Abstract
Aims
We performed a systematic review and meta-analysis on the effect of HIV infection and antiretroviral therapy (ART) on carotid intima-media thickness (cIMT) to elucidate the role of HIV infection and ART. Also, an analysis on the role of ethnicity and gender on cIMT in HIV-infected populations was performed.
Main methods
We searched the PubMed, Web of Science, the WHO websites and International AIDS Society for published observational studies were conducted by two independent reviewers for studies comparing HIV-infected antiretroviral-experienced patients and/or inexperienced with healthy controls on cIMT. The primary outcome was the standardized mean difference (SMD) of cIMT.
Findings
Twenty studies (five cohort, 15 cross-sectional, and two both cohort and cross-sectional studies) were identified comprising 7,948 subjects (4,656 HIV-infected; 3,292 controls). In cohort studies, the standardized mean 1-year change in cIMT between HIV-infected patients and uninfected controls was not significantly different (0.16 mm/yr; 95% CI, −0.16, 0.49; p=0.326). In 17 cross-sectional studies, the SMD in cIMT was significantly higher in HIV-infected than uninfected persons (0.27 mm; 95% CI, 0.04, 0.49; p=0.027). HIV-infected patients on ART exhibited significantly higher SMD in cIMT compared to those not on ART (0.75 mm; 95% CI, 0.30, 1.19; p=0.001). No confounding effect of gender and ethnicity could be established using meta-regression p> 0.05.
Significance
HIV infection itself and ART appear to influence the progression of cIMT and hence may be risk factors for cardiovascular events. No firm conclusions could be drawn on the effect of ethnic/race and gender differences on cIMT in HIV-infected populations.
Keywords: human-immunodeficiency virus (HIV), antiretroviral therapy (ART), carotid intima-media thickness (cIMT), ethnicity, gender
Introduction
As the HIV-infected population continues to age, cardiovascular disease is becoming an increasingly important problem. This is particularly true as emerging data indicate that even after controlling for traditional cardiovascular risk factors, HIV-infected patients have increased rates of atherosclerosis-related disease [1–2]. There are concerns that the long-term use of antiretroviral drugs may promote atherogenesis, as these drugs can adversely affect cardiovascular risk factors [3–4]. Advances in antiretroviral therapy have greatly improved the survival of patients infected with the human immunodeficiency virus. However, in recent years, the incidence of premature myocardial infarction and cerebrovascular disease in HIV-infected patients receiving antiretroviral therapy has increased [5–7]. It is conceivable that this increase may be, at least in part, a consequence of the metabolic syndrome that clusters with antiretroviral therapy. On the other hand, HIV infection may set in motion atherogenic mechanisms, irrespective of antiretroviral therapy and the associated metabolic sequelae [8–10]. Other studies, however, have associated both HIV and antiretroviral therapy (ART) with the increased cardiovascular disease (CVD) risk [11].
Contradictory findings have been reported on the difference in carotid intima-media thickness (cIMT) between individuals of African ethnicity/race in contrast with non-African HIV-uninfected populations. For example, some studies have reported higher cIMT in individuals of African origin than in non-African counterparts [12–17] while one study did not find significant differences [18]. Also, studies have demonstrated that persons of male gender have higher cIMT than female in non-HIV-infected populations [16, 19–22]. It is unclear whether such ethnicity/race or gender differences on cIMT also exist in HIV-infected persons.
cIMT is a valid measure of subclinical atherosclerosis, which has consistently been related to future cardiovascular events in population studies [23–24], and also correlated with the extent of coronary atherosclerosis [23, 25–28]. The use of cIMT as a surrogate marker of CVD risk among HIV-infected patients has been widely investigated [1, 29, 30] and accepted for assessing large numbers of HIV-infected individual for CVD risk, especially young populations, due to its non-invasive nature [31]. It has been alluded that, because HIV and atherosclerosis are both inflammatory in nature, arterial wall markers of inflammation (cIMT) are increased in HIV-infected patients. Moreover, studies have shown that the magnitude of association between HIV infection with cIMT is similar to that of traditional risk factors (e.g. smoking and diabetes) [29] and larger observational studies have attested that the association of increased cIMT to CVD risks are similar disregarding the site of measurement [31, 32] though at different rates [30]. In this review we have collected papers written in English language that have studied cIMT in HIV. We have attempted to interpret the different results in published studies to dissect out the precise role of this surrogate marker in different populations. Here, we systematically reviewed and carried out meta-analysis on the effects of HIV infection and ART on cIMT to provide additional information on the role of HIV infection per se, and the effect of antiretroviral therapy. We also analyzed the role of ethnicity/race and gender on cIMT in HIV-infected populations.
Methods
Publication Search
We searched PubMed, Web of Science and relevant websites such as those of the WHO and International AIDS Society during the year 2014. Additionally, we conducted a manual search by screening the references of pertinent articles and identifying any additional relevant publications that were not previously included.
We included observational studies; both prospective cohort and cross-sectional studies. Details of the search strategy are shown in Table 1.
Table 1.
Search strategy used in PubMed
| Search # | PubMed search terms |
|---|---|
| ________ | |
| #5 | Search #3 AND #4 |
| #4 | Search #1 OR #2 |
| #3 | carotid artery stiffness [title/abstract] OR carotid stiffness [title/abstract] OR carotid intima media thickness [title/abstract] OR cIMT [title/abstract] |
| #2 | antiretroviral therapy, highly active [MesH] OR combination antiretroviral therapy [title/abstract] OR antiretroviral drug [title/abstract] |
| #1 | HIV [MesH] OR human immunodeficiency virus [title/abstract] OR HIV-infected [title/abstract] OR HIV infection title/abstract] |
Limits: Humans, English, German, Dutch, Young adults, Adults, Middle aged and Middle aged + aged.
Inclusion criteria
The selected studies met the following criteria:
Observational study, that is, cross-sectional/case control or prospective cohort
Evaluation of effect of HIV and /or combination anti retroviral therapy (cART) on cIMT
Outcome variable is cIMT presented as mean ± standard deviation or median ± interquartile range
Article published in English
Publication quality score according to Newcastle-Ottawa scale of at least five stars [33]
Publications published between year 2000 and 2014
Follow-up period of at least one year for cohort studies
Exclusion criteria
Duplicate publications or multiple articles reporting identical outcomes measured over the same time period on the same population.
Articles including patient age of under 17 or above 65 years because cIMT analyses have shown that cIMT is age-related and more so from age 65 and above in men and women [34]. Also, studies have alluded that carotid arterial wall is not affected by either age or gender up to the age of about 18 years [35–36].
Data extraction
Two authors independently conducted the literature search and extraction of relevant articles. Articles were independently judged for quality by the two authors using the Newcastle-Ottawa scale. This instrument is recommended for use by the Cochrane Collaboration Review Group on HIV infection and AIDS [37]. Articles extracted had to meet the following criteria: 1. Description of patient selection (four criteria); 2. Study-control group comparability (one criterion); and 3. Outcome assessment (three criteria). Assigning a star for each qualifying item scored each item in the three groups of criteria. A Quality Assessment tool for Diagnostic Accuracy Studies 2 (QUADAS 2) was used to evaluate the risk of bias and applicability of primary diagnostic accuracy studies [33]. Any arising differences between the two independent authors on article selection were resolved by consensus.
From the articles identified, we registered study design, sample size, technique of measuring cIMT, use or non-use of cART, matched variables, mean age, site measured, and follow-up period for cohort studies.
Studies with no follow-up but that compared HIV-infected patients on cART or not with HIV-uninfected controls were classified as cross-sectional. Also, in this category were studies comparing HIV-infected patients on cART with HIV-infected cART-naïve patients. Studies with follow-up comparing HIV-infected patients and HIV-uninfected controls were categorized as cohort.
Statistical analysis
After extracting the individual study results, pooling was performed by weighting/standardizing the mean of cIMT by the study size. The sum of the products of mean of cIMT and sample size for all studies was determined which was then divided by the sum of the sample sizes of all studies involved to obtain the weighted/standardized mean cIMT. Standardized mean differences (SMD) together with corresponding 95% confidence intervals (CI) comparing HIV-infected and uninfected patients, and cART use and non-use were computed and used to construct forest plots. The presence or absence of publication bias was determined using a funnel plot (graphical representation of a measure of study size as a function of effect size). To test the publication bias, the Egger’s regression test was performed to determine I-square (index for heterogeneity). MedCalc Statistical Software version 16.8 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2016) was used to complete the meta-analysis. Subgroup and meta-regression analysis was conducted on cross-sectional studies using R software version 3.3.3 to investigate the sources of heterogeneity. We conducted subgroup analyses for duration of cART use, age, duration of HIV, current CD4 count, nadir CD4 count, viral load, and smoking predominance as these have been identified as effect modifiers for cIMT [11, 19, 34]. Furthermore, subgroup analysis was conducted on gender and ethnicity/race predominance since these have been reported to have cIMT differences in healthy populations [12–21]. Sensitivity analysis by omitting one study at a time and meta-regression were also conducted on cross-sectional studies to determine sources of heterogeneity. All significance tests were done at 95% confidence interval and 5% level of significance.
