Abstract
Background:
Many patients with inflammatory bowel disease (IBD) have transitioned from an infliximab originator to a biosimilar. However, some patients retransition to the originator (i.e. stop biosimilar and reinitiate the originator). Whether this sign of potential unsatisfactory treatment response is specifically related to the infliximab biosimilar or the patient and/or the disease including patients’ beliefs on the biosimilar is unclear.
Objectives:
We aimed to compare the risk of and reasons for infliximab discontinuation between retransitioned patients and those remaining on biosimilar.
Design:
Non-interventional, multicentre cohort study.
Methods:
IBD patients who transitioned from infliximab originator to biosimilar between January 2015 and September 2019 in two Dutch hospitals were eligible for this study. Retransitioned patients (retransitioning cohort) were matched with patients remaining on biosimilar (biosimilar remainder cohort). Reasons for discontinuation were categorised as the unwanted response (i.e. loss of effect or adverse events) or remission. Risk of unwanted discontinuation was compared using Cox proportional hazards models.
Results:
Patients in the retransitioning cohort (n = 44) were younger (median age 39.9 versus 44.0 years), more often female (65.9% versus 48.9%) and had shorter dosing intervals (median 48.5 versus 56.0 days) than in the biosimilar remainder cohort (n = 127). Infliximab discontinuation due to unwanted response was 22.7% in the retransitioning and 13.4% in the biosimilar remainder cohort, and due to remission was 2.3% and 9.4%, respectively. Retransitioned patients are at increased risk of discontinuing due to unwanted response compared with biosimilar remainder patients (adjusted HR 3.7, 95% CI: 1.0–13.9). Patients who retransitioned due to an increase in objective disease markers had higher discontinuation rates than patients who retransitioned due to symptoms only (66.7% versus 23.7%).
Conclusion:
Retransitioned patients are at increased risk of infliximab discontinuation due to unwanted response. Retransitioning appeared related to the patient and/or disease and not the product. Clinicians might switch patients opting for retransitioning to other treatment regimens.
Keywords: biosimilar, inflammatory bowel disease, infliximab
Introduction
Tumour necrosis factor (TNF)α inhibitors have enriched the treatment of patients with inflammatory bowel disease (IBD). These agents made clinical and endoscopic remission realistic treatment targets.1,2 However, the drawback of treatment with TNFα inhibitors used to be their high price compared with conventional treatment, such as thiopurines, and this has placed a financial burden on health care systems and limited patients’ access. Several years ago, the loss of market exclusivity for some of these blockbusters resulted in the introduction of biosimilars and thus in lower prices with improved patient access. A biosimilar is a ‘biological medicinal product that contains a version of the active substance of an already authorised biological medicinal product (originator)’. 3 Biosimilars approved by the European Medicines Agency have proven to be as safe and effective as the originators, and are considered interchangeable with their corresponding originators, meaning that a biosimilar can be used instead of its originator. 4
In 2014, the first infliximab biosimilar entered the European market. 5 Since then, many patients treated with originator infliximab in clinical practice have transitioned to the biosimilar, mainly because the biosimilar was lower-priced.6,7 When transitioning from originator to biosimilar, patients are still treated with the molecule infliximab. Thus, transitioning differs from switching to another biological (with another active substance), for example when patients have an inadequate response to infliximab.
Transitioning has been proven effective and safe in double-blind studies, such as the NOR-SWITCH study, which reported that the incidence of disease worsening in the transitioned patients [36.5% for Crohn’s disease (CD) and 11.9% for ulcerative colitis (UC)] was more frequent, but within the predefined absolute margin set for non-inferiority of 15% to the incidence in patients who remained on originator (21.2% for CD and 9.1% for UC). 8
Despite the fact that many patients in clinical practice successfully transitioned from infliximab originator to biosimilar, studies have demonstrated that on average 7% of patients with IBD who transitioned subsequently retransitioned to originator infliximab (i.e. they stopped the biosimilar and reinitiated the originator). 9 Retransitioning is mainly due to either a perceived or objective increase in disease activity or the occurrence of (subjective) adverse events after transitioning to the biosimilar.10–12 However, no clear pharmacotherapeutic rationale exists for retransitioning, and furthermore, it is not recommended in clinical guidelines. 6
After retransitioning to the originator, patients could regain efficacy or adverse events could resolve, which might indicate that they have experienced the nocebo effect. The nocebo effect refers to ‘an unexplained, unfavourable therapeutic effect subsequent to a non-medical switch from originator infliximab to biosimilar infliximab with regaining of the beneficial effects after reinitiating the originator’. 13 Retransitioning could also be related to a general lack of confidence in biosimilars by patients and/or prescribers.14,15 Thus, it is unclear if retransitioning is related to the drug product or to the patient and his/her disease.
The aim of this study was to compare the risk of and reasons for infliximab discontinuation between patients who retransitioned to the originator and those who remained on biosimilar in a study base of patients with IBD who had transitioned from infliximab originator to the corresponding biosimilar.
Method
Setting
This study was conducted at two large teaching hospitals in the Netherlands: Spaarne Gasthuis (SG; Haarlem and Hoofddorp, The Netherlands) and Medisch Spectrum Twente (MST; Enschede, the Netherlands). SG has 601 beds, 34,000 clinical hospitalisations and 160 IBD patients treated with biologicals annually. MST has 547 beds, 30,000 clinical hospitalisations and 230 IBD patients treated with biologicals annually.
