Abstract
The development of tunable, ultrasound-responsive hydrogels that can deliver protein payload on-demand when exposed to focused ultrasound is described in this study. Reversible Diels–Alder linkers, which undergo a retro reaction when stimulated with ultrasound, were used to cross-link chitosan hydrogels with entrapped FITC-BSA as a model protein therapeutic payload. Two Diels–Alder linkage compositions with large differences in the reverse reaction energy barriers were compared to explore the influence of linker composition on ultrasound response. Selected physicochemical properties of the hydrogel construct, its basic degradation kinetics, and its cytocompatibility were measured with respect to Diels–Alder linkage composition. Focused ultrasound initiated the retro Diels–Alder reaction, controlling the release of the entrapped payload while also allowing for real-time visualization of the ongoing process. Additionally, increasing the focused ultrasound amplitude and time correlated with an increased rate of protein release, indicating stimuli responsive control.
Keywords: chitosan, hydrogels, ultrasound, controlled release, click chemistry, Diels–Alder
Graphical Abstract
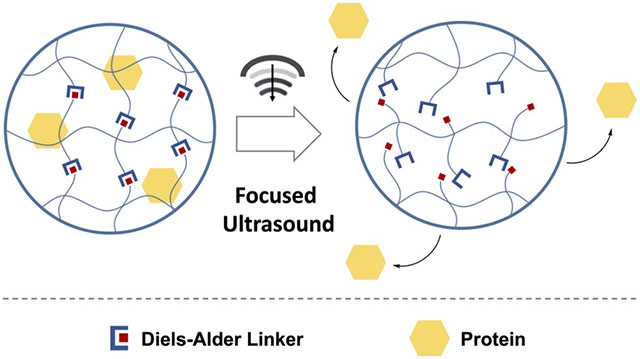
INTRODUCTION
Spatiotemporally controlled drug delivery systems allow for fewer side effects and better patient outcomes in regenerative medicine by precisely modulating payload release.1,2 Conventional polymer-based systems for drug delivery typically rely on passive diffusion and polymer degradation, providing spatial localization but not temporal control of the payload release.3,4 By contrast, recently developed platforms for on-demand drug delivery provide a means to modulate the intensity and frequency of drug release via either the application of external stimulation (ultrasound, light, magnetic field) or chemical triggers.4,5
Specifically, focused ultrasound has been demonstrated as a potent and safe external stimulus, allowing for deep tissue penetration while being nonionizing and noninvasive.6 Focused ultrasound, delivering a spatiotemporal pressure wave, can be used to interact locally with hydrogels, releasing drugs through thermal or mechanical mechanisms.1,3,4,7,8 Ultrasound can achieve temperatures from mild hyperthermia (<6 °C) up to boiling temperatures at the focus; it can also create, oscillate, and collapse acoustic cavitation bubbles within the focus. Focused ultrasound can be accurately targeted and monitored in real time, with temperature fluctuations most often monitored with magnetic resonance imaging, while cavitation is primarily monitored with ultrasound imaging.9
“Click chemistry” reactions have become increasingly popular for the preparation of hydrogels due to their selective reactivity and rapid reaction kinetics.10,11 Among them, the Diels–Alder reaction occurs orthogonally, in water, and without catalysts.12,13 Consequently, Diels–Alder linkers have been used to cross-link several different types of hydrogels such as hyaluronic acid, gelatin, chitosan, PEG, and polyurethane, providing enhanced stability and gelation kinetics.14–21 Furthermore, the Diels–Alder reaction is reversible at higher temperature, yielding back the original diene and dienophile reactants and thus acting as a stimuli-responsive switch releasing payloads after localized heat induction.22,23 Reversible Diels–Alder linkers have successfully been used in photocontrolled and alternating magnetic field activated drug delivery applications to provide spatiotemporally controlled release from nanoparticles.23–28
Hence, we sought to develop ultrasound-responsive hydrogels using reversible Diels–Alder linkages that could deliver a drug on demand when exposed to focused ultrasound. Chitosan was chosen to prepare hydrogels due to its biocompatibility and ease of functionalization.29–31 Bovine serum albumin labeled with fluorescein (FITC-BSA) was entrapped as a model payload. Diels–Alder linkers, which undergo a reversible retrograde reaction when stimulated with ultrasound, were used to cross-link these hydrogels. When initiated by focused ultrasound, the retro Diels–Alder reaction restructured the hydrogel, releasing the entrapped protein. We demonstrate the capability for focused ultrasound excitation to control the release of entrapped BSA protein payloads in Diels–Alder cross-linked hydrogels while allowing for real-time visualization of the ongoing process. The physiochemical properties of the hydrogel as well as the basic degradation kinetics and cytocompatibility of the hydrogel construct are described.
MATERIALS AND METHODS
Materials.
Chitosan (75–85% deacetylated, 50–190 kDa), FITC-BSA (fluorescein isothiocyanate conjugate, bovine albumin), 2-thiophenecarboxylic acid (99%), 2-furoic acid (98%), 6-maleimidohexanoic acid (90%), methanol (anhydrous, 99.8%), glutaraldehyde (solution, 25% in H2O), hydrochloric acid (HCl, 37%), N-hydroxysuccinimide (NHS, 98%), and agarose (BioReagent) were obtained from Millipore Sigma (St. Louis, MO). Tegaderm was acquired from 3M Health Care (St. Paul, MN). HeLa cells were provided by the Sartorius Cell Culture Facility of the Pennsylvania State University (University Park, PA). Fetal bovine serum (FBS) was purchased from Corning (Corning, NY). Dulbecco’s phosphate buffered saline (DPBS) was obtained from Cytiva (Pittsburgh, PA). Disposable biopsy punches (Miltex, 4 and 8 mm diameter) were acquired from Integra LifeSciences (Princeton, NJ). Tissue culture plate inserts (polycarbonate membrane, translucent, 0.4 μm pore size) were bought from VWR (Radnor, PA). Dulbecco’s modified Eagle’s medium (DMEM), 1-ethyl-3-(3-(dimethylamino)propyl)-carbodiimide hydrochloride (EDC), LIVE/DEAD Viability/Cytotoxicity kit, alamarBlue HS cell viability reagent, antibiotic–antimycotic, and Quant-iT PicoGreen dsDNA assay kit were acquired from Thermo Fisher Scientific (Waltham, MA). The 24-well no-bottom plates were obtained from Greiner Bio-One (Monroe, NC). Reagents were used as received.
