders. Int J Mol Sci. 2020;21(15):5568. doi: 10.3390/ijms21155568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeyda M, Gollinger K, Todoric J, Kiefer FW, Keck M, Aszmann O, et al. Osteopontin is an activator of human adipose tissue macrophages and directly affects adipocyte function. Endocrinology. 2011;152(6):2219–27. doi: 10.1210/en.2010-1328. [DOI] [PubMed] [Google Scholar]
- 35.Wang P-y, Feng J-y, Zhang Z, Chen Y, Qin Z, Dai X-m, et al. The adipokine orosomucoid alleviates adipose tissue fibrosis via the AMPK pathway. Acta Pharmacol Sin. 2022;43(2):367–75. doi: 10.1038/s41401-021-00666-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen H, Huang X, Zhao Y, Wu D, Xue K, Yao J, et al. The Hippo pathway links adipocyte plasticity to adipose tissue fibrosis. Nat Commun. 2022;13(1):6030. doi: 10.1038/s41467-022-33800-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu S, Chen X, Chen SY, Wang A, Wu S, Wu YY, et al. Hesperetin derivative decreases CCl(4) -induced hepatic fibrosis by Ptch1-dependent mechanisms. J Biochem Mol Toxicol. 2022;36(10):e23149. doi: 10.1002/jbt.23149. [DOI] [PubMed] [Google Scholar]
- 38.Li JJ, Jiang HC, Wang A, Bu FT, Jia PC, Zhu S, et al. Hesperetin derivative-16 attenuates CCl(4)-induced inflammation and liver fibrosis by activating AMPK/SIRT3 pathway. Eur J Pharmacol. 2022;915:174530. doi: 10.1016/j.ejphar.2021.174530. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Wang T, Liu P, Yang F, Wang X, Zheng W, et al. Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD. Food Funct. 2021;12(9):3898–918. doi: 10.1039/D0FO02736G. [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Li XF, Chen Y, Zhu S, Li HD, Chen SY, et al. Hesperetin derivative attenuates CCl(4)-induced hepatic fibrosis and inflammation by gli-1-dependent mechanisms. Int Immunopharmacol. 2019;76:105838. doi: 10.1016/j.intimp.2019.105838. [DOI] [PubMed] [Google Scholar]
- 41.Abdel-Rahman RF, Fayed HM, Ogaly HA, Hussein RA, Raslan MA. Phytoconstituents of Sansevieria suffruticosa N.E.Br. Leaves and its Hepatoprotective Effect via activation of the NRF2/ARE signaling pathway in an Experimentally Induced Liver Fibrosis Rat Model. Chem Biodivers. 2022;19(4):e202100960. doi: 10.1002/cbdv.202100960. [DOI] [PubMed] [Google Scholar]
- 42.Hu X, Sun A, Chen H, Yan X, Ding F, Zheng P, et al. Saponins from Panax japonicus alleviate adipose tissue fibrosis and metabolic dysfunction in high-fat-diet-induced obese mice. Biomarkers. 2022;27(8):784–94. doi: 10.1080/1354750X.2022.2122566. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Ye X, Hua Y, Song Y. Berberine alleviates adipose tissue fibrosis by inducing AMP-activated kinase signaling in high-fat diet-induced obese mice. Biomed Pharmacother. 2018;105:121–9. doi: 10.1016/j.biopha.2018.05.110. [DOI] [PubMed] [Google Scholar]
- 44.Yoshinori N, Yasuharu W, Hiroe H, Kiyoshi T, Isoliquiritigenin . A unique component that attenuates adipose tissue inflammation and fibrosis by targeting the Innate Immune Sensors. In: Hiroshi S, editor. Licorice ingredients. Rijeka: IntechOpen; 2017. [Google Scholar]
- 45.Sezgin SBA, Bayoglu B, Ersoz F, Sarici M, Niyazoglu M, Dirican A, et al. Downregulation of MMP-2 and MMP-9 genes in obesity patients and their relation with obesity-related phenotypes. Turkish J Biochem. 2022;47(4):425–33. doi: 10.1515/tjb-2021-0124. [DOI] [Google Scholar]
- 46.Boumiza S, Chahed K, Tabka Z, Jacob M-P, Norel X, Ozen G. MMPs and TIMPs levels are correlated with anthropometric parameters, blood pressure, and endothelial function in obesity. Sci Rep. 2021;11(1):20052. doi: 10.1038/s41598-021-99577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu D, Li J, Hu X, Ma J, Dong W. Hesperetin inhibits Eca-109 cell proliferation and invasion by suppressing the PI3K/AKT signaling pathway and synergistically enhances the anti-tumor effect of 5-fluorouracil on esophageal cancer in vitro and in vivo. RSC Adv. 2018;8(43):24434–43. doi: 10.1039/C8RA00956B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MAO C-M. Effect and mechanism of hesperetin on P-selectin mediated breast cancer MDA-MB-231 metastasis. Chin Traditional Herb Drugs. 2017:714–21.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available from corresponding author (Mitra Nourbakhsh, nourbakhsh.m@iums.ac.ir) upon reasonable request.