Abstract
In this study, we utilized selectively modified, biodegradable polymer-based polyplexes to deliver custom, exogenous miR-148b mimics to induce apoptosis in human lung cancer (A549) cells. The gene regulatory effects of the payload miRNA mimics (miR-148b-3p) were first evaluated through bioinformatic analyses to uncover specific gene targets involved in critical carcinogenic pathways. Hyperbranched poly(β amino ester) polyplexes (hPBAE) loaded with custom miR-148b mimics were then developed for targeted therapy. When evaluated in vitro, these hPBAE-based polyplexes sustained high intracellular uptake, low cytotoxicity, and efficient escape from endosomes to deliver functionally intact miRNA mimics to the cytosol. High-resolution confocal microscopy revealed successful intracellular uptake, cell viability was assessed through qualitative fluorescence microscopy and fluorescence-based DNA quantification, and successful cytosolic delivery of intact miRNA mimics was evaluated using real-time polymerase chain reaction (RT-PCR) to demonstrate target gene knockdown. The hPBAE-miRNA mimic polyplexes were shown to induce apoptosis among A549 cells through direct modulation of intracellular protein expression, targeting multiple potential carcinogenic pathways at the gene level. These results indicated that spatially controlled miR-148b mimic delivery can promote efficient cancer cell death in vitro and may lead to an enhanced therapeutic design for in vivo application.
Graphical Abstract

INTRODUCTION
Despite decades of research, lung cancer rates have continued to rise over the last 50 years and today is the leading cause of cancer-related deaths.1,2 Specifically, there were an estimated 228,150 new cases, leading to an estimated 142,670 deaths in the United States in 2019.1 The majority of these lung cancer cases are categorized under two major subtypes, either small cell lung cancer (SCLC) or non-small-cell lung cancer (NSCLC).3 More than 85% of these underlying pathologies are consistent with the NSCLC subtype.3 These cancers are further categorized into adenocarcinoma (ADC), squamous cell carcinoma (SCC), and large cell carcinoma (LCC).4
Current, state-of-the-art cancer therapies often exhibit off-target effects that cause debilitating side effects in patients; thus, the development of novel methods to selectively kill cancer cells while reducing side effects is a critical need.5–7 Small ribonucleic acids (sRNAs) such as small interfering-RNAs (siRNAs) or micro-RNA (miRNAs) have recently gained attention for their prognostic and therapeutic capabilities in treating cancer due to their specificity and biocompatibility.8–10 Micro-RNAs are single-stranded short 20 to 23 nucleotide RNAs that negatively regulate the translation and stability of mRNAs.8 Recent advances in cancer biology have shown the importance of miRNAs in tumor growth, invasion, angiogenesis, and immune invasion.9–14 Efficient and effective delivery of specific anti-cancer miRNA, whose target genes are differentially expressed in tumors compared to surrounding tissue, pushes the boundaries in the development of innovative gene-level therapies. For example, recent studies have demonstrated that dysregulation of let-7,15,16 miR-34,17,18 miR-148b,19,20 and miR-200c16,21,22 may promote oncogenesis, and these miRNAs have been shown to be downregulated across multiple cancers.23–30 Therefore, restoring these tumorsuppressing miRNAs has the potential to improve current cancer treatments.31–35 We have explored the therapeutic potential of multiple customized miRNA mimics across a multitude of models, including cancer therapies, and directed differentiation of progenitor cells.36–41 For this study, past observation of the involvement of miR-148b-3p in hepatic cancer, gastric cancer, colorectal cancer, pancreatic cancer, and skin cancer models led to our examination using non-small-cell lung cancer.23–30
Multiple strategies have been developed to modulate miRNA function by delivering either exogenous miRNA mimics or miRNA inhibitors via viruses and liposomes.31,32,42–44 While cationic or neutral lipid/polymer-based nanoparticles can suppress tumor growth in a variety of cancer models,33,45 many of them lack efficient intracellular uptake required for delivery of viable miRNA mimic therapeutics.46 Thus, miRNA mimic-based therapeutics may provide a more targeted and effective way of treating these poor prognosis cancers. Because these miRNAs have demonstrated the ability to modulate abnormal protein levels in cancerous cells to mitigate disease, understanding the differences in gene expression has major implications in understanding the pathogenesis of diseases and the subsequent therapies that could be developed to combat their progression at the genetic level. Poly(β-amino esters) that utilize hyperbranching functional groups (hyperbranched poly(β amino ester) (hPBAEs)) form nanoscale polyplexes when introduced in solution containing single-stranded small ribonucleic acids (siRNAs).47–49. These complexes allow for efficient delivery of the class of siRNA known as micro-RNAs (miRNA) mimics.47–49 The hPBAE polyplexes are pH-sensitive, allowing the payload miRNA mimics to release from the nanoparticle upon intracellular uptake due to changes in the environment within the later-stage endosomes.50–52 These hPBAE polyplexes can be loaded with specific, stabilized miRNA mimics that target cancer-associated gene pathways to disrupt lung cancer cell survival and proliferation in a tunable and differential manner.47–52 Developing novel therapeutics and the methods essential to their optimal delivery in cancer cell models is crucial to advancing research in oncology.
In this study, we developed a noninvasive, nanoscale approach to induce apoptosis in lung cancer cells utilizing hPBAE-based polyplexes carrying small, gene-regulating nucleic acid therapeutics. We demonstrated that hPBAE-based polyplexes could serve as effective vectors for the release of therapeutic miRNA mimic payloads due to the well-defined hPBAE-miRNA mimic biodegradable, electrostatic interactions that disassemble under intracellular uptake and cytosolic entry conditions.
MATERIALS AND METHODS
Materials and Reagents.
Custom-modified miR-148b (5′–6-Cy5–2′Ome–[UCA GUG CAU CAC AGA ACU UUG U]–3′) and custom-modified noncoding control miRNA oligo mimics (5′–[CGG UAC GAU CGC GGC GGG AUA]–3′) were acquired through Integrated DNA Technologies, Inc. (Coralville, Iowa). Primary antibodies for the active and inactive forms of the caspase-3 protein, actCASP-3, and proCASP-3 were purchased from ABCAM (Cambridge, CB2 0AX, U.K.) and Cell Signaling Technology (Danvers, Massachusetts), respectively. A549 cells (Human Caucasian Male Lung Adenocarcinoma), Proteinase K, and sodium acetate buffer solution (pH 5.2) were purchased from Millipore Sigma (St. Louis, MO). Cell culture reagents including Dulbecco’s modified Eagle’s medium (DMEM) for mammalian cell culture, OptiMEM media, fetal bovine serum (FBS), penicillin–streptomycin (PS), and trypsin ethylenediamine tetraacetic acid (EDTA), as well as the NucBlue staining kit, the Verso cDNA Synthesis kit, and the PowerUp SYBR Green Master Mix, were all purchased from Thermo Fisher Scientific (Waltham, Massachusetts). The LIVE/DEAD Viability/Cytotoxicity kit, the Quant-iT PicoGreen dsDNA fluorescence assay kit, and the Trizol RNA isolation kit were purchased from Invitrogen (Carlsbad, California).
Cell Bioinformatics and miRNA Mimic Target Selection.