Preparation of Diels–Alder Linkers.
Two cycloadducts were synthesized: FDA (2-(5-carboxypentyl)-1,3-dioxo-1,2,3,3a,7,7a-hexahydro-4H-4,7-epoxyisoindole-4-carboxylic acid), which was the product of the cycloaddition between 2-furoic acid and 6-maleimidohexanoic acid, and TDA (2-(5-carboxypentyl)-1,3-dioxo-1,2,3,3a,7,7a-hexahydro-4H-4,7-epithioisoindole-4-carboxylic acid), which was the product between 2-thiophenecarboxylic acid and 6-maleimidohexanoic acid (Figure 1). For the preparation of FDA, 0.93 g of 2-furoic acid and 1.75 g of 6-maleimidohexanoic acid were dissolved into 15 mL of methanol. The glass vial was then sealed, kept in the dark at room temperature, and agitated for 7 days. TDA was synthesized by combining 1.06 g of 2-thiophenecarboxylic and 1.75 g of 6-maleimidohexanoic acid with 15 mL of methanol. The vial used for the reaction was then sealed, kept in the dark, and left for 3 days in a 60 °C oil bath.
Figure 1.
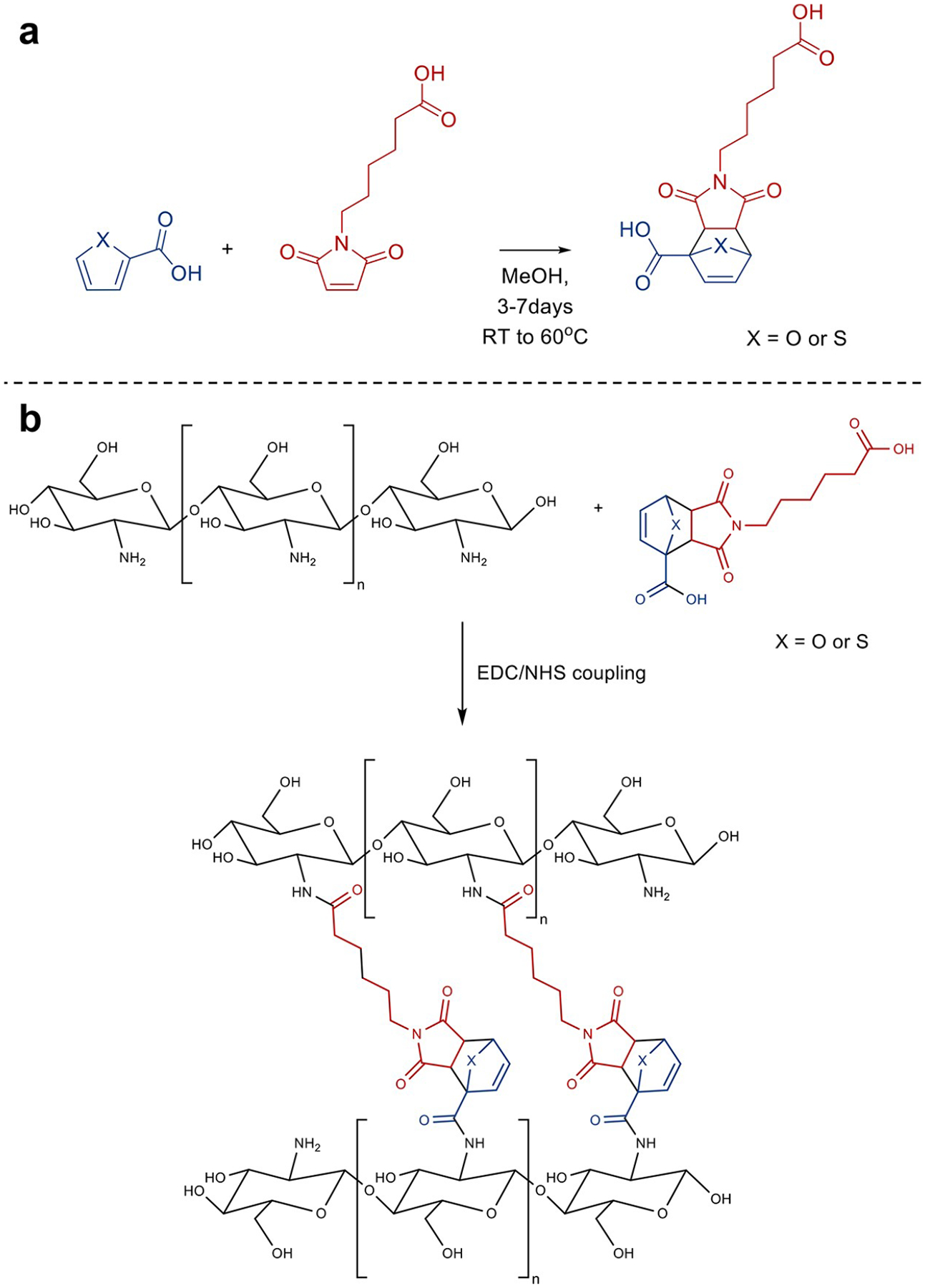
(a) Diels–Alder reaction between 6-maleimidohexanoic acid and a thiophene or furan-based diene. (b) Cross-linking of chitosan with a Diels–Alder linker (FDA or TDA) via EDC/NHS coupling reaction.
Preparation of Chitosan Hydrogels.
Chitosan (0.25 g) was dissolved in water (5 mL) with 17 μL of 1 M HCl. EDC/NHS (100 μL of a 100 μM solution) were added and reacted with the chitosan for 15 min. Then 500 μL of the Diels–Alder linker (either FDA or TDA) was added to the mixture prior to casting it in a 35 mm diameter Petri dish. The hydrogels were left to cross-link overnight and were lyophilized before characterization. Control chitosan hydrogels without a thermally labile Diels linker were prepared with glutaraldehyde (100 μL of a 25% solution in water) as previously described.32–34 For protein release studies, FITC-BSA (100 μL, 0.5 mg/mL) was added to the chitosan prior to cross-linking.
Characterization of Hydrogels.
A Bruker Vertex 70 instrument was used to record FTIR spectra in the 4000 to 550 cm−1 range. A DSC Q2000 calorimeter (TA Instruments, New Castle, DE) was used for differential scanning calorimetry (DSC) analyses. Thermal properties were recorded at a heating rate of 10 °C/min between 25 and 200 °C using nitrogen as the purge gas.
