Abstract
Objectives
Inferior vena cava (IVC) diameter may be a surrogate for volume status in acute decompensated heart failure (ADHF). The utility of IVC diameter measurement is under studied. The aim of this study was to assess the relationship between IVC diameter, clinical variables and ADHF rehospitalisations.
Methods
Retrospective chart review of 200 patients admitted for ADHF from 2018 to 2019 with transthoracic echocardiogram during index hospitalisation. Charts were assessed for ADHF rehospitalisation within 1 year.
Results
The median age was 64, 30.5% were female, and average left ventricular ejection fraction was 41%±20%. IVC diameter correlated to pulmonary arterial (PA) pressure (R=0.347, p<0.001) and body surface area (BSA) (R=0.424 p<0.001). IVC diameter corrected for BSA correlated to PA pressure (R=0.287, p<0.001) and log N-terminal B-type natriuretic peptide (NT-proBNP) (R=0.247, p≤0.01). Patients rehospitalised within 1 year had significantly greater mean IVC diameter compared with those not rehospitalised (p<0.001) while there was no difference in mean net weight lost during index hospitalisation or mean log NT-proBNP. Patients with IVC diameter greater than 2.07 cm had significantly increased ADHF rehospitalisation (85.6% vs 49.3%, log rank p<0.001) with HR 2.44 (95% CI 1.85 to 3.23, p<0.001). In multivariable Cox regression only IVC diameter (p<0.001), presence of tricuspid regurgitation (p=0.02) and NYHA class III/IV (p<0.001) independently predicted ADHF rehospitalisation within 1 year.
Conclusions
IVC diameter is predictive of rehospitalisation in patients with ADHF and may identify patients in need of greater monitoring and diuresis.
Keywords: Echocardiography; HEART FAILURE; Outcome Assessment, Health Care
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Ultrasound inferior vena cava (IVC) diameter may be a surrogate for intravascular volume status in heart failure patients, but there are limited studies on the utility of IVC diameter assessment in acute decompensated heart failure (ADHF).
WHAT THIS STUDY ADDS
IVC diameter measured at any point during index hospitalisation is an independent predictor of ADHF rehospitalisation up to 1 year in patients with NYHA class I–IV and heart failure with both preserved and reduced ejection fraction.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
IVC diameter guided heart failure management may reduce ADHF hospitalisations and rehospitalisations, help guide diuretic dosing and reduce overall mortality. We are conducting a prospective study to validate this hypothesis.
Introduction
The prevalence of heart failure continues to rise along with rates of hospitalisation for acute decompensated heart failure (ADHF). By 2030, more than 8 million people in the USA alone will have heart failure with projected associated medical cost of US $70 billion dollars.1 The vast majority of ADHF admissions are due to volume overload rather than low cardiac output.2 3 Despite this, many patients are discharged persistently volume overloaded. Accurate volume status determination in heart failure patients is crucial to direct medical management.
Physical examination findings such as peripheral oedema, jugular venous distention and rales are routinely used to assess volume status. It has been shown, however, that many heart failure patients have ultrasound indices of elevated intravascular volume in absence of these signs.4 5 Body weight trends are also frequently used but difficult to interpret as patients may gain non-fluid weight or lose skeletal muscle mass over time and daily weights in the hospital setting are prone to error. More recently, biomarkers B‐type natriuretic peptide (BNP) and N-terminal BNP (NT-proBNP) have gained utility. Natriuretic peptides rise in ADHF and relate to disease severity, but are not specific to heart failure and are influenced by renal function and body mass.6 Further, it has been shown that BNP does not correlate with haemodynamic measures of congestion in ADHF.7
Invasive pulmonary artery pressure guided heart failure management with CardioMEMS device (Abbott, Santa Clara, California, USA) has been shown to reduce hospitalisations and mortality in patients with heart failure with reduced ejection fraction on guideline directed medical therapy.8 CardioMEMS, however, requires an implant, is expensive and is only used in a small number of patients. In the inpatient setting, pulmonary arterial (PA) catheter guided therapy failed to show benefit over clinical assessment in patients with heart failure and had higher risk of procedural complications.9
Non-invasive haemodynamic monitoring using ultrasound inferior vena cava (IVC) diameter measurement may be an alternate surrogate for volume status, particularly intravascular volume, in patients with heart failure both in the inpatient and outpatient setting. IVC diameter can be used to approximate right atrial pressure at least as precisely as complex prediction methods and changes in IVC diameter correlate with changes in pulmonary capillary wedge pressure.10 11 IVC diameter has also been shown to decrease with diuretics during treatment for ADHF.12 13 There are limited studies on the utility of IVC diameter assessment in heart failure patients and relationship to clinical variables. Most of these studies are in outpatients with chronic heart failure with only 1–3 months follow-up. We, therefore, sought to collect a large sample size of inpatients with ADHF with 1-year follow-up.
Methods
We conducted retrospective chart review for 200 patients admitted to University of California San Diego Health hospitals from 2018 to 2019 for primary diagnosis of ADHF, with both reduced and preserved ejection fraction, who had a transthoracic echocardiogram during the index hospitalisation. Charts were assessed for ADHF rehospitalisation within 1 year. Patients were excluded if they required mechanical respiratory or circulatory support, were on inotropic agents, were on dialysis, had mechanical assist devices, cardiac transplant patients or congenital heart disease patients as IVC diameter may not correlate with RA pressures in these subsets.14 15 Patients who died during the index hospitalisation were excluded.
IVC diameter as reported on formal transthoracic echocardiogram during index hospitalisation was recorded along with other echocardiographic parameters including left ventricular ejection fraction (LVEF), PA pressure estimated from tricuspid regurgitation velocity, tricuspid regurgitation and mitral regurgitation. Right ventricular (RV) failure was determined by tricuspid annular plane systolic excursion less than 16 mm or as qualitatively assessed by the reader.16 IVC image acquisition was conducted using American Society of Echocardiography guidelines in subcostal long axis view at end-expiration proximal to the junction of the hepatic veins that lie 0.5–3 cm proximal to the ostium of the right atrium. The images were obtained by 1 of 15 University of California San Diego (UCSD) cardiac sonographers using Siemens Acuson SC2000, Philips EPIQ CVx or GE Vivid E95 machines. Echocardiograms were read by 1 of 15 UCSD cardiologists using Siemens Syngo Dynamics software. Body surface area (BSA) corrected IVC diameter was calculated by dividing IVC diameter by BSA derived from Du Bois method.17 Ideal BSA corrected IVC diameter was calculated by dividing IVC diameter by ideal BSA based on body mass index (BMI) of 25 kg/m2. Height corrected IVC diameter was calculated by dividing IVC diameter by height. Vital signs, laboratory results including NT pro-BNP and physical exam findings within 24 hours of IVC diameter measurement were noted. Weight and cardiac medications were recorded from admission and discharge. Data from repeat transthoracic echocardiograms within 1 year were recorded, if available, along with volume status at that time as determined using documented weight, physical examination findings and symptoms of congestion. Electronic medical records were reviewed for ADHF rehospitalisation within 1 year of index hospitalisation.
