Abstract
Culicoides-borne viruses are an important arbovirus group causing bovine diseases. During 2012–2019, 2,525 pools consisting of 108,937 specimens of vectors were subjected to PCR detection of bovine arbovirus belonging to Orthobunyavirus, Orbivirus, and Ephemerovirus. Twelve virus RNAs, of which 6, that is, Shuni virus, Shamonda virus, and Sathuperi virus in Orthobunyavirus and Sathuvachari virus and epizootic hemorrhagic disease virus serotypes 4 and 7 in Orbivirus were detected for the first time in the area. Potential vector species were evaluated by the minimum infection rate, and the population abundance of Culicoides oxystoma, Culex tritaeniorhynchus, and Anopheles sinensis indicated that they were the main potential vector species in dairy farms in Taiwan.
Keywords: Culicoides biting midge, mosquito, arbovirus, cattle
Introduction
Arthropod-borne viruses (arboviruses) are an influential group of viruses transmitted by arthropod vectors that pose rising threats to humans, livestock, and wild animals (Weaver and Reisen 2010). Culicoides biting midges (Diptera: Ceratopogonidae) play a vital role in loss of production through annoyance and transmission of arboviruses to livestock (Purse et al. 2015). In the livestock industry, Culicoides-borne viruses have a significant economic impact. Bluetongue virus (BTV, Seodoreoviridae: Orbivirus) is a well-known Culicoides-borne virus that causes billion-dollar losses in the livestock industry of Europe, America, and Australia annually (Alkhamis et al. 2020). In East Asia, bovine ephemeral fever caused by bovine ephemeral fever virus (BEFV, Rhabdoviridae: Ephemerovirus) is a severe bovine febrile disease that causes serious illness and even death. The frequency of BEF epidemics has increased in Taiwan since 2000, with a shorter interval of 1–2 yr compared to the previous interval of 5-6 yr. In Japan, BEF reemerged in 2015 after 23 yr of no BEF epidemics (Hirashima et al. 2017, Lee 2019).
In East Asia, at least 23 bovine arboviruses belong to the genera Orthobunyavirus, Orbivirus, and Ephemerovirus (Yanase et al. 2020). Culicoides biting midges are considered the principal vectors for the transmission of these viruses to host animals, and a few mosquito species might also be involved in the natural circulation of these viruses (Hubalek et al. 2014). Due to their relatively small body size, 1–3 mm, Culicoides are easily transported oversea by wind currents (Gale et al. 2015, Aguilar-Vega et al. 2019). The wind transportation of virus-infected midges onto islands can be led to the introduction and establishment of the virus onto the island (Sellers and Herniman 1981, Sedda and Rogers 2013). This phenomenon is an important mechanism for the transboundary movement of Culicoides-borne viruses onto an island such as Taiwan. BEFV and Akabane virus (AKAV, Peribunyaviridae: Orthobunyavirus) have been reported to be exchanged between Taiwan and nearby localities (Lee 2019, Tzeng et al. 2022), which indicated that the candidate virus list for Taiwan surveillance could be expanded by including surveillance results from nearby localities, such as the Yaeyama Islands and the southeastern part of China. Currently, 7 viruses have been only identified in Taiwan (Tzeng et al. 2019, Yanase et al. 2020). However, 10 viruses in the above genus were identified or isolated in Culicoides biting midges/mosquitoes/ruminant hosts in the Yaeyame Islands, and 24 viruses have been found in China (Yanase et al. 2020). Thus, the number of bovine Culicoides-borne viruses might be greater than that currently known in Taiwan.
In this study, 19 Culicoides-borne viruses that are occurrence records in nearby localities were targeted and surveyed using RT-PCR in Taiwan (Kato et al. 2016, Yanase et al. 2020; Supplementary Table S2). To detect numerous viruses from over 2,000 pools, we designed a screening method called the 2-step PCR strategy to conduct this surveillance. In the first step, universal screening of 3 target genera using universal primers in PCR was conducted. The second step was that the positive specimens in the first step were subjected to a second round of PCR using specific primers to detect the target viruses. Before detection, the specimen’s species were identified, and their population abundance was calculated; then, the above information was combined with the detection results to understand the potential vectors and occurrence of bovine arboviruses on dairy farms in Taiwan.
