Abstract
Objective
We conducted a systematic review to evaluate associations between influenza vaccination during pregnancy and adverse birth outcomes and maternal non-obstetric serious adverse events (SAEs), taking into consideration confounding and temporal biases.
Methods
Electronic databases (Ovid MEDLINE ALL, Embase Classic+Embase and the Cochrane Central Register of Controlled Trials) were searched to June 2021 for observational studies assessing associations between influenza vaccination during pregnancy and maternal non-obstetric SAEs and adverse birth outcomes, including preterm birth, spontaneous abortion, stillbirth, small-for-gestational-age birth and congenital anomalies. Studies of live attenuated vaccines, single-arm cohort studies and abstract-only publications were excluded. Records were screened using a liberal accelerated approach initially, followed by a dual independent approach for full-text screening, data extraction and risk of bias assessment. Pairwise meta-analyses were conducted, where two or more studies met methodological criteria for inclusion. The Grading of Recommendations, Assessment, Development and Evaluation approach was used to assess evidence certainty.
Results
Of 9443 records screened, 63 studies were included. Twenty-nine studies (24 cohort and 5 case–control) evaluated seasonal influenza vaccination (trivalent and/or quadrivalent) versus no vaccination and were the focus of our prioritised syntheses; 34 studies of pandemic vaccines (2009 A/H1N1 and others), combinations of pandemic and seasonal vaccines, and seasonal versus seasonal vaccines were also reviewed. Control for confounding and temporal biases was inconsistent across studies, limiting pooling of data. Meta-analyses for preterm birth, spontaneous abortion and small-for-gestational-age birth demonstrated no significant associations with seasonal influenza vaccination. Immortal time bias was observed in a sensitivity analysis of meta-analysing risk-based preterm birth data. In descriptive summaries for stillbirth, congenital anomalies and maternal non-obstetric SAEs, no significant association with increased risk was found in any studies. All evidence was of very low certainty.
Conclusions
Evidence of very low certainty suggests that seasonal influenza vaccination during pregnancy is not associated with adverse birth outcomes or maternal non-obstetric SAEs. Appropriate control of confounding and temporal biases in future studies would improve the evidence base.
Keywords: PUBLIC HEALTH, Epidemiology, Infection control, IMMUNOLOGY
STRENGTHS AND LIMITATIONS OF THIS STUDY.
We used robust systematic review methods and restricted meta-analyses to studies that adjusted for confounding and accounted for temporal biases while focusing syntheses on outcome definitions that matched accepted criteria.
The available evidence was associated with limitations related to between-study inconsistencies in methods for adjustments for confounding and immortal time bias and variability in outcome definitions.
Outcomes assessed using the Grading of Recommendations, Assessment, Development and Evaluation framework were judged to have very low certainty of evidence.
Introduction
Maternal influenza vaccination during pregnancy can reduce influenza and associated complications among pregnant women and newborn children throughout the first months of life.1 The WHO has recommended vaccination of pregnant women since 2005,2 and many countries have adopted guidance advising vaccination of all pregnant women with a licensed, recommended, age appropriate, inactivated influenza vaccine, regardless of trimester.3–8 The safety profile of influenza vaccines during pregnancy is considered favourable9; however, the evidence base is continually evolving, warranting ongoing synthesis of new data.
Most studies on this topic have used observational designs. Such studies are susceptible to bias, including confounding bias, if appropriate methods are not used to control for critical confounders (eg, maternal age, sociodemographic characteristics), as well as temporal biases, such as immortal time bias, if appropriate study design or statistical methods are not used.10–13 Confounding and immortal time bias can substantially impact both the magnitude and direction of results if not adequately addressed.13 14 Additionally, other complex temporal issues may further distort results, such as the seasonality of the influenza disease in many countries, the restricted availability of influenza vaccinations to specific times of the year, annual changes to the viral strains circulating and present in vaccines, and reduced opportunity for vaccination during pregnancy in women with shorter gestations (ie, preterm birth (PTB)).
Previous systematic reviews of influenza vaccination during pregnancy have generally used all-or-nothing approaches to synthesis and have elected to either (A) pool all data, often without considering study design, interventions (eg, multivalent seasonal influenza vaccines and monovalent 2009–2010 A/H1N1 influenza pandemic vaccines), confounding bias, immortal time bias or reporting format15–18 or (B) summarise all data descriptively due to concerns about heterogeneity of study designs, interventions and reporting formats reducing the validity of pooled results.19 20 Neither approach is ideal—robust strategies are needed to restrict meta-analysis to studies meeting important methodological and statistical considerations on an outcome-by-outcome basis and to address pooling of different measures of effect. We conducted a systematic review and meta-analyses to address the following review question, taking into consideration the potential impacts of confounding and temporal biases, and stratifying by seasonal and 2009 pandemic A/H1N1 influenza vaccines: Are influenza vaccines received at any time during pregnancy safe for women and their unborn children, compared with no influenza vaccination?
Methods
Protocol, registration and reporting
A review protocol was developed a priori, guided by established systematic review methodology,21 and registered on the Open Science Framework (OSF; doi 10.17605/OSF.IO/XEY2K) and with PROSPERO (CRD42020159030). The original protocol included a second review question regarding the efficacy/effectiveness of influenza vaccination during pregnancy against influenza outcomes; the focus of this manuscript is solely on vaccine safety. A full technical report describing our findings for both review questions is available on the OSF (https://osf.io/xey2k/). A summary of minor protocol amendments has been provided in online supplemental file 1, section 1.
bmjopen-2022-066182supp001.pdf (10.2MB, pdf)
This manuscript has been written in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis 2020 statement.22
Patient and public involvement
No patients or the public were involved in the performance of this systematic review.
Study eligibility criteria
Table 1 summarises the study eligibility criteria. We sought observational studies that assessed the safety of any seasonal or pandemic influenza vaccine not contraindicated for pregnant women, given at any time during pregnancy. Randomised controlled trials (RCTs) were excluded for this review of safety outcomes, given the rarity of most birth outcomes of interest (ie, RCTs were underpowered for these outcomes23) and given the different settings of RCTs (lower-to-middle-income countries) on this topic compared with observational studies (high-income countries)14 that would increase clinical and statistical heterogeneity. All birth outcome data from RCTs have previously been pooled in other recent publications23 24 and are summarised in the Discussion section.
Table 1.
Study eligibility criteria
| PICOS domain | Inclusion criteria |
| Population | Pregnant women and their unborn children |
| Interventions | Seasonal and pandemic influenza vaccines of any valency administered during pregnancy that were not contraindicated during pregnancy (eg, live-attenuated vaccines) |
| Comparators | No influenza vaccine, active comparators (eg, other influenza vaccine, meningococcal or pneumococcal vaccines), placebo |
| Outcomes | Dichotomous measures of the following: Maternal
Birth outcomes:
|
| Study designs | Prospective and retrospective cohort studies, case–control and test-negative studies Excluded: single-arm cohort studies, studies based on databases of voluntary reporting or surveillance of adverse events, abstracts |
| Language | English and French |
Description of methods
A brief description of the most pertinent review methods is provided in table 2, while a complete description of our methods is reported in online supplemental file 1, section 2. Briefly, we searched multiple literature databases, using a comprehensive search strategy. A liberal accelerated approach was used for title/abstract screening, while dual independent methods were used for full-text screening, data extraction and risk of bias (ROB) assessment. Pairwise meta-analyses were conducted, where two or more studies met strict inclusion criteria for meta-analysis, including accounting for confounding and/or temporal biases. ORs and risk ratios (RRs) were considered to approximate each other, given the rarity of events and small anticipated effects of vaccination. Assessment of the certainty of evidence followed the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework.25 Outcomes reported in the main manuscript were prioritised according to GRADE methods as detailed in table 2, and syntheses for these outcomes have been reported in the main text, along with secondary syntheses that provide context to the prioritised findings.
