Abstract
Objective
An increasing number of studies have explored the clinical effects of antiglaucoma surgical procedures; however, economic evidence was scarce. We aimed to compare the cost-effectiveness between maximal medical treatment (MMT) and commonly used surgical procedures (trabeculectomy, Ahmed glaucoma valve implantation, gonioscopy-assisted transluminal trabeculotomy and ab interno canaloplasty).
Design and setting
A Markov model study.
Participants
A hypothetical cohort of 100 000 patients with mild-to-moderate primary open-angle glaucoma (POAG).
Outcomes
Data were obtained from public sources. The main outcomes were incremental cost–utility ratios (ICURs) using quality-adjusted life-years (QALYs). Sensitivity analyses were conducted to verify the robustness and sensitivity of base-case results.
Main results
Both cumulative costs and QALYs gained from surgical procedures (US$6045–US$13 598, 3.33–6.05 QALYs) were higher than those from MMT (US$3117–US$6458, 3.14–5.66 QALYs). Compared with MMT, all surgical procedures satisfied the cost-effectiveness threshold (lower than US$30 501 and US$41 568 per QALY gained in rural and urban settings, respectively). During the 5-year period, trabeculectomy produced the lowest ICUR (US$21 462 and US$15 242 per QALY gained in rural and urban settings, respectively). During the 10-year-follow-up, trabeculectomy still produced the lowest ICUR (US$13 379 per QALY gained) in urban setting; however, gonioscopy-assisted transluminal trabeculotomy (US$19 619 per QALY gained) and ab interno canaloplasty (US$18 003 per QALY gained) produced lower ICURs than trabeculectomy (US$19 675 per QALY gained) in rural areas. Base-case results were most sensitive to the utilities and costs of initial treatment and maintenance.
Conclusions
The long-term cost-effectiveness of commonly used surgical procedures could be better than the short-term cost-effectiveness for mild-to-moderate POAG patients in China. Health economic studies, supported by more rigorous structured real-world data, are needed to assess their everyday cost-effectiveness.
Keywords: Public health, Health economics, SURGERY
STRENGTHS AND LIMITATIONS OF THIS STUDY.
From a societal perspective, this study used cost–utility analysis to compare the cost-effectiveness between t commonly used antiglaucoma surgical technologies and maximal medical treatment in China.
The rural–urban and long-term and short-term subgroup analyses comprehensively assessed the impact of social, geographical and temporal factors on the results.
Our limitations include that our findings are only representative of China and need to be carefully extrapolated to other low-income and middle-income countries.
Introduction
Eye health is a globally important health and development priority, and the annual productivity loss associated with visual impairment was approximately US$411 billion, with the highest cost in East Asia.1 The delaying of primary open-angle glaucoma (POAG) treatment imposes serious irreversible blindness and economic burden.2 3 Except for maximal medical treatment (MMT), classic trabeculectomy (TRAB) and an increasing number of novel antiglaucoma surgeries (including minimally invasive glaucoma surgeries) have become popular during the past few decades.4 5 Nevertheless, the management of glaucoma is a chronic and lifelong process, and each intervention method has different disadvantages.
Health economics study results help policy-makers and healthcare providers fully understand the clinical value and cost-effectiveness of emerging interventions. The results of a recent study indicated that TRAB produced lower costs and more quality-adjusted life-years (QALYs) than MicroShunt; thus, TRAB seemed to be a more cost-effective surgical procedure than MicroShunt from the perspective of the US Medicare system.6 However, there is insufficient economic evidence from low-income and middle-income countries (LMICs) to make specific recommendations for the optimal use and funding of novel antiglaucoma surgeries according to local conditions.7
Therefore, to compare the cost-effectiveness of several commonly used surgical procedures (TRAB, Ahmed glaucoma valve (AGV) implantation, gonioscopy-assisted transluminal trabeculotomy (GATT), and ab interno canaloplasty (ABiC)) and MMT in Chinese patients, we enrolled a hypothetical cohort of 100 000 patients with mild-to-moderate POAG older than 40 years old from a societal perspective. In addition, further analyses were conducted from the perspectives of urban and rural settings, and short-term (5-year) and long-term (10-year) outcomes.
Methods
Model overview
We simulated a hypothetical cohort of Chinese patients with mild-to-moderate POAG who were over age 40 and conducted 5-year or 10 1-year Markov cycles using TreeAge Pro software (TreeAge Software; Williamstown, Massachusetts, USA). Due to the slightly different settings of cost and treatment compliance between rural and urban patients, we established Markov models for both environments. The classification of POAG was based on the following International Society of Geographical and Epidemiologic Ophthalmology glaucoma classification: mild POAG, moderate POAG, severe POAG, POAG-related unilateral blindness and POAG-related bilateral blindness.8 9 Patients were assumed to receive initial intervention only in the first year. The model selected the four most widely used and representative antiglaucoma surgeries in China, including TRAB, AGV implantation, GATT and ABiC. Since laser trabeculoplasty has been proven to be a low-cost, widely used and repeatable intervention, it did not meet the inclusion criteria of this model and was not included.