To model regulation of intracellular mRNA and small RNA in an initial in vivo model, a mouse model of ADC was assessed utilizing basic RNA sequencing (RNAseq) techniques. Due to the link between smoking and lung cancer, tobacco-related carcinogen chemical induction of tumors in mouse models has been widely studied, frequently exhibiting activated KRAS-pathway (Kirsten rat sarcoma viral oncogene homologue) mutations.53 These KRAS-mutated mouse models exhibit tumors that mimic features in NSCLC, and more specifically ADC.54 The BioProject PRJNA431556 (https://www.ncbi.nlm.nih.gov/) was accessed to download the three replicates of ADC and WT RNAseq data from the SRA study SRP131222. These data were imported into Galaxy (Version 2.10.4 + Galaxy1) as two separate text files containing the three RNAseq accession numbers per file as a list. These two text files were accessed through Galaxy with the tool Faster Download and Extract Reads in FASTQ format from NCBI SRA (Galaxy Version 2.10.4 + Galaxy1). The history was kept clean by removing the empty/unnecessary paired-end data or single-end data, and other empty data files that are created through this process. The newly imported SRA raw data was analyzed for quality using the FastQC Read Quality Reports (Galaxy Version 0.72 + Galaxy1) tool to make sure the data were clear before proceeding. After this qualitative measure, the data for the genome (imported the homo sapiens genome fasta format file from the Ensembl website ftp://ftp.ensembl.org/pub/release-99/fasta/homo_sapienss/dna/) were imported into Galaxy. The Kallisto Quant (Galaxy Version 0.46.0.4) tool was used to quantify read count data from the cDNA transcriptome accessed from Ensembl (ftp://ftp.ensembl.org/pub/release-99/fasta/mus_musculus/dna/) and uploaded into Galaxy. The data output by the Kallisto Quant analysis was fed into the DESeq. 2 (Galaxy Version 2.11.40.6 + Galaxy1) tool on Galaxy to provide all of the differential expression data from the totalRNAseq accessions. The homo sapiens reference genome and the SRA raw data were aligned using the HISAT2, a fast and sensitive alignment program (Galaxy Version 2.1.0 + Galaxy5) in Galaxy. HISAT2 created the BAM files for the ADC and WT RNAseq data individually, which were then merged into compound, separate files for subsequent visualization and quantification. The merged BAM and BAM index files were downloaded to the local device for visualization in Integrative Genomics Viewer (IGV) software (https://software.broadinstitute.org/software/igv/download). IGV software was loaded on the local device, and the homo sapiens gene annotation file (.gff3) was uploaded for gene identification searchability (ftp://ftp.ensembl.org/pub/release-99/gff3/homo_sapiens/). Once the correct genome was loaded into IGV with the gene annotation file loaded as a track, the relevant BAM files produced through HISAT2 were imported as separate tracks for gene coverage visualization. Differentially expressed genes, determined through the RNAseq read alignment analysis in Galaxy previously described, were utilized as the input data for gene ontology classification from the gene ontology website (Version 2020–04-2). The biological process, molecular function, and cellular component ontologies of both the upregulated and downregulated genes were investigated for pathway-related features suggested to be critical in cancer cell proliferation. The biological process, molecular function, and cellular component ontologies were filtered relative to the lowest false discovery rate (FDR) for annotation quality prior to being considered for further assessment. The three filtered ontological lists for both upregulated and downregulated genes were then saved on the local device for future identification of the documented ADC-significant pathologies following the miRNA expression results achieved in this study. The same BioProject PRJNA431556 was accessed to download the three replicates of ADC and WT sRNAseq data from the SRA study SRP131222 for the miRNA analysis. These data were imported into Galaxy as two separate text files containing the three RNAseq accession numbers per file as a list just as the RNAseq data. The known homo sapiens miRNA hairpin structures were downloaded from miRbase, Release 22.1 (http://www.mirbase.org/summary.shtml?org=has) for Galaxy read alignment and feature counts procedures using HISAT2 as above.55 Samtools idxstats (Galaxy Version 2.0.3) was used to gather the feature count data from the BAM files in tabular format for further analysis. The feature counts and Log2FC data were calculated through statistical analysis of the alignment of miRNAseq data to the annotated version of known miRNA sequences in homo sapiens (from the hsa.gff3 file located at http://www.mirbase.org/ftp.shtml) and the same human genome files that were used in the totalRNAseq analysis. All tabular manipulations carried out in Galaxy were through the use of the Table Compute (Galaxy Version 0.9.2), Cut (Galaxy Version 1.1.0), Filter (Galaxy Version 1.1.0), and Sort (Galaxy Version 1.1.0) tools in Galaxy. The differentially expressed miRNA, determined through the miRNAseq read alignment analysis in Galaxy previously described, were utilized as the input data for the prediction of miRNA gene targets through TargetScan website (release 8.0).56 These miRNA gene target results were then compared against the genes found to be differentially expressed through tabular data manipulation in Microsoft Excel. This entire process was then repeated using a dataset from transcriptomic analyses of a human model of NSCLC (PRJNA485691). This in vitro model, along with the initial data from the in vivo model was dissected and interpreted in the coming bioinformatics data.
Synthesis of Poly(β Amino Ester).
DD90–118 hyperbranched PBAE (DD90–118 hPBAE) was synthesized using a previously published approach with slight changes,47 as illustrated in Figure 1A. In brief, acrylate (DD), backbone amine (90), and trifunctional amine monomers were reacted in a ratio of 1:0.5:0.2. Monomers were agitated for 4 h at 40 °C in anhydrous dimethylformamide at a 150 mg/mL concentration, then for 48 h at 90 °C. The solutions were allowed to cool to 30 °C before adding 1.5 molar equivalent end cap amine (118) to the excess acrylate and stirred for another 24 h. Dropwise precipitation into cold anhydrous diethyl ether spiked with glacial acetic acid was used to purify the polymers, then vortexed, and centrifuged at 1250 G for 2 min to pellet the polymer. To obtain DD90–118 hPBAE, the supernatant was removed and the polymer was washed twice more in fresh diethyl ether and dried under vacuum for 48 h. The schematic outlining the synthesis of the custom hPBAE is shown in Figure 1A. The final DD90–118 product was characterized by 1H NMR spectroscopy as reported in the Supporting Information (Figure S1). The polymers were maintained at a temperature of –20 °C.
Figure 1.

Detailed schematic of hPBAE-miRNA mimic polyplex synthesis. (A) Depiction of poly(β amino ester) building blocks used in the assembly of custom DD90–118 hPBAE. (B) Representation of intracellular miRNA processing required to form mature intracellular miRNAs, of which custom, exogenous miRNA mimics were used to synthesize hPBAE-miRNA mimic polyplexes.
Synthesis of hPBAE-miRNA Mimic Polyplexes.
The necessary amount of hPBAE-miRNA polyplexes was determined using a bottom-up approach, calculating how much miRNA mimics would be necessary for all treatments and analyses. Individual hPBAE and miRNA mimic aliquots were prepared in equal volumes of sodium acetate buffer (25 mM, pH 5.2) at a 50:1 hPBAE-to-miRNA mimic mass ratio. The hPBAE and miRNA mimic aliquots were then combined in solution 1:1 (v:v) to reach a final solution concentration of 10 ng/μL (miRNA mimic content).47 The hPBAE-miRNA mimic treatment samples were cyclically pipetted for 10 min and allowed to sit for a total of 30 min prior to further use or analysis. Representation of the final solution mixture, containing the hPBAE-miRNA mimic polyplex treatment groups, can be seen in Figure 1B.
Characterization of hPBAE-miRNA Polyplexes.
The hPBAE-miRNA mimic polyplexes were prepared in sodium acetate buffer solution (25 mM, pH 5.2) under sterile conditions and evaluated in DTS0012 disposable polystyrene cuvettes along with DTS1070 reusable cuvettes. The size, dispersity, and surface charge of the hPBAE-miRNA nanoparticle complexes were analyzed using a Malvern Zetasizer (Malvern Panalytical Ltd, Malvern WR14 1XZ, United Kingdom) as previously described.36,41,57,58 The size and morphology of the hPBAE-miRNA complexes were further verified through the use of an FEI Titan Krios (Thermo Fisher Scientific, Inc.) outfitted with a Cs: spherical aberration corrector, Volta Phase Plate for enhanced contrast, Falcon 3EC direct electron detector camera, and a Quantum GIF (K2) energy filter with K2 direct electron detector. Samples were prepared in the VitrobotTM Mark IV (Thermo Fisher Scientific, Inc.) on carbon-coated copper transmission electron microscopy (TEM) grids using a highly concentrated hPBAE-miRNA mimic polyplex solution.