On the 1st of January 2012, reimbursement regulations were implemented in the Netherlands that required all outpatient-administered biologicals registered for IBD to be exclusively dispensed by the outpatient pharmacies of the hospitals where the patients are treated. All in-hospital administered biologicals are dispensed by the hospital pharmacy and administered at the day-care clinic. Consequently, the hospital pharmacy has a complete overview of all biologicals used by a patient with IBD. 16
Dispensing data (brand name, ATC code, dose, dosing interval and dispensing date) from the outpatient pharmacy (outpatient used medication) from SG and MST were obtained from the outpatient pharmacy system CompuGroup Medical (CGM; Echt, the Netherlands). Dispensing data [brand name, anatomical therapeutic chemical (ATC) code, dose, administration date, indication and prescriber] and patient information (gender and date of birth) from SG hospital pharmacy (in-hospital administered medication) were obtained from the pharmacy information system Centrasys (Nexus; Vianen, the Netherlands). Prescription data (brand name, ATC code, dose, administration date and prescriber) and patient information (gender and date of birth) from MST were obtained from Vipharma (HI Systems; Oosterhout, the Netherlands), the hospital’s electronic prescription system (in-hospital administered medication). Indication, reasons for retransitioning to infliximab originator and discontinuing infliximab treatment were obtained by manually searching electronic patient files from Epic (Epic; Verona, WI, USA) (SG) and Hix (ChipSoft; Amsterdam, the Netherlands) (MST). Outpatient and in-hospital data were linked using patients’ social security number (SG) or unique patient identification number (MST).
The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology statement 17 (Supplemental File 1).
Study design and patients
This was a matched cohort study in a study base of patients with IBD who had transitioned from infliximab originator (Remicade) to infliximab biosimilar (including Remsima, Inflectra and Flixabi) between 1 January 2015 and 30 September 2019. In the Netherlands, transitioning patients from infliximab originator to biosimilar is controlled by the individual hospitals, and transitioning is directed by treating physicians and hospital pharmacists. Patients were informed on the transition by the treating gastroenterologist or the IBD nurse. In principle, all patients with IBD treated with infliximab were eligible for transitioning. Transitioning was strongly encouraged, but not mandatory. Patients could object to transitioning and then remained treated with originator infliximab. The latter group was not included in this study. The date of transitioning was assigned as the patient’s transition date. Patients with less than 1 year of follow-up from the transition date were excluded.
From this study base, all patients who retransitioned during the study period to the originator were identified and included in the retransitioning cohort. Retransitioning from infliximab biosimilar to originator was defined as having at least one dispensing of the originator following transitioning, thus after having at least one dispensing of the biosimilar. To ensure retransitioning was intended, and not due to, for example, an accidental prescription error, the electronic health record (EHR) file notes of the patients were checked. If retransitioning was also mentioned in the file notes, patients were considered to be retransitioned. If there was not any mention, patients were considered as solely transitioned. For the patients in the retransitioning cohort, the date of retransitioning was assigned as their index date.
Reasons for retransitioning were extracted from the EHR file notes and were classified as loss of effect, adverse events (potentially) related to infliximab treatment, remission, other or unknown. Loss of effect included increased calprotectin, gastrointestinal complaints including abdominal pains, changes in defaecation (frequency and/or composition) and intestinal complaints and loss of effect in general. Adverse events were further subdivided into skin complaints including redness, eczema, psoriasis, itching and hives; joint complaints including joint pains and stiffness, fatigue or other adverse events.
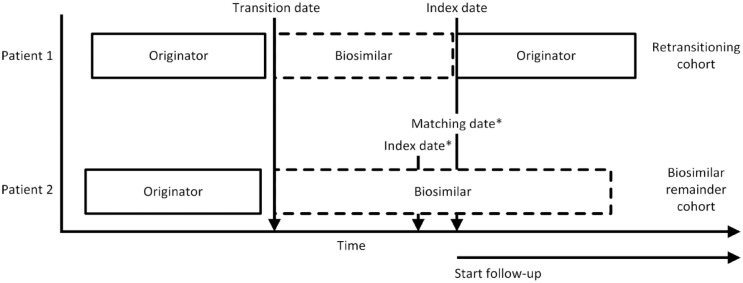
Retransitioned patients were matched with up to three patients 18 from the study base who had transitioned from originator to biosimilar and not retransitioned. These patients formed the biosimilar remainder cohort. Patients in the biosimilar remainder cohort could only be matched once to a patient in the retransitioning cohort. 19 Retransitioned patients who could not be matched were excluded. Matching was performed based on the following criteria: (i) treatment in the same hospital, as treatment policies may differ between hospitals; (ii) transition date in the same 6-month calendar period, accounting for changes in treatment policies and treatment options over time; and (iii) duration of biosimilar use from transition date: patients were matched on the duration of biosimilar use, 20 defined as the time from transition date until the match date, as depicted in Figure 1 where patient 2 is matched with patient 1. Patient 1 received infliximab on the index date, thus the patient cannot discontinue infliximab for the next 8 weeks (standard infliximab dosing interval 21 ). To account for this in the biosimilar remainder cohort, their index date was set on their infliximab administration date closest prior to the match date.
Figure 1.
Matching of patients.
*For patients in the biosimilar remainder cohort, the infliximab biosimilar administration date closest prior to the matching date was assigned as their index date.
Patients were followed from their index date until discontinuation of biological treatment, censoring, death, loss to follow-up, or the end of data collection (30 September 2020), whichever came first. In case retransitioned patients were accidentally reintroduced on the biosimilar without any mention of this change in their EHR file notes, this was considered a prescription error and these patients were still considered retransitioned and continuing their infliximab originator treatment.
Outcomes
The primary outcome of this study was infliximab treatment discontinuation. To identify discontinuation, treatment episodes of infliximab treatment were first constructed for each patient. A treatment episode was defined as the time between the first infliximab administration until the last administration. A maximum gap of 8 weeks between the theoretical end date of the previous administration and the next one was permitted to account for small adjustments in dosing schedules for non-medical reasons (e.g. holidays). Patients were considered to have discontinued infliximab treatment if they did not receive an infliximab administration within the maximum permissible gap (total of 16 weeks after the date of the last administration, considering a standard dosing interval of 8 weeks 21 ).
We specifically assessed retransitioned patients by analysing whether the reason for retransitioning affected infliximab discontinuation rates, and if discontinued patients either switched to other biological treatment or discontinued biological treatment. The retransitioning cohort was divided into two cohorts according to the patients’ reason for retransitioning: objective disease markers (elevated calprotectin and/or active disease seen on endoscopy) or only (subjective) symptoms, without changes in objective disease markers.
Reasons for discontinuing were extracted from the EHR file notes and were classified according to the same classification as for reasons for retransitioning described earlier.