Categorical data are presented as percentages and continuous data are presented as mean±SD. Log transformed values for NT-pro BNP were used to reduce skew. The relationships between IVC diameter and other variables were assessed by Pearson correlation coefficients. Patients were grouped as those readmitted for ADHF within 1 year and those not readmitted for ADHF within 1 year. Unpaired t-test was used to compare continuous variables between groups and χ2 test was used for categorical variables. Associations between variables and ADHF rehospitalisation were assessed with univariable and multivariable Cox proportional hazards regression analysis with forwards and backwards procedures to determine independent predictors. We tested variables which have been shown to be related to ADHF rehospitalisation and mortality and are used in several risk prediction models.18 19 Subgroup analysis was conducted between patients with reduced and preserved ejection fraction, NYHA class and RV dysfunction. Receiver operating curve was calculated for IVC diameter as a continuous variable in predicting rehospitalisation within 1 year. Optimal IVC diameter cut-off was determined by Youden index. Kaplan-Meier curve with log-rank statistic was used to assess rehospitalisation within 1 year in patients above and below optimal IVC diameter cut-off. Wald χ2 testing was used to assess the goodness-of-fit. Analyses were performed using IBM SPSS Statistics for Windows, V.27 (IBM), a two-sided p value less than 0.05 was considered statistically significant.
Patient and public involvement
Patients or the public were not involved in this study.
Results
Patient characteristics
Two hundred patients were included in the study with characteristics as noted in table 1. The only missing data was BNPP for 12 patients and PA pressure for 17 patients due to insufficient tricuspid regurgitation. The mean patient age was 66±14 with 30.5% female. A total of 122 patients had heart failure with reduced ejection fraction less than 50%, 78 patients had heart failure with preserved ejection fraction greater than or equal to 50%, and 109 patients had RV dysfunction. Most patients had NYHA class II (37.5%) and III (49%) heart failure with mean LVEF of 41%±20%. Patients were on a variety of outpatient cardiac medications, loop diuretics (92%) and beta blockers (76%) were the most common. Diabetes (40.5%) and hypertension (58.5%) were the most common comorbidities.
Table 1.
Patient characteristics
| Variable | Total N=200 | Not rehospitalised within 1 year N=56 | Rehospitalised within 1 year N=144 | P value |
| IVC diameter | 2.25 (0.57) | 1.90 (0.49) | 2.39 (0.54) | <0.001 |
| BSA | 2.02 (0.34) | 1.96 (0.29) | 2.05 (0.35) | 0.11 |
| Corrected IVC diameter | 1.12 (0.27) | 0.98 (0.25) | 1.18 (0.25) | <0.001 |
| Age | 66 (14) | 69 (15) | 65 (14) | 0.09 |
| Sex | 0.51 | |||
| Women | 61 (30.5%) | 19 (33.9%) | 42 (29.2%) | |
| Men | 139 (69.5%) | 37 (66.1%) | 102 (70.8%) | |
| NYHA functional class | <0.001 | |||
| I | 2 (1.0%) | 2 (3.6%) | 0 (0.0%) | |
| II | 75 (37.5%) | 41 (73.2%) | 34 (23.6%) | |
| III | 98 (49.0%) | 10 (17.9%) | 88 (61.1%) | |
| IV | 25 (12.5%) | 3 (5.4%) | 22 (15.3%) | |
| Afib/aflutter | 74 (37.0%) | 20 (35.7%) | 54 (37.5%) | 0.81 |
| Ischaemic heart disease | 64 (32.0%) | 22 (39.3%) | 42 (29.2%) | 0.17 |
| HLD | 51 (25.5%) | 16 (28.6%) | 35 (24.3%) | 0.53 |
| HTN | 117 (58.5%) | 31 (55.4%) | 86 (59.7%) | 0.57 |
| PAD | 8 (4.0%) | 3 (5.4%) | 5 (3.5%) | 0.54 |
| CVA | 17 (8.5%) | 3 (5.4%) | 14 (9.7%) | 0.32 |
| Diabetes | 81 (40.5%) | 22 (39.3%) | 59 (41.0%) | 0.83 |
| CKD | 53 (26.5%) | 14 (25.0%) | 39 (27.1%) | 0.76 |
| COPD | 24 (12.0%) | 5 (8.9%) | 19 (13.2%) | 0.41 |
| Delta weight | 15.21 (12.33) | 16.63 (12.58) | 14.66 (12.23) | 0.31 |
| Systolic BP | 125 (23) | 128 (26) | 124 (22) | 0.27 |
| Diastolic BP | 74 (17) | 75 (20) | 74 (15) | 0.70 |
| HR | 88 (20) | 85 (21) | 89 (20) | 0.19 |
| Creatinine | 1.55 (0.86) | 1.41 (0.54) | 1.60 (0.96) | 0.08 |
| eGFR | 58.95 (27.44) | 58.12 (24.14) | 59.27 (28.69) | 0.79 |
| Sodium | 138 (4) | 139 (4) | 138 (4) | 0.18 |
| Potassium | 4.2 (0.5) | 4.2 (0.4) | 4.2 (0.6) | 0.95 |
| Haemoglobin | 11.7 (2.5) | 12.6 (2.4) | 11.3 (2.4) | <0.01 |
| NT-proBNP | 6530 (8106) | 5075 (5993) | 7106 (8756) | 0.12 |
| Elevated JVP | 125 (62.5%) | 37 (66.1%) | 88 (61.1%) | 0.52 |
| Crackles | 93 (46.5%) | 26 (46.4%) | 67 (46.5%) | 0.99 |
| Peripheral oedema | 162 (81.0%) | 46 (82.1%) | 116 (80.5%) | 0.80 |
| Medications | ||||
| Beta blocker | 152 (76.0%) | 46 (82.1%) | 106 (73.6%) | 0.21 |
| ACE/ARB | 96 (48.0%) | 29 (51.8%) | 67 (46.5%) | 0.50 |
| Aldosterone antagonist | 51 (25.5%) | 11 (19.6%) | 40 (27.8%) | 0.24 |
| ARNI | 12 (6.0%) | 7 (12.5%) | 5 (3.5%) | 0.02 |
| Loop diuretic | 184 (92.0%) | 51 (91.1%) | 133 (92.4%) | 0.76 |
| Statin | 113 (56.5%) | 39 (69.6%) | 74 (51.4%) | 0.02 |
| Antiplatelet | 21 (10.5%) | 7 (12.5%) | 14 (9.7%) | 0.57 |
| Warfarin | 24 (12.0%) | 5 (8.9%) | 19 (13.2%) | 0.41 |
| DOAC | 58 (29.0%) | 19 (33.9%) | 39 (27.1%) | 0.34 |
| Digoxin | 17 (8.5%) | 5 (8.9%) | 12 (8.3%) | 0.89 |
| ICD | 17 (8.5%) | 3 (5.4%) | 14 (9.7%) | 0.32 |
| CRT | 5 (2.5%) | 2 (3.6%) | 3 (2.1%) | 0.55 |
| CardioMEMS | 3 (1.5%) | 0 (0.0%) | 3 (2.1%) | 0.28 |
| Echocardiographic findings | ||||
| LVEF | 41 (20) | 43 (19) | 40 (20) | 0.25 |
| PA pressure | 46 (14) | 42 (15) | 48 (14) | 0.01 |
| Tricuspid regurgitation | <0.01 | |||
| None/trace | 49 (24.5%) | 22 (39.3%) | 27 (18.8%) | |
| Mild | 79 (39.5%) | 21 (37.5%) | 58 (40.3%) | |
| Moderate/severe | 72 (36.0%) | 13 (23.2%) | 59 (41.0%) | |
| Mitral regurgitation | 0.02 | |||
| None/trace | 65 (32.5%) | 25 (44.6%) | 40 (27.8%) | |
| Mild | 77 (38.5%) | 15 (26.8%) | 62 (43.1%) | |
| Moderate/severe | 58 (29.0%) | 16 (28.6%) | 42 (29.2%) | |
| RV dysfunction | 109 (54.5%) | 25 (44.6%) | 84 (58.3%) | 0.06 |
| Race/ethnicity | ||||
| White | 96 (48.0%) | 27 (48.2%) | 69 (47.9%) | 0.97 |
| Black | 46 (23.0%) | 10 (17.9%) | 36 (25.0%) | 0.28 |
| Hispanic (any race) | 29 (14.5%) | 7 (12.5%) | 22 (15.3%) | 0.62 |
| Asian | 19 (9.5%) | 7 (12.5%) | 12 (8.3%) | 0.37 |
| Other | 39 (19.5%) | 12 (21.4%) | 27 (18.8%) | 0.67 |
Values are mean±SD for continuous variables and number and percent of patients for categorical variables. The statistical difference between variables is given for the comparison between patients with and without ADHF readmission within 1 year. Corrected IVC diameter refers to IVC diameter corrected for BSA.