Cell Culture.
A549 cells (Human Caucasian Male Lung Adenocarcinoma) were cultured at 37 °C, 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) for mammalian cell culture enhanced with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin–streptomycin.40 A549 cells were cultured to passage 3 and utilized in experimental analyses through passage 10 before discontinuing further split cycles.
Cell Viability and Proliferation Assays.
The A549 cells were transfected with hPBAE-miRNA mimic treatment groups and then allowed to further incubate for 24 h. Post-incubation, cell viability was assessed using the LIVE/DEAD Viability/Cytotoxicity kit and imaged with an Olympus IX73 fluorescence microscope (Olympus, Center Valley, PA).40,59 For the LIVE/DEAD assay, the cells were washed with phosphate-buffered saline (PBS) and incubated at 37 °C for 30 min with a 2 μM calcein-AM and 4 μM EthD-1 working solution. ImageJ (NIH, Bethesda, MD) was used for image processing. Proteinase K (0.5 mg/mL) was used to lyse and digest cell culture samples (16 h at 55 °C) beginning 24 h following transfection with the hPBAE-miRNA mimic treatment groups. Cell proliferation was quantified using direct DNA quantification using the Quant-iT PicoGreen dsDNA fluorescence assay kit to quantify the amount of dsDNA per sample according to the manufacturer’s protocols. Total DNA content was used to determine the cell count.40,59,60 Equal volumes of Quant-iT PicoGreen dsDNA reagent were combined with the volumes of the wells and the fluorescence intensity was then measured in each well at 480/520 nm (Excitation/Emission) using a Spectramax iD3 Multi-Mode Microplate Reader (Molecular Devices).
Confocal Microscopy for Intracellular Uptake and Release.
A549 cells were seeded on 35 mm dishes and maintained in cell culture at a density of 500,000 cells per dish using OptiMEM medium. After 16 h, 100 μL of hPBAE polyplexes carrying Cy5-tagged miR-148b-3p mimics (10 ng/μL) was added to the dish. The cell nuclei were stained with NucBlue stain according to the protocol. The cells were allowed to incubate with hPBAE-miRNA mimic polyplexes, and 2 h after transfection, the samples were imaged using the 645/665 nm (excitation/emission) and 405/420–480 nm (excitation/emission) channels as previously described.36,40,41 A Zeiss LSM 880 confocal microscope with FLIM (Zeiss, Oberkochen, Germany) was used to take images of the intracellular delivery of hPBAE-miRNA polyplexes. ImageJ processing techniques to extract quantitative data from pixels of bio-format images were used to generate data corresponding to cell viability analyses.39,61,62
Intracellular Uptake via Flow Cytometry.
Human lung adenocarcinoma (A549) cells were cultured, transfected, stained, and analyzed using flow cytometry as previously described.36,41 Briefly, A549 was grown in 35 mm cell culture dishes to 80% confluency (106 cells total) and transfected with the hPBAE polyplexes in OptiMEM for 4 h. The cells were then washed with Dulbecco’s phosphate-buffered saline (DPBS) and detached from culture plates using Trypsin EDTA (0.25%, 2.21 mM) to remove the adherent cell culture. The suspended cells were centrifuged (250g, 5 min) to remove the wash supernatant, followed by direct resuspension in the fixative (4% paraformaldehyde) for 15 min at 4 °C. Samples were once again washed and resuspended in DPBS before flow cytometry analysis. The fixed cells were loaded into a 96-well plate (250 μL per well, 4 wells/sample). The cells were analyzed using an LSRFortessa cytometer and the software FlowJo (version 10). PBS was used to measure the background noise and cells without hPBAE nanoparticles were used as the negative control. A flow rate of 10 μL/min was used and 7,000–10,000 events per second were recorded in all samples. The optimal threshold of the FIFC channel was set at 3,000.
Gene Expression Analysis.
Total RNA was isolated from cells using Trizol, and the expression levels were measured by real-time quantitative polymerase chain reaction (RT-qPCR) (Applied Biosystems) using Verso cDNA Synthesis kit and PowerUp SYBR Green Master Mix as previously described.36,41 The sequences of primers were selected from PrimerBank and analyzed using Primer-BLAST to ensure their specificity as previously described.36 The gene expression was normalized with the housekeeping gene 18S using the –ΔΔCt method,36,63 and the primers used in this study are listed in the Supporting Information (Figure S2).
Western Blot Analysis.
The presence of apoptotic signals via analysis of intracellular protein content within the A549 treatment groups was detected as previously described.36,41 Cells were washed in ice-cold PBS and lysed in radioimmunoprecipitation assay (RIPA) buffer with protease/phosphatase inhibitors. Protein (50 μg) was separated using 4–8% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS/PAGE) gels and transferred to nitrocellulose using the Trans-Blot TURBO transfer system (Bio-Rad), according to the manufacturer’s instruction. Primary antibodies for the active and inactive forms of the caspase-3 protein, actCASP-3, and proCASP-3 respectively, were utilized to detect apoptotic signal among A549 groups treated with hPBAE-miRNA mimic polyplexes. Primary antibodies against the common intracellular protein β-actin (Millipore) were used as the loading control.
Statistical Analyses.
Quantitative results were expressed as mean ± standard deviation (SD). Sample size (n) is indicated in the figure descriptions. Statistical analysis was performed via two-way analysis of variance (ANOVA), followed by Tukey’s post hoc testing. Statistical significance was set at p < 0.05. The software Prism GraphPad (version 9.2.0) was used for all statistical analyses.
RESULTS AND DISCUSSION
Bioinformatic Identification of Advantageous miRNAs for Gene Modulation, and Analysis of Cascading Consequences among Target Pathways.
The feature counts and Log2FC data from the alignment of miRNAseq data to the annotated version of known miRNA sequences indicated downregulated expression of miR-148b-3p in both the mouse model of ADC and among NSCLC cell lines including A549 cells specifically. The culmination of tabular data obtained from alignment analyses depicting attached associations connecting gene targets to molecular function and pathogenesis can be seen in Figure 2.
Figure 2.

Identification and selection of miR-148b as a potential therapeutic modulation of carcinogenic gene pathways in A549 cells to drive selective apoptosis. (A) Additional analyses outlining significant consequences of supplementary miR-148b on the predicted target CEACAM, (B) ALCAM, (C) SOX2, and (D) ITGA5. Data represented for target-based and experimentally reported connection (p < 0.0001). Green plus symbols indicate cascades reported to amplify lung cancer progression. Red octagon symbols indicate cascades reported to diminish normal cellular function (p < 0.0001). Additional analyses are available in Supporting Information Figures S3, S4, and S5.
Synthesis and Characterization of hPBAE-miR148b Polyplexes.