Table 2.
Overview of review methods
| Review stage | Details |
| Literature search | As per protocol for review of both efficacy and safety outcomes, separate search strategies were developed for observational studies (MEDLINE and Embase), RCTs (Ovid MEDLINE ALL, Embase Classic+Embase and the Cochrane Central Register of Controlled Trials), and systematic reviews (OVID platform: Ovid MEDLINE ALL, Embase Classic+Embase, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects and Health Technology Assessment). Study design filters were applied for RCTs and systematic reviews. No language or date restrictions were used. Search strategies are provided in online supplemental file 1, section 3. Searches were peer reviewed using Peer Review of Electronic Search Strategies (PRESS) Checklist.125 Searches were run 11 December 2019 and updated to 3 June 2021. Bibliographies of included systematic reviews and primary studies were screened. Grey literature searching (June 2020) included clinical trial registries and other platforms, and was guided by the Canadian Agency of Drugs and Technologies in Health (CADTH) Grey Matters Checklist.126 |
| Study selection | Search results were deduplicated in Reference Manager* (original search) or EndNote† (update) prior to being uploaded to online review management software DistillerSR.‡ Two levels of screening were performed (title/abstract and full text), with pilots at each level. A liberal accelerated approach was used to screen titles/abstracts (one reviewer to include, two to exclude), while dual independent screening was used for full texts. Conflict resolution was through discussion. |
| Data extraction | Dual independent data extraction was performed in DistillerSR, using standardised piloted forms. Conflict resolution was reached through discussion. Data extraction files have been made available online (see online supplemental files 2–4). Study characteristics collected: author, year of publication, language, design, objective, funding, whether conflicts of interest were declared, country of conduct, study period and influenza seasons, longest follow-up, sample size, inclusion criteria, method of measurement of gestational age. Demographic data collected: maternal age, race, gestational age at vaccination, maternal comorbidities, whether specific maternal risk factors were reported, proportion vaccinated. Intervention data collected: vaccine type (seasonal or pandemic), valency, name, presence of adjuvant, strains, setting of vaccine delivery. Outcome data (all outcome definitions and time points were extracted): numbers of events and participants per group, and, if reported, point estimate with 95% CI, whether confounding was controlled and the primary approach (eg, matching, inclusion as covariates, propensity score), and the confounders that were controlled, including three critical confounders: maternal age, smoking during pregnancy, and socioeconomic status. Country income level was determined for each study as per The World Bank World Development Indicators.127 |
| Risk of bias | Dual independent assessment using adaptations of the Newcastle-Ottawa Scale (NOS)128 for cohort and case–control studies, with conflict resolution through discussion. NOS scores can range from 0 to 9, with lower scores representing higher ROB. Suggested score cut-offs are as follows: low ROB (7–9), some concerns (4–6), high ROB (0–3). Details regarding the different ROB domains are provided in online supplemental file1, section 4. |
| Data synthesis/ Statistical methods | Data were cleaned and collated in Microsoft Excel, and tables summarising study characteristics were developed separately for cohort and case–control studies. Results data were tabulated to assess feasibility of meta-analyses. ORs and risk ratios (RRs) were considered equivalent, given the rarity of the outcomes of interest and anticipated small effects of vaccination. HRs were considered approximate to RRs, ORs and incidence RRs, given short follow-up periods (eg, duration of gestation), rarity of events and small effects. Pairwise meta-analyses using random effects models were considered when two or more studies reported findings that were adjusted for confounding (and immortal time bias, where appropriate; see online supplemental file 1, section 2 for a brief discussion of immortal time bias) for a given outcome definition, time point and comparison, and when the available evidence was not clinically heterogeneous. Approaches to handle confounding that were of interest included matching, multivariable adjustment and propensity score methods. Where study effect estimates for meta-analysis were heterogeneous (eg, both RRs and ORs), meta-analyses were conducted and reported using RRs. Effect estimates <1 indicated reduced risk of event for vaccinated subjects (ie, greater safety). Statistical heterogeneity was assessed with the I2 statistic, where values ≥50% suggested important heterogeneity. Statistical heterogeneity was explored using stratified analyses, and studies were pooled if no important differences in study characteristics were found. All meta-analyses were conducted in Comprehensive Meta-analysis Software (V.3.3.070, Biostat, Englewood, New Jersey, USA). Descriptive summaries were developed for prioritised definitions, when meta-analysis was not feasible, guided by the Synthesis without Meta-analysis statement.129 |
| Assessment of level of evidence | Strength and certainty of evidence were assessed following the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework,25 using GRADEpro GDT online software (https://gradepro.org/). Syntheses were prioritised for assessment based on outcome definition (as per the GAIA Network and Brighton Collaboration Network124; see online supplemental file1, section 2, intervention/comparison (trivalent inactivated influenza vaccine or quadrivalent inactivated influenza vaccine vs no vaccine), content expert prioritisation exercise, and analysis type (full-sample analyses only). Dual independent assessment was conducted, with discussion of conflicts. |
| Reporting | Only findings from GRADE-prioritised syntheses and secondary syntheses that provide additional context have been reported in the main text; all other seasonal influenza vaccine results are reported in online supplemental file 1, section 5 or as raw data in online supplemental files 2–4. RoB has been summarised across studies, using each study’s primary outcome with the lowest RoB. Study-level RoB data have been provided in online supplemental file 1,section 6. GRADE lay statements129 and Summary of Findings tables have been reported in the main text, with an Evidence Profile table provided in online supplemental file 1, section 7. Syntheses and/or data for vaccines other than seasonal influenza (eg, pandemic vaccines, combinations of seasonal and pandemic vaccines) have been provided in online supplemental file 1, sections 8–11 and online supplemental files 2–4. |
*Reference Manager, V.12.0.3, Thomson Reuters.
†EndNote, V.X9.3.3, Clarivate.
‡DistillerSR, V.2.35. Evidence Partners; 2021. Accessed December 2019–November 2021. https://www.evidencepartners.com.
RoB, risk of bias.
bmjopen-2022-066182supp002.xlsx (126.4KB, xlsx)
bmjopen-2022-066182supp003.xlsx (583.8KB, xlsx)
bmjopen-2022-066182supp004.xlsx (144.5KB, xlsx)
Results
Availability of the literature
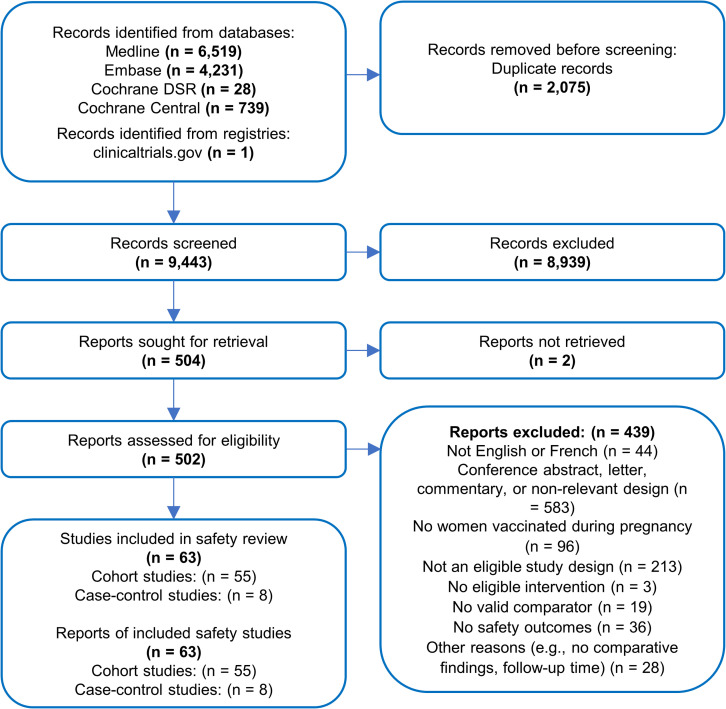
Of 9443 unique references identified by our searches, 504 full texts were assessed and 93 were included in the original review. Eight RCTs reported in 16 references,1 26–40 8 cohort studies41–48 and 6 case–control studies49–54 were removed because they focused solely on vaccine efficacy/effectiveness outcomes, leaving 63 studies in the current review of vaccine safety (figure 1; 55 cohort studies13 55–108 and 8 case–control studies109–116). Citations of the 411 studies excluded at full-text screening have been provided in online supplemental file 1, section 12, along with reasons for exclusion.