ADHF, acute decompensated heart failure; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; BP, blood pressure; BSA, body surface area; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronisation therapy; CVA, cerebrovascular accident; DOAC, direct oral anticoagulant; eGFR, estimated glomerular filtration rate; HLD, hyperlipidaemia; HR, heart rate; HTN, hypertension; ICD, implantable cardioverter defibrillator; IVC, inferior vena cava; JVP, jugular venous pulse; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal B-type natriuretic peptide; NYHA, New York Heart Association; PA, pulmonary arterial; PAD, peripheral arterial disease; RV, right ventricular.
Patients with preserved ejection fraction were more likely to have hypertension (67.9% vs 52.5%, p=0.03) and chronic kidney disease (35.9% vs 20.5%, p=0.02). Those with reduced ejection fraction were more likely to have elevated jugular venous pressure (71.3% vs 48.7%, p<0.01) whereas there was no difference in IVC diameter between preserved and reduced ejection fraction (2.31 cm vs 2.16 cm, p=0.06). Patients with RV dysfunction had significantly greater mean IVC diameter compared with those without RV dysfunction (2.39 cm vs 2.10 cm P<0.001).
Correlates of IVC diameter
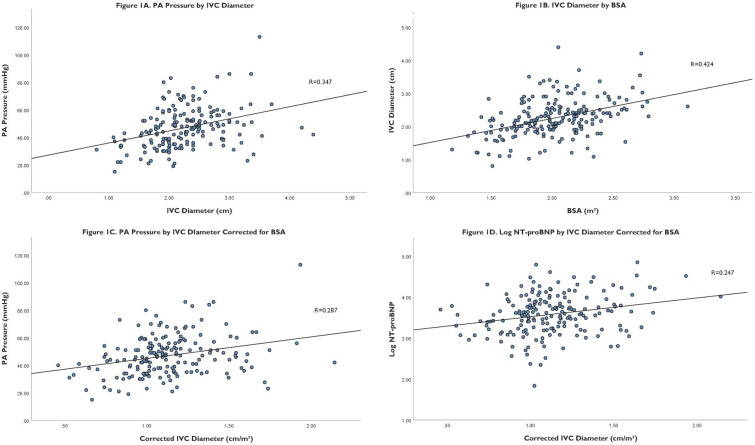
The relationship between variables associated with IVC diameter and corrected IVC diameter is shown in table 2. IVC diameter correlated to PA pressure (R=0.347, p<0.001) (figure 1A) and BSA (R=0.424, p<0.001) (figure 1B). IVC diameter also correlated to height (R=0.350, p<0.001), ideal BSA based on BMI 25 kg/m2 (R=0.350, p<0.001), age (R=−0.315, p<0.001) and LVEF (R=−0.200, p≤0.01). IVC diameter corrected for BSA correlated to PA pressure (R=0.287, p<0.001) (figure 1C) and log NT-proBNP (R=0.247, p≤0.01) (figure 1D). IVC diameter corrected for BSA also correlated to haemoglobin (R=−0.159, p<0.03) and LVEF (R=−0.192, p≤0.01). IVC diameter corrected for ideal BSA based on BMI 25 kg/m2 correlated to age (R=−0.194, p≤0.01), PA pressure (R=0.329, p≤0.001) and creatinine (R=0.148, p=0.04). IVC diameter corrected for height correlated to age (R=−0.247, p<0.001), PA pressure (R=0.345, p≤0.001) and LVEF (R=−0.150, p=0.03). IVC diameter and corrected IVC diameter did not correlate with glomerular filtration rate.
Table 2.
Variables associated with IVC diameter and corrected IVC diameter
| IVC diameter | BSA corrected IVC diameter | Ideal BSA (BMI 25 kg/m2) Corrected IVC diameter | Height corrected IVC diameter | ||||||||
| Variable | Correlation Coefficient | P value | Variable | Correlation Coefficient | P value | Variable | Correlation Coefficient | P value | Variable | Correlation Coefficient | P value |
| Height | 0.35 | <0.001 | Height | -- | -- | Height | -- | -- | Height | -- | -- |
| BSA | 0.424 | <0.001 | BSA | -- | -- | BSA | -- | -- | BSA | 0.245 | <0.001 |
| Ideal BSA (BMI 25 kg/m2) | 0.35 | <0.001 | Ideal BSA (BMI 25 kg/m2) | -- | -- | Ideal BSA (BMI 25 kg/m2) | -- | -- | Ideal BSA (BMI 25 kg/m2) | -- | -- |
| Age | −0.315 | <0.001 | Age | −0.034 | 0.63 | Age | −0.194 | <0.01 | Age | −0.247 | <0.001 |
| Echocardiographic findings | Echocardiographic findings | Echocardiographic findings | Echocardiographic findings | ||||||||
| LVEF | −0.200 | 0.01 | LVEF | −0.192 | <0.01 | LVEF | −0.112 | 0.12 | LVEF | −0.150 | 0.03 |
| PA pressure | 0.347 | <0.001 | PA pressure | 0.287 | <0.001 | PA pressure | 0.329 | <0.001 | PA pressure | 0.345 | <0.001 |
| Laboratory values | Laboratory values | Laboratory values | Laboratory values | ||||||||
| Log NT-proBNP | 0.102 | 0.16 | Log NT-proBNP | 0.247 | <0.01 | Log NT-proBNP | 0.072 | 0.33 | Log NT-proBNP | 0.085 | 0.25 |
| Creatinine | 0.139 | 0.05 | Creatinine | 0.080 | 0.26 | Creatinine | 0.148 | 0.04 | Creatinine | ||
| eGFR | 0.044 | 0.54 | eGFR | 0.024 | 0.73 | eGFR | 0.007 | 0.92 | eGFR | 0.022 | 0.76 |
| Sodium | −0.127 | 0.07 | Sodium | −0.080 | 0.26 | Sodium | −0.087 | 0.22 | Sodium | −0.104 | 0.14 |
| Potassium | 0.014 | 0.85 | Potassium | 0.005 | 0.94 | Potassium | −0.026 | 0.71 | Potassium | −0.012 | 0.87 |
| Haemoglobin | −0.091 | 0.21 | Haemoglobin | −0.159 | 0.03 | Haemoglobin | −0.139 | 0.06 | Haemoglobin | −0.123 | 0.09 |
| Vital signs | Vital signs | Vital signs | Vital signs | ||||||||
| Systolic BP | −0.079 | 0.27 | Systolic BP | −0.034 | 0.63 | Systolic BP | −0.066 | 0.35 | Systolic BP | −0.073 | 0.30 |
| Diastolic BP | 0.140 | 0.05 | Diastolic BP | 0.003 | 0.97 | Diastolic BP | 0.033 | 0.64 | Diastolic BP | 0.076 | 0.29 |
| Heart rate | 0.101 | 0.16 | Heart rate | 0.033 | 0.64 | Heart rate | 0.051 | 0.47 | Heart rate | 0.073 | 0.31 |
Bolded values are significant at p<0.05. Linear relationship assessed using Pearson correlation coefficient.
