Abstract
Objective
Precision medicine in rheumatoid arthritis (RA) requires a good understanding of treatment outcomes and often collaborative efforts that call for data harmonisation. We aimed to describe how harmonisation across study cohorts can be achieved and investigate how the observed proportions reaching remission vary across remission criteria, study types, disease-modifying antirheumatic drugs (DMARDs) and countries, and how they relate to other treatment outcomes.
Methods
We used data from eight existing large-scale, clinical RA registers and a pragmatic trial from Sweden, Denmark and Norway. In these, we defined three types of treatment cohorts; methotrexate monotherapy (as first DMARD), tumour necrosis factor inhibitors (TNFi) (as first biological DMARD) and rituximab. We developed a harmonised study protocol defining time points during 36 months of follow-up, collected clinical visit data on treatment response, retention, persistence and six alternative definitions of remission, and investigated how these outcomes differed within and between cohorts, by treatment.
Results
Cohort sizes ranged from ~50 to 22 000 patients with RA. The proportions reaching each outcome varied across outcome metric, but with small to modest variations within and between cohorts, countries and treatment. Retention and persistence rates were high (>50% at 1 year), yet <33% of patients starting methotrexate or TNFi, and only 10% starting rituximab, remained on drug without other DMARDs added and achieved American Congress of Rheumatology/European Alliance of Associations for Rheumatology or Simplified Disease Activity Index remission at 1 year.
Conclusion
Harmonisation of data from different RA data sources can be achieved without compromising internal validity or generalisability. The low proportions reaching remission, point to an unmet need for treatment optimisation in RA.
Keywords: Rheumatoid Arthritis, Methotrexate, Tumor Necrosis Factor Inhibitors, Rituximab
WHAT IS ALREADY KNOWN ON THIS TOPIC
Many patients with rheumatoid arthritis (RA) do not reach treatment targets (remission), but how remission relates to other treatment outcomes (eg, treatment retention) and how remission rates vary with treatment context remains less well understood. Further, studies of treatment outcomes often use different definitions of this target, making comparisons difficult.
Studies on personalised medicine in RA often require collaboration and harmonisation of data to reach sufficient size and may be limited to subsets of all available data, for example, as defined by the availability of blood samples for biomarker analyses, the representability of which is often neither investigated nor disclosed.
WHAT THIS STUDY ADDS
We provide concrete examples of how data harmonisation may be achieved, and comprehensive data on observed estimates for different definitions of remission and other treatment outcomes with standard RA therapies as used in clinical practice, and how remission related to, for example, retention.
Two out of three patients on methotrexate as first disease-modifying antirheumatic drug (DMARD), two out of three patients on TNFi as first biological DMARD, and three-quarters of patients initiating rituximab, remained on the drug with no switch or other DMARDs added at 12 months. At the same time point, only some 20% of methotrexate initiators, 15%–20% of TNFi initiators and around 10% of rituximab initiators, met the American Congress of Rheumatology/European Alliance of Associations for Rheumatology, or Simplified Disease Activity Index, respectively, remission criteria.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY.
Harmonisation of research data for collaborative studies can be achieved without compromising internal validity or generalisability of baseline, follow-up and outcome data.
Collaborative research into RA treatment outcomes including precision medicine should use and disclose the harmonisation methods used and employ multiple outcome definitions.
The discrepancy between high retention rates, but low remission rates observed across remission metrics, cohort types, treatments and countries points to unmet clinical needs.
Introduction
With increasing treatment options and a treat-to-target approach to rheumatoid arthritis (RA), remission has become a realistic treatment goal in clinical practice, and, when not reached, reason for treatment modification.1–3
Remission has also become a central concept for personalised medicine research. So far, however, attempts to identify predictive clinical variables for remission have yielded few such predictors.4–6 This may in part be due to the variety of outcome definitions used (that limits comparability across studies) but also due to our limited understanding of the underlying biology. Indeed, the actual predictive capacity of granular biological and other data risks being lost by the use of measures of remission that, by their overly holistic or inadequate design, may blur important temporal or interindividual differences.7 This underscores the importance of clarifying what exact ‘remission’ or other treatment outcome we are trying to predict.
In addition, personalised medicine has an inherent focus on subsetting and clustering, and therefore, puts increasing demands on study size. Collaboration and pooling across data collections and countries are often needed. Such collaborative approaches call for harmonisation of definitions to ensure comparability across data sources. This harmonisation may be more or less thorough, but its impact is seldom described (potentially causing unnecessary interstudy variability). Similarly, often only a subset of a theoretical study population (eg, a research cohort) may be available for a specific study, for instance, due to the requirement for particular patients (eg, new-onset RA), clinical variables or stored blood samples.8 Information on how well such subsets represent their underlying populations is fundamental for the generalisability of any results, but far from always investigated or reported.
Therefore, the aims of this study were as follows: (1) to describe how data on typical disease-modifying antirheumatic drug (DMARD) therapies in RA from different sources may be harmonised for collaborative analysis, including the level of consistency across and within data sources, for example, as defined by calendar period or availability of blood samples and (2) to use these harmonised data to comprehensively investigate the proportions of patients reaching remission, and in particular how these proportions vary across remission metrics, raw data type, individual DMARDs and countries, and how they relate to other measures of treatment outcomes such as treatment retention.
Subjects and methods
Setting
In Sweden, Denmark and Norway healthcare is public and tax based. Patients with RA are typically managed within specialised rheumatology (hospital-based outpatient clinics or other outpatient facilities). The national RA treatment guidelines in each country are largely similar and adhere to the European Alliance of Associations for Rheumatology (EULAR) RA treatment guidelines.9–11 Access to biological DMARDs (bDMARDs) has been relatively similar, and from an international perspective, liberal.12 In terms of access to the treatments under study and to rheumatologist visits, all data sources in this study reflect standard care, which was similar across the countries.
Data sources
In each country, we used existing large-scale and clinically integrated RA data sources (routine care clinical RA cohorts, research cohorts and (Norwegian) pragmatic trials) to define populations of patients with RA. In brief, in Sweden, we used the Epidemiological Investigation of RA (EIRA) study,13 the Swedish Rheumatology Quality (SRQ) register and the subset that had provided blood samples for SRQ biobank (SRQb).14 In Denmark, we used the Danish nationwide clinical registry (Danish Registry for Biologic Therapies in Rheumatology, DANBIO)15 where a subset of patients had provided samples for studies within the Danish Rheumatologic Biobank (DRB, the Biomarker Protocol16). In Norway, we used the Norwegian Antirheumatic Drug Register (NOR-DMARD),17 the Norwegian Very Early Arthritic Clinic (NOR-VEAC)18 and ULtrasound in Rheumatoid Arthritis patients starting BIologic Treatment (ULRABIT)19 cohorts, and the Aiming for Remission in rheumatoid arthritis: a randomised trial examining the benefit of ultrasound in a Clinical TIght Control regimen (ARCTIC) randomised trial.20 Table 1 and online supplemental methods provide a description of each data source and the healthcare use in the Nordic countries.
Table 1.
Characteristics of the Swedish, Danish and Norwegian data collections used to derive the analytical RA treatment cohorts
| Country | Sweden | Denmark | Norway | ||||||
| Source cohort | EIRA | SRQ | SRQ biobank | DANBIO | DRB* | NOR-DMARD | ULRABIT | ARCTIC | NOR-VEAC |
| Years of operation | 1996 and onwards | 1995 and onwards | 2012 and onwards | 2000 and onwards | 2015 and onwards | 2000 and onwards | 2010–2013 | 2010–2015 | 2010–2016 |
| Early or Established RA | Early | Early and established | Early and established | Early and established | Early and established | Early and established | Established | Early | Early |
| Coverage | Central and southern parts of Sweden | Nationwide | Some 14 participating centres across the country | Nationwide | 10–12 participating centres across the country | Currently 4 (previously 6) geographically spread hospitals in Norway | From single site | 13 Norwegian Rheumatology clinics | Seven RA clinics in central and southern Norway |
| RA inclusion criteria | Newly diagnosed RA diagnosed by a rheumatologist (EULAR/ACR 2010 or ACR 1987) | Clinical diagnosis of RA by a rheumatologist (EULAR/ACR 2010/ACR 1987) | Inclusion in SRQ, plus: early RA, or starting or having stared a b/tsDMARD during the last 2 years. | Clinical diagnosis of RA by a rheumatologist (EULAR/ACR 2010/ACR 1987) | Inclusion in DANBIO | Clinical diagnosis of RA by a rheumatologist (EULAR/ACR 2010 or ACR 1987) | Clinical diagnosis of RA by a rheumatologist (ACR 1987) | Clinical diagnosis of RA by a rheumatologist (EULAR/ACR 2010 or ACR 1987) | Clinical diagnosis of RA by a rheumatologist (EULAR/ACR 2010 or ACR 1987) |
| Study design | Research project. Incident case–control study with RA as incident cases and population-based controls | Nationwide prospective clinically integrated quality of care register with data entry by the rheumatologist and the patient | Research project. Cohort study. | Nationwide prospective clinically integrated quality of care register with data entry by the rheumatologist and the patient | Research project affiliated to DANBIO | Longitudinal observational study | Longitudinal observational study | Randomised controlled trial | Longitudinal observational study |
| Data capture | Questionnaires and blood samples | Data entered by the rheumatologist and the patient | One-time blood sample | Data entered by the rheumatologist and the patient | Blood sample (one or several per patient) | Data entered by the rheumatologist and the patient, 1–2 blood samples on subset of patients | Data entered by the rheumatologist and the patient, multiple blood samples per patient | Data entered by the rheumatologist and the patient, multiple blood samples per patient | Data entered by the rheumatologist and the patient, multiple blood samples per patient |
*DRB is a subcohort of the DANBIO cohort.
