Abstract
Prostaglandins are lipid mediators involved in physiological processes, such as constriction or dilation of blood vessels, but also pathophysiological processes, which include inflammation, pain and fever. They are produced by almost all cell types in the organism by activation of Prostaglandin endoperoxide synthases/Cyclooxygenases. The inducible Prostaglandin Endoperoxide Synthase 2/Cyclooxygenase 2 (PTGS2/COX2) plays an important role in pathologies associated with inflammatory signaling. The main product derived from PTGS2/COX2 expression and activation is Prostaglandin E2 (PGE2), which promotes a wide variety of tissue-specific effects, pending environmental inputs. One of the major sources of PGE2 are infiltrating inflammatory cells - the production of this molecule increases drastically in damaged tissues. Immune infiltration is a hallmark of type 1 diabetes mellitus, a multifactorial disease that leads to autoimmune-mediated pancreatic beta cell destruction. Controversial effects for the PTGS2/COX2-PGE2 signaling cascade in pancreatic islet cells subjected to diabetogenic conditions have been reported, allocating PGE2 as both, cause and consequence of inflammation. Herein, we review the main effects of this molecular pathway in a tissue-specific manner, with a special emphasis on beta cell mass protection/destruction and its potential role in the prevention or development of T1DM. We also discuss strategies to target this pathway for future therapies.
Keywords: Beta cells, Cyclooxygenases, Inflammation, Pancreatic Islets, Prostaglandin, Type 1 Diabetes Mellitus
1. Introduction
Type 1 Diabetes Mellitus (T1DM) is a disease caused by the selective destruction of pancreatic islet beta cells by aberrant activation of the immune system, characterized by a subsequent unresolved proinflammatory status within the pancreas 1, 2. To date, no effective therapies have been developed to cure this autoimmune disorder, which indeed, apart from the beta cell death and subsequent lack of insulin, leads to long-term complications that substantially impact on life quality and shorten life expectancy 2. Type 1 Diabetes Mellitus is a T cell-mediated autoimmune disease - the main cause is an imbalance between T regulatory and autoreactive CD4+ and CD8+ T effector cells, which make them react specifically against pancreatic islet-associated self-antigens, leading to beta cell mass destruction. In this context, infiltrating macrophages and T effector cells secrete different pro-inflammatory cytokines, promoting a chronic pro-inflammatory microenvironment within the pancreas 3-5. However, we and others have reported that some level of inflammation is beneficial for the regeneration of beta cell mass as it induces a dialogue between pancreatic and immune cells aimed at protecting beta cells 6, 7. This fact could explain the failure of potential reported treatments for T1DM based on either blocking the immune attack or aimed at the preservation of the beta cell mass 8. Based on this, we have recently proposed a model in which T1DM can be conceived as an 'unresolved wound healing process', where the pro-inflammatory phase persists and cannot be resolved 2. According to this model, pathways that may modulate inflammation, instead of supressing the immune system, may be promising therapeutic targets for T1DM.
1.1 Prostaglandins: General overview on their physiological roles
Cyclooxygenases (COXs) are the enzymes that catalyse the first rate-limiting step in prostaglandins (PGs) synthesis from arachidonic acid (AA). There are 2 main COXs isoforms; the constitutive cyclooxygenase 1, also known as (a.k.a.) Prostaglandin Endoperoxidase Synthase 1 (COX1/PTGS1) widely expressed in most tissues, and the stress-inducible cyclooxygenase 2, a.k.a. Prostaglandin Endoperoxidase Synthase 2 (COX2/PTGS2) normally expressed at very low levels under physiological conditions 9, 10. PTGS1/COX1 is a housekeeping enzyme - it is required for maintaining basal PGs levels for tissue/cell homeostasis 9. On the other hand, PTGS2/COX2 is tightly regulated, and its expression and activation are directly induced by pro-inflammatory cytokines and growth factors that activate intracellular inflammation-related pathways 11. Prostaglandin E2 (PGE2) is the main biologically-active PG-derived from the metabolization of AA 12 and it is mainly involved in inflammation-related processes 13. There is a third reported isoform, Cyclooxygenase 3/ Prostaglandin Endoperoxide Synthase 3 (COX3/PTGS3), although this one has been barely studied, as it is a splice variant of PTGS1/COX1, encoding a truncated protein lacking enzymatic activity 14.
Focusing on PTGS2/COX2, overwhelming opposite effects have been reported for this molecular pathway: Due to its expression and activation during inflammation, the PTGS2/COX2-PGE2 signalling axis has been widely thought to be a driver of inflammation, induced as a direct cause of it 10, 15. However, subsequent studies reported that both, endogenous production or repletion with PGE2 analogues restored tissue homeostasis upon stress or proinflammatory conditions 16, 17. As such, the probable involvement of PTGS2/COX2-PGE2 in the resolution phase of the inflammatory process points at this signalling pathway as a potential therapeutic target for pathologies displaying high levels of inflammation. We presently review the effects of PTGS2/COX2-PGE2 cascade in different inflammatory contexts, focusing on pancreatic beta cells subjected to T1DM conditions and discuss its potential exploitation as therapeutic target for the autoimmune pathology.