Materials and Methods
Specimen Collections, RNA Extraction, and Reverse Transcription
Specimens were collected from 10 farms distributed in Yunlin County, Chiayi County, Tainan City, Kaohsiung City, Pingtung County, and Hualien County in Taiwan, during 2012–2019 (Fig. 1A). Each farm was set a modified 365-nm UVA fan trap (Fig. 1B), and the collection was conducted for 2 h after sunset per month during the surveillance period. The species/genus- and blood-fed/unfed status of all alive female specimens were identified, and then all identified specimens, for example, species/blood unfed, genus/blood fed, etc. were pooled together, with the maximum specimens per pool consisting of 100 mosquitoes or 200 Culicoides biting midges. The specimens were homogenized using a TissueLyser II (Qiagen GmbH, Hilden, Germany), and the total RNA was extracted by a PureLink RNA Mini Kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Reverse transcription was performed using an iScript cDNA synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). All detailed procedures of extraction and cDNA synthesis were described in a previous study (Tzeng et al. 2019).
Fig. 1.

A) The localization of sampling farms in Taiwan. YL, Yunlin County; CY, Chiayi County; TN, Tainan City; KH, Kaohsiung City; PT, Pingtung County; HL, Hualien County. B) A modified 365-nm UVA fan trap was set in each farm. The collection bottle was connected for collecting alive hematophagous insects under the net on the trap, which could improve the survival of collected samples and avoid blowing dry by the fan at the same time.
Virus Detection and Sequencing
Nineteen Culicoides-borne viruses were detected by 2-step RT-PCR using KAPA HiFi HotStart ReadyMix (F. Hoffmann-La Roche Ltd, Basel, Switzerland) (Supplementary Table S1). All RT-PCRs were conducted by using an ABI 2720 PCR instrument (Thermo Fisher Scientific, Inc.). The first step is universal screening at the genus level, and then the second step is specific detection of target viruses with positive detections in the first step. The universal primers and specific primers are listed in Supplementary Tables S1 and S2. The PCR mix was composed of 10 μl of KAPA HiFi HotStart ReadyMix, 1 μl of cDNA, 1 μl of each primer at 10 μM, and enough nuclease-free water to reach a final volume of 20 μl. The PCR thermal conditions are listed in Supplementary Table S3. The PCR products were analyzed by electrophoresis on a 1.5% agarose gel. The amplicons were sequenced by a sequencing service (Genomic Ltd., Taipei, Taiwan). The sequencing results were transferred into VectorNTI v. 10 (Thermo Fisher Scientific, Inc.) and trimmed. The sequence identity was verified by BLAST, and each verified sequence was uploaded to GenBank at the National Center of Biotechnology Information (NCBI).
Minimum Infection Rates
Minimum infection rates (MIRs) of detected arboviruses were calculated through the following equation, with only one infected individual determined in each positive blood-unfed pool.
Climate Data Source, Statistics, and Graph Creation
Climate data, including temperature, rainfall, and wind, were obtained from an open data resource known as the CWB Observation Data Inquiry System Version 7.2 (CODiS) (https://e-service.cwb.gov.tw/HistoryDataQuery/), and data from the nearest weather stations to the sampling farms were selected. The incidence of detected viruses was calculated using the following equation, and the incidences from each sampling time and farm were used to determine the month-average or 8-yr incidence for a particular virus or all viruses. The R 4.2.2 with its packages, ggplot2, ggpmisc, and openair was used to create graphs (Carslaw and Ropkins 2012, Wickham 2016).
Results
A total of 85,093 Culicoides biting midges in 1,484 pools and 23,844 mosquitoes in 1,041 pools were collected in this study. Overall, 8 species of Culicoides biting midges and 16 species of mosquitoes in 5 genera were identified. The pools of unengorged specimens accounted for 54.2% (n = 1,371), pools of engorged specimens accounted for 31.3% (n = 791), and the pools of species-unidentified specimens accounted for 14.4% (n = 363) of the total. Eight Culicoides species were identified in 32,351 Culicoides specimens. Culicoides oxystoma was the dominant species (n = 30,313, 93.7%), followed by C. arakawae (n = 791, 2.4%), C. nipponensis (n = 566, 1.7%), C. actoni (n = 414, 1.2%), C. paregrinus (n = 186, 0.6%), C. jacobsoni (n = 37, 0.1%), C. bubalus (n = 25, 0.07%), and C. humeralis (n = 19, 0.06%). Of the 16 mosquito species identified within the genera Anopheles, Aedes, Armigeres, Culex, and Mansonia, 8 belonged to the genus Culex. Culex tritaeniorhynchus was the most abundant mosquito species (n = 18,639, 90.1%), followed by Anopheles sinensis (n = 546, 2.6%), Armigeres subalbatus (n = 441, 2.1%), Cx. quinquefasciatus (n = 410, 2.0%), and Cx. fuscocephala (n = 315, 1.5%), a further 11 mosquito species accounted for <1% of the mosquitoes identified (Table 1).
Table 1.