BMI, body mass index; BP, blood pressure; BSA, body surface area; eGFR, estimated glomerular filtration rate; IVC, inferior vena cava; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal B-type natriuretic peptide; PA, pulmonary arterial.
Figure 1.
(A) Scatter plot of PA pressure by IVC diameter. (B) Scatter plot of IVC diameter by BSA. (C) Scatter plot of PA pressure by IVC diameter corrected for BSA. (D) Scatter plot of log NT-proBNP by IVC diameter corrected for BSA. BSA, body surface area; IVC, inferior vena cava; NT-proBNP, N-terminal B-type natriuretic peptide; PA, pulmonary arterial.
Rehospitalisation for ADHF
Of the 200 patients studied, 144 patients were readmitted for ADHF within 1 year of index hospitalisation. There were nine deaths within 1 year of index hospitalisation, all after rehospitalisation for ADHF. Patients readmitted within 1 year had significantly greater mean IVC diameter compared with those not readmitted (2.39 cm vs 1.90 cm, p<0.001) (online supplemental figure 1). They also had higher NYHA functional class (p<0.001) and presence of tricuspid (p≤0.01) or mitral (p=0.02) regurgitation. Patients not readmitted were more likely to be on statins (p=0.02) and angiotensin receptor neprilysin inhibitors (ARNI) (p=0.02). There was no difference in net weight lost during hospitalisation, NT-proBNP or LVEF between those readmitted within 1 year and those not readmitted. There was also no difference in age, sex, race/ethnicity, medical history or physical exam findings between groups (table 1). Patients who died within 1 year of index hospitalisation had significantly greater mean IVC diameter (2.92 cm vs 2.22 cm, p<0.001) and PA pressure (60 mm Hg vs 46 mm Hg, p≤0.01) than those that did not die within 1 year.
openhrt-2023-002331supp001.pdf (136.4KB, pdf)
Receiver operating curve for IVC diameter as a continuous variable in predicting rehospitalisation within 1 year demonstrated area under the curve of 0.758 (95% CI 0.680 to 0.835, p<0.001) with 2.07 cm (sensitivity 76% specificity 68%) as ideal cut point (figure 2). Uncorrected IVC diameter had greater predictive value for rehospitalisation within 1 year than IVC diameter corrected for BSA which had area under the curve of 0.728 (95% CI 0.648 to 0.809, p<0.001) with 1.03 cm/m2 (sensitivity 71% specificity 68%) as ideal cut point. Uncorrected IVC diameter also had greater predictive value for rehospitalisation within 1 year than IVC diameter corrected for ideal BSA which had area under the curve of 0.743 (95% CI 0.660 to 0.825, p<0.001) and IVC diameter corrected for height which had area under the curve of 0.751 (95% CI 0.670 to 0.832, p<0.001).
Figure 2.
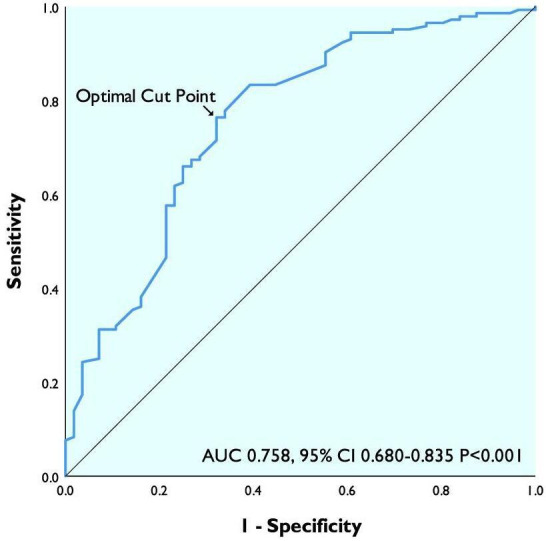
ROC curve of IVC diameter in predicting 1-year rehospitalisation. AUC, area under the curve; IVC, inferior vena cava; ROC, receiver operating curve.
Those with IVC diameter greater than 2.07 cm had greater BSA (2.12 m2 vs 1.86 m2, p<0.001), higher PA pressure (41 mm Hg vs 50 mm Hg, p<0.001) and lower LVEF (38% vs 45%, p=0.03). They were also more likely to be men (p=0.001) and had greater NYHA functional class (p=0.02).
Patients with IVC diameter greater than optimal cut point 2.07 cm had significantly increased ADHF rehospitalisation within 1 year (85.6% vs 49.3%, log rank p<0.001) with HR 2.44 (95% CI 1.85 to 3.23, p<0.001) (figure 3).
Figure 3.

IVC diameter and rehospitalisation after ADHF hospitalisation. ADHF, acute decompensated heart failure; IVC, inferior vena cava.
In univariable Cox regression, IVC diameter (p<0.001), IVC diameter corrected for BSA (p<0.001), IVC diameter corrected for ideal BSA (p<0.001), IVC diameter corrected for height (p<0.001), NYHA functional class (p<0.001), log NT-proBNP (p≤0.01), tricuspid regurgitation (p≤0.01) and mitral regurgitation (p=0.03) predicted ADHF rehospitalisation within 1 year (table 3). Uncorrected IVC diameter had the highest Wald χ2 value for goodness-of-fit.
Table 3.