Figure 1.
Flow of evidence in a systematic review of the safety of influenza vaccination during pregnancy.
Study characteristics
Twenty-nine studies compared a seasonal influenza vaccine to no vaccine and are the focus of the main text (trivalent inactivated influenza vaccine (TIIV), n=2455 57 58 61 68 77 83–94 97 102 103 108 110 113; TIIV or quadrivalent inactivated influenza vaccine (QIIV), n=564 104 111 115 116). Thirty-four studies that evaluated other vaccines (eg, 2009 monovalent A/H1N1 pandemic influenza vaccine) or vaccine combinations, or had influenza vaccine comparators have been summarised in online supplemental file 1, sections 8–11.13 56 59 60 62 63 65–67 69–76 78–82 95 96 98–101 105–107 109 112 114
Characteristics of the 29 TIIV/QIIV studies are presented in table 3, and an evidence map of reported outcomes has been provided in the online supplemental file 1, section 13. Five were case–control studies110 111 113 115 116 and 24 used a cohort design.55 57 58 61 64 68 77 83–94 97 102–104 108 Most studies were conducted in high-income countries (ie, Australia, Canada, Japan, the USA; n=25),55 57 61 64 68 77 83–85 87–92 94 97 102 104 108 110 111 113 115 116 while three were conducted in lower-middle-income countries (ie, India, Laos, Nicaragua),58 93 103 and one in South Africa, an upper-middle-income country.86 Sample sizes ranged from 346 to 407 745 women (nmedian=11 225). All but two studies61 88 were published after 2010 (range: 2004–2020).
Table 3.
Characteristics of the 24 cohort studies and 5 case–control studies that compared seasonal influenza vaccination (TIIV and or QIIV) to no vaccination
| Study Country of conduct; design; funding |
Recruitment period | No of women (infants) | Interventions (cohort studies)/exposure window (case–control studies) | Safety endpoints |
| Cohort studies | ||||
| McMorrow et al, 202086 South Africa; RC; Non-industry |
Women who sought antenatal care between 14 May 2015–16 August 2015 and 1 April 2016–25 August 2016 | 4084 (4084) | Seasonal vaccine (TIIV) No vaccine |
Stillbirths, PTB, LBW, SGA |
| Mohammed et al, 202087 Australia; PC; No funding |
First antenatal appointment between March 2015 and December 2017 | 1253 (1201) | Seasonal vaccine (TIIV) No vaccine |
PTB, LBW, SGA, SAB, CA |
| Ohfuji et al, 202091 Japan; PC; Non-industry |
Attended antenatal clinic between October and December 2013, prior to the 2013/2014 influenza season | 10 330 (NR) | Seasonal vaccine (TIIV) No vaccine |
SAB, stillbirths, PTB, LBW, CA |
| Speake et al, 2020104 Australia; RC; Non-industry |
Date of conception estimated to have occurred between December and July, from April 2012 to July 2015 | 70 838 (70 838) | Seasonal vaccine (TIIV and QIIV) No vaccine |
Stillbirths, PTB, SGA |
| McHugh et al, 201984 Australia; RC; Non-industry |
Pregnant between 1 December 2003 and 31 December 2006 or between 1 January 2009 and 31 June 2011 | 576 (697) | Seasonal vaccine (TIIV) No vaccine |
PTB, LBW, SGA |
| McHugh et al, 201985 Australia; PC, post hoc analysis; Non-industry |
Within 55 days of birth from 1 April 2012 to 31 December 2015; recruitment maximised during influenza seasons (April–October) | 8827 (8,827) | Seasonal vaccine (TIIV) No vaccine |
PTB, LBW, SGA |
| Singh et al, 2019103 India; RC; NR |
Pregnant and vaccinated or refused vaccination between January 2016 and March 2018 | 346 (348) | Seasonal vaccine (TIIV) No vaccine |
Maternal SAEs, PTB |
| Ohfuji et al, 201892 Japan; PC; Non-industry |
Pregnant between September and December 2013 | 3044 (3044) | Seasonal vaccine (TIIV) No vaccine |
PTB, LBW, CA |
| Arriola et al, 201758 Nicaragua; PC/RC; Non-industry |
Births from 21 July 2014 to 4 December 2014 | 3268 (3268) | Seasonal vaccine (TIIV) No vaccine |
PTB, LBW, SGA |
| McHugh et al, 201783 Australia; Nested RC; Non-industry |
Births from 1 April 2012 to 31 December 2014 | 7126 (7126) | Seasonal vaccine (TIIV) No vaccine |
PTB, LBW |
| Zerbo et al, 2017108 USA; RC; Non-industry |
Births from 1 January 2010 to 31 December 2015 | 145 869 (145 869) | Seasonal vaccine (TIIV) No vaccine |
PTB, LBW, SGA |
| Chambers et al, 201664 USA and Canada; PC; Mixed |
Pregnant between 2010 and the end of 2014 | 1730 (1707) | Seasonal vaccine (TIIV and QIIV) No vaccine |
SAB, stillbirths, PTB, SGA |
| Olsen et al, 201693 Laos; PC; Non-industry |
Births from April 2014 to February 2015 | 5103 (5103) | Seasonal vaccine (TIIV by Afluria) No vaccine |
PTB, SGA |
| Regan et al, 201697 Australia; RC; Non-industry |
Births from 1 April 2012 to 31 December 2013 | 58 008 (57 660) | Seasonal vaccine (TIIV) No vaccine |
Stillbirths |
| Ahrens et al, 201457 USA; RC (control group from a case–control study); Non-industry |
Pregnant and vaccinated or matched by LMP to vaccinated group from 2006 to 2010 | 1619 (1619) | Seasonal vaccine (TIIV) No vaccine |
PTB, SGA |
| Legge et al, 201477 Canada; RC; No funding |
Births from 1 November 2010 to 31 March 2012 | 12 223 (11 293) | Seasonal vaccine (TIIV) No vaccine |
PTB, LBW, SGA |
| Nordin et al, 201490 USA; RC; Non-industry and Insurance |
Pregnant during influenza seasons between 2004/2005 and 2008/2009 | 150 089 (150 089) | Seasonal vaccine (TIIV) No vaccine |
PTB, SGA |
| Adedinsewo et al, 201355 USA; RC; Non-industry and Insurance |
Births from 1 January 2005 to 31 December 2008 | 5422 (NR) | Seasonal vaccine (TIIV) No vaccine |
PTB, SGA |
| Nordin et al, 201389 USA; RC; Non-industry and Insurance |
Pregnant between 1 June 2002 and 31 July 2009 and vaccinated or matched | 223 898 (NA) | Seasonal vaccine (TIIV) No vaccine |
Maternal SAEs |
| Dodds et al, 201268 Canada; RC; Non-industry |
Births from 1 April 2006 to 31 October 2009 | 9647 (9647) | Seasonal vaccine (TIIV) No vaccine |
PTB, LBW, SGA |
| Sheffield et al, 2012102 USA; PC; NR |
Pregnant between October and March in 2003–2008 | 84 843 (85 783) | Seasonal vaccine (TIIV) No vaccine |
CA, stillbirths, PTB, LBW |
| Omer et al, 201194 USA; RC; Non-industry |
Births from 1 June 2004 to 30 September 2006 | 4168 (NR) | Seasonal vaccine (TIIV) No vaccine |
PTB, SGA |
| Munoz et al, 200588 USA; RC; Non-industry |
