Abstract
American Indian and Alaska Natives (AI/ANs) are disproportionately impacted by gestational diabetes mellitus (GDM), subsequent type 2 diabetes, and food insecurity. It is prudent to decrease risk of GDM prior to pregnancy to decrease the intergenerational cycle of diabetes in AI/AN communities. The purpose of this project is to describe and examine food insecurity, healthy eating self-efficacy, and healthy eating behaviors among AI/AN females (12–24 years old) as related to GDM risk reduction. Methods included: secondary analysis of healthy eating self-efficacy and behaviors, and household-level food insecurity measures from an randomized controlled trial that tested the effect of engagement in a GDM risk reduction educational intervention on knowledge, behavior, and self-efficacy for GDM risk reduction from baseline to 3-month follow-up. Participants were AI/AN daughters (12–24 years old) and their mothers (N = 149 dyads). Researchers found that more than one-third (38.1%) reported food insecurity. At baseline food insecurity was associated with higher levels of eating vegetables and fruit for the full sample (p = .045) and cohabitating dyads (p = .002). By 3 months healthy eating self-efficacy (p = .048) and limiting snacking between meals (p = .031) improved more in the control group than the intervention group only for cohabitating dyads. For the full sample, the intervention group had increases in times eating vegetables (p = .022) and fruit (p = .015), whereas the control group had declines. In the full sample, food insecurity did not moderate the group by time interaction for self-efficacy for healthy eating (p ≥ .05) but did moderate the group by time interaction for times drinking soda (p = .004) and days eating breakfast (p = .013). For cohabitating dyads, food insecurity did moderate self-efficacy for eating 3 meals a day (p = .024) and days eating breakfast (p = .012). These results suggest food insecurity is an important factor regarding the efficacy of interventions designed to reduce GDM risk and offer unique insight on “upstream causes” of GDM health disparities among AI/AN communities.
Keywords: Gestational diabetes, Risk reduction, American Indian and Alaska Native, Food insecurity, Healthy eating, Self-efficacy
Limited access to healthful food (e.g., food insecurity) is a social determinant of health that impacts gestational diabetes risk and related disparities for American Indian and Alaska Native females.
Implications.
Given the intergenerational implications of gestational diabetes (GDM), it is prudent that public health and healthcare organizations work with American Indian and Alaska Native (AI/AN) communities to support healthful eating environments and practices among female AI/AN adolescent and young adults (AYAs). This effort includes cross-sector collaborations—which can differ in urban and rural (including reservation) AI/AN communities, and both types of communities need policy and increased awareness in the general community that support healthy eating environments, recognize tribal food sovereignty, and enforce rights to reclaim traditional food systems and tribally owned food retail outlets. Both rural and urban-dwelling AI/ANs need improved access to healthful food, safe places to engage in physical activity, affordable, safe housing, and improved economic opportunities to sustain these healthful practices.
INTRODUCTION
Gestational diabetes mellitus (GDM) is the most common complication of pregnancy in the USA, affecting 2%–10% of pregnancies annually [1]. GDM increases the risk of preeclampsia, preterm birth, cesarean section, and stillbirth [1–3]. GDM is also a significant risk factor for developing type 2 diabetes (T2D). American Indian and Alaska Native (AI/AN) women are twice as likely to have GDM and subsequent diagnosis of T2D than non-Hispanic White females [4, 5]. AI/ANs already have the highest prevalence of T2D among all racial and ethnic groups in the USA [6]. In addition to causing severe complications for both the mother and baby, GDM and obesity represent significant risk factors for both to develop T2D [7], perpetuating a vicious intergenerational cycle of diabetes in AI/AN communities [2]. AI/AN adolescents and young adults (AYAs) are disproportionately affected by adolescent pregnancy and GDM; both with nearly twice the overall U.S. prevalence [5, 8, 9]. Reducing the risk of GDM in AI/AN women is imperative to reducing diabetes health disparities among AI/AN communities and breaking this intergenerational cycle.
The United States Department of Agriculture (USDA) defines food insecurity as the lack of consistent access to enough food for an active, healthy life [10]. AI/AN peoples have higher rates of food insecurity when compared with non-AI/ANs [11–14] and are more likely to live in food deserts than any other racial/ethnic group [15–17]. Map the Meal Gap data from 2014 indicates counties with American Indian reservations have substantially higher rates of food insecurity than neighboring counties [18, 19]. In 2018, food insecurity among AI/AN communities was more than double that of general U.S. population (24.0% vs. 11.8%, respectively) [20]. Food insecurity is typically measured at the household level, and so the validated USDA’s Household Food Security Scale [21], the gold standard for measuring food insecurity, does not capture “severity” of food insecurity for any given family member within a single household. Food insecurity and limited access to healthful food can give individuals no choice but to rely on calorie-dense, carbohydrate-rich, processed foods, which negatively impact blood sugar in the general population [22–24] and AI/AN populations alike [25–27]. Further, as reflected in the adapted National Institutes of Minority Health and Health Disparities (NIMHD) Research Framework [28], food insecurity is exacerbated in AI/AN communities by contributors to barriers in physical and built environments, such as water insecurity [29, 30], stolen ancestral homelands, forced relocation, and environmental pollution, all of which have devastated AI/ANs traditional healthy food practices [10, 31]. Further complicating AI/ANs disparate access to healthy food, AI/AN communities often experience barriers to acquiring healthy traditional foods (such as wild game, fish, fresh produce, and nuts) [31], which further worsen food security [11–14]. Food insecurity is an independent risk factor for poor blood sugar management [24, 32–34], negatively impacts a person’s ability to manage blood sugar [24, 32, 35], and can contribute to unwanted weight gain in both adults and children [12, 36, 37].
Women deserve special consideration in discussions of food insecurity and its effects on health, nutrition, and behavior [38]. Among women of reproductive age, living in a food insecure household may increase risk of greater weight gain and perinatal complications [39]. Among adolescent females, food insecurity is associated with elevated body mass index [40], increased depressive symptoms [41], and smoking [41], and is a strong predictor of poor pregnancy outcomes including large for gestational age babies [42]. Further, adult women and pregnant adolescent females [43] who live in food insecure households experience macro- and micronutrient deficiencies, most notably iron and folate, nutrients especially important during the preconception period and pregnancy, with major implications for fetal and infant health and development [44]. Finally, AI/AN women with GDM have multiple maternal risk factors and their birth outcomes demonstrate the need for further research to improve care in this population [45]. Reducing the risk of GDM for AI/AN girls prior to their first pregnancy may effectively decrease diabetes disparities among AI/AN communities [2].
To help reduce the risk of GDM in AI/AN communities, our research team developed a GDM risk reduction intervention entitled Stopping Gestational Diabetes Mellitus in Daughters and Mothers (Stopping GDM) [46, 47]. Stopping GDM is an online theory- and evidence-based GDM risk reduction and preconception counseling program for AI/AN AYA who have a family history of diabetes or elevated body weight prior to pregnancy. The grounding theoretical framework for Stopping GDM is the Expanded Health Belief Model [48, 49]. Stopping GDM includes an online eBook, educational video, mother–daughter communication booklet, and online toolkit [46, 47] and is intended to serve AI/AN AYA at risk for GDM as well as their adult female family member (e.g., mother). The intention of prioritizing both the AI/AN AYA and their adult female caregiver is because of the sensitive nature of much of the information in Stopping GDM, specifically related to reproductive health and addressing elevated body weight and the importance of a positive mother/daughter relationship in navigating such sensitive information [50–52]. The online eBook includes two parts: “GDM and GDM Prevention” and “Taking Care of Your Body: Balancing Mind, Body, and Spirit.” The educational video is ~45 min in length and narrated by a female American Indian physician. The Stopping GDM team conducted a randomized controlled trial (RCT) to evaluate the effect of dyadic (e.g., mother and daughter) engagement in Stopping GDM on GDM knowledge, self-efficacy, and GDM risk reduction behaviors, such as healthy eating and physical activity, reproductive health choices, and family planning. The team also recognized the role of multilevel social determinants of health on risk factors of GDM, including food security [53]. The team collected data on self-reported food insecurity using a validated household-level food security survey at several time points during the Stopping GDM intervention [21]. While Stopping GDM currently does not specifically address food insecurity as a content area of focus, it recognizes that women tend to make the majority of food-related decisions and are known as the “nutritional gatekeepers” in a household [54–57].
Given the potential role of food insecurity in shaping future risk of GDM among AI/AN AYA, the purpose of this study is to (a) describe food insecurity and healthy eating self-efficacy and behaviors among AI/AN AYA in the Stopping GDM dataset at baseline; (b) examine the association of food insecurity with self-efficacy and healthy eating behaviors at baseline; and (c) explore the extent to which food insecurity may moderate the effect of the Stopping GDM intervention on self-efficacy for healthy eating and healthy eating behaviors using baseline and 3-month follow-up data. We hypothesized that AI/AN AYA who lived in food secure households would have greater self-efficacy for healthy eating and more positive changes in healthy eating behavior after participating in the Stopping GDM intervention than AI/AN AYA who lived in food insecure households.
METHODS
Conceptual framework
Most GDM risk reduction efforts focus on reducing the risk for women who are already pregnant [58, 59] or on risk reduction of future diagnoses of T2D among women who had GDM during a prior pregnancy [58, 60]. Unlike other GDM risk reduction interventions, Stopping GDM focuses on supporting healthy GDM risk reduction behaviors among AI/AN AYA prior to the first pregnancy, in order to break the intergenerational cycle of diabetes. The conceptual framework for this study on GDM risk reduction behaviors (healthy eating) is contextualized within multilevel domains of influence and social determinants of health (Fig. 1) [39]. In this study, we build on Laraia’s conceptual framework (white boxes) [39], which suggests the direct influence of food insecurity on GDM weight gain and pregnancy complications. We contextualized Laraia’s associations between food insecurity, individual characteristics, mediating behaviors, and pregnancy complications within multilevel frameworks guided by the National Institutes of Health Research Framework [28] and the Social Ecological Model [61, 62]. This adapted conceptual model helps to understand multidomain barriers and facilitators to healthy eating and weight management.
Study design
This secondary analysis used existing internal, deidentified data from the parent RCT study, Stopping GDM. The purpose was to address new research aims to describe food insecurity and explore food insecurity as a potential moderator of the effect of the Stopping GDM intervention on healthy eating behaviors and self-efficacy among AI/AN AYAs. Details of the parent study are reported elsewhere [63]. For the parent RCT, residing in the same household was not an eligibility criterion as many of the AYA were college students. In addition, the food insecurity module measures household-level food insecurity, but was food security was not the primary focus of this RCT.
Stopping GDM intervention
Briefly, in October 2019 our team concluded data collection from a five-site RCT with 3-month follow-up of female AI/AN AYA (12–24 years old) and their mothers (or other adult female caregivers) (N = 149 dyads). For clarity, the adult females in the dyad will be referred to as “mothers” through this manuscript. Data for the RCT were collected from March 2018 to October 2019. Residing in the same household was not an eligibility criterion for study enrollment as many of the AYA were college students. Participants were recruited across five collaborating AI/AN sites across the USA through site-based study coordinators efforts, which included word-of-mouth, use of diabetes registries, social media, and school-based connections. Both members of each dyad received between $20 and $40 for each study visit. The present study uses data collected at baseline and the 3-month follow-up visit. After completing the baseline assessment, dyads were randomized to either an immediate intervention or wait-list control group. Those who were randomized to the immediate intervention group watched the Stopping GDM video (~45 min) at the first (baseline) visit. At subsequent study visits, the intervention group dyads read the first and second half of the Stopping GDM eBook. At each visit dyad members completed the computer-based intervention and assessments independently from one another. Those dyads who were randomized to the wait-list control group received standard of care materials at baseline, which included March of Dimes reproductive health education materials [64]. As with the immediate intervention group, dyad members in the wait-list control group completed assessments independently at each visit. Data were collected at the same time points for both immediate intervention and wait-list control group dyads. This includes survey-based data for both mother and daughter and clinical metrics for the daughter. The latter are not included in the present study and findings are reported elsewhere [63]. This study was approved by the University of Pittsburgh IRB, Oklahoma City Area Indian Health Service IRB, Navajo Nation IRB, as well as two tribal review boards prior to human subjects research commencing.