Cell Culture.
HeLa cells were cultured in DMEM supplemented with 1% antibiotic–antimycotic and 10% fetal bovine serum. Cell were kept at 37 °C in a humidified incubator with 5% CO2.
Cell Proliferation and Viability Assays.
Hydrogels were sectioned using disposable biopsy punches to obtain samples with a uniform geometry and volume. For cytocompatibility assessment, HeLa cells were seeded in 24-well plates (0.05 × 106 cells per well), and hydrogels samples were added to the different wells using tissue culture plate inserts. Assays were performed at days 1, 3, and 7 after exposing the cells to the hydrogels. The metabolic activity of the cells was measured using an alamarBlue assay, cell viability was assessed using a LIVE/DEAD Viability/Cytotoxicity kit, and the total DNA content was determined using a PicoGreen dsDNA assay kit as previously described.28,35
Immersion Heating.
Hydrogels containing FITC-BSA were sectioned using 4 mm diameter disposable biopsy punches to obtain samples with a uniform geometry and volume. Each hydrogel sample was placed in a microcentrifuge tube containing 1 mL of DPBS. Sealed microcentrifuge tubes were then heated at either 20, 37, or 60 °C for 1 h. The 37 °C temperature was achieved with a water bath, and the 60 °C temperature was achieved with an oil bath to avoid water evaporation. Microcentrifuge tubes containing the samples were then centrifuged for 10 min at 1200g. The amount of FITC-BSA released by the immersion heating was evaluated by measuring the fluorescence intensity at 495/520 nm (excitation/emission) of the supernatant (three 150 μL aliquots per sample) using a Spectramax M5 microplate reader (Molecular Devices, San Jose, CA).
Ultrasound-Mediated Protein Release.
Hydrogels containing FITC-BSA were sectioned using 8 mm diameter disposable biopsy punches to obtain samples with a uniform geometry and volume. Hydrogels samples were placed in 24-well no-bottom plates sealed with Tegaderm on both sides. Each well was filled with DPBS and contained an hydrogel sample secured at its bottom with agarose (1%). After ultrasound targeting, the fluorescence intensity of the DPBS contained in each well was determined. These measurements were obtained similarly to the protocol described for the immersion heating experiment. Ultrasound targeting was applied with a spherically curved single-element focused ultrasound H-234 transducer modified with a 42 mm diameter larger center opening (Sonic Concepts, Bothell, WA). The ultrasonic energy was focused to a depth of 45 mm by the geometrically curved body of the transducer. Full width at half-maximum beam profiles of the focused ultrasound transducer were measured at a peak positive pressure of 37 MPa to be 0.6 mm transversely and 5 mm axially.36 The transducer was driven at an operational frequency of 1.5 MHz by using a 33600A Series waveform generator (Keysight, Santa Rosa, CA) and a 55 dB A500 linear radiofrequency power amplifier (Electronic Navigation Industries, Rochester, NY).36 Gels were targeted at the focus with repeated pulses (20 ms, 1 Hz) with increasing peak positive pressures of 37, 57, and 75 MPa (peak negative pressures of 16, 29, and 32 MPa, respectively). A HFO-690 fiber optic probe hydrophone (ONDA, Sunnyvale, CA) was used to measure focal pressures in the lowest-pressure case. A HIFU-beam simulator37 was used to estimate the two higher pressure amplitudes, since they caused cavitation damage to the tip of the fiber optic probe hydrophone. Focused ultrasound treatments were applied for 1 or 5 min. Bubble activity and transducer alignment were monitored coaxially with B-mode ultrasound imaging using a Verasonics research ultrasound system (Vantage-128, Kirkland, WA) and a P4–2 imaging transducer (Philips/ATL, Bothell, WA).38 The focus was aligned 1.5 cm in depth at one center location in the gel. A diagram and pictures of the setup used for focused ultrasound are available in the Supporting Information (Figures S1 and S2).
Statistical Analysis.
Sample size (n) is indicated in the legend of each figure. Results were expressed as mean ± standard deviation (SD). Data were analyzed using two-way ANOVA and Tukey’s posthoc test. Statistical significance was set at p < 0.05. GraphPad Prism 8 was used for data analysis.
RESULTS AND DISCUSSION
FTIR and DSC Characterization of the Diels–Alder Chitosan Hydrogels.
The FTIR spectra for unmodified chitosan (Ch) and chitosan cross-linked with FDA (Ch-FDA) and TDA (Ch-TDA) are displayed in Figure 2. The characteristic absorption bands of the polysaccharide are present with bands at 897, 1033, 1072, and 1153 cm−1 as reported in the literature.29,30,39 The characteristic peak of the carbonyl (C=O) groups present on both Diels–Alder linkers is also noticeable at 1692 cm−1.
Figure 2.