Pregnant during influenza seasons from 1 July 1998 to 30 June 2003 | 1051 (1051) | Seasonal vaccine (TIIV by Aventis Pasteur or Wyeth) No vaccine |
Maternal SAEs, PTB, CA |
| Black et al, 200461 USA; RC; NR |
Births between November and February from November 1997 to February 2002 | 49 585 (48 639) | Seasonal vaccine (TIIV) No vaccine |
PTB |
| Case–control studies | ||||
| Panagiotakopopulos et al, 2020116 USA; RCC; Non-industry |
Pregnancies ending in live or stillbirth from 1 January 2012 to 30 September 2015 | 3975 | Seasonal vaccine (TIIV, with or without Tdap), 14 days after last menstrual period through 7 days before index date | Stillbirths |
| Donahue et al., 2019111 USA; RCC; Non-industry |
Pregnant during the 2012–2013, 2013–2014 or 2014–2015 influenza seasons | 2472 | Seasonal vaccine (TIIV) 1–28 days after conception, >28 days after conception and 1–28 days before SAB | SAB |
| Donahue et al, 2017110 USA; RCC; Non-industry |
During the 2010–2011 or 2011–2012 influenza seasons | 970 | Seasonal vaccine (TIIV) 1–28 days before SAB | SAB |
| Louik et al, 2016115 USA; RCC; Mixed |
During the 2011–2012, 2012–2013 or 2013–2014 influenza vaccine seasons | 1910 | Seasonal vaccine (TIIV or QIIV) during first trimester | CA |
| Irving et al, 2013113 USA; RCC; Non-industry |
During the 2005–2006 or 2006–2007 influenza seasons | 486 | Seasonal vaccine (TIIV) any time during pregnancy and 1–28 days before SAB | SAB |
CA, congenital anomalies; LBW, low birth weight; NR, not reported; PC, prospective cohort; PTB, preterm birth; QIIV, quadrivalent inactivated influenza vaccine; RC, retrospective cohort; RCC, retrospective case–control; SAB, spontaneous abortion; SAE, serious adverse events; SGA, small-for-gestational-age birth; Tdap, tetanus toxoid, reduced diphtheria toxoid and acellular pertussis; TIIV, trivalent inactivated influenza vaccine.
Eight studies (28%) reported no adjusted analyses.61 83–85 91 92 102 103 In the remaining studies, confounding was most commonly addressed with multivariable regression models that included either (A) potential confounders as covariates,64 68 77 83 87–89 93 94 108 110 111 113 116 (B) propensity score as a single covariate55 57 64 or (C) both individual and propensity score covariates.97 Substantial heterogeneity was observed in the sets of covariates used to adjust for confounding. All 15 studies included in the prioritised syntheses reported below adjusted for critical confounders (ie, maternal age, smoking during pregnancy and socioeconomic status (SES)), except 3 cohort studies evaluating small-for-gestational-age (SGA) that did not adjust for smoking during pregnancy58 90 93 and 1 case–control study evaluating stillbirths that did not adjust for SES.116 Additional details can be found in online supplemental files 2–4.
ROB assessment
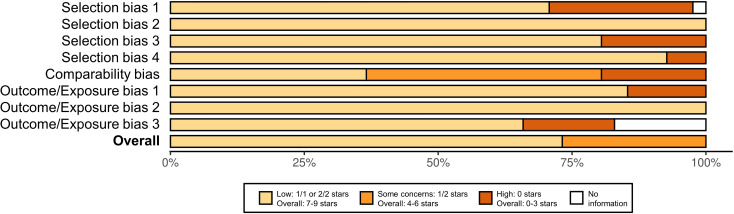
Across all studies, the most prevalent ROB concerns were related to lack of appropriate adjustment for confounding (‘comparability bias’ in 62% of studies; figure 2). Twenty-one studies (72%) were judged to have an overall low ROB, while eight were judged to have a moderate ROB. A domain-level summary of ROB by study and the original outcome-level ROB data files have been provided in online supplemental file 1, section 6 and online supplemental files 2–4.
Figure 2.
Summary of risk of bias assessments by domain of the Newcastle Ottawa Scale for observational studies comparing seasonal influenza vaccine (TIIV and/or QIIV) to no vaccine, when safety outcomes with the lowest risk of bias were included. QIIV, quadrivalent inactivated influenza vaccine; TIIV, trivalent inactivated influenza vaccine
Syntheses of prioritised outcomes
Table 4 summarises all synthesis findings for the prioritised outcomes. Numbers of mothers or infants included in individual study analyses and overall in each meta-analysis are provided in the associated forest plots (see figures 3 and 4).
Table 4.
Summary of findings table of GRADE-prioritised outcomes synthesised from 29 included observational studies that compared seasonal influenza vaccination (TIIV and or QIIV) to no vaccination
| Outcome no of participants (studies) |
Relative effect (95% CI) |
Anticipated absolute effects (95% CI) | Certainty | GRADE lay statement | ||
| Without seasonal influenza vaccine | With seasonal influenza vaccine | Difference | ||||
| Preterm birth (<37 completed weeks) No of participants: 152 476 (5 observational studies) |
HR 1.08 (0.89 to 1.30) |
Low baseline risk | ⨁〇〇〇 Very low*† |
The evidence is very uncertain about the effect of seasonal influenza vaccination during pregnancy on the risk of preterm birth <37 completed weeks. | ||
| 3.2% | 3.5% (2.9 to 4.2) |
0.3% more (0.3 fewer to 1 more) |
||||
| High baseline risk | ||||||
| 15.0% | 16.2% (13.5 to 19.4) |
1.2% more (1.5 fewer to 4.4 more) |
||||
| Spontaneous abortion (<20 gestational weeks) No of participants: 1900 (2 observational studies) |
HR 0.77 (0.31 to 1.89) |
Moderate baseline risk | ⨁〇〇〇 Very low‡§¶ |
Seasonal influenza vaccination during pregnancy may reduce spontaneous abortion <20 gestational weeks, but the evidence is very uncertain. | ||
| 5.0% | 3.9% (1.6 to 9.2) |
1.1% fewer (3.4 fewer to 4.2 more) |
||||
| Stillbirths (≥ 18–22 gestational weeks or ≥500 g) No of participants: 71 602 (2 observational studies) |
Regan et al, 2016,97 reported an adjusted HR=0.49 (0.29 to 0.84) in 58 008 pregnancies (5076 exposed and 52 932 unexposed) in a cohort study evaluating influenza vaccination at any trimester of pregnancy. In a case–control study evaluating influenza vaccination at any trimester, Panagiotakopoulos et al,116 2020 found an aOR=0.98 (0.82 to 1.18) among 1475 cases and 12 119 controls. These two studies could not be pooled due to extremely high heterogeneity (I2=82.85). | ⨁〇〇〇 Very low** |
The evidence is very uncertain about the effect of seasonal influenza vaccine during pregnancy on the risk of stillbirth ≥18–22 gestational weeks or ≥500 g. | |||
| Small-for-gestational-age birth (<10th percentile) No of participants: 297 424 (11 observational studies) |