Biting midges and mosquito pools by type and feeding status provided for virus detection
| Species | Numbers, n | Pools, n | Blood-fed pools, n | |||
|---|---|---|---|---|---|---|
| Unfed | Fed | Unidentified | ||||
| Biting midges | Culicoides spp. | 52,742 | 398 | 34 | 75 | 329 |
| C. oxytoma | 30,313 | 779 | 491 | 288 | 0 | |
| C. arakawae | 791 | 121 | 93 | 28 | 0 | |
| C. nipponensis | 566 | 93 | 31 | 62 | 0 | |
| C. actoni | 414 | 39 | 33 | 6 | 0 | |
| C. paregrinus | 186 | 22 | 18 | 4 | 0 | |
| C. jacobsoni | 37 | 10 | 9 | 1 | 0 | |
| C. bubalus | 25 | 16 | 16 | 0 | 0 | |
| C. humeralis | 19 | 6 | 5 | 1 | 0 | |
| Mosquitoes | Anopheles spp. | 214 | 63 | 26 | 31 | 6 |
| An. sinensis | 546 | 151 | 81 | 70 | 0 | |
| An. tessellatus | 14 | 6 | 5 | 1 | 0 | |
| An. ludlowae | 12 | 2 | 1 | 1 | 0 | |
| Aedes spp. | 4 | 4 | 1 | 2 | 1 | |
| Ae. albopictus | 26 | 9 | 6 | 3 | 0 | |
| Ae. vexans | 19 | 4 | 3 | 1 | 0 | |
| Armigeres spp. | 154 | 22 | 9 | 11 | 2 | |
| Ar. subalbatus | 441 | 71 | 39 | 32 | 0 | |
| Ar. baiasi | 3 | 2 | 2 | 0 | 0 | |
| Culex spp. | 2,739 | 109 | 49 | 36 | 24 | |
| Cx. tritaeniorhynchus | 18,639 | 431 | 289 | 142 | 0 | |
| Cx. quinquefasciatus | 410 | 60 | 49 | 10 | 1 | |
| Cx. fuscocephala | 315 | 46 | 35 | 11 | 0 | |
| Cx. annulus | 131 | 33 | 20 | 13 | 0 | |
| Cx. pipiens molestus | 100 | 21 | 19 | 2 | 0 | |
| Cx. sitiens | 5 | 1 | 1 | 0 | 0 | |
| Cx. sinensis | 3 | 2 | 2 | 0 | 0 | |
| Cx. fuscanus | 3 | 2 | 2 | 0 | 0 | |
| Mansonia uniformis | 10 | 2 | 2 | 0 | 0 | |
Six viral RNAs were detected for the first time in Taiwan. These viruses were Shamonda virus (SHAV, Peribunyaviridae: Orthobunyavirus), Shuni virus (SHUV, Peribunyaviridae: Orthobunyavirus), and Sathuperi virus (SATV, Peribunyaviridae: Orthobunyavirus), which belong to the genus Orthobunyavirus, and Sathuvachari virus (SVIV, Seodoreoviridae: Orbivirus) and epizootic hemorrhagic disease virus serotypes 4 and 7 (EHDV-4 and EHDV-7, Seodoreoviridae: Orbivirus), which belong to Orbivirus (Table 2). However, the Orthobunyavirus species cannot be determined solely by specific amplification using nucleoprotein sequences on the S segment of the RNA genome, as the structural protein sequences on the M segment also need to be considered. Thus, the Orthobunyavirus species is only suggestive in this study. The detected arbovirus RNAs were deposited under accession numbers KF676987–KF676999, MF083618–MF083684, and OP976209–OP976212 in GenBank. The positive prevalence was 4.8% (121/2525 pools), where 34.4% (44/121) of pools showed more than one virus. In the RNA detection rate of 3 virus genera in 6 sampling districts, Orthobunyavirus had 40–60%, Orbivirus had 22–41%, and Ephemerovirus had 16–30% in the total (Fig. 2A). The AKAV, aino virus (AINOV), EHDV-7, and chuzan virus (CHUV, Seodoreoviridae: Orbivirus) could be detected in all sampling districts. The SHUV and SVIV were only distributed in Yunlin County and Tainan City, respectively (Fig. 2B).
Table 2.