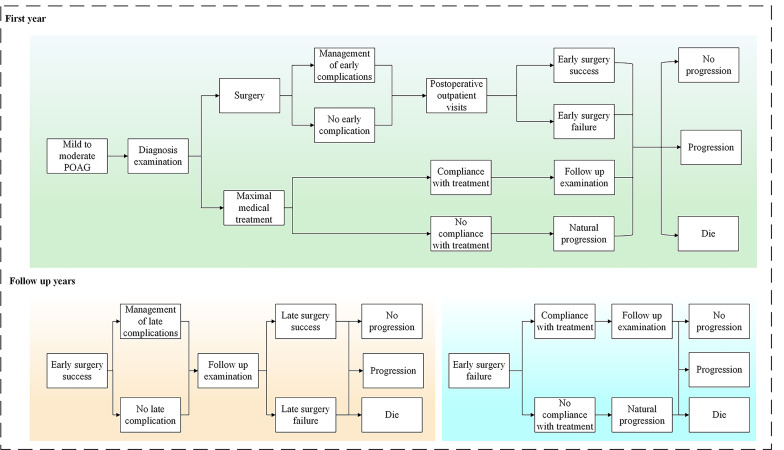
Patients who entered the model were in a mild or moderate state of POAG and were allowed to receive treatment. In the first year, they received one of the above treatments (MMT, TRAB, AGV implantation, GATT or ABiC) and faced the following risks: (1) early complications, (2) early success with or without disease progression, (3) early failure with or without disease progression and (4) death. In the following years, patients received routine follow-up and maintenance treatment and faced the risk of (1) late complications, (2) late success with or without disease progression, (3) late failure with or without disease progression and (4) death. At the end of each cycle, patients either stayed at the same stage or progressed to a more serious stage, depending on the specified transition probabilities. At the same time, they could transition to death from any healthy state. The Markov model for POAG progression and medical care is shown in figure 1.
Figure 1.
Healthcare pathways of different treatments for patients with mild-to-moderate primary open-angle glaucoma. Patients who entered the model were in a mild or moderate state of POAG and may have previously received the maximal medical treatment or antiglaucoma surgery. In the first year, patients received initial treatment, follow-up and management of complications. During the follow-up years, patients with successful surgery received regular follow-up and management of late complications, while patients with failed surgery received maximal medical treatment. POAG, primary open-angle glaucoma.
Parameters
Relevant parameters were obtained from published literature. We gave priority to research conducted in China, followed by studies conducted in Asian countries, then in other countries. The main parameters used, the setting of all interventions and complications are listed in online supplemental tables S1–S4. A brief introduction and complications associated with each antiglaucoma method are listed in online supplemental table S5.
bmjopen-2023-073219supp001.pdf (716.2KB, pdf)
Surgical failure was defined as two consecutive follow-ups of intraocular pressure (IOP)>21 mm Hg or a decrease of less than 20% compared with baseline, with or without maximal medication, reoperation for uncontrolled IOP or loss of light perception vision.5 10 The early and late failure rates were obtained from clinical follow-up studies, and the cumulative failure rate was converted into the annual failure rate by the following verified formula: r=−[ln(1–p)]/t, where r denotes the late annual surgical failure rate, and p represents the cumulative probability over the length of interval t.9 It was assumed that patients who had failed surgeries received maximum medical treatment in the subsequent years.5 The probabilities of early and late complications were obtained from other cost-effectiveness analyses and clinical studies.
For POAG progression, we divided the patients into three groups. First, patients who did not receive MMT followed the natural progression of POAG.9 Second, the transition rate for patients with successful surgeries was mainly derived from a published glaucoma screening programme in China.9 Since there was no transition rate from mild to moderate POAG in this study, we made a reasonable assumption that patients who had successful surgeries had a 50% reduction in disease progression.11 Third, we also assumed that the disease progression rate of the group with failed surgeries was 1.5 times higher than that in the successful surgery group based on previous studies.5
Mortality may occur at any stage. Therefore, our model considered both the natural age-specific mortality rate and the increased odds of mortality.9 12–14
Costs
We considered both direct and indirect costs from a social perspective, and there were differences in cost composition between rural and urban areas. The medical costs for glaucoma are controlled by the Chinese government and set uniformly by the Medical Insurance Bureau, and there is little difference between institutions at the same level. Under the guidance of the Chinese Glaucoma Study Alliance, we determined the average medical costs of rural and urban POAG patients. All costs were collected in Chinese yuan and converted into US dollars according to the average exchange rate in 2021 (¥6.45to US1). Costs were discounted at 3.5% per annum in the base-case analysis.15 16 A similar cost collection method was derived from previous models.17 All data related to costs are listed in table 1 and online supplemental tables S2 and S6, S7.
Table 1.
