Abstract
Context
Individuals who undergo anterior cruciate ligament reconstruction (ACLR) are at higher risk of posttraumatic osteoarthritis. Altered joint tissue loading caused by aberrant gait biomechanics leads to deleterious changes in joint health linked to the onset of posttraumatic osteoarthritis. Knee braces have been used to modify joint tissue loading in individuals with joint injury, yet the effects of walking with a brace after ACLR on biomechanical, biochemical, and structural cartilage outcomes are unknown.
Objective
To compare biomechanical, biochemical, and structural outcomes between braced and nonbraced walking in individuals with ACLR.
Design
Crossover study.
Setting
Research laboratory.
Patients or Other Participants
A total of 34 individuals with unilateral ACLR (18 females, 16 males; time since ACLR = 50.1 ± 36.8 months).
Intervention(s)
Gait biomechanics were assessed during braced and unbraced conditions on separate days.
Main Outcome Measure(s)
Vertical ground reaction force, knee-flexion angle, and internal knee-extension moment waveforms were evaluated throughout the stance phase and compared between conditions. Percentage changes in serum cartilage oligomeric matrix protein (%ΔCOMP) and femoral cartilage cross-sectional area (%ΔCSA) measured via ultrasound were calculated after a 3000-step walking protocol.
Results
Braced walking increased the knee-flexion angle (largest difference = 3.56°; Cohen d effect size = 1.72) and knee-extension moment (largest difference = −0.48% body weight × height; Cohen d effect size = −1.14) compared with nonbraced walking but did not influence vertical ground reaction force. Whereas no difference (P = .20) in %ΔCOMP existed between the braced and nonbraced conditions in the entire cohort (n = 30 with complete blood data), a larger increase (P = .04) in %ΔCOMP was seen during nonbraced than braced walking in individuals who demonstrated increased COMP during nonbraced walking. No difference (P = .86) in %ΔCSA was present between the braced and nonbraced conditions.
Conclusions
Braced walking may improve sagittal-plane gait biomechanics and %ΔCOMP in a subset of individuals who demonstrate a typical increased COMP response to load (ie, increase in COMP) after nonbraced walking.
Keywords: stiffened-knee gait strategy, knee-flexion angle, knee-extension moment, ultrasound, cartilage oligomeric matrix protein
Key Points
Compared with nonbraced walking, braced walking after anterior cruciate ligament reconstruction may mitigate the stiffened-knee strategy by increasing the peak knee-flexion angle early in the stance phase of gait.
Braced walking may decrease the acute serum cartilage oligomeric matrix protein response in individuals with anterior cruciate ligament reconstruction who typically demonstrate increased serum cartilage oligomeric matrix protein concentrations after nonbraced walking.
Ultrasound measures of femoral cartilage cross-sectional area were not different between the braced and nonbraced walking conditions.
Individuals who sustain anterior cruciate ligament (ACL) injury and undergo ACL reconstruction (ACLR) are at increased risk of developing posttraumatic osteoarthritis (OA).1 Posttraumatic OA develops, in part, because of the changes in gait biomechanics that occur after ACLR. Aberrant gait biomechanics, such as a stiffened-knee gait strategy (ie, lesser sagittal knee-flexion angle [KFA] and peak internal knee-extension moment [KEM]) and lesser lower extremity loading, are common in individuals after ACLR compared with uninjured control participants.2 Researchers3 have hypothesized that a stiffened-knee gait strategy negatively influences force attenuation and localizes forces to cartilage regions ill-adapted to withstand such loads, especially during early stance phase, when contact force may be highest. In addition, accumulating evidence suggests that lesser vertical ground reaction force (vGRF) early after ACLR is associated with worse outcomes. Specifically, lesser vGRF, as well as a stiffened-knee gait strategy, has been linked to greater serum biomarkers of joint tissue inflammation and extracellular articular cartilage breakdown,4,5 altered articular cartilage composition,6 and worse patient-reported outcomes.7 It is not clear if interventions that modify aberrant gait biomechanics after ACLR could slow or reverse these deleterious changes in articular cartilage health. A multifaceted approach incorporating biomechanical, biochemical, and structural outcomes is important for understanding the ability of an intervention to influence multiple factors linked to posttraumatic OA development.8
Knee braces have been used to modify loading of articular cartilage during gait in individuals with idiopathic knee OA.9,10 An external 3-point bending force is often applied to the tibiofemoral joint to decrease tibiofemoral compartment loading.9 Three-point bending braces resist femoral cartilage deformation11 and increase peak KFAs during gait,9,10 indicating that bracing may influence the sagittal-plane gait biomechanics that are critical for force attenuation after ACLR. Therefore, using a 3-point bending brace to decrease the propensity to rely on the stiffened-knee gait strategy without further decreasing vGRF may be an effective intervention model to assist in restoring normal loading across the tibiofemoral joint during gait after ACLR. However, investigators have not comprehensively evaluated the effects of 3-point bending braces on biomechanical, biochemical, and structural outcomes related to articular cartilage health.
Researchers have used serum biomarker cartilage oligomeric matrix protein (COMP) and ultrasound (US) measures of femoral cartilage cross-sectional area (CSA) after an acute standardized loading protocol to evaluate the acute biochemical12–15 and structural16–18 responses, respectively, to walking. Cartilage oligomeric matrix protein is a mechanosensitive biomarker of cartilage breakdown19 associated with OA severity and progression.13 In individuals with knee OA, larger increases in COMP after a 30-minute walking protocol are associated with larger decreases in medial femoral and tibial cartilage thickness over the next 5 years.13 Ultrasound measures of the anterior femoral cartilage CSA are similarly responsive to loading during an acute bout of walking.18 Although walking may cause dynamic changes in anterior femoral cartilage CSA, most individuals demonstrate decreased anterior femoral cartilage CSA after 3000 steps.16
Therefore, the primary purpose of our study was to determine if gait biomechanics related to lower extremity loading and a stiffened-knee strategy (ie, vGRF, KFA, and KEM), changes in serum COMP concentrations, and US measures of femoral cartilage CSA differed between 3000-step braced and nonbraced walking conditions in individuals with ACLR. We hypothesized that the braced condition would result in increased sagittal KFA and KEM but would not alter vGRF. Additionally, we hypothesized that serum COMP would increase less and femoral cartilage CSA would decrease less in the braced than the nonbraced condition.
METHODS
Study Design
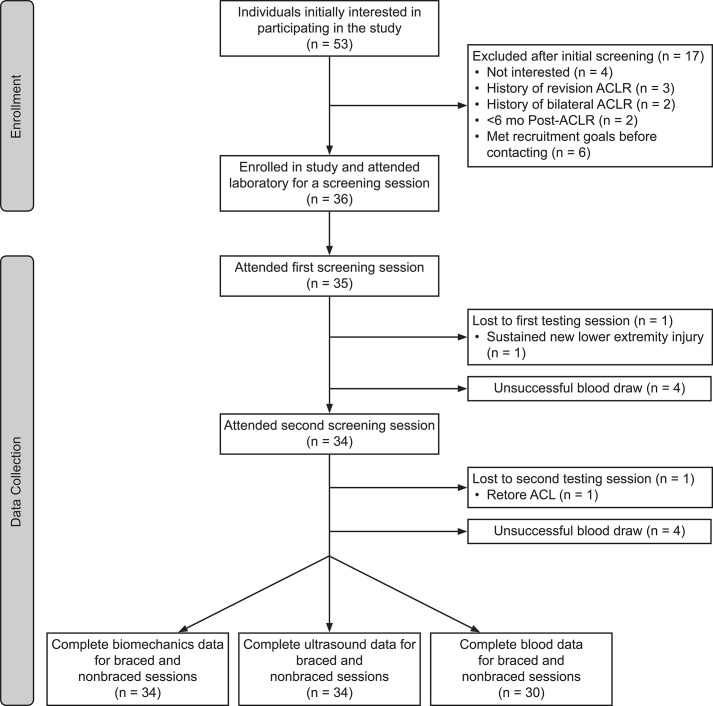
We conducted a crossover study with 3 sessions that occurred on separate days (Figure 1). All sessions started at the same time of day (±1 hour) to account for possible diurnal variations in articular cartilage physiology. The first visit was a screening session during which we collected descriptive data and determined self-selected walking speed. During testing sessions 2 (19 ± 10 days after session 1) and 3 (17 ± 8 days after session 2), participants completed the braced and nonbraced walking conditions. Outcomes of gait biomechanics were assessed at the beginning of each testing session (braced and nonbraced), and COMP and US outcome measures were collected before (baseline) and immediately after (posttest) a standardized walking protocol. The order of conditions was block randomized (blocks of 6). In the braced condition, participants wore a valgus unloader brace (model Rebound Cartilage; Ossur Inc) on the ACL-reconstructed limb.