1.2 Biosynthesis of PGE2 and its downstream signalling pathways
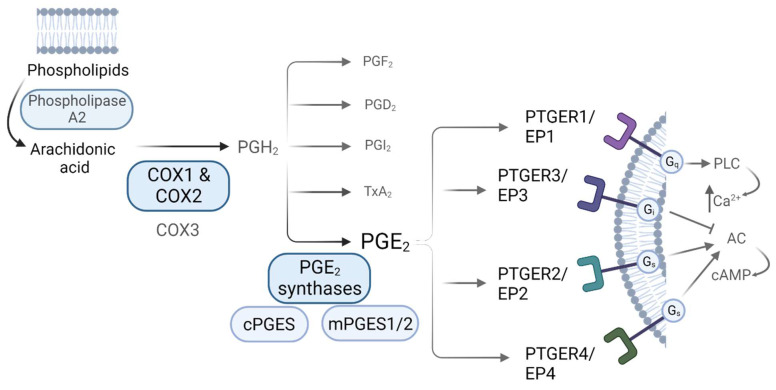
Prostaglandins are synthetized via the arachidonic acid (AA) pathway. Arachidonic acid is liberated from membrane phospholipids by the action of phospholipase A2 (PLA2), and then converted into the unstable precursor Prostaglandin H2 (PGH2) 13. This conversion constitutes the first rate-limiting step in PGs synthesis, and it is performed by the action of the COXs 9, 10. Once PGH2 is formed, it is converted into different prostaglandins by tissue-specific Prostaglandin Synthases (PGS). PGE2 can be produced by the action of both, cytosolic Prostaglandin E Synthases (cPGES) and microsomal Prostaglandin E Synthases1/2 (mPGES1/2) 13. COXs-derived PGs mediate their actions via binding to receptors coupled to different G (guanine nucleotide-binding) proteins, denoted as G-protein coupled receptors (GPCRs), which are widely expressed throughout the body and whose activation induce distinct intracellular signalling cascades. Regarding PGE2, there are 4 known GPCR subtypes: PTGER1 (a.k.a. EP1) that employs Gq (G protein heterotrimeric, that activates beta isoforms of phospholipase C (PLC) and induces calcium mobilization), PTGER3 (a.k.a. EP3) that employs Gi (G inhibitory protein, which decreases the cAMP levels by inhibiting adenylyl cyclase (AC) activity), and PTGER2 and PTGER4 (a.k.a. EP2 and EP4), which both utilize Gs (G stimulatory protein, that stimulates the cAMP dependent pathway by activating AC) (Figure 1) 11, 18. The affinity of PGE2 for these receptors, along with the expression levels of each of them in a specific environment, leads to different physiological outcomes. Both PTGER3/EP3 and PTGER4/EP4 possess the highest affinities for the prostaglandin (dissociation constant (Kd) values of 0.33-2.9nM and 0.59-1.27nM, respectively) while PTGER1/EP1 exhibits the lowest affinity (Kd of 16-25nM). The affinity of PTGER2/EP2 for PGE2 differs between species - the rat PTGER2/EP2 exhibits higher affinity for PGE2 as compared to mouse and human ones 18.
Figure 1.
PGE2 synthesis via the Arachidonic Acid pathway. Biosynthesis of prostaglandins. AA is liberated from membrane phospholipids by the action of Phospholipase A2, that is then converted into PGH2 by the action of the Cyclooxygenases, a rate-limiting step for prostaglandin biosynthesis. Prostaglandin H2 (PGH2) is the unstable precursor of Prostaglandin F2 (PGF2), Prostaglandin D2 (PGD2), Prostaglandin I2 (PGI2), Thromboxane A2 (TxA2) and Prostaglandin E2 (PGE2). PGE2 receptors and their related downstream signaling are also shown.
2. PTGS2/COX2-PGE2 role upon inflammatory signaling: tissue-specific crosstalk with immune cells
Despite widely foreseen as an inducible gene, exceptions for constitutive PTGS2/COX2 expression include testes 19, kidney - macula densa cells 20 and the central nervous system (CNS) 21, 22. For instance, in the brain, PTGS2/COX2 expression was shown in specific subsets of neurons dispersed throughout the tissue, where it participates in synaptic activity and memory consolidation. Indeed, aberrant upregulation of Ptgs2/Cox2 expression in vascular endothelial cells from the CNS has been linked to neurotoxicity and chronic neurodegenerative processes in rats treated with Carrageenan, a well-known acute inflammation model 23, 24.
Several studies have attempted to address whether PTGS2/COX2 expression, and specially PGE2 production, convey either beneficial or detrimental effects using different models of inflammation. Initial studies performed in Caco-2 colorectal cells treated with exogenous PGE2 showed that the lipid mediator was able to upregulate proinflammatory pathways such as NF-κB 25. Remarkably, PTGS2/COX2 exhibits a specific NF-κB binding site in its promoter region, therefore, a feedback loop involving PTGS2/COX2 expression and further endogenous PGE2 synthesis upon proinflammatory conditions could be taking place, leading to increased NF-κB signalling 26. However, subsequent studies reported overwhelming opposite effects. For instance, repletion with PGE2 analogues has been reported to restore homeostasis during inflammation in vivo, in a mouse model of immune arthritis 16. This phenotype appeared to be facilitated by PGE2-mediated inhibition of NF-κB, therefore decreasing the pro inflammatory cytokines production 27. This event reveals a very interesting therapeutic loop that could resolve inflammation and restore tissue homeostasis: PGE2 production may induce NF-κB, but later on, as it is necessary to resolve the inflammatory process, the final outcome is decreased NF-κB activation. Indeed, it has been widely reported that conventional nonsteroidal anti-inflammatory drugs (NSAIDs), the most common inhibitors of PTGS2/COX2, exhibit palliative (rather than curative) effects 28, 29, a fact that could be explained by the impossibility to resolve inflammation upon PTGS2/COX2 inhibition.
Focusing on the immune system, PGE2 treatment has been shown to alter macrophage polarization and metabolism towards an M2/anti-inflammatory phenotype. This event has been observed in white adipose tissue, in the ob/ob mouse model of obesity 30. PGE2 synthesis was also shown to be upregulated in human T-cells as an early inducible gene upon T-cell activation 31 and to exert opposite effects in a 'concentration-dependent' manner, leading to different scenarios of homeostasis and inflammation during T-cell responses. At high concentration, PGE2 was shown to promote an anti-inflammatory environment by suppressing the T helper 1 (Th1) subpopulation while activating the T helper 2 (Th2) subset of T-cells 32, and by inducing the differentiation of FOXP3+CD4+CD25+ adaptive regulatory T-cells that inhibit T-cell effector responses. The aforementioned suppressive properties were conveyed via binding to PTGER2/EP2 and PTGER4/EP4, the PTGERs expressed by T-cells 33. In contrast, low concentrations of PGE2 have been shown to induce Th1 differentiation patterns, conveying a proinflammatory phenotype, via PTGER4/EP4 signaling 34. These findings highlight the tight regulation of the PTGS2/COX2-PGE2 signaling cascade that can lead to opposite scenarios in contexts of inflammation.