To achieve efficient and controlled intracellular uptake, hyperbranched poly(β amino ester) polymers were utilized as the vehicles to deliver miR-148b-3p mimics that were electrostatically coupled to the polymer to form the nanosized polyplexes when combined at specific ratios that exploit the nitrogen/phosphorous (N/P) intermolecular interactions.47 The hPBAE-miRNA mimic polyplexes were prepared at an N/P ratio of 10, and they exhibited an average diameter size of 146.16 ± 0.60 nm as indicated by the average diameter measured determined through dynamic light scattering (DLS) and shown in Figure 3A, which is firmly within the optimal range for intracellular uptake.47,64,65 These polyplexes also had a narrow size distribution of 0.118 PDI as determined by DLS and shown in Figure 3B. An increasingly cationic ζ-potential was also reported, as shown in Figure 3C, as a result of miRNA mimic addition within the hPBAEs in a predictable manner based on the N/P ratio. Although the miRNA that we are adding would add a negative charge to the overall polyplex, the ζ-potential measurement is indicative of the surface charge on the nanoparticles. At specific N/P ratios, the overall surface charge of the polyplexes is negative due to efficient internalization of the miRNA mimics within the hPBAE, where positively charged functional groups are maintained around the exterior surface. The ζ-potential of the hPBAE-miR148b mimic polyplexes was 52.93 ± 0.40 mV, as shown in Figure 3C, demonstrating excellent colloidal stability in solution, in agreement with the literature.47,66 The concentration of the encapsulated miRNA mimic was confirmed by fluorescence measurements of the Cy5 tag conjugated to the miRNA mimic following our previously established methods and standard curves.36,40,41 An additional experiment was also performed to confirm that no free miRNA mimics were present in solution after encapsulation (Supporting Information Figure S6). Cryo-transmission electron microscopy (cryo-TEM) analysis showed that hPBAE-miR148b polyplexes were spherical in morphology and supported the DLS-based size results previously mentioned and can be seen in Figure 3D,E.
Figure 3.

Characterization of hPBAE polyplexes. (A) Nanoparticle size characterization via dynamic light scattering (DLS). (B) Polydispersity of hPBAE polyplexes in solution (n = 3 per group). ***Significant difference (p < 0.001). (C) Surface charge of hPBAE polyplexes through ζ-potential analysis (n = 3 per group). ***Significant difference (p < 0.001). (D) Size and morphology of hPBAE polyplexes (hPBAE-miR148b) through cryo-transmission electron microscopy (cryo-TEM). (E) Partial zoom of (D) with scale bar (50 nm).
The average diameter, charge, and morphology of the hPBAE-miRNA mimic polyplexes as measured via DLS, ζ-potential, and cryo-TEM were in agreement with previously reported studies.47–49 Both ζ-potential and DLS indicated successful polyplex formation and miR-148b mimic loading due to the predictable stabilization in the hydrodynamic radius and polydispersity index. The size, morphology, and stability of the hPBAE-miRNA mimic system indicated successful polyplex formation and miRNA mimic incorporation. Minimal fluorescently tagged miRNA mimics were detected upon centrifugation and analysis of supernatant fluorescence when compared against miRNA-stripped hPBAE polyplexes, indicating efficient encapsulation and little leakage of the miRNA mimics within the hPBAE delivery system.
hPBAE-miRNA Mimic Nanoparticle Cellular Uptake.
Confocal imaging of human non-small-cell carcinoma cells (A549) was performed to validate cellular uptake and cytosolic entry of miRNA mimic-loaded polyplexes via fluorescent detection of the Cy5-tagged miR-148b mimics (Figure 4A). The DAPI channel (420 nm excitation/480 nm emission) was used to observe the stained (with DAPI) nuclei of A549 cells from a nontreatment control compared against treatment groups that had been transfected with the hPBAE-miR148b and subsequently washed to confirm intracellular delivery. The Cy5 channel (645 nm excitation/665 nm emission) was used to observe the Cy5-tagged exogenous miR-148b-3p mimics within the cells in proximity to the nucleus. Much of this fluorescence signal was identified in perinuclear regions of the cytoplasm for hPBAE-miR148b treated cells, without requiring the use of additional transfection reagents, ultrasonication, or electroporation. After longer treatment periods, the fluorescence of the Cy5-tagged exogenous miR-148b-3p mimics began to disperse within the cytosol and to reduce significantly prior to cell death. Flow cytometry results also indicated verification of the qualitative analyses, with greater than 62% uptake efficiency of FAM-tagged hPBAE-miR148b polyplexes in vitro when compared against nontreated controls, as seen in Figure 4B.
Figure 4.

Intracellular uptake of hPBAE polyplexes in A549 lung cancer cells. (A) Qualitative uptake of hPBAE polyplexes (left: nontreatment control, right: treatment group) showing perinuclear (blue fluorescence) localization of miR148b (pink fluorescence) containing polyplexes under confocal microscopy. Scale bar 20 μm. Additional confocal and bright-field images are available in Supporting Information Figure S7. (B) Quantitative uptake of hPBAE polyplexes through fluorescence (FAM) detection of cells containing hPBAE-miRNA polyplexes using flow cytometry.
These results mirror prior nanoparticle-based miRNA mimic delivery experiments performed by our group demonstrating strong perinuclear localization of oligonucleotides after intracellular release,36–38,40 as well as reflecting similar results from other groups examining the delivery of other cargo via hPBAE-based polyplexes.47–52,66–69 The driving pH-stimulated mechanism provides control over the therapeutic release profile in cells/tissues allowing for a tunable, gene-level approach to controlled drug delivery and release in vitro as well as in vivo.47 This drove the hypothesis that if the hPBAE polyplexes could deliver functional miRNA mimics that target dysregulated gene pathways critical to the uncontrolled proliferation of cancer cells, then one could exploit genetic variances among cell lines to controllably disrupt tumor viability and growth.
Efficacy of hPBAE-miR148b Mimic Polyplexes In Vitro.
Intracellular uptake and viability post-transfection with the hPBAE-miRNA mimic polyplexes were analyzed to determine the dose-dependent dynamics capable of sustaining significant therapeutic response due to direct modulation of downstream gene targets in vitro. To evaluate the ability of miR-148b-3p mimic to kill oncogenic cells, we analyzed the effects of our nanoparticle treatment on the highly annotated A549 non-small-cell lung cancer cell line. Cell death was measured using LIVE/DEAD cell staining kit and the Quant-iT PicoGreen assay kit 24 h after initial transfection of A549 cells with the hPBAE-miR148b mimic polyplexes. To rule out the effects of adverse toxicity, a scrambled RNA sequence (hPBAE-miRNC) was implemented as a negative control to detect cytotoxicity of hPBAE polyplexes carrying “off-target” miRNA mimic payloads, along with another negative control to detect hPBAE-specific cytotoxicity (hPBAE) containing no miRNA mimic therapeutics. A549 cell viability was assessed in vitro through fluorescence microscopy and imaging of cultured cells via the LIVE/DEAD fluorescent detection assay kit that directly corresponded to treatment with the hPBAE-miR148b mimic polyplexes against the relevant controls, as shown in Figure 5A (green: calcein-AM/red: ethidium homodimer 1). Cell numbers were indirectly measured via dsDNA quantification. The qualitative LIVE/DEAD assay images of Figure 5A were analyzed using ImageJ to quantify the relative green/red intensity, and the results are shown in Figure 5C.
Figure 5.

Cytotoxicity and cell viability in vitro. (A) Qualitative cell viability assessment through LIVE/DEAD assay. (B) Quantitative cell viability assessment through direct DNA detection using PicoGreen dsDNA assay; the dotted line represents the initial seeding density of A549 cells (n = 3 per group). ***Significant difference (p < 0.001). ****Significant difference (p < 0.0001). (C) Image analysis corresponding to the qualitative data seen in (A) using ImageJ measurement techniques. *Significant difference (p < 0.1). ***Significant difference (p < 0.001).