RNA Detection of bovine arboviruses during 2012–2019
| Year | Orthobunyavirus a | Orbivirus | Ephemerovirus | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AKAV | AINOV | PEAV | SHAV | SHUV | SATV | CHUV | BTV-2 | EHDV-4 | EHDV-7 | SVIV | BEFV | |
| 2012 | ● | ● | ● | ● | ● | ● | ||||||
| 2013 | ● | ● | ● | ● | ● | |||||||
| 2014 | ● | ● | ● | ● | ● | ● | ● | |||||
| 2015 | ● | ● | ● | ● | ● | ● | ||||||
| 2016 | ● | ● | ● | |||||||||
| 2017 | ● | ● | ● | ● | ● | |||||||
| 2018 | ● | ● | ● | ● | ● | ● | ● | |||||
| 2019 | ● | ● | ● | ● | ● | ● | ||||||
AKAV, akabane virus; AINOV, aino virus; PEAV, peaton virus; SATV, sathuperi virus; SHAV, shamonda virus; SHUV, shuni virus; CHUV, chuzan virus; BTV-2, bluetongue virus serotype 2; EHDV-4/-7, epizootic hemorrhagic disease virus serotype 4/serotype 7; SVIV, Sathuvachari virus; BEFV, bovine ephemeral fever virus.
aPartial nucleoprotein sequences on S segment RNA genome were only detected.
Fig. 2.
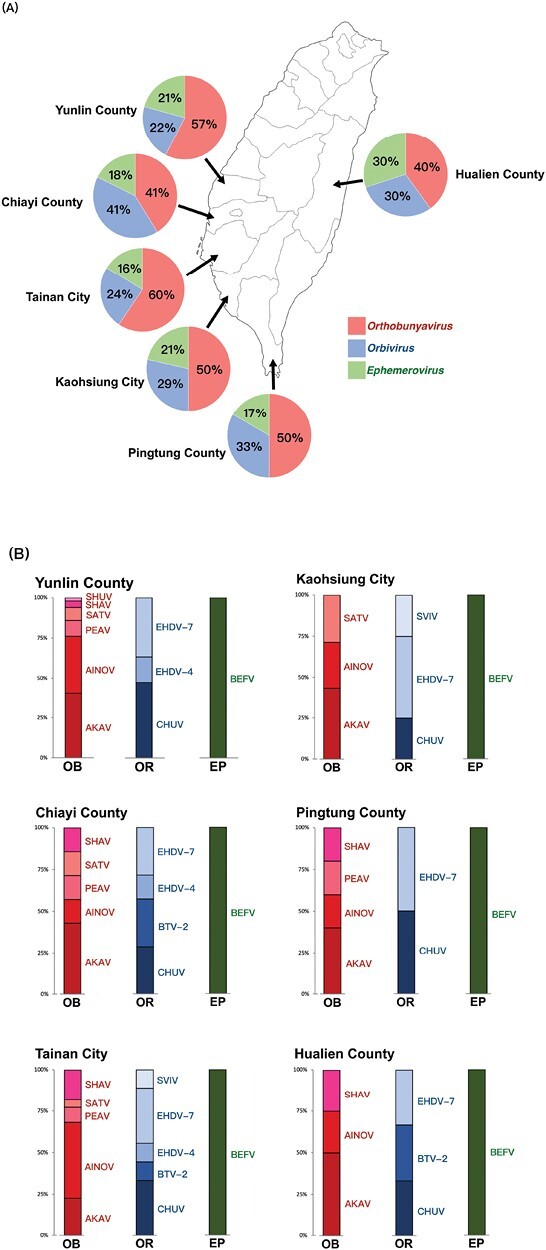
The geographic distribution and detection rate of 12 viruses in 6 sampling areas. A) The detection rate of viruses in 3 genera. B) The detection rate of each virus in the sampling farms. OB, orthobunyavirus; OR, orbivirus; EP, ephemerovirus; AKA, akabane virus; AINOV, aino virus; PEAV, peaton virus; SATV, sathuperi virus; SHAV, shamonda virus; SHUV, shuni virus; CHUV, chuzan virus; BTV-2, bluetongue virus serotype 2; EHDV-4/-7, epizootic hemorrhagic disease virus serotype 4/serotype 7; SVIV, Sathuvachari virus; BEFV, bovine ephemeral fever virus.
During the surveillance of Orthobunyavirus RNA, AKAV was detected annually from 2012 to 2019, while SHUV, SATV, and SHAV were first detected in 2012, 2014, and 2015, respectively. Other appearances are listed in Table 2. Regarding the surveillance of Orbivirus RNA, EHDV-4, and EHDV-7 were first detected in 2014, and SVIV was detected only in 2018. In the detection of Ephemerovirus RNA, BEFV was detected in 2012, 2014–2015, and 2017–2019 (Table 2). The 8-yr average incidence of AKAV, AINOV, CHUV, and BEFV was higher than the overall average (Fig. 3A). The monthly incidence showed a higher occurrence of viruses from July to December compared to January to June (Fig. 3B). The 4 viruses with above-average incidence, AKAV, CHUV, and BEFV, showed high incidence from August to October, while EHDV-7 showed high incidence from March to May (Fig. 3). Peaton virus was only detected in November during 2012–2014, and SHUV appeared in October in both 2012 and 2017 (Table 2 and Fig. 3B).