Results
Study characteristics and quality
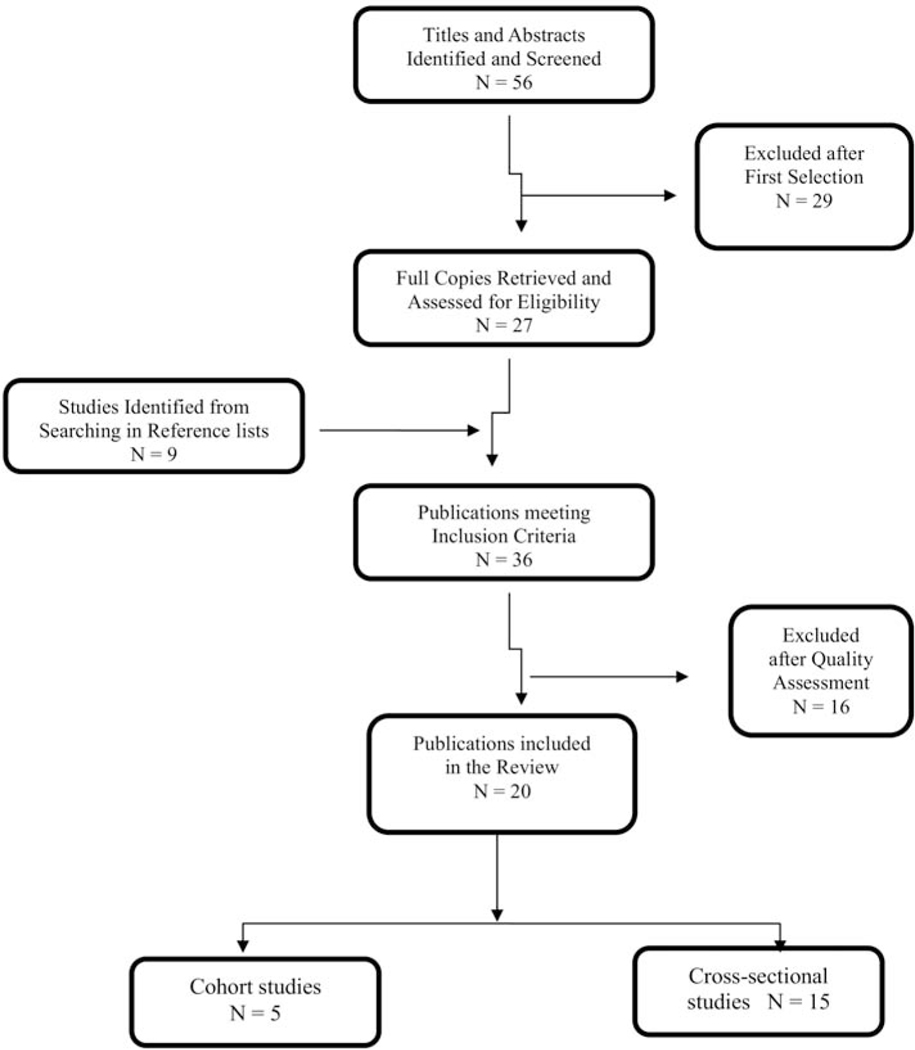
Table 2 shows the study characteristics. The systematic literature search identified 56 articles. At first selection 29 articles were excluded because they were duplicate publications reporting identical outcomes measured over the same time period on the same population, had no control group or study population involved elderly older than 65 years; thus, 27 articles remained. An additional 9 articles were identified through searches of references. Thirty-six articles met the general study criteria, but only 22 met the minimum of 5-star quality criteria. Two studies were excluded because they were systematic reviews, thus remaining with 20 studies in this review (Figure 1). Fifteen studies were cross-sectional and five cohort. Two studies were cross-sectional derived from cohort studies and therefore included in both cohort and cross-sectional studies. Studies used different anatomic sites to measure cIMT; seven studies measured cIMT at one site of the unilateral carotid artery, three studies at multiple sites of the bilateral common carotid artery, three studies used mean of 12 sites of the bilateral carotid artery, six studies measured cIMT at 2 sites of the bilateral carotid artery, and one study did not specify the site of measurement (Table 2). Most patients on cART used a combination of two nucleoside reverse transcriptase inhibitors (NRTI’s) plus a protease inhibitor (PI) or non-nucleoside reverse transcriptase inhibitors (NNRTI).
Table 2.
Study characteristics
| Author | Study Design | No. | HIV+ | HIV+/ART− | HIV+/ART+ | HIV− | Follow-up period (months) | Site measured | Variables Adjusted |
|---|---|---|---|---|---|---|---|---|---|
| Hsue et al.2004[1] | PCO | 211 | 148 | 0 | 148 | 63 | 12 | Mean of 12 sites | Age and Sex |
| Hsue et al.2012 [39] | PCO | 347 | 300 | 300 | 0 | 47 | 28 | CCA,ICA,BCC | Demographics, Traditional risk factors, hsCRP, IMT progression |
| Kelesidis et al.2012 [38] | PCO | 91 | 55 | 26 | 29 | 36 | 144 | RCC | Age, sex, race/ethnicity, smoking, BP and menopause |
| Hileman et al.2013 [40] | PCO | 130 | 85 | 85 | 0 | 45 | 48 | CCA,BCC | CVD risk factors, Age, sex and BMI |
| Hileman et al.2014 [41] | PCO | 83 | 42 | 42 | 41 | 96 | CCA, BCC | CVD risk factors, Age, sex and BMI | |
| Papita et al.2011 [51] | CS | 99 | 63 | 7 | 56 | 36 | RCC,CBA | Age and Sex | |
| Lorenz et al.2008[3] | CS | 1460 | 292 | 0 | 292 | 1168 | CCA/BIF | Age, sex, BMI, DM, BP, Cholesterol, use of statins and smoking | |
| Seminari et al.2002 [54] | CS | 59 | 43 | 15 | 28 | 16 | MCCA/CCCA | Age, Weight, Height, BP and HIV risk factors | |
| van Vonderen et al.2006[42] | CS | 129 | 77 | 22 | 55 | 52 | RCC/CFA/CBA | Age, BMI, Mean arterial BP, Smoking and Createnine clearance | |
| Oliviero et al.2009 [52] | CS | 79 | 38 | 38 | 0 | 41 | Mean of 12 sites | Age, sex, BMI and smoking | |
| Mondy et al.2008 [53] | CS | 100 | 50 | 0 | 50 | 50 | BCC | Age, Sex, race and BMI | |
| Jung et al.2015 [45] | CS | 1792 | 1282 | 0 | 0 | 510 | BCC | Age, race/ethnicity, Serum lipids, BP and Smoking | |
| Cristofaro et al.2011 [47] | CS | 421 | 286 | 64 | 222 | 135 | CCA | Unadjusted | |
| Desvarieux et al.2013 [43] | CS | 150 | 100 | 50 | 50 | 50 | Mean of 12 sites | Age,smoking,DM,HT | |
| Fitch at el.2013 [48] | CS | 318 | 166 | 0 | 0 | 152 | RCC& ICC | Age, race, gender, traditional CVD risks, use of statins, Ant-HT, ART use CD4 and VL | |
| Karim et al.2013 [46] | CS | 584 | 170 | 0 | 0 | 414 | RCC | Age, race, BMI, DM, Smoking, alcohol intake, IDU, non-IDU, ART, HIV and VL | |
| Monroe et al.2012 [44] | CS | 856 | 534 | 0 | 0 | 322 | RCC/ICA/CBA | Unadjusted | |
| Vigano et al.2010[11] | CS | 42 | 23 | 0 | 0 | 19 | CCA | Age, gender and BMI | |
| Zormpala et al.2012 [49] | CS | 229 | 105 | 0 | 0 | 124 | R&L CCA | Age, sex and Conversional CVD frisk factors | |
| Kristoffersen et al.2013 [50] | CS | 210 | 105 | 0 | 105 | 105 | Did not specify | Age, gender and Smoking |
PCO – Prospective cohort study; CS- Cross-sectional; BCC – Bifurcation common carotid; CFA – Common femoral artery; CBA – Common brachial artery; LCC – Left common carotid; ICA – Internal carotid artery; RCC – Right common carotid; hsCRP-high sensitive C-reactive protein; IMT-Intima Media Thickness; BP-Blood Pressure; CVD- Cardiovascular Disease; BMI-Body Mass Index; DM-Diabetes Mellitus; HT- Hypertension; ART-Antiretroviral Therapy; IDU- Intravenous Drug Users; VL- Viral Load
Figure 1.
Search strategy chart. The systematic literature search identified 56 articles. After exclusion 22 studies met the minimum of 5-star quality criteria. 2 studies were cross-sectional derived from cohort studies and therefore included in both cohort and cross-sectional studies. 15 studies were cross-sectional and 5 cohort. In the final, 20 studies were included in this review
Cohort studies
Within the 5 cohort studies there were 862 patients (630 HIV-infected and 232 HIV-uninfected). Mean age was 42.5 and 40.7 years for HIV-infected and uninfected patients, respectively. All cohort studies except one [39] were matched by gender, age and body mass index (BMI). In two studies [39, 40], there were more African Americans in the HIV-infected group. In three studies the HIV-infected patients were on cART [1, 38–39] with mean duration of 3 years while the remaining two studies compared ART-naïve HIV-infected with HIV-uninfected control [40, 41]. The sites of measurement of carotid artery are indicated in Table 2. One study [1] used a mean of 12 measurements at the common carotid artery; another [38] measured only at the right common carotid artery while three studies [39, 40, 41] measured at the common carotid artery and the bulb and/or internal carotid artery. Mean duration of HIV infection was 7.2 years. Mean duration of follow-up was 44.5 months ranging from 12 to 144 months.