Cost composition and calculation of management of complications
| Rural settings (US$/person) | Urban settings (US$/person) | |||||||
| Direct medical costs* | Direct non-medical costs† | Indirect costs‡ | Societal costs per person | Direct medical costs* | Direct non-medical costs | Indirect costs‡ | Societal costs per person | |
| Vitrectomy | 2677 | 124 | 574 | 3375 | 2677 | 2 | 784 | 3463 |
| Suprachoroidal haemorrhage drainage | 575 | 124 | 82 | 781 | 575 | 2 | 112 | 689 |
| Bleb leak revision | 707 | 124 | 82 | 913 | 707 | 2 | 112 | 720 |
| Penetrating keratoplasty | 5411 | 124 | 574 | 6109 | 5411 | 2 | 784 | 6197 |
| Endothelial keratoplasty | 4560 | 124 | 574 | 5258 | 4560 | 2 | 784 | 5346 |
| Tube revision or reinforcement | 606 | 124 | 82 | 812 | 606 | 2 | 112 | 720 |
| Correction of strabismus | 1380 | 124 | 574 | 2078 | 1380 | 2 | 784 | 2166 |
| Anterior chamber irrigation | 56 | 0 | 0 | 56 | 56 | 0 | 0 | 56 |
We considered both direct and indirect costs from a social perspective. All costs were collected in Chinese yuan and converted into US dollars according to the average exchange rate in 2021 (¥6.45 to US$1). Costs were discounted at 3.5% per annum.
*Direct costs consist of direct medical costs and direct non-medical costs. Patients undergoing vitrectomy, penetrating keratoplasty, endothelial keratoplasty and correction of strabismus need to be hospitalised for 7 days. Patients undergoing suprachoroidal haemorrhage drainage, bleb leak revision and tube revision or reinforcement only need to be hospitalised for 1 day. Patients with IOP spike caused by massive hyphema received anterior chamber irrigation during hospitalisation.
†Direct non-medical costs includes food, transportation and accommodation expenses for patients and one family member.
‡The human capital method was used to calculate indirect costs such as wage loss of patients and family members in the process of disease diagnosis and treatment.
IOP, intraocular pressure.
Direct costs consist of direct medical costs and direct non-medical costs, which were obtained under the guidance of the Chinese Glaucoma Study Alliance and interviews with POAG patients. The human capital method was used to calculate indirect costs such as wage loss of patients and family members in the process of disease diagnosis and treatment. In our model, the annual indirect economic burden of each unilateral or bilateral blind patient included the costs of loss of labour resources, the loss of productivity of family members, modification, low vision and so on.9 18
Main outcomes
Utilities were used to calculate QALYs. The utility value of POAG patients in different stages came from an economic study of glaucoma screening programmes in China.9 Additionally, utilities for patients who failed surgery and received MMT were reduced by 10%.5 The main outcome was incremental cost–utility ratios (ICURs) using QALYs. All included surgical procedures (TRAB, AGV implantation, GATT or ABiC) were compared with MMT using the following calculation formula:
The WHO stipulated that the project was considered highly cost-effective when the cost was lower than the per capita gross domestic product (GDP); cost-effective when the cost was between 1 and 3 times the per capita GDP; and not cost-effective when the cost exceeded 3 times the per capita GDP.19 Based on the overall per capita national GDP (US$12 551), urbanisation rate (0.65) and urban–rural ratio of per capita disposable income (2.5) in 2021, we calculated the per capita GDP of rural and urban China to be US$10 167 and US$13 856, respectively. Thus, China’s willingness-to-pay (WTP) threshold was US$30 501 and US$41 568 per QALY gained in rural and urban settings, respectively.
Sensitivity analysis
We performed both one-way deterministic and probability sensitivity analyses to verify the robustness of the base-case results.9 16 The tornado diagrams revealed five factors that had the greatest impact on ICURs. Probability sensitivity analysis could make the uncertainty of all variables change together. By recalculating 10 000 randomly selected ICURs, the probability uncertainties were evaluated.
Patient and public involvement
None.
Results
Base-case results
We conducted cost–utility analyses in both rural and urban settings to compare the cost-effectiveness of commonly used surgical procedures and MMT (tables 2–3). During the 5-year mode, the total expected medical cost for a 40-year-old rural patient with mild-to-moderate POAG was US$3570, and the total expected QALYs gained was 3.14; the figures for an urban patient were US$3117 and US$3.14, respectively. For patients in both settings, although the cumulative costs of surgical procedures (TRAB, AGV implantation, GATT and ABiC) increased, the cumulative QALYs gained also increased accordingly, and ABiC produced the highest costs (US$10 471 for rural settings and US$8 920 for urban settings) and QALYs (3.38 for rural settings and 3.38 for urban settings). Compared with MMT, the ICURs produced by all surgical procedures in both rural (US$21 462–US$29 178 per QALY gained) and urban (US$15 242–US$24 619 per QALY gained) settings satisfied the cost-effectiveness threshold (US$30 501 per QALY gained in rural settings, and US$41 568 per QALY gained in urban settings). Among these surgical procedures, TRAB produced the lowest ICUR (US$21 462 in rural settings and US$15 242 in urban settings).