RR 0.99 (0.95 to 1.04) |
Low baseline risk | ⨁〇〇〇 Very low†† |
Seasonal influenza vaccination during pregnancy may have little to no effect on the risk of small-for-gestational-age birth (<10th percentile for gestational age and sex of a valid reference control group), but the evidence is very uncertain. | ||
| 3.7% | 3.7% (3.5 to 3.8) |
0.0% fewer (0.2 fewer to 0.1 more) |
||||
| Moderate baseline risk | ||||||
| 6.0% | 5.9% (5.7 to 6.2) |
0.1% fewer (0.3 fewer to 0.2 more) |
||||
| High baseline risk | ||||||
| 13.6% | 13.5% (12.9 to 14.1) |
0.1% fewer (0.7 fewer to 0.5 more) |
||||
| Congenital anomalies from birth to 6 months of age No of participants: 4277 (one observational study) |
Single case–control study reporting adjusted findings from 2866 cases and 1411 controls, indicating no significant effect of TIIV on congenital anomalies up to 6 months of age (aOR=1.01 (0.85 to 1.21)). | ⨁〇〇〇 Very low‡‡ |
The evidence is very uncertain about the effects of seasonal influenza vaccination during the first trimester of pregnancy on the risk of congenital anomalies identified at birth or up to six months of age. | |||
| Congenital anomalies from birth to discharge No of participants: 1207 (1 observational study) |
Single cohort study found an aRR=0.33 (0.04 to 2.73), with 2 events in 141 vaccinated pregnancies and 21 events in 1066 unvaccinated pregnancies. | ⨁〇〇〇 Very low§§ |
||||
| Maternal serious non-obstetrical adverse events: events unrelated to pregnancy causing hospitalisation No of participants: 1051 (1 observational study) |
Single study Munoz et al,88 2005 found no apparent difference in risk between TIIV vaccinated (2 in 225) and unvaccinated women (3 in 826). | ⨁〇〇〇 Very low¶¶ |
The evidence is very uncertain regarding the effect of seasonal influenza vaccination during pregnancy on hospitalisation events within 42 days and non-obstetric SAEs, generally, for an undetermined time after vaccination. | |||
| Maternal serious non-obstetrical adverse events: not defined and time of follow-up not reported No of participants: 346 (one observational study) |
No events found in a single study Singh et al,103 2019 of 288 TIIV vaccinated and 58 unvaccinated women. | ⨁〇〇〇 Very low¶¶***††† |
||||
| Maternal serious non-obstetrical adverse events: inpatient Guillain-Barré syndrome No of participants: 223 898 (1 observational study) |
Single study Nordin et al,95 2013 found no significant difference between TIIV vaccinated (0 in 75 906) and unvaccinated women (1 in 147 992) (p=0.34). | ⨁〇〇〇 Very low††† |
Seasonal influenza vaccine may have little to no effect on maternal serious non-obstetrical adverse events (inpatient Guillain-Barré syndrome), but the evidence is very uncertain. | |||
GRADE working group grades of evidence: High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: ur confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
*Four of five studies scored ≥7 and the fourth scored 6; however, there were concerns regarding the representativeness of the exposed cohort (selection bias) in four studies, the adequacy of follow-up in two studies, and assessment of the exposure in one study and assessment of the outcome in two studies.
†The 95% CIs of the most heavily weighted study does not encompass the effect estimates of the three moderately weighted studies.
‡The total sample size met the criteria for optimal information size, but the total number of events did not.
§There were concerns regarding selection bias in both of the included studies.
¶Initial postpandemic study found significantly increased risk, whereas no other study found significant effects.
**Neither of the CIs of the two studies contains the other’s effect estimate.
††The p value of the Q estimate for the meta-analysis was >0.05 and two heavily weighted studies were consistent; however, most of the minimally weighted studies had point estimates that did not fall within all other confidence intervals.
‡‡Serious ROB due to inadequate case definition, the unclear representativeness of the cases and unclear non-response rate.
§§Concerns regarding biases related to selection and outcome assessment.
¶¶Total sample size is much lower than the optimal information size for the control group risk.
***Potential serious bias due to lack of reporting of follow-up time and follow-up methods in unvaccinated group, leading to potential bias for the following domains: ‘Could outcome be present at the start of study for unvaccinated women?,’ ‘Was follow-up time long enough for outcome to occur?’ and ‘Were unvaccinated women followed adequately?’.
†††Although country/countries of conduct was/were low income to middle income, the outcome shouldn't be substantially different in the Canadian context.
aOR, adjusted OR; aRR, adjusted risk ratio; GRADE, Grading of Recommendations, Assessment, Development and Evaluation; QIIV, trivalent inactivated influenza vaccine; SAE, serious adverse event; TIIV, trivalent inactivated influenza vaccine.
Figure 3.
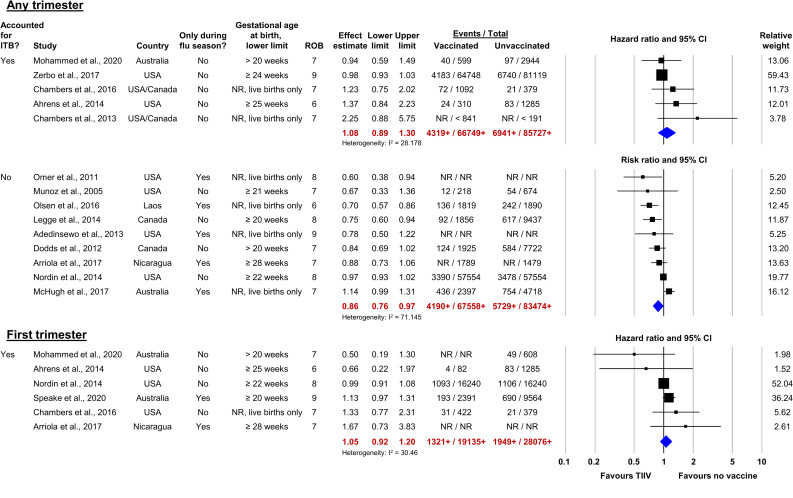
Forest plots of studies reporting adjusted analyses for preterm birth, by timing of vaccine administration.*Denotes outcomes for which meta-analysis could not be conducted. Forest plots for these outcomes illustrate effects reported in studies that were descriptively summarized. Risk of bias was assessed using the 9-point Newcastle-Ottawa Scale. Higher values are associated with lower risk of bias. Numbers followed by plus signs (+) are lower than the actual total numbers included in the meta-analysis due to unreported raw data in one or more studies. aOR, adjusted odds ratio; aRR, adjusted risk ratio; NR, not reported; ROB, risk of bias; TIIV, trivalent inactivated influenza vaccine.
Figure 4.
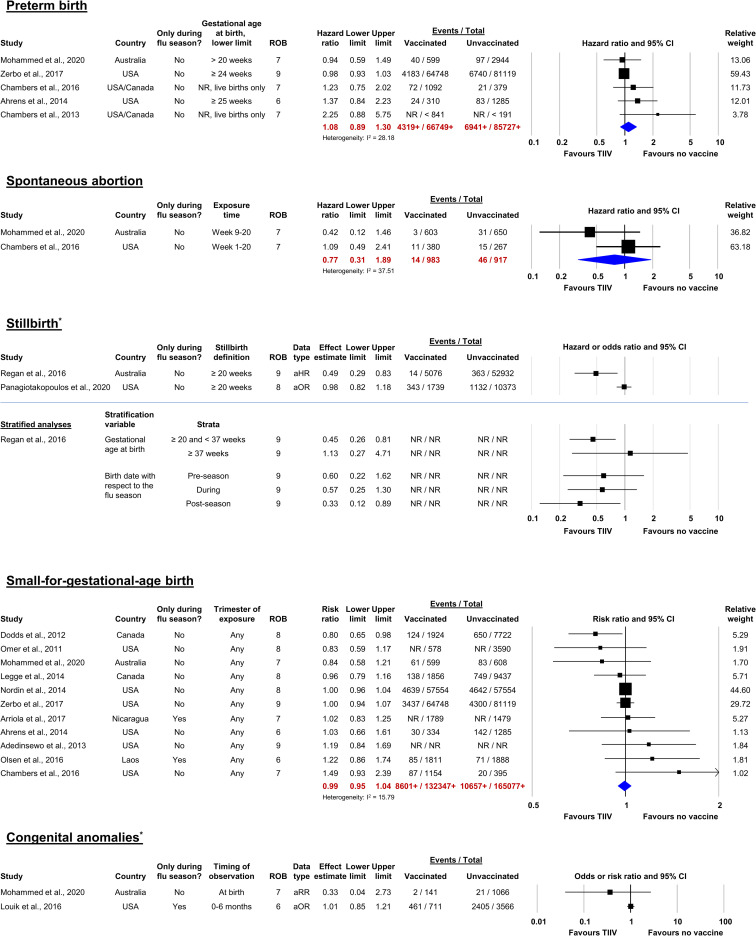
Forest plots of studies reporting adjusted analyses for the prioritised adverse birth outcomes. ITB, immortal time bias; NR, not reported; ROB, risk of bias; TIIV, trivalent inactivated influenza vaccine. Numbers followed by plus signs (+) are lower than the actual total numbers included in the meta-analysis due to unreported raw data in one or more studies.