Significant cell death as measured by loss of calcein-AM signal, and increasing ethidium homodimer 1 red fluorescence was observed in hPBAE-miR148b treated as well as in the dead controls when compared against the negative control treatment groups. The viability of A549 lung adenocarcinoma cells was not significantly reduced among the hPBAE nor hPBAE-miRNC negative controls when compared against the no treatment group, demonstrating sufficient cytocompatibility. A control experiment has been performed to demonstrate the selective apoptosis of miR-148b mimic (Supporting Information Figure S8). Human adipose-derived stem cells were transfected with either hPBAE-miRNC or hPBAE-miR148b, similarly to that reported with the A549 lung adenocarcinoma cells in Figure 5. No visible differences were apparent among the different groups on the LIVE/DEAD fluorescent images. Similarly, no significant difference in cell numbers could be measured between the no treatment, hPBAE-miRNC, and hPBAE-miR148b groups for the adipose-derived stem cells (Supporting Information Figure S8).
Target Gene Knockdown.
Despite having verified intracellular uptake of the hPBAE-miRNA nanoparticles, the successful delivery of bioactive miRNA mimics needed to be determined as there are multiple additional factors known to hinder miRNA efficacy in vitro.46 After nanoparticles are endocytosed by cells, likely due to clatherin-mediated mechanisms,32,41,66–68,70,71 the miRNA mimics must escape from endosomes intact to interact with RNA-induced silencing complexes (RISCs)10–13,18–20 within the cytosol to modulate intracellular mRNA levels. Moreover, a decrease in endosomal pH during maturation and potential lysosome fusion could lead to unintended degradation of the miRNA mimics.48–51,72,73 Therefore, validation for the presence of functional miRNA mimics and successful modulation of the RNA-induced silencing complex was achieved through analyzing target gene expression. To determine potential molecular mechanisms of miR-148b-3p-induced apoptosis, four predicted genes that have demonstrated tumor suppression capabilities were selected for PCR analysis, where one or multiple combinations of these genes might be responsible for the specific cancer cell apoptosis indicated by this study.20,74 Specifically, the genes CEACAM, ALCAM, ITGA5, and SOX2 were identified as direct targets of miR-148b-3p based upon previous literature TargetScan verification, and the bioinformatic analyses that produced Figure 2 as previously shown.20,56,74 Downregulation of these target genes in response to the hPBAE-miR148b mimic polyplex treatments was confirmed in the A549 cells and is shown in Figure 6A–D.
Figure 6.
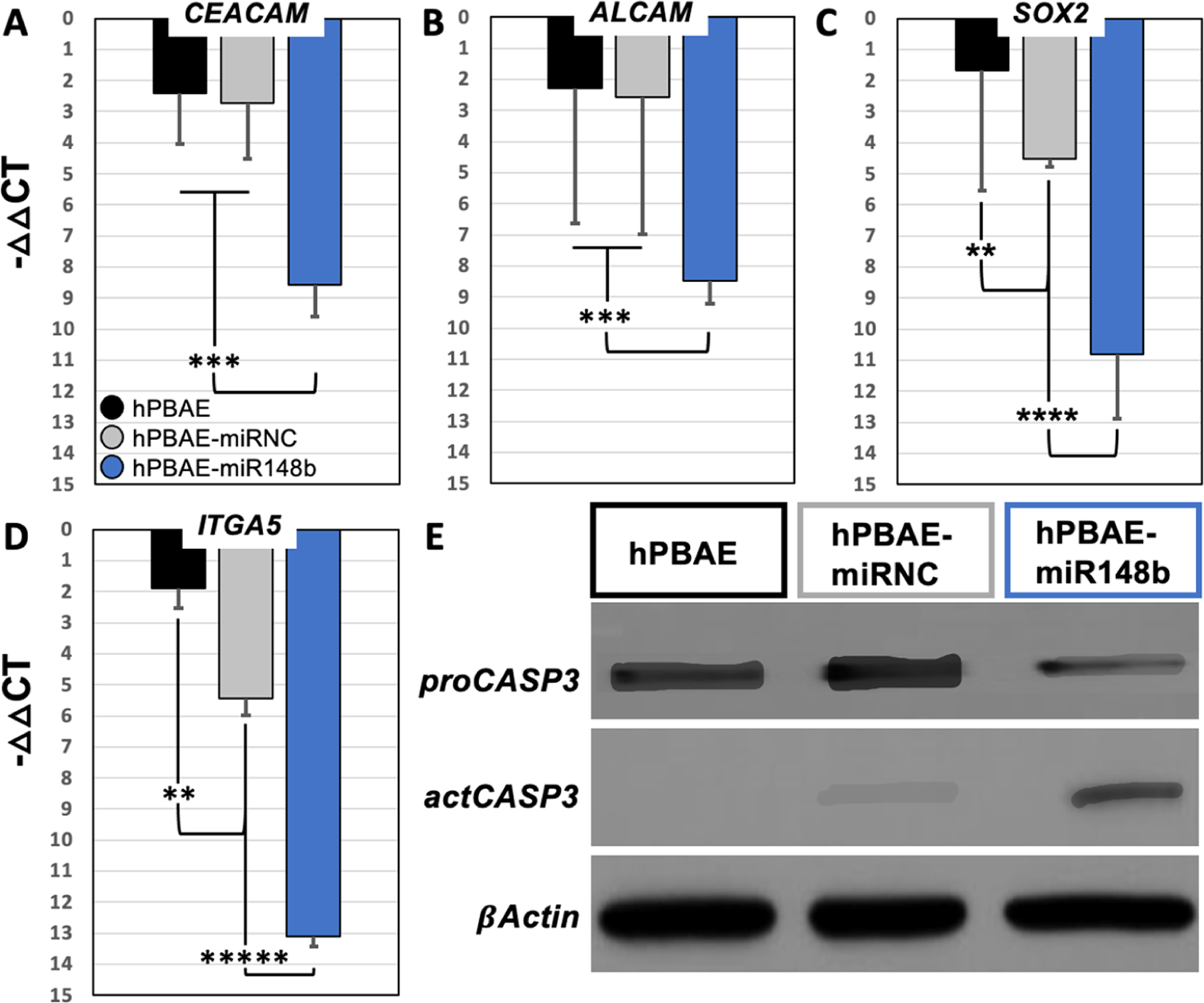
Gene expression and target suppression analysis in vitro. Quantitative gene expression analysis of predicted targets of miR-148b-3p using RT-PCR to measure intracellular levels of the predicted target (A) CEACAM, (B) ALCAM, (C) SOX2, and (D) ITGA5. Qualitative analysis of intracellular levels of caspase-3 known to direct apoptotic cell signaling pathways (E); analysis of western blot showing the presence of proCASP-3: inactive caspase-3; actCASP-3: active caspase-3 in samples collected 16 h post-transfection.
It is widely reported that epithelial cells are difficult to transfect; however, the hPBAE-based polyplexes demonstrated significantly enhanced intracellular uptake compared to previous cationic nanoparticle systems.41,75 The increased transfection efficiency of A549 cells with these hPBAE-miR148b mimic polyplexes is believed to account for the significantly downregulated expression among the target genes selected.
Markers of Cellular Apoptosis.
Additional evidence from cytosolic extracts isolated from A549 cells treated with hPBAE-miR148b mimic polyplexes, shown in Figure 6E, marking an increase in cleaved-caspase-3 proteins, suggesting activation of apoptotic mechanisms, remains consistent with the previous LIVE/DEAD and Quant-iT PicoGreen assay results reported in Figure 5. Together, these results show that delivery of miR-148b-3p mimics via the hPBAE polyplexes explicitly caused cell death among non-small-cell lung adenocarcinoma cells. Further, these results suggest that cancer cell death was specific to treatment with the miR-148b-3p mimic-loaded nanoparticles rather than nonspecific miRNA mimic effects or exposure to hPBAE polymers alone. It also remains plausible that treatment of the A549 cells with hPBAE-miR148b mimic polyplexes directly activated caspase-3 proteins, affecting conversion from the pro form to cleaved form of the protein, to promote direct tumor suppression as has been shown in previous studies of melanomas and hepatocellular carcinomas. Collectively, these results indicate that this hPBAE-based delivery system can efficiently deliver functional miRNA mimics to cells, successfully unload intact miRNA mimics upon cytosolic entry, and modulate intracellular expression of target genes.