ACR, American Congress of Rheumatology; ARCTIC, Aiming for Remission in rheumatoid arthritis: a randomised trial examining the benefit of ultrasound in a Clinical TIght Control regimen; DANBIO, Danish Registry for Biologic Therapies in Rheumatology; DMARD, disease-modifying antirheumatic drug; DRB, Danish Rheumatologic Biobank; EIRA, Epidemiological Investigation of RA; EULAR, European Alliance of Associations for Rheumatology; NOR-DMARD, The Norwegian Antirheumatic Drug Register; NOR-VEAC, Norwegian Very Early Arthritic Clinic; RA, rheumatoid arthritis; SRQ, Swedish Rheumatology Quality register; ULRABIT, ULtrasound in Rheumatoid Arthritis patients starting BIologic Treatment.
rmdopen-2023-003027supp001.pdf (521.4KB, pdf)
Harmonisation of treatment cohorts
Within each country and data source, we assembled three treatment cohorts based on available information on treatment initiations: all patients with RA starting (1) methotrexate in DMARD monotherapy and (where such information was available:) as first ever DMARD within 6 months of RA diagnosis and with less than 1 year of symptom duration at the time of RA diagnosis (for details, see table 2), (2) a tumour necrosis factor inhibitor (TNFi) as first ever bDMARD (concomitant csDMARD allowed if started within 30 days of TNFi) and (3) rituximab (irrespective of previous DMARD treatment history, concomitant csDMARD allowed if started within 30 days of rituximab). In these cohorts, oral or other cotreatment with corticosteroids was allowed.
Table 2.
Patients with early RA initiating methotrexate as the first ever DMARD
| Country | Sweden | Denmark | Norway | |||||
| Source cohort | EIRA* | SRQ* | SRQb* | DANBIO† | DRB† | NOR-DMARD† | ARCTIC* | NOR-VEAC* |
| N patients | 2428 | 17 385 | 1930 | 12 493 | 1286 | 1783 | 230 | 404 |
| Characteristics at treatment start | ||||||||
| N women (%) | 1673 (69%) | 11 770 (68%) | 1336 (69%) | 8225 (66%) | 837 (65%) | 1225 (69%) | 141 (61%) | 269 (67%) |
| Median age at baseline (IQR) | 57 (47–65) | 62 (51–71) | 60 (48–69) | 62 (51–71) | 58 (49–68) | 58 (48–66) | 54 (42–62) | 57 (45–66) |
| Median calendar year of baseline (IQR) | 2008 (2004–2013) | 2012 (2007–2016) | 2015 (2011–2017) | 2016 (2013–2018) | 2014 (2013–2017) | 2006 (20032008) | 2011 (2011–2012) | 2013 (2011–2014) |
| Median disease duration in years (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–1) | 0 (0–1) | 0 (0–0) |
| Seropositive disease‡ (%) | 1609 (68%) | 10 974 (65%) | 1184 (63%) | 7972 (66%) | 877 (71%) | 1221 (68%) | 186 (81%) | 287 (71%) |
| Current smoker (%) | 512 (25%) | 2228 (16%) | 298 (16%) | 1266 (28%) | 149 (31%) | 525 (30%) | 55 (24%) | 89 (23%) |
| Cotreatment with oral steroids (%) | 1188 (49%) | 9138 (53%) | 962 (50%) | 1482 (12%) | 174 (14%) | 1783 (100%) | 230 (100%) | 404 (100%) |
| Visit-based clinical data | ||||||||
| Treatment start | ||||||||
| Median SJC(28) (IQR) | 8 (5–12) | 7 (4–11) | 6 (3–10) | 4 (1– 7) | 4 (1– 7) | 6 (3–10) | 6 (3–11) | 4 (1–8) |
| Median TJC (IQR) | 7 (4–12) | 6 (3–10) | 6 (3–10) | 5 (2– 10) | 5 (2– 10) | 6 (3–12) | 6 (3–11) | 4 (1–8) |
| Median CRP (IQR) | 13 (6–30) | 12 (5–31) | 11 (4–27) | 9 (3– 23) | 9 (3– 22) | 10 (5–27) | 7 (3–18) | 7 (3–20) |
| Median ESR (IQR) | 28 (15–45) | 27 (14–46) | 26 (14–45) | NA | NA | 22 (12–40) | 20 (11–32) | 19 (11–36) |
| Median Patient Global VAS (IQR) | 50 (30–70) | 52 (31–71) | 51 (32–71) | 58 (33– 79) | 60 (33– 80) | 47 (29–66) | 50 (31–70) | 45 (25–66) |
| Median HAQ (IQR) | 1.00 (0.63–1.38) | 1.00 (0.50–1.38) | 1.00 (0.63–1.50) | 0.88 (0.38– 1.38) | 0.88 (0.38– 1.38) | 0.62 (0.25–1.00) | NA | 0.75 (0.38–1.25) |
| Three months | ||||||||
| Retention (%) | 2114 (99%) | 12 709 (98%) | 1526 (97%) | 9209 (98%) | 1019 (97%) | 1464 (94%) | 230 (100%) | 331 (96%) |
| Persistence (%) | 2102 (98%) | 12 606 (97%) | 1495 (95%) | 8960 (95%) | 995 (95%) | 1464 (94%) | 223 (97%) | 331 (96%) |
| EULAR good response | 910 (46%) | 5103 (47%) | 696 (53%) | 2815 (47%) | 269 (37%) | 447 (32%) | 126 (55%) | 59 (43%) |
| Six months | ||||||||
| Retention (%) | 1812 (93%) | 9401 (93%) | 1162 (92%) | 7373 (93%) | 848 (93%) | 1255 (83%) | 209 (100%) | 275 (90%) |
| Persistence (%) | 1540 (79%) | 8177 (81%) | 976 (77%) | 6429 (82%) | 712 (78%) | 1255 (83%) | 176 (84%) | 275 (90%) |
| EULAR good response | 850 (49%) | 3670 (43%) | 481 (45%) | 2051 (45%) | 205 (36%) | 449 (39%) | 141 (68%) | 52 (47%) |
| 12 months | ||||||||
| Retention (%) | 1921 (89%) | 11 027 (89%) | 1402 (88%) | 8372 (89%) | 991 (87%) | 1129 (70%) | 182 (99%) | 262 (82%) |
| Persistence (%) | 1470 (68%) | 8687 (70%) | 1037 (65%) | 6588 (70%) | 698 (61%) | 1129 (70%) | 133 (72%) | 261 (82%) |
| EULAR good response | 889 (47%) | 4396 (43%) | 559 (41%) | 2446 (47%) | 276 (41%) | 498 (47%) | 142 (77%) | 51 (52%) |
Characteristics of Swedish, Danish and Norwegian treatment cohorts at the start of methotrexate (baseline) and outcomes during 1 year of follow-up. Percentages are calculated base on available data, for proportions of missing data, see online supplemental table S4.
*For EIRA, SRQ, SRQb, ARCTIC and NOR-VEAC data pertain specifically to patients diagnosed with RA within 12 months of symptom onset.
†For DANBIO, DRB and NORDMARD, information on RA symptom onset at diagnosis was not uniformly registered. For these cohorts, the data here presented refer to methotrexate treatment start within 6 months of RA diagnosis (irrespective of information on RA symptom duration at RA diagnosis).
‡Seropositivity defined as diagnosed with ICD code M05.8 or M05.9 and seronegative defined as diagnosed with M06.0 or M06.8 (and, if available, negative for anti-CCP and IgM RF).
ACR, American Congress of Rheumatology; ARCTIC, Aiming for Remission in rheumatoid arthritis: a randomised trial examining the benefit of ultrasound in a Clinical TIght Control regimen; CRP, C reactive protein; DANBIO, Danish Registry for Biologic Therapies in Rheumatology; DMARD, disease-modifying antirheumatic drug; DRB, Danish Rheumatologic Biobank; EIRA, Epidemiological Investigation of RA; ESR, erythrocyte sedimentation rate; EULAR, European Alliance of Associations for Rheumatology; ICD, International Classification of Diseases; NOR-DMARD, The Norwegian Antirheumatic Drug Register; NOR-VEAC, Norwegian Very Early Arthritic Clinic; RA, rheumatoid arthritis; SJC(28), swollen 28 joint count; SRQb, SRQ biobank; VAS, Visual Analogue Scale.