Figure 1.
The experimental protocol for the initial laboratory visit and both the braced and nonbraced conditions. Although the braced condition is depicted first, the order of the testing conditions was randomized. Abbreviations: ACLR, anterior cruciate ligament reconstruction; ACL, anterior cruciate ligament.
Participants
Participants were recruited from the university community and surrounding community using flyers, email, and word of mouth (Figure 2). Only individuals who met the following criteria were included: (1) age between 16 and 35 years, (2) body mass index between 18 and 35, (3) unilateral ACLR ≥6 months before testing, (4) no history of neurologic disorder, (5) no lower extremity joint injury in the 6 months before the study, (6) cleared by a physician for return to physical activity, (7) currently engaged in ≥20 minutes of physical activity 3 times per week, and (8) no previous diagnosis of any diseases that affect joint tissue. The study was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill, and all participants provided written informed consent.
Figure 2.
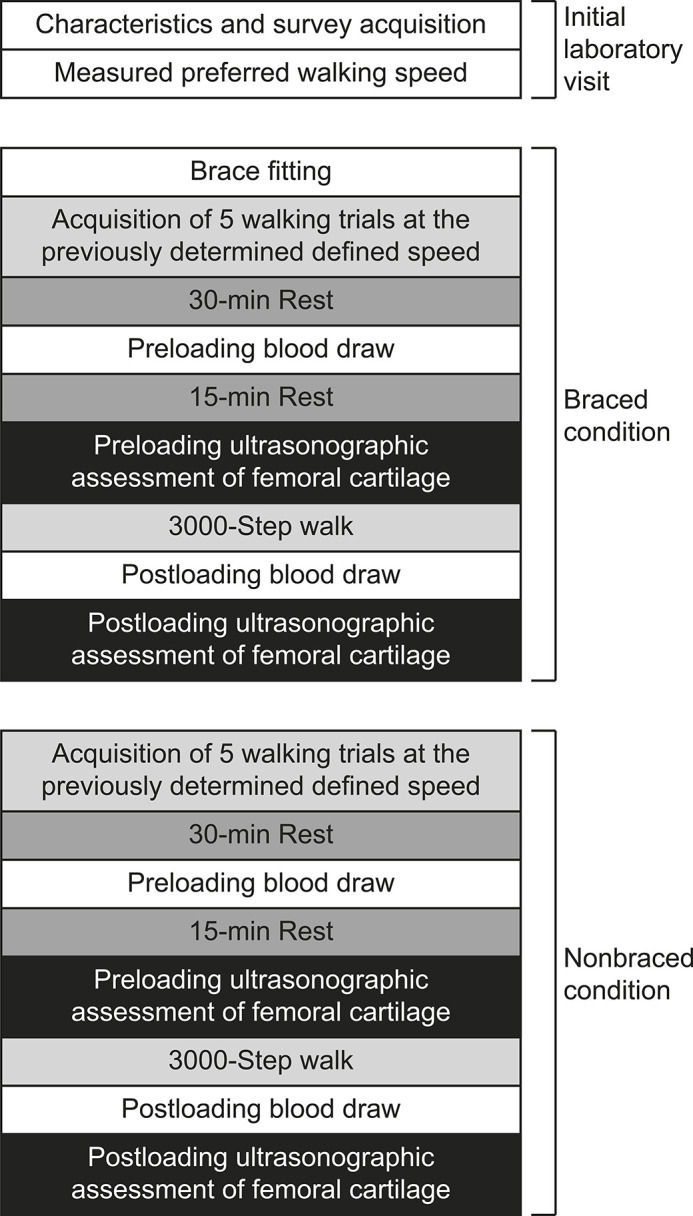
The recruitment, enrollment, and data collection of participants. The initial laboratory visit, the braced condition, and the nonbraced condition each occurred on separate days.
We estimated braced walking would have a moderate effect (Cohen d = 0.6) for a change in biomechanics based on a previous study20 that assessed acute modifications in gait biomechanics due to bracing in individuals with ACLR. The authors of another study11 reported a moderate effect (Cohen d = 0.6) for differences in US measures of femoral CSA after a similar walking protocol between braced and nonbraced conditions in uninjured individuals with varus alignment. Finally, we estimated that we would find a moderate effect size (Cohen d = 0.6) for a change in COMP based on earlier work that involved evaluating the acute effects of a real-time gait biofeedback intervention in modifying acute measures of COMP.21 We estimated that we would need to test 24 individuals to detect differences using a dependent t test (2-tailed α = .05; 1 − β = 0.8) for changes in COMP and CSA if similar between-conditions effect sizes were found. We estimated that at least 9 participants would be needed to detect a moderate difference (Cohen d = 0.6) in biomechanics using a functional waveform analysis. Therefore, accounting for the potential removal of outliers or missing data, we recruited 35 individuals to enable us to detect differences with a moderate effect (Cohen d = 0.5) using the same rigorous approach (2-tailed α = .05; 1 − β = 0.8).
Procedures
During the screening session, we determined self-selected walking speed by instructing participants to walk over a 6-m distance between 2 infrared timing gates (model TF100; Trac Tronix) at the pace they would “comfortably walk over a sidewalk.” After participants were comfortable walking in the laboratory, the speeds of 5 walking trials were averaged and used to set the speed of the treadmill during the subsequent testing conditions. For the braced condition and before any data collection, all participants were fitted with the brace using the manufacturer’s guidelines. For both conditions, we collected gait biomechanics before a 45-minute rest period in which participants were positioned supine on a padded plinth. The brace was removed during the rest period. Baseline blood samples and US images were collected after 30 and 45 minutes of rest, respectively.18,22 The brace was refitted after the baseline blood collection and US imaging were performed. For both conditions, participants then completed the 3000-step walking protocol. Immediately afterward, they were seated on a plinth for posttest blood sample collection and US cartilage imaging. The brace was removed immediately after the walking protocol and before the posttest US cartilage imaging and blood sample collection.
Brace-Fitting Protocol
At the beginning of the braced condition, participants walked 40 m, after which any needed readjustments to the fit of the brace were made. This brace readjustment and 40-m walk were repeated 3 times. A permanent marker was used to outline the brace on the skin to assist with reapplication as required. Participants underwent the same fitting procedures after the rest period and the collection of the baseline blood and US images to ensure brace comfort during the standardized walking protocol. After the rest period during the nonbraced condition, participants covered the same walking distance to ensure consistency of lower extremity loading between conditions.
Walking Gait Biomechanics Collection, Processing, and Analysis
Participants were outfitted with 26 retroflective markers and 1 rigid cluster of 3 markers placed over the sacrum.23 For both testing sessions, a static trial was collected without the brace and used to create the segment-linkage model. For the braced condition only, the lateral epicondyle retroreflective marker was then removed to allow proper refitting of the brace. When refitting the brace using the previously drawn outlines, we replaced the lateral epicondyle retroreflective marker using the outline of its previous location. Data from 5 successful walking trials were then collected. Trials were considered successful if participants did each of the following: (1) individually struck a single force plate with each foot, (2) maintained a walking speed ±5% of the predetermined self-selected walking speed, and (3) did not undergo any visible alterations to gait during the trial (eg, trip or stutter step).
We evaluated kinematic and kinetic outcomes from the ACL-reconstructed limb. Marker positions were collected at 120 Hz using a 10-camera motion-capture system (version 1.8.5; Vicon Industries, Inc), and force data were sampled at 1200 Hz. All data were low-pass filtered at 10 Hz using a fourth-order recursive Butterworth filter. Biomechanical outcomes during the stance phase of walking were analyzed on a global coordinate system using Visual3D software (version 2020.09.1; C-Motion, Inc). Hip-joint centers were estimated using the Bell and Brand hip-joint CODA coordinate system.24 Knee- and ankle-joint centers were identified as the midpoint between the medial and lateral femoral epicondyles and the malleoli, respectively. Knee kinematics were calculated as motion of the shank relative to the thigh using Euler angles (sagittal-frontal-transverse sequence). Internal joint moments were calculated using anthropometrics, synchronized kinematic and ground reaction force data, and a standard inverse-dynamics approach via Visual3D software. Vertical ground reaction force was normalized to body weight (BW). Internal moments were normalized to the product of BW (in newtons) and height (in meters). Vertical ground reaction force, KFA, and internal KEM data during stance were time normalized to 101 data points before analysis.