Preterm birth
Fifteen cohort studies assessed TIIV at any time during pregnancy on the occurrence of PTB (<37 completed gestational weeks; table 4).55 57 58 63 64 68 77 83 86–88 90 93 94 108 Five studies57 63 64 87 108 accounted for immortal time bias and confounding and were included in the prioritised meta-analysis, while nine studies55 58 68 77 83 88 90 93 94 were pooled in a secondary synthesis to evaluate differences in pooled results when temporal biases were not considered. A final study was descriptively summarised in online supplemental file 1, section 5 due to insufficient details about its analytic methods.86
Prioritised synthesis
The estimated adjusted HR (aHR) of PTB for women who received TIIV during pregnancy was 1.08 (95% CI 0.89 to 1.30, I2=28.2; 5 studies, >152 476 women; figure 3). The evidence was assessed to be of very low certainty due to concerns regarding to ROB and inconsistency (table 4). One large high-quality study108 that reported an association close to the null value of 1 was weighted heavily (55.6%; n=145 867). There was a trend of decreasing effect size over time, based on date of publication.
Secondary syntheses
In the meta-analysis of nine studies (n>151 032) that did not consider temporal biases, the estimated adjusted RR (aRR) of PTB for women who received TIIV during pregnancy was 0.86 (95% CI 0.76 to 0.97, I2=71.1; figure 4, Any trimester, lower plot). Additionally, statistical heterogeneity was high, potentially due to differences in the background risk of PTB (range: 6%–16%) in different study populations and variation in the outcome definitions (eg, lower limits of gestational age at birth).
Six studies (n>47 211) reported adjusted findings for first trimester vaccination and were included in a meta-analysis.57 58 64 87 90 104 The lower gestational age limit of the PTB definitions ranged from >20 weeks to ≥28 weeks. All studies were at a reduced risk of temporal bias because first-trimester vaccination, by definition, occurred before the at-risk period for PTB. Four studies further reduced temporal bias through statistical methods57 64 87 104 and two did not.58 90 The estimated aHR of PTB for women who received TIIV during their first trimester was 1.05 (95% CI 0.92 to 1.20, I2=30.5; figure 4, First trimester).
Spontaneous abortion
Five studies evaluated the effect of TIIV during pregnancy on the occurrence of spontaneous abortion (SAB) under 2064 87 110 111 and 2291 gestational weeks. However, because one study did not adjust for confounding91 and different exposure windows were assessed,110 111 only two studies64 87 were pooled.
Prioritised synthesis
Both studies64 87 (n=1900) assessed an exposure window of up to 20 gestational weeks and accounted for immortal time bias. The estimated aHR of SAB for women who received TIIV during pregnancy was 0.77 (95% CI 0.31 to 1.89; I2=37.5; figure 4). This evidence was of very low certainty due to serious concerns regarding ROB, inconsistency and imprecision (table 4). Neither study restricted events to the influenza season, therefore, some of the included women may not have had the opportunity for exposure to either influenza vaccine or virus.
Secondary syntheses
Two case–control studies conducted by the same investigators used an exposure window of 1–28 days before the index date (ie, date of SAB for cases or the equivalent time postconception for controls).110 111 The initial study (n=782) was conducted during the first two influenza seasons following the 2009 A/H1N1 pandemic and found a significant association between SAB and TIIV received within 1–28 days before the index date during the first postpandemic season (2010–11 adjusted OR (aOR) 3.70, 95% CI 1.40 to 9.40), but not during the second season (2011–2012 aOR=1.40, 95% CI 0.60 to 3.30). When women were stratified by receipt of monovalent A/H1N1 2009 pandemic vaccine in the previous year, the association remained significant only for those who had received pandemic vaccine. A second case–control study (n=1934) spanning the three influenza seasons between 2012 and 2015 was conducted to evaluate the interaction between previous year vaccination and TIIV or QIIV exposure during pregnancy. No interaction was found and, overall, the estimated aOR of SAB for women who received TIIV or QIIV during pregnancy was 0.80 (95% CI 0.60 to 1.10).111 Very high statistical heterogeneity (I2=86.0%) precluded meta-analysis of the two studies.
Stillbirth
Seven studies reported data for stillbirth occurring at ≥18–22 gestational weeks or with birth weight ≥500 g.64 86 91 97 102 104 116 Two studies that evaluated vaccination at any time during pregnancy and reported results of adjusted analyses that accounted for immortal time bias were pooled97 116; however, very high statistical heterogeneity was found (I2=82.9), potentially due to differences in study designs, covariates considered to be confounders, and methods used to control confounding. The high heterogeneity suggested that pooling was inappropriate due to differences between the two studies; thus, the pooled results have not been reported and the studies have been descriptively summarised.
Prioritised synthesis
In a cohort study,97 the estimated aHR of stillbirth for women who received TIIV during pregnancy was 0.49 (95% CI 0.29 to 0.83; n=50 008), while in a case–control study,116 the estimated aOR of stillbirth for TIIV or QIIV received between 14 days after the last menstrual period and 7 days before the index date was 0.98 (95% CI 0.81 to 1.17; n=3975; did not adjust for SES; figure 4). A very low certainty of evidence was assessed due to concerns regarding inconsistency between the two studies (table 4).
Secondary syntheses
The aforementioned cohort study97 also reported stratified analyses for gestational age at birth (preterm and full term) and for timing of events with respect to the influenza season (before, during, after). Sample sizes were not reported. This study was conducted year-round in Australia and spanned two influenza seasons of differing durations. Among PTB, the estimated aHR of stillbirth for women who received TIIV during pregnancy was 0.45 (95% CI 0.26 to 0.81), but among term births, the estimated aHR of stillbirth was 1.13 (95% CI 0.27 to 4.71; figure 4). As well, in the 2.5–3 months after the influenza season, the estimated aHR of stillbirth for women who received TIIV during pregnancy was 0.33 (95% CI 0.12 to 0.88), but the effect was not significant in the 3–4 months during (aHR 0.57, 95% CI 0.25 to 1.31) or the 2–6.5 months before the influenza season (aHR 0.60, 95% CI 0.22 to 1.61; figure 4).
Another cohort study104 that accounted for immortal time bias through design and assessed only first trimester vaccination found the estimated aRR of stillbirth to be 1.18 (95% CI 0.64 to 2.18; n=11 955).