Measures
Key study variables were collected at baseline and at 3-month follow-up, as there was significant loss to follow-up at the 6-month time point. Food insecurity was assessed using a modified version of the validated USDA Household Food Security Survey Module: 6-item Short Form [21]. This measure is based on self-reported household food security. It is the most commonly used scale for epidemiologic surveillance and produces almost all national estimates of food insecurity. Households with scores 0–1 are described as food secure. Households with scores 2–4 (“low food security”) and 5–6 (“very low food security”) together comprise households considered “food insecure.” In our modified version of this scale, the measure was dichotomized so that scores 0–1 indicated food security and scores 2–6 indicated food insecurity because of an error in the participant-facing version of the measure. Mothers completed this survey with respect to their household at baseline and 3-month visits. Daughters completed the “Self-Efficacy for Healthy Eating” Questionnaire and for this investigation the 5-item healthy eating subscale was used with 10-point Likert-type scaling (summation score range 5–50) whereby higher scores indicate greater self-efficacy to eat healthfully (Cronbach’s α = 0.96 and 0.74 in the current sample) [65]. Daughters also completed the “Eating Healthy and Physical Activity” section of the Centers for Disease Control and Prevention Youth Risk Behavior Surveillance System (YRBSS) [66]. Nine items from YRBSS assess healthy eating behaviors focusing on the intake of fruits, vegetables, milk, breakfast, and sugar-sweetened beverages, over a 7-day period. For this investigation, a 4-item vegetable subscale and the remaining five individual healthy eating items from the YRBSS were examined. Individual items range from 0 to 6 regarding the daily frequency of intake, except Item 9 regarding number of days per week eating breakfast ranges from 0 to 7; and the vegetable subscale ranges from 0 to 24; higher values or subscale scores indicate greater weekly intake. Internal consistency for the vegetable subscale was 0.71 in the current sample. Daughters’ demographic characteristics collected at baseline included age, ethnicity/race, employment (self), highest education attained, and marital status. Mothers’ demographic characteristics that were collected at baseline included employment status, highest education attained, household income, and marital status. These mothers’ demographic characteristics are known predictors of food insecurity [67–69].
Statistical analysis
Data were analyzed using IBM SPSS Statistics (version 28, IBM Corp., Armonk, NJ). Data were first screened for anomalies (e.g., outliers, data missingness) considering randomized treatment group assignment for the parent study (Stopping GDM vs. wait-list control) and food security status (food secure vs. food insecure). Missing data were handled using all available information for univariate, bivariate, and longitudinal analyses. Assuming data were missing at random, maximum likelihood methods were used. In particular, for longitudinal, repeated measures modeling full information maximum likelihood was employed through the predictive modeling. Descriptive statistics were calculated for the total sample and by treatment group assignment for the demographic characteristics of mothers and daughters and the baseline values of the targeted outcomes for daughters using frequency counts and percentages for categorical variables and means and standard deviations for continuous type variables. In particular, regarding the first aim, for daughter’s healthy eating self-efficacy (subscale score and the five items that makeup this subscale) and healthy eating behaviors (4-item vegetable subscale score and other five items from healthy eating portion of the YRBSS), the mean and 95% confidence interval were estimated. With existing data from 149 dyads we anticipated having a margin of error (in terms of the half-width of the confidence interval) of at most 0.083 when estimating proportions (or 8.3% for percentages) for a particular category for food security status (conservatively assuming 0.50 for a proportion) and 0.162σ (where σ is the standard deviation of outcome variable in the population) when estimating a mean based on the interval-scaled summary or item scores for daughter’s healthy eating self-efficacy and healthy eating behaviors. To compare daughter and mother characteristics and baseline values of the daughter’s outcomes between the treatment groups, standard group comparative analyses were performed, such as two-sample t-tests (or Wilcoxon rank-sum tests, if non-normality was encountered) for continuous type variables and chi-square tests of independence (or Fisher’s exact tests, if sparse cells occurred) for categorical variables.
To examine the association of food security with the daughter’s healthy eating self-efficacy and healthy eating behaviors at baseline (Aim 2), group comparative analyses for continuous type variables were again applied. With existing data from 149 dyads, we projected having at least 80% power to detect small to moderate sized correlations as small as r = .227 or mean differences of d = 0.463 when conducting nondirectional hypothesis testing at a two-tailed significance level of .05.
To explore the efficacy of the Stopping GDM intervention on daughter’s healthy eating self-efficacy and healthy eating behavior and food security as a possible moderator of the short-term efficacy of the intervention (i.e., treatment modification) (Aim 3), we used generalized linear mixed-effect regression modeling assuming normally distributed model errors and an identity link. All participants were analyzed as randomized, per an intention to treat approach. For each outcome variable, models included the fixed design effects of treatment group assignment (Stopping GDM intervention vs. wait-list control), time, and the interaction of treatment group assignment with time. To explore food insecurity as a possible moderator of the effect of the intervention, the main effect of food insecurity and its interactions with the design effects were added to the model. In addition to F-tests and p values for the model effects, least square means with 95% confidence intervals for modeling main effects and interactions and the within-group change were reported to describe possible treatment efficacy and treatment modification by food insecurity. Residual analysis with influence diagnostics was performed for all fitted models. As the modeling of the efficacy of the Stopping GDM intervention on daughter’s healthy eating self-efficacy and healthy eating behavior and food security as a possible moderator of the short-term efficacy of the intervention (Aim 3) was viewed as more exploratory, power analysis or the determination of the minimum detectable effect size for the intervention and its possible modification by perceived food insecurity was not performed.
For all analyses, we present results for both the full sample (N = 149 dyads) and for the subsample (n = 95 dyads) of mother–daughter dyads who shared a household. We include results for the full sample as it reflects the universe of participants who received the intervention and because food security is a dimension of socioeconomic status that may contribute to healthy eating self-efficacy and behaviors even outside of a currently common household living situation. Because household food security was reported by the mother (i.e., adult female member of the dyad) and dyad members were not required to live in the same household, all analyses were also conducted limiting the sample to dyad members who lived in the same household (n = 95 dyads).
RESULTS
Based on descriptive and test statistics reported in Table 1, the treatment groups were similar in terms of daughters’ and mothers’ characteristics as well as on daughters’ outcomes of self-efficacy for healthy eating and their actual healthy eating behaviors for both the total sample (N = 149) and the subsample (n = 95) of dyad members living in the same household (p ≥ .05). Most daughters were 18 years or younger (78% in the full sample, 83% in the subsample), with a mean age of 16.7 years (16.3 years in the subsample). The majority of daughters reported being American Indian (79% in the full sample, 76% in the subsample), nearly all in school (89% in full sample, 92% in subsample), none were married, and most were not employed (72% in full sample, 71% in subsample). Among mothers, the average age across both the full sample and subsample was 44 years and most had more than a high school education (83% in full sample, 85% in subsample), were in union/partnership (58% in full sample, 62% in subsample), and were employed (70% in full sample, 79% in subsample). More than one-third of households (38.1%, 95% CI = [30.2, 46.0]) in the full sample (and 34.7%, 95% CI = [25.1, 44.3] in the subsample) reported food insecurity. Daughters’ mean scores on self-efficacy for healthy eating subscale and healthy eating behaviors vegetable subscale were 29.7 (95% CI = [28.2, 31.3]) and 5.5 (95% CI = [5.3, 5.7]), respectively, in the full sample and 31.1 (95% CI = [29.2, 32.9]) and 5.5 (95% CI = [4.7, 6.3]), respectively, in the subsample. These baseline scores indicate a moderate level of self-efficacy for healthy eating yet a low vegetable intake per week.
Table 1.
Characteristics of daughters and mothers and daughters’ outcomes at the baseline visit (total, by treatment group)
| Characteristic | Full sample | Dyad members living together | ||||||
|---|---|---|---|---|---|---|---|---|
| Total (N = 149) Mean ± SD or n (%) |
Intervention (n = 79) Mean ± SD or n (%) |
Control (n = 70) Mean ± SD or n (%) |
p | Total (n = 95) Mean ± SD or n (%) |
Intervention (n = 57) Mean ± SD or n (%) |
Control (n = 38) Mean ± SD or n (%) |
p | |
| Daughter’s characteristics | ||||||||
| Age (years) | 16.7 ± 3.0 | 16.3 ± 2.7 | 17.2 ± 3.3 | .093 | 16.3 ± 2.9 | 15.9 ± 2.7 | 16.8 ± 3.1 | .180 |
| <19 | 116 (77.9) | 66 (56.9) | 50 (43.1) | .113 | 79 (83.2) | 49 (86.0) | 30 (78.9) | |
| ≥19 | 33 (22.1) | 13 (39.4) | 20 (60.6) | 16 (16.8) | 8 (14.0) | 8 (21.1) | ||
| Race (self-identified as best applies) | .725a | .606a | ||||||
| American Indian | 118 (79.2) | 60 (75.9) | 58 (82.9) | 72 (75.8) | 40 (70.2) | 32 (84.2) | ||
| White | 8 (5.4) | 6 (7.6) | 2 (2.9) | 7 (7.4) | 6 (10.5) | 1 (2.6) | ||
| Black/African American | 14 (9.4) | 9 (11.4) | 5 (7.1) | 10 (10.5) | 7 (12.3) | 3 (7.9) | ||
| Hispanic/Latino | 2 (1.3) | 1 (1.3) | 1 (1.4) | 2 (2.1) | 1 (1.8) | 1 (2.6) | ||
| Native Hawaiian or Pacific Islander | 2 (1.3) | 1 (1.3) | 1 (1.4) | 1 (1.1) | 1 (1.8) | 0 (0.0) | ||
| Other | 5 (3.4) | 2 (2.5) | 3 (4.3) | 3 (3.2) | 2 (3.5) | 1 (2.6) | ||
| Education | .109 | .709 | ||||||
| In school | 133 (89.3) | 74 (93.7) | 59 (84.3) | 87 (91.6) | 53 (93.0) | 34 (89.5) | ||
| Out of school | 16 (10.7) | 5 (6.3) | 11 (15.7) | 8 (8.4) | 4 (7.0) | 4 (10.5) | ||
| Educational attainment | .473 | .933 | ||||||
| Less than high school | 105 (70.5) | 59 (74.7) | 46 (65.7) | 74 (77.9) | 45 (78.9) | 29 (76.3) | ||
| High school graduate/GED | 21 (14.1) | 9 (11.4) | 12 (17.1) | 10 (10.5) | 6 (10.5) | 4 (10.5) | ||
| Beyond high school | 23 (15.4) | 11 (13.9) | 12 (17.1) | 11 (11.6) | 6 (10.5) | 5 (13.2) | ||
| Marital status | NA | NA | ||||||
| In union (married/cohabiting) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Not in union | 149 (100) | 79 (100) | 70 (100) | 95 (100) | 57 (100) | 38 (100) | ||
| Employment status | .714 | 1.000 | ||||||
| Working | 41 (27.7) | 23 (29.5) | 18 (25.7) | 27 (28.7) | 16 (28.6) | 11 (28.9) | ||
| Not working | 107 (72.3) | 55 (70.5) | 52 (74.3) | 67 (71.3) | 40 (71.4) | 27 (71.1) | ||
| Mother’s characteristics | ||||||||
| Age (years) | 44.14 ± 9.29 | 43.30 ± 8.35 | 45.09 ± 10.22 | .242 | 43.81 ± 7.11 | 42.81 ± 6.34 | 45.34 ± 7.97 | .089 |
| Educational attainment | .086 | .205a | ||||||
| Less than high school | 5 (3.4) | 3 (3.8) | 2 (2.9) | 3 (3.2) | 2 (3.5) | 1 (2.6) | ||
| High school graduate/GED | 20 (13.4) | 6 (7.6) | 14 (20.0) | 11 (11.6) | 4 (7.0) | 7 (18.4) | ||
| Beyond high school | 124 (83.2) | 70 (88.6) | 54 (77.1) | 81 (85.3) | 51 (89.5) | 30 (78.9) | ||
| Marital status | .067 | .831 | ||||||
| In union (married/cohabiting) | 87 (58.4) | 52 (65.8) | 35 (50.0) | 59 (62.1) | 36 (63.2) | 23 (60.5) | ||
| Not in union | 62 (41.6) | 27 (34.2) | 35 (50.0) | 36 (37.9) | 21 (36.8) | 15 (39.5) | ||
| Employment | .157 | .442 | ||||||
| Working | 103 (69.6) | 59 (74.7) | 44 (63.8) | 75 (78.9) | 47 (82.5) | 28 (78.9) | ||
| Not working | 45 (30.4) | 20 (25.3) | 25 (36.2) | 20 (21.1) | 10 (17.5) | 10 (26.3) | ||
| Household food security | 1.000 | .827 | ||||||
| Secure | 91 (61.9) | 48 (62.3) | 43 (61.4) | 62 (65.3) | 38 (66.7) | 24 (63.2) | ||
| Insecure | 56 (38.1) | 29 (37.7) | 27 (38.6) | 33 (34.7) | 19 (33.3) | 14 (36.8) | ||
| Daughter’s outcomesb | ||||||||
| Self-efficacy for healthy living | .878 | .311 | ||||||
| Healthy eating subscale | 29.72 ± 9.61 | 29.83 ± 9.78 | 29.59 ± 9.48 | 31.07 ± 9.34 | 32.88 ± 9.13 | 29.88 ± 9.65 | ||
| Healthy eating behaviors | .958 | .830 | ||||||
| Vegetable subscale | 5.50 ± 1.51 | 5.49 ± 3.90 | 5.52 ± 4.20 | 5.48 ± 4.04 | 5.41 ± 3.74 | 5.59 ± 4.49 | ||
aFisher–Freeman–Halton exact test.
bThe possible range of response for self-efficacy for health living was 5–50 for the healthy eating subscale score. For healthy eating behaviors, the range of response was 0–24 for the vegetable subscale score.