Serum COMP Collection, Processing, and Analysis
We collected 5 mL of blood from an antecubital vein using a standard vacutainer serum separator tube fitted with a 21-gauge needle. Serum vacutainers were kept cool until being centrifuged at 4°C for 10 minutes at 3000g.25 Serum was pipetted equally into two 1.5-mL cryovials and stored at −80°C in a freezer until all serum samples could be batch analyzed at the end of the study. Serum was assessed for COMP concentrations using commercially available enzyme-linked immunosorbent assays (Human COMP Quantikine ELISA kit; R & D Systems) according to the manufacturer’s protocols. All standards, samples, and controls were performed in duplicate determinations. All assays demonstrated an average intra-assay variability of 1.92%, and all intra-assay variabilities were <7.56%.
Collection of Femoral Cartilage CSA Using US
Just before US image acquisition, participants sat on a plinth with their back against a wall and their ACL-reconstructed knee positioned in 140° of flexion using a manual goniometer. A measuring tape was secured along the length of the plinth so the position of the posterior calcaneus could be recorded to allow for consistent positioning between measurements. At each time point, 3 US images of the anterior femoral cartilage were obtained using a LOGIQe US System (General Electric Co) and a 12-MHz linear probe. The probe was placed transversely in line with the medial and lateral femoral condyles just superior to the patella and rotated in the sagittal plane to maximize sound reflection off the femoral articular cartilage. To further improve reproducibility of the US image, we placed a transparent numbered grid over the US screen.17 The US images were deidentified and segmented by a blinded and trained investigator (H.C.D.W.) using ImageJ software (version 1.52q; National Institutes of Health). To obtain CSA (in millimeters squared), we segmented the femoral cartilage by identifying the cartilage–bone and soft tissue–cartilage interfaces over the medial and lateral femur. This method has demonstrated strong intrasession (intraclass correlation coefficient [ICC] for medial femoral cartilage = 0.99, ICC for lateral femoral cartilage = 0.99) and intersession (ICC for medial femoral cartilage = 0.98, ICC for lateral femoral cartilage = 0.98) reliability.17
Walking Protocol
Participants walked on a treadmill (model 4Front; WOODWAY) at the predetermined self-selected speed for 3000 steps. Steps were measured using a pedometer. A similar protocol has been used to elicit femoral articular cartilage deformation.11 During the braced condition, participants wore the brace throughout the entire 3000 steps.
Statistical Analyses
Before data analysis, a single investigator (A.E.P.) inspected all biomechanical outcome curves to ensure quality control (ie, to ensure individuals stepped entirely on the force plate). Before data analysis, for discrete outcome measures, we defined participants with point measures >3 SDs from the mean as statistical outliers and planned to subsequently remove their data points from the analyses.
Biomechanical Data Analysis
We compared biomechanical outcomes between the braced and nonbraced conditions at each percentile of the stance phase using a separate functional waveform analysis26 for each outcome (vGRF, KFA, and internal KEM). We also compared knee-adduction moments (KAMs) between the braced and nonbraced conditions (see Supplemental Figure, available online at http://dx.doi.org/10.4085/1062-6050-0700.20.S1). The functional waveform gait analysis facilitates comparison of the biomechanical outcomes at each percentile of the stance phase rather than only at discrete time points.26 Functional waveform gait analyses were performed as previously reported27 using the functional data analysis package in R statistical computing software (version 2.2.6; The R Foundation). We calculated 95% CIs for each biomechanical waveform for each group. The comparisons were considered different at any percentile of the stance phase in which the mean differences and corresponding 95% CIs did not cross zero.26 We reported the largest difference between ensemble curves and corresponding between-groups effect sizes (Cohen d) within the proportions of stance demonstrating differences for each primary comparison as described. Effect size magnitude was interpreted as no effect (0–0.19), small (0.20–0.49), medium (0.50–0.79), or large (≥0.80).
The COMP and Femoral Cartilage CSA Analysis
The COMP response due to each walking condition was calculated as the percentage change score from baseline to posttest (%ΔCOMP; posttest − baseline). The CSAs from the 3 femoral cartilage US images at each time point (baseline and posttest) were averaged. Femoral cartilage deformation due to each loading condition was calculated as the percentage change from the average baseline to posttest CSA (%ΔCSA; posttest − baseline). Paired t test and Cohen d between-groups effect-size calculations were conducted to compare %ΔCOMP and %ΔCSA between the braced and nonbraced conditions.
Post Hoc Analyses
An increase in serum COMP concentration after a walking protocol is associated with future cartilage thinning.13 Researchers28 have shown greater effects in loading interventions in patients who displayed an increase in COMP during normal walking. Therefore, we performed a separate post hoc analysis to determine if differences in %ΔCOMP existed between conditions in a subset of participants who exhibited increased COMP from baseline to posttest (>0-μg/mL increase in COMP during the nonbraced condition). Cohen d between-groups effect sizes were calculated for differences between conditions. All analyses were conducted using SPSS (version 26; IBM Corp).
To identify potential associations between changes in gait biomechanics and changes in %ΔCOMP and %ΔCSA, we conducted separate Pearson r correlations between the differences in biomechanics and the differences in %ΔCOMP and %ΔCSA between the braced and nonbraced conditions (nonbraced − braced). Pearson r correlation coefficients and associated P values (significant at P < .05) were calculated using SPSS.
RESULTS
A total of 53 active individuals who were cleared for unrestricted physical activity were screened, and 36 individuals were enrolled in the study (Table 1). Biomechanics and US data were collected for 34 participants, whereas blood was collected from 30 participants in both the braced and nonbraced conditions (Figure 2). Percentiles of the stance phase showing mean differences and corresponding effect sizes for vGRF, KFA, and internal KEM are listed in Table 2. One statistical outlier was identified and removed from subsequent femoral cartilage CSA analysis; thus, data from 33 participants were used in the final CSA analysis.
Table 1.
Participant Characteristics
|
Characteristic |
No. |
| Sex | |
| Female | 18 |
| Male | 16 |
| Graft type | |
| Patellar tendon autograft | 18 |
| Quadriceps tendon autograft | 14 |
| Cadaveric allograft | 2 |
|
Mean ± SD |
|
| Age, y | 22.1 ± 4.2 |
| Height, cm | 172.9 ± 9.2 |
| Mass, kg | 71.7 ± 12.7 |
| Body mass index | 23.9 ± 2.8 |
| Knee injury and Osteoarthritis Outcome Score Quality of Life | 78.1 ± 16.5 |
| Self-selected gait speed, m/s | 1.31 ± 0.16 |
|
Mean ± SD (Range) |
|
| Time since surgery, mo | 50.1 ± 36.8 (9–146) |
Table 2.
Portions of Stance Demonstrating Relevant Differences Between Braced and Nonbraced Conditions
|
|
Portions of Stance With Differences Between Ensemble Curves, % |
Largest Difference Between Ensemble Curves |
Cohen d Effect Size (95% CI) of Largest Difference Between Ensemble Curves |
| Vertical ground reaction force | 4–10 | 3.56% BW | 0.91 (0.68, 1.14) |
| 55–71 | 2.46% | 0.78 (0.55, 1.01) | |
| 86–95 | −2.23% | −0.61 (−0.83, −0.39) | |
| Knee-flexion angle | 1–99 | 3.56° | 1.72 (1.46, 1.97) |
| Internal knee-extension moment | 1–12 | −0.19% BW × height | −0.72 (−0.94, −0.49) |
| 53–91 | −0.48% BW × height | −1.14 (−1.38, −0.91) | |
| 95–100 | −0.11% BW × height | −1.00 (−1.23, −0.76) |
Abbreviation: BW, body weight.
Biomechanical Data
Compared with the nonbraced condition, the braced condition demonstrated greater vGRF between 4% and 10% and between 55% and 71% of stance and lesser vGRF between 86% and 95% of stance (Figure 3). Compared with the nonbraced condition, KFA was greater throughout most of the stance phase (1%–99% of stance; Figure 4) during the braced condition. The KEM was greater between 1% and 12%, 53% and 91%, and 95% and 100% of stance (Figure 5) in the braced than the nonbraced condition. Compared with the nonbraced condition, the braced condition displayed greater KAM between 9% and 23% and between 31% and 38% of stance (see Supplemental Figure) and lesser vGRF between 91% and 99% of stance.
Figure 3.