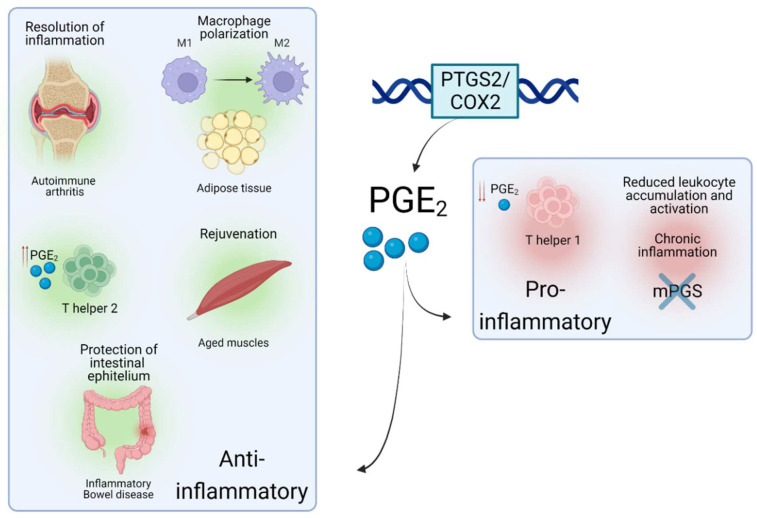
Interestingly, a recent study reported that PGE2 levels were decreased in muscles upon aging, a progressive and degenerative multifactorial condition, finely intertwined with inflammatory responses. In mice, depletion of PGE2 directly correlated with muscle wasting, while restoration of PGE2 levels via inhibition of the PGE2-degrading enzyme (15- PGDH) ameliorated the effect of age-related processes in muscle. This was achieved via a complete 'rejuvenation' of mitochondrial function and downregulation of the ubiquitin and TGFβ pathways. Interestingly, a tendency towards upregulated 15- PGDH enzymatic activity and decreased levels of PGE2 was observed in tissue-resident macrophages 35 which led the authors to conclude that these cells are a major site of PGE2 degradation and as such, drivers of the dysfunction of the aged environment. This study highlights a potential paracrine interaction between immune and tissue-specific cell types via PTGS2/COX2-PGE2 signaling, which could be targeted in inflammation-related pathologies. Such crosstalk via PTGS2/COX2-PGE2 has been documented in several cellular contexts. A subtype of tissue-resident innate lymphoid cells was recently shown to protect the intestinal epithelium from Tumour Necrosis Factor (TNF)-induced cell death by producing a heparin-binding epidermal growth factor promoted by PGE2 production in inflammatory bowel disease, a chronic inflammation-related disorder of the intestinal tract 36. Similarly, in a mouse model of chronic inflammation, a novel interaction between the pro-inflammatory Interleukin-17 (IL-17) and the microsomal PGE2 synthase (Figure 1) generated by macrophages was shown to reduce leukocyte infiltration and myeloperoxidase activity 37. These data substantiate that the PTGS2/COX2-PGE2 signalling promotes different immune-related effects, which mainly depends on the context of the local proinflammatory injury and levels of PGE2. In some cases, PGE2 would be involved in paracrine communication within different cell types, mainly immune and tissue-specific cells - a summary of several roles of the PTGS2/COX2-PGE2 pathway in different inflammatory scenarios is shown in Figure 2. In this line, some studies have emerged attempting to determine the role of this signalling pathway upon the immune-tissue specific crosstalk that characterizes autoimmune-related diseases, such as T1DM 2.
Figure 2.
Roles of PTGS2/COX2-PGE2 in different inflammatory situations. Schematic view of the role and effects of PTGS2/COX2-PGE2 upon different inflammation-related environments.
3. PTGS2/COX2-PGE2; beneficial or detrimental for type 1 diabetes development?
The specific impact of PTGS2/COX2-PGE2 signalling on beta cell health and functions upon diabetes features has not been fully established, yet a few studies aimed at dissecting the role of this cascade within this context have been performed. Interestingly, a study dating back to 1876, demonstrated that administration of aspirin, a potent PTGS/COX inhibitor, was proficient in normalizing blood glucose and reverting diabetes 38. These anti-diabetic effects were confirmed 100 years later in individuals with type 2 diabetes mellitus (T2DM): high-dose aspirin therapy reduced fasting glucose levels in blood, decreased total cholesterol and triglycerides and improved peripheral insulin-stimulated glucose uptake 39. However, rather than depending exclusively on PTGS/COX inhibition, the authors highlighted that the treatment with aspirin/salicylates blunted the activity of the serine kinase IKKbeta 40, which plays a key role in the pathogenesis of insulin resistance 41. In the same line, Goldfine and colleagues confirmed the robust anti-hyperglycaemic effect of a salicylate-derivative, salsalate (a dimeric pro-drug of salicylate), in individuals with T2DM 42. Whether, these beneficial effects of aspirin/salsalate are mediated via inhibition of PTGS/COX, rather than the well-defined target IKKbeta, remains to be determined. Interestingly, such studies have not been reported for T1DM. In contrast, other studies have linked the expression of the PTGS2/COX2 isoform in beta cells to the development of pathological processes in diabetes, e.g., exposure of mouse and human islets to hyperglycaemic conditions, in culture in case of human, and in vivo, in diabetic mice, induced PTGS2/COX2 expression and activation 43, 44. Remarkably, Ptgs2/Cox2-induced expression and PGE2 production in islet beta cells was shown to promote hyperglycaemia in vivo, in transgenic mice overexpressing Ptgs2/Cox2 and Prostaglandin E Synthase (Pges) in beta cells under the rat insulin promoter (RIP). This effect was mainly linked to reduced proliferation of beta cells 45. These findings raised the question whether hyperglycaemia was the cause, or just a consequence of PTGS2/COX2 expression, which in any case, was apparently linked to detrimental effects. In this regard, mice treated with a high dose of streptozotocin (STZ, a chemically-induced diabetes model), displayed an increase in Ptgs2/Cox2 expression in whole pancreas and isolated islets, but strikingly, these authors found that constitutive Ptgs2/Cox2 knock out animals displayed significantly a higher incidence of hyperglycaemia, both after a single high dose and after several low doses of STZ 46.