Subcohorts of each treatment cohort
To enable investigation of changes in cohort characteristics and the observed proportions of patients achieving remission when additional inclusion criteria were added, we defined several subcohorts, nested within each treatment cohort. An example of the data extraction and processing is provided in online supplemental figure S1. Mainly, criteria for (the timing of) sampling of blood for research biobanking were added.
Harmonisation of the handling of clinical variables
We used a predefined study protocol and code-book to define all time points and time intervals (=windows) in which clinical visit data were collected. For instance, we defined a baseline visit among visits occurring in an evaluation window from −90 to +30 days from the treatment start date, with a preference for the visit closest to time 0 (=date of treatment start, baseline). The 3-month visit was defined as any visits occurring from 31 to 149 days from the treatment start date, with a preference for the visit closest to 90 days, and the 6-month visit was defined as the visit occurring from 150 to 269 days from the treatment start date, with a preference for the visit closest to 180 days. In case there were several visits within each time interval, we defined a hierarchy among these (table 3). In an iterative process, each of these operational definitions was first imposed on each raw dataset, and the resultant numbers or proportions were then inspected and seeming inconsistencies across cohorts were further investigated until any uncertainty regarding the implementation of each definition or algorithm was resolved (typically after 2–3 such iterations) among all investigators.
Table 3.
Hierarchy for selection of an evaluation visit in case multiple visits were available within the time period of interest
| Priority | Criteria |
| 1 | Select the visit with the highest completeness of the outcome measures included (ie, swollen 28 joint count, tender 28 joint count, C reactive protein, erythrocyte sedimentation rate, patient global health on a Visual Analogue Scale). |
| 2 | Select the visit closest to the time point of interest by calculating the absolute difference between the visit and the target number of days (3 months: 90 days, 6 months: 180 days, 1 year: 365 days) For baseline visits, visits occurring before the start of treatment are prioritised. |
| 3 | In case a patient stops treatment within the given time interval, select only visits that actually occur during treatment. Thus, visits at a given time point are only available if the patient is still on treatment within the time-window for data-capture of that time point. |
Definitions and harmonisation of clinical outcomes
In each treatment (subcohort and at each time point of clinical evaluation (3, 6 and 12 months) we captured information on: (1) remission through six alternative definitions of remission: the original American Congress of Rheumatology (ACR)/EULAR Boolean remission, Simplified Disease Activity Index (SDAI) remission,21 ACR/EULAR three-item remission, Disease Activity Score 28 joints (DAS28) remission, swollen 28 joint count (SJC) SJC(28)=0 and patient global health ≤10 mm (see online supplemental table S2 for definitions), (2) response as defined by EULAR DAS28-erythrocyte sedimentation rate (ESR) or DAS28-C reactive protein (CRP), (3) retention defined as remaining on the drug at the evaluation time point and (4) persistence defined as retention but with no changes/additions of other DMARDs allowed (not including corticosteroids). In some data sources, data on retention and persistence could be extracted even if there was no recorded visit in the evaluation window. Thus, treatment episodes with no clinical data but information on treatment start and stop dates could still contribute to analyses of retention and persistence.
For all assessments of treatment outcomes, treatments discontinued before the clinical evaluation visit in question (eg, methotrexate treatment stopped month 4, with the next intended evaluation visit at month 6) we applied the following rules: (1) patients changing treatment (stopping due to lack of effect, death, patient decision or adding another DMARD) were categorised as non-responders/not in remission, (2) If the reason for withdrawal was an adverse event or pregnancy, the individuals were excluded from the analysis. (3) If the treatment was stopped due to remission or inactive disease and the patient did not start any other DMARD and fulfilled the outcome criteria at the evaluation time point (in the above: the 6-month visit), the patient was considered to fulfil the remission criteria under study, otherwise categorised as a non-responder.
Descriptive statistics
For each treatment cohort, subcohort and evaluation time point, we calculated the number and proportion of patients with an available visit, and the proportions reaching each treatment outcome. These data were then tabulated but since we did not address any specific hypothesis regarding differences across cohorts, and since the cohorts differed considerably in size, we did not perform any formal statistical comparisons across cohorts.
Each country uploaded their data to a central remote access data storage infrastructure at Karolinska Institutet, where the analyses were performed.
Exploratory analysis
As an exploratory analysis, we used our largest cohort (SRQ, methotrexate treatment cohort) and compared the 2012 EULAR/ACR Boolean remission criteria to the very recently updated version that uses 20 mm as compared with the original 10 mm as threshold for patient’s global assessment.22 Further, among the patients in the SRQ MTX cohort with available data on all remission outcomes at 3 months, we cross-tabulated the proportion of patients reaching each remission criteria at that time point to investigate how the different criteria correlated to each other. Finally, for each of the remission criteria (apart from the criterion SJC(28)=0), we further attempted to quantify the proportion of patients who did not reach that criterion but who at least seemingly were in inflammatory remission (defined as SJC(28)=0 and CRO below 0 mg/mL or ESR below 20 mg/mL for women and 15 mg/mL for men).
Results
Initiators of methotrexate
Online supplemental table S1 displays the numbers of eligible patients in each of the source cohorts when successive criteria to define each treatment cohort and subcohorts were added. Demographics and clinical characteristics for each treatment cohort, and the number and proportion meeting each outcome definition (other than remission) at the different evaluation time points are summarised in table 2 (online supplemental table S3 provides information on additional subcohorts). Missing data are shown in online supplemental table S4.
The smallest methotrexate cohort comprised 230 patients and the largest encompassed over 17 000 patients. The proportion of women (two-thirds), age at treatment start (typically: late 50s), the proportion of seropositive disease (around two-thirds) and the median calendar year of inclusion (around 2013–2014) showed a non-negligible variation across cohorts and their subcohorts (table 2, online supplemental table S3). By contrast, the proportion of current smokers (overall approximately 25%) displayed a larger range of variation across subcohorts from the same source cohort. The proportion of patients who were registered with oral corticosteroids varied markedly across the three countries, from 12% to 14% in the Danish cohorts to 50% in the Swedish and around 70% in the Norwegian cohorts, but less across cohorts and their subcohorts within each country. The clinical disease activity measures at treatment start were similar across countries, source cohorts and subcohorts.
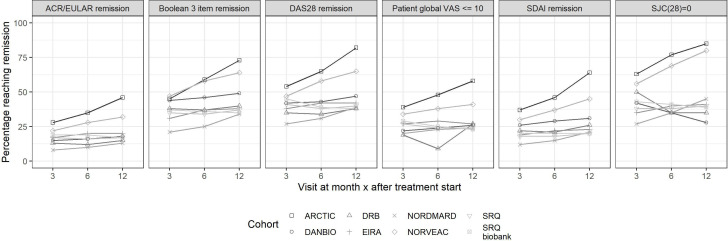
The proportion of patients in each of the eight cohorts reaching each remission outcome is depicted in figure 1. Detailed numbers for these cohorts and for the subcohorts of EIRA, SRQ, SRQb, DANBIO and DRB are provided in online supplemental table S5. At 3 months, the retention and persistence were both above 95%, and varied little across cohorts. The proportion of EULAR good responders was around 50% (higher (64%) in the Swedish SRQ subcohort sampled in conjunction with methotrexate start, online supplemental tables S3 and S5). For remission (online supplemental table S5), a much larger variability was observed, and two patterns emerged at 3 months and at the later time points: First, across remission definitions, there was a consistent difference in the proportions of patients reaching each definition, with the highest proportions achieving DAS28 remission, followed closely by remission defined as no SJC(28)=0, then by Patients Global Visual Analogue Scale (VAS)≤10 mm, SDAI and finally the original ACR/EULAR Boolean remission. Second, for each definition of remission, there was a reasonably high consistency in the proportions reaching the outcome under study across cohorts and their subcohorts, although the absolute proportions in the Norwegian cohorts were a few percentage points higher than the Swedish and Danish cohorts.
Figure 1.
Patients with early RA initiating methotrexate in Swedish, Danish and Norwegian treatment cohorts. Proportion reaching remission at 0, 3, 6 and 12 months according to six outcome definitions. ACR, American Congress of Rheumatology; ARCTIC, Aiming for Remission in rheumatoid arthritis: a randomised trial examining the benefit of ultrasound in a Clinical TIght Control regimen; DANBIO, Danish Registry for Biologic Therapies in Rheumatology; DAS28, Disease Activity Score 28; DRB, Danish Rheumatologic Biobank; RA, rheumatoid arthritis; NOR-VEAC, Norwegian Very Early Arthritic Clinic; SDAI, Simplified Disease Activity Index; SJC28, swollen 28 joint count; SRQ, Swedish Rheumatology Quality; VAS, Visual Analogue Scale.