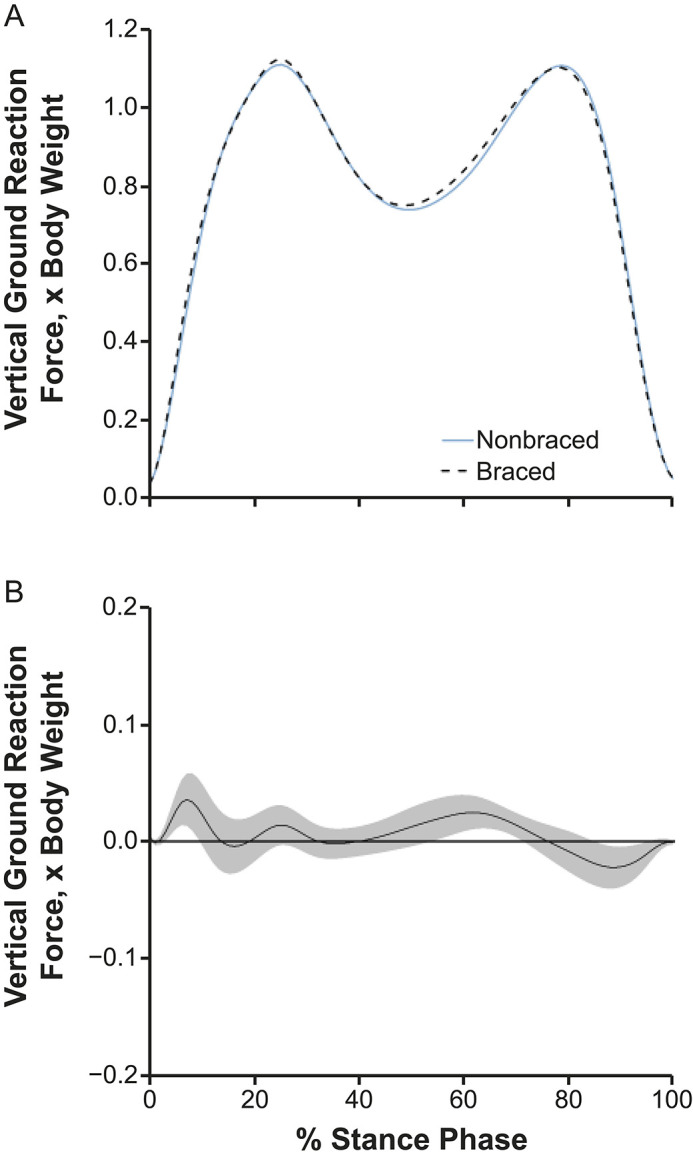
A, Vertical ground reaction forces and, B, mean difference curve of the braced and nonbraced conditions. A, Mean ensemble waveforms plotted over the stance phase of walking for mean vertical ground reaction force normalized to body weight for both the braced and nonbraced conditions. B, Corresponding pairwise comparison functions and associated 95% CIs (gray bands) indicating the mean differences between the braced and nonbraced conditions. Differences between conditions exist when the 95% CIs do not overlap zero.
Figure 4.
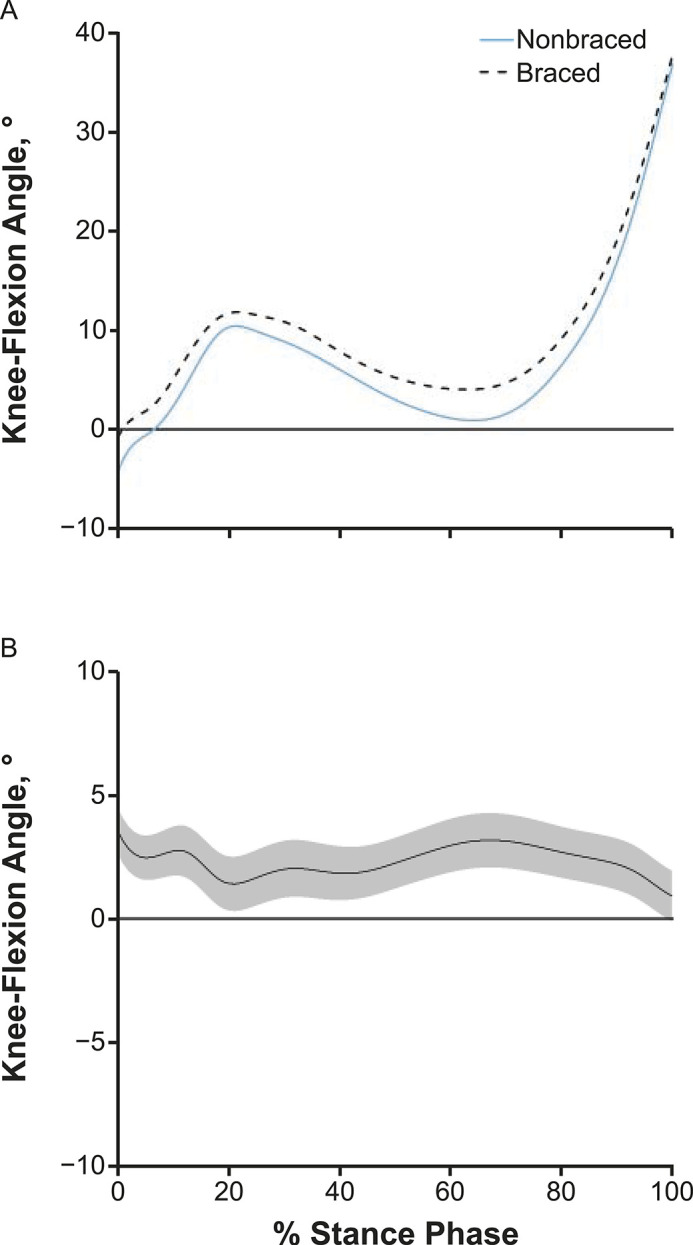
A, Knee-flexion angles and, B, mean difference curve of the braced and nonbraced conditions. A, Mean ensemble waveforms plotted over the stance phase of walking for mean knee-flexion angle for both the braced and nonbraced conditions. B, Corresponding pairwise comparison functions and associated 95% CIs (gray bands) indicating the mean differences between the braced and nonbraced conditions. Differences between conditions exist when the 95% CIs do not overlap zero.
Figure 5.
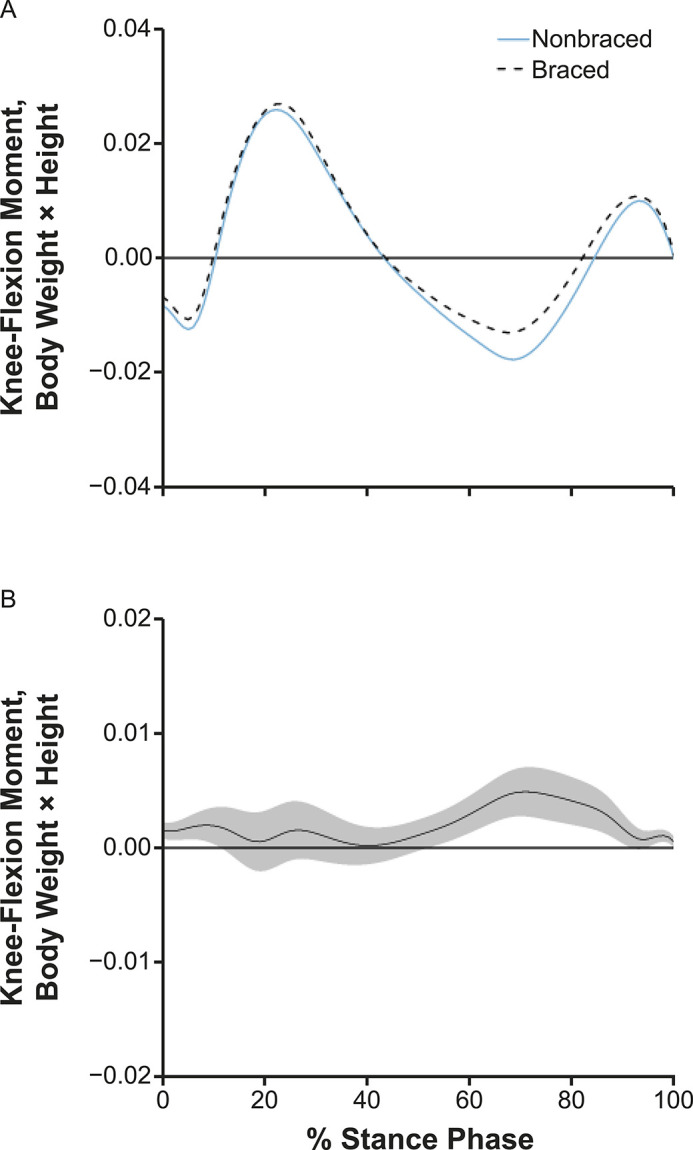
A, Internal knee-flexion moments and, B, mean difference curve of the braced and nonbraced conditions. A, Mean ensemble waveforms plotted over the stance phase of walking for mean internal knee-flexion moment for both the braced and nonbraced conditions. B, Corresponding pairwise comparison functions and associated 95% CIs (gray bands) indicating the mean differences between the braced and nonbraced conditions. Differences between conditions exist when the 95% CIs do not overlap zero.