Table 2.
Base-case cost–utility results for patients with mild-to-severe primary open angle glaucoma in rural setting
| Costs per person, US$ | QALYs per person | Incremental costs per person, US$ | Incremental QALYs per person | ICURs, US$ | |
| 5 years | |||||
| MMT | 3570 | 3.14 | – | – | – |
| TRAB | 7709 | 3.33 | 4139 | 0.19 | 21 462 |
| AGV implantation | 9017 | 3.34 | 5447 | 0.20 | 27 470 |
| GATT | 9848 | 3.37 | 6278 | 0.23 | 26 945 |
| ABiC | 10 471 | 3.38 | 6901 | 0.24 | 29 178 |
| 10 years | |||||
| MMT | 6458 | 5.65 | – | – | – |
| TRAB | 11 542 | 5.91 | 5084 | 0.26 | 19 675 |
| AGV implantation | 12 619 | 5.94 | 6161 | 0.29 | 21 151 |
| GATT | 12 613 | 5.97 | 6155 | 0.31 | 19 619 |
| ABiC | 13 598 | 6.05 | 7140 | 0.40 | 18 003 |
Costs are given in US dollars. ICURs were calculated against maximal medical treatment. The cost-effectiveness threshold was US$30 501 per QALY gained for rural setting and US$41 568 per QALY gained for urban setting.
ABiC, ab interno canaloplasty; AGV, Ahmed glaucoma valve; GATT, gonioscopy-assisted transluminal trabeculotomy; GDP, gross domestic product; ICUR, incremental cost–utility ratio; MMT, maximal medical treatment; QALY, quality-adjusted life-year; TRAB, trabeculectomy.
Table 3.
Base-case cost–utility results for patients with mild-to-severe primary open angle glaucoma in urban setting
| Costs per person, US$ | QALYs per person | Incremental costs per person, US$ | Incremental QALYs per person | ICURs, US$ | |
| 5 years | |||||
| MMT | 3117 | 3.14 | – | – | – |
| TRAB | 6045 | 3.33 | 2928 | 0.19 | 15 242 |
| AGV implantation | 7454 | 3.34 | 4337 | 0.20 | 21 955 |
| GATT | 8317 | 3.37 | 5200 | 0.23 | 22 400 |
| ABiC | 8920 | 3.38 | 5803 | 0.24 | 24 619 |
| 10 years | |||||
| MMT | 5761 | 5.66 | – | – | – |
| TRAB | 9196 | 5.91 | 3435 | 0.26 | 13 379 |
| AGV implantation | 10 459 | 5.95 | 4698 | 0.29 | 16 223 |
| GATT | 10 649 | 5.97 | 4888 | 0.31 | 15 675 |
| ABiC | 11 366 | 6.05 | 5605 | 0.39 | 14 203 |
Costs are given in US dollars. ICURs were calculated against maximal medical treatment. The cost-effectiveness threshold was US$30 501 per QALY gained for rural setting and US$41 568 per QALY gained for urban setting.
ABiC, ab interno canaloplasty; AGV, Ahmed glaucoma valve; GATT, gonioscopy-assisted transluminal trabeculotomy; GDP, gross domestic product; ICUR, incremental cost–utility ratio; MMT, maximal medical treatment; QALY, quality-adjusted life-year; TRAB, trabeculectomy.
At the 10-year follow-up mode, all surgical procedures remained cost-effective, and the ICURs were less than those of the 5-year horizon. In urban settings, the ICUR produced by TRAB was still the lowest (US$13 379 per QALY gained); however, the ICURs produced by GATT (US$19 619 per QALY gained) and ABiC (US$18 003 per QALY gained) were lower than those produced by TRAB (US$19 675 per QALY gained) in rural areas.
Sensitivity analyses
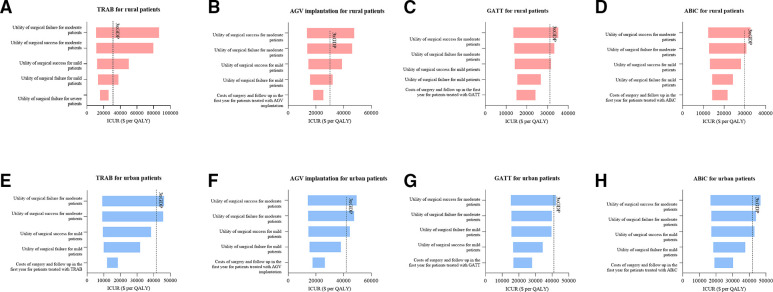
We performed both one-way deterministic and probabilistic sensitivity analyses to test the robustness of the base-case results. In one-way deterministic sensitivity analyses, the base-case results were mainly sensitive to the utility values of surgical success and failure in different disease stages, leading to ICURs exceeding three times the per capita GDP (figure 2).