Table 2 describes diabetes-nutrition-related constructs at the baseline visit, prior to any intervention delivery, and their association with food security status as reported by the mother; results are shown for the full sample and the subsample of dyad members living together. Overall, similarities were noted between the food secure and insecure groups with both groups reporting higher levels of self-efficacy for healthy eating. Namely, participants perceived self-confidence (e.g., self-efficacy) in their ability to eat 3 meals a day, limit snacks between meals, drink water, and avoid junk food and sugar-sweetened beverages. Item-specific scores tended to be higher on the ability to eat 3 meals a day, drink water, and avoid sugar-sweetened beverages than on avoiding junk food and limiting snacks. Although the overall average scores and the items that make up this subscale tended to be slightly higher in the food secure group, there were no significant differences between food secure and insecure groups on this construct for both the total sample and subsample of dyad members living in the same household (p ≥ .05).
Table 2.
Association of household food security with diabetes-nutrition-related constructs at baseline
| Outcomea | Full sample (N = 148) | Dyad members living together (n = 95) | ||||||
|---|---|---|---|---|---|---|---|---|
| Food security | p | Food security | p | |||||
| Secure Mean ± SD |
Insecure Mean ± SD |
Total Mean ± SD |
Secure Mean ± SD |
Insecure Mean ± SD |
Total Mean ± SD |
|||
| Self-efficacy for healthy living | ||||||||
| Healthy eating subscale (sum of Items 1, 2, 3, 4, and 8) | 30.3 ± 10.0 | 29.0 ± 8.8 | 29.8 ± 9.6 | .436 | 31.8 ± 9.5 | 29.7 ± 9.1 | 31.1 ± 9.3 | .307 |
| Eat 3 meals a day (Item 1) | 7.1 ± 3.2 | 6.7 ± 2.8 | 6.9 ± 3.0 | .476 | 7.4 ± 3.1 | 6.6 ± 2.9 | 7.1 ± 3.0 | .175 |
| Limit snacking in between meals (Item 2) | 5.4 ± 2.9 | 5.2 ± 2.8 | 5.3 ± 2.8 | .741 | 5.9 ± 3.0 | 5.2 ± 2.9 | 5.6 ± 2.9 | .316 |
| Drink water most of the time (Item 3) | 7.3 ± 2.8 | 7.4 ± 2.6 | 7.3 ± 2.7 | .883 | 7.3 ± 2.7 | 7.8 ± 2.5 | 7.5 ± 2.6 | .369 |
| Avoid junk food and fast food (Item 4) | 4.5 ± 2.6 | 4.3 ± 2.1 | 4.4 ± 2.4 | .580 | 5.1 ± 2.8 | 4.4 ± 2.3 | 4.8 ± 2.6 | .262 |
| Avoid drinking sugar-sweetened beverages such as soda, juice, and energy drinks (Item 8) | 6.0 ± 2.9 | 5.4 ± 2.4 | 5.8 ± 2.7 | .239 | 6.0 ± 2.7 | 5.7 ± 2.6 | 5.9 ± 2.7 | .617 |
| Healthy eating behaviors | ||||||||
| Times eating vegetables subscale (sum of Items 3–6) | 5.0 ± 3.9 | 6.3 ± 4.1 | 5.5 ± 4.0 | .057 | 4.6 ± 3.6 | 7.0 ± 4.4 | 5.5 ± 4.0 | .006 |
| Times drinking 100% fruit juice such as orange, apple, or grape juice (Item 1) | 1.4 ± 1.5 | 1.6 ± 1.5 | 1.4 ± 1.5 | .449 | 1.6 ± 1.6 | 1.7 ± 1.6 | 1.6 ± 1.6 | .662 |
| Times eating fruit (Item 2) | 1.8 ± 1.4 | 2.4 ± 1.8 | 2.0 ± 1.6 | .077 | 1.7 ± 1.2 | 2.7 ± 1.8 | 2.0 ± 1.5 | .007 |
| Times drinking a can, bottle, or glass of soda or pop (Item 7) | 1.5 ± 1.3 | 1.6 ± 1.4 | 1.5 ± 1.4 | .704 | 1.5 ± 1.3 | 1.5 ± 1.5 | 1.5 ± 1.4 | .872 |
| Glasses of milk drank (Item 8) | 1.2 ± 1.4 | 1.3 ± 1.4 | 1.2 ± 1.4 | .701 | 1.3 ± 1.5 | 1.3 ± 1.4 | 1.3 ± 1.5 | .892 |
| Days eating breakfast (Item 9) | 4.1 ± 2.4 | 3.9 ± 2.1 | 4.0 ± 2.3 | .757 | 3.9 ± 2.4 | 3.7 ± 2.2 | 3.9 ± 2.3 | .627 |
aThe possible range of response for self-efficacy for health living was 5 to 50 for the healthy eating subscale score, while for the individual items the possible range of response for was 1 to 10. For healthy eating behaviors, the range of response was 0 to 24 for the times eating vegetables subscale score, while for the individual items the possible range of response was 0 to 6 regarding the daily frequency of intake, except Item 9 regarding number of days per week eating breakfast which ranges from 0 to 7.
Similarities were also noted between treatment groups for actual healthy eating behavior at baseline (Table 2). However, the overall average scores tended to be slightly higher in the food insecure group compared with the food secure group, with significant differences by food security status for the times eating vegetables (4.6 among food secure vs. 7.0 among food insecure, p = .006) and times eating fruit (1.7 among food secure vs. 2.7 among food insecure, p = .007) but only in the subsample of dyad members living in the same household.
Table 3 focuses on the effect of the Stopping GDM intervention on AI/AN AYA self-efficacy for healthy eating and healthy eating behaviors from baseline to the 3-month follow-up. The full sample had significant increases over time from baseline to 3-month follow-up for each of the self-efficacy for healthy eating items and for its summation score (p < .05) with no significant differences in these changes between treatment groups (p ≥ .05). With regard to healthy eating behaviors, the full sample showed significant group by time interactions for times eating vegetables (pG×T = .022) and times eating fruit (pG×T = .015), whereby the times eating vegetables and times eating fruit tended to increase in the intervention group and decrease in the wait-list control group from baseline to 3-month follow-up. Additionally, a significant group main effect was found for times drinking 100% fruit juice, such as orange, apple, or grape juice (pGroup = .004). On average, the wait-list control group had lower scores at the 3-month follow-up than the intervention group.
Table 3.
Baseline, follow-up, and mean change scores in diabetes-nutrition-related constructs by study assignment
| Outcomea | Full sample (N = 149) | Dyad members living together (n = 95) | ||||||
|---|---|---|---|---|---|---|---|---|
| Time | p | Time | p | |||||
| Baseline pre Mean score (95% CI) |
3 months Mean score (95% CI) |
Mean change (95% CI) |
Baseline pre Mean score (95% CI) |
3 months Mean score (95% CI) |
Mean change (95% CI) |
|||
| Self-efficacy for health living | ||||||||
| Healthy eating subscale (sum of Items 1, 2, 3, 4, and 8) |
p
Group = .674 pTime < .001 pG×T = .255 |
p
Group = .969 pTime = .002 pG×T = .048 |
||||||
| Intervention | 29.8 (27.6 to 32.0) |
32.4 (30.2 to 34.7) |
2.6 (0.5 to 4.6)* |
31.9 (29.5 to 34.3) |
33.1 (30.5 to 35.6) |
1.2 (−1.2 to 3.6) |
||
| Control | 29.6 (27.4 to 31.8) |
33.9 (31.6 to 36.2) |
4.3 (2.2 to 6.4)* |
29.9 (26.8 to 32.9) |
34.9 (31.8 to 38.1) |
5.1 (2.1 to 8.0)* |
||
| Eat 3 meals a day (Item 1) |
p
Group = .664 pTime = .028 pG×T = .852 |
p
Group = .793 pTime = .058 pG×T = .995 |
||||||
| Intervention | 6.8 (6.1 to 7.5) |
7.4 (6.7 to 8.1) |
0.6 (−0.2 to 1.4) |
7.1 (6.3 to 7.8) |
7.7 (6.7 to 8.5) |
0.6 (−0.4 to 1.6) |
||
| Control | 7.0 (6.3 to 7.7) |
7.5 (6.8 to 8.2) |
0.5 (−0.1 to 1.1) |
7.2 (6.2 to 8.2) |
7.8 (6.9 to 8.7) |
0.60 (−0.1 to 1.3) |
||
| Limit snacking in between meals (Item 2) |
p
Group = .321 pTime = .003 pG×T = .070 |
p
Group = .403 pTime = .012 pG×T = .031 |
||||||
| Intervention | 5.3 (4.7 to 6.0) |
5.6 (5.0 to 6.3) |
0.3 (−0.4 to 1.0) |
5.7 (5.0 to 6.5) |
5.9 (5.1 to 6.6) |
0.12 (−0.7 to 0.9) |
||
| Control | 5.3 (4.6 to 6.0) |
6.5 (5.8 to 7.2) |
1.2 (0.5 to 2.0)* |
5.5 (4.6 to 6.4) |
7.0 (6.1 to 7.9) |
1.5 (0.5 to 2.5)* |
||
| Drink water most of the time (Item 3) |
p
Group = .643 pTime = .010 pG×T = .822 |
p
Group = .665 pTime = .107 pG×T = .334 |
||||||
| Intervention | 7.2 (6.6 to 7.8) |
7.7 (7.1 to 8.4) |
0.5 (−0.1 to 1.1) |
7.7 (7.0 to 8.3) |
7.8 (7.1 to 8.6) |
0.2 (−0.6 to 0.9) |
||
| Control | 7.3 (6.7 to 8.0) |
8.0 (7.4 to 8.5) |
0.6 (0.0 to 1.2)* |
7.2 (6.3 to 8.1) |
7.9 (7.1 to 8.7) |
0.7 (−0.1 to 1.5) |
||
| Avoid junk food and fast food (Item 4) |
p
Group = .364 pTime = .004 pG×T = .818 |
p
Group = .358 pTime = .101 pG×T = .222 |
||||||
| Intervention | 4.6 (4.1 to 5.2) |
5.3 (4.6 to 6.0) |
0.7 (0.0 to 1.4) |
5.2 (4.5 to 5.9) |
5.3 (4.5 to 6.2) |
0.1 (−0.7 to 1.0) |
||
| 4.3 (3.5 to 5.2) |
5.3 (4.4 to 6.3) |
1.0 (−0.1 to 2.1) |
||||||
| Control | 4.2 (3.7 to 4.8) |
5.0 (4.4 to 5.7) |
0.8 (0.1 to 1.5)* |
|||||
| Avoid drinking sugar-sweetened beverages such as soda, juice and energy drinks (Item 8) |
p
Group = .555 pTime = .017 pG×T = .637 |
p
Group = .953 pTime = .241 pG×T = .578 |
||||||
| Intervention | 5.7 (5.1 to 6.3) |
6.2 (5.5 to 6.9) |
0.5 (−0.3 to 1.2) |
6.0 (5.3 to 6.7) |
6.2 (5.4 to 7.0) |
0.2 (−0.7 to 1.1) |
||
| Control | 5.8 (5.2 to 6.5) |
6.5 (5.8 to 7.3) |
−0.7 (−1.4 to 0.0)* |
5.8 (4.9 to 6.7) |
6.4 (5.4 to 7.5) |
0.6 (−0.5 to 1.7) |
||
| Healthy eating behavior | ||||||||
| Times eating vegetables (sum of Items 3–6) |
p
Group = .130 pTime = .893 pG×T = .022 |
p
Group = .555 pTime = .904 pG×T = .258 |
||||||
| Intervention | 5.5 (4.6 to 6.4) |
6.5 (5.2 to 7.9) |
1.0 (−0.2 to 2.2) |
5.5 (4.5 to 6.5) |
6.0 (4.4 to 7.6) |
0.5 (−0.9 to 20) |
||
| Control | 5.5 (4.5 to 6.5) |
4.6 (3.6 to 5.6) |
−0.9 (−2.0 to 0.2) |
5.6 (4.1 to 7.1) |
4.9 (3.6 to 6.2) |
−0.7 (−2.2 to 0.9) |
||
| Times drinking 100% fruit juice, such as orange, apple, or grape juice (Item 1) |
p
Group = .004 pTime = .331 pG×T = .563 |
p
Group = .372 pTime = .095 pG×T = .965 |
||||||
| Intervention | 1.7 (1.3 to 2.0) |
1.6 (1.2 to 2.0) |
−0.1 (−0.5 to 0.4) |
1.7 (1.3 to 2.1) |
1.4 (0.9 to 1.8) |
−0.3 (−0.9 to 0.2) |
||
| Control | 1.2 (0.9 to 1.5) |
1.0 (0.7 to 1.2) |
−0.2 (−0.6 to 0.1) |
1.5 (1.0 to 2.0) |
1.1 (0.7 to 1.6) |
−0.3 (−0.9 to 0.3) |
||
| Times eating fruit (Item 2) |
p
Group = .008 pTime = .609 pG×T = .015 |
|
p
Group = .250 pTime = .579 pG×T = .093 |
|||||
| Intervention | 2.1 (1.8 to 2.5) |
2.6 (2.2 to 3.1) |
0.5* (0.6 to 0.9) |
2.0 (1.6 to 2.3) |
2.5 (2.0 to 3.0) |
0.5 (0.0 to 1.0)* |
||
| Control | 1.9 (1.6 to 2.3) |
1.6 (1.3 to 2.0) |
−0.3 (−0.8 to 0.2) |
2.1 (1.5 to 2.6) |
1.8 (1.3 to 2.3) |
−0.3 (−1.0 to 0.5) |
||
| Times drinking a can, bottle, or glass of soda or pop (Item 7) |
p
Group = .766 pTime = .381 pG×T = .375 |
p
Group = .576 pTime = .835 pG×T = .346 |
||||||
| Intervention | 1.5 (1.2 to 1.8) |
1.5 (1.1 to 1.9) |
0.0 (−0.4 to 0.4) |
1.5 (1.1 to 1.8) |
1.3 (0.8 to 1.7) |
−0.2 (−0.7 to 0.3) |
||
| Control | 1.5 (1.2 to 1.9) |
1.3 (0.9 to 1.7) |
−0.2 (−0.6 to 0.1) |
1.5 (1.0 to 2.0) |
1.6 (1.0 to 2.2) |
0.1 (−0.3 to 0.6) |
||
| Glasses of milk drank (Item 8) |
p
Group = .777 pTime = .683 pG×T = .293 |
p
Group = .676 pTime = .685 pG×T = .170 |
||||||
| Intervention | 1.1 (0.9 to 1.4) |
1.2 (0.8 to 1.6) |
0.1 (−0.3 to 0.5) |
1.2 (0.9 to 1.6) |
1.4 (0.9 to 1.9) |
0.2 (−0.4 to 0.7) |
||
| Control | 1.3 (1.0 to 1.7) |
1.1 (0.8 to 1.5) |
−0.2 (−0.6 to 0.2) |
1.4 (0.8 to 1.9) |
1.0 (0.6 to 1.5) |
−0.3 (−0.8 to 0.2) |
||
| Days eating breakfast (Item 9) |
p
Group = .183 pTime = .698 pG×T = .374 |
p
Group = .905 pTime = .595 pG×T = .230 |
||||||
| Intervention | 3.7 (3.2 to 4.2) |
3.8 (3.2 to 4.4) |
0.1 (−0.5 to 0.7) |
3.8 (3.2 to 4.4) |
4.1 (3.4 to 4.9) |
0.4 (−0.3 to 1.1) |
||
| Control | 4.4 (3.8 to 4.9) |
4.1 (3.5 to 4.7) |
−0.2 (−0.7 to 0.2) |
4.0 (3.2 to 4.7) |
3.8 (3.0 to 4.7) |
−0.1 (−0.7 to 0.4) |
||
CI confidence interval.