Univariable Cox regression for ADHF rehospitalisation within 1 year (N=200 patients with 144 events)
| Variable | HR (95% CI) | Wald χ2 | P value |
| IVC diameter | 2.44 (1.85 to 3.23) | 38.91 | <0.001 |
| BSA | 1.50 (0.89 to 2.51) | 2.36 | 0.13 |
| BSA corrected IVC diameter | 5.58 (3.11 to 10.01) | 33.21 | <0.001 |
| Ideal BSA (BMI 25 kg/m2) corrected IVC diameter | 4.50 (2.72 to 7.44) | 34.15 | <0.001 |
| Height corrected IVC diameter | 4.66 (2.84 to 7.65) | 37.25 | <0.001 |
| Days hospitalised | 1.05 (1.01 to 1.08) | 7.9 | <0.01 |
| Age | 0.99 (0.98 to 1.00) | 4.03 | 0.05 |
| Men | 1.23 (0.86 to 1.76) | 1.29 | 0.26 |
| NYHA functional class III/IV versus I/II | 3.39 (2.29 to 5.01) | 37.34 | <0.001 |
| Afib/aflutter | 0.99 (0.71 to 1.39) | 0.01 | 0.95 |
| Ischaemic heart disease | 0.74 (0.52 to 1.06) | 2.72 | 0.10 |
| HLD | 0.88 (0.60 to 1.28) | 0.47 | 0.50 |
| HTN | 1.09 (0.78 to 1.52) | 0.27 | 0.60 |
| PAD | 0.89 (0.37 to 2.18) | 0.06 | 0.80 |
| CVA | 1.24 (0.72 to 2.16) | 0.59 | 0.44 |
| Diabetes | 0.98 (0.70 to 1.36) | 0.02 | 0.89 |
| CKD | 1.05 (0.72 to 1.51) | 0.05 | 0.82 |
| COPD | 1.21 (0.74 to 1.96) | 0.58 | 0.45 |
| Delta weight | 0.99 (0.98 to 1.01) | 1.69 | 0.19 |
| Systolic BP | 1.00 (0.99 to 1.00) | 0.70 | 0.40 |
| Diastolic BP | 1.00 (0.99 to 1.01) | 0.09 | 0.77 |
| HR | 1.01 (1.00 to 1.02) | 5.94 | 0.02 |
| Creatinine | 1.20 (1.00 to 1.45) | 3.83 | 0.05 |
| eGFR | 1.00 (0.99 to 1.01) | 0.01 | 0.91 |
| Sodium | 0.95 (0.90 to 0.99) | 6.04 | 0.01 |
| Potassium | 0.96 (0.70 to 1.32) | 0.07 | 0.80 |
| Haemoglobin | 0.90 (0.84 to 0.96) | 8.88 | <0.01 |
| Log NT-proBNP | 1.63 (1.15 to 2.32) | 7.35 | <0.01 |
| Elevated JVP | 0.93 (0.66 to 1.30) | 0.19 | 0.66 |
| Crackles | 1.03 (0.75 to 1.43) | 0.04 | 0.85 |
| Peripheral oedema | 0.96 (0.64 to 1.45) | 0.04 | 0.85 |
| LVEF | 0.99 (0.98 to 1.00) | 4.93 | 0.03 |
| PA pressure | 1.02 (1.01 to 1.03) | 9.20 | <0.01 |
| Tricuspid regurgitation | 1.96 (1.29 to 2.98) | 9.84 | <0.01 |
| Mitral regurgitation | 1.49 (1.03 to 2.15) | 4.57 | 0.03 |
| RV dysfunction | 1.47 (1.05 to 2.04) | 5.09 | 0.02 |
ADHF, acute decompensated heart failure; BMI, body mass index; BP, blood pressure; BSA, body surface area; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; eGFR, estimated glomerular filtration rate; HLD, hyperlipidaemia; HR, heart rate; HTN, hypertension; IVC, inferior vena cava; JVP, jugular venous pulse; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal B-type natriuretic peptide; NYHA, New York Heart Association; PA, pulmonary arterial; PAD, peripheral arterial disease; RV, right ventricular.
In multivariable Cox regression using variables selected from forwards and backwards procedures only IVC diameter (p<0.001), presence of tricuspid regurgitation (p=0.02) and NYHA class III/IV (p<0.001) independently predicted ADHF rehospitalisation within 1 year (table 4). IVC diameter alone had Wald χ2 goodness-of-fit of 36.77 p<0.001 for predicting ADHF rehospitalisation. IVC diameter and NYHA functional class had Wald χ2 goodness-of-fit of 68.32 p<0.001 for predicting ADHF rehospitalisation. IVC diameter, NYHA functional class and presence of TR had Wald χ2 goodness-of-fit of 73.62 p<0.001 for predicting ADHF rehospitalisation. Log NT-proBNP was not a statistically significant predictor of rehospitalisation in multivariable Cox regression.
Table 4.
Multivariable Cox regression for ADHF rehospitalisation within 1 year (N=200 patients with 144 events)
| Variable | HR (95% CI) | Wald χ2 | P value |
| IVC diameter | 2.24 (1.63 to 3.08) | 24.76 | <0.001 |
| NYHA functional class III/IV versus I/II | 2.65 (1.76 to 3.99) | 21.95 | <0.001 |
| Delta weight | 0.98 (0.96 to 0.99) | 8.79 | <0.01 |
| HR | 1.01 (1.00 to 1.02) | 6.24 | 0.01 |
| Sodium | 0.95 (0.91 to 1.00) | 4.70 | 0.03 |
| Haemoglobin | 0.92 (0.85 to 0.99) | 4.73 | 0.03 |
| Tricuspid regurgitation | 1.65 (1.08 to 2.52) | 5.27 | 0.02 |
Multivariable Cox regression analysis based on independent predictor variables from forwards and backwards procedures.
ADHF, acute decompensated heart failure; HR, heart rate; IVC, inferior vena cava; NYHA, New York Heart Assocation.
Subgroup analysis
In univariable Cox regression IVC diameter, IVC diameter corrected for BSA, IVC diameter corrected for ideal BSA and IVC diameter corrected for height predicted ADHF rehospitalisation within 1 year in patients with NYHA class I/II, NYHA class III/IV, heart failure with preserved ejection fraction, heart failure with reduced ejection fraction and RV dysfunction (online supplemental tables 2,4,6,8,10,12).
In multivariable Cox regression for patients with NYHA class I/II, IVC diameter (p<0.001) and log NT-proBNP (p=0.02) independently predicted ADHF rehospitalisation within 1 year (online supplemental table 3). In multivariable Cox regression for patients with NYHA class III/IV, IVC diameter corrected for BSA (p<0.001), net weight lost (p=0.01), heart rate (p<0.001) and creatinine (p=0.03) independently predicted ADHF rehospitalisation within 1 year (online supplemental table 5).
In multivariable Cox regression for patients with heart failure with preserved ejection fraction, BSA corrected IVC diameter (p=0.01), presence of tricuspid regurgitation (p=0.01), haemoglobin (p≤0.01) and NYHA class III/IV (p<0.001) independently predicted ADHF rehospitalisation within 1 year (online supplemental table 7).In multivariable Cox regression for patients with heart failure with reduced ejection fraction, IVC diameter corrected for ideal BSA (p<0.001), net weight lost (p≤0.01), heart rate (p<0.001) and NYHA class III/IV (p≤0.01) independently predicted ADHF rehospitalisation within 1 year (online supplemental table 9).
In multivariable Cox regression for patients with RV dysfunction, height corrected IVC diameter (p<0.001), net weight lost (p=0.01), NYHA class III/IV (p=0.01) and LVEF (p=0.04) independently predicted ADHF reshospitalisation within 1 year (online supplemental table 11). In multivariable Cox regression for patients without RV dysfunction, BSA corrected IVC diameter (p=0.03), NYHA class III/IV (p<0.001), heart rate (p<0.01) and haemoglobin (p<0.01) independently predicted ADHF reshospitalisation within 1 year (online supplemental table 13).