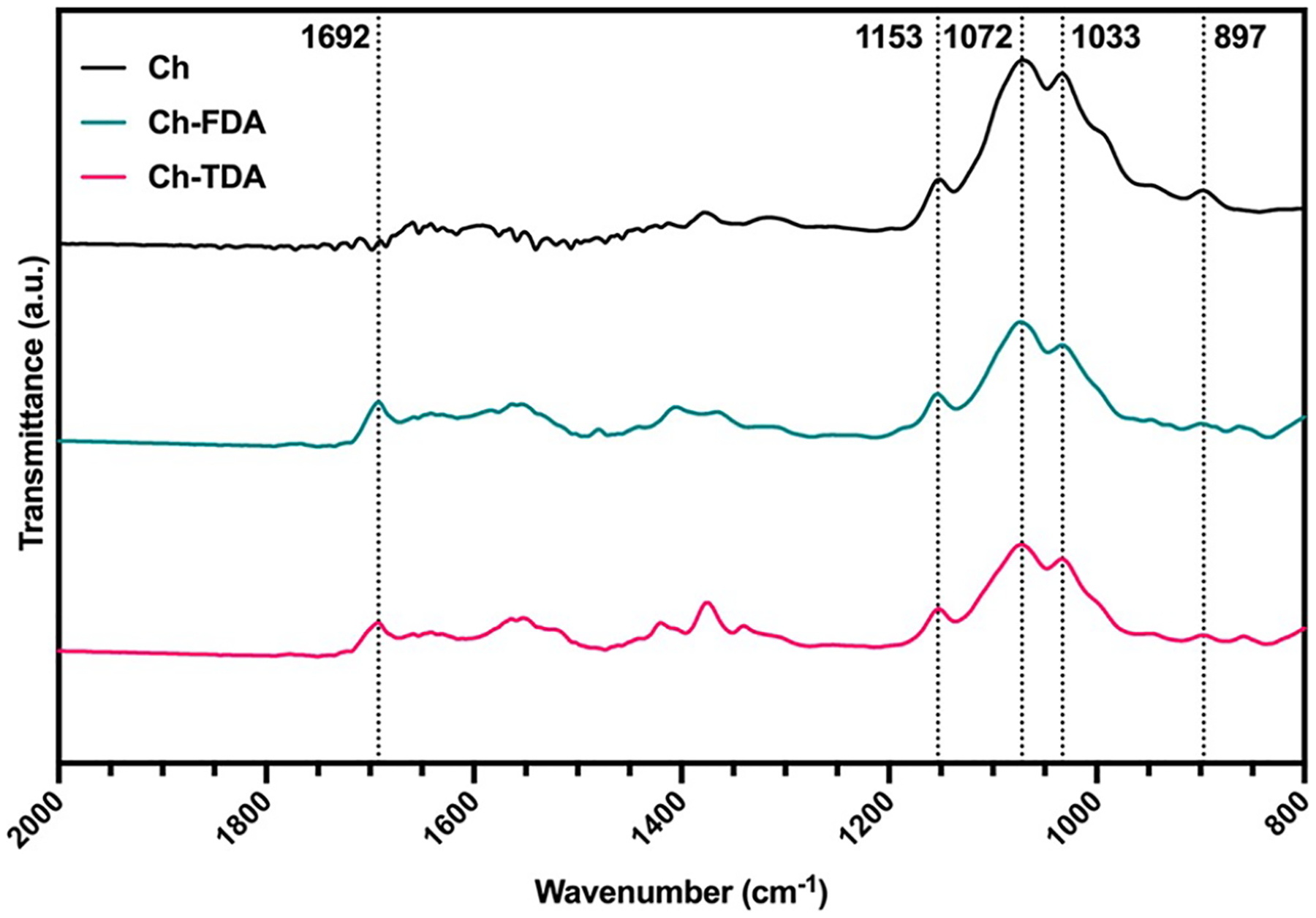
FTIR spectra of chitosan (Ch, black), chitosan cross-linked with FDA (Ch-FDA, blue), and chitosan cross-linked with TDA (Ch-TDA, red).
Ch-FDA and CH-TDA samples were also analyzed by DSC as show in Figure 3. Peaks in relation to the retro-Diels–Alder reaction energy barriers were observed at 101.9 °C for Ch-FDA and at 119.7 °C for Ch-TDA.39
Figure 3.
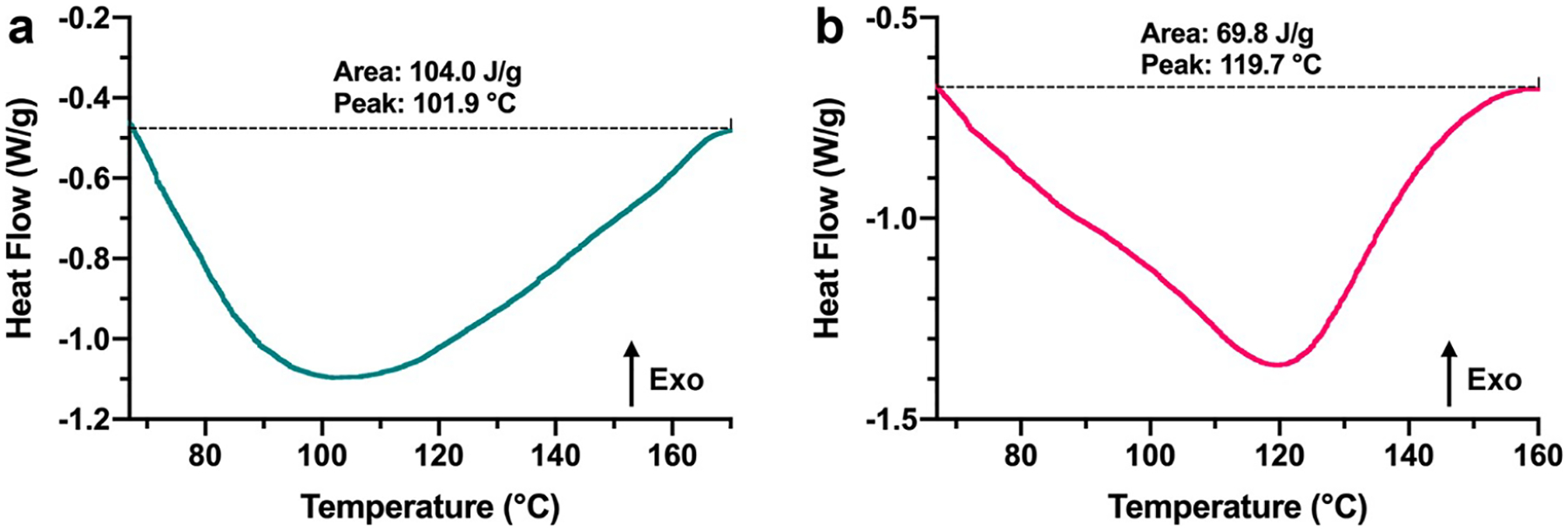
DSC traces of (a) Ch-FDA and (b) Ch-TDA. Additional DSC traces with an expanded x-axis to show lower and higher temperatures are available in Supporting Information Figure S3.
Immersion Heating.
Thermal release immersion studies were performed with chitosan hydrogels that contained FITC-BSA as a model protein. (Figure 4). Fluorescence intensity measurements were used to determine the amount of FITC-BSA released by the retro Diels–Alder reaction. A higher released protein amount was measured for the FDA linker compared to the TDA linker with the same thermal treatments. This result aligns with previous studies that used comparable Diels–Alder linkers for nucleic acid delivery from metal nanoparticles.23 The furan based cycloadduct was reported having a higher payload release rate than the thiophene-based linker, in agreement with the energy barriers calculated by density functional theory.23 Additionally, glutaraldehyde was used to prepare control hydrogels cross-linked without a Diels–Alder linker. The amount of FITC-BSA released from these control gels was significantly lower than the amount released from the gels cross-linked with thermoresponsive Diels–Alder linkers.