Potential confounders
Age, gender, duration of use of infliximab originator prior to transitioning (1 year or less, or more than 1 year 22 ), and the number of other biologicals that a patient used before initiating treatment with infliximab were assessed as potential confounders.22,23
Data analysis
The baseline characteristics of the patients were descriptively analysed. Reasons for retransitioning from biosimilar to originator infliximab were plotted in pie charts and reasons for discontinuing infliximab treatment were plotted in stacked bar charts. Reasons for discontinuing were classified as either due to remission or due to unwanted response, including loss of effect, adverse events and other unwanted responses. In the following analysis, discontinuing due to unwanted response was analysed, thus patients discontinuing due to remission were censored at the time of discontinuation. Kaplan–Meier curves were used to present the risk of overall infliximab treatment discontinuation, infliximab discontinuation due to remission and due to unwanted response for both cohorts. The hazard ratio (HR) of infliximab discontinuation was calculated using unadjusted and adjusted conditional Cox proportional hazards models. The model was adjusted for the aforementioned potential confounders summarising these in a propensity score and including this in the analysis. The data were analysed using R version 3.6.3 (R Foundation for Statistical Computing; Vienna, Austria).
Results
In total, 198 patients who had at least 1 year of follow-up transitioned from infliximab originator to the biosimilar. These patients had a median age of 39.9 years and 53.0% were female. Of the 198 patients who transitioned, 49 patients (24.7%) retransitioned to originator infliximab during follow-up. Retransitioning occurred after a median (interquartile range; IQR) of 8.6 (3.7–14.0) months after transitioning. There were no major differences between the two included hospitals.
After matching, the retransitioning cohort comprised 44 patients and the biosimilar remainder cohort comprised 127 patients; 2 patients from the retransitioning cohort could only be matched with 1 patient, while 1 patient from the retransitioning cohort could only be matched with 2 patients. Five retransitioned patients could not be matched with any patient; therefore, these patients were excluded from the retransitioning cohort. These patients were transitioned in calendar periods with an insufficient amount of biosimilar remainder patients to match all retransitioned patients.
The retransitioning and biosimilar remainder cohorts had some differences in baseline characteristics; patients in the retransitioning cohort were younger (median 39.9 versus 44.0 years in the biosimilar remainder cohort), were more often female (65.9% versus 48.9%), and had a shorter median dosing interval than patients in the biosimilar remainder cohort [48.5 days (IQR: 42–56) days versus 56 days (IQR: 45–56)], as depicted in Table 1.
Table 1.
Baseline characteristics of the retransitioning cohort and the biosimilar remainder cohort.
| Baseline characteristics | Retransitioning cohort | Biosimilar remainder cohort |
|---|---|---|
| n = 44 | n = 127 | |
| Median (IQR) age at transitioning (years) | 39.9 (28.4–52.8) | 44.0 (31.8–57.7) |
| Females (%) | 29 (65.9%) | 62 (48.9%) |
| Hospital | ||
| Medisch Spectrum Twente | 29 (65.9%) | 84 (66.2%) |
| Spaarne Gasthuis | 15 (34.1%) | 43 (33.8%) |
| Disease | ||
| Crohn’s disease | 25 (56.8%) | 75 (59.0%) |
| Ulcerative colitis | 12 (27.2%) | 34 (26.8%) |
| Unknown | 7 (16.0%) | 18 (14.2%) |
| Median (IQR) duration of infliximab originator prior to transitioning (years) | 4.6 (2.3–4.9) | 3.7 (2.5–4.8) |
| Median (IQR) dose at index date (mg) | 400 (300–500) | 400 (350–500) |
| Median (IQR) dosing interval at index date (days) | 48.5 (42–56) | 56 (45–56) |
| Biologicals prior to transitioning | ||
| Only infliximab | 34 (77.3%) | 114 (89.8%) |
| Adalimumab | 8 (18.1%) | 8 (6.3%) |
| Ustekinumab | 1 (2.3%) | 4 (3.1%) |
| Adalimumab and golimumab | 0 | 1 (0.8%) |
| Adalimumab and ustekinumab | 1 (2.3%) | 0 |
| Median (IQR) follow-up (years) | 2.8 (2.4–3.2) | 2.9 (2.4–3.2) |
IQR, interquartile range.
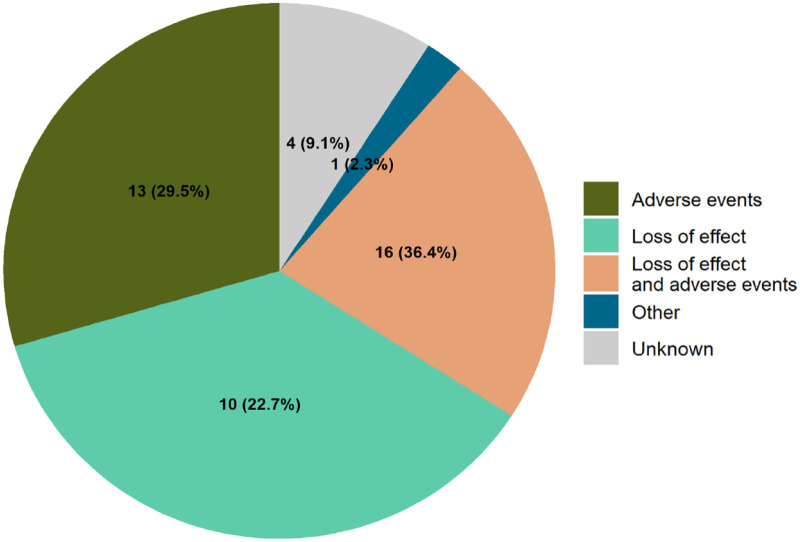
The main reasons for patients to retransition were loss of effect (36.4%), adverse events (29.5%) or both loss of effect and adverse events (22.7%) (Figure 2). One patient (2.3%) was retransitioned due to a lack of trust in the biosimilar, this was classified as ‘other’. For the other patients (9.1%), the reason for retransitioning was not explicitly specified in their EHR file notes. The most reported adverse events were fatigue (reported by 12 patients), skin complaints (8 patients) and joint complaints (7 patients).