Serum COMP Response and Associated Post Hoc Analysis
We observed no difference between the nonbraced (8.6% ± 13.6%) and braced (6.4% ± 15.3%) conditions for %ΔCOMP in the entire cohort (t29 = 1.323, P = .20; Cohen d = −0.30; 95% CI = −0.80, 0.22; Figure 6). Twenty-two participants who exhibited an increase in COMP after the nonbraced condition were included in the post hoc analysis. A smaller increase in COMP (t21 = 2.243, P = .04; Cohen d = −0.65; 95% CI = −1.26, −0.04) existed after the braced (8.9% ± 13.8%) than the nonbraced condition (13.6% ± 12.3%) in this subgroup (Figure 6).
Figure 6.
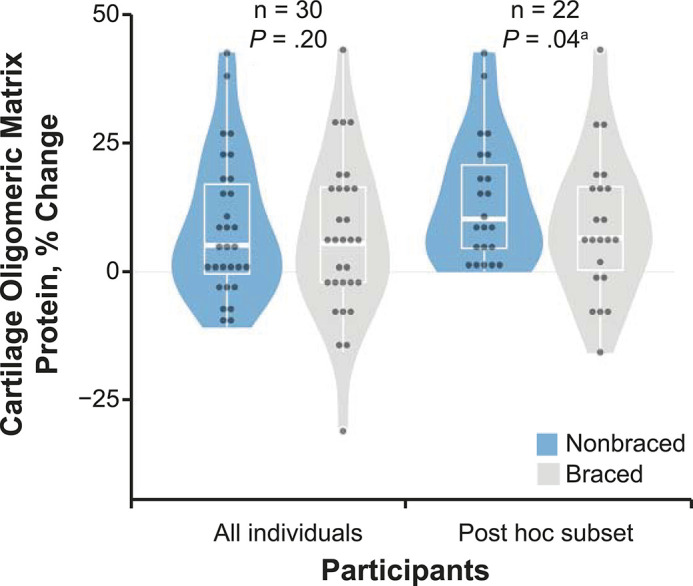
Percentage change in serum cartilage oligomeric matrix protein (COMP) due to the braced and nonbraced conditions in all participants and in a post hoc subset of participants. The percentage change in serum COMP due to a standardized 3000-step walking protocol both with (braced) and without (nonbraced) a knee brace designed to offload articular tissues both for all participants (n = 30) and in a subset of participants who demonstrated increased COMP (>0-μg/mL increase in COMP during nonbraced walking; n = 22). The horizontal line indicates the median. The upper and lower hinges correspond to the first (25th) and third (75th) quartiles, respectively. The interquartile range is the distance between the first and third quartiles. The whiskers indicate the smallest and largest data points within 1.5 × interquartile range of the respective hinge.
Femoral Cartilage Deformation
We found no difference (t32 = 0.183, P = .86; Cohen d = −0.05; 95% CI = −0.53, 0.43) in %ΔCSA between the nonbraced (−0.86% ± 3.46%) and braced (−0.65% ± 4.76%) conditions (Figure 7).
Figure 7.
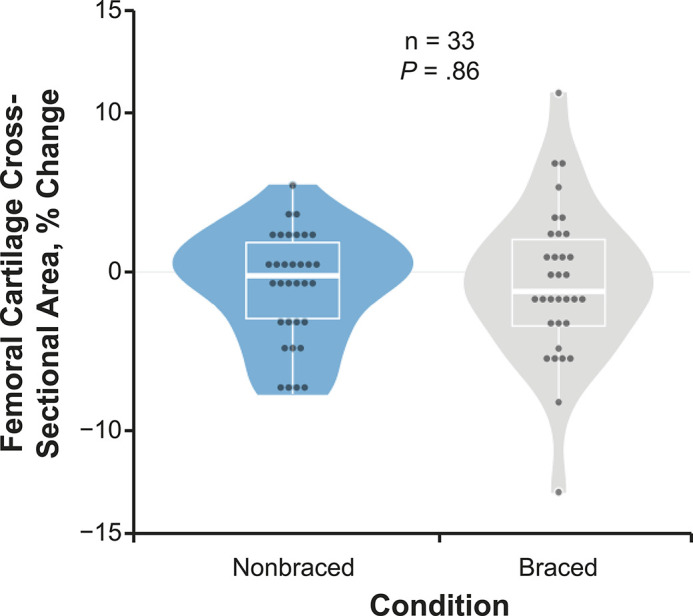
Percentage change in femoral cartilage cross-sectional area due to the braced and nonbraced conditions in all participants. The percentage change in ultrasonic femoral cartilage cross-sectional area due to a standardized 3000-step walking protocol both with (braced) and without (nonbraced) a knee brace designed to offload articular tissues for all individuals (n = 33). The horizontal line indicates the median. The upper and lower hinges correspond to the first (25th) and third (75th) quartiles, respectively. The interquartile range is the distance between the first and third quartiles. The whiskers indicate the smallest and largest data points within 1.5 × interquartile range of the respective hinge.
Correlations Between Biomechanical Changes and Changes in COMP and CSA Responses
A positive correlation existed between the change in vGRF impact peak (ΔvGRF-IP) and the change in the CSA responses. Specifically, a greater ΔvGRF-IP (ie, nonbraced vGRF-IP − braced vGRF-IP) was associated with a greater ΔCSA increase (ie, nonbraced %ΔCSA − braced %ΔCSA). We noted no correlations between KFA, KEM, or KAM values and changes in COMP or CSA responses. Similarly, no correlation was seen between ΔvGRF-IP and the change in the serum COMP response (see Supplemental Table, available online at http://dx.doi.org/10.4085/1062-6050-0700.20.S2).
DISCUSSION
Overall, individuals with ACLR demonstrated greater KFA throughout the stance phase and greater KEM in the first 12% of the stance phase during the braced condition, suggesting a propensity to minimize the stiffened-knee gait strategy early in stance. In the entire study cohort, walking with the 3-point bending brace did not influence acute serum COMP concentrations, yet a smaller increase in serum COMP was evident after walking with a brace in a subset of individuals (n = 22) whose serum COMP concentrations increased after the nonbraced condition. Contrary to our hypothesis, the braced condition did not acutely influence acute femoral cartilage deformation. Therefore, our laboratory-based findings suggested that the 3-point bending brace acutely modified the stiffened-knee strategy early in the stance phase and the acute biological response to walking in participants who displayed increased serum COMP concentration after a 3000-step walking protocol.
A stiffened-knee gait strategy (ie, less peak KFA and less sagittal moment)2 has been hypothesized to impede optimal energy attenuation across the tibiofemoral cartilage surface. Our results are consistent with those of previous researchers29 who reported that walking with a brace promoted increased KFA throughout stance compared with nonbraced walking in those with ACLR. Peak vGRFs (22%–23% of stance) remained similar between conditions; thus, the increased KFA during braced walking may have allowed force attenuation across more of the tibiofemoral joint.30 Further, an increase in KFA may be linked to the increased internal KEM in early stance (1%–12%) and the second half of stance (53%–91%) in the braced condition compared with the nonbraced condition. Individuals did show slightly greater vGRF early during the stance phase (4%–10%) when braced. Maintaining, or slightly increasing, vGRF during gait may also be beneficial for maintaining cartilage health, as greater vGRF in the first 50% of stance is associated with less type II collagen turnover.4 Our data indicated that alterations in the stiffened-knee gait strategy may have been caused by an acute increase in KFA throughout stance. Our findings also suggested that the brace may have acutely restricted full extension of the knee during midstance. Overall, our results provide evidence that using a 3-point bending brace may acutely elicit changes in sagittal-plane gait biomechanics that may be beneficial for cartilage health. The supplemental comparison of KAM biomechanics revealed that braced walking elicited greater KAM than did nonbraced walking in our cohort. In earlier studies, investigators observed that individuals with ACLR had lesser KAM,5,6,31 in addition to lesser KFAs and lesser KEMs than control participants.32–35 Although we did not compare gait biomechanics between individuals with ACLR and uninjured control individuals, the increases in KFA, KEM, and KAM in individuals with ACLR after acute bracing suggests that bracing may acutely assist in normalizing biomechanics in portions of the stance phase.