Assessment of publication bias was not performed in cohort studies as there was an inadequate number of studies (only 5) to properly assess a funnel plot or use more advanced regression-based methods.
Cross-sectional studies
HIV-infected vs. HIV-uninfected
Within the 17 cross-sectional studies there were 7,086 patients (4,026 HIV-infected and 3,060 HIV-negative). The mean age was 41.6 and 41.4 years for HIV-infected and non-infected, respectively. The BMI was 24.7 kg/m2 and 25.7 kg/m2 for HIV-infected and non-infected, respectively. Eight studies indicated the mean duration of HIV since diagnosis was 7.5 (range, 1.5–13) years. Three studies [42–44] investigated only male groups while two [45–46] included only female. Thirteen studies [1, 39, 42, 44, 46–54] reported smokers in both groups but with a significantly higher proportion of HIV-infected smokers in seven studies [1, 39, 42, 44, 47–49]. Nine studies indicated ethnicity/race [11, 39, 42–46, 48, 53].
Eleven studies [1, 3, 38, 40–41, 48–53] were matched for age and gender. Some studies were also matched for BMI [3, 11, 40–41, 52], race [38, 48, 52] and smoking habits [3, 38, 50, 52]. One study was matched for age, smoking habits and HIV infection risk factors [54]. One study investigated only HIV-infected ART-naïve vs. HIV-uninfected controls [48] while one study [11] included adolescents (17–23 years) only. Three studies [1, 38, 53] involved HIV-infected exclusively on cART vs. HIV-uninfected controls.
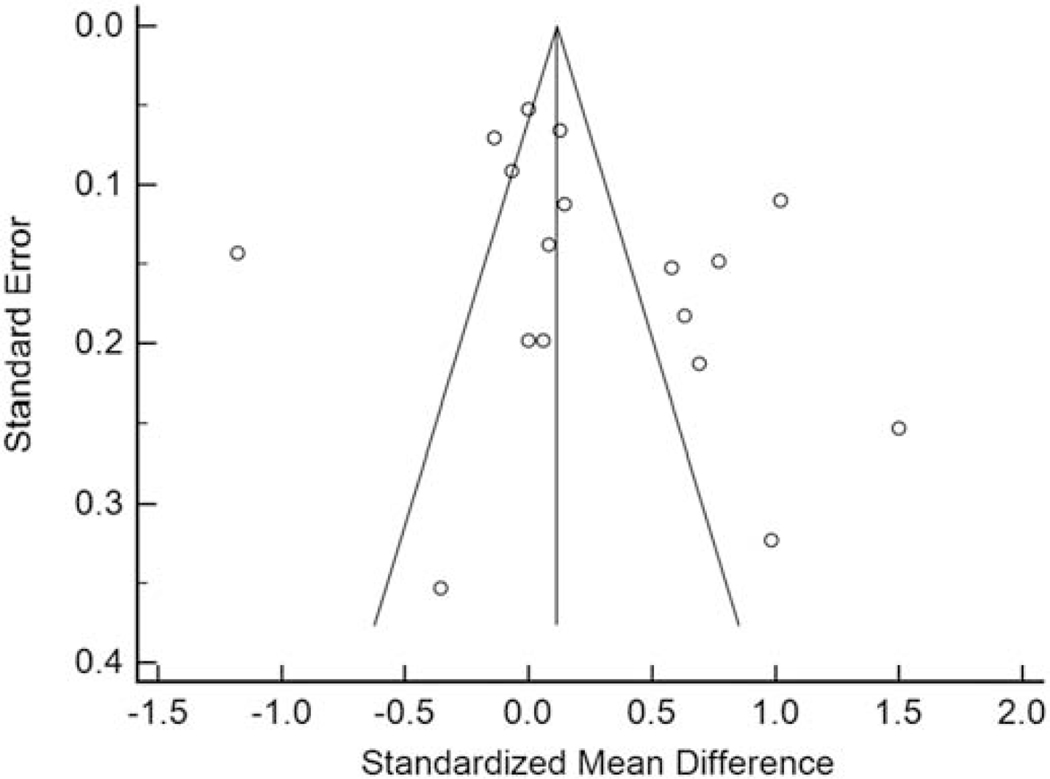
Review of the funnel plot demonstrated asymmetry with 9 studies to the right of the combined effect size and 8 studies to the left (Figure 2). Quantitatively, Egger’s regression indicated the presence of publication bias (intercept=5.28467, 95% CI: 1.97 to 8.59, t=3.39, P=0.00198).
Figure 2.
Funnel plot for the mean differences vs. standard errors in carotid intima-media thickness (mm) in HIV-infected and uninfected groups among cross-sectional studies. Funnel plot demonstrated asymmetry with 9 studies to the right of the combined effect size and 8 studies to the left. Egger’s regression indicated the presence of publication bias (intercept=5.28467, 95% CI: 1.97 to 8.59, t=3.39, P=0.00198).
HIV-infected on cART vs. HIV-infected cART-naïve
Four studies compared cART-experienced and naïve HIV-infected patients [42–43, 48, 54]. In two studies [42, 43], three cART regimens - namely, NNRTI, NRTI and PI were used while in one study [54] only PI was used and the other study [47] did not specify the type of regimen used. For all studies, patients had used cART for at least one year, and the mean duration of cART was 3.7 years. One study [43] involved only men. Two studies [42, 47] were not matched while one study [54] was matched for age, risk factors for HIV infection, cigarette use and CD4+ cell count and another [43] matched for age and non-smoking groups.
Data synthesis
Cohort studies
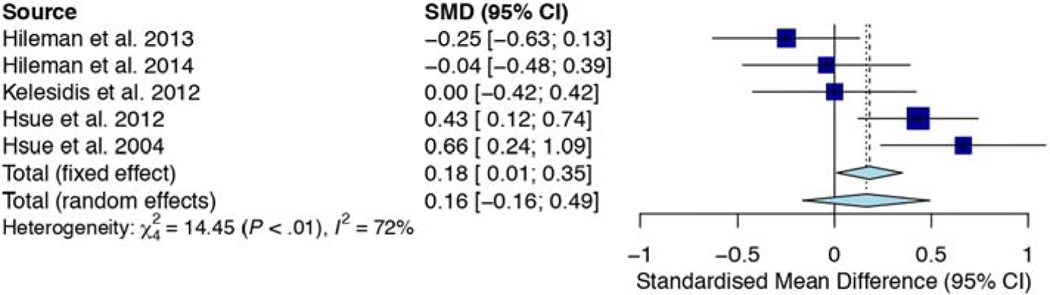
All five studies compared HIV-infected and uninfected groups on the 1-year progression of cIMT. Three studies compared HIV-infected on cART [1, 38, 39] while two studies compared HIV-infected cART-naïve [40, 41] with healthy controls. Overall, SMD of 1-year cIMT progression between HIV-infected patients and uninfected controls was not significant (SMD, 0.16mm; 95% CI, −0.16, 0.49; p=0.326). Heterogeneity between the studies was moderate (I2, 72.3%; p=0.006). Of the three studies, which compared HIV-infected on cART with healthy controls, two studies [1, 39] had higher 1-year cIMT progression than controls. On the contrary, the two studies [40, 41], which compared HIV-infected ART-naïve with HIV-uninfected controls, exhibited higher 1-year cIMT progression for controls than HIV-infected ART-naïve patients (Figure 3).
Figure 3.
Forest plot showing the 1-year change in carotid intima-media thickness (mm/yr.) in HIV-infected and uninfected groups among the prospective cohort studies. SMD of 1-year cIMT progression between HIV-infected patients and uninfected controls was not significant (SMD, 0.16mm; 95% CI, −0.16, 0.49; p=0.326). Heterogeneity between the studies was moderate (I2, 72.3%; p=0.006).
Cross-sectional studies
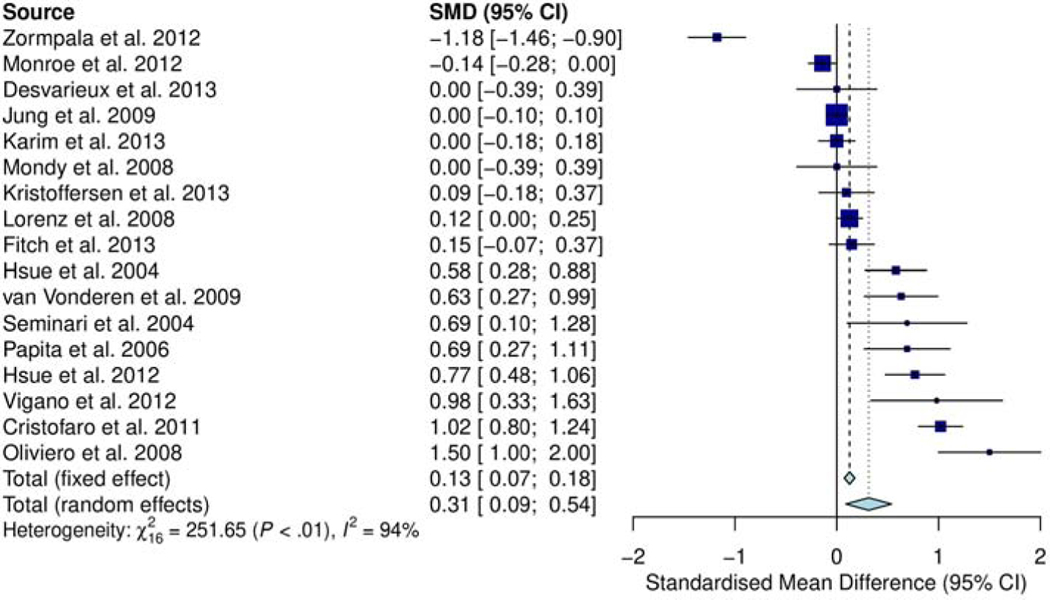
Using the 17 cross-sectional studies comparing SMD in cIMT for HIV-infected and uninfected, the pooled SMD of cIMT is shown in Figure 4. Significant increase in standardized mean of cIMT was observed in HIV-infected compared to HIV-uninfected patients (0.27 mm; 95% CI, 0.04, 0.49; p=0.027). Of 17 studies, eight [1, 11, 39, 42, 47, 51, 52, 54] demonstrated greater elevation in cIMT in HIV-infected than uninfected. Five of these studies [11, 42, 47, 52, 54] had small numbers of HIV-infected cases (<80 patients), which were characterized by wide 95% CI (standard error (SE) ranging from ±0.72 to ±1.30 mm). Of the remaining nine studies, seven studies [24, 43, 45, 46, 48, 50, 53] were close to or at the line of no difference (SMD ≈ 0.0); most of which were relatively large (>200 study cases). Of these, two studies [43, 53] had relatively small sample sizes (≤150 cases) with wide CI. (SE ±0.39mm each). Two studies [44, 49] depicted greater cIMT in HIV-uninfected than infected patients.