CONCLUSIONS
In brief, hPBAE-miRNA polyplexes with translational potential to advance the field of targeted cancer therapies were shown to promote inducible apoptosis in human lung adenocarcinoma cells via modulation of specific gene pathways implicated in carcinogenesis. This strategy provides an effective approach for tunable therapeutics to be delivered to lung tissue for the treatment of various forms of lung cancer with reduced side effects in healthy tissue. Cytosolic delivery of gene modulating miRNA mimics that are shown to be differentially expressed among cell types, such as miR-148b-3p, is anticipated to lead to targeted apoptosis of cancer cells. This cell death is expected to vary between tumorigenic cell lines due to the specificity of miRNA mimic targets in the cytosol. Further pharmacological studies will provide important preclinical insight for hPBAE-miRNA mimic polyplex systems as potential therapeutics. We identified a specific micro-RNA sequence that was demonstrated to specifically target critical pathways in cells expressing the Ras oncogene, which is one of the most frequently mutated genes in human cancer, has successfully implemented therapeutic strategies utilizing this miRNA, and now further demonstrates the potential for miRNA-based therapeutics for the targeted treatment in lung cancer models utilizing hPBAE-miRNA mimic polyplexes.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank all who were involved in data collection, writing, editing, and mentorship toward publication of this manuscript, including Drs. Arun K. Sharma, and Dhimant H. Desai. This work was supported partially by the National Institute of Dental and Craniofacial Research of the National Institutes of Health under award number (RDE024790A), the Office of the Assistant Secretary of Defense for Health Affairs through the Peer Reviewed Medical Research Program under Award No. W81XWH-18-1-0115, the USDA National Institute of Food and Federal Appropriations under Project PEN04607 with Accession number 1009993, and Penn State Institute of Energy and the Environment.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.langmuir.2c00913.
Additional experimental details, materials, and methods (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.langmuir.2c00913
The authors declare no competing financial interest.
Contributor Information
Nick A. Alden, The Department of Biomedical Engineering, The Pennsylvania State University, University Park, Pennsylvania 16802, United States
Julien H. Arrizabalaga, The Department of Biomedical Engineering, The Pennsylvania State University, University Park, Pennsylvania 16802, United States
Yiming Liu, The Department of Biomedical Engineering, The Pennsylvania State University, University Park, Pennsylvania 16802, United States.
Shantu Amin, Penn State Hershey Cancer Institute and The Department of Pharmacology, The Pennsylvania State University, College of Medicine, Hershey, Pennsylvania 17033, United States.
Krishne Gowda, Penn State Hershey Cancer Institute and The Department of Pharmacology, The Pennsylvania State University, College of Medicine, Hershey, Pennsylvania 17033, United States.
Shun Yao, The Department of Biology, The Pennsylvania State University, University Park, Pennsylvania 16802, United States.
Marco Archetti, The Department of Biology and The Huck Institute of the Life Sciences, Millennium Science Complex, The Pennsylvania State University, University Park, Pennsylvania 16802, United States.
Adam B. Glick, The Huck Institute of the Life Sciences, Millennium Science Complex, Department of Veterinary and Biomedical Sciences, and The Center for Molecular Toxicology and Carcinogenesis, The Pennsylvania State University, University Park, Pennsylvania 16802, United States
Daniel J. Hayes, The Department of Biomedical Engineering, The Huck Institute of the Life Sciences, Millennium Science Complex, and Materials Research Institute, Millennium Science Complex, The Pennsylvania State University, University Park, Pennsylvania 16802, United States
REFERENCES
- (1).Siegel RL; Miller KD; Fuchs HE; Jemal A Cancer Statistics, 2021. Ca-Cancer J. Clin 2021, 71, 7–33. [DOI] [PubMed] [Google Scholar]
- (2).Seung SJ; Hurry M; Hassan S; Walton RN; Evans WK Cost-of-Illness Study for Non-Small-Cell Lung Cancer Using Real-World Data. Curr. Oncol 2019, 26, 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Travis WD; Brambilla E; Nicholson AG; Yatabe Y; Austin JHM; Beasley MB; Chirieac LR; Dacic S; Duhig E; Flieder DB; Geisinger K; Hirsch FR; Ishikawa Y; Kerr KM; Noguchi M; Pelosi G; Powell CA; Tsao MS; Wistuba I The 2015 World Health Organization Classification of Lung Tumors. J. Thorac. Oncol 2015, 10, 1243–1260. [DOI] [PubMed] [Google Scholar]
- (4).Chen Z; Fillmore CM; Hammerman PS; Kim CF; Wong K-K Non-small-cell lung cancers: a heterogeneous set of diseases. Nat. Rev. Cancer 2014, 14, 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Urruticoechea A; Alemany R; Balart J; Villanueva A; Viñals F; Capellá G Recent advances in cancer therapy: an overview. Curr. Pharm. Des 2010, 16, 3–10. [DOI] [PubMed] [Google Scholar]
- (6).Trotti A; Byhardt R; Stetz J; Gwede C; Corn B; Fu K; Gunderson L; McCormick B; Morrisintegral M; Rich T; Shipley W; Curran W Common toxicity criteria: version 2.0. an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int. J. Radiat. Oncol. Biol. Phys 2000, 47, 13–47. [DOI] [PubMed] [Google Scholar]
- (7).Carelle N; Piotto E; Bellanger A; Germanaud J; Thuillier A; Khayat D Changing patient perceptions of the side effects of cancer chemotherapy. Cancer 2002, 95, 155–163. [DOI] [PubMed] [Google Scholar]
- (8).Lim LP; Glasner ME; Yekta S; Burge CB; Bartel DP Vertebrate microRNA genes. Science 2003, 299, 1540. [DOI] [PubMed] [Google Scholar]
- (9).Lewis BP; Burge CB; Bartel DP Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [DOI] [PubMed] [Google Scholar]
- (10).Grimson A; Farh KK; Johnston WK; Garrett-Engele P; Lim LP; Bartel DP MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol. Cell 2007, 27, 91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Esquela-Kerscher A; Slack FJ Oncomirs - microRNAs with a role in cancer. Nat. Rev. Cancer 2006, 6, 259–269. [DOI] [PubMed] [Google Scholar]
- (12).Garzon R; Calin GA; Croce CM MicroRNAs in Cancer. Annu. Rev. Med 2009, 60, 167–179. [DOI] [PubMed] [Google Scholar]
- (13).Croce CM; Calin GA miRNAs, cancer, and stem cell division. Cell 2005, 122, 6–7. [DOI] [PubMed] [Google Scholar]
- (14).Hanahan D; Weinberg RA Hallmarks of cancer: the next generation. Cell 2011, 144, 646–674. [DOI] [PubMed] [Google Scholar]
- (15).Yu F; Yao H; Zhu P; Zhang X; Pan Q; Gong C; Huang Y; Hu X; Su F; Lieberman J; Song E let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell 2007, 131, 1109–1123. [DOI] [PubMed] [Google Scholar]