aThe possible range of response for self-efficacy for health living was 5 to 50 for the healthy eating subscale score, while for the individual items the possible range of response for was 1 to 10. For healthy eating behaviors, the range of response was 0 to 24 for the times eating vegetables subscale score, while for the individual items the possible range of response was 0 to 6 regarding the daily frequency of intake, except Item 9 regarding number of days per week eating breakfast which ranges from 0 to 7.
* p < .05.
The subsample of dyad members living together had significant time main effects and group by time interaction effects for self-efficacy for both the healthy eating summation score (pTime = .002 and pG×T = .048, respectively) and limiting snacking in between meals item (pTime = .012 and pG×T = .031, respectively). In both instances, the wait-list control group had greater improvements in scores from baseline to 3 months. There were no significant group or time main effects or interactions for the healthy eating vegetable subscale or individual eating healthy behaviors in the subsample (p ≥ .05).
Table 4 summarizes the extent to which food security moderated the effect of Stopping GDM intervention on AI/AN AYA’s self-efficacy for healthy eating and actual healthy eating behaviors from baseline to the 3-month follow-up. In the full sample, food security did not moderate the group by time interaction for self-efficacy for healthy eating (p ≥ .05) but did moderate the interaction of group by time for two individual healthy eating behaviors: frequency of drinking soda or pop (pFS×G×T = .004) and days eating breakfast (pFS×G×T = .013). For those reporting food security at baseline, the intervention group showed small, yet nonsignificant decrease in their times drinking soda or pop, while the wait-list control group showed little change from the baseline to the 3-month follow-up. In contrast, for those reporting food insecurity at baseline, the intervention group tended to increase their times drinking soda or pop from baseline to the 3-month follow-up, whereas the wait-list control group significantly decreased their frequency of soda/pop consumption (mean change = −0.7, 95% CI = [−1.1, −0.3], p < .05). For those reporting food security at baseline, the intervention group significantly increased the mean number of days eating breakfast from baseline to 3 months (mean change = 0.8, 95% CI = [0.0, 1.5], p < .05), while the wait-list control group demonstrated a small, but nonsignificant decrease. For those reporting food insecurity at baseline, the intervention group had a significant decrease in the mean days eating breakfast from baseline to 3 months (mean change = −1.1, 95% CI = [−2.0, −0.2], p < .05) and the wait-list control group had a small, nonsignificant decrease.
Table 4.
The effect of baseline food insecurity with change in diabetes-nutrition-related constructs by treatment group assignment
| Outcomea | Full sample (N = 148) | Dyad members living together (n = 95) | ||||||
|---|---|---|---|---|---|---|---|---|
| Time | Test statistics | Time | Test statistics | |||||
| Baseline Mean score (95% CI) |
3 months Mean score (95% CI) |
Mean change (95% CI) |
p | Baseline Mean score (95% CI) |
3 months Mean score (95% CI) |
Mean change (95% CI) |
p | |
| Self-efficacy for healthy living | ||||||||
| Healthy eating subscale (sum of Items 1, 2, 3, 4, and 8) |
p
Group = .584 pTime < .001 pG×T = .292 pFS = .750 pFS×G = .372 pFS×T = .024 pFS×G×T = .438 |
p
Group = .833 pTime < .001 pG×T = .113 pFS = .627 pFS×G = .203 pFS×T = .012 pFS×G×T = .539 |
||||||
| Food secure | ||||||||
| Intervention | 31.2 (28.1 to 34.3) |
31.9 (28.8 to 34.9) |
0.7 (−1.9 to 3.2) |
33.4 (30.4 to 36.4) |
32.7 (29.5 to 36.0) |
−0.6 (−3.5 to 2.3) |
||
| Control | 29.3 (26.6 to 32.0) |
32.7 (30.0 to 35.4) |
3.4 (0.6 to 6.1)* |
29.4 (25.6 to 33.1) |
32.8 (29.5 to 36.2) |
3.4 (−0.2 to 7.0) |
||
| Food insecure | ||||||||
| Intervention | 28.1 (25.4 to 30.8) |
33.3 (29.8 to 36.7) |
5.2 (2.1 to 8.2)* |
28.9 (25.3 to 32.6) |
34.2 (30.0 to 38.5) |
5.3 (2.1 to 8.5)* |
||
| Control | 30.0 (26.2 to 33.8) |
35.6 (31.6 to 39.5) |
5.6 (2.4 to 8.7) |
30.8 (25.5 to 36.1) |
37.9 32.4 to 43.4) |
7.1 (2.3 to 11.9)* |
||
| Eat 3 meals a day (Item 1) |
p
Group = .931 pTime = .020 pG×T = .569 pFS = .717 pFS×G = .321 pFS×T = .403 pFS×G×T = .078 |
p
Group = .949 pTime = .015 pG×T = .454 pFS = .451 pFS×G = .469 pFS×T = .200 pFS×G×T = .024 |
||||||
| Food secure | ||||||||
| Intervention | 7.0 (6.1 to 7.9) |
7.1 (6.2 to 8.0) |
0.1 (−0.8 to 1.0) |
7.5 (6.5 to 8.4) |
7.4 (6.4 to 8.3) |
−0.1 (−1.2 to 1.0) |
||
| Control | 7.1 (6.2 to 8.1) |
7.8 (6.9 to 8.8) |
0.7 (−0.1 to 1.5) |
7.4 (6.1 to 8.6) |
8.3 (7.1 to 9.5) |
0.9 (−0.1 to 1.9) |
||
| Food insecure | ||||||||
| Intervention | 6.6 (5.6 to 7.6) |
8.0 (6.9 to 9.1) |
1.4 (0.0 to 2.9) |
6.3 (5.0 to 7.5) |
8.5 (7.1 to 9.9) |
2.3 (0.3 to 4.2)* |
||
| Control | 6.8 (5.7 to 7.9) |
7.0 (6.1 to 7.9) |
0.2 (−0.6 to 1.1) |
6.9 (5.3 to 8.5) |
7.2 (6.0 to 8.4) |
0.2 (−0.8 to 1.3) |
||
| Limit snacking in between meals (Item 2) |
p
Group = .301 pTime = .001 pG×T = .048 pFS = .663 pFS×G = .474 pFS×T = .150 pFS×G×T = .466 |
p
Group = .355 pTime = .002 pG×T = .011 pFS = .985 pFS×G = .476 pFS×T = .020 pFS×G×T = .073 |
||||||
| Food secure | ||||||||
| Intervention | 5.5 (4.7 to 6.3) |
5.6 (4.8 to 6.5) |
0.1 (−0.7 to 1.0) |
5.9 (5.0 to 6.9) |
5.9 (5.0 to 6.9) |
0.0 (−1.0 to 1.0) |
||
| Control | 5.3 (4.4 to 6.2) |
6.1 (5.2 to 6.9) |
0.8 (−0.3 to 1.8) |
5.8 (4.6 to 7.0) |
6.3 (5.2 to 7.4) |
0.5 (−0.7 to 1.7) |
||
| Food insecure | ||||||||
| Intervention | 5.2 (4.2 to 6.2) |
5.7 (4.7 to 6.6) |
0.5 (−0.6 to 1.5) |
5.4 (4.1 to 6.7) |
5.7 (4.4 to 7.0) |
0.3 (−0.8 to 1.5) |
||
| Control | 5.3 (4.2 to 6.3) |
7.1 (6.0 to 8.2) |
1.8 (0.9 to 2.8)* |
5.0 (3.6 to 6.4) |
7.8 (6.5 to 9.2) |
2.8 (1.7 to 4.0)* |
||
| Drink water most of the time (Item 3) |
p
Group = .740 pTime = .002 pG×T = .889 pFS = .163 pFS×G = .981 pFS×T = .036 pFS×G×T = .770 |
p
Group = .728 pTime = .022 pG×T = .626 pFS = .032 pFS×G = .338 pFS×T = .134 pFS×G×T = .277 |
||||||
| `Food secure | ||||||||
| Intervention | 7.3 (6.4 to 8.1) |
7.4 (6.5 to 8.2) |
0.1 (−0.7 to 1.0) |
7.7 (6.9 to 8.5) |
7.5 (6.6 to 8.4) |
−0.2 (−1.2 to 0.8) |
||
| Control | 7.3 (6.5 to 8.1) |
7.6 (6.8 to 8.3) |
0.3 (−0.5 to 1.1) |
6.7 (5.5 to 7.8) |
7.2 (6.2 to 8.3) |
0.6 (−0.6 to 1.8) |
||
| Food insecure | ||||||||
| Intervention | 7.3 (6.4 to 8.2) |
8.4 (7.5 to 9.3) |
1.1 (0.3 to 1.9)* |
7.6 (6.6 to 8.7) |
8.7 (7.7 to 9.7) |
1.1 (0.5 to 1.6)* |
||
| Control | 7.4 (6.4 to 8.5) |
8.5 (7.7 to 9.3) |
1.01 (0.2 to 1.9)* |
8.1 (6.7 to 9.4) |
8.9 (7.8 to 9.9) |
0.8 (−0.2 to 1.8) |
||
| Avoid junk food and fast food (Item 4) |
p
Group = .674 pTime = .004 pG×T = .951 pFS = .938 pFS×G = .053 pFS×T = .410 pFS×G×T = .234 |
p
Group = .661 pTime = .060 pG×T = .346 pFS = .983 pFS×G = .081 pFS×T = .214 pFS×G×T = .647 |
||||||
| Food secure | ||||||||
| Intervention | 5.1 (4.3 to 5.9) |
5.3 (4.5 to 6.2) |
0.2 (−0.7 to 1.1) |
5.7 (4.8 to 6.6) |
5.4 (4.5 to 6.4) |
−0.3 (−1.2 to 0.7) |
||
| Control | 3.9 (3.2 to 4.6) |
4.8 (4.0 to 5.5) |
0.8 (0.0 to 1.7) |
4.1 (3.0 to 5.2) |
4.8 (3.8 to 5.9) |
0.7 (−0.6 to 2.1) |
||
| Food insecure | ||||||||
| Intervention | 3.9 (3.2 to 4.6) |
5.1 (4.0 to 6.3) |
1.2 (0.1 to 2.3)* |
4.2 (3.3 to 5.2) |
5.2 (3.5 to 6.9) |
1.0 (−0.5 to 2.4) |
||
| Control | 4.7 (3.9 to 5.5) |
5.4 (4.3 to 6.5) |
0.7 (−0.5 to 1.8) |
4.7 (3.4 to 6.0) |
6.0 (4.3 to 7.7) |
1.3 (−0.5 to 3.1) |
||
| Avoid drinking sugar-sweetened beverages such as soda, juice and energy drinks (Item 8) |
p