Figure 4.
Forest plot showing 17 cross-sectional studies comparing SMD in cIMT for HIV-infected and uninfected. Significant increase in SMD of cIMT was observed in HIV-infected compared to HIV-uninfected patients (0.27 mm; 95% CI, 0.04, 0.49; p=0.027)
Heterogeneity among studies was significant (I2, 94%; p<0.01) (Figure 4). Subgroup analysis for age, duration of HIV, duration of cART use, nadir CD4, current CD4 count, viral load, and smoking predominance revealed that heterogeneity for all subgroup analyses was high (Shown in supplementary figures 1–7). This implies that there are studies with variable observed effects than would be expected by chance alone. Sensitivity by omission of one study at a time and meta-regression showed that only nadir CD4 and smoking were important confounders (Shown in supplementary tables S1-S2 and figures S4 and S7). Studies reporting relatively higher SMD were likely to include cases in the smokers predominant group [1, 39, 42, 47, 51, 52] and with nadir CD4 count of 200 cells/mL or lower [1, 39, 42] (Figure 4, Figure S7, Figure S4).
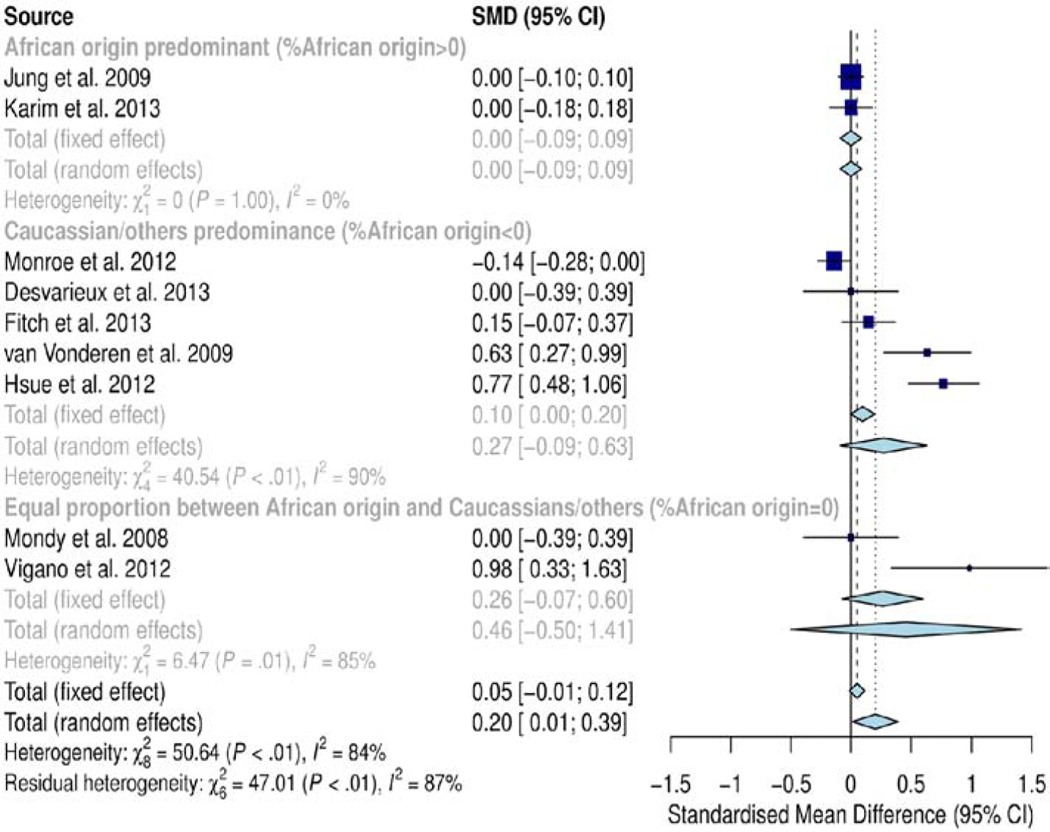
Subgroup analysis by ethnicity/race showed significant difference in SMD of cIMT between subgroups (SMD=0.20mm, CI=0.01, 0.39; p<0.01). The subgroup dominated by individuals of African origin exhibited no significant difference (SMD=0.0mm; p=1.0), the subgroup dominated by Caucasians/others had significantly higher SMD of cIMT (0.27 mm; p<0.01) and the subgroup matched for race (equal proportion) also showed significantly higher SMD of cIMT (0.46mm; p<0.01) (Figure 5). Overall, heterogeneity in this subgroup analysis was high (I2, 84%; p<0.01). Meta-regression analysis could not establish the confounding effect of race (Table S2). However, a smaller number of studies and cases contributed data to the African origin predominant subgroup (680 cases, 2 studies) and equal proportion subgroup (142 cases, 2 studies) compared to the Caucasian/other race predominant subgroup (1800 cases, 5 studies), implying that the analysis is unlikely to produce conclusive results on the effect of ethnicity/race on cIMT.
Figure 5.
Forest plot for race/ethnicity. Subgroup analysis by ethnicity/race showed significant difference in SMD of cIMT between subgroups (SMD=0.20mm, CI=0.01, 0.39; p<0.01). The subgroup dominated by individuals of African origin exhibited no significant difference (SMD=0.0mm; p=1.0), the subgroup dominated by Caucasians/others had significantly higher SMD of cIMT (0.27 mm; p<0.01) and the subgroup matched for race (equal proportion) also showed significantly higher SMD of cIMT (0.46mm; p<0.01). Overall, heterogeneity in this subgroup analysis was high (I2, 84%; p<0.01).
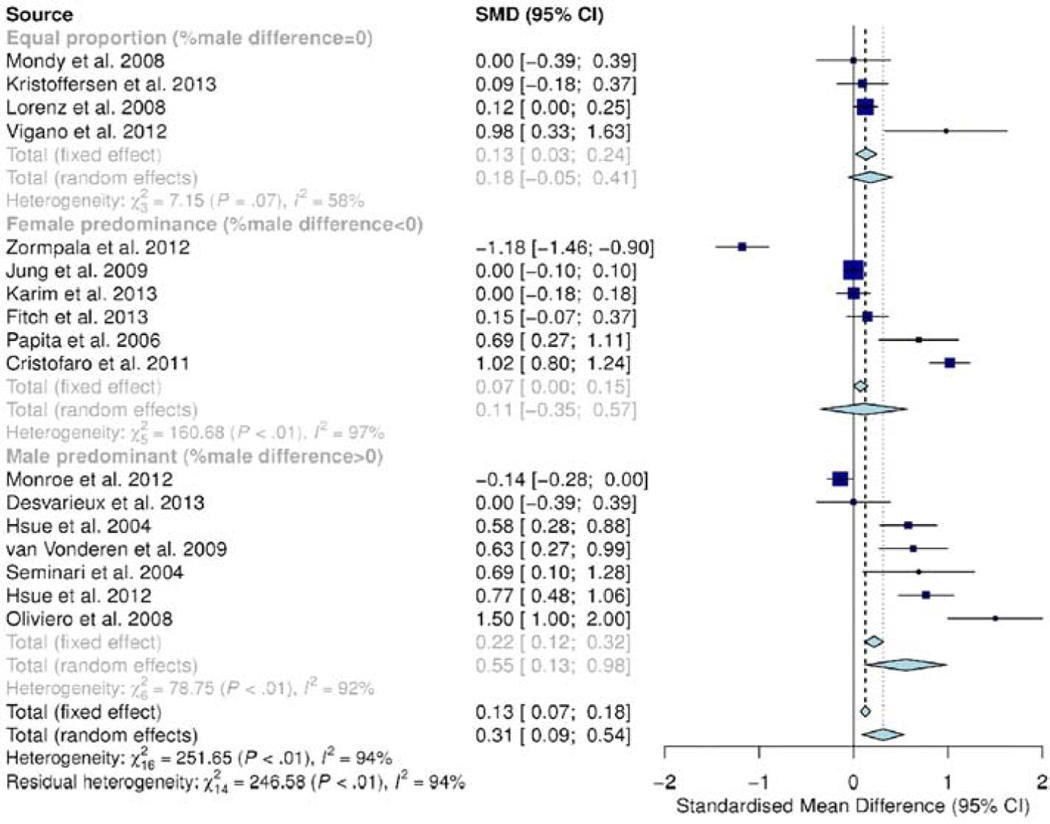
Subgroup analysis by gender (Figure 6) demonstrated statistically significantly subgroup effect (p<0.01) entailing that gender influences cIMT. A sufficient number of studies (4–7) and cases (>1800) in each subgroup, hence covariate distribution is of little concern. However, there was moderate to substantial unexplained heterogeneity between studies in each subgroup (equal gender proportion group: I2 = 58%; male dominated subgroup: I2 = 92% and female dominated subgroup: I2 = 97%), meaning that validity of the effect estimate in each subgroup is uncertain. The subgroup “Equal proportion” had moderate heterogeneity, but by omitting Vigano et al. [11] the heterogeneity would drop to 0%. The estimate would also change from 0.18 to 0.11 and would be more consistent (lower p-value). This study included only adolescents (age 17–23 years) and had few study cases (42 cases).