IVC diameter from subsequent echocardiograms
A total of 128 patients had repeat transthoracic echocardiogram within 1 year of index ADHF hospitalisation. Fifty-one of these patients were clinically euvolaemic and 77 were clinically hypervolaemic at time of subsequent transthoracic echocardiogram. Volume status was determined using documented weight, physical examination findings and symptoms of congestion. Those that were hypervolaemic had significantly greater IVC diameter compared with those that were euvolaemic (2.43 cm vs 1.44 cm, p<0.001). Regardless of volume status at time of subsequent IVC diameter measurement, patients who were readmitted for ADHF within 1 year had significantly greater IVC diameter than patients who were not readmitted (2.16 cm vs 1.61 cm, p≤0.001).
Discussion
Symptomatic volume overload necessitating intravenous diuretics in the absence of reduced cardiac output is the primary cause of ADHF admissions accounting for more than 90% of cases.3 Serial volume status assessment is crucial in ADHF yet routinely used measures such as jugular venous distention and weight are inexact. Our study shows that IVC diameter may be an alternate surrogate for volume status which predicts ADHF rehospitalisation.
This study is one of the largest sample sizes of inpatients hospitalised for ADHF, both reduced and preserved ejection fraction, with comprehensive consideration of covariates and the longest follow-up time in assessing rehospitalisation. Several previous studies only included patients with reduced ejection fraction, solely assessed mortality, dichotomised IVC diameter at 2.1 cm and did not consider NT-proBNP or clinical signs in analyses. We evaluated both IVC diameter and clinical variables and their correlation with rehospitalisation in a broad group of patients with ADHF.
In our study, patients rehospitalised for ADHF within 1 year had significantly greater IVC diameter during index hospitalisation. This is consistent with previous studies that associated greater IVC diameter during ADHF hospitalisation with poor outcomes.20–22 Analogous studies in outpatients with chronic heart failure showed that greater IVC diameter is associated with increased hospital admissions and mortality.4 23–25 Conversely, a single centre study found that IVC diameter was suboptimal to differentiate acute dyspnoea due to heart failure versus other causes likely due to a heterogenous population with numerous confounders.26 We also found that those rehospitalised within 1 year had significantly lower haemoglobin which has been shown as a poor prognostic indicator in heart failure.27 28 This may reflect anaemia of chronic disease or renal dysfunction from chronic heart failure or pseudo anaemia from increased intravascular volume in acute decompensation.29 Those rehospitalised were more likely to have tricuspid regurgitation and mitral regurgitation consistent with previous studies.30–32 There was no difference in LVEF among those rehospitalised within 1 year and those not rehospitalised within 1 year. There were significantly more patients on statins and ARNIs in the not readmitted group. Previous studies reported decreased heart failure hospitalisation and rehospitalisation rates on these medications.33 34 We found no difference in NT-proBNP between those rehospitalised and not rehospitalised. This may be because we compared values from various time points during hospitalisation. Previous studies showed a difference in predischarge natriuretic peptides in patients who were readmitted compared with those that were not readmitted but no difference in admission values.20 35–38
There was no difference in net weight lost or physical exam findings between those rehospitalised and not rehospitalised, highlighting the limitations of these parameters. Those with reduced ejection fraction were more likely to have jugular venous distention whereas there was no difference in IVC diameter between patients with preserved and reduced ejection fraction. IVC diameter may be more indicative of volume status independent of LVEF.
Our study shows a significant correlation between IVC diameter and BSA indicating that patients may have individual ‘dry weight’ IVC diameters. This supports a previous study which found that patients with low and high BSA had statistically different IVC diameter cutoffs for detecting RA pressure greater than 10 mm Hg.39 The relation between IVC diameter and BSA may be confounded by obesity, thus we also indexed IVC diameter to height and ideal BSA based on height. Regardless, we found that uncorrected IVC diameter had greater predictive value for rehospitalisation within 1 year than IVC diameter corrected for BSA.
Only IVC diameter corrected for BSA correlated to log NT-proBNP. Previous studies in outpatients with heart failure found a correlation between IVC diameter and log NT-proBNP.23 Studies in inpatients with ADHF found poor correlation between IVC diameter and log NT-proBNP, a stronger correlation in those with systolic heart failure, and a lack of correlation among women.20 40 Our findings may be explained by factors known to impact NT-proBNP such as age, sex, renal function and body mass.6 Natriuretic peptide levels are also not specific to heart failure. IVC diameter and natriuretic peptides may provide complimentary data in clinical settings. When there are heart failure signs and symptoms in absence of LV wall stress, NT-proBNP may not be elevated and IVC diameter may be a better direct measure of cardiac filling pressures. Unlike NT-proBNP, we found that IVC diameter is independent of glomerular filtration rate and may quantify congestion similarly in presence or absence of renal dysfunction.
IVC diameter, IVC diameter corrected for BSA, IVC diameter corrected for ideal BSA and IVC diameter corrected for height correlated to PA pressure supporting IVC diameter as method of non-invasive haemodynamic monitoring. IVC diameter may even be a more sensitive measure of volume status change than pressure. Studies of canine and human IVC demonstrate that volume changes precede pressure changes during distention.41 42 As intravascular volume initially increases there is a considerable shape change of the IVC from elliptical to circular with minimal associated pressure change. During this period, there is a large change in volume with minimal change in pressure and IVC diameter may be more reflective of volume status. Further, central venous pressure and wedge pressure have poor correlation with blood volume.43 Whereas, changes in IVC diameter have been shown to reflect blood volume changes during haemodialysis and blood donation.44 45 Measuring volume directly using IVC diameter may be more effective than central venous pressure.
This study identifies IVC diameter as an independent predictor for ADHF rehospitalisation within 1 year in patients with NYHA class I–IV and heart failure with both preserved and reduced ejection fraction. NYHA class III/IV was also a significant predictor of rehospitalisation with similar effect measures as IVC diameter. IVC diameter and NYHA class provide complimentary information to risk stratify patients. IVC diameter is both a predictor of rehospitalisation and a potential therapeutic target in ADHF. Patients readmitted for ADHF had significantly greater IVC diameter on subsequent echocardiograms within 1 year of index hospitalisation regardless of clinical volume status at that time. Patients may be undertreated with subclinical intravascular volume elevation at time of discharge which predisposes them to ADHF rehospitalisation.29 IVC diameter may be used to detect subclinical congestion and allow for interventions which prevent hospitalisations.
Serial IVC diameter measurement may be useful to guide ADHF management. Previous studies have shown that IVC diameter decreases from ADHF admission to discharge and demonstrated reproducible IVC image acquisition by healthcare providers with limited ultrasound training.46–49 We determined an optimal cut-off value for IVC diameter which can be used to assess whether patients need ongoing diuresis or can be discharged and identify those in need of frequent follow-up.
Study limitations
This is a single centre retrospective study with a modest sample size. IVC diameter measurements are from various times points during index ADHF hospitalisation and were not standardised among patients. This would bias towards the null hypothesis, but still our data indicates that any evidence of large IVC diameter obtained during the hospitalisation is clinically significant in all subgroups of patients with decompensated heart failure. Given that this was a retrospective study we were unable to determine whether IVC diameter guided ADHF management improves outcomes compared with standard of care, a topic for future study.