Figure 2.
Deterministic one-way sensitivity analysis for surgical procedures compared with maximal medical treatment at the 10-year follow-up. Costs are given in US dollars. The top five parameters that caused the greatest impact on ICURs are listed. We performed a deterministic one-way sensitivity analysis for surgical procedures (TRAB, AGV implantation, GATT and ABiC) compared with maximal medical treatment at the 10-year follow-up for rural (A–D) and urban (E–H) settings. Intervention was considered cost-effective when the cost was less than three times the per capita GDP (US$41 568 for urban patients, US$30 501 for rural patients) and highly cost-effective when the cost was less than one times the per capita GDP (US$13 856 for urban patients, US$10 167 for rural patients). AGV, Ahmed glaucoma valve; ABiC, ab interno canaloplasty; GATT, gonioscopy-assisted transluminal trabeculotomy; GDP, gross domestic product; ICURs, incremental cost–utility ratios; MIGS, minimally invasive glaucoma surgery; QALY, quality-adjusted life-year; TRAB, trabeculectomy.
The results of probabilistic sensitivity analyses showed that, when compared with MMT, TRAB, AGV implantation, GATT and ABiC were cost-effective in 70%, 73%, 82% and 85% of simulations, respectively, and the figures were 84%, 89%, 94% and 95% of simulations for urban POAG patients (online supplemental figure S1). In online supplemental figure S2, with the increase in the WTP threshold, the dominant proportion of MMT gradually decreased. TRAB was more likely to be the first surgical treatment of choice when WTP values were low. When the WTP threshold reached three times the per capita GDP (US$30 501 per QALY gained in rural settings and US$41 568 per QALY gained in urban settings), the proportions of MMT, TRAB, AGV implantation, GATT and ABiC as the preferred strategy were 13%, 4%, 1%, 9% and 72% for rural patients, and 4%, 1%, 0%, 4% and 91% for urban patients.
Discussion
Our study indicated that all included surgical procedures were more cost-effective than MMT for early-to-moderate POAG patients in both rural and urban China. Although the cumulative costs of surgeries were higher than those of MMT, they produced correspondingly more QALYs. Extensive sensitivity analyses indicated that the most important indicators that affected the base-case analysis were patients’ utilities and costs.
The main results of our study showed that surgical procedures incurred both higher incremental costs and effectiveness than MMT. According to the 2021 Chinese Health Statistical Yearbook, the total per capita medical costs per glaucoma patient was US$1041 in a public hospital, accounting for 21% of the per capita annual income, and the disability-adjusted life-years of blindness and vision loss due to glaucoma was US$112 528 in 2019.20 Kaplan et al5 compared the cost-effectiveness of 350 mm2 Baerveldt implants, TRAB with mitomycin and MMT within a 5-year time horizon. The results found that tube insertion and TRAB with mitomycin were both cost-effective options for POAG patients in the United States at a WTP of US$50 000 per QALY, but the cost per QALY of TRAB was substantially lower than that of tube insertion. Another study conducted in the USA showed that TRAB was superior to MicroShunt within 2–20 years, producing lower costs (US$4260 for TRAB and US$6318 for MicroShunt) and more QALYs, 0.86 QALYs for TRAB and 0.85 QALYs for MicroShunt).6 Several other cost-effectiveness analyses validated that iStent injection of a trabecular bypass stent for patients with mild-to-moderate OAG was a cost-effective option in France, Canada and Colombia.21–23
However, the available evidence on the cost-effectiveness of Micro-Invasive Glaucoma Surgery is limited, and most of these studies were conducted in developed countries and included only one novel surgical option.24 25 Furthermore, the popularity of various surgical methods, clinician proficiency in surgeries and the prices of procedures vary by country, and these large differences among countries limit the generalisability and comparability of previous findings in countries with greater heterogeneity. Our model simultaneously included four surgical procedures that have been widely used in China for a relatively long time, and proved the long-term cost-effectiveness and its possible positive financial implications for individuals, families and society.
We used both 5-year and 10-year time horizons. The results showed that all surgical procedures met the cost-effectiveness threshold in both short-term and long-term follow-up modes, the ICURs produced over the 10-year period were less than those produced over the 5-year period, and the ICURs produced by GATT and ABiC in rural areas were lower than those produced by TRAB over the course of 10 years. Medication, laser trabeculectomy and TRAB are currently the classic antiglaucoma interventions commonly used in China. However, long-term topical IOP-lowering drugs face challenges such as poor compliance and ongoing costs, the effect of laser trabeculoplasty decreases with the passage of time and the incidence of bleb-related complications after TRAB is high and unpredictable. However, it is possible that the high initial costs of surgical procedures are partially offset over the follow-up years by lower medication use, higher QALY gained, more years of blindness avoided, and reduced time-related productivity losses.21–23 25–27 Therefore, providing high-quality, cost-effective and sustainable interventions for glaucoma patients is an urgent and global challenge addressed by the International Agency for the Prevention of Blindness in its strategy, In Sight, closely tied to the WHO 2030 Sustainable Development Goals.