Figure 2.
Reasons for retransitioning from infliximab biosimilar to originator (n = 44).
Six months after the index date, none of the patients in the retransitioning cohort discontinued their infliximab treatment compared with 9.4% in the biosimilar remainder cohort, which increased to 9.1% in the retransitioning cohort and 11.8% in the biosimilar remainder cohort after 1 year, and to 25.0% in the retransitioning cohort and 22.8% in the biosimilar remainder cohort at the end of follow-up (Table 2).
Table 2.
Proportion of infliximab discontinuation of the retransitioning cohort and the biosimilar remainder cohort.
| Infliximab discontinuation | No. patients | 6 months | 1 year | End of follow-up |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Overall infliximab discontinuation | ||||
| Retransitioning cohort | 44 | 0 (0%) | 4 (9.1%) | 11 (25.0%) |
| Biosimilar remainder cohort | 127 | 12 (9.4%) | 15 (11.8%) | 29 (22.8%) |
| Discontinuation due to remission | ||||
| Retransitioning cohort | 44 | 0 (0%) | 0 (0%) | 1 (2.3%) |
| Biosimilar remainder cohort | 127 | 6 (4.7%) | 6 (4.7%) | 12 (9.4%) |
| Discontinuation due to unwanted response | ||||
| Retransitioning cohort | 44 | 0 (0%) | 4 (9.1%) | 10 (22.7%) |
| Biosimilar remainder cohort | 127 | 6 (4.7%) | 9 (7.1%) | 17 (13.4%) |
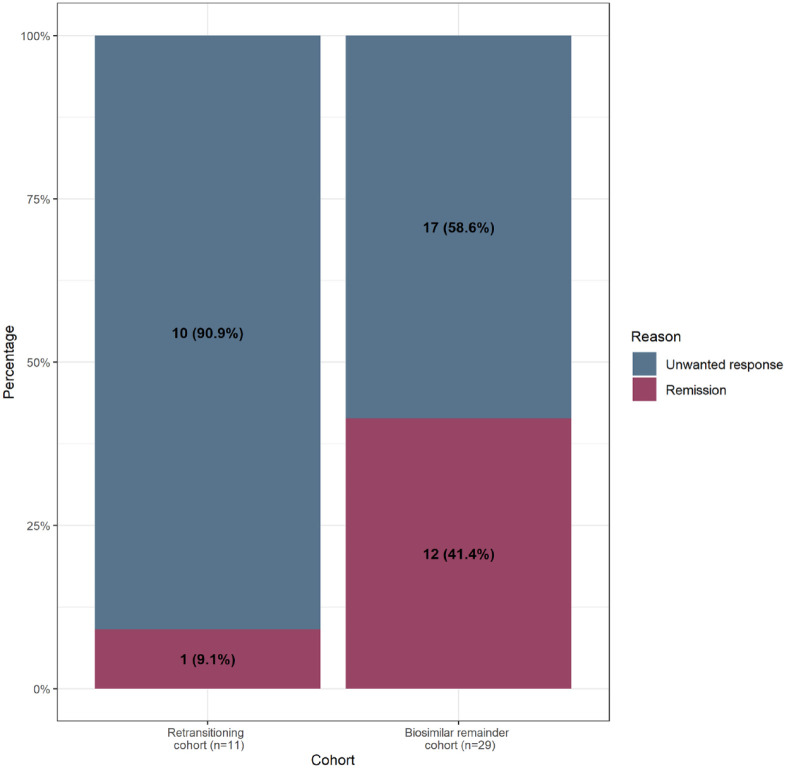
At the end of follow-up, 11 and 29 of all patients in the retransitioning cohort and the biosimilar remainder cohort, respectively, had discontinued their infliximab treatment (Table 2, Supplemental Figure S1), due to remission or unwanted response (Figure 3). Within the subgroup of patients who discontinued, patients in the biosimilar remainder cohort discontinued more often due to remission (41.4% versus 9.1%, Figure 3, Supplemental Figure S2).
Figure 3.
Overall reasons for discontinuing infliximab per cohort.
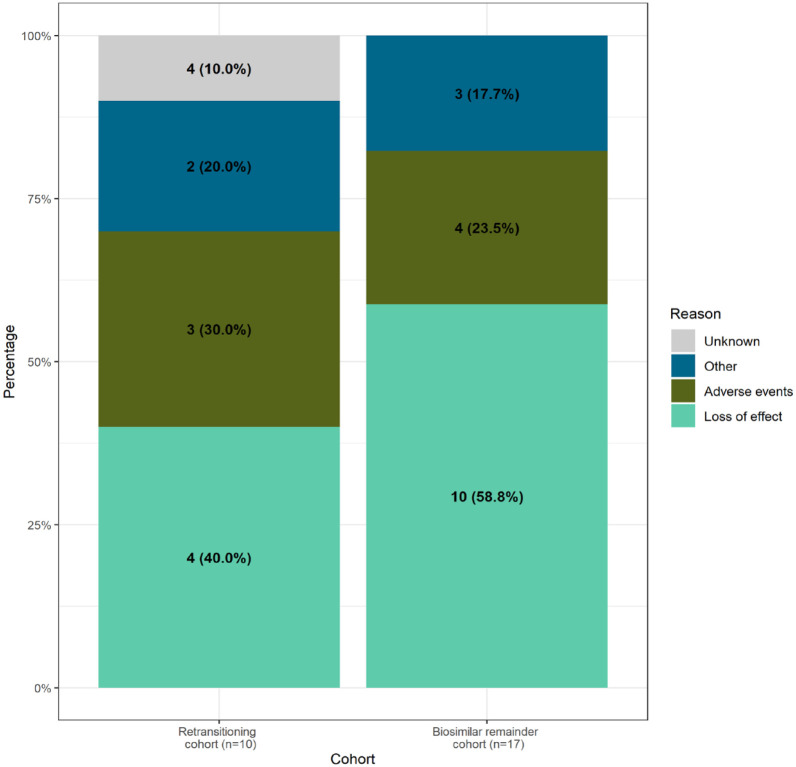
In total, 10 patients who retransitioned (22.7%) and 17 patients (13.4%) who remained on biosimilar discontinued infliximab due to unwanted response (Table 2, Figure 3). Discontinuing due to unwanted responses was further specified in Figure 4. Patients in both cohorts discontinued mainly due to loss of effect and adverse events (40.0% and 30% in the retransitioning cohort, 58.8% and 23.5% in the biosimilar remainder cohort, respectively). In total, three patients in the retransitioning cohort discontinued due to adverse events, mainly due to skin complaints (reported twice), and four patients in the biosimilar remainder cohort, categorised as other (depression, dyspnoea), skin complaints and unknown adverse event (both reported once). Other reasons for discontinuing included discontinuing due to a general loss of trust in infliximab, patients transferring from regular to alternative medicine and nonadherence to infliximab (without remission).