In line with the reported role of PTGS2/COX2 activation upon inflammation, sometimes beneficial/anti-inflammatory, and some others detrimental/pro-inflammatory, these results pinpointed to a controversy on the impact of PTGS2/COX2 expression in the pathogenesis of diabetes, especially in beta cells. Indeed, when moving to the immune field, PTGS2/COX2 expression has been detected in monocyte-cell cultures from diabetic patients, and linked to an increase in advanced glycated end products and their receptor (AGE-RAGE) expression 47. Advanced glycated end product deposition is known to take place in diabetic blood vessels, driven by hyperglycaemia and exacerbating proinflammatory signaling 47, 48. Similarly, microarray analyses performed in peripheral blood mononuclear cells (PBMCs) from individuals with T1DM revealed an upregulation of PTGS2/COX2 expression compared to control individuals 49. Furthermore, other studies showed that monocytes from individuals at high risk of T1DM development as well as from individuals with established T1DM expressed higher levels of PTGS2/COX2 when compared to monocytes from control normoglycemic individuals 50, 51.
Taken together, these studies indicate that PTGS2/COX2 expression is likely involved in the pathogenesis of diabetes, taking part in the persistent proinflammatory status and potentially leading to detrimental consequences. Nevertheless, in the past decade, further research and several discoveries led to a re-evaluation on the concept that PTGS2/COX2 is a factor contributing to the pathogenesis and the proinflammatory environment in a diabetic context.
4. The effect of the PTGS2/COX2-PGE2 signaling is contingent on the PTGERs/EPs in islet beta cells
As previously mentioned, and contrary to previous evidence, Ptgs2/Cox2 was subsequently shown to partially protect mouse islets from STZ-induced diabetogenic toxicity in vivo, using Ptgs2/Cox2-deficient mouse models. While no differences were reported between Ptgs1/Cox1 knock out and control mice in terms of blood glucose levels after short-term STZ administration, Ptgs2/Cox2 knock out animals exhibited a stronger induction of hyperglycaemia. This phenotype was further confirmed using a pharmacological approach, a selective PTGS2/COX2 inhibitor (SC-236) that also increased hyperglycaemia susceptibility in wild type (WT) animals 46. Moreover, PTGS2/COX2 was also linked to enhanced mouse and human islet survival in the presence of pro-inflammatory cytokines ex vivo 52. These beneficial effects appeared to be mediated by PGE2 interaction with one or multiple PTGERs/EPs and their subsequent activation, leading to different physiological outcomes 46, 52. This would be a plausible explanation of discrepancies reported in terms of the role of this pathway during inflammation. Both PTGER3/EP3 and PTGER4/EP4 have received the most attention, as they exhibit the highest affinities for PGE2 18 and remarkably, in terms of immunomodulation, opposite effects have been reported for these two receptors: In phagocytes from different tissues, PTGER3/EP3 activation has been linked to immunostimulatory signalling, while PTGER4/EP4 activation was linked to the opposite effect, conveying an immunosuppressive environment 53.
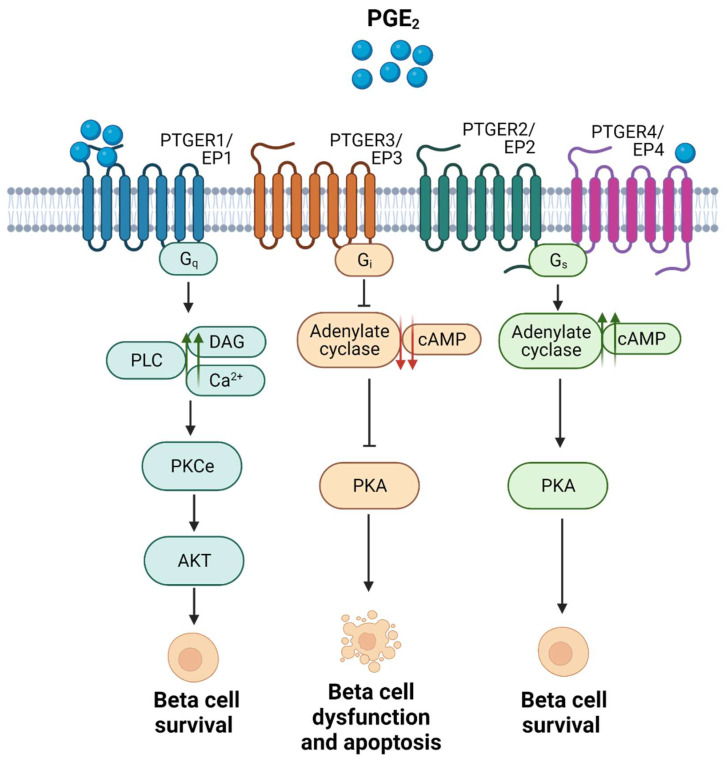
Focusing on the pancreatic niche, expression of PTGER3/EP3 was shown to be increased in pancreatic islets from individuals with T2DM, as well as in MIN6 cells exposed to palmitate. Activation of this receptor in beta cells by PGE2 binding was associated with beta cell dysfunction and apoptosis 52, 54. In addition, PTGER3/EP3 activation has been linked to inhibition of glucose stimulated insulin secretion (GSIS) caused by a decrease in cAMP levels, via inhibition of adenylyl cyclase (AC) activity (Figure 3). The latter was shown in a glucose-responsive beta cell line (HIT cells), and subsequently in rodent islets 55, 56. These detrimental effects have been confirmed in functional studies, e.g., blockade of PTGER3/EP3 in the db/db mouse model (non-obese diabetic mice) prevented beta cell death and enhanced beta cell mass and identity. The authors found that the systemic PTGER3/EP3 antagonism promoted an increase in beta cell proliferation, restored normal islet morphology and promoted expression of genes involved in beta cell identity and function. Moreover, they encountered activation of the Nrf2 antioxidant pathway and partial restoration of GLP-1R protein expression, which was undetectable in db/db islets from untreated mice 57. Since GLP-1 agonism based therapies have been a major focus of promising strategies for individuals with T2DM, albeit not always showing high efficiency 58, this study supports that a combinatory treatment could provide beneficial outcomes.