The results of subgroup analyses indicated that the cumulative costs in urban areas were lower than those in rural areas, and QALYs were higher. Extensive sensitivity analyses also indicated that both the clinical outcomes and intervention costs were the most important indicators that affected the base-case analysis. The reason could be explained by the huge heterogeneity between rural and urban areas.28 First, rural residents are often required to seek healthcare in cities, which inevitably increases the demand for medical resources in urban areas, leading to a vicious cycle. Second, a large part of expensive consumables related to ophthalmic treatment are out-of-pocket consumables. People living in rural communities or remote areas may not be able to afford the high costs and therefore may not obtain the services. Third, low awareness and demand for eye health, lack of confidence in emerging ophthalmic services, and poor adherence to lifelong management further hinder the promotion and use of new healthcare services in rural areas.
Targeted measures are needed to narrow the urban–rural gap. First, governments need to balance the equal distribution of medical resources among all populations and regions, develop a diverse and resilient ophthalmic workforce by increasing capacity training to maximise workforce productivity and team efficiency, and narrow the skills gap between regions.28 Second, although the effectiveness and safety of novel anti-glaucoma procedures have been proven, expensive imported devices are a barrier to adoption, and newer and more affordable surgical equipment and procedures are required to meet the growing needs; further, cost-effectiveness analysis to understand whether low-cost devices translate into lower overall costs should be a priority.29 Third, in view of the longer hospitalisation time of patients, shared medical care and virtual medical care policies can help to ensure the intervention effect while reducing the length of stay and subsequent economic burden.30 Fourth, a well-functioning hierarchical blindness prevention and treatment network is the key to managing the full range of glaucoma.1 31 With the help of advanced technologies, primary eye care staff at primary eye care institutes could master the ability to diagnose and treat mild eye conditions, conduct regular re-examinations of patients in a stable condition, and make timely referrals for emergencies. Secondary and tertiary hospitals should be proficient in various surgical methods, develop and explore new surgical methods, and improve surgical success rates and good outcomes to truly meet patients’ needs for high-quality eye health services.
High-quality health economic studies provide a valuable economical basis to help policymakers fully understand the costs and values of interventions and play a vital role in the process of policy formulation and medical insurance negotiation.32 In future, on the one hand, the comprehensive assessment of health economics should fully consider the level of socioeconomic development, people’s WTP and regional differences to make this approach a powerful tool for maintaining social equity. On the other hand, collecting patient history costs and disease progression information through structured electronic health records will facilitate more rigorous and realistic cost-effectiveness assessments.33
Our research has unique strengths. First, our study is the first to evaluate the cost-effectiveness of implementing novel glaucoma surgical technologies in LMICs represented by China, and it provides an economic reference for the widespread promotion of highly cost-effective antiglaucoma interventions in these countries. When sharing these results with other LMICs, it is necessary to consider the applicability and transferability of our results. Second, whereas most previous economic studies included only one novel surgical procedure of concern, our study fully considered a variety of representative surgical procedures widely used in China. Third, considering the large regional and geographical differences in China and the complexity and long-term characteristics of glaucoma management, we analysed the economic benefits for rural and urban areas as well as short-term and long-term management from an economic perspective.
Nonetheless, there are several limitations in our study. First, due to the lack of available data, some data come from studies conducted in other countries or validated formulas (eg, utility values). High-quality and cohesive studies are needed to evaluate the effects of glaucoma interventions.34 Second, our study only included four representative POAG surgical procedures widely used in China. Combined cataract surgery and other emerging procedures were not included in our study since these procedures lacked reliable clinical follow-up data, and many new procedures were not widely promoted in China. However, the evaluation of more novel surgical procedures and combined surgeries should be fully considered in future studies. Third, only the POAG type was included in our study, and no other glaucoma subtypes were considered.35 Future studies should focus on the economic benefits of different surgical procedures in different glaucoma subtypes. Fourth, in our study, the reason for not considering the serious harms with GATT and ABiC was the under-reporting of withdrawals or losses to follow-up because of adverse effects. However, with the introduction of novel surgical techniques, the use of severity classification system and total harm score to adequately quantify postoperative harm severity requires further attention in glaucoma clinical trials.36–38
Conclusion
In conclusion, our study verified the long-term cost-effectiveness of surgical procedures for POAG patients in China, and our results provide economic evidence for the formulation of health policies for the surgical treatment of glaucoma in China. More evidence-based, efficient, affordable and high-quality interventions that contribute to universal eye health should be explored in the future.