Figure 4.
Specification of discontinuing infliximab due to unwanted response per cohort.
Of these discontinued patients, 5 (50.0%) out of the retransition cohort switched to another biological for their IBD treatment (adalimumab, golimumab, vedolizumab or ustekinumab) and 5 (50.0%) discontinued without switching to another biological. In the biosimilar remainder cohort, 3 (17.6%) of the patients who discontinued switched to another biological and 14 (82.4%) discontinued without switching.
The cumulative incidence of discontinuation of infliximab due to unwanted response was also compared between the cohorts in a Kaplan–Meier curve (Figure 5). As the lines of the cumulative incidence curves crossed, HRs were calculated for the period prior to the lines crossing (at 11.2 months, Figure 5) and after. In both the unadjusted and adjusted Cox proportional hazard models up to 11.2 months of follow-up, patients in the retransitioning cohort had a similar risk of overall infliximab discontinuation due to an unwanted response compared with patients in the biosimilar remainder cohort (unadjusted HR 1.1, 95% CI: 0.3–4.3; adjusted HR 1.0, 95% CI: 0.3–4.2). After 11.2 months, patients in the retransitioning cohort were at increased risk for overall infliximab discontinuation (unadjusted HR 2.1, 95% CI: 0.7–6.2; adjusted HR 3.7, 95% CI: 1.0–13.9).
Figure 5.
Cumulative incidence of infliximab discontinuation due to unwanted response, the dashed vertical line at 11.2 months indicates the moment of lines crossing.
We further zoomed in on the retransitioned patients. Of the 44 retransitioned patients, for 6 (13.6%) patients changes in objective disease markers were mentioned as the reason for retransitioning. For the other 38 (86.3%) only symptoms were mentioned. Overall, infliximab continuation rates were higher in the patients who retransitioned due to symptoms only (76.3% versus 33.3% of the patients who retransitioned based on objective disease markers, Table 3).
Table 3.
Infliximab discontinuation rates in retransitioned patients (n = 44).
| Infliximab discontinuation | Retransitioned due to objective markers (n = 6) | Retransitioned due to symptoms only (n = 38) |
|---|---|---|
| Infliximab continuation | 2 (33.3%) | 29 (76.3%) |
| Overall infliximab discontinuation | 4 (66.7%) | 9 (23.7%) |
Within the subgroup of patients who discontinued infliximab treatment after retransitioning, three (75%) out of four patients who retransitioned due to objective disease markers discontinued infliximab treatment without switching to another biological. This is a higher percentage compared to the five out of nine patients (55.5%) in the subgroup of patients who retransitioned due to symptoms only (Table 4).
Table 4.
Specification of discontinuation in retransitioned patients.
| Infliximab discontinuation | Retransitioned due to objective markers (n = 4) | Retransitioned due to symptoms only (n = 9) |
|---|---|---|
| Discontinuing infliximab with switch to other biological treatment | 1 (25.0%) | 4 (44.5%) |
| Discontinuing infliximab without switch | 3 (75.0%) | 5 (55.5%) |
Discussion
In this study, we found a similar overall proportion of patients who discontinue infliximab between patients who retransitioned compared with patients who remained on biosimilars. However, patients who retransitioned discontinued infliximab treatment more often due to unwanted response compared with patients who remained on biosimilar (22.7% versus 13.4%), whereas patients who remained on biosimilar discontinued infliximab more often due to remission (9.4 versus 2.3%). Patients who retransitioned have after 11.2 months of treatment over a three-fold increased risk for discontinuing infliximab due to unwanted response compared with patients who remained on biosimilar (adjusted HR 3.7, 95% CI: 1.0–13.9). This was in contrast with the similar risk of discontinuation between the two cohorts in the first 11.2 months of treatment (adjusted HR 1.0, 95% CI: 0.3–4.2). This aligned with our finding that the proportion of patients who discontinued infliximab due to unwanted response within 1 year was moderately increased in the retransitioning cohort compared with the biosimilar remainder cohort (9.1% versus 7.1%), but this further diverged at the end of follow-up (22.7% in the retransitioning cohort versus 13.4% in the biosimilar remainder cohort).
In total, 24.7% of patients in our study who initially transitioned from originator to biosimilar subsequently retransitioned, which is much higher than the 7% reported in an earlier systematic review. 9 Studies included in the systematic review had a median follow-up of 12 months. However, as patients in our study retransitioned after a median of 8.6 (3.7–14.0) months, the long follow-up time of our study (median 3.6–years from transitioning) allowed for more patients to retransition, which shows that retransitioning might also occur after a longer period of time.
A previous study reported similar infliximab discontinuation rates between patients who retransitioned and patients who remained on biosimilar, which was under 10% in both cohorts after 1-year follow-up. 24 In this previous study, both patients who remained on biosimilar and those who retransitioned were followed from the moment of transitioning to the infliximab biosimilar. Following the latter cohort from transitioning onwards might induce immortal time bias, as these patients are not yet exposed to the originator and thus cannot discontinue originator treatment from the moment follow-up started, whereas patients who remained on the biosimilar could discontinue directly after transitioning. In our analysis, patients who retransitioned were followed from the moment of retransitioning to overcome this bias. Therefore, we believe that the method used in our study provides a less biased comparison between patients who retransitioned and patients who remained on biosimilar treatment.