Fig. 3.

The incidence of 12 bovine arboviruses. A) Each virus incidence was shown by normalization of viral incidence. B) The heatmap of the monthly average incidence of 12 bovine arboviruses in the surveillance, with deeper colors indicating higher incidence. All graphs were created using the ggplot2 package in R 4.2.2.
For the analysis of virus incidence and climate factors, 3 groups of viruses were defined. First group consisted of identified endemic viruses, such as AKAV, the second one was no endemic virus strain such as BEFV, while the other group included uncertainly endemic viruses that were found in the analysis results (Supplementary Figs. S1 and S2). The virus incidence showed no correlations with the average temperature in the current month, rainfall in the current month, or the preceding month (Supplementary Figs. S1 and S2). The wind direction analysis showed that the north wind blew during September to the following April, and then it transitioned to the south wind from May to June in most of the sampling areas in west Taiwan. A higher north wind speed occurred from September to December than from January to April. In east Taiwan, the northeast wind showed a regular seasonal change throughout the year (Supplementary Fig. S3).
The MIRs of detected viruses in the field-collected potential vectors were calculated in blood unfed specimens only for representing virus-infected vector insects. It was possible to calculate the MIR for 12 detected viruses in vector species because 4 of them did not have positive detection in unengorged specimens. Culicoides oxystoma exhibited MIRs of 8 arboviruses between 0.037 and 0.371, and MIRs of 5 arboviruses between 0.069 and 0.414 were found in Cx. tritaeniorhynchus. The MIRs of other vector species were calculated and are shown in Table 3.
Table 3.
MIR in the vector species
| Vector species | MIRa, ‰ | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| BEFV | AKAVb | AINOVb | PEAVb | SATVb | SHAVb | CHUV | EHDV-4 | ||
| Biting midge | C. oxystoma | 0.148 | 0.371 | 0.371 | 0.037 | 0.037 | 0.074 | 0.259 | 0.037 |
| C. arakawae | 1.736 | — | — | — | — | — | — | — | |
| C. actoni | — | — | 2.577 | — | — | — | — | — | |
| Mosquito | An. Sinensis | 9.645 | 3.215 | — | — | — | — | — | — |
| Ar. Subalbatus | — | — | 3.460 | — | — | — | 3.460 | — | |
| Cx. tritaeniorhynchus | 0.414 | 0.069 | 0.343 | — | — | 0.069 | 0.138 | — | |
| Cx. quinquefasciatus | — | 2.865 | 2.865 | — | — | — | — | — | |
| Cx. pipiens molestus | — | — | — | — | — | — | 10.309 | — | |
| Cx. Fuscocephala | — | — | — | — | — | — | 3.367 | — | |
AKAV, akabane virus; AINOV, aino virus; PEAV, peaton virus; SATV, sathuperi virus; SHAV, shamonda virus; CHUV, chuzan virus; EHDV-4, epizootic hemorrhagic disease virus serotype 4; BEFV, bovine ephemeral fever virus.
aThe MIR represents the virus prevalence in each 1,000 mosquitoes or biting midges.
bOnly partial nucleoprotein sequences on S segment RNA genome were only detected in orthobunyaivirus.
Discussion
This study introduces the 12 arboviruses detected in the study, with 6 of them being newly identified in Taiwan (Liao et al. 1996, Ting et al. 2005a, Tzeng et al. 2019). AKAV and CHUV are found to be endemic bovine viruses in Taiwan with high serum prevalence rates (Ting et al. 2005b), while IBAV, previously considered endemic (Liao et al. 1996, Ting et al. 2005b), was not detected using regular testing methods (Tzeng et al. 2019). The potential for missed vectors cannot be excluded in vector surveillance, and the cross-reactivity between EHDV-7 and Ibaraki virus in some sera makes distinguished diagnosis challenging (Anthony et al. 2009). Despite this, the detection rate of EHDV-7 was found to be 0.55%, which is close to the 8-yr incidence of all viruses (0.68%), suggesting that EHDV-7 may be prevalent in Taiwan. Also, its distribution in East Asia also includes Japan, Korea, and China (Yanase et al. 2020), making further investigation of the relationship between EHDV-7 in Taiwan and neighboring countries worthwhile.