Small-for-gestational-age birth
Thirteen studies evaluated the association between TIIV received during any trimester and SGA birth.55 57 58 64 68 77 85–87 90 93 94 108 One reported findings from unadjusted analyses85 and 1 did not report statistical methods sufficiently,86 leaving 11 studies for meta-analysis.55 57 58 64 68 77 87 90 93 94 108
Prioritised synthesis
Two90 108 of 11 studies included in the meta-analysis comprised almost 75% of the pooled estimate by weight and reported aRRs of 1.00; the estimated pooled aRR of SGA for women who received TIIV during any trimester was 0.99 (95% CI 0.95 to 1.04, I2=15.8; n>297 424; figure 4). Three studies did not adjust for smoking during pregnancy.58 90 93 A very low certainty of evidence was assessed due to concerns regarding inconsistency (table 4).
Congenital anomalies
Four studies87 91 102 115 evaluated the association between TIIV or QIIV during the first trimester and the occurrence of congenital anomalies identified either at birth87 91 102 or up to 6 months of age.115 Two studies (one cohort87 and one case–control115) adjusted for confounding but used differing periods to ascertain anomalies in infants (ie, at birth and up to 6 months of age), precluding meta-analysis.
Prioritised synthesis
TIIV or QIIV during the first trimester was not significantly associated with congenital anomalies in either of the studies, whether ascertainment occurred at birth87 (aRR 0.33, 95% CI 0.04 to 2.73; n=1207) or up to 6 months of age115 (aOR 1.01, 95% CI 0.85 to 1.21; n=4277; figure 4). Both studies included both live and stillborn infants from multiple seasons.87 115 The certainty of the evidence was assessed to be very low due to ROB at both time points (table 4).
Maternal non-obstetric serious adverse events
Three cohort studies reported non-obstetric serious adverse events (SAEs).88 89 103 One study89 adjusted for confounders and found no significant association with Guillain-Barre syndrome within 42 days postvaccination (p=0.34; vaccinated: 0 events/75 906 women, unvaccinated: 1 event/147 992 women). The other two studies reported raw data only: no significant associations with non-obstetric hospitalisations within 42 days postvaccination88 (vaccinated: 2 events/225 women, unvaccinated: 3 events/826 women; RR 2.45, 95% CI 0.41 to 14.56) or with SAEs over an unknown follow-up period103 (vaccinated: 0 events/288 women, unvaccinated: 0 events/58 women). Events were rare for all outcomes and sample sizes potentially too small to detect significant differences between groups. All maternal SAE outcomes were assessed with a very low certainty of evidence due to concerns regarding imprecision (table 4).
Discussion
Our systematic review of the safety of seasonal influenza vaccination during pregnancy was designed to ensure our findings were relevant to currently used multivalent products that may have a potentially different safety profile than monovalent influenza vaccines and to ensure reporting of least biased estimates. We identified no significant harmful or beneficial effects of TIIV or QIIV on birth outcomes or maternal non-obstetric SAEs among 29 studies. However, clinical, methodological and statistical heterogeneity and lack of adjustment for confounding and/or immortal time bias precluded meta-analysis for stillbirth, congenital anomalies and maternal SAEs, and reduced the number of studies available in meta-analyses of PTB, SAB and SGA. Meta-analyses for PTB and SGA were driven by one or two heavily weighted studies each. Syntheses of the effects of 2009 A/H1N1 pandemic vaccination on adverse birth outcomes demonstrated similar results (see online supplemental file 1, section 8).
Research regarding the safety of seasonal influenza vaccination during pregnancy is ongoing, with five new studies published in 2020 alone,86 87 91 104 116 warranting continued review of the literature. Recent systematic reviews have opted to either pool all available studies, regardless of vaccine type (ie, seasonal and 2009 A/H1N1 pandemic) or the presence of confounding,15 16 or not to pool any studies due to differences in vaccine types and effect estimates and the presence of confounding bias.19 To the best of our knowledge, no previous review has attempted to reduce temporal and confounding biases. Pharmacoepidemiological studies can be biased by immortal time117 if each participant’s times of enrolment, treatment start, and event occurrence are not measured, and the duration of unexposed and exposed time not accounted for through either analytical methods10 or study design.10 11 Observational studies of vaccination during pregnancy that report time-dependent birth outcomes (eg, PTB and stillbirth10 13) are susceptible to immortal time bias if the time at risk prior to vaccination is misclassified as being exposed. Approaches, such as use of Cox proportional hazards models with a time-varying exposure variable for vaccination status correctly account for each woman’s exposed and unexposed time, reducing immortal time bias.10 13 118 Our meta-analyses for PTB demonstrated that immortal time bias can have a substantial impact on study results. When immortal time bias was not accounted for, a significant protective effect of seasonal influenza vaccination during pregnancy on PTB was observed. However, when appropriate analytical methods were used, seasonal influenza vaccination was not associated with PTB. Similar impacts of temporal biases were found in meta-analyses of the effects of 2009 A/H1N1 pandemic vaccine during pregnancy on stillbirth (see online supplemental file 1, section 8). These differences may have important implications for decision-makers and knowledge users.
Four RCTs have been conducted in pregnant women, with objectives to evaluate seasonal influenza vaccine efficacy to prevent infant lab-confirmed influenza1 36 38 40 and maternal influenza-like illness36 as primary outcomes. Data on adverse birth outcomes were also collected as secondary outcomes; however, due to the inherent rarity of these events, the individual studies were underpowered to detect differences between groups for these outcomes.23 Omer et al23 pooled individual patient data from three of these RCTs1 36 38 (one excluded due to small sample size40) and found that within the combined dataset of 10 000 women no significant associations with adverse birth outcomes were found.23 They concluded that the pooled dataset was still substantially underpowered to detect a meaningful difference between groups for the stillbirth outcome, and moderately underpowered for all other adverse birth outcomes.23 Regan and Munoz24 pooled fetal death data from the same three RCTs1 36 38 and included data from the fourth RCT37 40 in meta-analyses of PTB, LBW and SGA birth. No increased risks of any adverse birth outcomes were found. We reported two meta-analyses that were in common with the above publications,23 24 and our findings were similar: no significant associations with PTB (aHRcurrent=1.09 (95% CI 0.89 to 1.33) vs RROmer=0.97 (95% CI 0.87 to 1.08) vs RRRegan=0.95 (95% CI 0.85 to 1.06)) or SGA (aRRcurrent=0.99 (95% CI 0.95 to 1.04) vs RROmer=0.99 (95% CI 0.93 to 1.06) vs RRRegan=0.96 (95% CI 0.90 to 1.02)). Furthermore, Regan and Munoz descriptively summarised maternal adverse events postinfluenza-vaccination reported in RCTs,31 119 prospective cohort studies,120 121 and postmarketing surveillance studies122 123 and found no evidence of increased safety signals beyond those found in the general public or compared with pertussis vaccine.24 Vaccination for seasonal influenza during pregnancy is recommended by the WHO, therefore, ethical approval of a future large-scale (>10 000 women) RCT powered for birth outcomes is unlikely. It is encouraging that data pooled from methodologically sound observational studies can approximate findings from pooled individual patient data.
Our review has several strengths, including a broad search and use of robust systematic review methods. To reduce clinical heterogeneity and to align with currently available vaccines, we did not pool seasonal and monovalent 2009 A/H1N1 pandemic influenza vaccines. Entry into meta-analyses was restricted to least-biased estimates that were adjusted for confounding and accounted for temporal biases, where appropriate. As well, we prioritised synthesis of very focused outcome definitions that matched published accepted criteria.124 As a limitation, we made assumptions regarding equivalence of effect estimates (ie, RR=OR=hour); however, only the meta-analysis for SGA included heterogeneous effect estimates, and for this outcome, the assumptions behind the approximation of effect estimates were met. The available evidence was limited by inconsistent adjustment for confounding and immortal time bias and by high variability of outcome definitions and time points, which precluded pooling of some of the identified data. As well, poor reporting of detailed characteristics of the seasonal influenza vaccines in use in the study location during the study periods prevented the development of inferences regarding the effects of vaccine adjuvant or production technology (eg, egg based, cell based, recombinant). Finally, there were limitations in the use of GRADE in that only observational designs were considered for inclusion. GRADE assessment of syntheses of observational studies begins with a rating of low certainty, and evidence is only rated upward to moderate certainty if a large statistically significant effect is found. When the review objective is to assess safety (ie, no significant difference between exposure groups), large significant effects are not expected and, thus, evidence would be considered at low certainty, at best, even when well-conducted studies are included.