At 6 months, the retention to methotrexate was above 90%, but the persistence decreased to 80%–85%, with little variation across subcohorts. The proportion of EULAR good responders remained around or just below 50% and the pattern of remission was largely similar to that at 3 months, though the proportions reaching remission were slightly higher in some (Norwegian) cohorts.
At 12 months, retention had dropped to just below 90% and persistence was down to around 70%, again with relatively low variation across cohorts. Close to 50% of patients remained on drug and had achieved EULAR good response by month 12 (online supplemental table S3). For remission, the variation between the different definitions remained similar to that at 6 months, as did the absolute proportions in each cohort, although the proportions reaching the various remission criteria during follow-up increased more in the Norwegian than in the Swedish and Danish cohorts. For instance, at 12 months between one third (Denmark), via 40% (Sweden) to up to 80% (Norway) reached no swollen joints. At the same time point, the proportion of patients with patient global health score on a VAS (patient global VAS) ≤10 mm was generally lower than those reaching remission as defined by SJC28=0 and varied from around 10% to around 50% (online supplemental table S5). Save for the Norwegian trial cohort, less than 20% had reached ACR/EULAR remission by month 12.
Initiators of TNF inhibitors
Table 4 summarises key elements for patients who initiated TNF inhibitors (TNFi) as first ever bDMARD treatment, and online supplemental table S6 summarises all subcohorts. Missing data are presented in online supplemental table S7. The (sub)cohorts ranged from 52 to 22 486 patients. The proportion of females was just above three-quarters, the median age at treatment start was around 55 years, and in most cohorts (except the Norwegian NORDMARD cohort) the median RA disease duration was relatively short (2–3 years). The proportion of seropositive patients varied from close to 90% (Norwegian cohorts), via 80% (Danish cohorts) to three-quarters (Swedish cohorts). The proportion of patients treated with oral corticosteroids concomitantly with TNFi was around 45% in Sweden and Norway, but considerably lower in Denmark (one quarter or less).
Table 4.
Patients with RA initiating TNF inhibitors as the first ever biological DMARD
| Source cohort | Sweden | Denmark | Norway | ||||||
| EIRA | SRQ | SRQ biobank | DANBIO | DRB | NOR-DMARD | ULRABIT | NOR-VEAC | ARCTIC | |
| N patients | 1628 | 22 486 | 2674 | 9312 | 2127 | 2036 | 141 | 96 | 52 |
| Characteristics at treatment start | |||||||||
| N women (%) | 1242 (76%) | 16 970 (75%) | 2060 (77%) | 6913 (74%) | 1601 (75%) | 1463 (72%) | 112 (79%) | 58 (60%) | 35 (67%) |
| Median age at baseline (IQR) | 55 (43,63) | 57 (46,65) | 56 (45,64) | 57 (48, 66) | 55 (46, 63) | 55 (44,63) | 53 (41,62) | 52 (40,63) | 53 (42,61) |
| Median calendar year of baseline (IQR) | 2010 (2006, 2015) | 2010 (2005, 2016) | 2013 (2011, 2016) | 2011 (2007, 2017) | 2011 (2007, 2016) | 2010 (2005, 2014) | 2011 (2010, 2012) | 2014 (2013, 2015) | 2012 (2012, 2013) |
| Median disease duration (IQR) | 2 (0,7)* | 3 (1,7)* | 3 (0,7)* | 5 (1, 12) | 5 (1, 12) | 5 (2,13) | 7 (3,13) | 1 (1,2) | 1 (1,2) |
| Seropositive disease† (%) | 1256 (78%) | 16 563 (77%) | 1922 (73%) | 7140 (80%) | 1661 (81%) | 1622 (80%) | 72 (76%) | 81 (84%) | 45 (87%) |
| Current smoker (%) | 361 (24%) | 2651 (14%) | 340 (13%) | 915 (26%) | 214 (25%) | 401 (23%) | 19 (21%) | 19 (21%) | 11 (21%) |
| Co-treatment with oral steroids (%) | 707 (43%) | 9821 (44%) | 1145 (43%) | 2376 (26%) | 496 (23%) | 966 (50%) | 65 (46%) | 40 (42%) | 20 (38%) |
| Visit-based clinical data | |||||||||
| Treatment start | |||||||||
| Median SJC(28) (IQR) | 6 (3–10) | 6 (3–10) | 5 (2–8) | 4 (2– 8) | 4 (2– 8) | 5 (2–9) | 4 (2–8) | 1 (0–3) | 0 (0–3) |
| Median TJC(28) (IQR) | 6 (3–10) | 6 (3–10) | 6 (3–10) | 6 (3– 12) | 6 (3– 11) | 5 (2–11) | 3 (1–7) | 1 (0–3) | 2 (0–6) |
| Median CRP (IQR) | 9 (4–21) | 10 (4–28) | 8 (3–20) | 11 (4– 25) | 10 (4– 24) | 8 (3–22) | 5 (2–13) | 3 (1–6) | 3 (1–6) |
| Median ESR (IQR) | 21 (11–36) | 22 (12–40) | 18 (9–32) | NA | NA | 19 (9–34) | 20 (10–33) | 9 (5–19) | 12 (6–16) |
| Median Patient Global VAS (IQR) | 54 (33–72) | 59 (40–75) | 56 (35–73) | 67 (47– 82) | 65 (45– 81) | 50 (29–71) | 47 (23–67) | 20 (10–51) | 18 (8–50) |
| Median HAQ (IQR) | 1.00 (0.5–1.38) | 1.13 (0.63–1.50) | 1.00 (0.50–1.38) | 1.12 (0.62– 1.75) | 1.00 (0.62– 1.62) | 0.62 (0.25–1.00) | 0.50 (0.13–0.88) | 0.50 (0.13–1.00) | NA |
| Three months | |||||||||
| Retention (%) | 1127 (94%) | 13 883 (95%) | 1676 (95%) | 7206 (96%) | 1774 (96%) | 1527 (90%) | 138 (100%) | 39 (57%) | 37 (100%) |
| Persistence (%) | 1122 (94%) | 13 825 (95%) | 1672 (95%) | 7149 (95%) | 1764 (95%) | 1492 (88%) | 138 (100%) | 38 (54%) | 36 (97%) |
| EULAR good response | 469 (47%) | 5045 (42%) | 662 (47%) | 2492 (41%) | 677 (43%) | 502 (35%) | 43 (32%) | 2 (10%) | 30 (81%) |
| Six months | |||||||||
| Retention (%) | 881 (84%) | 10 113 (84%) | 1184 (79%) | 5280 (83%) | 1359 (83%) | 1181 (72%) | 114 (100%) | 36 (38%) | 30 (100%) |
| Persistence (%) | 852 (81%) | 9752 (81%) | 1146 (77%) | 5118 (80%) | 1328 (81%) | 1152 (70%) | 114 (100%) | 36 (38%) | 30 (100%) |
| EULAR good response | 397 (44%) | 3872 (38%) | 482 (40%) | 1931 (44%) | 562 (47%) | 447 (41%) | 42 (37%) | 3 (17%) | 24 (80%) |
| 12 months | |||||||||
| Retention (%) | 943 (73%) | 11 268 (73%) | 1337 (66%) | 5311 (71%) | 1362 (72%) | 1050 (61%) | 103 (100%) | 48 (52%) | 23 (100%) |
| Persistence (%) | 888 (69%) | 10 494 (68%) | 1271 (62%) | 4950 (66%) | 1278 (68%) | 1002 (58%) | 103 (100%) | 44 (47%) | 20 (87%) |
| EULAR good response | 444 (42%) | 4548 (36%) | 564 (35%) | 2228 (50%) | 632 (52%) | 420 (43%) | 43 (43%) | 2 (9%) | 20 (87%) |
Characteristics of Swedish, Danish and Norwegian treatment cohorts at the start of TNF inhibitor (baseline), outcomes during 1 year and retention and persistence during 1 year of follow-up. Percentages are calculated based on available data, for proportions of missing data, see online supplemental table S7.
*Calculated on the subset of patients starting TNFi treatment in year 2006 or later.
†Seropositivity defined as diagnosed with ICD code M05.8 or M05.9 and seronegative defined as diagnosed with M06.0 or M06.8 (and, if available, negative for anti-CCP and IgM RF).
ACR, American Congress of Rheumatology; ARCTIC, Aiming for Remission in rheumatoid arthritis: a randomised trial examining the benefit of ultrasound in a Clinical TIght Control regimen; CRP, C reactive protein ; DANBIO, Danish Registry for Biologic Therapies in Rheumatology; DMARD, disease-modifying antirheumatic drug; DRB, Danish Rheumatologic Biobank; EIRA, Epidemiological Investigation of RA; ESR, erythrocyte sedimentation rate; EULAR, European Alliance of Associations for Rheumatology; ICD, International Classification of Diseases; NOR-DMARD, The Norwegian Antirheumatic Drug Register; NOR-VEAC, Norwegian Very Early Arthritic Clinic; RA, rheumatoid arthritis; SJC(28), swollen 28 joint count; SRQ, Swedish Rheumatology Quality; TJC(28), tender 28 joint count; ULRABIT, ULtrasound in Rheumatoid Arthritis patients starting BIologic Treatment; VAS, Visual Analogue Scale.