Figure 4.
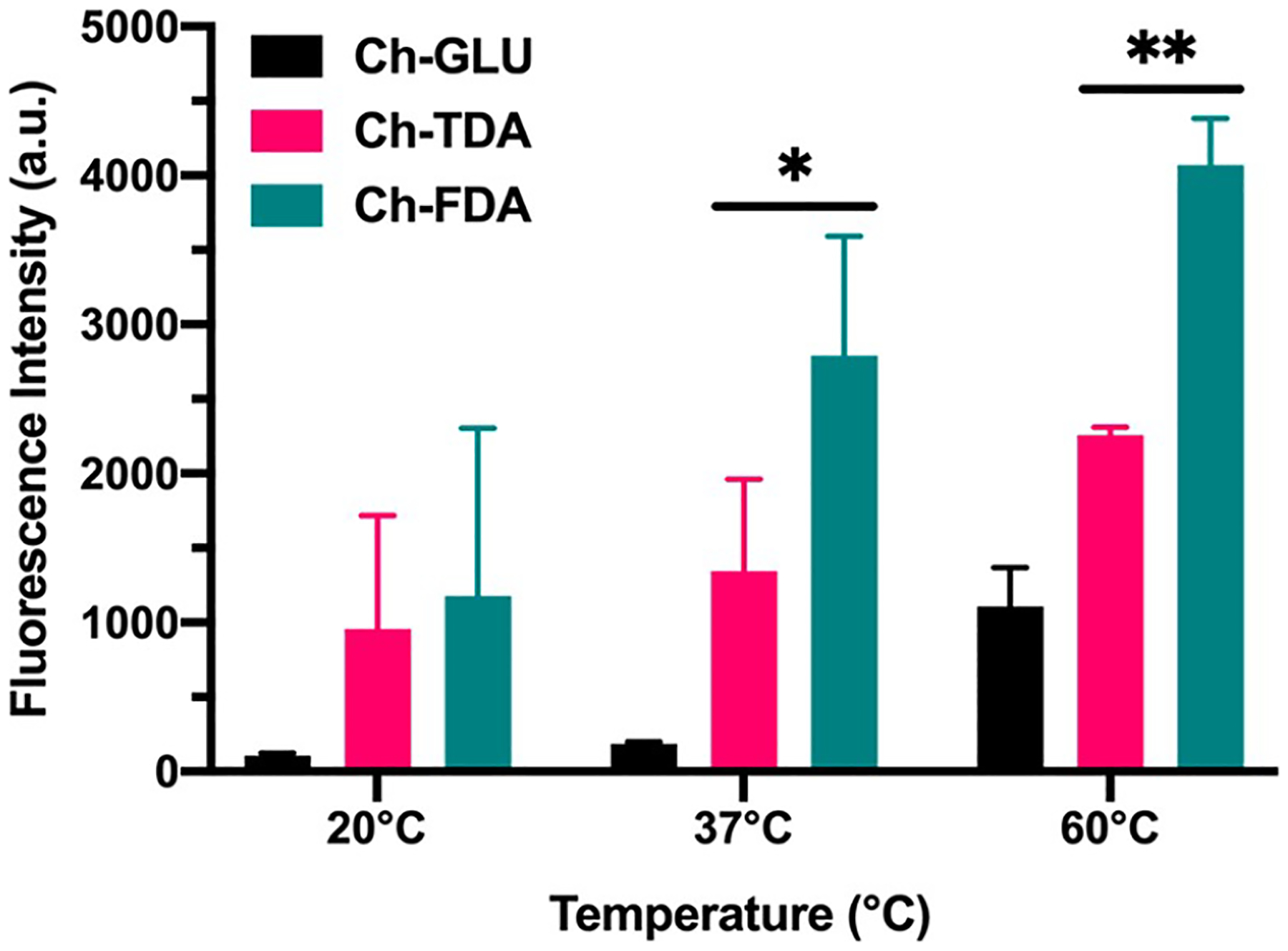
Protein release from chitosan hydrogels by immersion heating (n = 4). *Significant difference (p < 0.05). **Significant difference (p < 0.01).
Ultrasound-Mediated Protein Release.
The synthesis and characterization of different Diels–Alder hydrogels have been previously described,16,17,40 but the interaction with focused ultrasound, hydrogel reorganization, and resulting controlled drug release has not been explored. Our results indicate the capability for focused ultrasound to drive the retro Diels–Alder reaction which resulted in the controlled release of entrapped protein payloads in Diels–Alder cross-linked hydrogels while allowing for real-time visualization of the ongoing process (Figure 5). Increasing the focused ultrasound amplitudes and treatment time correlated with an increased rate of protein release, indicating stimuli responsive control. The protein release rate for Ch-FDA was higher than that observed for Ch-TDA, in agreement with the data from the thermal immersion study (Figure 4). These results suggest that the retro Diels–Alder reaction energy barriers correlate with the protein release rates, providing a means to tune the hydrogel stability upon exposure to focused ultrasound, through manipulation of the diene and dienophile composition. The amount of FITC-BSA released from the control hydrogels cross-linked with glutaraldehyde was also significantly lower than the amount released from the gels cross-linked with thermoresponsive Diels–Alder linkers. A control experiment (Supporting Information Figure S4) was also performed to verify that the chitosan present in DPBS after ultrasound treatment did not interfere with the FITC-BSA fluorescence in the range of concentrations used for this study.
Figure 5.