Other previous studies, including between 74 and 260 patients, have described the effect of retransitioning anecdotally and with conflicting outcomes. Some studies have reported that patients who retransitioned were treated successfully with at least 2–4 administrations of infliximab originator,14,25 whereas another study has reported patients discontinuing infliximab originator shortly after retransitioning. 11 The findings in our study demonstrate that the risk for discontinuing infliximab in patients who retransitioned compared with patients who remained on biosimilar appeared to increase over time. Retransitioning is done due to complaints on the biosimilar, such as loss of effect and adverse events, intending to regain effects and/or dispose of adverse events. Thus, patients and clinicians might first try a few administrations to wait for the effect of the reintroduced originator. However, as the infliximab biosimilar is similar to the originator in terms of efficacy and safety, it is expected that these patients did not benefit from retransitioning to originator and discontinued infliximab treatment. This is supported by the finding that patients in the retransitioning cohort had used more other biologicals prior to infliximab initiation and had a shorter infliximab dosing interval, which puts them at higher risk of switching to another biological 26 and might indicate that these patients already had more disease complaints. 27 Moreover, less patients in the retransitioning cohort discontinued infliximab treatment due to remission, which also suggests less treatment benefit.
However, a subset of patients who retransitioned persisted treatment with the originator infliximab, which suggested that these patients benefitted from retransitioning. Three-quarters of the patients who retransitioned due to symptoms without objectively confirmed disease worsening continued their infliximab originator treatment. This might, although the sample size was limited, indicate that these patients experienced a nocebo effect (an unexplained, unfavourable therapeutic effect subsequent to a non-medical switch from originator to biosimilar accompanied by the regaining of beneficial effects after reinitiating the originator 13 ). Another explanation is that these patients might have attributed their complaints to the biosimilar; for example, they could coincidentally have experienced disease worsening at the time of transition. This is supported by the finding that three patients who retransitioned also increased their infliximab dose, which could also (partly) explain the regained effect.
Five patients (all from the same hospital) who retransitioned received alternately both infliximab originator and biosimilar, and since this was not mentioned in their EHR file notes, this could have been due to prescribing errors. When these patients were consciously transitioned to infliximab biosimilar, they experienced complaints such as fatigue, abdominal pains and changes in defaecation. However, when they were unconsciously alternating originator and biosimilar, no complaints were mentioned in their dossiers, indicating that these patients alternated between infliximab originator and biosimilar without any reported issues. Despite the number of these patients is low, this finding might indicate that consciously transitioning from originator to biosimilar induces complaints in certain patients.
As the route of administration and excipients of Remicade and infliximab biosimilar are identical21,28–31 (except for Zessly, which was not used in this study), contrary to subcutaneously administered TNFα inhibitors, issues such as allergy for excipients and difficulties with administration devices should not contribute to retransitioning for infliximab.
Retransitioning from biosimilar to originator has similarities with generic-to-brand retransitioning in small-molecule treatment, which has been extensively studied for antiepileptic brand-to-generic transitioning. Such studies have demonstrated that patients who retransitioned from generic to brand were at an increased risk of hospitalisation or of a dose increase of their antiepileptics; furthermore, they had more comorbidities compared with patients who remained on their generic antiepileptics.32–34 This finding was not related to differences in the pharmacokinetic properties of generics, 35 but rather it reflects patients’ attitudes towards generics and their anxiety regarding disease flares. 36 Similar to small-molecule treatment, retransitioning from biosimilar to originator appears to be more related to the patient and his/her disease than to the product itself.
For clinicians, patients who wish to retransition can be troublesome, as doing so is not recommended in the IBD treatment guidelines1,2,37 and no pharmacotherapeutic rationale exists for retransitioning to infliximab originator. Our results demonstrated that patients who retransition might have an increased risk of discontinuing infliximab due to loss of effect or adverse events, which could indicate that retransitioning is mainly related to the patient and/or to problems with his/her disease, and that it is less likely related to the infliximab biosimilar itself. As patients do not seem to benefit from retransitioning, clinicians might – after a thorough investigation to confirm active disease – consider switching patients who opt for retransitioning to another treatment regimen. However, in clinical practice, classifying certain subjective complaints, for example, joint pains, as an adverse event or as a nocebo effect can be difficult as these are often not objectively measurable. As shown in Tables 3 and 4, the reason for retransitioning might have an impact on the course of the infliximab originator treatment and could thus be of importance in clinical decision-making.
The strengths of our study include its comprehensive strategy for matching patients and its data analysis. By matching patients who retransitioned with patients who remained on biosimilar by calendar time and hospital, patients were similar in terms of treatment policies and the availability of options for switching treatment. Moreover, by matching them on the time of biosimilar treatment, patients were followed from the same moment in their treatment trajectory. Our thorough matching strategy allowed for a fair comparison of the two cohorts.
However, this study also had some limitations. The number of included patients was small, this should be kept in mind in interpreting our results. Therefore, it was not feasible to perform subgroup analyses, for example, stratification on indication (Crohn’s disease or ulcerative colitis). Furthermore, data on disease activity was not available, as in Dutch clinical care disease activity scores are not routinely collected. In addition, we were unable to retrieve the specific indication of 25 patients. The hospital these patients were treated in implemented a new EHR, and disease information was only stored in the archives of the old EHR which we could not access. However, as the proportions of patients with unknown indication was similar between the cohorts, we are confident that this did not affect our findings. Moreover, this study was performed in patients with IBD only. However, as both the nocebo effect and the attribution effect, which are both possibly the main drivers of retransitioning, are patient-related but not indication-related, we believe that our results are generalisable to other indications as well. Furthermore, biosimilars for other biologicals for long-term use are and will become available. We believe that the results from this study will be applicable to those biosimilars as well.