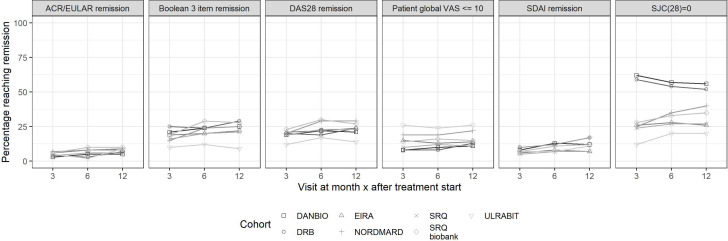
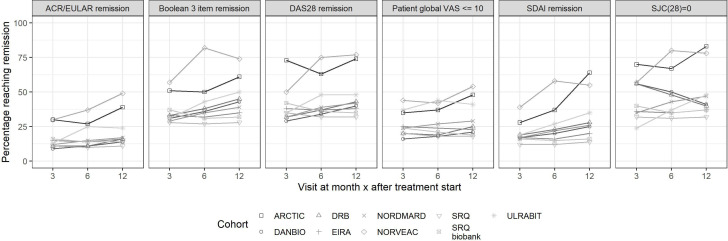
The clinical characteristics at start of TNFi were similar, apart from lower levels of disease activity in the Norwegian trial cohort of early RA (vs all other cohorts) as defined by the SJC(28), tender 28 joint count, CRP, ESR and patient global VAS. The patterns for retention, persistence, response and remission (online supplemental table S8) mirrored those observed in the cohorts of patients initiating methotrexate therapy (both regarding the internal ranking among remission definitions and the intercountry pattern). At 12 months, about two-thirds of patients remained on drug with no new DMARD added. The proportions reaching the remission outcomes at each time point are shown in figure 3 (exact numbers in online supplemental table S8), and included 10%–25% reaching ACR/EULAR remission at 1 year (higher in the Norwegian trial cohort).
Figure 3.
Patients with RA initiating rituximab in the Swedish, Danish and Norwegian treatment cohorts. Proportion reaching remission at 0, 3, 6 and 12 months according to six outcome definitions. ACR, American Congress of Rheumatology; ARCTIC, Aiming for Remission in rheumatoid arthritis: a randomised trial examining the benefit of ultrasound in a Clinical TIght Control regimen; DANBIO, Danish Registry for Biologic Therapies in Rheumatology; DAS28, Disease Activity Score 28; DMARD, disease-modifying antirheumatic drug; DRB, Danish Rheumatologic Biobank; NOR-VEAC, Norwegian Very Early Arthritic Clinic; RA, rheumatoid arthritis; SDAI, Simplified Disease Activity Index; SJC28, swollen 28 joint count; SRQ, Swedish Rheumatology Quality; ULRABIT, ULtrasound in Rheumatoid Arthritis patients starting BIologic Treatment; VAS, Visual Analogue Scale.
Initiators of rituximab
Table 5 summarises key characteristics of the rituximab treatment cohorts and online supplemental table S9 summarises their subcohorts. Missing data are shown in online supplemental table S10. The rituximab initiator (sub)cohorts ranged from 43 through 5232 patients. The proportion of females was above three-quarters. The median age, disease duration and the proportions of seropositive disease were all higher than for methotrexate and TNFi initiators. Likewise, higher proportions were smokers and on oral corticosteroids. Also, the clinical disease activity metrics appeared higher than those in the TNFi and methotrexate initiator cohorts.
Table 5.
Patients with RA initiating rituximab
| Country | Sweden | Denmark | Norway | ||||
| Source cohort | EIRA | SRQ | SRQ biobank | DANBIO | DRB | NOR-DMARD | ULRABIT |
| N patients | 432 | 5232 | 942 | 1462 | 346 | 411 | 43 |
| Characteristics at treatment start | |||||||
| N women (%) | 338 (78%) | 3969 (76%) | 713 (76%) | 1134 (78%) | 276 (80%) | 315 (77%) | 35 (81%) |
| Median age at baseline (IQR) | 61 (51–68) | 63 (53–71) | 62 (52–70) | 62 (54–70) | 59 (50–66) | 57 (48–65) | 57 (53–62) |
| Median calendar year of baseline (IQR) | 2014 (2010–2017) | 2013 (2010–2016) | 2014 (2012–2017) | 2013 (2010–2017) | 2015 (2011–2018) | 2011 (2008–2014) | 2011 (2010–2012) |
| Median disease duration (IQR) | 6 (3–11)* | 5 (2–9)* | 6 (2–11)* | 10 (4–18) | 10 (5–17) | 10 (5–19) | 10 (4–17) |
| Seropositive disease† (%) | 372 (87%) | 4467 (88%) | 794 (86%) | 1197 (86%) | 273 (84%) | 386 (94%) | 14 (82%) |
| Current smoker (%) | 129 (31%) | 645 (14%) | 118 (13%) | 188 (23%) | 46 (21%) | 90 (26%) | 7 (22%) |
| Cotreatment with oral steroids (%) | 218 (50%) | 2559 (49%) | 435 (46%) | 491 (34%) | 100 (29%) | 264 (68%) | 33 (77%) |
| Visit-based clinical data | |||||||
| Clinical data at treatment start | |||||||
| Median SJC(28) (IQR) | 6 (3–9) | 6 (3–9) | 5 (2–8) | 4 (2– 8) | 4 (2– 7) | 5 (2–10) | 8 (3–12) |
| Median TJC(28) (IQR) | 6 (3–10) | 6 (3–10) | 5 (2–9) | 7 (3– 13) | 7 (3– 12) | 6 (2–12) | 6 (0–9) |
| Median CRP (IQR) | 8 (3–23) | 11 (5–28) | 8 (4–22) | 11 (4– 27) | 9 (4– 21) | 10 (4–25) | 7 (2–13) |
| Median ESR (IQR) | 24 (13–42) | 28 (14–46) | 22 (11–41) | NA | NA | 26 (13–44) | 26 (14–42) |
| Median Patient Global VAS (IQR) | 60 (38–75) | 61 (42–76) | 60 (35–74) | 73 (52– 87) | 72 (53– 86) | 61 (38–77) | 59 (30–72) |
| Median HAQ (IQR) | 1.13 (0.75–1.50) | 1.25 (0.88–1.75) | 1.13 (0.63–1.63) | 1.50 (1.00– 2.00) | 1.38 (0.88– 2.00) | 0.88 (0.50–1.13) | 0.63 (0.19–1.00) |
| 3 months | |||||||
| Retention (%) | 233 (92%) | 2472 (95%) | 411 (96%) | 973 (90%) | 268 (91%) | 294 (97%) | 42 (100%) |
| Persistence (%) | 229 (90%) | 2454 (94%) | 407 (95%) | 960 (89%) | 266 (90%) | 286 (94%) | 42 (100%) |
| EULAR good response | 49 (26%) | 460 (24%) | 73 (23%) | 230 (30%) | 74 (33%) | 70 (25%) | 5 (12%) |
| 6 months | |||||||
| Retention (%) | 226 (88%) | 2767 (91%) | 500 (92%) | 927 (84%) | 251 (88%) | 277 (89%) | 41 (100%) |
| Persistence (%) | 211 (82%) | 2664 (87%) | 485 (89%) | 882 (80%) | 242 (85%) | 265 (85%) | 41 (100%) |
| EULAR good response | 56 (28%) | 651 (28%) | 145 (35%) | 222 (29%) | 60 (28%) | 95 (37%) | 12 (29%) |
| 12 months | |||||||
| Retention (%) | 277 (82%) | 3138 (82%) | 583 (81%) | 913 (74%) | 238 (76%) | 269 (81%) | 37 (100%) |
| Persistence (%) | 250 (74%) | 2906 (76%) | 547 (76%) | 858 (70%) | 228 (73%) | 254 (77%) | 37 (100%) |
| EULAR good response | 69 (27%) | 816 (28%) | 171 (32%) | 241 (32%) | 74 (36%) | 99 (40%) | 7 (20%) |
Characteristics of Swedish, Danish and Norwegian treatment cohorts at the start of rituximab (baseline), outcomes during 1 year and retention and persistence during 1 year of follow-up. Percentages are calculated based on available data, for proportions of missing data, see online supplemental table 10.
*Calculated on the subset of patients starting rituximab treatment year 2006 or later.
†Seropositivity defined as diagnosed with ICD code M05.8 or M05.9 and seronegative defined as diagnosed with M06.0 or M06.8 (and, if available, negative for anti-CCP and IgM RF).