Conclusions
IVC diameter is an independent predictor of rehospitalisation in patients with ADHF and may identify patients in need of greater monitoring and diuresis. IVC diameter guided heart failure management in the inpatient and outpatient setting may reduce ADHF hospitalisations and rehospitalisations, help guide diuretic dosing, and reduce overall mortality. Prospective studies are necessary to test this hypothesis.
Acknowledgments
This research was presented in abstract form by Dr. Revathy Sampath-Kumar at the Heart Failure Society of America conference in Denver in September, 2021 cited below. Revathy Sampath-Kumar, Ori Ben-Yehuda, Inferior Vena Cava Diameter Independently Predicts Acute Decompensated Heart Failure Rehospitalizations, Journal of Cardiac Failure, Volume 28, Issue 5, Supplement, 2022, Page S116, ISSN 1071-9164, https://doi.org/10.1016/j.cardfail.2022.03.297.
Footnotes
Contributors: Conceptualisation, investigation and writing—original draft preparation: RS-K, conceptualisation, writing—reviewing and editing, supervision, and guarantor: OB-Y.
Funding: This work was supported by a gift from the Malcolm Wiener Foundation.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The Institutional Review Board of the University of California, San Diego approved the study #200527.
References
- 1.Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke Statistics—2020 update: A report from the American heart Association. Circulation 2020;141:e139–596. 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 2.Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J 2007;154:260–6. 10.1016/j.ahj.2007.01.041 [DOI] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Follath F, Ponikowski P, et al. Assessing and grading congestion in acute heart failure: A scientific statement from the acute heart failure committee of the heart failure Association of the European society of cardiology and endorsed by the European society of intensive care medicine. European Journal of Heart Failure 2010;12:423–33. 10.1093/eurjhf/hfq045 [DOI] [PubMed] [Google Scholar]
- 4.Pellicori P, Shah P, Cuthbert J, et al. Prevalence, pattern and clinical relevance of ultrasound indices of congestion in outpatients with heart failure. Eur J Heart Fail 2019;21:904–16. 10.1002/ejhf.1383 [DOI] [PubMed] [Google Scholar]
- 5.Saha NM, Barbat JJ, Fedson S, et al. Outpatient use of focused cardiac ultrasound to assess the inferior vena cava in patients with heart failure. Am J Cardiol 2015;116:1224–8. 10.1016/j.amjcard.2015.07.040 [DOI] [PubMed] [Google Scholar]
- 6.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol 2007;50:2357–68. 10.1016/j.jacc.2007.09.021 [DOI] [PubMed] [Google Scholar]
- 7.Omar HR, Guglin M. A single BNP measurement in acute heart failure does not reflect the degree of congestion. J Crit Care 2016;33:262–5. 10.1016/j.jcrc.2016.02.023 [DOI] [PubMed] [Google Scholar]
- 8.Givertz MM, Stevenson LW, Costanzo MR, et al. Pulmonary artery pressure-guided management of patients with heart failure and reduced ejection fraction. J Am Coll Cardiol 2017;70:1875–86. 10.1016/j.jacc.2017.08.010 [DOI] [PubMed] [Google Scholar]
- 9.Shah MR, O’Connor CM, Sopko G, et al. Evaluation study of congestive heart failure and pulmonary artery Catheterization effectiveness (ESCAPE): design and rationale. Am Heart J 2001;141:528–35. 10.1067/mhj.2001.113995 [DOI] [PubMed] [Google Scholar]
- 10.Tsutsui RS, Borowski A, Tang WHW, et al. Precision of echocardiographic estimates of right atrial pressure in patients with acute decompensated heart failure. J Am Soc Echocardiogr 2014;27:1072–8. 10.1016/j.echo.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 11.Blair JE, Brennan JM, Goonewardena SN, et al. Usefulness of hand-carried ultrasound to predict elevated left ventricular filling pressure. Am J Cardiol 2009;103:246–7. 10.1016/j.amjcard.2008.08.061 [DOI] [PubMed] [Google Scholar]
- 12.Ramasubbu K, Deswal A, Chan W, et al. Echocardiographic changes during treatment of acute decompensated heart failure: insights from the ESCAPE trial. J Card Fail 2012;18:792–8. 10.1016/j.cardfail.2012.08.358 [DOI] [PubMed] [Google Scholar]
- 13.Tchernodrinski S, Lucas BP, Athavale A, et al. Inferior vena cava diameter change after intravenous furosemide in patients diagnosed with acute decompensated heart failure. J Clin Ultrasound 2015;43:187–93. 10.1002/jcu.22173 [DOI] [PubMed] [Google Scholar]
- 14.Seif D, Mailhot T, Perera P, et al. Caval Sonography in shock a noninvasive method for evaluating Intravascular volume in critically ILL patients. J Ultrasound Med 2012;31:1885–90. 10.7863/jum.2012.31.12.1885 [DOI] [PubMed] [Google Scholar]
- 15.Ciozda W, Kedan I, Kehl DW, et al. The efficacy of Sonographic measurement of inferior vena cava diameter as an estimate of central venous pressure. Cardiovasc Ultrasound 2016;14:33. 10.1186/s12947-016-0076-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American society of echocardiography. Journal of the American Society of Echocardiography 2010;23:685–713. 10.1016/j.echo.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 17.Du Bois D D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Nutrition 1989. [PubMed] [Google Scholar]
- 18.Levy WC, Mozaffarian D, Linker DT, et al. The Seattle heart failure model: prediction of survival in heart failure. Circulation 2006;113:1424–33. 10.1161/CIRCULATIONAHA.105.584102 [DOI] [PubMed] [Google Scholar]
- 19.Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: A systematic review. JAMA 2011;306:1688–98. 10.1001/jama.2011.1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goonewardena SN, Gemignani A, Ronan A, et al. Comparison of hand-carried ultrasound assessment of the inferior vena cava and N-terminal pro-brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging 2008;1:595–601. 10.1016/j.jcmg.2008.06.005 [DOI] [PubMed] [Google Scholar]
- 21.Palazzuoli A, Ruocco G, Franci B, et al. Ultrasound indices of congestion in patients with acute heart failure according to body mass index. Clin Res Cardiol 2020;109:1423–33. 10.1007/s00392-020-01642-9 [DOI] [PubMed] [Google Scholar]
- 22.Lee HF, Hsu LA, Chang CJ, et al. Prognostic significance of dilated inferior vena cava in advanced decompensated heart failure. Int J Cardiovasc Imaging 2014;30:1289–95. 10.1007/s10554-014-0468-y [DOI] [PubMed] [Google Scholar]
- 23.Pellicori P, Carubelli V, Zhang J, et al. IVC diameter in patients with chronic heart failure: relationships and Prognostic significance. JACC Cardiovasc Imaging 2013;6:16–28. 10.1016/j.jcmg.2012.08.012 [DOI] [PubMed] [Google Scholar]