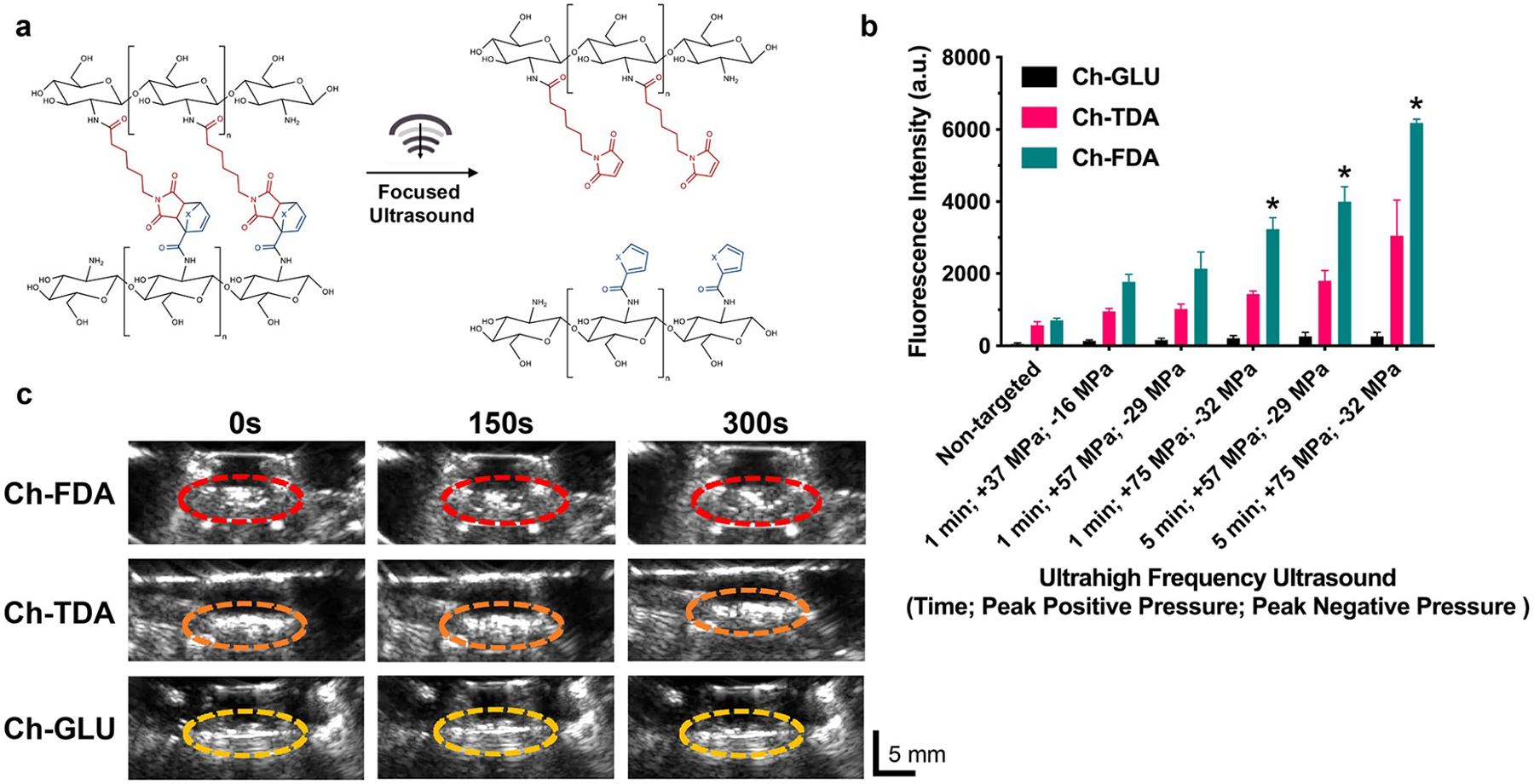
Ultrasound-mediated protein release from hydrogels. (a) Retro Diels–Alder reaction prompted by focused ultrasound for chitosan hydrogels cross-linked with either FDA or TDA. (b) Focused ultrasound dependent release of FITC-BSA from Ch-GLU, Ch-FDA, and Ch-TDA hydrogels (n = 3). *Significant difference (p < 0.05). (c) Real-time B-mode ultrasound imaging during focused ultrasound (5 min with a positive peak pressure of 37 MPa and peak negative pressure of 16 MPa) treatment of Ch-GLU, Ch-FDA, and Ch-TDA. A diagram and pictures of the setup used for focused ultrasound are available in the Supporting Information (Figures S1 and S2).
Biocompatibility.
The cytocompatibility of the chitosan hydrogels cross-linked with Diels–Alder linkers was assessed by exposing HeLa cells directly to hydrogels and their decomposition products. The metabolic activity, viability, and total cell numbers were measured after 1, 3, and 7 days (Figure 6). The hydrogels did not appear to induce any cytotoxicity in these direct exposure in vitro studies. No visible differences were apparent among the different groups on the LIVE/DEAD fluorescent images. Similarly, no significant difference could be measured between the Ch, Ch-FDA, and Ch-TDA groups for the metabolic activity and cell numbers over time. These results align with the literature, where chitosan has been described as a biomaterial with an excellent biocompatibility supporting robust cell proliferation.41–43 Likewise, other hydrogels cross-linked with Diels–Alder linkers have also been reported as being suitable for cell culture.40,44
Figure 6.

(a) Representative LIVE/DEAD staining of HeLa cells at days 1, 3, and 7. Green indicates live cells, and red indicates dead cells. Scale bar = 200 μm. (b) Metabolic activity relative to cells cultivated without exposure to hydrogels (n = 3). No significant difference found when comparing groups at the same time point. (c) Total cell number relative to cells cultivated without exposure to hydrogels (n = 3). No significant difference found when comparing groups at the same time point.
CONCLUSIONS
In this study, the successful cross-linking of chitosan hydrogels with two distinct Diels–Alder linkers was reported. When targeted with focused ultrasound, these reversible linkers underwent a retro reaction, restructuring the hydrogel and releasing the entrapped model protein therapeutic payload. This release correlated with predicted retro reaction energy barriers as calculated by density functional theory. Focused ultrasound proved to be an effective external trigger to control the payload release while also providing monitoring in real time. Increasing the focused ultrasound amplitude and time correlated with an increased rate of protein release, indicating stimuli responsive control. Additionally, these hydrogels did not induce any cytotoxicity in vitro. Taken together, these findings suggest that this payload delivery system could be further developed for clinical applications requiring on-demand or precisely controlled drug delivery.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported partially by the National Institute of Dental and Craniofacial Research of the National Institutes of Health under award number (RDE024790A), the Office of the Assistant Secretary of Defense for Health Affairs through the Peer Reviewed Medical Research Program under Award No. W81XWH2110052. The opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the National Institutes of Health or the Department of Defense.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsabm.2c00192.
Additional experimental details including photographs of experimental setup (PDF)
The authors declare no competing financial interest.
Complete contact information is available at: https://pubs.acs.org/10.1021/acsabm.2c00192
Contributor Information
Julien H. Arrizabalaga, Department of Biomedical Engineering, The Pennsylvania State University, University Park, Pennsylvania 16802, United States;
Molly Smallcomb, Graduate Program in Acoustics, The Pennsylvania State University, University Park, Pennsylvania 16802, United States;.