SHUV and EHDV-4 were surprisingly detected in East Asia, as they had only been detected in the Middle East and Africa before this study (Hubalek et al. 2014). The partial VP2 sequence of Taiwanese EHDV-4 RNA showed 94.6–95.3% nucleotide identity to currently known EHDV-4 VP2 sequences, and the neighbor-joining (NJ) tree showed that Taiwanese EHDV-4 still grouped with EHDV-4 (Supplementary File S1). The NP sequence (334 bps) of Taiwanese SHUV RNA showed 97.05–97.6% nucleotide identity to currently known SHUV NP sequences, and the NJ tree showed that Taiwanese SHUV clustered with other SHUV (Supplementary File S2). The NP gene represents a conserved sequence region and serves as a suitable target for a 2-step PCR approach. However, it is a challenge for virus determination due to the potential occurrence of reassortment in orthobunyavirus. In order to overcome the difficulty in virus determination, it is necessary to identify and develop a PCR primer set targeting the conserved sequences of 2 structural proteins, Gn and Gc. To better understand the occurrence of SHUV and EHDV-4, further sequencing and phylogenetic analysis of both viruses are needed to understand their origins and migration into East Asia.
Current surveillance for bovine arboviral diseases or pathogens is regularly conducted in Taiwan. The major system is passive monitoring, conducted by the Animal Health Research Institute or the animal epidemic prevention unit of the local government to detect clinical animals with illness. The other project is to collect blood-sucking vector insects in cattle farms and detect the viruses carried by them to monitor the prevalence of arboviruses in sampling farms. Although these surveillances provided data on the prevalence of the virus in domestic cattle areas, the bovine virus can infect not only domestic cattle but also other domestic or wild ruminants (Hubalek et al. 2014); thus, for bovine viral epidemiology, such monitoring is still insufficient. So, despite ongoing surveillance efforts, more work is needed to improve the understanding of arbovirus communities in domestic and wild animals in Taiwan. Additionally, an effective surveillance strategy should be established and integrated into different human, livestock, and wildlife systems.
Since 2012, 6 Culicoides-borne viruses have been newly detected from vectors, which has led to an interest in understanding the island biogeography of arboviruses with transboundary properties between Taiwan and nearby islands and continents. Four of the 6 newly recorded viruses in this study were isolated in the Yaeyama Islands of Okinawa Prefecture, Japan, which are located approximately 70 nautical miles from Taiwan (Kato et al. 2016, Murota et al. 2021, Yamamoto et al. 2021). Among these viruses, only EHDV-7 has been isolated in Japan and China (Yanase et al. 2020). Further sequencing of the viruses of Taiwan and the Yaeyama Islands is necessary to understand the relationship between the 2 places. Transboundary migration by wind is a mechanism for the long-distance transmission of carrier viruses on Culicoides biting midges (Aguilar-Vega et al. 2019). This might be especially important for ecological interactions of Culicoides-borne diseases between Taiwan and nearby islands. Studies of BEFV and AKAV showed the possibility of transboundary transmission by Culicoides-borne diseases that could communicate in both directions between Taiwan and the Yaeyama Islands using meteorological analysis, surveillance, and phylogenetic evidence (Hayama et al. 2016, Tzeng et al. 2019, 2022). However, the identification of transboundary events requires information exchange to link the spatiotemporal and phylogenetic relationships between 2 or more epidemic events. Thus, further studies and the establishment of an information exchange platform between Taiwan and nearby places are needed to understand the detailed mechanisms.
Among 24 species of collected mosquitoes or Culicoides biting midges, 9 species were virus positive, suggesting that these species might be potential vectors in the field. The MIRs of the bovine viruses in the field-collected blood-unfed Culicoides/mosquitoes showed that 2 dominant hematophagous insects are important potential vectors of arboviruses in the dairy farms in Taiwan: C. oxystoma, which showed the ability to carry 8 arbovirus RNAs, and Cx tritaeniorhynchus, which showed the ability to carry 5 arbovirus RNAs. This indicated that orthobunyaviruses, orbiviruses, and ephemeroviruses might be easily transmitted in dairy farms in Taiwan. A detected arbovirus, BEFV, is a serious etiologic agent of bovine ephemeral fever in Taiwan, and the MIRs from this surveillance work suggested C. oxystoma, Culicoides arakawae, Cx. tritaeniorhynchus, and An. sinensis as potential vectors of BEFV. However, C. arakawae is an ornithophilic species, and its occurrence might be related to wild birds or poultry farms, indicating that it might be less involved in the BEFV epidemic in Taiwan. Culicoides oxystoma was demonstrated to transmit AKAV in Japan (Yanase et al. 2019), and Taiwanese Cx. tritaeniorhynchus is able to disseminate BEFV in laboratory tests (unpublished data). Thus, we suggest that experiments on susceptibilities and dissemination should be conducted to confirm vector competence or vectorial capacity.