Figure 6.
Subgroup analysis for gender demonstrated statistically significantly subgroup effect (p<0.01) entailing that gender influences cIMT. There was moderate to substantial unexplained heterogeneity between studies in each subgroup (equal gender proportion group: I2 = 58%; male dominated group: I2= 92% and female dominated group: I2 = 97%), meaning that validity of the effect estimate in each subgroup is uncertain.
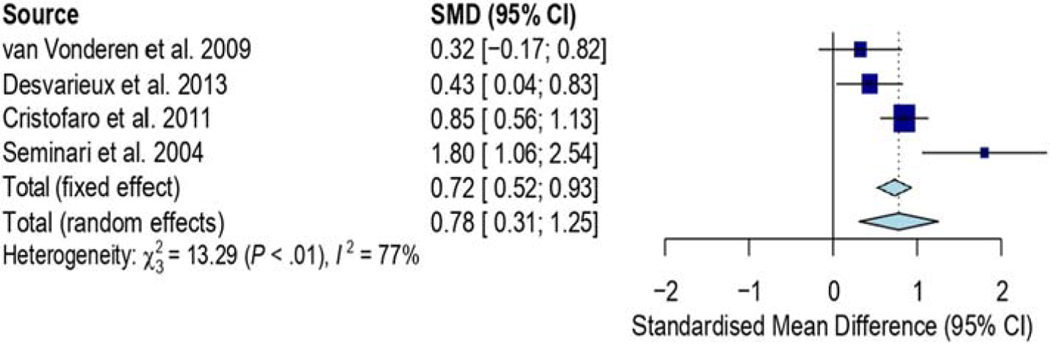
Figure 7 shows four studies comparing HIV-infected on cART and cART-naïve patients. The results show significantly increased standardized mean difference of cIMT in the group of HIV-infected on cART compared to the cART-naïve group (SMD, 0.75mm; 95% CI, 0.30, 1.19; p=0.001). Heterogeneity for these studies was substantial with I2 of 75.2% (p=0.007). However, most of these studies (three out of four) were mostly small; with HIV-infected cases less or equal to 150.
Figure 7.
shows four studies comparing HIV-infected on cART and cART-naïve patients. The results show significantly increased SMD of cIMT in the group of HIV-infected on cART compared to the cART-naïve group (SMD, 0.75mm; 95% CI, 0.30, 1.19; p=0.001). Heterogeneity for these studies was significant with I2 of 75.2% (p=0.007).
Discussion
This review summarizes 20 studies examining cIMT, an early marker for atherosclerosis in HIV-infected patients. We tried to find evidence for the influence of HIV infection (causing a chronic inflammatory state) and cART on this marker. Furthermore, subgroup analyses were conducted to find out existence of cIMT differences according to ethnicity/race and gender in HIV-infected populations.
The findings in this review show that HIV-infected patients have significantly higher values of SMD of cIMT than HIV-uninfected for cross-sectional studies but not significant in cohort studies. Also, HIV-infected patients on cART exhibited markedly higher values than cART-naïve. Furthermore, ethnicity/race subgroup analysis had few studies in the African origin dominated and equal proportion subgroups leading to inconclusive findings on the effect of ethnicity/race on cIMT. Likewise, though the difference was significant between gender subgroups, due to considerable unexplained heterogeneity within subgroups, the effect of gender on cIMT is uncertain.
Controversial results on effect of HIV per se and HIV-associated factors such as cART on cIMT have been reported. Several studies have shown association between HIV-infection and modest increase in cIMT in both observational [55–57] and longitudinal studies [1]. Most meta-analysis studies also have reported increased cIMT in HIV-infected individuals compared to healthy controls [58, 59]. However, some studies have reported no association of HIV infection with cIMT in both observational [60, 61] and prospective studies [62].
The explanation given for higher cIMT in the HIV-infected group compared to uninfected is that any immunosuppressive condition, including HIV infection, may have an impact on both systemic inflammation and other human environmental factors, such as co-infections that may influence atherogenesis [63]. Arterial stiffness is increased in chronic inflammatory disorders and is related to disease duration, cholesterol, and the inflammatory mediator C-reactive protein (CRP) and the cytokine that stimulates production of IL-6 [64]. These findings suggest that HIV infection itself may increase the risk for cardiovascular disease [59] although mechanisms have not been fully explained by differences in cIMT.
The results show increased cIMT in HIV-infected on cART than in cART-naïve patients. It is argued that thicker cIMT could be associated with HIV-related risk factors, such as HIV viremia, immune activation and cART, and classical cardiovascular risk factors such as smoking, hypertension and dyslipidemia [65–67]. Some cART regimens have been reported to be independently associated with CVD [68]. HIV infection per se may change the lipid metabolism by decreasing high-density lipoprotein (HDL) cholesterol, and at the same time, by increasing low density lipoprotein (LDL) cholesterol and total cholesterol which are known to be CVD risk factors [63]. It remains to be determined whether these modifications are due to direct effect of cART or caused indirectly, by their metabolic side-effects [69]. It is also controversial whether specific cART regimens have greater influence on cIMT than others. Thus, while two studies Seminari et al. [54] and Sun et al. [58] demonstrated association with PI use, other studies have found no association between PI-regimen use and cIMT [1,64].
On the contrary, various studies have demonstrated that traditional cardiovascular risk factors, rather than HIV per se and HIV-related factors, independently predict increase in cIMT among HIV-infected individuals [60–62, 70–71]. One recent study in Uganda reported no association between high sensitivity C-reactive protein (hsCRP) and cIMT at common carotid artery (CCA) segment but cIMT was correlated with traditional CVD risk factors such as waist circumference, triglycerides and total cholesterol [68]. If this is true, then the inflammatory effect of HIV on cIMT could be obscured by effect of traditional risk factors such as age for predicting cIMT. On the other hand, Grunfeld et al. [30], Pacheco et al. [60] and Hanna et al. [62] argue that carotid artery segment measurements can be affected differently in HIV-infected patients and since cIMT is not uniformly measured as per consensus in cIMT measurements in the studies [72], conflicting results are imminent. Moreover, cIMT measured at common carotid artery is regarded a weak predictor of atherosclerosis when plaque measurement is missing [73]. Furthermore, though most meta-analyses based on observational studies report an association between HIV and HIV-related factors and cIMT [58, 59], it is argued that small studies tend to report positive results while larger studies cluster around the line of no difference [60].
It is worthy to note that; while cross-sectional studies showed significantly elevated cIMT in HIV-infected than uninfected individuals, such significance was non-existent in cohort studies. Several possible explanations for this discrepancy are possible. One possible explanation could be that cross-sectional studies are characterized by confounding effects compared to cohort studies, which could lead to the divergence in the results [74]. Also, due to the fact that the effects of HIV and cART on cIMT [68, 73] are dynamic and cumulative, such discrepancies in results may be expected [30]. Moreover, since three out of the five cohort studies [38, 40–41] were small while the other two studies [1, 39] had a small number of controls (HIV-uninfected individuals), the divergence in results between cross-sectional and cohort studies may arise.
In our study, subgroup analysis was inconclusive on the effect of gender on cIMT, and this is inconsistent with findings in other studies [29, 72–73], which demonstrated significantly higher cIMT in HIV-infected male compared to their female counterparts. However, upon multivariate analysis in the Albuquerque study [72], after accounting for traditional cardiovascular risk factors, male gender was an independent predictor of elevated cIMT only in the age below 40 years. Jarauta et al. [19] and Sinning et al. [75], studying atherosclerosis in healthy HIV-uninfected persons with no cardiovascular risk factors, found that cIMT in males was higher than in females up to the age less than 55 years. A recent study comparing cIMT between predominantly male HIV-infected patients with mean age of 49.4±10.5 years and reference values of age- and gender-matched uninfected persons reported greater cIMT compared to controls [76]. Another more recent study conducted in Thailand comparing cIMT between virologically suppressed HIV-infected patients and uninfected individuals, upon multivariate analysis in the HIV-infected group, found that cIMT greater than or equal to 0.9mm was predicted by male gender [77]. Several studies have attributed this gender difference in cIMT to gender hormones, specifically the protective effect of estrogen on coronary atherosclerosis [78–80].