- (16).Peter ME Let-7 and miR-200 microRNAs: guardians against pluripotency and cancer progression. Cell Cycle 2009, 8, 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Agostini M; Knight RA miR-34: from bench to bedside. Oncotarget 2014, 5, 872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Hermeking H The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010, 17, 193–199. [DOI] [PubMed] [Google Scholar]
- (19).Orso F; Quirico L; Virga F; Penna E; Dettori D; Cimino D; Coppo R; Grassi E; Elia AR; Brusa D; Deaglio S; Brizzi MF; Stadler MB; Provero P; Caselle M; Taverna D miR-214 and miR-148b Targeting Inhibits Dissemination of Melanoma and Breast Cancer. Cancer Res. 2016, 76, 5151–5162. [DOI] [PubMed] [Google Scholar]
- (20).Penna E; Orso F; Cimino D; Vercellino I; Grassi E; Quaglino E; Turco E; Taverna D miR-214 coordinates melanoma progression by upregulating ALCAM through TFAP2 and miR-148b downmodulation. Cancer Res. 2013, 73, 4098–4111. [DOI] [PubMed] [Google Scholar]
- (21).Ceppi P; Mudduluru G; Kumarswamy R; Rapa I; Scagliotti GV; Papotti M; Allgayer H Loss of miR-200c expression induces an aggressive, invasive, and chemoresistant phenotype in non-small cell lung cancer. Mol Cancer Res 2010, 8, 1207–1216. [DOI] [PubMed] [Google Scholar]
- (22).Hamano R; Miyata H; Yamasaki M; Kurokawa Y; Hara J; Moon JH; Nakajima K; Takiguchi S; Fujiwara Y; Mori M; Doki Y Overexpression of miR-200c induces chemoresistance in esophageal cancers mediated through activation of the Akt signaling pathway. Clin. Cancer Res 2011, 17, 3029–3038. [DOI] [PubMed] [Google Scholar]
- (23).Ge H; Li B; Hu W-X; Li R-J; Jin H; Gao M-M; Ding C-M MicroRNA-148b is down-regulated in non-small cell lung cancer and associated with poor survival. Int. J. Clin. Exp. Pathol 2015, 8, 800–805. [PMC free article] [PubMed] [Google Scholar]
- (24).Lu L; Liu Q; Wang P; Wu Y; Liu X; Weng C; Fang X; Li B; Cao X; Mao H; Wang L; Guan M; Wang W; Liu G MicroRNA-148b regulates tumor growth of non-small cell lung cancer through targeting MAPK/JNK pathway. BMC Cancer 2019, 19, No. 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Zhang J-G; Shi Y; Hong D-F; Song M; Huang D; Wang C-Y; Zhao G MiR-148b suppresses cell proliferation and invasion in hepatocellular carcinoma by targeting WNT1/β-catenin pathway. Sci. Rep 2015, 5, No. 8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Song Y-X; Yue Z-Y; Wang Z-N; Xu Y-Y; Luo Y; Xu H-M; Zhang X; Jiang L; Xing C-Z; Zhang Y MicroRNA-148b is frequently down-regulated in gastric cancer and acts as a tumor suppressor by inhibiting cell proliferation. Mol. Cancer 2011, 10, No. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Song Y; Xu Y; Wang Z; Chen Y; Yue Z; Gao P; Xing C; Xu H MicroRNA-148b suppresses cell growth by targeting cholecystokinin-2 receptor in colorectal cancer. Int. J. Cancer 2012, 131, 1042–1051. [DOI] [PubMed] [Google Scholar]
- (28).Wang G; Cao X; Lai S; Luo X; Feng Y; Wu J; Ning Q; Xia X; Wang J; Gong J; Hu J Altered p53 regulation of miR-148b and p55PIK contributes to tumor progression in colorectal cancer. Oncogene 2015, 34, 912–921. [DOI] [PubMed] [Google Scholar]
- (29).Azizi M; Teimoori-Toolabi L; Arzanani MK; Azadmanesh K; Fard-Esfahani P; Zeinali S MicroRNA-148b and microRNA-152 reactivate tumor suppressor genes through suppression of DNA methyltransferase-1 gene in pancreatic cancer cell lines. Cancer Biol. Ther 2014, 15, 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Zhao G; Zhang JG; Liu Y; Qin Q; Wang B; Tian K; Liu L; Li X; Niu Y; Deng SC; Wang CY miR-148b functions as a tumor suppressor in pancreatic cancer by targeting AMPKα1. Mol. Cancer Ther 2013, 12, 83–93. [DOI] [PubMed] [Google Scholar]
- (31).Pereira DM; Rodrigues PM; Borralho PM; Rodrigues CM Delivering the promise of miRNA cancer therapeutics. Drug Discovery Today 2013, 18, 282–289. [DOI] [PubMed] [Google Scholar]
- (32).Chen Y; Gao DY; Huang L In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv. Drug Delivery Rev 2015, 81, 128–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Deng X; Cao M; Zhang J; Hu K; Yin Z; Zhou Z; Xiao X; Yang Y; Sheng W; Wu Y; Zeng Y Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials 2014, 35, 4333–4344. [DOI] [PubMed] [Google Scholar]
- (34).Rupaimoole R; Slack FJ MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discovery 2017, 16, 203–222. [DOI] [PubMed] [Google Scholar]
- (35).Ren Y; Wang R; Gao L; Li K; Zhou X; Guo H; Liu C; Han D; Tian J; Ye Q; Hu YT; Sun D; Yuan X; Zhang N Sequential co-delivery of miR-21 inhibitor followed by burst release doxorubicin using NIR-responsive hollow gold nanoparticle to enhance anticancer efficacy. J. Controlled Release 2016, 228, 74–86. [DOI] [PubMed] [Google Scholar]
- (36).Liu Y; Bailey JT; Abu-Laban M; Li S; Chen C; Glick AB; Hayes DJ Photocontrolled miR-148b nanoparticles cause apoptosis, inflammation and regression of Ras induced epidermal squamous cell carcinomas in mice. Biomaterials 2020, 256, No. 120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Brown PK; Qureshi AT; Moll AN; Hayes DJ; Monroe WT Silver Nanoscale Antisense Drug Delivery System for Photoactivated Gene Silencing. ACS Nano 2013, 7, 2948–2959. [DOI] [PubMed] [Google Scholar]
- (38).Qureshi AT; Monroe WT; Dasa V; Gimble JM; Hayes DJ miR-148b-Nanoparticle conjugates for light mediated osteogenesis of human adipose stromal/stem cells. Biomaterials 2013, 34, 7799–7810. [DOI] [PubMed] [Google Scholar]
- (39).Celik N; Kim MH; Hayes DJ; Ozbolat IT miRNA induced co-differentiation and cross-talk of adipose tissue-derived progenitor cells for 3D heterotypic pre-vascularized bone formation. Biofabrication 2021, 13, 044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Arrizabalaga JH; Casey JS; Becca JC; Liu Y; Jensen L; Hayes DJ Development of magnetic nanoparticles for the intracellular delivery of miR-148b in non-small cell lung cancer. Biomed. Eng. Adv 2022, 3, No. 100031. [Google Scholar]
- (41).Abu-Laban M; Hamal P; Arrizabalaga JH; Forghani A; Dikkumbura AS; Kumal RR; Haber LH; Hayes DJ Combinatorial Delivery of miRNA-Nanoparticle Conjugates in Human Adipose Stem Cells for Amplified Osteogenesis. Small 2019, 15, No. 1902864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Beg MS; Brenner AJ; Sachdev J; Borad M; Kang YK; Stoudemire J; Smith S; Bader AG; Kim S; Hong DS Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest. New Drugs 2017, 35, 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Hatakeyama H; Murata M; Sato Y; Takahashi M; Minakawa N; Matsuda A; Harashima H The systemic administration of an anti-miRNA oligonucleotide encapsulated pH-sensitive liposome results in reduced level of hepatic microRNA-122 in mice. J. Controlled Release 2014, 173, 43–50. [PubMed] [Google Scholar]