Researchers4 have hypothesized that aberrant joint loading contributes to harmful biological changes to knee-joint tissues. As such, it is surprising that walking with a brace did not result in a smaller COMP increase compared with walking without a brace in the entire cohort. It is possible, however, that in attempting to unload the medial compartment, braced walking increased the lateral tibiofemoral contact stresses, thereby creating a net-neutral articular cartilage load that may have resulted in the same serum COMP results as those of nonbraced walking. Future investigators should measure the shifting of contact stresses between the medial and lateral compartments due to braced walking in individuals with ACLR. In addition, braced walking may result in biological changes in individuals who demonstrate the most deleterious responses to joint loading. Similar to the data from previous authors,21 approximately 65% of our cohort experienced increased serum COMP after habitual walking. In individuals whose serum COMP increased after the nonbraced condition, the braced condition resulted in a smaller increase in serum COMP concentration. Our results indicated that walking with a brace may reduce acute cartilage breakdown during walking in individuals with ACLR who displayed more breakdown during activities of daily living, such as walking. Furthermore, the acute effects of braced walking on COMP concentrations in individuals whose serum COMP increased after usual walking in our study were similar to those found when patients with ACLR were cued to normalize gait patterns with real-time gait biofeedback.21 Whereas our study was not powered to conduct the additional analyses to evaluate multiple covariates, it is possible that this subgroup of individuals with greater breakdown during activities of daily living may present with additional factors that influence the biological response to load. Static lower extremity posture is a factor associated with knee OA.36 Authors of multiple studies have specifically sought to understand the link between gait biomechanics and medial tibiofemoral cartilage unloading37–39 attributed to varus malalignment, as cartilage degeneration is also common in the lateral tibiofemoral cartilage after ACL injury.40 As such, future researchers should consider personalizing the direction of the 3-point bending that the brace applies to the knee based on an individual’s baseline static posture. Moreover, it will be important to determine the effect of other covariates (eg, meniscal injury, articular cartilage lesions, sex, time since surgery or injury) on the COMP response in individuals with ACLR.
The results of the post hoc analyses between the biomechanics and changes in CSA and COMP response suggested that a higher ΔvGRF-IP was associated with a higher ΔCSA response. Although our work was not powered to characterize the different CSA responses associated with an increase in vGRF-IP, we speculate that individuals’ biomechanical responses to bracing may have affected ΔCSA differently. Future authors should elucidate the factors associated with the femoral CSA response to braced walking. Specifically, researchers may choose to characterize the effect of covariates (eg, meniscal injury, articular cartilage lesions, sex, time since surgery or injury, baseline concentrations of additional biomarkers of articular cartilage breakdown and metabolism) on the COMP and CSA response in individuals with ACLR to subsequently improve the clinical outcomes for the entire group of individuals with ACLR.
We are the first, to our knowledge, to evaluate the effects of an unloader brace on femoral cartilage CSA in individuals with ACLR. Healthy individuals who displayed measurable deformation during walking demonstrated lesser cartilage deformation when walking with a valgus unloader brace than without a brace.11 Conversely, we did not find differences in cartilage deformation (ie, %ΔCSA) between the braced and nonbraced conditions in individuals with ACLR, suggesting that wearing a brace during a single session of walking may not influence the structural response of femoral articular cartilage. However, it is possible that changes in femoral cartilage structure not evaluated using US imaging were modified by braced walking. The US imaging procedures we used were restricted to the anterior femoral cartilage; therefore, the central and posterior femoral cartilage regions that may be deformed during walking were not assessed. In future studies, investigators may seek to incorporate other imaging modalities (eg, magnetic resonance imaging) to understand the changes in articular cartilage structure across the entire femoral condyle after braced walking.
The effects of postoperative bracing on individuals with ACLR are controversial.41–43 Whereas postoperative bracing is still relatively common clinical practice, the authors41,44 of recent systematic reviews have proposed that bracing for ACLR should not be recommended routinely. Yet these reviews were based only on knee functional and joint stability outcomes.41,44 We are the first, to our knowledge, to evaluate how knee bracing affects biomechanical, biochemical, and structural outcomes related to articular cartilage health, and our results suggested that bracing may benefit some physically active individuals with ACLR who have been cleared for unrestricted physical activity. As such, we suggest future efforts to consider other benefits of postoperative bracing beyond traditional knee function and joint stability outcome measures.
Limitations to this study can inform future research. Although Pollo et al45 reported that medial unloader braces decreased medial compartment tibiofemoral loads in individuals with OA, whether this remains true for individuals with ACLR using the specific valgus unloader brace used in our study is unknown, as we did not collect or estimate contact stresses. We recruited a convenience sample of physically active individuals at 50.1 ± 36.8 months post-ACLR. The time since ACLR and baseline femoral cartilage structure have been associated with gait mechanics46,47 and may have contributed to the femoral cartilage structural response to the walking conditions. Whereas our research was not powered to run the analyses with additional covariates (eg, meniscal injury, articular cartilage lesions, sex, time since surgery or injury), we believe we have provided justification to conduct future large-scale projects that consider the influence of these covariates on the effectiveness of the intervention. Additionally, fewer steps per day have been associated with the cartilage response to load.48 Future authors should evaluate the effect of physical activity, time since ACLR, and baseline femoral cartilage structure on cartilage response to load. Further, we did not obtain information regarding concomitant injuries (eg, meniscal and chondral injures) from the surgical records. Given the established associations between concomitant injury and articular cartilage health,49 determining if concomitant injury influences changes after braced walking will be beneficial. We did not assess compartment-specific changes; researchers may seek to identify if personalized evaluation of cartilage changes and individualized varus versus valgus unloader bracing improves the biomechanical, biochemical, and structural responses to bracing. Additionally, we measured only the immediate and acute changes after bracing, and it is possible that alterations may be more pronounced after a longer time postwalking (eg, 30 minutes postwalking) or repeated brace wear. Moreover, because serum COMP also originates from anatomic sources outside of the knee-joint capsule,50 we were unable to determine the extent to which the change in serum COMP specifically represented the knee COMP response. Quantifying COMP directly from the knee synovial fluid would provide a more specific measure of COMP changes in the involved joint tissue. However, knee aspirations in noneffused joints are often unreliable, making these measurements unfeasible for this study. Furthermore, serum COMP is associated with the development of knee OA,51 and the acute change in serum COMP is the most common biomarker used to measure an acute response to lower extremity loading.12,14,15,21,22,52–56 As such, we chose to analyze serum COMP to represent the biochemical response to load. The time range from ACLR to participation was long (9–146 months post-ACLR) and could have influenced individuals’ responses to bracing. Therefore, future researchers should consider controlling for the time post-ACLR. Whether another device (eg, a compression strap around the thigh and shank) could be used to alter walking-gait biomechanics and CSA in individuals with ACLR also remains unknown. Finally, given the nature of the study, which prioritized evaluating the immediate effects of bracing on CSA and COMP responses, we were unable to assess nonbraced biomechanics after the 3000-step braced walking protocol. Walking with the brace for 3000 steps may have amplified or altered the gait changes described herein and should be taken into account when drawing conclusions and designing future studies. Overall, further investigation is needed to determine the effect of bracing during walking on structural femoral cartilage changes in individuals post-ACLR.
CONCLUSIONS
Walking with a 3-point bending brace resulted in differences in sagittal-plane gait biomechanics as well as decreased %ΔCOMP in a subset of individuals who demonstrated an increase in serum COMP concentrations after nonbraced walking. Braced walking did not cause changes in %ΔCSA in the anterior femoral articular cartilage compared with nonbraced walking. Our results indicated that walking with a brace may improve walking biomechanics in individuals who are active and cleared for unrestricted physical activity. Also, a subset of these individuals who displayed an increase in serum COMP after nonbraced walking exhibited less %ΔCOMP during braced walking, which may reflect lesser cartilage breakdown or turnover in individuals post-ACLR. It remains unknown if similar responses to bracing would be found in the acute stages of rehabilitation.
SUPPLEMENTAL MATERIAL
Supplemental Figure. A, External knee-adduction moments and, B, mean difference curve of the braced and nonbraced conditions. A, Mean ensemble waveforms plotted over the stance phase of walking for mean external knee-adduction moment for both the braced and nonbraced conditions. B, Corresponding pairwise comparison functions and associated 95% CIs (gray bands) indicating the mean differences between the braced and nonbraced conditions. Differences between conditions exist when the 95% CIs do not overlap zero.