Furthermore, the subgroup analysis by ethnicity/race could not substantiate drawing conclusions on the effect of ethnicity/race on cIMT because of fewer studies in two out of three subgroups. On the contrary, the study by Albuquerque [73] reported increased cIMT among nonwhite HIV-infected adults but significantly higher in the age group under 40 years. Other studies have also demonstrated higher cIMT in non-HIV-infected blacks compared to whites in young adulthood [14]. The study by Rosero et al. [18] found significantly higher mean arterial wall thickness in black women compared to Hispanic and white women, though an insignificant difference was observed in men. The reasons for the race/ethnicity differences in cIMT are not clear. Hao et al. [14] and Bennet et al. [13] failed to explain the race/ethnicity difference in cIMT by differences in cardiovascular risk factors.
It is important to note that the results of this review should be interpreted with caution. Firstly, biases inherent to the study design of the original cross-sectional and cohort studies must still be regarded as significant in the final analysis. Secondly, the meta-analysis was characterized by high heterogeneity between studies, sources of which could not be identified even after subgroup and sensitivity analyses. The high between-study heterogeneity was also reported in the study by Sun et al. [57]. However, Alba et al. [81] attest that meta-analyses evaluating continuous outcomes are inherent of substantially higher heterogeneity than those of binary outcomes. Thirdly, only articles written in English were included in the meta-analysis, which could lead to language bias (location bias) and also non-inclusion of grey literature. Fourthly, the meta-analysis included a small number of studies with high variability of sample sizes among studies. Fifthly, the gender and ethnicity/race differences on cIMT was based on subgroup analyses using predominance of these factors between HIV-infected and HIV-uninfected groups in varying proportions rather than on studies among these specific groups (that is, exclusively male vs. exclusively female and exclusively Caucasian vs. exclusively African) and hence the results from the subgroup analyses may not reflect the true differences between these groups. Lastly, most subgroup analyses that were conducted had uneven distribution/inadequate number of studies between subgroups (ethnicity/race, smoking, viral load, ART duration, current CD4 count) jeopardizing drawing valid conclusions on their confounding effect on cIMT.
In a global perspective, the small negative impact of cART on premature atherosclerosis and increased cardiovascular risk is of a different scale compared to the improved outcomes with the effective treatment of HIV and many patients are living healthy lives for decades after diagnosis. Data from randomized controlled trials of cART drugs may further clarify cardiovascular adverse effects but are difficult to assess because the HIV trials may not be powered to assess cardiovascular outcomes.
Conclusion
This review shows evidence for enhanced premature atherosclerosis caused by chronic HIV infection and cART use, measured by cIMT. Whether the negative effect of chronic HIV infection on cIMT is reversed with longstanding effective treatment is likely, further studies in chronically suppressed patients are needed. Ethnic/race and gender differences could not be established in this meta-analysis, probably due to limitations mentioned above.
Supplementary Material
Acknowledgments
The NUFFIC Scholarship programme, The Netherlands, and an International Fellows Program award from the University of Wisconsin System supported this study. JB receives salary support from the US National Institutes of Health Awards P30AI64518, U01AI067854, D43CA153722, and D43TW06732, and from the Health Resources and Services Administration Award T84HA21123. All authors designed the study, contributed and revised manuscript drafts, and approved the final manuscript. MVA, MVF, TF and JB supervised data collection procedures, patient recruitment, and human subject protection. TF and GV performed data collection procedures and developed the dataset. All authors completed the review of literature, data analysis, and prepared text.
Footnotes
Disclaimer: The authors declared no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hsue PY, Lo JC, Franklin A, Bolger AF, Martin JN, Deeks SG et al. Progression of Atherosclerosis as Assessed by Carotid Intima-Media Thickness in Patients With HIV Infection. Circulation 2004;109:1603–1608. [DOI] [PubMed] [Google Scholar]
- 2.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased Acute Myocardial Infarction Rates and Cardiovascular Risk Factors among Patients with Human Immunodeficiency Virus Disease. Journal of Clinical Endocrinology & Metabolism 2007; 92: 2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorenz MW, Stephan C, Harmjanz A, Staszewski S, Buehler A, Bickel M et al. Both long-term HIV infection and highly active antiretroviral therapy are independent risk factors for early carotid atherosclerosis. Atherosclerosis 2008; 196: 720–726 [DOI] [PubMed] [Google Scholar]
- 4.Holmberg SD, Moorman AC, Williamson JM, Tong TC, Ward DJ, Wood KC, et al. Protease inhibitors and cardiovascular outcomes in patients with HIV-1. The Lancet 2002; 360: 1747–1748. [DOI] [PubMed] [Google Scholar]
- 5.Bozzette SA, Ake CF, Tam HK, Chang SW, Louis TA. Cardiovascular and Cerebrovascular Events in Patients Treated for Human Immunodeficiency Virus Infection. New England Journal of Medicine 2003; 348: 702–710. [DOI] [PubMed] [Google Scholar]
- 6.The D: A: D Study Group. Combination Antiretroviral Therapy and the Risk of Myocardial Infarction. New England Journal of Medicine 2003; 349: 1993–2003. [DOI] [PubMed] [Google Scholar]
- 7.d’Arminio A, Sabin CA, Phillips AN, Reiss P, Weber R, Kirk O, et al. Cardio- and cerebrovascular events in HIV-infected persons. AIDS 2004; 18: 1811–7. [DOI] [PubMed] [Google Scholar]
- 8.van Wijk JPH, de Koning EJP, Cabezas MC, Joven J, op’t Roodt J, Rabelink TJ, et al. Functional and Structural Markers of Atherosclerosis in Human Immunodeficiency Virus-Infected Patients. Journal of the American College of Cardiology 2006; 47: 1117–1123. [DOI] [PubMed] [Google Scholar]
- 9.Dhawan S, Puri RK, Kumar A, Duplan H, Masson JM, Aggarwal BB, et al. Human Immunodeficiency Virus-1–Tat Protein Induces the Cell Surface Expression of Endothelial Leukocyte Adhesion Molecule-1, Vascular Cell Adhesion Molecule-1, and Intercellular Adhesion Molecule-1 in Human Endothelial Cells. Blood 1997; 90: 1535–1544. [PubMed] [Google Scholar]
- 10.Park I-W, Ullrich CK, Schoenberger E, Ganju RK, Groopman JE. HIV-1 Tat Induces Microvascular Endothelial Apoptosis Through Caspase Activation. The Journal of Immunology 2001;167: 2766–2771. [DOI] [PubMed] [Google Scholar]
- 11.Vigano A, Bedogni G, Cerini C, Meroni L, Giacomet V, Stucchi S, et al. Both HIV-Infection and Long-Term Antiretroviral Therapy are Associated with Increased Common Carotid Intima-Media Thickness in HIV-Infected Adolescents and Young Adults. Current HIV Research, 2010, 8, 411–417 [DOI] [PubMed] [Google Scholar]
- 12.Casella IB, Sotelo FJB, Yamazaki Y, Presti C, Vassoler A, Melo HAH. Comparison of common carotid artery intima-media thickness between brazilian euro-descendants and afro-descendants with atherosclerosis risk factors. CLINICS 2009; 64 (7):657–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett PC, Gill PS, Silverman S, Blann AD and Lip GYH.Ethnic differences in common carotid intima–media thickness, and the relationship to cardiovascular risk factors and peripheral arterial disease: the Ethnic-Echocardiographic Heart of England Screening Study. Q J Med 2011; 104:245–254 [DOI] [PubMed] [Google Scholar]
- 14.Hao G, Wang X, Treiber FA, Davis H, Leverett S, Su S, Kapuku G. Growth of Carotid Intima-Media Thickness in Black and White Young Adults.J Am Heart Assoc. 2016;5:e004147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erqou S, Kip KE, Mulukutla SR, Aiyer AN and Reis SE. Racial differences in the burden of coronary artery calcium and carotid intima media thickness between Blacks and Whites.Neth Heart J (2015) 23:44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams JE, Couper DJ, Din-Dzietham R, Nieto FJ, and Folsom AR. Race-Gender Differences in the Association of Trait Anger with Subclinical Carotid Artery Atherosclerosis. The Atherosclerosis Risk in Communities Study. Am J Epidemiol 2007; 165:1296–1304 [DOI] [PubMed] [Google Scholar]