The expansion of the livestock industry in tropical regions, as a result of improving economies, raises concerns about the spread of arboviruses (Wu et al. 2017). Information sharing is critical for preventing and controlling arboviral diseases, and developing an affordable and efficient detection method for monitoring a wide range of virus species is essential. In this study, we propose a 2-step strategy for the broad screening of numerous and unknown virus species in various virus taxa, which could be applied in a surveillance system. Additionally, integrating surveillance data and establishing models in the tropical area of East Asia will be essential for monitoring the outbreak and transboundary migration of Culicoides-borne viruses and controlling emerging arboviral diseases in livestock in the future.
Supplementary Material
Acknowledgments
This study was supported by a research grant from the Bureau of Animal and Plant Health Inspection and Quarantine, Taiwan, ROC (Project Nos. 105AS-10.10.3-BQ-B1, 106AS-9.9.3-BQ-B1, 107-AS-8.7.2-BQ-B1, 108-AS-8.6.2-BQ-B1).
Contributor Information
Hau-You Tzeng, Department of Entomology, National Chung Hsing University, Taichung City 40225, Taiwan.
Lu-Jen Ting, Animal Health Research Institute, Council of Agriculture, Executive Yuan, New Taipei City 25158, Taiwan.
Chin-Ing Chiu, Department of Entomology, National Chung Hsing University, Taichung City 40225, Taiwan.
Nien-Nung Lin, Council of Agriculture, Bureau of Animal and Plant Health Inspection and Quarantine, Taipei City 100060, Taiwan.
Kuei-Min Liao, Department of Entomology, National Chung Hsing University, Taichung City 40225, Taiwan; National Mosquito-Borne Diseases Control Research Center, National Health Research Institutes, Kaohsiung City 801301, Taiwan.
Wu-Chun Tu, Department of Entomology, National Chung Hsing University, Taichung City 40225, Taiwan; National Mosquito-Borne Diseases Control Research Center, National Health Research Institutes, Kaohsiung City 801301, Taiwan; School of Life Sciences and Technology, Bandung Institute of Technology, Bandung, West Java 40132, Indonesia.
References
- Aguilar-Vega C, Fernandez-Carrion E, Sanchez-Vizcaino JM.. The possible route of introduction of bluetongue virus serotype 3 into Sicily by windborne transportation of infected Culicoides spp. Transbound Emerg Dis. 2019:66(4):1665–1673. 10.1111/tbed.13201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhamis MA, Aguilar-Vega C, Fountain-Jones NM, Lin K, Perez AM, Sanchez-Vizcaino JM.. Global emergence and evolutionary dynamics of bluetongue virus. Sci Rep. 2020:10(1):21677. 10.1038/s41598-020-78673-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony SJ, Maan S, Maan N, Kgosana L, Bachanek-Bankowska K, Batten C, Darpel KE, Sutton G, Attoui H, Mertens PP.. Genetic and phylogenetic analysis of the outer-coat proteins VP2 and VP5 of epizootic haemorrhagic disease virus (EHDV): comparison of genetic and serological data to characterise the EHDV serogroup. Virus Res. 2009:145(2):200–210. 10.1016/j.virusres.2009.07.012 [DOI] [PubMed] [Google Scholar]
- Carslaw DC, Ropkins K.. openair—an R package for air quality data analysis. Environ Model Softw. 2012:27–28:52–61. 10.1016/j.envsoft.2011.09.008 [DOI] [Google Scholar]
- Gale P, Kelly L, Snary EL.. Pathways for entry of livestock arboviruses into Great Britain: assessing the strength of evidence. Transbound Emerg Dis. 2015:62(2):115–123. 10.1111/tbed.12317 [DOI] [PubMed] [Google Scholar]
- Hayama Y, Moriguchi S, Yanase T, Suzuki M, Niwa T, Ikemiyagi K, Nitta Y, Yamamoto T, Kobayashi S, Murai K, et al. Epidemiological analysis of bovine ephemeral fever in 2012–2013 in the subtropical islands of Japan. BMC Vet Res. 2016:12:47. 10.1186/s12917-016-0673-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima Y, Nojiri M, Ohtsuka Y, Kato T, Shirafuji H, Kurazono M, Imafuji T, Yanase T.. Resurgence of bovine ephemeral fever in mainland Japan in 2015 after a 23-year absence. J Vet Med Sci. 2017:79(5):904–911. 10.1292/jvms.16-0345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubalek Z, Rudolf I, Nowotny N.. Arboviruses pathogenic for domestic and wild animals. Adv Virus Res. 2014:89:201–275. 10.1016/B978-0-12-800172-1.00005-7 [DOI] [PubMed] [Google Scholar]