Group = .377 pTime = .013 pG×T = .490 pFS = .741 pFS×G = .181 pFS×T = .146 pFS×G×T = .571 |
p
Group = .634 pTime = .212 pG×T = .536 pFS = .607 pFS×G = .074 pFS×T = .237 pFS×G×T = .355 |
||||||
| Food secure | ||||||||
| Intervention | 6.0 (5.2 to 6.9) |
6.3 (5.4 to 7.2) |
0.2 (−0.7 to 1.2) |
6.2 (5.4 to 7.1) |
6.4 (5.4 to 7.4) |
0.1 (−0.9 to 1.2) |
||
| Control | 5.9 (5.0 to 6.7) |
6.2 (5.3 to 7.0) |
0.3 (−0.5 to 1.1) |
5.7 (4.6 to 6.9) |
5.6 (4.5 to 6.6) |
−0.1 (−1.2 to 1.0) |
||
| Food insecure | ||||||||
| Intervention | 5.1 (4.4 to 5.9) |
5.8 (4.9 to 6.9) |
0.7 (−0.3 to 1.8) |
5.5 (4.5 to 6.5) |
5.8 (4.5 to 7.1) |
0.3 (−1.3 to 1.9) |
||
| Control | 5.7 (4.7 to 6.8) |
7.1 (5.9 to 8.3) |
1.4 (0.1 to 2.6)* |
6.1 (4.5 to 7.7) |
7.6 (5.7 to 9.4) |
1.5 (−0.5 to 3.5) |
||
| Healthy eating behavior | ||||||||
| Times eating vegetables (sum of Items 3–6) |
p
Group = .127 pTime = .893 pG×T = .045 pFS = .160 pFS×G = .160 pFS×T = .545 pFS×G×T = .986 |
p
Group = .561 pTime = .812 pG×T = .343 pFS = .041 pFS×G = .873 pFS×T = .566 pFS×G×T = .901 |
||||||
| Food secure | ||||||||
| Intervention | 4.9 (3.8 to 5.9) |
6.0 (4.6 to 7.4) |
1.1 (0.2 to 2.4) |
4.8 (3.6 to 5.9) |
5.5 (4.1 to 6.9) |
0.8 (−0.5 to 2.0) |
||
| Control | 5.2 (3.9 to 6.5) |
4.5 (3.3 to 5.7) |
−0.7 (−2.27 to 0.8) |
4.6 (3.0 to 6.2) |
4.3 (2.9 to 5.6) |
−0.3 (−2.0 to 1.4) |
||
| Food insecure | ||||||||
| Intervention | 6.6 (5.1 to 8.1) |
7.2 (4.3 to 10.1) |
0.6 (−1.9 to 3.0) |
6.9 (5.0 to 8.7) |
7.1 (2.3 to 11.9) |
0.2 (−4.1 to 4.5) |
||
| Control | 6.0 (4.4 to 7.6) |
4.7 (3.2 to 6.3) |
−1.3 (−3.0 to 0.5) |
7.2 (4.7 to 9.8) |
5.9 (3.5 to 8.4) |
−1.3 (−4.1 to 1.6) |
||
| Times drinking 100% fruit juice such as orange, apple, or grape juice (Item 1) |
p
Group < .001 pTime = .562 pG×T = .475 pFS = .043 pFS×G = .031 pFS×T = .268 pFS×G×T = .604 |
p
Group = .120 pTime = .371 pG×T = .755 pFS = .141 pFS×G = .078 pFS×T = .188 pFS×G×T = .657 |
||||||
| Food secure | ||||||||
| Intervention | 1.5 (1.0 to 1.9) |
1.2 (0.9 to 1.6) |
−0.2 (−0.7 to 0.2) |
1.6 (1.0 to 2.1) |
1.0 (0.7 to 1.3) |
−0.6 (−1.0 to −0.1)* |
||
| Control | 1.2 (0.8 to 1.7) |
0.9 (0.6 to 1.3) |
−0.3 (−0.8 to 0.2) |
1.6 (0.9 to 2.3) |
1.1 (0.6 to 1.6) |
−0.5 (−1.2 to 0.2) |
||
| Food insecure | ||||||||
| Intervention | 2.1 (1.4 to 2.7) |
2.4 (1.5 to 3.2) |
0.3 (−0.7 to 1.3) |
2.1 (1.3 to 2.8) |
2.3 (1.0 to 3.7) |
0.3 (−1.2 to 1.7) |
||
| Control | 1.1 (0.7 to 1.6) |
1.0 (0.5 to 1.5) |
−0.1 (−0.7 to 0.5) |
1.3 (0.6 to 2.0) |
1.2 (0.4 to 2.0) |
−0.1 (−1.1 to 0.9) |
||
| Times eating fruit (Item 2) |
p
Group = .012 pTime = .962 pG×T = .051 pFS = .180 pFS×G = .940 pFS×T = .254 pFS×G×T = .792 |
p
Group = .012 pTime = .962 pG×T = .051 pFS = .180 pFS×G = .940 pFS×T = .254 pFS×G×T = .792 |
||||||
| Food secure | ||||||||
| Intervention | 1.9 (1.5 to 2.3) |
2.6 (2.0 to 3.1) |
0.6* (0.2 to 1.1) |
|||||
| Control | 1.8 (1.4 to 2.2) |
1.6 (1.2 to 1.9) |
−0.2 (−0.7 to 0.3) |
|||||
| Food insecure | ||||||||
| Intervention | 2.5 (1.9 to 3.2) |
2.6 (1.8 to 3.5) |
0.1 (−0.8 to 1.0) |
|||||
| Control | 2.2 (1.5 to 2.9) |
1.7 (1.0 to 2.4) |
−0.5 (−1.5 to 0.5) |
|||||
| Times drinking a can, bottle, or glass of soda or pop (Item 7) |
p
Group = .473 pTime = .533 pG×T = .098 pFS = .636 pFS×G = .116 pFS×T = .773 pFS×G×T = .004 |
p
Group = .910 pTime = .941 pG×T = .730 pFS = .960 pFS×G = .179 pFS×T = .787 pFS×G×T = .053 |
||||||
| Food secure | ||||||||
| Intervention | 1.5 (1.1 to 1.8) |
1.1 (0.8 to 1.5) |
−0.3 (−0.7 to 0.1) |
1.4 (1.1 to 1.8) |
1.0 (0.6 to 1.4) |
−0.4 (−0.9 to 0.1) |
||
| Control | 1.5 (1.1 to 1.9) |
1.5 (1.0 to 2.1) |
0.1 (−0.4 to 0.5) |
1.5 (0.9 to 2.1) |
2.0 (1.2 to 2.7) |
0.5 (−0.2 to 1.1) |
||
| Food insecure | ||||||||
| Intervention | 1.5 (1.0 to 2.0) |
2.1 (1.3 to 2.9) |
0.6 (−0.2 to 1.4) |
1.6 (0.9 to 2.2) |
1.8 (0.7 to 2.8) |
0.2 (−0.8 to 1.3) |
||
| ` Control | 1.6 (1.0 to 2.2) |
0.9 (0.3 to 1.5) |
−0.7 (−1.1 to −0.3)* |
1.4 (0.5 to 2.3) |
1.1 (0.0 to 2.1) |
−0.4 (−0.9 to 0.2) |
||
| Glasses of milk drank (Item 8) |
p
Group = .826 pTime = .823 pG×T = .164 pFS = .386 pFS×G = .920 pFS×T = .624 pFS×G×T = .177 |
p
Group = .630 pTime = .852 pG×T = .104 pFS = .873 pFS×G = .618 pFS×T = .966 pFS×G×T = .158 |
||||||
| Food secure | ||||||||
| Intervention | 1.2 (0.8 to 1.5) |
1.1 (0.6 to 1.5) |
−0.1 (−0.6 to 0.4) |
1.3 (0.9 to 1.7) |
1.3 (0.8 to 1.8) |
0.0 (−0.6 to 0.6) |
||
| Control | 1.2 (0.7 to 1.7) |
1.1 (0.7 to 1.5) |
−0.1 (−0.5 to 0.3) |
1.3 (0.6 to 2.0) |
1.3 (0.7 to 1.8) |
−0.1 (−0.6 to 0.5) |
||
| Food insecure | ||||||||
| Intervention | 1.1 (0.6 to 1.6) |
1.5 (0.7 to 2.3) |
0.4 (−0.3 to 1.1) |
1.1 (0.6 to 1.6) |
1.7 (0.5 to 2.8) |
0.6 (−0.5 to 1.7) |
||
| Control | 1.6 (0.9 to 2.2) |
1.2 (0.6 to 1.8) |
−0.4 (−1.0 to 0.3) |
1.4 (0.5 to 2.4) |
0.8 (0.2 to 1.3) |
−0.7 (−1.6 to 0.2) |
||
| Days eating breakfast (Item 9) |
p
Group = .252 pTime = .307 pG×T = .857 pFS = .128 pFS×G = .597 pFS×T = .018 pFS×G×T = .013 |
p
Group = .959 pTime = .799 pG×T = .777 pFS = .146 pFS×G = .827 pFS×T = .067 pFS×G×T = .012 |
||||||
| Food secure | ||||||||
| Intervention | 3.5 (2.9 to 4.2) |
4.3 (3.6 to 5.0) |
0.8 (0.0 to 1.5)* |
3.7 (2.9 to 4.4) |
4.6 (3.8 to 5.3) |
`0.9 (0.0 to 1.8)* |
||
| Control | 4.7 (3.9 to 5.4) |
4.4 (3.6 to 5.2) |
−0.2 (−0.7 to 0.2) |
4.3 (3.3 to 5.3) |
4.1 (2.9 to 5.3) |
−0.2 (−0.9 to 0.4) |
||
| Food insecure | ||||||||
| Intervention | 4.1 (3.3 to 4.9) |
3.0 (2.0 to 4.1) |
−1.1 (−2.0 to −0.2)* |
3.9 (2.9 to 5.0) |
3.1 (1.6 to 4.5) |
−0.9 (−1.7 to −0.1)* |
||
| Control | 3.9 (3.1 to 4.7) |
3.7 (2.8 to 4.5) |
−0.2 (−1.1 to 0.6) |
3.4 (2.3 to 4.4) |
3.4 (2.4 to 4.4) |
0.0 (−0.8 to 0.8) |
||
CI confidence interval.
aThe possible range of response for self-efficacy for health living was 5 to 50 for the healthy eating subscale score, while for the individual items the possible range of response for was 1 to 10. For healthy eating behaviors, the range of response was 0 to 24 for the times eating vegetables subscale score, while for the individual items the possible range of response was 0 to 6 regarding the daily frequency of intake, except Item 9 regarding number of days per week eating breakfast which ranges from 0 to 7.
* p < .05.
For the subsample of dyad members living together, food security moderated the group by time interaction for self-efficacy for eating 3 meals daily (pFS×G×T = .024) and the healthy eating behavior of days eating breakfast (pFS×G×T = .012). For those reporting food security at baseline, participants in the intervention group had a slight mean decline in their self-efficacy in eating 3 meals daily yet wait-list control participants tended to increase over the 3-month follow-up. For those reporting food insecurity at baseline, participants randomized to the intervention group had a significant mean increase in self-efficacy for eating 3 meals a day (mean change= 2.3, 95% CI = [0.3, 4.2], p < .05), whereas those in the wait-list control group showed a modest improvement. The moderation effect of food security on the group by time interaction for days eating breakfast was similar in the subsample of dyad members living together as seen in the full sample. Specifically, among those reporting food security at baseline, participants in the intervention group increased their mean days eating breakfast over the 3-month period (mean change = 0.9, 95% CI = [0.0, 1.8], p < .05); however, among those reporting food insecurity at baseline, the intervention group showed a significant decrease (mean change = −0.9, 95% CI = [−1.7, −0.1], p < .05). The wait-list control participants with food insecurity showed no change, while wait-list control participants with food security had a slight decrease.