Found at DOI: https://doi.org/10.4085/700.20.S1
Supplemental Table. Correlation Between Changes in Discrete Biomechanical Variables and Changes to Both the Cartilage Oligomeric Matrix Protein and Femoral Cartilage Cross-Sectional Area Responses.
Found at DOI: http://dx.doi.org/10.4085/1062-6050-0700.20.S2
REFERENCES
- 1. Barenius B, Ponzer S, Shalabi A, Bujak R, Norlén L, Eriksson K. Increased risk of osteoarthritis after anterior cruciate ligament reconstruction: a 14-year follow-up study of a randomized controlled trial. Am J Sports Med. 2014;42((5)):1049–1057. doi: 10.1177/0363546514526139. [DOI] [PubMed] [Google Scholar]
- 2. Davis HC, Pfeiffer SJ, Johnston CD, et al. Walking biomechanics six and twelve months following anterior cruciate ligament reconstruction compared to healthy controls Med Sci Sports Exerc 2019. 51 (Suppl 6) 265. 10.1249/01.mss.0000561300.58804.30 [DOI] [Google Scholar]
- 3. Farrokhi S, O’Connell M, Gil AB, Sparto PJ, Fitzgerald GK. Altered gait characteristics in individuals with knee osteoarthritis and self-reported knee instability. J Orthop Sports Phys Ther. 2015;45((5)):351–359. doi: 10.2519/jospt.2015.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pietrosimone B, Blackburn JT, Harkey MS, et al. Greater mechanical loading during walking is associated with less collagen turnover in individuals with anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44((2)):425–432. doi: 10.1177/0363546515618380. [DOI] [PubMed] [Google Scholar]
- 5. Pietrosimone B, Loeser RF, Blackburn JT, et al. Biochemical markers of cartilage metabolism are associated with walking biomechanics six-months following anterior cruciate ligament reconstruction. J Orthop Res. 2017;35((10)):2288–2297. doi: 10.1002/jor.23534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pfeiffer SJ, Spang J, Nissman D, et al. Gait mechanics and T1ρ MRI of tibiofemoral cartilage 6 months after ACL reconstruction. Med Sci Sports Exerc. 2019;51((4)):630–639. doi: 10.1249/MSS.0000000000001834. [DOI] [PubMed] [Google Scholar]
- 7. Pietrosimone B, Seeley MK, Johnston C, Pfeiffer SJ, Spang JT, Blackburn JT. Walking ground reaction force post-ACL reconstruction: analysis of time and symptoms. Med Sci Sports Exerc. 2019;51((2)):246–254. doi: 10.1249/MSS.0000000000001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chu CR, Andriacchi TP. Dance between biology, mechanics, and structure: a systems-based approach to developing osteoarthritis prevention strategies. J Orthop Res. 2015;33((7)):939–947. doi: 10.1002/jor.22817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petersen W, Ellermann A, Zantop T, et al. Biomechanical effect of unloader braces for medial osteoarthritis of the knee: a systematic review (CRD 42015026136) Arch Orthop Trauma Surg. 2016;136((5)):649–656. doi: 10.1007/s00402-015-2388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramsey DK, Russell ME. Unloader braces for medial compartment knee osteoarthritis: implications on mediating progression. Sports Health. 2009;1((5)):416–426. doi: 10.1177/1941738109343157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pfeiffer SJ, Valentine JA, Goodwin JS, Nissman DB, Blackburn T, Pietrosimone B. Effects of a knee valgus unloader brace on medial femoral articular cartilage deformation following walking in varus-aligned individuals. Knee. 2019;26((5)):1067–1072. doi: 10.1016/j.knee.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 12. Herger S, Vach W, Liphardt A-M, Egloff C, Nüesch C, Mündermann A. Dose-response relationship between ambulatory load magnitude and load-induced changes in COMP in young healthy adults. Osteoarthritis Cartilage. 2019;27((1)):106–113. doi: 10.1016/j.joca.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 13. Erhart-Hledik JC, Favre J, Asay JL, et al. A relationship between mechanically-induced changes in serum cartilage oligomeric matrix protein (COMP) and changes in cartilage thickness after 5 years. Osteoarthritis Cartilage. 2012;20((11)):1309–1315. doi: 10.1016/j.joca.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 14. Luc-Harkey BA, Franz J, Hackney AC, et al. Immediate biochemical changes after gait biofeedback in individuals with anterior cruciate ligament reconstruction. J Athl Train. 2020;55((10)):1106–1115. doi: 10.4085/1062-6050-0372.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hyldahl RD, Evans A, Kwon S, et al. Running decreases knee intra-articular cytokine and cartilage oligomeric matrix concentrations: a pilot study. Eur J Appl Physiol. 2016;116((11–12)):2305–2314. doi: 10.1007/s00421-016-3474-z. [DOI] [PubMed] [Google Scholar]
- 16. Pfeiffer SJ, Davis-Wilson HC, Pexa B, et al. Assessing step count–dependent changes in femoral articular cartilage using ultrasound. J Ultrasound Med. 2020;39((5)):957–965. doi: 10.1002/jum.15180. [DOI] [PubMed] [Google Scholar]
- 17. Harkey MS, Blackburn JT, Hackney AC, et al. Comprehensively assessing the acute femoral cartilage response and recovery after walking and drop-landing: an ultrasonographic study. Ultrasound Med Biol. 2018;44((2)):311–320. doi: 10.1016/j.ultrasmedbio.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 18. Harkey MS, Blackburn JT, Davis H, Sierra-Arévalo L, Nissman D, Pietrosimone B. Ultrasonographic assessment of medial femoral cartilage deformation acutely following walking and running. Osteoarthritis Cartilage. 2017;25((6)):907–913. doi: 10.1016/j.joca.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 19. Neidhart M, Hauser N, Paulsson M, DiCesare PE, Michel BA, Häuselmann HJ. Small fragments of cartilage oligomeric matrix protein in synovial fluid and serum as markers for cartilage degradation. Br J Rheumatol. 1997;36((11)):1151–1160. doi: 10.1093/rheumatology/36.11.1151. [DOI] [PubMed] [Google Scholar]
- 20. Lu TW, Lin HC, Hsu HC. Influence of functional bracing on the kinetics of anterior cruciate ligament-injured knees during level walking. Clin Biomech (Bristol, Avon) 2006;21((5)):517–524. doi: 10.1016/j.clinbiomech.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 21. Luc-Harkey BA, Franz J, Hackney AC, Blackburn JT, Padua DA. Immediate biochemical changes after gait biofeedback in individuals with anterior cruciate ligament reconstruction. J Athl Train. 2020;55((10)):1106–1115. doi: 10.4085/1062-6050-0372.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luc-Harkey BA, Franz JR, Hackney AC, Blackburn JT, Padua DA, Pietrosimone B. Lesser lower extremity mechanical loading associates with a greater increase in serum cartilage oligomeric matrix protein following walking in individuals with anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon) 2018;60:13–19. doi: 10.1016/j.clinbiomech.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 23. Luc-Harkey BA, Harkey MS, Stanley LE, Blackburn JT, Padua DA, Pietrosimone B. Sagittal plane kinematics predict kinetics during walking gait in individuals with anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon) 2016;39:9–13. doi: 10.1016/J.CLINBIOMECH.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 24. Bell AL, Brand RA, Pedersen DR. Prediction of hip joint centre location from external landmarks. Hum Mov Sci. 1989;8((1)):3–16. doi: 10.1016/0167-9457(89)90020-1. [DOI] [Google Scholar]
- 25. Hackney AC, Viru A. Research methodology: endocrinologic measurements in exercise science and sports medicine. J Athl Train. 2008;43((6)):631–639. doi: 10.4085/1062-6050-43.6.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park J, Seeley MK, Francom D, Reese CS, Hopkins JT. Functional vs. traditional analysis in biomechanical gait data: an alternative statistical approach. J Hum Kinet. 2017;60:39–49. doi: 10.1515/hukin-2017-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Son SJ, Kim H, Seeley MK, Hopkins JT. Movement strategies among groups of chronic ankle instability, coper, and control. Med Sci Sports Exerc. 2017;49((8)):1649–1661. doi: 10.1249/MSS.0000000000001255. [DOI] [PubMed] [Google Scholar]