CRP, C reactive protein; DANBIO, Danish Registry for Biologic Therapies in Rheumatology; DRB, Danish Rheumatologic Biobank; EIRA, Epidemiological Investigation of RA; ESR, erythrocyte sedimentation rate; EULAR, European Alliance of Associations for Rheumatology; ICD, International Classification of Diseases; NOR-DMARD, The Norwegian Antirheumatic Drug Register; RA, rheumatoid arthritis; SJC28, swollen 28 joint count; ULRABIT, ULtrasound in Rheumatoid Arthritis patients starting BIologic Treatment; VAS, Visual Analogue Scale.
Figure 3 shows the proportion reaching remission in each cohort (exact figures in online supplemental table S11) and online supplemental table S9 the proportion reaching remission in each subcohort. The proportions reaching remission with rituximab during follow-up were generally somewhat lower than for patients with RA initiating TNFi and methotrexate, as were the retention and persistence, but the pattern of small or modest variations between cohorts and subcohorts and larger variations across remission definitions remained the same with rituximab as that observed for TNFi and methotrexate. For instance, at 12 months, some two-thirds of patients remain on drug with no other DMARD added, but less than 15% met the ACR/EULAR remission criteria.
Exploratory analysis using data from the Swedish SRQ methotrexate cohort
When changing the definition of EULAR/ACR Boolean remission criteria to patient’s global assessment up to 20 mm instead of 10 mm of the 0–100 VAS scale, the proportion meeting this remission definition in the SRQ methotrexate cohort increased from 17% at 3 months to 26%, from 16% to 22% at 6 months and from 17% to 24% at 12 months.
When cross-tabulating the different remission criteria, patients reaching ACR/EULAR Boolean remission or SDAI remission had in general the highest proportion of patients meeting also other criteria (67%–99%), while the proportion of patients meeting patient’s global remission or having no swollen joints generally fulfilled less other remission criteria (37%–80%), online supplemental figure S2.
Among those not reaching a specific remission criterion (and among patients not reaching any of the remission criteria) the proportion who were in inflammatory remission (defined as SJC(28)=0 and a normal acute phase reactant) was around 10%.
Discussion
We provide examples and results of how raw data from eight RA data sources from three countries may be harmonised for collaborative analyses. Using these data, we provide a comprehensive overview of rates of remission with standard DMARD therapies for RA and their relation to other treatment outcomes in RA and demonstrate that (1) the proportions reaching each treatment outcome varied by treatment and by outcome metric, but, within and between (sub)cohorts of the same treatment and metric, the variation was small to modest, (2) patients reaching more stringent remission criteria such as ACR/EULAR Boolean remission or SDAI remission in general fulfilled most remission criteria. By contrast, less than half of patients who reached SJC(28)=0 fulfilled remission based on patient’s global assessment, SDAI or ACR/EULAR Boolean remission, (3) around 10% of patients (on methotrexate as first DMARD in early RA (Swedish data) who did not achieve remission by any given criterion had no swollen joints and normal acute phase reactants at that same time point, (4) the modest difference between (the generally high) drug retention (remaining on drug) and persistence (remaining on drug with no new DMARDS added) was in sharp contrast to the much lower proportions reaching remission, not only at 3 months, but throughout the first year. For instance, while around two-thirds of the patients who had started a first-line methotrexate monotherapy remained on that drug and had no other DMARD added 1 year later, only 20%–30% were in remission at that time point. Similar discrepancies between retention/persistence and various measures of remission were noted for TNF inhibitors and for rituximab, (5) although the absolute proportions reaching the treatment outcomes under study varied by drug, the above patterns of similarities and discrepancies was a consistent finding across methotrexate, TNFi and rituximab, and also across the three countries and (6) the treatment outcomes from the protocolised clinical trial data source were superior to those from observational cohorts reflecting a higher effectiveness of the same intervention (methotrexate as first treatment in early RA) in a similar source population.
With respect to baseline characteristics in the RA cohorts, these were inconspicuous (compared with the existing literature) within each treatment but displayed some variation across country, data source and subcohorts. With a code-book and harmonised definitions, this variation was generally low (a few percentage units). Since our harmonisation was mainly achieved by agreement on the definitions and implementation of operational measures, for example, of time-windows, response and of individuals at risk, it does not readily provide quantitative information on the counterfactual situation (where all investigators would have used the same raw data to compile treatment cohorts at their own discretion). Importantly, however, restrictions to subcohorts defined by, for example, the availability of blood samples generally only had a modest, if any, effect on their aggregate-level baseline characteristics, indirectly testifying to the robustness of the level of harmonisation. We did, however, observe a difference in the use of oral corticosteroids at baseline between the countries, with a range 12%–16% in Denmark, 40%–60% in Sweden and 57%–75% use in Norway (excluding ARCTIC where prednisolone was added to MTX in all patients as part of the treatment algorithm). This is likely to represent a true difference between the countries.9–11 Thus, in Denmark, there is widespread use of intra-articular steroid injections based on the tradition and results from previous randomised controlled trials (RCTs) successfully applying this approach.23–28 This observed and remaining difference exemplifies the importance of harmonisation of data within and between countries and cohorts, specifically of involving clinical experts from each country familiar with the clinical work-flow, as well as data specialists with knowledge of the variables and their representations and definitions, in all analytical steps, from data preparation to interpretation.
With respect to remission and other treatment outcomes, previous studies have indicated that the observed proportions of patients reaching remission vary substantially depending on the definition of remission,29–31 which may be a result of artificial differences related to handling of the data within each study. Our results negate the hypothesis that lack of study harmonisation is a main explanation of these intermetric differences. Instead, such variation is likely a result of the handling (here: inclusion or exclusion) of individual components of composite-measure definitions of remission.1 For instance, VAS pain and global health capture not only RA-specific inflammation but also capture other health aspects, related or not to concurrent RA inflammatory activity. Similarly, the inclusion or exclusion of components for which there may be drug-specific effects, such as CRP, may impact the observed comparative effectiveness of DMARDs.30 32 33 Our exploratory analysis in which we applied the very recently updated ACR/EULAR Boolean remission criteria22 to the SRQ methotrexate cohort demonstrated that the proportion reaching remission with the new criteria went from about one in six patients to closer to one in four, also resulting in better alignment with the other remission criteria under study. Our exploratory analyses further demonstrated that around 10% of patients not achieving any given composite remission criteria had normal ESR/CRP and no swollen joints in the SJC(28). The latter observation does of course not preclude that these patients had other manifestations (eg, MTP-joint involvement) of active RA, but offers a rough quantification of the proportion of patients whose failure to meet remission might primarily reside with other factors than concomitant RA-related inflammatory activity.
The internal variation in, and ranking of, proportions reaching each of the six remission criteria in our study (figures 1–3) is largely in line with previous observations.34 35 With regard to the absolute percentages reaching each of the treatment outcomes under study, we note that the proportion of patients reaching remission with methotrexate was comparatively high compared with other studies. For TNFi and rituximab (that are more often used as second, third or more DMARD) the observed proportions were more in keeping with those reported from previous studies (eg, as compiled in).35 The protocol-driven Norwegian RA data collections (the ARCTIC trial) had treatment outcomes superior to those cohorts reflective of treatment decisions as in clinical practice. Whether this reflects the advantage of protocol-based treat-to-target strategies, selection of patients into clinical trials, or both, remains unclear and beyond the scope of this study.
Figure 2.
Patients with RA initiating TNFi as first ever biological DMARD in Swedish, Danish and Norwegian treatment cohorts. Proportion reaching remission at 0, 3, 6 and 12 months according to six outcome definitions. ACR, American Congress of Rheumatology; ARCTIC, Aiming for Remission in rheumatoid arthritis: a randomised trial examining the benefit of ultrasound in a Clinical TIght Control regimen; DANBIO, Danish Registry for Biologic Therapies in Rheumatology; DAS28, Disease Activity Score 28; DMARD, disease-modifying antirheumatic drug; DRB, Danish Rheumatologic Biobank; NOR-VEAC, Norwegian Very Early Arthritic Clinic; RA, rheumatoid arthritis; SDAI, Simplified Disease Activity Index; SJC28, swollen 28 joint count; SRQ, Swedish Rheumatology Quality; ULRABIT, ULtrasound in Rheumatoid Arthritis patients starting BIologic Treatment; VAS, Visual Analogue Scale.
With respect to variation within and across cohorts, we noted only modest variation in the observed treatment outcomes across subcohorts defined based on, for example, a stored blood sample, versus the entire cohort. This was somewhat surprising since for some of these patients and samplings, the methotrexate cohorts in particular, there was an implicit conditioning on non-response to methotrexate. For instance, in Sweden, patients were eligible for biobanking in SRQb at start of a bDMARD (often after failing methotrexate), and in Denmark inclusion in the DRB was conditioned on survival/follow-up up until a certain calendar year. Importantly, this similarity in treatment outcomes across subcohorts suggests that, for example, biomarker analyses performed in (these) subcohort of patients with available samples might be extrapolated to their whole underlying cohorts.