Figure 3.
Summary of the main signaling pathways activated upon the 4 subtypes of PTGERs/EPs and the physiological outcomes in terms of beta cell survival. Proposed model of shift in receptor selectivity upon elevated production of PGE2.
In contrast, activation of PTGER4/EP4 has been reported to enhance beta cell survival in mouse and human islets ex vivo, in response to cytokines 59, potentially via downstream activation of the Phospho Kinase A (PKA) signalling pathway, which promotes an increase in the levels of cAMP by activating AC and subsequent phosphorylation of the cAMP response element binding (CREB) factor (Figure 3) 53, 59.
These data clearly show a major role for PTGS2/COX2-PGE2 in the beta cell physiology and pathophysiology, which can be detrimental or beneficial for beta cell function and survival depending on the PTGER(s)/EP(s) mediating the effect.
As the various outputs of this pathway seem to mainly depend on the expression levels and activity of the 4 PTGERs/EPs, interventions such as blockade of the proapoptotic PTGER3/EP3 or activation of the anti-apoptotic PTGER4/EP4 have been explored in terms of conveying protection of the beta cell mass against cytokines-induced apoptosis 57, 59. However, the specific impact of these receptors, along with those towards which PGE2 exhibits less affinity, has been barely studied in T1DM pathogenesis. Both PTGER1/EP1 and PTGER2/EP2 exhibit lower affinities for the prostaglandin, therefore, research focusing on them under diabetogenic conditions has been scarcely performed. However, in vivo global Ptger2/Ep2 knock out was shown to exacerbate STZ pathology, remarkably, only upon PTGER4/EP4 pharmacological inactivation, showing potential pro survival properties for this receptor 46. Similarly, our group has recently shown anti-apoptotic effects via inhibition of the intrinsic apoptosis pathway mediated via PTGER1/EP1 signaling ex vivo, in mouse islets challenged with pro inflammatory cytokines. Our study showed a major increase in PGE2 synthesis in mouse islets after cytokines exposure, and an unexpected massive production of the prostaglandin when combining the cytokines treatment with BL001 17, a small chemical agonist of the nuclear receptor Liver Receptor Homolog-1 (LRH1/NR5A2) developed by our group as a potential anti-diabetic drug 6. We uncovered a new pro-survival effect of PGE2 mainly mediated by PTGER1/EP1 upon BL001 treatment 17, instead of PTGER4/EP4 as reported by other studies performed both in T2DM 59 and T1DM models 46. Indeed, when blocking PTGER4/EP4, we still reported protection from cytokines-induced apoptosis upon BL001 treatment, showing that the latter was not the receptor mediating the protection of LRH-1/NR5A2 activation 17. Similar to the findings of Bosma and colleagues 57, our results shed light on potentially combinatory treatments, i.e., BL001+PTGER1/EP1 agonist(s), aimed at prompting additional antidiabetic benefits.
As mentioned before, PTGER1/EP1 activation has been reported to increase diacylglycerol (DAG) production and Ca2+ levels, and to promote protein kinase B (AKT) phosphorylation via protein kinase C (PKC) activation (Figure 3) 11, 18. Remarkably, phospho-AKT-mediated signalling has been associated with beta cell survival and proliferation 60, 61, and PKC activators such as yeRACK improve islet survival during their isolation procedure, as well as their functionality after transplantation in mice 62. Since PGE2 exhibits the lowest affinity for PTGER1/EP1 and the highest for PTGER4/EP4 18, we proposed that high concentrations of PGE2 upon a post transcriptional regulation of Ptgs2/Cox2 may favour a shift in pro-survival receptors selectivity, from PTGER4/EP4 towards PTGER1/EP1 (Figure 3) 17. This hypothesis needs to be further confirmed. Noteworthy, PGE2 has been also correlated with the expression of inhibitor-of-apoptosis proteins such as SURVIVIN through PTGER1/EP1 receptor signaling in a cancer context 63. Taken together, these studies emphasise on the high complexity of the interaction network between PTGS2/COX2-PGE2 and the various PTGERs/EPs under proinflammatory/stress conditions and other environmental factors.
5. Concluding remarks
Herein, we discuss the complex PTGS2/COX2-PGE2 signaling cascade upon different inflammatory scenarios. We focused on its role in inflammation and on the crosstalk between the immune system and different tissue-specific cell types, especially on pancreatic beta cells under diabetes pathophysiology. We describe reported phenotypes observed upon activation of the different PTGERs/EPs in immune cells and with special emphasis, in beta cells, which further supports the promiscuity of this signaling axis, and conclude that fine-tunning PGE2 production and promoting activation/blocking of the different receptors would be mandatory interventions to achieve a desirable output during stress or inflammation. Moreover, we emphasize on the fact that it is essential to consider the effect of all the 4 PTGERs/EPs, including the ones towards which the prostaglandin exhibits less affinity, such as PTGER1/EP1 17. Since PTGS2/COX2-PGE2 signaling cascade has been widely shown to play a fundamental role within immune cells 30, 31, 33, 34, 36, 37, exploring the potential interaction (especially between pancreatic beta cells and immune cell types) and outcomes upon modulation of this signaling axis could be a promising strategy to find new therapies to treat T1DM. However, further studies are needed to dissect the factors involved in this paracrine communication, in order to ensure that the outcome of modulating the pathway will be beneficial/anti-inflammatory. To sum up, modulation of PTGS2/COX2-PGE2 signaling pathway may be a promising strategy to treat different inflammation-related pathologies. However, it is vital to determine the role of PGE2 in each individual context, since depending on the main PTGERs/EPs mediating the effects, PGE2 may lead to very distinct phenotypes. In the case of T1DM, since preliminary research performed up to date has shown promising outcomes when targeting this pathway, it is conceivable that new cellular mechanisms will be revealed in the future, which could be targeted in addition to the canonical pathways with therapeutic purposes.