Mohammad Abu-Laban, Department of Biomedical Engineering, The Pennsylvania State University, University Park, Pennsylvania 16802, United States.
Yiming Liu, Department of Biomedical Engineering, The Pennsylvania State University, University Park, Pennsylvania 16802, United States.
Tyus J. Yeingst, Department of Biomedical Engineering, The Pennsylvania State University, University Park, Pennsylvania 16802, United States;
Aman Dhawan, Department of Orthopaedics and Rehabilitation, Penn State College of Medicine, Milton S. Hershey Medical Center, Hershey, Pennsylvania 17033, United States.
Julianna C. Simon, Department of Biomedical Engineering, The Pennsylvania State University, University Park, Pennsylvania 16802, United States; Graduate Program in Acoustics, The Pennsylvania State University, University Park, Pennsylvania 16802, United States
Daniel J. Hayes, Department of Biomedical Engineering, The Pennsylvania State University, University Park, Pennsylvania 16802, United States; Materials Research Institute, Millennium Science Complex, The Pennsylvania State University, University Park, Pennsylvania 16802, United States; The Huck Institute of the Life Sciences, Millennium Science Complex, The Pennsylvania State University, University Park, Pennsylvania 16802, United States;
REFERENCES
- (1).Li J; Mooney DJ Designing hydrogels for controlled drug delivery. Nat. Rev. Mater 2016, 1 (12), 16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Bayer EA; Gottardi R; Fedorchak MV; Little SR The scope and sequence of growth factor delivery for vascularized bone tissue regeneration. J. Controlled Release 2015, 219, 129–140. [DOI] [PubMed] [Google Scholar]
- (3).Huebsch N; Kearney CJ; Zhao X; Kim J; Cezar CA; Suo Z; Mooney DJ Ultrasound-triggered disruption and self-healing of reversibly cross-linked hydrogels for drug delivery and enhanced chemotherapy. Proc. Natl. Acad. Sci. U. S. A 2014, 111 (27), 9762–9767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Brudno Y; Mooney DJ On-demand drug delivery from local depots. J. Controlled Release 2015, 219, 8–17. [DOI] [PubMed] [Google Scholar]
- (5).Spiller KL; Vunjak-Novakovic G Clinical translation of controlled protein delivery systems for tissue engineering. Drug Delivery and Translational Research 2015, 5 (2), 101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Mitragotri S Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat. Rev. Drug Discovery 2005, 4 (3), 255–260. [DOI] [PubMed] [Google Scholar]
- (7).Epstein-Barash H; Orbey G; Polat BE; Ewoldt RH; Feshitan J; Langer R; Borden MA; Kohane DS A microcomposite hydrogel for repeated on-demand ultrasound-triggered drug delivery. Biomaterials 2010, 31 (19), 5208–5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Kost J; Leong K; Langer R Ultrasound-enhanced polymer degradation and release of incorporated substances. Proc. Natl. Acad. Sci. U. S. A 1989, 86 (20), 7663–7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Elhelf IAS; Albahar H; Shah U; Oto A; Cressman E; Almekkawy M High intensity focused ultrasound: The fundamentals, clinical applications and research trends. Diagnostic and Interventional Imaging 2018, 99 (6), 349–359. [DOI] [PubMed] [Google Scholar]
- (10).Kolb HC; Finn MG; Sharpless KB Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem., Int. Ed 2001, 40 (11), 2004–2021. [DOI] [PubMed] [Google Scholar]
- (11).Echalier C; Valot L; Martinez J; Mehdi A; Subra G Chemical cross-linking methods for cell encapsulation in hydrogels. Mater. Today Commun 2019, 20, 100536. [Google Scholar]
- (12).Perera MM; Ayres N Dynamic covalent bonds in self-healing, shape memory, and controllable stiffness hydrogels. Polym. Chem 2020, 11 (8), 1410–1423. [Google Scholar]
- (13).Oz Y; Sanyal A The Taming of the Maleimide: Fabrication of Maleimide-Containing ‘Clickable’ Polymeric Materials. Chem. Rec 2018, 18 (6), 570–586. [DOI] [PubMed] [Google Scholar]
- (14).Madl CM; Heilshorn SC Bioorthogonal Strategies for Engineering Extracellular Matrices. Adv. Funct. Mater 2018, 28 (11), 1706046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Garcia-Astrain C; Guaresti O; Gonzalez K; Santamaria-Echart A; Eceiza A; Corcuera MA; Gabilondo N Click gelatin hydrogels: Characterization and drug release behaviour. Mater. Lett 2016, 182, 134–137. [Google Scholar]
- (16).Smith LJ; Taimoory SM; Tam RY; Baker AEG; Binth Mohammad N; Trant JF; Shoichet MS Diels–Alder Click-Cross-Linked Hydrogels with Increased Reactivity Enable 3D Cell Encapsulation. Biomacromolecules 2018, 19 (3), 926–935. [DOI] [PubMed] [Google Scholar]
- (17).Stewart SA; Backholm M; Burke NAD; Stöver HDH Cross-Linked Hydrogels Formed through Diels–Alder Coupling of Furan- and Maleimide-Modified Poly(methyl vinyl ether-alt-maleic acid). Langmuir 2016, 32 (7), 1863–1870. [DOI] [PubMed] [Google Scholar]
- (18).Stewart SA; Coulson MB; Zhou C; Burke NAD; Stöver HDH Synthetic hydrogels formed by thiol–ene crosslinking of vinyl sulfone-functional poly(methyl vinyl ether-alt-maleic acid) with α,ω-dithio-polyethyleneglycol. Soft Matter 2018, 14 (41), 8317–8324. [DOI] [PubMed] [Google Scholar]
- (19).Kirchhof S; Brandl FP; Hammer N; Goepferich AM Investigation of the Diels–Alder reaction as a cross-linking mechanism for degradable poly(ethylene glycol) based hydrogels. J. Mater. Chem. B 2013, 1 (37), 4855. [DOI] [PubMed] [Google Scholar]
- (20).Koehler KC; Anseth KS; Bowman CN Diels–Alder Mediated Controlled Release from a Poly(ethylene glycol) Based Hydrogel. Biomacromolecules 2013, 14 (2), 538–547. [DOI] [PubMed] [Google Scholar]