Our study has some limitations. Several of the included data sources, such as SRQ and DANBIO, are clinically integrated systems that capture data as part of standard care in a quality-controlled manner.15 As such they include visits as they occur rather than at predefined time points specific to each cohort, and capture only the information available to the patient and rheumatologist at the time point of the visit. To maximise comparability and to minimise missing data, we used harmonised algorithms to define the visits to be used for this study. While the use of such algorithms should not have led to a selective inclusion of visits with high or low disease activity, we cannot formally exclude this possibility. Further, although several of the treatment cohorts represent some of the world’s largest longitudinal clinical RA data collections, others were small limiting precision and the possibility to compare across cohorts. We initially set out to employ strict definitions of certain cohorts (eg, ‘newly diagnosed RA’) which might have excluded many patients for whom the necessary information was not uniformly collected. This would have reduced in particular the Danish and NOR-DMARD MTX cohorts, but it was eventually decided to relax this criterion for these specific cohorts (it was kept in the Swedish cohorts). With respect to treatment data, our study was based on clinical visit and registered start/stop dates, which may or may not equal actual drug intake or use. Finally, it should be noted that although some of the included data sources (such as DANBIO and SRQ) have a high national coverage, not all cohorts should be seen as reflective of the characteristics of the average patients with RA in each of the three Scandinavian countries. For example, in the ARCTIC RCT, the visit frequency was higher (nine visits during the first year) than that of standard of care in the Nordic countries (around twice) as well as obligation to follow a strict algorithm for treatment escalation. However, it is important to note that access to treatment/drugs did not differ between any of the cohorts. Further, data on retreatment with rituximab were not available within the data sources, making distinctions between retreated and not retreated for the longer-term rituximab data uncertain. Common to many observational studies, our methods for data collection were based on clinical data on treatment, which is not necessarily similar to actual drug intake (compliance).
Certain strengths should be mentioned. We included multiple cohorts, from several sources and countries, and performed a detailed harmonisation of their data to ensure that the study data would be conceptually comparable and that any variation across datasets would not simply be due to differences in data handling and definitions. Our inclusion of cohorts from both routine care and randomised strategy trials is of value as previous studies have demonstrated that patients in trials and in clinical practice differ on a range of parameters.36 37 Further, we could include and compare the pattern across cohorts and subcohorts for three commonly used DMARDs, and the performance and internal relation of several different definitions of treatment outcomes (retention, persistence, response and six definitions of remission). For future studies, addition of information from the national patient registries38 in the Nordic countries on, for instance, death, hospitalisations (comorbidities and adverse outcomes) and socioeconomics, combined with biological material samples for biomarker analyses, promises huge potentials for precision medicine research in RA.
In conclusion, we exemplify how different RA raw data sources can be harmonised for joint analyses and provide comprehensive results on response, remission, retention and persistence to methotrexate, TNFi and rituximab in (tens of) thousands of patients from clinical practice-based and other RA cohorts. With regard to harmonisation, our results demonstrate a variation related to the choice of outcome metric, for example, a twofold variation between the proportions reaching different remission criteria. Our results further demonstrate that judicious harmonisation of the raw data can bring down variation across datasets to a minimum, for example, for many metrics less than a 10% unit variation across cohorts and subcohorts, with regard to baseline, follow-up and response data, and therefore, without compromising generalisability. With regard to the observed treatment outcomes, our results quantify the (modest) variations in each treatment outcomes measure across cohorts and countries, and the (marked) variation across remission metrics. The high retention rates but low remission rates underscore the large unmet need for treatment optimisation: only 15%–20% of patients initiating first-line methotrexate monotherapy or TNFi treatment, and only 1 in 10 patients on rituximab, remain on drug and in ACR/EULAR or SDAI remission at 12 months.
With no single gold standard for remission in RA, it will now be an important task for collaborative research in RA precision medicine to investigate commonalities and differences in the predictors and prediction models for different definitions (and thus, types) of treatment-induced remission.
Acknowledgments
The authors wish to thank all clinicians and patients contributing to the RA cohorts used for this investigation, and to past or present PIs of the RA data collection used, namely Lars Alfredsson (EIRA), Lars Klareskog (EIRA), the SRQ biobank group, Espen Haavardsholm (ARCTIC), Maria Dahl Mjaavatten (NOR-VEAC) and Tore K. Kvien (NORD-MARD). Thank you to all departments and patients contributing to the Danish DANBIO registry. We also thank colleagues contributing the Danish Rheumatologic Biobank (DRB): Oliver Hendricks, Estrid Høgdall, Søren Jacobsen, Dorte Vendelbo Jensen, Salome Kristensen, Asta Linauskas, Anne Gitte Loft, Heidi Lausten Munk, Inge Juul Sørensen, Torkell Ellingsen.
Footnotes
Contributors: Study idea and concept: JA. Data collection: BG, E-MH, HBH, HW, JA, JS, MLH and NSK. Data analysis: HW, IMT, JS and NSK. BG, HBH, HW and JA drafted the article. JA acts as guarantor of the study. All authors interpreted the results, critically revised the manuscript and approved the final version.
Funding: This project was supported by Vinnova, Innovationsfonden and The Research Council of Norway, under the frame of Nordforsk (Grant agreement no. 90825, Project NORA), the Swedish Research Council, the Region Stockholm-Karolinska Institutet research funds (ALF).
Competing interests: SS is a part-time employee at deCODE genetics. E-MH has received institutional grants or contracts from Novo Nordic Foundation, Danish Rheumatism Association, Danish Regions Medicine Grants, Rocke, Novartis or Abbvie, payment or honoraria for lecture, presentations, speakers bureaus, manuscript writing or educational events from Sobi, support from attending meetings and/or travel from Pfizer, Sobi and Abbvie. participated on data safety monitoring board or advisory board for Novartis, Abbvie and Sanofi and is principal investigator in trials by SynACT Pharma, site principal investigator in trials by AbbVie, Novartis, Novo and Sanofi. HBH has received honoraria for lectures from AbbVie, Novartis, Lilly and UCB, and participated on advisory board for AbbVie, UCB and Novarties. Karolinska Institutet has entered into agreements with the following companies, with JA as PI: Abbvie, BMS, Eli Lilly, Galapagos, Janssen, Pfizer, Roche, Samsung Bioepis and Sanofi. BG has received research grant from Pfizer, AbbVie, BMS and Sandoz. The remaining authors have nothing to declare.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Due to ethical and legal considerations, data is not publicly available. Please contact the authors on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by the Swedish Ethics Review Authority (2022-03969-02). According to Danish legislation, no ethical approvals were needed for registry analyses. For Norway, the NORA study was approved by the Norwegian ethics authorities (REC south-east ref 97026). All data transfer and storage were approved by data protection authorities. Patient consents were collected according to the requirements and ethical permits of the contributing subcohorts.
References
- 1.Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA 2018;320:1360–72. 10.1001/jama.2018.13103 [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS. Insights into the treatment of rheumatoid arthritis: a paradigm in medicine. J Autoimmun 2020;110:102425. 10.1016/j.jaut.2020.102425 [DOI] [PubMed] [Google Scholar]
- 3.Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying Antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. 10.1136/annrheumdis-2019-216655 [DOI] [PubMed] [Google Scholar]
- 4.Giacomelli R, Afeltra A, Alunno A, et al. Guidelines for biomarkers in autoimmune rheumatic diseases - evidence based analysis. Autoimmun Rev 2019;18:93–106. 10.1016/j.autrev.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 5.Khader Y, Beran A, Ghazaleh S, et al. Predictors of remission in rheumatoid arthritis patients treated with Biologics: a systematic review and meta-analysis. Clin Rheumatol 2022;41:3615–27. 10.1007/s10067-022-06307-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu C, Jin S, Wang Y, et al. Remission rate and predictors of remission in patients with rheumatoid arthritis under treat-to-target strategy in real-world studies: a systematic review and meta-analysis. Clin Rheumatol 2019;38:727–38. 10.1007/s10067-018-4340-7 [DOI] [PubMed] [Google Scholar]
- 7.Lin CMA, Cooles FAH, Isaacs JD. Precision medicine: the precision gap in rheumatic disease. Nat Rev Rheumatol 2022;18:725–33. 10.1038/s41584-022-00845-w [DOI] [PubMed] [Google Scholar]
- 8.He J, Morales DR, Guthrie B. Exclusion rates in randomized controlled trials of treatments for physical conditions: a systematic review. Trials 2020;21:228. 10.1186/s13063-020-4139-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlemmer A, Uhrenholt L, Ammitzboll Danielsen M, et al. National behandlingsvejledning for seropositiv og seronegativ reumatoid artritis (RA) [Dansk Reumatologi]. 2022. Available: https://danskreumatologi.dk/nbv/sygdomme/ra [Accessed 29 Aug 2022].
- 10.Gjertsson I, Kastbom A, Lampa J, et al. Riktlinjer för läkemedelsbehandlings vid reumatoid artrit [Svensk Reumatologisk Förening]. 2022. Available: https://svenskreumatologi.se/srfs-riktlinjer/ [Accessed 29 Aug 2022].