In addition, significant time effects were seen in both the full sample and subsample for self-efficacy for healthy eating, and the following self-efficacy items: “eating 3 meals a day,” “limit snacking in between meals,” and “drink water most of the time”; and in the full sample only for “avoid junk food and fast food” and “avoid drinking sugar-sweetened beverages such as soda, juice and energy drinks.” No significant time effects were noted for healthy eating behaviors.
DISCUSSION
Multilevel social determinants influence the ability to access and consume nutritious food for weight management [70] and a healthy pregnancy [71, 72]. Food insecurity has been shown to increase risk for GDM [39]. This study found that one third of the overall AI/AN sample reported food insecurity. Food insecurity status was associated with higher levels of self-efficacy for healthy eating, and with some more frequent individual healthy eating behaviors, such as, eating fruits and vegetables. In this study, we hypothesized that food insecurity may moderate the effect of a GDM risk reduction intervention (i.e., Stopping GDM) on self-efficacy for healthy eating and individual healthy eating behaviors. One finding of note, was that food security had a moderating effect on frequency of eating breakfast. Those who reported food security in the treatment group ate breakfast more frequently than those with food insecurity. Breakfast eating is associated with healthy body weight among adolescents [73]. Though findings from this secondary analysis are mixed as to whether or not they supported the original moderating effect hypotheses, these unanticipated findings are contextualized by existing literature and our theoretical framework. Food insecurity is known to alter adolescent’s eating behavior, though the bulk of extant literature focuses on binge eating disorder among adolescents who live in food insecure environments [74–76]. Our findings suggest that food security status had some moderating effect over time in individual healthy eating behaviors for both the intervention and wait-list control groups. The Hawthorne effect may contribute to the improvement for the wait-list control group [77]; however, research also suggests healthy eating behaviors are linked to healthy eating knowledge among adolescents [78, 79], and the AYA participants in the wait-list control arm of this study may have learned about nutrition after taking the “pre” comprehensive survey assessments alone. As supported by the Stopping GDM’s theoretical framework, the Expanded Health Belief Model, significant time effects were seen in both the full sample and subsample for self-efficacy for healthy eating—and self-efficacy is a key construct to predict health-related behavior change [48, 49].
These findings also indicate that both individual healthy eating behaviors and self-efficacy for healthy eating improved more among the AYA who experienced food insecurity at baseline, which is contrary to our hypothesis that AYA who lived in food secure environments would have greater improvements in both. One possible reason for this unexpected finding is that our study design required the adult female in the dyad to complete the USDA food security module, while the AYA in the dyad responded to healthy eating behavior and self-efficacy measures. In other words, asking the AYA participant herself about her experience of food security may have yielded different results. Literature on adolescent food insecurity suggests there are discrepancies in adults’ versus adolescents’ assessment of food insecurity and adolescents should be included in the conversation [41, 80]. One published literature review recommended that adolescents should be directly involved in food security research since they are often willing and reliable participants who can speak accurately about their own experiences [41]. Additionally, adults in a household are known to take on the brunt of the implications of living in a food insecure environment, and siphon resources (e.g., food) to their children [81, 82]. Therefore, even if the adult in the dyad indicated her household experienced food insecurity, it may be that the effects of this food insecure environment were shielded by the adults, and had less effect on the AYA and children in the household. As research on food insecurity grows, there may be additional opportunities to engage AYA themselves on this topic as to recognize the perspective of their experience with food insecurity.
Specific to AI/AN communities, there are mixed reports on the relationship between food insecurity and healthy eating behaviors. One recent literature review aimed to synthesize the research on food insecurity among AI/AN communities, and concluded that standardized measures for food insecurity and healthy eating behaviors may not be culturally relevant nor might they capture the nuances in food security among AI/AN households [83]. For example, households who rely on hunting, fishing, and gathering may not respond to questions specific to “having enough money to buy food” as phrased in the USDA food security module, and may be more impacted by subsistence lifestyles, sharing of food within families, and seasonality of traditional food acquisition habits [37]. Further and of particular importance, in the present study, our measures did not include systematic collection of food aid resources utilized by each household, such as the USDA Supplemental Nutrition Assistance Program (e.g., SNAP) [84] or the Food Distribution Program on Indian Reservations (e.g., FDPIR or “commodity foods”) [85].
Another research team aimed to evaluate whether AI/AN households who experienced food insecurity differed in nutritional quality or dietary diversity according to 24-hr dietary recalls and were unable to identify any significant differences. The authors of this paper worked closely with a community advisory board to understand these findings and were informed that even for households who were determined “food secure”—accessing healthy food was challenging due to a myriad of factors, not limited to transportation and distance to travel to healthy food retailers [86]. Future iterations of Stopping GDM can address food security and other multilevel (e.g., community) barriers to healthful GDM risk reduction behaviors by including resources within the Stopping GDM education materials that are tailored to any given community (e.g., the location in any given community regarding FDPIR or SNAP registration) and include information on strengths-based resources such as traditional food acquisition resources, and tribally or Native-run food systems resources [31, 87, 88].
Of note, the COVID-19 pandemic exacerbated most social determinants of health for already under-resourced communities. This includes increases in food insecurity for AI/AN communities across the USA [89]. Though data for this analysis were collected prior to the COVID-19 pandemic, it would be remiss not to remind readers that more research is needed regarding the implications of the COVID-19 emergency food aid packages such as free school meals for all public school children, expanded summer meals programs for children, and the USDA’s The Emergency Food Assistance Program (TEFAP). As these emergency response programs to the COVID-19 pandemic expire, it will be crucial to document effects on household food security and downstream implications of diet quality. Further, given the vast implications of colonization, racist policies, and systematic oppression that AI/AN peoples experience, it is noteworthy that individual-level education (e.g., GDM risk reduction education) alone will not suffice to decrease GDM health disparities. Traditional AI/AN foodways have been devastated by colonization and racist policies [31, 90, 91]. In AI/AN communities, food insecurity is intimately tied to decimation of traditional and cultural practices due to attempted genocide—in addition to disparate rates of poverty, transportation issues, and lack of retail stores selling fresh food [12, 13, 15, 16, 81, 92]. In these communities food insecurity is exacerbated by water insecurity [29, 30], stolen Native land, forced relocation, and environmental pollution, which have devastated their traditional healthy food practices [10, 31].
A key strength of the parent study is the female-based, dyadic nature of engagement in Stopping GDM. In this secondary analysis, we recognize that women are well known to be the gatekeepers of nutrition and food [93, 94] and healthcare “managers” for the household [95]. As supported by our theoretical framework (Fig. 1), interpersonal health is a key level of influence that can support and improve individual health in any given household. The benefits of adult women supporting and influencing younger women in the home, especially around reproductive and women’s health, have positive implications for reducing intergenerational trauma related to food insecurity [96, 97] and mitigating consequences of food insecurity on risk for GDM and subsequent T2D. Another strength of this study is the focus on multilevel, upstream causes for GDM health disparities among AI/AN adolescents. Multilevel, multisector diabetes prevention programs among AI/AN communities are a promising approach to addressing diabetes health disparities—provided these programs are wanted by and developed in partnership with the priority audience [98, 99]. By focusing on AYA and their adult female caregivers (e.g., mother), and prioritizing education, empowerment, and intervention prior to conception, Stopping GDM is unique in acknowledging both the context in which healthful eating occurs and that there is critical window during which health behaviors can be shaped that will have effects beyond the AYA herself, into her own progeny and future generations. Traditionally, Native peoples value responsibility for seven future generations, and Native women value that practicing self-care and healthful lifestyles will pass health to their daughters and future female generations [100–102]. Given the outsized burden of diabetes in many AI/AN communities, prioritizing primary prevention for AYA is critical in breaking the intergenerational cycle of diabetes in AI/AN communities [2].
Fig 1.
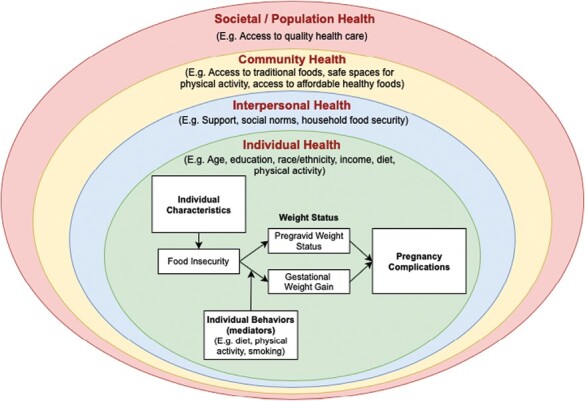
Conceptual framework embedding Laraia’s conceptual framework of the influence of food security status on gestational weight gain and pregnancy complications [39] within multilevel influences on health behavior.
Limitations
Limitations to this study were severalfold and will inform our subsequent studies around food insecurity and GDM risk reduction for AI/AN AYA females. First, because of an error in the language of question 6 on the USDA food insecurity module on the participant-facing survey portal, we conservatively decided to omit that question but were still able to generate a valid dichotomy of “food secure” or “food insecure” for analyses involving food security. This adaptation caused us to lose information as to “level” or severity of food insecurity and how that may have impacted the findings. Second, we had no systematic way to know if the daughters and mothers in the full sample lived together, as in some cases, the dyad may have participated together but the daughter lived with her father or grandparents for part of the week. This has implications for the validity of the food security measure, which is based on USDA household-level food insecurity. Therefore, we conducted all analyses with both the full sample and the subsample, the latter of which includes dyads that we were confident lived together because of the way both members of the dyad responded to baseline survey question “With whom do you live?” Third, none of the measures were specifically validated for AI/AN audiences, which is a persistent and challenging issue in research focused on AI/AN populations. For example, questions in the dietary screener related to milk may not be relevant in measuring nutrition as many AI/ANs are lactose intolerant [103]. There is also debate as to whether the USDA food insecurity module measures culturally diverse communities’ food insecurity accurately, as there may be concerns of stigma, use of nontraditional food acquisition (e.g., hunting, gathering, fishing), and “household” can be difficult to assess for large extended families who experience flux in their living arrangements [83]. A limitation of the YRBSS Healthy Eating Behaviors measure indicator is that it is a frequency tool and known to overestimate actual intakes [104]; however this indicator is able to rank behaviors/intakes by providing the number of times certain foods are consumed/day versus servings or grams/day [105]. Fourth, because of challenges with recruitment in the parent study, recruitment continued until the last day of RCT data collection, which means some dyads “timed out” of the study. Because of this, and the large decrease in participants completing the 6-month follow-up, we opted to only examine baseline to 3-month data in this study. Longer measures of the impact of living in food insecure environments are warranted.
Public health implications
Despite these limitations, we believe the study offers unique insight on the “upstream causes” of GDM health disparities among AI/AN communities. Given the intergenerational implications of GDM, it is prudent that public health and healthcare organizations work with AI/AN communities to support healthful eating environments and practices among AI/AN AYAs. This effort includes cross-sector collaborations—which can differ in urban and rural (including reservation) AI/AN communities. Rural and urban Indian communities need policy and increased awareness in the general community that support healthy eating environments, recognize tribal food sovereignty, and enforce rights to reclaim traditional food systems and tribally owned food retail outlets. Both rural and urban-dwelling AI/ANs, as with other underserved communities who are impacted by systemic racism and impacts of past and modern-day colonization [88, 106–108], need improved access to healthful food, safe places to engage in physical activity, affordable, safe housing and improved economic opportunities to sustain these healthful practices.
Acknowledgments
The Stopping GDM Study Group includes: A. Akers, A. Brega, S. Beirne, L. Chalmers, D. Charron-Prochownik, A. Fischl, H. Garrow, K. Gonzales, J. Howe, G. Marshall, K. McNealy, K. Moore, K.J. Nadeau, N. O’Banion, J. Powell, E. Seely, S. Sereika, H. Stein, S. Stotz, M. Terry, S. Thorkelson, and X. Uribe-Rios.
Contributor Information
Sarah A Stotz, University of Colorado Anschutz Medical Campus, Colorado School of Public Health, Centers for American Indian and Alaska Native Health, Aurora, CO, USA.
Luciana E Hebert, Institute for Research and Education Advancing Community Health (IREACH) at the Elson S. Floyd College of Medicine at Washington State University, Seattle, WA, USA.