Acknowledgments
We acknowledge the support of the pancreatic islet study group of the Spanish Association of Diabetes as well as all our colleagues with whom we have collaborated over the years. Figures were created with BioRender.com.
Funding
The authors are supported by grants from the Consejería de Salud, Fundación Pública Andaluza Progreso y Salud, Junta de Andalucía (PI-0727-2010 to B.R.G., PI-0085-2013 to P.I.L.), the Consejería de Economía, Innovación y Ciencia (P10.CTS.6359 to B.R.G and DOC_00652 to L.L.N.), the Ministerio de Ciencia E Innovación co-funded by Fondos FEDER (PI10/00871, PI13/00593 and BFU2017-83588-P, PID2021-123083NB-I00 to B.R.G), Vencer el Cancer (B.R.G), DiabetesCero (B.R.G.) and the Juvenile Diabetes Research Foundation (17-2013-372, 2-SRA-2019-837-S-B, 3-SRA-2023-1307-S-B to B.R.G.). E.M.V. is recipient of a Fellowship from the Ministerio de Ciencia E Innovación co-funded by Fondos FEDER (PRE2018-084907).
Author contributions
E.M.-V., N.C.-V., L.L.-N, P.I.-L. and B.R.-G. conceived the article, reviewed the literature, and wrote the text. The final version was revised and approved by all authors.
Review criteria
We selected relevant publications by searching the PubMed database. Please note, the literature on PTGS2/COX2-PGE2 in islet beta cells is quite limited. All papers cited herein were English-language, and review articles were also cited to provide the readers with further references.
References
- 1.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5:219–26. doi: 10.1038/nrendo.2009.21. [DOI] [PubMed] [Google Scholar]
- 2.Cobo-Vuilleumier N, Gauthier BR. Time for a paradigm shift in treating type 1 diabetes mellitus: coupling inflammation to islet regeneration. Metabolism. 2020;104:154137. doi: 10.1016/j.metabol.2020.154137. [DOI] [PubMed] [Google Scholar]
- 3.Lu J, Liu J, Li L, Lan Y, Liang Y. Cytokines in type 1 diabetes: mechanisms of action and immunotherapeutic targets. Clin Transl Immunology. 2020;9:e1122. doi: 10.1002/cti2.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arif S, Tree TI, Astill TP, Tremble JM, Bishop AJ, Dayan CM. et al. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest. 2004;113:451–63. doi: 10.1172/JCI19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arif S, Moore F, Marks K, Bouckenooghe T, Dayan CM, Planas R. et al. Peripheral and islet interleukin-17 pathway activation characterizes human autoimmune diabetes and promotes cytokine-mediated beta-cell death. Diabetes. 2011;60:2112–9. doi: 10.2337/db10-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cobo-Vuilleumier N, Lorenzo PI, Rodriguez NG, Herrera Gomez IG, Fuente-Martin E, Lopez-Noriega L. et al. LRH-1 agonism favours an immune-islet dialogue which protects against diabetes mellitus. Nat Commun. 2018;9:1488. doi: 10.1038/s41467-018-03943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eizirik DL, Sammeth M, Bouckenooghe T, Bottu G, Sisino G, Igoillo-Esteve M. et al. The human pancreatic islet transcriptome: expression of candidate genes for type 1 diabetes and the impact of pro-inflammatory cytokines. PLoS Genet. 2012;8:e1002552. doi: 10.1371/journal.pgen.1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perakakis N, Mantzoros CS. Immune therapy in type 1 diabetes mellitus - Attempts to untie the Gordian knot? Metabolism. 2016;65:1278–85. doi: 10.1016/j.metabol.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB. et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–73. [PubMed] [Google Scholar]
- 10.Alexanian A, Sorokin A. Cyclooxygenase 2: protein-protein interactions and posttranslational modifications. Physiol Genomics. 2017;49:667–81. doi: 10.1152/physiolgenomics.00086.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein T, Shephard P, Kleinert H, Komhoff M. Regulation of cyclooxygenase-2 expression by cyclic AMP. Biochim Biophys Acta. 2007;1773:1605–18. doi: 10.1016/j.bbamcr.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Kawahara K, Hohjoh H, Inazumi T, Tsuchiya S, Sugimoto Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim Biophys Acta. 2015;1851:414–21. doi: 10.1016/j.bbalip.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Cheng H, Huang H, Guo Z, Chang Y, Li Z. Role of prostaglandin E2 in tissue repair and regeneration. Theranostics. 2021;11:8836–54. doi: 10.7150/thno.63396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS. et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002;99:13926–31. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shanmugam N, Figarola JL, Li Y, Swiderski PM, Rahbar S, Natarajan R. Proinflammatory effects of advanced lipoxidation end products in monocytes. Diabetes. 2008;57:879–88. doi: 10.2337/db07-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan MM, Moore AR. Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibition and restored by prostaglandin E2-mediated lipoxin A4 production. J Immunol. 2010;184:6418–26. doi: 10.4049/jimmunol.0903816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin Vazquez E, Cobo-Vuilleumier N, Araujo Legido R, Marin-Canas S, Nola E, Dorronsoro A. et al. NR5A2/LRH-1 regulates the PTGS2-PGE2-PTGER1 pathway contributing to pancreatic islet survival and function. iScience. 2022;25:104345. doi: 10.1016/j.isci.2022.104345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dey I, Lejeune M, Chadee K. Prostaglandin E2 receptor distribution and function in the gastrointestinal tract. Br J Pharmacol. 2006;149:611–23. doi: 10.1038/sj.bjp.0706923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winnall WR, Ali U, O'Bryan MK, Hirst JJ, Whiley PA, Muir JA. et al. Constitutive expression of prostaglandin-endoperoxide synthase 2 by somatic and spermatogenic cells is responsible for prostaglandin E2 production in the adult rat testis. Biol Reprod. 2007;76:759–68. doi: 10.1095/biolreprod.106.053124. [DOI] [PubMed] [Google Scholar]