- 11.Norwegian treatment guidelines for rheumatoid arthritis. 2022. Available: https://metodebok.no/index.php?action=topic&item=kh5aS3AF [Accessed 23 Sep 2022].
- 12.Putrik P, Ramiro S, Kvien TK, et al. Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann Rheum Dis 2014;73:198–206. 10.1136/annrheumdis-2012-202603 [DOI] [PubMed] [Google Scholar]
- 13.Stolt P, Bengtsson C, Nordmark B, et al. Quantification of the influence of cigarette smoking on rheumatoid arthritis: results from a population based case-control study, using incident cases. Ann Rheum Dis 2003;62:835–41. 10.1136/ard.62.9.835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saevarsdottir S, Stefansdottir L, Sulem P, et al. Multiomics analysis of rheumatoid arthritis yields sequence variants that have large effects on risk of the Seropositive subset. Ann Rheum Dis 2022;81:1085–95. 10.1136/annrheumdis-2021-221754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibfelt EH, Jensen DV, Hetland ML. The Danish nationwide clinical register for patients with rheumatoid arthritis: DANBIO. Clin Epidemiol 2016;8:737–42. 10.2147/CLEP.S99490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kringelbach TM, Glintborg B, Hogdall EV, et al. Identification of new biomarkers to promote Personalised treatment of patients with inflammatory rheumatic disease: protocol for an open cohort study. BMJ Open 2018;8:e019325. 10.1136/bmjopen-2017-019325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kvien TK, Heiberg MS, Lie E, et al. A Norwegian DMARD register: prescriptions of DMARDs and biological agents to patients with inflammatory rheumatic diseases. Clin Exp Rheumatol 2005;23:S188–94. [PubMed] [Google Scholar]
- 18.Mjaavatten MD, Uhlig T, Haugen AJ, et al. Positive anti-citrullinated protein antibody status and small joint arthritis are consistent predictors of chronic disease in patients with very early arthritis: results from the NOR-VEAC cohort. Arthritis Res Ther 2009;11:R146. 10.1186/ar2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammer HB, Uhlig T, Kvien TK, et al. Pain catastrophizing, subjective outcomes, and inflammatory assessments including ultrasound: results from a longitudinal study of rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2018;70:703–12. 10.1002/acr.23339 [DOI] [PubMed] [Google Scholar]
- 20.Haavardsholm EA, Aga A-B, Olsen IC, et al. Ultrasound in management of rheumatoid arthritis: ARCTIC randomised controlled strategy trial. BMJ 2016;354:i4205. 10.1136/bmj.i4205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aletaha D, Ward MM, Machold KP, et al. Remission and active disease in rheumatoid arthritis: defining criteria for disease activity States. Arthritis Rheum 2005;52:2625–36. 10.1002/art.21235 [DOI] [PubMed] [Google Scholar]
- 22.Studenic P, Aletaha D, de Wit M, et al. American college of rheumatology/EULAR remission criteria for rheumatoid arthritis: 2022 revision. Ann Rheum Dis 2023;82:74–80. 10.1136/ard-2022-223413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hetland ML, Stengaard-Pedersen K, Junker P, et al. Combination treatment with methotrexate, cyclosporine, and intraarticular betamethasone compared with methotrexate and Intraarticular betamethasone in early active rheumatoid arthritis: an investigator-initiated, multicenter, randomized, double-blind, parallel-group, placebo-controlled study. Arthritis Rheum 2006;54:1401–9. 10.1002/art.21796 [DOI] [PubMed] [Google Scholar]
- 24.Hetland ML, Stengaard-Pedersen K, Junker P, et al. Aggressive combination therapy with intra-articular glucocorticoid injections and conventional disease-modifying anti-rheumatic drugs in early rheumatoid arthritis: second-year clinical and radiographic results from the CIMESTRA study. Ann Rheum Dis 2008;67:815–22. 10.1136/ard.2007.076307 [DOI] [PubMed] [Google Scholar]
- 25.Hetland ML, Østergaard M, Ejbjerg B, et al. Short- and long-term efficacy of intra-articular injections with betamethasone as part of a treat-to-target strategy in early rheumatoid arthritis: impact of joint area, repeated injections, MRI findings, anti-CCP, Igm-RF and CRP. Ann Rheum Dis 2012;71:851–6. 10.1136/annrheumdis-2011-200632 [DOI] [PubMed] [Google Scholar]
- 26.Hørslev-Petersen K, Hetland ML, Junker P, et al. Adalimumab added to a treat-to-target strategy with methotrexate and intra-articular triamcinolone in early rheumatoid arthritis increased remission rates, function and quality of life. The OPERA study: an investigator-initiated, randomised, double-blind, parallel-group, placebo-controlled trial. Ann Rheum Dis 2014;73:654–61. 10.1136/annrheumdis-2012-202735 [DOI] [PubMed] [Google Scholar]
- 27.Hørslev-Petersen K, Hetland ML, Ørnbjerg LM, et al. Clinical and radiographic outcome of a treat-to-target strategy using methotrexate and intra-articular glucocorticoids with or without adalimumab induction: a 2-year investigator-initiated, double-blinded, randomised, controlled trial (OPERA). Ann Rheum Dis 2016;75:1645–53. 10.1136/annrheumdis-2015-208166 [DOI] [PubMed] [Google Scholar]
- 28.Glinatsi D, Heiberg MS, Rudin A, et al. Head-to-head comparison of aggressive conventional therapy and three biological treatments and comparison of two de-escalation strategies in patients who respond to treatment: study protocol for a multicenter, randomized, open-label, blinded-assessor, phase 4 study. Trials 2017;18:161. 10.1186/s13063-017-1891-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Studenic P, Aletaha D, de Wit M, et al. American college of rheumatology/EULAR remission criteria for rheumatoid arthritis: 2022 revision. Arthritis Rheumatol 2023;75:15–22. 10.1002/art.42347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira RJO, Duarte C, Ndosi M, et al. Suppressing inflammation in rheumatoid arthritis: does patient global assessment blur the target? A practice-based call for a paradigm change. Arthritis Care Res (Hoboken) 2018;70:369–78. 10.1002/acr.23284 [DOI] [PubMed] [Google Scholar]
- 31.Ferreira RJO, Welsing PMJ, Jacobs JWG, et al. Revisiting the use of remission criteria for rheumatoid arthritis by excluding patient global assessment: an individual meta-analysis of 5792 patients. Ann Rheum Dis 2021;80:293–303. 10.1136/annrheumdis-2020-217171 [DOI] [PubMed] [Google Scholar]
- 32.Boers M. Patient global assessment to define remission in rheumatoid arthritis:quo Vadis Ann Rheum Dis 2021;80:277–9. 10.1136/annrheumdis-2020-218802 [DOI] [PubMed] [Google Scholar]
- 33.Brites L, Rovisco J, Costa F, et al. High patient global assessment scores in patients with rheumatoid arthritis otherwise in remission do not reflect subclinical inflammation. Joint Bone Spine 2021;88:105242. 10.1016/j.jbspin.2021.105242 [DOI] [PubMed] [Google Scholar]
- 34.Felson D, Lacaille D, LaValley MP, et al. Reexamining remission definitions in rheumatoid arthritis: considering the twenty-eight-joint disease activity score, C-reactive protein level and patient global assessment. RMD Open 2021;7:e002034. 10.1136/rmdopen-2021-002034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferreira RJO, Welsing PMJ, Jacobs JW, et al. “Correspondence on 're-examining remission definitions in rheumatoid arthritis: considering the 28-joint disease activity score, C reactive protein level and patient global assessment' by Felson et al” Ann Rheum Dis 2023;82:e183. 10.1136/annrheumdis-2021-221917 [DOI] [PubMed] [Google Scholar]
- 36.Aaltonen KJ, Ylikylä S, Tuulikki Joensuu J, et al. Efficacy and effectiveness of tumour necrosis factor inhibitors in the treatment of rheumatoid arthritis in randomized controlled trials and routine clinical practice. Rheumatology (Oxford) 2017;56:725–35. 10.1093/rheumatology/kew467 [DOI] [PubMed] [Google Scholar]
- 37.Vashisht P, Sayles H, Cannella AC, et al. Generalizability of patients with rheumatoid arthritis in biologic agent clinical trials. Arthritis Care Res (Hoboken) 2016;68:1478–88. 10.1002/acr.22860 [DOI] [PubMed] [Google Scholar]
- 38.Chatzidionysiou K, Hetland ML, Frisell T, et al. Opportunities and challenges for real-world studies on chronic inflammatory joint diseases through data enrichment and collaboration between national registers: the Nordic example. RMD Open 2018;4:e000655. 10.1136/rmdopen-2018-000655 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003027supp001.pdf (521.4KB, pdf)
Data Availability Statement
Data are available on reasonable request. Due to ethical and legal considerations, data is not publicly available. Please contact the authors on reasonable request.