Conclusion
Our study demonstrated that patients who retransitioned discontinued infliximab treatment more often due to unwanted response compared with patients who remained on biosimilar, whereas patients who remained on biosimilar discontinued infliximab more often due to remission. Patients who retransitioned have, over time, over a three-fold increased risk for discontinuing infliximab due to unwanted response compared with patients who remained on biosimilar. These findings indicate that retransitioning is mainly related to the patient and/or his/her disease including patients’ beliefs on the biosimilar and less likely related to the infliximab biosimilar itself. Clinicians could consider patients who opt for retransitioning to another treatment option.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848231197923 for Discontinuation of infliximab treatment in patients with inflammatory bowel disease who retransitioned to originator and those who remained on biosimilar by Rosanne W. Meijboom, Helga Gardarsdottir, Matthijs L. Becker, Kris L. L. Movig, Johan Kuijvenhoven, Toine C. G. Egberts and Thijs J. Giezen in Therapeutic Advances in Gastroenterology
Acknowledgments
None.
Footnotes
ORCID iD: Rosanne W. Meijboom  https://orcid.org/0000-0002-7370-0695
https://orcid.org/0000-0002-7370-0695
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Rosanne W. Meijboom, Pharmacy Foundation of Haarlem Hospitals, Haarlem, the Netherlands Division of Pharmacoepidemiology & Clinical Pharmacology, Utrecht Institute for Pharmaceutical Sciences, Utrecht University, Utrecht, the Netherlands.
Helga Gardarsdottir, Division of Pharmacoepidemiology & Clinical Pharmacology, Utrecht Institute for Pharmaceutical Sciences, Utrecht University, Utrecht, the Netherlands; Department of Clinical Pharmacy, University Medical Center Utrecht, Utrecht, the Netherlands; Department of Pharmaceutical Sciences, University of Iceland, Reykjavik, Iceland.
Matthijs L. Becker, Pharmacy Foundation of Haarlem Hospitals, Haarlem, the Netherlands Department of Clinical Pharmacy, Spaarne Gasthuis, Haarlem, the Netherlands.
Kris L. L. Movig, Department of Clinical Pharmacy, Medisch Spectrum Twente, Enschede, the Netherlands
Johan Kuijvenhoven, Department of Gastroenterology and Hepatology, Spaarne Gasthuis, Haarlem, the Netherlands.
Toine C. G. Egberts, Division of Pharmacoepidemiology & Clinical Pharmacology, Utrecht Institute for Pharmaceutical Sciences, Utrecht University, Utrecht, the Netherlands Department of Clinical Pharmacy, University Medical Center Utrecht, Utrecht, the Netherlands.
Thijs J. Giezen, Pharmacy Foundation of Haarlem Hospitals, Boerhaavelaan 24, Haarlem 2035 RC, the Netherlands; Division of Pharmacoepidemiology & Clinical Pharmacology, Utrecht Institute for Pharmaceutical Sciences, Utrecht University, Utrecht, the Netherlands; Department of Clinical Pharmacy, Spaarne Gasthuis, Haarlem, the Netherlands.
Declarations
Ethics approval and consent to participate: The protocol of this study was approved by the Institutional Review Board of the Spaarne Gasthuis (Adviescommissie Lokale Uitvoerbaarheid) (reference number: 2020 0116) and the Institutional Review Board of the Medisch Spectrum Twente (reference number: KH22-15). Requirement for informed consent was waived by the Institutional Review Boards.
Consent for publication: Not applicable.
Author contributions: Rosanne W. Meijboom: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Visualization; Writing – original draft; Writing – review & editing.
Helga Gardarsdottir: Conceptualization; Formal analysis; Investigation; Methodology; Writing – review & editing.
Matthijs L. Becker: Investigation; Writing – review & editing.
Kris L. L. Movig: Conceptualization; Investigation; Writing – review & editing.
Johan Kuijvenhoven: Data curation; Writing – review & editing.
Toine C. G. Egberts: Conceptualization; Formal analysis; Methodology; Writing – review & editing.
Thijs J. Giezen: Conceptualization; Formal analysis; Investigation; Methodology; Validation; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: The research data cannot be shared.
References
- 1. Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis 2020; 14: 4–22. [DOI] [PubMed] [Google Scholar]
- 2. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis 2017; 11: 769–784. [DOI] [PubMed] [Google Scholar]
- 3. European Medicines Agency. Guideline on similar biological medicinal products, https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-rev1_en.pdf (2014, accessed 15 February 2023).
- 4. European Medicines Agency. Statement on the scientific rationale supporting interchangeability of biosimilar medicines in the EU, https://www.ema.europa.eu/en/documents/public-statement/statement-scientific-rationale-supporting-interchangeability-biosimilar-medicines-eu_en.pdf (2022, accessed 19 December 2022). [DOI] [PMC free article] [PubMed]
- 5. European Medicines Agency. Remsima: EPAR – Product information, https://www.ema.europa.eu/en/documents/product-information/remsima-epar-product-information_en.pdf (2019, accessed 9 November 2021).