Denise Charron-Prochownik, Department of Health Promotion and Development, University of Pittsburgh School of Nursing, Pittsburgh, PA, USA.
Lisa Scarton, University of Florida, School of Nursing, Department of Family, Community and Health Systems Science, Gainsville, FL, USA.
Kelly R Moore, University of Colorado Anschutz Medical Campus, Colorado School of Public Health, Centers for American Indian and Alaska Native Health, Aurora, CO, USA.
Susan M Sereika, Department of Health Promotion and Development, University of Pittsburgh School of Nursing, Pittsburgh, PA, USA.
The Stopping GDM Study Group:
A Akers, A Brega, S Beirne, L Chalmers, D Charron-Prochownik, A Fischl, H Garrow, K Gonzales, J Howe, G Marshall, K McNealy, K Moore, K J Nadeau, N O’Banion, J Powell, E Seely, S Sereika, H Stein, S Stotz, M Terry, S Thorkelson, and X Uribe-Rios
Funding
This work was supported by the National Institute on Minority Health and Health Disparities (grant number R21MD016126; PI Stotz) and National Institute of Nursing Research (grant number 1R01NR014831-01A1; MPIs Charron-Prochownik and Moore). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Compliance with Ethical Standards
Conflict of Interest: None declared.
Ethical Approval: This study was conducted according to the guidelines outlined in the Declaration of Helsinki and all procedures involving research study participants were approved by the University of Colorado Multiple Institutional Review Board, University of Pittsburgh Institutional Review Board, Navajo Nation Institutional Review Board, Oklahoma City Area Indian Health Service Institutional Review Board, and the National Indian Health Service Institutional Review Board.
Informed Consent: Written informed consent and assent was obtained from all participants.
Welfare of Animals: This article does not contain any studies with animals performed by any of the authors.
Transparency Statements
1.Study registration: This study was not formally registered as it was secondary analysis only.
2.Analytic plan preregistration: The analysis plan was not formally preregistered.
3.Analytic code availability: Analytic code used to conduct the analyses presented in this study is not available in a public archive. They may be available by emailing the corresponding author.
4.Materials availability: The parent intervention, Stopping GDM, is publicly available and free of charge. It can be found at www.stoppinggdm.com.
Data Availability
Data for this study are not available to the public as data are owned by sovereign American Indian tribes and it is their discretion as to how data is shared.
REFERENCES
- 1. Centers for Disease Control and Prevention. Gestational diabetes [Internet]. 2019. [cited June 8, 2020]. Available at https://www.cdc.gov/diabetes/basics/gestational.html. Accessed on April 28, 2023.
- 2. Pettitt D, Jovanovic L.. The vicious cycle of diabetes and pregnancy. Curr Diab Rep. 2007;7(4):295–297. [DOI] [PubMed] [Google Scholar]
- 3. Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30(suppl 2):S141–S146. [DOI] [PubMed] [Google Scholar]
- 4. Fujimoto W, Wotring A.. Gestational diabetes in high risk populations. Clin Diabetes. 2013;31(2):90–94. [Google Scholar]
- 5. Garrett BE, Dube SR, Winder C, Caraballo RS.. CDC health disparities and inequalities report—United States. MMWR Morb Mortal Wkly Rep. 2013;62(3):81–84. Available at http://www.ncbi.nlm.nih.gov/pubmed/2426448723388551 [Google Scholar]
- 6. Centers for Disease Control and Prevention. National Diabetes Statistics Report. Atlanta, GA: Centers for Disease Control and Prevention; 2017. [Google Scholar]
- 7. American Diabetes Association. American Diabetes Association, Proceeding of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):251–260. [DOI] [PubMed] [Google Scholar]
- 8. Ventura SJ, Mathews TJ, Hamilton BE.. Births to teenagers in the United States, 1940–2000. Natl Vital Stat Rep. 2001;49(10):1–23. Available at http://www.ncbi.nlm.nih.gov/pubmed/11593890 [PubMed] [Google Scholar]
- 9. The State of Obesity. Better policies for a healthier America [Internet]. 2017. Available at http://healthyamericans.org/assets/files/TFAH-2017-ObesityReport-FINAL.pdf. Accessed on April 28, 2023.
- 10. USDA Economic Research Service. Definitions of food security [Internet].2018. [cited August 23, 2022]. Available at https://www.ers.usda.gov/topics/food-nutrition-assistance/food-security-in-the-us/definitions-of-food-security/. Accessed on April 28, 2023.
- 11. Jernigan VBB, Huyser KR, Valdes J, Simonds VW.. Food insecurity among American Indians and Alaska Natives: a national profile using the Current Population Survey–Food Security Supplement. J Hunger Environ Nutr. 2017;12(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bauer KW, Widome R, Himes JH, et al. High food insecurity and its correlates among families living on a rural American Indian reservation. Am J Public Health. 2012;102(7):1346–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pardilla M, Prasad D, Suratkar S, Gittelsohn J.. High levels of household food insecurity on the Navajo Nation. Public Health Nutr. 2014;17(1):58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gundersen C. Measuring the extent, depth, and severity of food insecurity: an application to American Indians in the USA. J Popul Econ. 2008;21(1):191–215. [Google Scholar]
- 15. Jernigan VBB, Garroutte E, Krantz EM, Buchwald D.. Food insecurity and obesity among American Indians and Alaska Natives and Whites in California. J Hunger Environ Nutr. 2013;8(4):458–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O’Connell M, Buchwald DS, Duncan GE.. Food access and cost in American Indian communities in Washington State. J Am Diet Assoc. 2011;111(9):1375–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaufman P, Dicken C, Williams R.. Measuring access to healthful, affordable food in American Indian and Alaska Native Tribal areas. 2014;(131):1– 29. Available at https://permanent.access.gpo.gov/gpo55515/eib131.pdf
- 18. Gundersen C, Dewey A, Crumbaugh A, Kato M, Engelhard E.. Map the Meal Gap 2016: Food Insecurity and Child Food Insecurity Estimates at the County Level. Chicago, IL, USA: Feeding America; 2016. [Google Scholar]
- 19. Gundersen C, Ziliak JP.. Food insecurity research in the United States: where we have been and where we need to go. Appl Econ Perspect Policy. 2018;40(1):119–135. [Google Scholar]
- 20. Coleman-Jensen A, Rabbitt MP, Gregory C, Singh A.. Household Food Security in the United States in 2018. Washington, DC, USA: US Dep Agric Econ Res Serv; 2019:1–56. [Google Scholar]
- 21. Blumberg J, Bialostosky K, Hamilton WL, Briefel RR.. U.S. Household Food Security Survey Module: six-item short form. Am J Public Health. 1999;89:1231–1234. Available at https://www.ers.usda.gov/media/8282/short2012.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bawadi HA, Ammari F, Abu-Jamous D, Khader YS, Bataineh S, Tayyem RF.. Food insecurity is related to glycemic control deterioration in patients with type 2 diabetes. Clin Nutr. 2012;31(2):250–254. [DOI] [PubMed] [Google Scholar]
- 23. Dyson P. Food insecurity: what does it mean to people with diabetes? Pract Diabetes. 2012;29(3):89–91. [Google Scholar]
- 24. Lyles CR, Wolf MS, Schillinger D, et al. Food insecurity in relation to changes in hemoglobin A1c, self-efficacy, and fruit/vegetable intake during a diabetes educational intervention. Diabetes Care. 2013;36(6):1448–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Edwards K, Patchell B.. A cultural view of Native Americans and diabetes prevention. J Cult Divers. 2009;16(1):32–35. Available at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2905172/pdf/nihms120326.pdf [PMC free article] [PubMed] [Google Scholar]
- 26. Teufel-Shone NI, Jiang L, Beals J, et al. Changes in food choices of participants in the Special Diabetes Program for Indians–Diabetes Prevention Demonstration Project, 2006–2010. Prev Chronic Dis. 2015;12(4):150266. Available at http://www.cdc.gov/pcd/issues/2015/15_0266.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Daley C, Hale J, Berryhill K, et al. Diabetes self-management behaviors among American Indians in the Midwestern United States. J Diabetes Endocrinol. 2017;3(1):34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manson SM. National Institute for Minority Health and Health Disparities Research Framework Adaptation—American Indian [Internet] [cited September 16, 2021]. Available at https://www.nimhd.nih.gov/about/overview/research-framework/adaptation-framework.html. Accessed on April 28, 2023.
- 29. Mitchell FM. Water (in)security and American Indian health: social and environmental justice implications for policy, practice, and research. Public Health. 2019;176:98–105. [DOI] [PubMed] [Google Scholar]
- 30. Deitz S, Meehan K.. Plumbing poverty: mapping hot spots of racial and geographic inequality in U.S. household water insecurity. Ann Am Assoc Geogr. 2019;109(4):1092–1109. [Google Scholar]
- 31. Gurney RM, Caniglia BS, Mix TL, Baum KA.. Native American food security and traditional foods: a review of the literature. Ann Am Assoc Geogr. 2015;8:681–693. [Google Scholar]
- 32. Seligman HK, Laraia B, Kushel MB.. Food insecurity is associated with chronic disease among low-income. J Nutr. 2009;140:304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seligman HK, Jacobs EA, Lopez A, Tschann J, Fernandez A.. Food insecurity and glycemic control among low-income patients with type 2 diabetes. Diabetes Care. 2012;35(2):233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mayer VL, McDonough K, Seligman H, Mitra N, Long JA.. Food insecurity, coping strategies and glucose control in low-income patients with diabetes. Public Health Nutr. 2016;19(6):1103–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ippolito MM, Lyles CR, Prendergast K, Marshall MB, Waxman E, Seligman HK.. Food insecurity and diabetes self-management among food pantry clients. Public Health Nutr. 2017;20(1):183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tomayko EJ, Kim K, Prince R J, et al. Predictors of overweight and obesity in American Indian families with young children. J Nutr Educ Behav. 2018;51(2):190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tomayko EJ, Mosso KL, Cronin KA, et al. Household food insecurity and dietary patterns in rural and urban American Indian families with young children. BMC Public Health. 2017;17(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ivers LC, Cullen KA.. Food insecurity: special considerations for women. Am J Clin Nutr. 2011;94(6):1740S–1744S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Laraia B, Siega-Riz AM, Gundersen C.. Household food insecurity is associated with self-reported pregravid weight status, gestational weight gain, and pregnancy complications. J Am Diet Assoc. 2010;110(5):692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lohman BJ, Neppl TK, Lee Y, Diggs ON, Russell D.. The association between household food insecurity and body mass index: a prospective growth curve analysis. J Pediatr. 2018;202:115–120.e1. [DOI] [PubMed] [Google Scholar]
- 41. Dush JL. Adolescent food insecurity: a review of contextual and behavioral factors. Public Health Nurs. 2020;37(3):327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. US Department of Health and Human Services and Centers of Disease Control and Prevention. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention; 2006. [Google Scholar]
- 43. Grilo SA, Earnshaw VA, Lewis JB, et al. Food matters: food insecurity among pregnant adolescents and infant birth outcomes. J Appl Res Child. 2015;6(2). [PMC free article] [PubMed] [Google Scholar]
- 44. Johnson CM, Sharkey JR, Lackey MJ, et al. Relationship of food insecurity to women’s dietary outcomes: a systematic review. Nutr Rev. 2018;76(12):910–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. LaVallie DL, Gabbe SG, Grossman DC, Larson EB, Baldwin L, Andrilla CHA.. Birth outcomes among American Indian/Alaska Native women with diabetes in pregnancy. J Reprod Med. 2003;48(8):610–616. Available at http://ezproxy.library.dal.ca/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=c8h&AN=2004061781&site=ehost-live [PubMed] [Google Scholar]
- 46. Moore K, Charron-Prochownik D, Stotz SA.. Daughter and Mothers Stopping GDM: Balancing Mind, Body and Spirit—Video. Pittsburgh: University of Pittsburgh; 2017. Copyrighted. Developed in collaboration with the University of Colorado Denver, University Invention Disclosure Number 04149. [Google Scholar]
- 47. Charron-Prochownik D, Moore K, Stotz SA.. Daughter and Mothers Stopping GDM: Balancing Mind, Body and Spirit. Pittsburgh, PA: University of Pittsburgh; 2017. Copyrighted. Developed in collaboration with the University of Colorado Denver, University Invention Disclosure Number 04149. [Google Scholar]
- 48. Charron-Prochownik D, Sereika S, Becker D, et al. Reproductive health beliefs and behaviors in teens with diabetes: application of the Expanded Health Belief Model. Pediatr Diabetes. 2001;2:30–39. [DOI] [PubMed] [Google Scholar]
- 49. Burns A. The expanded health belief model as a basis for enlightened preventive health care practice and research. J Health Care Mark. 1992;12(3):32–45. [PubMed] [Google Scholar]
- 50. Bornstein MH, Putnick DL.. Dyadic development in the family: stability in mother-child relationship quality from infancy to adolescence. J Fam Psychol. 2021;35(4):445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. La Sorsa VA, Fodor IG.. Adolescent daughter/midlife mother dyad: a new look at separation and self-definition. Psychol Women Q. 1990;14(4):593–606. [Google Scholar]
- 52. Smith JE, Erickson SJ, Austin JL, Winn JL, Lash DN, Amrhein PC.. Mother–daughter relationship quality and body image in preadolescent girls. J Child Fam Stud. 2016;25(9):2683–2694. [Google Scholar]
- 53. Office of Disease Prevention and Health Promotion. Social determinants of health: know what affects health [Internet].Healthy People 2020; 2019. [cited September 16, 2021]. Available at https://www.cdc.gov/socialdeterminants/index.htm. Accessed on April 28, 2023. [Google Scholar]
- 54. Burton M, Reid M, Worsley A, Mavondo F.. Food skills confidence and household gatekeepers’ dietary practices. Appetite. 2017;108:183–190. [DOI] [PubMed] [Google Scholar]
- 55. Wansink B. Nutritional gatekeepers and the 72% solution. J Am Diet Assoc. 2006;106(9):1324–1327. [DOI] [PubMed] [Google Scholar]
- 56. Kuhns A, Saksena M.. Food Purchase Decisions of Millennial Households Compared to Other Generations. Washington, DC, USA: US Dep Agric Econ Res Serv; 2017:1–51. [Google Scholar]
- 57. Raskind IG, Woodruff RC, Ballard D, et al. Decision-making processes shaping the home food environments of young adult women with and without children. Appetite. 2017;113:124–133. [DOI] [PubMed] [Google Scholar]
- 58. Shepherd E, Gomersall JC, Tieu J, Han S, Crowther CA, Middleton P.. Combined diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2017;2017(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Koivusalo SB, Rönö K, Klemetti MM, et al. Gestational diabetes mellitus can be prevented by lifestyle intervention: the Finnish Gestational Diabetes Prevention Study (RADIEL): a randomized controlled trial. Diabetes Care. 2016;39(1):24–30. [DOI] [PubMed] [Google Scholar]
- 60. Ratner RE, Christophi CA, Metzger BE, et al. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93(12):4774–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gregson J, Foerster SB, Orr R, et al. System, environmental, and policy changes: using the social-ecological model as a framework for evaluating nutrition education and social marketing programs with low-income audiences. J Nutr Educ. 2001;33(suppl 1):S4–S15. [DOI] [PubMed] [Google Scholar]
- 62. McLeroy K, Bibeau D, Steckler A, Glanz K.. An ecological perspective on health promotion programs. Health Educ Q. 1988;15:351–377. [DOI] [PubMed] [Google Scholar]
- 63. Charron-Prochownik D, Sereika S, Stotz S, et al. Awareness, knowledge, health beliefs of American Indian/Alaska Native (AIAN) girls and their mothers regarding risk reduction of gestational diabetes mellitus. Diabetes Care. 2019;68(S1):882-P. [Google Scholar]
- 64. March of Dimes. Gestational diabetes [Internet].2019. [cited November 28, 2019]. Available at https://www.marchofdimes.org/complications/gestational-diabetes.aspx. Accessed on April 28, 2023.