- 24.Nath J, Vacek JL, Heidenreich PA. A dilated inferior vena cava is a marker of poor survival. Am Heart J 2006;151:730–5. 10.1016/j.ahj.2005.04.023 [DOI] [PubMed] [Google Scholar]
- 25.Khandwalla RM, Birkeland KT, Zimmer R, et al. Usefulness of serial measurements of inferior vena cava diameter by VscanTM to identify patients with heart failure at high risk of hospitalization. Am J Cardiol 2017;119:1631–6. 10.1016/j.amjcard.2017.02.007 [DOI] [PubMed] [Google Scholar]
- 26.Sforza A, Carlino MV, Guarino M, et al. Diagnostic accuracy of inferior vena cava evaluation in the diagnosis of acute heart failure among Dyspneic patients. Monaldi Arch Chest Dis 2020;90:704–7. 10.4081/monaldi.2020.1375 [DOI] [PubMed] [Google Scholar]
- 27.Scrutinio D, Passantino A, Santoro D, et al. The Cardiorenal anaemia syndrome in systolic heart failure: prevalence, clinical correlates, and long-term survival. Eur J Heart Fail 2011;13:61–7. 10.1093/eurjhf/hfq167 [DOI] [PubMed] [Google Scholar]
- 28.Anand I, McMurray JJV, Whitmore J, et al. Anemia and its relationship to clinical outcome in heart failure. Circulation 2004;110:149–54. 10.1161/01.CIR.0000134279.79571.73 [DOI] [PubMed] [Google Scholar]
- 29.Miller WL. Fluid volume overload and congestion in heart failure. Circ: Heart Failure 2016;9:1–9. 10.1161/CIRCHEARTFAILURE.115.002922 [DOI] [PubMed] [Google Scholar]
- 30.De la Espriella R, Santas E, Chorro FJ, et al. Functional Tricuspid regurgitation and recurrent admissions in patients with acute heart failure. Int J Cardiol 2019;291:83–8. 10.1016/j.ijcard.2019.03.051 [DOI] [PubMed] [Google Scholar]
- 31.Bruch C, Klem I, Breithardt G, et al. Diagnostic usefulness and Prognostic implications of the mitral E/E′ ratio in patients with heart failure and severe secondary mitral regurgitation. Am J Cardiol 2007;100:860–5. 10.1016/j.amjcard.2007.03.108 [DOI] [PubMed] [Google Scholar]
- 32.Kajimoto K, Sato N, Takano T, et al. Functional mitral regurgitation at discharge and outcomes in patients hospitalized for acute decompensated heart failure with a preserved or reduced ejection fraction. Eur J Heart Fail 2016;18:1051–9. 10.1002/ejhf.562 [DOI] [PubMed] [Google Scholar]
- 33.Desai AS, Claggett BL, Packer M, et al. Influence of Sacubitril/valsartan (Lcz696) on 30-day readmission after heart failure hospitalization. J Am Coll Cardiol 2016;68:241–8. 10.1016/j.jacc.2016.04.047 [DOI] [PubMed] [Google Scholar]
- 34.Lee MMY, Sattar N, McMurray JJV, et al. Statins in the prevention and treatment of heart failure: a review of the evidence. Curr Atheroscler Rep 2019;21:41. 10.1007/s11883-019-0800-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng V, Kazanagra R, Garcia A, et al. A rapid bedside test for B-type peptide predicts treatment outcomes in patients admitted for decompensated heart failure: A pilot study. J Am Coll Cardiol 2001;37:386–91. 10.1016/s0735-1097(00)01157-8 [DOI] [PubMed] [Google Scholar]
- 36.Dokainish H, Zoghbi WA, Lakkis NM, et al. Incremental predictive power of B-type natriuretic peptide and tissue Doppler echocardiography in the prognosis of patients with congestive heart failure. J Am Coll Cardiol 2005;45:1223–6. 10.1016/j.jacc.2005.01.025 [DOI] [PubMed] [Google Scholar]
- 37.Gackowski A, Isnard R, Golmard JL, et al. Comparison of echocardiography and plasma B-type natriuretic peptide for monitoring the response to treatment in acute heart failure. Eur Heart J 2004;25:1788–96. 10.1016/j.ehj.2004.07.038 [DOI] [PubMed] [Google Scholar]
- 38.Logeart D, Thabut G, Jourdain P, et al. Predischarge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J Am Coll Cardiol 2004;43:635–41. 10.1016/j.jacc.2003.09.044 [DOI] [PubMed] [Google Scholar]
- 39.Taniguchi T, Ohtani T, Nakatani S, et al. Impact of body size on inferior vena cava parameters for estimating right atrial pressure: A need for standardization. J Am Soc Echocardiogr 2015;28:1420–7. 10.1016/j.echo.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 40.Hebl V, Zakharova MY, Canoniero M, et al. Correlation of natriuretic peptides and inferior vena cava size in patients with congestive heart failure. VHRM 2012;8:213–8. 10.2147/VHRM.S30001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moreno AH, Katz AI, Gold LD, et al. Mechanics of distension of dog veins and other very thin-walled tubular structures. Circ Res 1970;27:1069–80. 10.1161/01.res.27.6.1069 [DOI] [PubMed] [Google Scholar]
- 42.Seo Y, Iida N, Yamamoto M, et al. Estimation of central venous pressure using the ratio of short to long diameter from cross-sectional images of the inferior vena cava. J Am Soc Echocardiogr 2017;30:461–7. 10.1016/j.echo.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 43.Shippy CR, Appel PL, Shoemaker WC. Reliability of clinical monitoring to assess blood volume in critically ill patients. Critical Care Medicine 1984;12:107–12. 10.1097/00003246-198402000-00005 [DOI] [PubMed] [Google Scholar]
- 44.Katzarski KS, Nisell J, Randmaa I, et al. A critical evaluation of ultrasound measurement of inferior vena cava diameter in assessing dry weight in normotensive and hypertensive Hemodialysis patients. Am J Kidney Dis 1997;30:459–65. 10.1016/s0272-6386(97)90302-4 [DOI] [PubMed] [Google Scholar]
- 45.Pasquero P, Albani S, Sitia E, et al. Inferior vena cava diameters and Collapsibility index reveal early volume depletion in a blood donor model. Crit Ultrasound J 2015;7:17. 10.1186/s13089-015-0034-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akiyama E, Cinotti R, Čerlinskaitė K, et al. Improved cardiac and venous pressures during hospital stay in patients with acute heart failure: an echocardiography and biomarkers study. ESC Heart Fail 2020;7:996–1006. 10.1002/ehf2.12645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gundersen GH, Norekval TM, Haug HH, et al. Adding point of care ultrasound to assess volume status in heart failure patients in a nurse-led outpatient clinic. A randomised study. Heart 2016;102:29–34. 10.1136/heartjnl-2015-307798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yavaşi Ö, Ünlüer EE, Kayayurt K, et al. Monitoring the response to treatment of acute heart failure patients by ultrasonographic inferior vena cava Collapsibility index. Am J Emerg Med 2014;32:403–7. 10.1016/j.ajem.2013.12.046 [DOI] [PubMed] [Google Scholar]
- 49.Laffin LJ, Patel AV, Saha N, et al. Focused cardiac ultrasound as a Predictor of readmission in acute decompensated heart failure. Int J Cardiovasc Imaging 2018;34:1075–9. 10.1007/s10554-018-1317-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2023-002331supp001.pdf (136.4KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.