- 6. Danese S, Fiorino G, Raine T, et al. ECCO position statement on the use of biosimilars for inflammatory bowel disease-an update. J Crohns Colitis 2017; 11: 26–34. [DOI] [PubMed] [Google Scholar]
- 7. Fiorino G, Caprioli F, Daperno M, et al. Use of biosimilars in inflammatory bowel disease: a position update of the Italian Group for the Study of Inflammatory Bowel Disease (IG-IBD). Dig Liver Dis 2019; 51: 632–639. [DOI] [PubMed] [Google Scholar]
- 8. Jørgensen KK, Olsen IC, Goll GL, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet 2017; 389: 2304–2316. [DOI] [PubMed] [Google Scholar]
- 9. Meijboom RW, Gardarsdottir H, Egberts TCG, et al. Patients retransitioning from biosimilar TNFα inhibitor to the corresponding originator after initial transitioning to the biosimilar: a systematic review. BioDrugs 2022; 36: 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmitz EMH, Boekema PJ, Straathof JWA, et al. Switching from infliximab innovator to biosimilar in patients with inflammatory bowel disease: a 12-month multicentre observational prospective cohort study. Aliment Pharmacol Ther 2018; 47: 356–363. [DOI] [PubMed] [Google Scholar]
- 11. Binkhorst L, Sobels A, Stuyt R, et al. Short article: switching to a infliximab biosimilar: short-term results of clinical monitoring in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol 2018; 30: 699–703. [DOI] [PubMed] [Google Scholar]
- 12. Razanskaite V, Bettey M, Downey L, et al. Biosimilar infliximab in inflammatory bowel disease: outcomes of a managed switching programme. J Crohns Colitis 2017; 11: 690–696. [DOI] [PubMed] [Google Scholar]
- 13. Boone NW, Liu L, Romberg-Camps MJ, et al. The nocebo effect challenges the non-medical infliximab switch in practice. Eur J Clin Pharmacol 2018; 74: 655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peyrin-Biroulet L, Lönnfors S, Roblin X, et al. Patient perspectives on biosimilars: a survey by the European federation of Crohn’s and ulcerative colitis associations. J Crohns Colitis 2017; 11: 128–133. [DOI] [PubMed] [Google Scholar]
- 15. Danese S, Fiorino G, Michetti P. Changes in biosimilar knowledge among European Crohn’s Colitis Organization [ECCO] members: an updated survey. J Crohns Colitis 2016; 10: 1362–1365. [DOI] [PubMed] [Google Scholar]
- 16. Vlieland ND, Gardarsdottir H, Bouvy ML, et al. The majority of patients do not store their biologic disease-modifying antirheumatic drugs within the recommended temperature range. Rheumatology (Oxford) 2016; 55: 704–709. [DOI] [PubMed] [Google Scholar]
- 17. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 18. Brazauskas R, Logan BR. Observational studies: matching or regression? Biol Blood Marrow Transplant 2016; 22: 557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iwagami M, Shinozaki T. Introduction to matching in case-control and cohort studies. Ann Clin Epidemiol 2022; 4: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Desai RJ, Kim SC, Curtis JR, et al. Methodologic considerations for noninterventional studies of switching from reference biologic to biosimilars. Pharmacoepidemiol Drug Saf 2020; 29: 757–769. [DOI] [PubMed] [Google Scholar]
- 21. European Medicines Agency. Remicade: EPAR – Product information, https://www.ema.europa.eu/en/documents/product-information/remicade-epar-product-information_en.pdf (2009, accessed 9 November 2022).
- 22. Fleischmann R, Jairath V, Mysler E, et al. Nonmedical switching from originators to biosimilars: does the nocebo effect explain treatment failures and adverse events in rheumatology and gastroenterology? Rheumatol Ther 2020; 7: 35–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mahmoud R, van Lieshout C, Frederix GWJ, et al. Continuation of anti-TNF in patients with ulcerative colitis in remission is not cost-effective compared to treatment withdrawal: a Markov model. J Crohns Colitis 2021; 15: 709–718. [DOI] [PubMed] [Google Scholar]
- 24. Mahmmod S, Schultheiss JPD, van Bodegraven AA, et al. Outcome of reverse switching from CT-P13 to originator infliximab in patients with inflammatory bowel disease. Inflamm Bowel Dis 2021; 27: 1954–1962. [DOI] [PubMed] [Google Scholar]
- 25. Avouac J, Moltó A, Abitbol V, et al. Systematic switch from innovator infliximab to biosimilar infliximab in inflammatory chronic diseases in daily clinical practice: the experience of Cochin University Hospital, Paris, France. Semin Arthritis Rheum 2018; 47: 741–748. [DOI] [PubMed] [Google Scholar]
- 26. Meijboom RW, Gardarsdottir H, Becker ML, et al. Switching TNFα inhibitors: patterns and determinants. Pharmacol Res Perspect 2021; 9: e00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Armuzzi A, DiBonaventura MD, Tarallo M, et al. Treatment patterns among patients with moderate-to-severe ulcerative colitis in the United States and Europe. PLoS One 2020; 20: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim NH, Lee JH, Hong SN, et al. Long-term efficacy and safety of CT-P13, a biosimilar of infliximab, in patients with inflammatory bowel disease: a retrospective multicenter study. J Gastroenterol Hepatol (Australia) 2019; 34: 1523–1532. [DOI] [PubMed] [Google Scholar]
- 29. European Medicines Agency. Inflectra: EPAR – Product information, https://www.ema.europa.eu/en/documents/product-information/inflectra-epar-product-information_en.pdf (2019, accessed 9 November 2021).
- 30. European Medicines Agency. Flixabi: EPAR – Product information, https://www.ema.europa.eu/en/documents/product-information/flixabi-epar-product-information_en.pdf (2019, accessed 29 November 2021).
- 31. European Medicines Agency. Zessly: EPAR – Product information, https://www.ema.europa.eu/en/documents/product-information/zessly-epar-product-information_en.pdf (2020, accessed 9 November 2021).
- 32. LeLorier J, Duh MS, Paradis PE, et al. Clinical consequences of generic substitution of lamotrigine for patients with epilepsy. Neurology 2009; 72: 1876. [DOI] [PubMed] [Google Scholar]
- 33. Hansen RA, Qian J, Berg R, et al. Comparison of generic-to-brand switchback rates between generic and authorized generic drugs. Pharmacotherapy 2017; 37: 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Duh MS, Paradis PE, Latrémouille-Viau D, et al. The risks and costs of multiple-generic substitution of topiramate. Neurology 2009; 72: 2122–2129. [DOI] [PubMed] [Google Scholar]
- 35. Olsson P, Reimers A, Källén K. Quality of life after switching to generic levetiracetam: a prospective comparative study. Epilepsy Behav 2019; 96: 169–174. [DOI] [PubMed] [Google Scholar]
- 36. Andermann F, Duh MS, Gosselin A, et al. Compulsory generic switching of antiepileptic drugs: high switchback rates to branded compounds compared with other drug classes. Epilepsia 2007; 48: 464–469. [DOI] [PubMed] [Google Scholar]
- 37. Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol 2018; 113: 481–517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848231197923 for Discontinuation of infliximab treatment in patients with inflammatory bowel disease who retransitioned to originator and those who remained on biosimilar by Rosanne W. Meijboom, Helga Gardarsdottir, Matthijs L. Becker, Kris L. L. Movig, Johan Kuijvenhoven, Toine C. G. Egberts and Thijs J. Giezen in Therapeutic Advances in Gastroenterology