Supplementary Material
Footnotes
Contributors: Conception and design: HaL and NW. Acquisition or interpretation of data: RL, JW, DM and HuL. Drafting of the manuscript: RL. Critical revision of the manuscript for important intellectual content: HaL and NW. Statistical analysis: RL, KZ, ZL, SF and HuL. Obtained funding: HaL. HaL and NW accepted full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: National Natural Science Foundation of China, NSFC (82171051).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. The parameters that we used in our model (text, tables, figures, models and appendices) are available on reasonable request from the corresponding author (HL; hanruo.liu@hotmail.co.uk) under certain conditions (with the consent of all participating centres and with a signed data access agreement).
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Burton MJ, Ramke J, Marques AP, et al. The lancet global health commission on global eye health: vision beyond 2020. Lancet Glob Health 2021;9:e489–551. 10.1016/S2214-109X(20)30488-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA, Cassard SD, Gower EW, et al. The cost of glaucoma care provided to medicare beneficiaries from 2002 to 2009. Ophthalmology 2013;120:2249–57. 10.1016/j.ophtha.2013.04.027 [DOI] [PubMed] [Google Scholar]
- 3.Dirani M, Crowston JG, Taylor PS, et al. Economic impact of primary open-angle glaucoma in Australia. Clin Exp Ophthalmol 2011;39:623–32. 10.1111/j.1442-9071.2011.02530.x [DOI] [PubMed] [Google Scholar]
- 4.Kirwan JF, Lockwood AJ, Shah P, et al. Trabeculectomy in the 21st century: a multicenter analysis. Ophthalmology 2013;120:2532–9. 10.1016/j.ophtha.2013.07.049 [DOI] [PubMed] [Google Scholar]
- 5.Kaplan RI, De Moraes CG, Cioffi GA, et al. Comparative cost-effectiveness of the Baerveldt implant trabeculectomy with mitomycin, and medical treatment. JAMA Ophthalmol 2015;133:560–7. 10.1001/jamaophthalmol.2015.44 [DOI] [PubMed] [Google Scholar]
- 6.Atik A, Fahy ET, Rhodes LA, et al. Comparative cost-effectiveness of trabeculectomy versus microshunt in the US medicare system. Ophthalmology 2022;129:1142–51. 10.1016/j.ophtha.2022.05.016 [DOI] [PubMed] [Google Scholar]
- 7.CADTH . CADTH optimal use reports. In: Optimal use of minimally invasive glaucoma surgery: recommendations. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health, 2019. [PubMed] [Google Scholar]
- 8.Foster PJ, Buhrmann R, Quigley HA, et al. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol 2002;86:238–42. 10.1136/bjo.86.2.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang J, Liang Y, O’Neill C, et al. Cost-effectiveness and cost-utility of population-based glaucoma screening in China: a decision-analytic Markov model. Lancet Glob Health 2019;7:e968–78. 10.1016/S2214-109X(19)30201-3 [DOI] [PubMed] [Google Scholar]
- 10.Grover DS, Smith O, Fellman RL, et al. Gonioscopy-assisted transluminal trabeculotomy: an AB interno circumferential trabeculotomy: 24 months follow-up. J Glaucoma 2018;27:393–401. 10.1097/IJG.0000000000000956 [DOI] [PubMed] [Google Scholar]
- 11.Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol 2003;121:48–56. 10.1001/archopht.121.1.48 [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Wei M. The evaluation of the mortality and life expectancy of Chinese population. Popul J 2016;38:18–28. 10.16405/j.cnki.1004-129X.2016.03.002 [DOI] [Google Scholar]
- 13.Xu L, Wang YX, Jonas JB. Glaucoma and mortality in the Beijing eye study. Eye (Lond) 2008;22:434–8. 10.1038/sj.eye.6703072 [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Sun D, Liu P, et al. Visual impairment and mortality in a rural adult population (the Southern Harbin eye study). Ophthalmic Epidemiol 2011;18:54–60. 10.3109/09286586.2010.545503 [DOI] [PubMed] [Google Scholar]
- 15.Guide to the methods of technology appraisal 2013. London: National Institute for Health and Care Excellence (NICE); 2013. [PubMed] [Google Scholar]
- 16.Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics 2000;17:479–500. 10.2165/00019053-200017050-00006 [DOI] [PubMed] [Google Scholar]
- 17.Li R, Yang Z, Zhang Y, et al. Cost-effectiveness and cost-utility of traditional and telemedicine combined population-based age-related macular degeneration and diabetic retinopathy screening in rural and urban China. Lancet Reg Health West Pac 2022;23:100435. 10.1016/j.lanwpc.2022.100435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein JD, Kim DD, Peck WW, et al. Cost-effectiveness of medications compared with laser trabeculoplasty in patients with newly diagnosed open-angle glaucoma. Arch Ophthalmol 2012;130:497–505. 10.1001/archophthalmol.2011.2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutubessy R, Chisholm D, Edejer T-T. Generalized cost-effectiveness analysis for national-level priority-setting in the health sector. Cost Eff Resour Alloc 2003;1:8. 10.1186/1478-7547-1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Y, Chen A, Zou M, et al. Disease burden of glaucoma in China: findings from the global burden of disease 2019 study. Clin Epidemiol 2022;14:827–34. 10.2147/CLEP.S357188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel V, Ahmed I, Podbielski D, et al. Cost-effectiveness analysis of standalone trabecular micro-bypass stents in patients with mild-to-moderate open-angle glaucoma in Canada. J Med Econ 2019;22:390–401. 10.1080/13696998.2019.1572013 [DOI] [PubMed] [Google Scholar]