- 65. Charron-Prochownik D, Wang S, Sereika S, Kim Y, Janz N.. A theory-based reproductive health and diabetes instrument. Am J Health Behav. 2006;30(2):208–220. [DOI] [PubMed] [Google Scholar]
- 66. Brener ND, Kann L, Shanklin S, et al. Methodology of the youth risk behavior surveillance system—2013. MMWR Recomm Rep. 2013;62(1):1–23. [PubMed] [Google Scholar]
- 67. Bartfeld J, Dunifon R.. State-level predictors of food insecurity among households with children. J Policy Anal Manag. 2006;25(4):921–942. [Google Scholar]
- 68. Laraia BA, Siega-Riz AM, Gundersen C, Dole N.. Psychosocial factors and socioeconomic indicators are associated with household food insecurity among pregnant women. J Nutr. 2006;136(1):177–182. [DOI] [PubMed] [Google Scholar]
- 69. Franklin B, Jones A, Love D, Puckett S, Macklin J, White-Means S.. Exploring mediators of food insecurity and obesity: a review of recent literature. J Community Health. 2012;37(1):253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Larson NI, Story MT.. Food insecurity and weight status among U.S. children and families: a review of the literature. Am J Prev Med. 2011;40(2):166–173. [DOI] [PubMed] [Google Scholar]
- 71. Yee LM, Lezia K, Jackson J, Niznik C, Simon MA.. Health care providers’ perspectives on barriers and facilitators to care for low-income pregnant women with diabetes. Diabetes Spectr. 2000;33(2):190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yee LM, McGuire JM, Taylor SM, Niznik CM, Simon MA.. Social and environmental barriers to nutrition therapy for diabetes management among underserved pregnant women: a qualitative analysis. J Nutr Educ Behav. 2016;48(3):170–180.e1. [DOI] [PubMed] [Google Scholar]
- 73. Timlin MT, Pereira MA, Story M, Neumark-Sztainer D.. Breakfast eating and weight change in a 5-year prospective analysis of adolescents: Project EAT (Eating Among Teens). Pediatrics. 2008;121(3):e638–e645. [DOI] [PubMed] [Google Scholar]
- 74. Hazzard VM, Hooper L, Larson N, Loth KA, Wall MM, Neumark-Sztainer D.. Associations between severe food insecurity and disordered eating behaviors from adolescence to young adulthood: findings from a 10-year longitudinal study. Prev Med (Baltim). 2022;154:106895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hooper L, Telke S, Larson N, Mason SM, Neumark-Sztainer D.. Household food insecurity: associations with disordered eating behaviours and overweight in a population-based sample of adolescents. Public Health Nutr. 2020;23(17):3126–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hazzard VM, Loth KA, Hooper L, Becker CB.. Food insecurity and eating disorders: a review of emerging evidence. Curr Psychiatry Rep. 2020;22(12):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. McCambridge J, Witton J, Elbourne DR.. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67(3):267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Thakur S, Mathur P.. Nutrition knowledge and its relation with dietary behaviour in children and adolescents: a systematic review. Int J Adolesc Med Health. 2021;34(6):381–392. doi: 10.1515/ijamh-2020-0192 [DOI] [PubMed] [Google Scholar]
- 79. Brown R, Seabrook J, Stranges S, et al. Examining the correlates of adolescent food and nutrition knowledge. Nutrients. 2001;13(2044). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Duke NN. Adolescent-reported food insecurity: correlates of dietary intake and school lunch behavior. Int J Environ Res Public Health. 2021;18(12):6647–6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mullany B, Neault N, Tsingine D, et al. Food insecurity and household eating patterns among vulnerable American-Indian families: associations with caregiver and food consumption characteristics. Public Health Nutr. 2013;16(4):752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ridberg RA, Marpadga S, Akers MM, Bell JF, Seligman HK.. Fruit and vegetable vouchers in pregnancy: preliminary impact on diet & food security. J Hunger Environ Nutr. 2021;16(2):149–163. [Google Scholar]
- 83. Nikolaus CJ, Johnson S, Benally T, et al. Food insecurity among American Indian and Alaska Native people: a scoping review to inform future research and policy needs. Adv Nutr. 2022;13(5):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. United States Department of Agriculture Food and Nutrition Service. Supplemental Nutrition Assistance Program [Internet].2020. [cited June 15, 2020]. Available at https://www.fns.usda.gov/snap/SNAP-Ed. Accessed on April 28, 2023.
- 85. United States Department of Agriculture. Food Distribution Program on Indian Reservations. Washington, DC, USA: Food and Nutrition Service; 2020. [Google Scholar]
- 86. Byker Shanks C, Ahmen S, Dupuis V, et al. Dietary quality varies among adults on the Flathead Nation of the Confederated Salish and Kootenai Tribes in Montana. J Community Health. 2020;45(2):388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Manson SM. Strength-based approaches to wellness in Indian country. Am Indian Alaska Nativ Ment Heal Res. 2016;23(3). [Google Scholar]
- 88. Weiler AM, Hergesheimer C, Brisbois B, Wittman H, Yassi A, Spiegel JM.. Food sovereignty, food security and health equity: a meta-narrative mapping exercise. Health Policy Plan. 2015;30(8):1078–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hake M, Dewey A, Engelhard E, et al. The impact of the coronavirus on food insecurity in 2020 [Internet]. Feeding America; 2020:1–8. Available at https://www.feedingamerica.org/sites/default/files/2020-10/Brief_LocalImpact_10.2020_0.pdf [Google Scholar]
- 90. Warne D, Wescott S.. Social determinants of American Indian nutritional health. Curr Dev Nutr. 2019;3(suppl 2):12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Smith-Morris CM. Reducing diabetes in Indian country: lessons from the three domains influencing Pima diabetes. Hum Organ. 2015;63(1):34–46. [Google Scholar]
- 92. Tarasuk V, Cheng J, de Oliveria C, Dachner N, Gunderson C, Kurdyak P.. Association between household food insecurity and annual health care costs. Can Med Assoc J. 2015:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kurz KM, Johnson-Welch C.. Enhancing women’s contributions to improving family food consumption and nutrition. Food Nutr Bull. 2001;22(4):443–453. [Google Scholar]
- 94. Reid M, Worsley A, Mavondo F.. The obesogenic household: factors influencing dietary gatekeeper satisfaction with family diet. Psychol Mark. 2015;32(5):544–557. [Google Scholar]
- 95. Matoff-Stepp S, Applebaum B, Pooler J, Kavanagh E.. Women as health care decision-makers: implications for health care coverage in the United States. J Health Care Poor Underserved. 2014;25(4):1507–1513. [DOI] [PubMed] [Google Scholar]
- 96. Sun J, Knowles M, Patel F, Frank DA, Heeren TC, Chilton M.. Childhood adversity and adult reports of food insecurity among households with children. Am J Prev Med. 2016;50(5):561–572. [DOI] [PubMed] [Google Scholar]
- 97. Chilton M, Chyatte M, Breaux J.. The negative effects of poverty & food insecurity on child development. Indian J Med Res. 2007;126(4):262–272. [PubMed] [Google Scholar]
- 98. Stotz SA, McNealy K, Begay RL, DeSanto K, Manson SM, Moore KR.. Multi-level diabetes prevention and treatment interventions for Native people in the USA and Canada: a scoping review. Curr Diab Rep. 2021;21(11). doi: 10.1007/s11892-021-01414-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jernigan VB, D’Amico EJ, Duran B, Buchwald D.. Multilevel and community-level interventions with Native Americans: challenges and opportunities. Prev Sci. 2018:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Jacob MM, Gonzales KL, Chappell Belcher D, Ruef JL, RunningHawk Johnson S.. Indigenous cultural values counter the damages of white settler colonialism. Environ Sociol. 2020:1–13. doi: 10.1080/23251042.2020.1841370 [DOI] [Google Scholar]
- 101. Walters K, Simoni J.. Reconceptualizing Native women’s health: an “indigenist ” stress-coping model. Am Public Health. 2002;92(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Walters KL, Johnson-Jennings M, Stroud S, et al. Growing from our roots: strategies for developing culturally grounded health promotion interventions in American Indian, Alaska Native, and Native Hawaiian communities. Prev Sci. 2020;21:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Newcomer AD, Thomas PJ, McGill DB, Hofmann AF.. Lactase deficiency: a common genetic trait of the American Indian. Gastroenterology. 1977;72(2):234–237. [PubMed] [Google Scholar]
- 104. Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 105. Eaton DK, Olsen E, Brener ND, et al. A comparison of fruit and vegetable intake estimates from three survey question sets to estimates from 24-hour dietary recall interviews. J Acad Nutr Diet. 2013;113(9):1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Odoms-Young A, Bruce MA.. Examining the impact of structural racism on food insecurity: implications for addressing racial/ethnic disparities. Fam Community Health. 2018;41:S3–S6. Available at https://journals.lww.com/familyandcommunityhealth/Fulltext/2018/04001/Examining_the_Impact_of_Structural_Racism_on_Food.2.aspx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Coté C. “Indigenizing” food sovereignty—revitalizing Indigenous food practices and ecological knowledges in Canada and the United States. Humanities. 2016;5(3):57. Available at http://www.mdpi.com/2076-0787/5/3/57 [Google Scholar]
- 108. Walker JD, Slater M, Jones CR, et al. Diabetes prevalence, incidence and mortality in First Nations and other people in Ontario, 1995–2014: a population-based study using linked administrative data. Can Med Assoc J. 2020;192(6):E128–E135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for this study are not available to the public as data are owned by sovereign American Indian tribes and it is their discretion as to how data is shared.