- 20.Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN, Breyer MD. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest. 1994;94:2504–10. doi: 10.1172/JCI117620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yaksh TL, Dirig DM, Conway CM, Svensson C, Luo ZD, Isakson PC. The acute antihyperalgesic action of nonsteroidal, anti-inflammatory drugs and release of spinal prostaglandin E2 is mediated by the inhibition of constitutive spinal cyclooxygenase-2 (COX-2) but not COX-1. J Neurosci. 2001;21:5847–53. doi: 10.1523/JNEUROSCI.21-16-05847.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller N, Riedel M, Schwarz MJ. Psychotropic effects of COX-2 inhibitors-a possible new approach for the treatment of psychiatric disorders. Pharmacopsychiatry. 2004;37:266–9. doi: 10.1055/s-2004-832682. [DOI] [PubMed] [Google Scholar]
- 23.Ibuki T, Matsumura K, Yamazaki Y, Nozaki T, Tanaka Y, Kobayashi S. Cyclooxygenase-2 is induced in the endothelial cells throughout the central nervous system during carrageenan-induced hind paw inflammation; its possible role in hyperalgesia. J Neurochem. 2003;86:318–28. doi: 10.1046/j.1471-4159.2003.01848.x. [DOI] [PubMed] [Google Scholar]
- 24.Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004;63:901–10. doi: 10.1093/jnen/63.9.901. [DOI] [PubMed] [Google Scholar]
- 25.Poligone B, Baldwin AS. Positive and negative regulation of NF-kappaB by COX-2: roles of different prostaglandins. J Biol Chem. 2001;276:38658–64. doi: 10.1074/jbc.M106599200. [DOI] [PubMed] [Google Scholar]
- 26.Kellogg AP, Cheng HT, Pop-Busui R. Cyclooxygenase-2 pathway as a potential therapeutic target in diabetic peripheral neuropathy. Curr Drug Targets. 2008;9:68–76. doi: 10.2174/138945008783431691. [DOI] [PubMed] [Google Scholar]
- 27.Gomez PF, Pillinger MH, Attur M, Marjanovic N, Dave M, Park J. et al. Resolution of inflammation: prostaglandin E2 dissociates nuclear trafficking of individual NF-kappaB subunits (p65, p50) in stimulated rheumatoid synovial fibroblasts. J Immunol. 2005;175:6924–30. doi: 10.4049/jimmunol.175.10.6924. [DOI] [PubMed] [Google Scholar]
- 28.Dickman A, Ellershaw J. NSAIDs: gastroprotection or selective COX-2 inhibitor? Palliat Med. 2004;18:275–86. doi: 10.1191/0269216304pm894fd. [DOI] [PubMed] [Google Scholar]
- 29.Parisien M, Lima LV, Dagostino C, El-Hachem N, Drury GL, Grant AV. et al. Acute inflammatory response via neutrophil activation protects against the development of chronic pain. Sci Transl Med. 2022;14:eabj9954. doi: 10.1126/scitranslmed.abj9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luan B, Yoon YS, Le Lay J, Kaestner KH, Hedrick S, Montminy M. CREB pathway links PGE2 signaling with macrophage polarization. Proc Natl Acad Sci U S A. 2015;112:15642–7. doi: 10.1073/pnas.1519644112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sreeramkumar V, Hons M, Punzon C, Stein JV, Sancho D, Fresno M. et al. Efficient T-cell priming and activation requires signaling through prostaglandin E2 (EP) receptors. Immunol Cell Biol. 2016;94:39–51. doi: 10.1038/icb.2015.62. [DOI] [PubMed] [Google Scholar]
- 32.Sreeramkumar V, Fresno M, Cuesta N. Prostaglandin E2 and T cells: friends or foes? Immunol Cell Biol. 2012;90:579–86. doi: 10.1038/icb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahic M, Yaqub S, Johansson CC, Tasken K, Aandahl EM. FOXP3+CD4+CD25+ adaptive regulatory T cells express cyclooxygenase-2 and suppress effector T cells by a prostaglandin E2-dependent mechanism. J Immunol. 2006;177:246–54. doi: 10.4049/jimmunol.177.1.246. [DOI] [PubMed] [Google Scholar]
- 34.Yao C, Sakata D, Esaki Y, Li Y, Matsuoka T, Kuroiwa K. et al. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med. 2009;15:633–40. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- 35.Palla AR, Ravichandran M, Wang YX, Alexandrova L, Yang AV, Kraft P, Inhibition of prostaglandin-degrading enzyme 15-PGDH rejuvenates aged muscle mass and strength. Science. 2021. 371. [DOI] [PMC free article] [PubMed]
- 36.Zhou L, Zhou W, Joseph AM, Chu C, Putzel GG, Fang B. et al. Group 3 innate lymphoid cells produce the growth factor HB-EGF to protect the intestine from TNF-mediated inflammation. Nat Immunol. 2022;23:251–61. doi: 10.1038/s41590-021-01110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raucci F, Saviano A, Casillo GM, Guerra-Rodriguez M, Mansour AA, Piccolo M. et al. IL-17-induced inflammation modulates the mPGES-1/PPAR-gamma pathway in monocytes/macrophages. Br J Pharmacol. 2022;179:1857–73. doi: 10.1111/bph.15413. [DOI] [PubMed] [Google Scholar]
- 38.Ebstein W. Invited comment on W. Ebstein: On the therapy of diabetes mellitus, in particular on the application of sodium salicylate. J Mol Med (Berl) 2002;80:618. discussion 9. [PubMed] [Google Scholar]
- 39.Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE. et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321–6. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 41.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M. et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–7. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 42.Goldfine AB, Fonseca V, Jablonski KA, Pyle L, Staten MA, Shoelson SE. et al. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2010;152:346–57. doi: 10.1059/0003-4819-152-6-201003160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Persaud SJ, Burns CJ, Belin VD, Jones PM. Glucose-induced regulation of COX-2 expression in human islets of Langerhans. Diabetes. 2004;53(Suppl 1):S190–2. doi: 10.2337/diabetes.53.2007.s190. [DOI] [PubMed] [Google Scholar]