- (21).Altinbasak I; Sanyal R; Sanyal A Best of both worlds: Diels–Alder chemistry towards fabrication of redox-responsive degradable hydrogels for protein release. RSC Adv. 2016, 6 (78), 74757–74764. [Google Scholar]
- (22).Gregoritza M; Brandl FP The Diels–Alder reaction: A powerful tool for the design of drug delivery systems and biomaterials. Eur. J. Pharm. Biopharm 2015, 97, 438–453. [DOI] [PubMed] [Google Scholar]
- (23).Abu-Laban M; Kumal RR; Casey J; Becca J; Lamaster D; Pacheco CN; Sykes DG; Jensen L; Haber LH; Hayes DJ Comparison of thermally actuated retro-diels-alder release groups for nanoparticle based nucleic acid delivery. J. Colloid Interface Sci 2018, 526, 312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Abu-Laban M; Hamal P; Arrizabalaga JH; Forghani A; Dikkumbura AS; Kumal RR; Haber LH; Hayes DJ Combinatorial Delivery of miRNA-Nanoparticle Conjugates in Human Adipose Stem Cells for Amplified Osteogenesis. Small 2019, 15 (50), 1902864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Casey JS; Arrizabalaga JH; Abu-Laban M; Becca JC; Rose BJ; Strickland KT; Bursavich JB; McCann JS; Pacheco CN; Jensen L; Attaluri A; Hayes DJ Alternating magnetic field mediated release of fluorophores from magnetic nanoparticles by hysteretic heating. J. Colloid Interface Sci 2020, 571, 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Liu Y; Bailey JT; Abu-Laban M; Li S; Chen C; Glick AB; Hayes DJ Photocontrolled miR-148b nanoparticles cause apoptosis, inflammation and regression of Ras induced epidermal squamous cell carcinomas in mice. Biomaterials 2020, 256, 120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Arrizabalaga JH; Casey JS; Becca JC; Jensen L; Hayes DJ Comparison of thermoresponsive Diels-Alder linkers for the release of payloads from magnetic nanoparticles via hysteretic heating. JCIS Open 2021, 4, 100034. [Google Scholar]
- (28).Arrizabalaga JH; Casey JS; Becca JC; Liu Y; Jensen L; Hayes DJ Development of magnetic nanoparticles for the intracellular delivery of miR-148b in non-small cell lung cancer. Biomedical Engineering Advances 2022, 3, 100031. [Google Scholar]
- (29).Guaresti O; Garcia-Astrain C; Aguirresarobe RH; Eceiza A; Gabilondo N Synthesis of stimuli-responsive chitosan-based hydrogels by Diels-Alder cross-linking ‘click’ reaction as potential carriers for drug administration. Carbohydr. Polym 2018, 183, 278–286. [DOI] [PubMed] [Google Scholar]
- (30).Guaresti O; Garcia-Astrain C; Palomares T; Alonso-Varona A; Eceiza A; Gabilondo N Synthesis and characterization of a biocompatible chitosan-based hydrogel cross-linked via ‘click’ chemistry for controlled drug release. Int. J. Biol. Macromol 2017, 102, 1–9. [DOI] [PubMed] [Google Scholar]
- (31).Sahariah P; Másson M Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18 (11), 3846–3868. [DOI] [PubMed] [Google Scholar]
- (32).Monteiro OAC; Airoldi C Some studies of crosslinking chitosan-glutaraldehyde interaction in a homogeneous system. Int. J. Biol. Macromol 1999, 26 (2–3), 119–128. [DOI] [PubMed] [Google Scholar]
- (33).Knaul JZ; Hudson SM; Creber KAM Crosslinking of chitosan fibers with dialdehydes: Proposal of a new reaction mechanism. J. Polym. Sci., Part B: Polym. Phys 1999, 37 (11), 1079–1094. [Google Scholar]
- (34).Wu X; Black L; Santacana-Laffitte G; Patrick CW Preparation and assessment of glutaraldehyde-crosslinked collagen–chitosan hydrogels for adipose tissue engineering. J. Biomed. Mater. Res 2007, 81A (1), 59–65. [DOI] [PubMed] [Google Scholar]
- (35).Arrizabalaga JH; Nollert MU Riboflavin-UVA crosslinking of amniotic membranes and its influence on the culture of adipose-derived stem cells. Journal of the Mechanical Behavior of Biomedical Materials 2020, 106, 103729. [DOI] [PubMed] [Google Scholar]
- (36).Smallcomb M; Elliott J; Khandare S; Butt AA; Vidt ME; Simon JC Focused Ultrasound Mechanical Disruption of Ex Vivo Rat Tendon. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control 2021, 68 (9), 2981–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Yuldashev PV; Karzova MM; Kreider W; Rosnitskiy PB; Sapozhnikov OA; Khokhlova VA “HIFU Beam:” A Simulator for Predicting Axially Symmetric Nonlinear Acoustic Fields Generated by Focused Transducers in a Layered Medium. IEEE Trans Ultrason Ferroelectr Freq Control 2021, 68 (9), 2837–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Khandare S; Smallcomb M; Butt AA; Elliott J; Simon JC; Vidt ME Effects of focused ultrasound and dry needling on tendon mechanical properties. J. Biomech 2022, 132, 110934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Chapelle C; Quienne B; Bonneaud C; David G; Caillol S Diels-Alder-Chitosan based dissociative covalent adaptable networks. Carbohydr. Polym 2021, 253, 117222. [DOI] [PubMed] [Google Scholar]
- (40).Madl CM; Heilshorn SC Rapid Diels–Alder Cross-linking of Cell Encapsulating Hydrogels. Chem. Mater 2019, 31 (19), 8035–8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Murugesan S; Scheibel T Chitosan-based nanocomposites for medical applications. J. Polym. Sci 2021, 59 (15), 1610–1642. [Google Scholar]
- (42).Wang Y; Liu S; Yu W Bioinspired Anisotropic Chitosan Hybrid Hydrogel. ACS Applied Bio Materials 2020, 3 (10), 6959–6966. [DOI] [PubMed] [Google Scholar]
- (43).Rodrigues S; Dionísio M; López CR; Grenha A Biocompatibility of Chitosan Carriers with Application in Drug Delivery. Journal of Functional Biomaterials 2012, 3 (3), 615–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Wang G; Zhu J; Chen X; Dong H; Li Q; Zeng L; Cao X Alginate based antimicrobial hydrogels formed by integrating Diels–Alder “click chemistry” and the thiol–ene reaction. RSC Adv. 2018, 8(20), 11036–11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