- 28. Luc-Harkey BA, Franz JR, Blackburn JT, Padua DA, Hackney AC, Pietrosimone B. Real-time biofeedback can increase and decrease vertical ground reaction force, knee flexion excursion, and knee extension moment during walking in individuals with anterior cruciate ligament reconstruction. J Biomech. 2018;76:94–102. doi: 10.1016/J.JBIOMECH.2018.05.043. [DOI] [PubMed] [Google Scholar]
- 29. Hart HF, Crossley KM, Ackland DC, Cowan SM, Collins NJ. Effects of an unloader knee brace on knee-related symptoms and function in people with post-traumatic knee osteoarthritis after anterior cruciate ligament reconstruction. Knee. 2016;23((1)):85–90. doi: 10.1016/j.knee.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 30. Cook TM, Farrell KP, Carey IA, Gibbs JM, Wiger GE. Effects of restricted knee flexion and walking speed on the vertical ground reaction force during gait. J Orthop Sports Phys Ther. 1997;25((4)):236–244. doi: 10.2519/jospt.1997.25.4.236. [DOI] [PubMed] [Google Scholar]
- 31. Wellsandt E, Gardinier ES, Manal K, Axe MJ, Buchanan TS, Snyder-Mackler L. Decreased knee joint loading associated with early knee osteoarthritis after anterior cruciate ligament injury. Am J Sports Med. 2016;44((1)):143–151. doi: 10.1177/0363546515608475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Davis-Wilson H, Pfeiffer SJ, Johnston CD, et al. Bilateral gait 6 and 12 months post-ACL reconstruction compared to controls. Med Sci Sports Exerc. 2020;52((4)):785–794. doi: 10.1249/MSS.0000000000002208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Noehren B, Wilson H, Miller C, Lattermann C. Long-term gait deviations in anterior cruciate ligament-reconstructed females. Med Sci Sports Exerc. 2013;45((7)):1340–1347. doi: 10.1249/MSS.0b013e318285c6b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kaur M, Ribeiro DC, Theis J-C, Webster KE, Sole G. Movement patterns of the knee during gait following ACL reconstruction: a systematic review and meta-analysis. Sports Med. 2016;46((12)):1869–1895. doi: 10.1007/s40279-016-0510-4. [DOI] [PubMed] [Google Scholar]
- 35. Slater LV, Hart JM, Kelly AR, Kuenze CM. Progressive changes in walking kinematics and kinetics after anterior cruciate ligament injury and reconstruction: a review and meta-analysis. J Athl Train. 2017;52((9)):847–860. doi: 10.4085/1062-6050-52.6.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sharma L, Song J, Dunlop D, et al. Varus and valgus alignment and incident and progressive knee osteoarthritis. Ann Rheum Dis. 2010;69((11)):1940–1945. doi: 10.1136/ard.2010.129742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Erhart-Hledik JC, Favre J, Andriacchi TP. New insight in the relationship between regional patterns of knee cartilage thickness, osteoarthritis disease severity, and gait mechanics. J Biomech. 2015;48((14)):3868–3875. doi: 10.1016/j.jbiomech.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 38. Teng H-L, Wu D, Su F, et al. Gait characteristics associated with a greater increase in medial knee cartilage T1ρ and T2 relaxation times in patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45((14)):3262–3271. doi: 10.1177/0363546517723007. [DOI] [PubMed] [Google Scholar]
- 39. Chang AH, Moisio KC, Chmiel JS, et al. External knee adduction and flexion moments during gait and medial tibiofemoral disease progression in knee osteoarthritis. Osteoarthritis Cartilage. 2015;23((7)):1099–1106. doi: 10.1016/j.joca.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Swärd P, Kostogiannis I, Neuman P, Von Porat A, Boegård T, Roos H. Differences in the radiological characteristics between post-traumatic and non-traumatic knee osteoarthritis. Scand J Med Sci Sport. 2010;20((5)):731–739. doi: 10.1111/j.1600-0838.2009.01000.x. [DOI] [PubMed] [Google Scholar]
- 41. Rodríguez-Merchán EC. Knee bracing after anterior cruciate ligament reconstruction. Orthopedics. 2016;39((4)):e602–e609. doi: 10.3928/01477447-20160513-04. [DOI] [PubMed] [Google Scholar]
- 42. Chen Z, Liu L, Xiao T. Letter to the editor regarding “Knee bracing after anterior cruciate ligament reconstruction.” Orthopedics 2017. 40(2) 70. 10.3928/01477447-20170302-01 [DOI] [PubMed] [Google Scholar]
- 43. Rodríguez-Merchán EC. Response to a letter to the editor regarding “Knee bracing after anterior cruciate ligament reconstruction.”. Orthopedics. 2016;39((4)):e602–e609. doi: 10.3928/01477447-20170302-01. [DOI] [PubMed] [Google Scholar]
- 44. Yang X-G, Feng J-T, He X, Wang F, Hu Y-C. The effect of knee bracing on the knee function and stability following anterior cruciate ligament reconstruction: a systematic review and meta-analysis of randomized controlled trials. Orthop Traumatol Surg Res. 2019;105((6)):1107–1114. doi: 10.1016/j.otsr.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 45. Pollo FE, Otis JC, Backus SI, Warren RF, Wickiewicz TL. Reduction of medial compartment loads with valgus bracing of the osteoarthritic knee. Am J Sports Med. 2002;30((3)):414–421. doi: 10.1177/03635465020300031801. [DOI] [PubMed] [Google Scholar]
- 46. Pamukoff DN, Montgomery MM, Holmes SC, Moffit TJ, Garcia SA, Vakula MN. Association between gait mechanics and ultrasonographic measures of femoral cartilage thickness in individuals with ACL reconstruction. Gait Posture. 2018;65:221–227. doi: 10.1016/j.gaitpost.2018.07.174. [DOI] [PubMed] [Google Scholar]
- 47. Goetschius J, Hertel J, Saliba SA, Brockmeier SF, Hart JM. Gait biomechanics in anterior cruciate ligament-reconstructed knees at different time frames postsurgery. Med Sci Sports Exerc. 2018;50((11)):2209–2216. doi: 10.1249/MSS.0000000000001693. [DOI] [PubMed] [Google Scholar]
- 48. Davis-Wilson H, Johnston CD, Evans-Pickett A, et al. Fewer steps per day associates with greater cartilage breakdown biomarkers post anterior cruciate ligament reconstruction Med Sci Sports Exerc 2020. 52(7S) 246. 10.1249/01.mss.0000676256.25165.8d [DOI] [Google Scholar]
- 49. Luc B, Gribble PA, Pietrosimone BG. Osteoarthritis prevalence following anterior cruciate ligament reconstruction: a systematic review and numbers-needed-to-treat analysis. J Athl Train. 2014;49((6)):806–819. doi: 10.4085/1062-6050-49.3.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Müller G, Michel A, Altenburg E. COMP (cartilage oligomeric matrix protein) is synthesized in ligament, tendon, meniscus, and articular cartilage. Connect Tissue Res. 1998;39((4)):233–244. doi: 10.3109/03008209809021499. [DOI] [PubMed] [Google Scholar]
- 51. Hoch JM, Mattacola CG, Medina McKeon JM, Howard JS, Lattermann C. Serum cartilage oligomeric matrix protein (sCOMP) is elevated in patients with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2011;19((12)):1396–1404. doi: 10.1016/j.joca.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Verma P, Dalal K. Serum cartilage oligomeric matrix protein (COMP) in knee osteoarthritis: a novel diagnostic and prognostic biomarker. J Orthop Res. 2013;31((7)):999–1006. doi: 10.1002/jor.22324. [DOI] [PubMed] [Google Scholar]
- 53. Celik O, Salci Y, Ak E, Kalaci A, Korkusuz F. Serum cartilage oligomeric matrix protein accumulation decreases significantly after 12 weeks of running but not swimming and cycling training - a randomised controlled trial. Knee. 2013;20((1)):19–25. doi: 10.1016/j.knee.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 54. Denning WM, Becker Pardo M, Winward JG, et al. Ambulation speed and corresponding mechanics are associated with changes in serum cartilage oligomeric matrix protein. Gait Posture. 2016;44:131–136. doi: 10.1016/j.gaitpost.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 55. Andersson MLE, Thorstensson CA, Roos EM, Petersson IF, Heinegård D, Saxne T. Serum levels of cartilage oligomeric matrix protein (COMP) increase temporarily after physical exercise in patients with knee osteoarthritis BMC Musculoskelet Disord 2006. 7 98. 10.1186/1471-2474-7-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chehab EF, Titchenal MR, Asay JL, Andriacchi TP. Gait characteristics that influence cartilage thickness are related to serum concentrations of cartilage oligomeric matrix protein before and after a mechanical stimulus. Osteoarthritis Cartilage. 2015;23((Suppl 2)):A90–A91. doi: 10.1016/j.joca.2015.02.798. [DOI] [Google Scholar]