- 44.Shanmugam N, Todorov IT, Nair I, Omori K, Reddy MA, Natarajan R. Increased expression of cyclooxygenase-2 in human pancreatic islets treated with high glucose or ligands of the advanced glycation endproduct-specific receptor (AGER), and in islets from diabetic mice. Diabetologia. 2006;49:100–7. doi: 10.1007/s00125-005-0065-7. [DOI] [PubMed] [Google Scholar]
- 45.Oshima H, Taketo MM, Oshima M. Destruction of pancreatic beta-cells by transgenic induction of prostaglandin E2 in the islets. J Biol Chem. 2006;281:29330–6. doi: 10.1074/jbc.M602424200. [DOI] [PubMed] [Google Scholar]
- 46.Vennemann A, Gerstner A, Kern N, Ferreiros Bouzas N, Narumiya S, Maruyama T. et al. PTGS-2-PTGER2/4 signaling pathway partially protects from diabetogenic toxicity of streptozotocin in mice. Diabetes. 2012;61:1879–87. doi: 10.2337/db11-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cipollone F, Iezzi A, Fazia M, Zucchelli M, Pini B, Cuccurullo C. et al. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: role of glycemic control. Circulation. 2003;108:1070–7. doi: 10.1161/01.CIR.0000086014.80477.0D. [DOI] [PubMed] [Google Scholar]
- 48.Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Du Yan S. et al. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem. 1999;274:31740–9. doi: 10.1074/jbc.274.44.31740. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi P, Xavier DJ, Lima J, Evangelista AF, Collares CVA, Foss-Freitas MC. et al. Transcript Expression Profiles and MicroRNA Regulation Indicate an Upregulation of Processes Linked to Oxidative Stress, DNA Repair, Cell Death, and Inflammation in Type 1 Diabetes Mellitus Patients. J Diabetes Res. 2022;2022:3511329. doi: 10.1155/2022/3511329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Litherland SA, Xie XT, Hutson AD, Wasserfall C, Whittaker DS, She JX. et al. Aberrant prostaglandin synthase 2 expression defines an antigen-presenting cell defect for insulin-dependent diabetes mellitus. J Clin Invest. 1999;104:515–23. doi: 10.1172/JCI4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Litherland SA, She JX, Schatz D, Fuller K, Hutson AD, Peng RH. et al. Aberrant monocyte prostaglandin synthase 2 (PGS2) expression in type 1 diabetes before and after disease onset. Pediatr Diabetes. 2003;4:10–8. doi: 10.1034/j.1399-5448.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- 52.Carboneau BA, Breyer RM, Gannon M. Regulation of pancreatic beta-cell function and mass dynamics by prostaglandin signaling. J Cell Commun Signal. 2017;11:105–16. doi: 10.1007/s12079-017-0377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Medeiros A, Peres-Buzalaf C, Fortino Verdan F, Serezani CH. Prostaglandin E2 and the suppression of phagocyte innate immune responses in different organs. Mediators Inflamm. 2012;2012:327568. doi: 10.1155/2012/327568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amior L, Srivastava R, Nano R, Bertuzzi F, Melloul D. The role of Cox-2 and prostaglandin E2 receptor EP3 in pancreatic beta-cell death. FASEB J. 2019;33:4975–86. doi: 10.1096/fj.201801823R. [DOI] [PubMed] [Google Scholar]
- 55.Robertson RP, Tsai P, Little SA, Zhang HJ, Walseth TF. Receptor-mediated adenylate cyclase-coupled mechanism for PGE2 inhibition of insulin secretion in HIT cells. Diabetes. 1987;36:1047–53. doi: 10.2337/diab.36.9.1047. [DOI] [PubMed] [Google Scholar]
- 56.Tran PO, Gleason CE, Robertson RP. Inhibition of interleukin-1beta-induced COX-2 and EP3 gene expression by sodium salicylate enhances pancreatic islet beta-cell function. Diabetes. 2002;51:1772–8. doi: 10.2337/diabetes.51.6.1772. [DOI] [PubMed] [Google Scholar]
- 57.Bosma KJ, Andrei SR, Katz LS, Smith AA, Dunn JC, Ricciardi VF. et al. Pharmacological blockade of the EP3 prostaglandin E2 receptor in the setting of type 2 diabetes enhances beta-cell proliferation and identity and relieves oxidative damage. Mol Metab. 2021;54:101347. doi: 10.1016/j.molmet.2021.101347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34(Suppl 2):S279–84. doi: 10.2337/dc11-s231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carboneau BA, Allan JA, Townsend SE, Kimple ME, Breyer RM, Gannon M. Opposing effects of prostaglandin E2 receptors EP3 and EP4 on mouse and human beta-cell survival and proliferation. Mol Metab. 2017;6:548–59. doi: 10.1016/j.molmet.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tuttle RL, Gill NS, Pugh W, Lee JP, Koeberlein B, Furth EE. et al. Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nat Med. 2001;7:1133–7. doi: 10.1038/nm1001-1133. [DOI] [PubMed] [Google Scholar]
- 61.Fatrai S, Elghazi L, Balcazar N, Cras-Meneur C, Krits I, Kiyokawa H. et al. Akt induces beta-cell proliferation by regulating cyclin D1, cyclin D2, and p21 levels and cyclin-dependent kinase-4 activity. Diabetes. 2006;55:318–25. doi: 10.2337/diabetes.55.02.06.db05-0757. [DOI] [PubMed] [Google Scholar]
- 62.Hamilton D, Rugg C, Davis N, Kvezereli M, Tafti BA, Busque S. et al. A preconditioning regimen with a PKCvarepsilon activator improves islet graft function in a mouse transplant model. Cell Transplant. 2014;23:913–9. doi: 10.3727/096368913X665567. [DOI] [PubMed] [Google Scholar]
- 63.Bai XM, Jiang H, Ding JX, Peng T, Ma J, Wang YH. et al. Prostaglandin E2 upregulates survivin expression via the EP1 receptor in hepatocellular carcinoma cells. Life Sci. 2010;86:214–23. doi: 10.1016/j.lfs.2009.12.009. [DOI] [PubMed] [Google Scholar]