- 22.Nieland K, Labbé A, Schweitzer C, et al. A cost-effectiveness analysis of iStent inject combined with phacoemulsification cataract surgery in patients with mild-to-moderate open-angle glaucoma in France. PLoS One 2021;16:e0252130. 10.1371/journal.pone.0252130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ordóñez JE, Ordóñez A, Osorio UM. Cost-effectiveness analysis of iStent trabecular micro-bypass stent for patients with open-angle glaucoma in Colombia. Curr Med Res Opin 2019;35:329–40. 10.1080/03007995.2018.1506022 [DOI] [PubMed] [Google Scholar]
- 24.Agrawal P, Bradshaw SE. Systematic literature review of clinical and economic outcomes of micro-invasive glaucoma surgery (MIGS) in primary open-angle glaucoma. Ophthalmol Ther 2018;7:49–73. 10.1007/s40123-018-0131-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bicket AK, Le JT, Azuara-Blanco A, et al. Minimally invasive glaucoma surgical techniques for open-angle glaucoma: an overview of Cochrane systematic reviews and network meta-analysis. JAMA Ophthalmol 2021;139:983–9. 10.1001/jamaophthalmol.2021.2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azuara-Blanco A, Burr J, Ramsay C, et al. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): a randomised controlled trial. Lancet 2016;388:1389–97. 10.1016/S0140-6736(16)30956-4 [DOI] [PubMed] [Google Scholar]
- 27.Gedde SJ, Feuer WJ, Lim KS, et al. Treatment outcomes in the primary tube versus trabeculectomy study after 3 years of follow-up. Ophthalmology 2020;127:333–45. 10.1016/j.ophtha.2019.10.002 [DOI] [PubMed] [Google Scholar]
- 28.Qiao C, Zhang H, Cao K, et al. Changing trends in glaucoma surgery over the past 5 years in China. J Glaucoma 2022;31:329–34. 10.1097/IJG.0000000000002004 [DOI] [PubMed] [Google Scholar]
- 29.Puthuran GV, Palmberg P, Wijesinghe HK, et al. Intermediate-term outcomes of an affordable aqueous drainage implant in adults with refractory glaucoma. Ophthalmol Glaucoma 2019;2:258–66. 10.1016/j.ogla.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 30.Simons A-S, Vercauteren J, Barbosa-Breda J, et al. Shared care and virtual clinics for glaucoma in a hospital setting. J Clin Med 2021;10:4785. 10.3390/jcm10204785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiao J, Wang Y, Li X, et al. A lancet commission on 70 years of women's reproductive, maternal, newborn, child, and adolescent health in China. Lancet 2021;397:2497–536. 10.1016/S0140-6736(20)32708-2 [DOI] [PubMed] [Google Scholar]
- 32.Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol 2016;10:189–206. 10.2147/OPTH.S80490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, Jia H, Duan X, et al. The Chinese glaucoma study consortium for patients with glaucoma: design, rationale and baseline patient characteristics. J Glaucoma 2019;28:974–8. 10.1097/IJG.0000000000001378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qureshi R, Azuara-Blanco A, Michelessi M, et al. What do we really know about the effectiveness of glaucoma interventions?: an overview of systematic reviews. Ophthalmol Glaucoma 2021;4:454–62. 10.1016/j.ogla.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chinese medical association . Guidelines for glaucoma in China (2020). Chin J Ophthalmol 2020;56:573–86. 10.3760/cma.j.cn112142-20200313-00182 [DOI] [Google Scholar]
- 36.Bonnar J, Azuara-Blanco A. Systematic review of the method and quality of reporting of complications from studies evaluating innovative glaucoma surgical procedures. Eye 2023;37:1774–7. 10.1038/s41433-022-02268-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Protasio JN, King A, Pasquale LR, et al. How can we quantify and compare harm in surgical trials? Am J Ophthalmol 2022;241:64–70. 10.1016/j.ajo.2022.04.020 [DOI] [PubMed] [Google Scholar]
- 38.Henein C, Fang CEH, Virgili G, et al. Adverse events associated with minimally invasive glaucoma surgeries (MIGS) including Bleb‐Forming microstent surgeries. Cochrane Database Syst Rev 2022;2022:12. 10.1002/14651858.CD015294 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-073219supp001.pdf (716.2KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information. The parameters that we used in our model (text, tables, figures, models and appendices) are available on reasonable request from the corresponding author (HL; hanruo.liu@hotmail.co.uk) under certain conditions (with the consent of all participating centres and with a signed data access agreement).