- (44).Pegtel DM; Cosmopoulos K; Thorley-Lawson DA; van Eijndhoven MA; Hopmans ES; Lindenberg JL; de Gruijl TD; Würdinger T; Middeldorp JM Functional delivery of viral miRNAs via exosomes. Proc. Natl. Acad. Sci. U.S.A 2010, 107, 6328–6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Yin H; Kanasty RL; Eltoukhy AA; Vegas AJ; Dorkin JR; Anderson DG Non-viral vectors for gene-based therapy. Nat. Rev. Genet 2014, 15, 541–555. [DOI] [PubMed] [Google Scholar]
- (46).Singh S; Narang AS; Mahato RI Subcellular fate and off-target effects of siRNA, shRNA, and miRNA. Pharm. Res 2011, 28, 2996–3015. [DOI] [PubMed] [Google Scholar]
- (47).Patel AK; Kaczmarek JC; Bose S; Kauffman KJ; Mir F; Heartlein MW; Derosa F; Langer R; Anderson DG Inhaled Nanoformulated mRNA Polyplexes for Protein Production in Lung Epithelium. Adv. Mater 2019, 31, No. 1805116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Song W; Tang Z; Li M; Lv S; Yu H; Ma L; Zhuang X; Huang Y; Chen X Tunable pH-Sensitive Poly(β -amino ester)s Synthesized from Primary Amines and Diacrylates for Intracellular Drug Delivery. Macromolecular Bioscience 2012, 12, 1375–1383. [DOI] [PubMed] [Google Scholar]
- (49).Zhou D; Cutlar L; Gao Y; Wang W; O’Keeffe-Ahern J; McMahon S; Duarte B; Larcher F; Rodriguez BJ; Greiser U; Wang W The transition from linear to highly branched poly(β-amino ester)s: Branching matters for gene delivery. Sci. Adv 2016, 2, No. e1600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Su X; Fricke J; Kavanagh DG; Irvine DJ In vitro and in vivo mRNA delivery using lipid-enveloped pH-responsive polymer nanoparticles. Mol. Pharmaceutics 2011, 8, 774–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Lakes AL; Puleo DA; Hilt JZ; Dziubla TD Highly Thiolated Poly (Beta-Amino Ester) Nanoparticles for Acute Redox Applications. Gels 2018, 4, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Cheng W; Wu D; Liu Y Michael Addition Polymerization of Trifunctional Amine and Acrylic Monomer: A Versatile Platform for Development of Biomaterials. Biomacromolecules 2016, 17, 3115–3126. [DOI] [PubMed] [Google Scholar]
- (53).Vikis HG; Rymaszewski AL; Tichelaar JW Mouse models of chemically-induced lung carcinogenesis. Front. Biosci 2013, E5, 939–946. [DOI] [PubMed] [Google Scholar]
- (54).Johnson L; Mercer K; Greenbaum D; Bronson RT; Crowley D; Tuveson DA; Jacks T Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature 2001, 410, 1111–1116. [DOI] [PubMed] [Google Scholar]
- (55).Griffiths-Jones S The microRNA Registry. Nucleic Acids Res. 2004, 32, 109D–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Agarwal V; Bell GW; Nam J-W; Bartel DP Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, No. e05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Casey JS; Arrizabalaga JH; Abu-Laban M; Becca JC; Rose BJ; Strickland KT; Bursavich JB; McCann JS; Pacheco CN; Jensen L; Attaluri A; Hayes DJ Alternating magnetic field mediated release of fluorophores from magnetic nanoparticles by hysteretic heating. J. Colloid Interface Sci 2020, 571, 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Arrizabalaga JH; Casey JS; Becca JC; Jensen L; Hayes DJ Comparison of thermoresponsive Diels-Alder linkers for the release of payloads from magnetic nanoparticles via hysteretic heating. JCIS Open 2021, 4, No. 100034. [Google Scholar]
- (59).Arrizabalaga JH; Smallcomb M; Abu-Laban M; Liu Y; Yeingst TJ; Dhawan A; Simon JC; Hayes DJ Ultrasound-Responsive Hydrogels for On-Demand Protein Release. ACS Appl. Bio Mater 2022, 5, 3212–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Arrizabalaga JH; Nollert MU Riboflavin-UVA crosslinking of amniotic membranes and its influence on the culture of adipose-derived stem cells. J. Mech. Behav. Biomed. Mater 2020, 106, No. 103729. [DOI] [PubMed] [Google Scholar]
- (61).Schindelin J; Arganda-Carreras I; Frise E; Kaynig V; Longair M; Pietzsch T; Preibisch S; Rueden C; Saalfeld S; Schmid B; Tinevez J-Y; White DJ; Hartenstein V; Eliceiri K; Tomancak P; Cardona A Fiji: an open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Schneider CA; Rasband WS; Eliceiri KW NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Livak KJ; Schmittgen TD Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- (64).Zhang YN; Poon W; Tavares AJ; McGilvray ID; Chan WCW Nanoparticle-liver interactions: Cellular uptake and hepatobiliary elimination. J. Controlled Release 2016, 240, 332–348. [DOI] [PubMed] [Google Scholar]
- (65).Li SD; Huang L Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharmaceutics 2008, 5, 496–504. [DOI] [PubMed] [Google Scholar]
- (66).Kaczmarek JC; Patel AK; Kauffman KJ; Fenton OS; Webber MJ; Heartlein MW; Derosa F; Anderson DG Polymer-Lipid Nanoparticles for Systemic Delivery of mRNA to the Lungs. Angew. Chem., Int. Ed 2016, 55, 13808–13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Green JJ; Zugates GT; Langer R; Anderson DG Poly(β-amino esters): procedures for synthesis and gene delivery. In Methods in Molecular Biology, Humana Press, 2009; Vol. 480, pp 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Sunshine JC; Sunshine SB; Bhutto I; Handa JT; Green JJ Poly(β-amino ester)-nanoparticle mediated transfection of retinal pigment epithelial cells in vitro and in vivo. PLoS One 2012, 7, No. e37543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Liu Y; Li Y; Keskin D; Shi L Poly(β-Amino Esters): Synthesis, Formulations, and Their Biomedical Applications. Adv. Healthcare Mater 2018, No. 1801359. [DOI] [PubMed] [Google Scholar]
- (70).Zhao QQ; Chen JL; Lv TF; He CX; Tang GP; Liang WQ; Tabata Y; Gao JQ N/P ratio significantly influences the transfection efficiency and cytotoxicity of a polyethylenimine/chitosan/DNA complex. Biol. Pharm. Bull 2009, 32, 706–710. [DOI] [PubMed] [Google Scholar]
- (71).Rui Y; Varanasi M; Mendes S; Yamagata HM; Wilson DR; Green JJ Poly(Beta-Amino Ester) Nanoparticles Enable Nonviral Delivery of CRISPR-Cas9 Plasmids for Gene Knockout and Gene Deletion. Mol. Ther.–Nucleic Acids 2020, 20, 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Treiber T; Treiber N; Meister G Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol 2019, 20, 5–20. [DOI] [PubMed] [Google Scholar]
- (73).Gebert LFR; MacRae IJ Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol 2019, 20, 21–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Cimino D; De Pittà C; Orso F; Zampini M; Casara S; Penna E; Quaglino E; Forni M; Damasco C; Pinatel E; Ponzone R; Romualdi C; Brisken C; De Bortoli M; Biglia N; Provero P; Lanfranchi G; Taverna D miR148b is a major coordinator of breast cancer progression in a relapse-associated microRNA signature by targeting ITGA5, ROCK1, PIK3CA, NRAS, and CSF1. FASEB J. 2013, 27, 1223–1235. [DOI] [PubMed] [Google Scholar]
- (75).Gustafson HH; Holt-Casper D; Grainger DW; Ghandehari H Nanoparticle Uptake: The Phagocyte Problem. Nano Today 2015, 10, 487–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