- 17.Wendell CR, Waldstein SR, Evans MK, Zonderman AB.Distributions of Subclinical Cardiovascular Disease in a Socioeconomically and Racially Diverse Sample. Stroke. 2017; 48:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosero EB, Peshock RM, Khera A, Clagett P, Lo H, and Timaran CH.Sex, race, and age distributions of mean aortic wall thickness in a multiethnic population-based Sample. J Vasc Surg 2011; 53:950–7 [DOI] [PubMed] [Google Scholar]
- 19.Jarauta E, Mateo-Gallego R, Bea A, Burillo E, Calmarza P,and Civeira F. Carotid Intima-Media Thickness in Subjects With No Cardiovascular Risk Factors. Rev Esp Cardiol. 2010; 63(1):97–102 [DOI] [PubMed] [Google Scholar]
- 20.Tzou WS, Douglas PS, Srinivasan SR, Bond MG, Tang R, Li S, et al.Distribution and Predictors of Carotid Intima-Media Thickness in Young Adults. Prev Cardiol. 2007; 10:181–189) [DOI] [PubMed] [Google Scholar]
- 21.Paul J, Shaw K, Dasgupta S, and Ghosh MK. Measurement of intima media thickness of carotid artery by B-mode ultrasound in healthy people of India and Bangladesh, and relation of age and sex with carotid artery intima media thickness: An observational study. J Cardiovasc Dis Res 2012; 3:128–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juonala M, Kähönen M, Laitinen T, Hutri-Kähönen N, Jokinen E, Taittonen L.Effect of age and sex on carotid intima-media thickness, elasticity and brachial endothelial function in healthy adults: The Cardiovascular Risk in Young Finns Study. European Heart Journal (2008) 29, 1198–1206 [DOI] [PubMed] [Google Scholar]
- 23.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common Carotid Intima-Media Thickness and Risk of Stroke and Myocardial Infarction : The Rotterdam Study. Circulation 1997; 96: 1432–1437. [DOI] [PubMed] [Google Scholar]
- 24.Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid Intima-Media Thickening Indicates a Higher Vascular Risk Across a Wide Age Range. Stroke 2006; 37: 87–92. [DOI] [PubMed] [Google Scholar]
- 25.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Carotid-Artery Intima and Media Thickness as a Risk Factor for Myocardial Infarction and Stroke in Older Adults. New England Journal of Medicine 1999;340(1): 14–22. [DOI] [PubMed] [Google Scholar]
- 26.Rohani M, Jogestrand T, Ekberg M, van der Linden J, Källner G, Jussila R, et al. Interrelation between the extent of atherosclerosis in the thoracic aorta, carotid intima-media thickness and the extent of coronary artery disease. Atherosclerosis 2005;179: 311–316. [DOI] [PubMed] [Google Scholar]
- 27.Kablak-Ziembicka A, Tracz W, Przewlocki T, Pieniazek P, Sokolowski A, Konieczynska M. Association of increased carotid intima-media thickness with the extent of coronary artery disease. Heart 2004; 90: 1286–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heiss G, Sharrett AR, Barnes R, Chambless LE, Szklo M, Alzola C. Carotid atherosis measured by B-mode ultrasound in populations: associations with cardiovascular risk factors in the ARIC study. Am J Epidermiology 1991;134:250–256. [DOI] [PubMed] [Google Scholar]
- 29.Hulten E, Mitchell J, Scally J, Gibbs B, Villines TC. HIV positivity, protease inhibitor exposure and subclinical atherosclerosis: a systematic review and meta-analysis of observational studies.Heart 2009,95:1826–1835.doi: 10.1136/hrt.2009.117774 [DOI] [PubMed] [Google Scholar]
- 30.Grunfeld C, Delaney JAC, Wanke C, Currier JS, Scherzer R, Biggs ML et al. Pre-Clinical Atherosclerosis due to HIV Infection: Carotid Intima-Medial Thickness Measurements from the FRAM Study. AIDS. 2009; 23(14): 1841–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.STEIN JH, KORCARZ CE, HURST RT, LONN E, KENDALL CB, MOHLER ER, NAJJAR SS, REMBOLD CM, POST WS. USE OF CAROTID ULTRASOUND TO IDENTIFY SUBCLINICAL VASCULAR DISEASE AND EVALUATE CARDIOVASCULAR DISEASE RISK: A CONSENSUS STATEMENT FROM THE AMERICAN SOCIETY OF ECHOCARDIOGRAPHY CAROTID INTIMA-MEDIA THICKNESS TASK FORCE. J AM SOCECHOCARDIOGR. 2008;21:93–111. □ [DOI] [PubMed] [Google Scholar]
- 32.FOLSOM AR, KRONMAL RA, DETRANO RC, O’Leary DH, BILD DE, BLUEMKE DA, BUDOFF MJ, LIU K, SHEA S, SZKLO M, TRACY RP, WATSON KE, BURKE GL. CORONARY ARTERY CALCIFICATION COMPARED WITH CAROTID INTIMA-MEDIA THICKNESS IN THE PREDICTION OF CARDIOVASCULAR DISEASE INCIDENCE: THE MULTI-ETHNIC STUDY OF ATHEROSCLEROSIS (MESA). ARCH INTERN MED. 2008; 168:1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goebell PJ, Kamat AM, Sylvester RJ, Black P, Droller M, Godoy G. et al. Assessing the quality of studies on the diagnostic accuracy of tumor markers. Urol Oncol. 2014; 32(7): 1051–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma SM, Wei CK, Liang CC, Chou JM, Lee SY The Age Correlation of the Carotid Intima-Media Thickness According to Sex and Side in Asymptomatic Subjects. Acta Neurol Taiwan 2011;20:29–34. [PubMed] [Google Scholar]
- 35.Sass C, Herbeth B, Chapet O, Siest G, Visvikis S, Zannad F. Intima-media thickness and diameter of carotid and femoral arteries in children, adolescents and adults from the Stanislas cohort: effect of age, sex, anthropometry and blood pressure. J Hypertens. 1998;16 (11):1593–602 [DOI] [PubMed] [Google Scholar]
- 36.Gooty VD, Sinaiko AR, Ryder JR, Dengel DR, Jacobs DR Jr., Steinberger J. Association Between Carotid intima media thickness, age, and cardiovascular risk factors in children and adolescents. Metabolic Syndrome and Related Disorders 16(3) 2018. DOI: 10.1089/met.2017.0149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The Cochrane Collaborative Review Group on HIV Infection and AIDS: Editorial Policy: Inclusion and Appraisal of Experimental and Non-experimental (Observational) Studies. [http://www.igh.org/Cochrane].Cited on 6/12/2014 [Google Scholar]
- 38.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of Microbial Translocation and Macrophage Activation: Association With Progression of Subclinical Atherosclerosis in HIV-1 Infection. The Journal of Infectious Diseases 2012;206:1558–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsue PY, Scherzer R, Hunt PW, Schnell A, Bolger AF, Kalapus SC., et al. Carotid Intima-Media Thickness Progression in HIV-Infected Adults Occurs Preferentially at the Carotid Bifurcation and Is Predicted by Inflammation. (J Am Heart Assoc. 2012;1:jah3-e000422 doi: 10.1161/JAHA.111.000422.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hileman CO, Scherzer R, Hunt PW, Schnell A, Bolger AF, Kalapus SC., et al.Rate and predictors of carotid artery intima mediathickness progression in antiretroviral-naïve HIV-infected and uninfected adults:a 48 weeks matched prospective cohort study.Antiviral Therapy 2013,18:921–929(doi: 10.3851/IMP2651) [DOI] [PubMed] [Google Scholar]
- 41.Hileman CO, Longenecker CT, Carman TL, and McComsey GA.C-Reactive Protein Predicts 96-Week Carotid Intima Media Thickness Progression in HIV-Infected Adults Naive to Antiretroviral Therapy. J Acquir Immune Defic Syndr 2014;65:340–344) [DOI] [PubMed] [Google Scholar]
- 42.van Vonderen MGA, Smulders YM, Stehouwer CDA, Danner SA, Gundy CM, Vos F, et al. Carotid Intima-Media Thickness and Arterial Stiffness in HIV-Infected Patients: The Role of HIV, Antiretroviral Therapy, and Lipodystrophy. JAIDS Journal of Acquired Immune Deficiency Syndromes 2009;50: 153–161 10.1097/QAI.1090b1013e31819367cd. [DOI] [PubMed] [Google Scholar]
- 43.Desvarieux M, Boccara F, Meynard J-L, Bastard J-P, Mallath Z, Charbiti B, et al. Infection duration and inflammatory imbalance are associated with atherosclerotic risk in HIV-infected never-smokers independent of antiretroviral therapy. AIDS 2013, Vol 27 No 16 [DOI] [PubMed] [Google Scholar]
- 44.Monroe AK, Dobs AS, Xu X, Palella FJ, Kingsley LA, Post WS, et al. Low free testosterone in HIV-infected men is not associated with subclinical cardiovascular disease. HIV Med. 2012. July; 13(6): 358–366. doi: 10.1111/j.1468-1293.2011.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung M, Parrinello CM, Xue X, Mack WJ, Anastos K, Lazar JM, et al. Echolucency of the Carotid Artery Intima-Media Complex and Intima-Media Thickness Have Different Cardiovascular Risk Factor Relationships: The Women’s Interagency HIV Study. (J Am Heart Assoc. 2015;4:e001405 doi: 10.1161/JAHA.114.001405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karim R, Mack WJ, Kono N, Tien PC, Anastos K, Lazar J, et al. Gonadotropin and Sex Steroid Levels in HIV-Infected Premenopausal Women and Their Association With Subclinical Atherosclerosis in HIV-Infected and -Uninfected Women in the Women’s Interagency HIV Study (WIHS). (J Clin Endocrinol Metab 98: E610–E618, 2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cristofaro M, Cicalini S, Rizzi EB, Schininà V, Petrosillo N, Bibbolino C.Ultrasonography in lesions of the carotid vessels in HIV positive patients. Radiol med (2011) 116:61–70 DOI 10.1007/s11547-010-0591-3 [DOI] [PubMed] [Google Scholar]