- Kato T, Shirafuji H, Tanaka S, Sato M, Yamakawa M, Tsuda T, Yanase T.. Bovine arboviruses in Culicoides biting midges and sentinel cattle in southern Japan from 2003 to 2013. Transbound Emerg Dis. 2016:63(6):e160–e172. 10.1111/tbed.12324 [DOI] [PubMed] [Google Scholar]
- Lee F. Bovine ephemeral fever in Asia: recent status and research gaps. Viruses. 2019:11(5). 10.3390/v11050412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao YK, Lu YS, Huang ST, Lee SH.. The etiological and epidemiological studies on the Ibaraki disease in Taiwan. J Chin Soc Vet Sci. 1996:22:183–191. [Google Scholar]
- Murota K, Ishii K, Mekaru Y, Araki M, Suda Y, Shirafuji H, Kobayashi D, Isawa H, Yanase T.. Isolation of Culicoides- and mosquito-borne orbiviruses in the southwestern islands of Japan between 2014 and 2019. Vector Borne Zoonotic Dis. 2021:21(10):796–808. 10.1089/vbz.2021.0001 [DOI] [PubMed] [Google Scholar]
- Purse BV, Carpenter S, Venter GJ, Bellis G, Mullens BA.. Bionomics of temperate and tropical Culicoides midges: knowledge gaps and consequences for transmission of Culicoides-borne viruses. Annu Rev Entomol. 2015:60:373–392. 10.1146/annurev-ento-010814-020614 [DOI] [PubMed] [Google Scholar]
- Sedda L, Rogers DJ.. The influence of the wind in the Schmallenberg virus outbreak in Europe. Sci Rep. 2013:3:3361. 10.1038/srep03361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers RF, Herniman KA.. Neutralising antibodies to Akabane virus in ruminants in Cyprus. Trop Anim Health Prod. 1981:13(1):57–60. 10.1007/BF02237891 [DOI] [PubMed] [Google Scholar]
- Ting LJ, Lee MS, Huang TS, Huang CC, Kuo ST, Lee F, Jong MH, Shiau JR, Lin SY.. Identification of bluetongue virus in goats in Taiwan. Vet Rec. 2005a:156(2):52. 10.1136/vr.156.2.52 [DOI] [PubMed] [Google Scholar]
- Ting LJ, Lee MS, Kuo ST, Shiau JR, Sung WHT.. Serological survey on arbovirus infections of cattle in Taiwan. Exp Rep Taiwan Anim Health Res Inst. 2005b:40:65–74. [Google Scholar]
- Tzeng HY, Tsai CL, Ting LJ, Liao KM, Tu WC.. Molecular epidemiology of Akabane virus in Taiwan. Vet Med Sci. 2022:8(5):2215–2222. 10.1002/vms3.887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng HY, Wu HH, Ting LJ, Chang NT, Chou YC, Tu WC.. Monitoring Taiwanese bovine arboviruses and non-arboviruses using a vector-based approach. Med Vet Entomol. 2019:33(2):195–202. 10.1111/mve.12345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC, Reisen WK.. Present and future arboviral threats. Antiviral Res. 2010:85(2):328–345. 10.1016/j.antiviral.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. ggplot2: elegant graphics for data analysis. New York (NY): Springer-Verlag; 2016. [Google Scholar]
- Wu T, Perrings C, Kinzig A, Collins JP, Minteer BA, Daszak P.. Economic growth, urbanization, globalization, and the risks of emerging infectious diseases in China: a review. Ambio. 2017:46(1):18–29. 10.1007/s13280-016-0809-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Hiromatsu R, Kaida M, Kato T, Yanase T, Shirafuji H.. Isolation of epizootic hemorrhagic disease virus serotype 7 from cattle showing fever in Japan in 2016 and improvement of a reverse transcription-polymerase chain reaction assay to detect epizootic hemorrhagic disease virus. J Vet Med Sci. 2021:83(9):1378–1388. 10.1292/jvms.20-0523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanase T, Kato T, Hayama Y, Shirafuji H, Yamakawa M, Tanaka S.. Oral susceptibility of Japanese Culicoides (Diptera: Ceratopogonidae) species to Akabane virus. J Med Entomol. 2019:56(2):533–539. 10.1093/jme/tjy201 [DOI] [PubMed] [Google Scholar]
- Yanase T, Murota K, Hayama Y.. Endemic and emerging arboviruses in domestic ruminants in East Asia. Front Vet Sci. 2020:7:168. 10.3389/fvets.2020.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