- 48.Fitch KV, Looby SE, Rope A, Eneh P, Hemphill L, Lee H, Grinspoon SK. Effects of Aging and Smoking on Carotid Intima Media Thickness in HIV-infection. AIDS. 2013. January 2; 27(1): 49–57. Doi: 10.1097/QAD.0b013e328358b29c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zormpala A, Sipsas NV, Moyssakis I, Georgiadou SP, Gamaletsou MN, Kontos AN, et al. Impaired distensibility of ascending aorta in patients with HIV infection. BMC Infectious Diseases 2012, 12:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kristoffersen US, Lebech A-M, Wiinberg N, Petersen CL, Hasbak P, Gutteet H, al. Silent Ischemic Heart Disease and Pericardial Fat Volume in HIV-Infected Patients: A Case-Control Myocardial Perfusion Scintigraphy Study. PLOS ONE | www.plosone.org 3 August 2013. | Volume 8 | Issue 8 | e7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papiţă A, Albu A, Fodor D, Itu C, Cârstina D. Arterial stiffness and carotid intima-media thickness in HIV infected patients. Medical Ultrasonography 2011, Vol. 13, no. 2, 127–134 [PubMed] [Google Scholar]
- 52.Oliviero U, Bonadiesb G, Apuzzia V, Foggiab M, Bossoa G, Nappab S, et al. Human immunodeficiency virus per se exerts atherogenic effect. Atherosclerosis 204 (2009) 586–589 [DOI] [PubMed] [Google Scholar]
- 53.Mondy KE, de las Fuentesa L, Waggonera A, Önena NF, Boppa CS,Lassa-Claxtona S, et al. Insulin resistance predicts endothelial dysfunction and cardiovascular risk in HIV-infected persons on long-term highly active antiretroviral therapy. AIDS. 2008. April 23; 22(7): 849 856.doi: 10.1097/QAD.0b013e3282f70694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seminari E, Pan A, Voltini G, Carnevale G, Maserati R, Minoli L, et al. Assessment of atherosclerosis using carotid ultrasonography in a coh1111ort of HIV-positive patients treated with protease inhibitors. Atherosclerosis 162 (2002) 433–438 [DOI] [PubMed] [Google Scholar]
- 55.Jericó C, Knobel H, Calvo N, Sorli ML, Guelar A, Gimeno-Bayón JL, et al. Subclinical carotid atherosclerosis in HIV-infected patients: role of combination antiretroviral therapy. Stroke. 2006; 37:812–7. [DOI] [PubMed] [Google Scholar]
- 56.Vohra S, Bharti V, Sharma A, Jaret PK, Marwaha R, Sachdeva A. Comparison of cimt among HIV seropositive and HIV seronegative subjects: A case-control study. International Journal of Current Advanced Research. 2017; 6 (10): 6442–6447 [Google Scholar]
- 57.Sun D, Wu Y, Yuan Y, Wang Y, Liu W, Yang J. Is the atherosclerotic process accentuated under conditions of HIV infection, antiretroviral therapy, and protease inhibitor exposure? Meta-analysis of the markers of arterial structure and function. Atherosclerosis 242 (2015) 109e116 [DOI] [PubMed] [Google Scholar]
- 58.Pacheco AG, Grinsztejn B, da Fonseca MdM, Griep RH, Lotufo P, Bensenor I, et al. HIV Infection Is Not Associated with Carotid Intima-Media Thickness in Brazil: A Cross-Sectional Analysis from the INI/ELSA-Brasil Study. PLoS ONE 2016; 11(7): e0158999. doi: 10.1371/journal.pone.0158999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeong SJ, Kim HW, Ku NS, Han SH, Kim CO, Choi JY, et al. Clinical Factors Associated with Carotid Plaque and Intima-Medial Thickness in HIV-Infected Patients. Yonsei Med J 2013; 54(4):990–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanna DB, Post WS, Deal JA, Hodis HN, Jacobson LP, Mack WJ, et al. HIV Infection Is Associated With Progression of Subclinical Carotid Atherosclerosis. Clinical Infectious Diseases 2015; 61(4):640–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barbaro G, Barbarini G, Pellicelli AM. HIV-Associated Coronary Arteritis in a Patient with Fatal Myocardial Infarction. New England Journal of Medicine 2001;344: 1799–1800. [DOI] [PubMed] [Google Scholar]
- 62.Ellis RW. Infection and coronary heart disease. Journal of Medical Microbiology 1997;46: 535–539. [DOI] [PubMed] [Google Scholar]
- 63.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ,et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis 2011; 203(4)452–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Merlini E, Luzi K, Suardi E, Barassi A, Cerrone M, Martínez JS, et al. T-cell phenotypes apoptosis and inflammation in HIV+ patients on virologically effective cART with early atherosclerosis. PLoS One. 2012;7(9):e46073. doi: 10.1371/j.journal.pone.0046073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baker JV, Henry WK, Patel P, Bush TJ, Conley LJ, Mack WJ, et al. Progression of carotid intima-media thickness in a contemporary human immunodeficiency virus cohort. Clin Infect Dis. 2011; 53(8):826–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friis-Møller N, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infraction. N Engl Med 2003;%20;349(21):1993–2003. doi. 10.1056/NEJMoa030218 [DOI] [PubMed] [Google Scholar]
- 67.Msoka TF, Van Guilder GP, Smulders YM, van Furth M, Bartlett JA, van Agtmael MA. Association of HIV-infection, antiretroviral treatment and metabolic syndrome with large artery stiffness: a cross-sectional study. BMC Infectious Diseases (2018) 18:708 10.1186/s12879-018-3637-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ssinabulya I, Kayima J, Longenecker C, Luwedde M, Semitala1 F, Kambugu A et al. Subclinical Atherosclerosis among HIV-Infected Adults Attending HIV/AIDS Care at Two Large Ambulatory HIV Clinics in Uganda. PLoS ONE 2014; 9(2): e89537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schoffelen AF, de Groot E, Tempelman HA, Visseren FLJ, Hoepelman AIM, andBarth RE. Carotid Intima Media Thickness in Mainly Female HIV-Infected Subjects in Rural South Africa: Association With Cardiovascular but Not HIV-Related Factors. Clinical Infectious Diseases 2015; 61(10):1606–14 [DOI] [PubMed] [Google Scholar]
- 70.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011.Cerebrovasc Dis. 2012; 34(4):290–296. doi: 10.1159/000343145000343145 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spence JD. Carotid Ultrasound Phenotypes Are Biologically Distinct. Arterioscler Thromb Vasc Biol.2015; 35(9):1910–3. [DOI] [PubMed] [Google Scholar]
- 72.Albuquerque VMG, Zírpoli JC, Miranda-Filho DB, Albuquerque MFPM, Montarroyos UR, Ximenes RAA, and Lacerda1 HR. Risk factors for subclinical atherosclerosis in HIV-infected patients under and over 40 years: a case–control study. BMC Infectious Diseases 2013, 13:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pacheco AG, Grinsztejn B, da Fonseca MJM, Moreira RI, Veloso VG, Friedman RK. et al. Traditional Risk Factors Are More Relevant than HIV-Specific Ones for Carotid Intima-Media Thickness (cIMT) in a Brazilian Cohort of HIV-Infected Patients. PLoS ONE 2015. 10(2): e0117461.doi: 10.1371/journal.pone.0117461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li M, Wang P, Yang C, Jiang W, Wei X, Mu X, Li X et al. A systematic review and meta-analysis: Does hepatitis C virus infection predispose to the development of chronic kidney disease? Oncotarget. 2017; (8) 10692–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sinning C, Wild PS, Echevarria FMO, Wilde S, Schnabel R, Lubos E. et al. Sex Differences in Early Carotid Atherosclerosis (from the Community-Based Gutenberg-Heart Study). Am J Cardiol 2011;107:1841–1847 [DOI] [PubMed] [Google Scholar]
- 76.KRIKKE M, ARENDS JE, VAN LELYVELD SFL, HOEPELMAN AIM AND VISSEREN FLJ GREATER CAROTID INTIMA MEDIA THICKNESS AT A YOUNGER AGE IN HIV-INFECTED PATIENTS COMPARED WITH REFERENCE VALUES FOR AN UNINFECTED COHORT. HIV MEDICINE 2017; (18): 275–283. DOI: 10.1111/hiv.I242 [DOI] [PubMed] [Google Scholar]
- 77.PUTCHAROEN O, PLEUMKANITKUL S, CHUTINET A, VONGSAYAN p, SAMAJARN J, Sophonphan J et al. Comparable carotid intima-media thickness AMONG LONG-TERM VIROLOGICALLY SUPPRESSED INDIVIDUALS WITH HIV AND THOSE WITHOUT HIV IN THAILAND. JOURNAL OF VIRUS ERADICATION 2019; (5): 23–27 [PMC free article] [PubMed] [Google Scholar]
- 78.Cicconea MM, Bilianoub E, Balbarinic A,Gesualdoa M, Ghiadonid L, Metrae M. et al. Task force on: ‘Early markers of atherosclerosis: influence of age and sex’ J Cardiovasc Med 2013, 14:757–766 [DOI] [PubMed] [Google Scholar]
- 79.Fairweather. Sex Differences in Inflammation during Atherosclerosis. Clinical Medicine Insights: Cardiology 2014:8(S3) 49–59 doi: 10.4137/CMC.S17068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yahagi K, Davis HR, Arbustini E, Virmani R. Sex differences in coronary artery disease: Pathological observations. Atherosclerosis 239 (2015) 260e267 [DOI] [PubMed] [Google Scholar]
- 81.Alba AC, Alexander PE, Chang J, MacIsaac J, DeFry S, Guyatt GH. High statistical heterogeneity is more frequent in meta-analysis of continuous than binary outcomes. J Clin Epidemiol. 2016;70:129–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.