In conclusion, our review suggests that vaccination against seasonal influenza during pregnancy is not associated with significant safety issues, with respect to adverse birth outcomes and maternal non-obstetric SAEs. A small but non-significant association with an increased risk of PTB was found in a meta-analysis of studies that accounted for immortal time bias. This was in contrast to a significant decrease in the risk of PTB, when studies that did not account for immortal time bias were pooled. Improvements in study design and analytical methods in future studies would advance the evidence base. Additionally, although no safety signals have been identified through routine pharmacovigilance, there is limited published peer-reviewed evidence regarding the safety of administration during pregnancy of more recently licensed inactivated influenza vaccines products that are based on different vaccine technologies, including quadrivalent mammalian cell-culture-based vaccines (eg, Flucelvax Quad) and recombinant influenza vaccines (eg, Flublok, Supemtek). Similarly, there is limited evidence regarding the safety of administration of influenza vaccine during the first trimester, specifically. Ongoing primary research in these areas is necessary, warranting future systematic review to inform guidance for healthcare providers counselling pregnant women about the benefits and safety of influenza vaccination.
Supplementary Material
Acknowledgments
We wish to thank Raymond Daniel for his consistently excellent support toward article retrieval and citation management throughout the review. We wish to acknowledge Matthew Tunis and Kelsey Young from the Public Health Agency of Canada for their input throughout the review.
Footnotes
Twitter: @cgarritty, @dmoher, @bh_epistat
Contributors: CG and BH conceptualised the design and initial protocol for this study. DF, CH, ACT andDM provided feedback on the study protocol. BS designed and executed the literature search. DW, CG, CH, CB, MH, NA, DBR, LE, AM, CS, MG and PAK were responsible for study selection and data collection. DW and BH performed and summarised findings from data analysis. DW and BH prepared the initial draft of the manuscript. All coauthors (DW, DF, CG, CH, CB, MH, NA, DBR, LE, AM, CS, MG, PAK, AS, BS, ACT, DM and BH) reviewed and approval the final draft of the manuscript. BH is guarantor of the review.
Funding: This study was funded by the Canadian Institutes of Health Research and the Drug Safety and Effectiveness Network. ACT is funded by a Tier 2 Canada Research Chair in Knowledge Synthesis.
Disclaimer: The funders had no role in the design, analysis, interpretations or reporting of this research.
Competing interests: BH has previously received honoraria from Eversana for methodologic advice related to the conduct of systematic reviews and meta-analysis. All remaining authors have no competing interests to declare.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. All relevant data are provided within the manuscript and its supporting files. Our supplemental files include additional detail regarding the methods and findings of our review, and we also have provided spreadsheets containing all of our gathered data.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Madhi SA, Nunes MC, Cutland CL. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med 2014;371:2340. 10.1056/NEJMc1412050 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Influenza vaccines: WHO position paper. Weekly Epidemiol Rec 2005:277–88. [Google Scholar]
- 3.on behalf of the National Advisory Committee on Immunization (NACI), Sinilaite A, Young K, et al. Summary of the National advisory committee on immunization (NACI) seasonal influenza vaccine statement for 2021-2022. CCDR 2021;47:372–80. 10.14745/ccdr.v47i09a04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grohskopf LA, Alyanak E, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices, United States, 2021-22 influenza season. MMWR Recomm Rep 2021;70:1–28. 10.15585/mmwr.rr7005a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizzo C, Rezza G, Ricciardi W. Strategies in recommending influenza vaccination in Europe and US. Hum Vaccin Immunother 2018;14:693–8. 10.1080/21645515.2017.1367463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Australian Technical Advisory Group on Immunisation . Statement on the administration of seasonal influenza vaccines in 2022. 2022. Available: https://www.health.gov.au/sites/default/files/documents/2022/02/atagi-advice-on-seasonal-influenza-vaccines-in-2022.pdf [Accessed 02 May 2022].
- 7.European Centre for Disease Prevention and Control . Vaccine Scheduler - influenza: recommended vaccinations. 2022. Available: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=15&SelectedCountryIdByDisease=-1#:~:text=Flu%20vaccine%20is%20strongly%20recommended,and%20older%20with%20specific%20conditions [Accessed 02 May 2022].
- 8.Joint Committee on Vaccination and Immunisation scientific secretariat . Joint Committee on vaccination and Immunisation: advice on influenza vaccines for 2022/23. 2021. Available: https://www.nitag-resource.org/sites/default/files/2021-10/JCVI%20Statement%20on%20Influenza%20Vaccines%202022-23.pdf [Accessed 02 May 2022].
- 9.Etti M, Calvert A, Galiza E, et al. Maternal vaccination: a review of current evidence and recommendations. Am J Obstet Gynecol 2022;226:459–74. 10.1016/j.ajog.2021.10.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Platt RW, Hutcheon JA, Suissa S. Immortal time bias in epidemiology. Curr Epidemiol Rep 2019;6:23–7. 10.1007/s40471-019-0180-5 [DOI] [Google Scholar]
- 11.Suissa S. The quasi-cohort approach in pharmacoepidemiology: upgrading the nested case-control. Epidemiology 2015;26:242–6. 10.1097/EDE.0000000000000221 [DOI] [PubMed] [Google Scholar]
- 12.Matok I, Azoulay L, Yin H, et al. Immortal time bias in observational studies of drug effects in pregnancy. Birth Defects Res A Clin Mol Teratol 2014;100:658–62. 10.1002/bdra.23271 [DOI] [PubMed] [Google Scholar]
- 13.Vazquez-Benitez G, Kharbanda EO, Naleway AL, et al. Risk of preterm or small-for-gestational-age birth after influenza vaccination during pregnancy: caveats when conducting retrospective observational studies. Am J Epidemiol 2016;184:176–86. 10.1093/aje/kww043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savitz DA, Fell DB, Ortiz JR, et al. Does influenza vaccination improve pregnancy outcome? Methodological issues and research needs. Vaccine 2015;33:6430–5. 10.1016/j.vaccine.2015.08.041 [DOI] [PubMed] [Google Scholar]
- 15.Giles ML, Krishnaswamy S, Macartney K, et al. The safety of Inactivated influenza vaccines in pregnancy for birth outcomes: a systematic review. Hum Vaccin Immunother 2019;15:687–99. 10.1080/21645515.2018.1540807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong S, Jang EJ, Jo J, et al. Effects of maternal influenza vaccination on adverse birth outcomes: a systematic review and Bayesian meta-analysis. PLoS ONE 2019;14:e0220910. 10.1371/journal.pone.0220910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bratton KN, Wardle MT, Orenstein WA, et al. Maternal influenza immunization and birth outcomes of stillbirth and spontaneous abortion: a systematic review and meta-analysis. Clin Infect Dis 2015;60:e11–9. 10.1093/cid/ciu915 [DOI] [PubMed] [Google Scholar]
- 18.Polyzos KA, Konstantelias AA, Pitsa CE, et al. Maternal influenza vaccination and risk for congenital malformations: a systematic review and meta-analysis. Obstet Gynecol 2015;126:1075–84. 10.1097/AOG.0000000000001068 [DOI] [PubMed] [Google Scholar]
- 19.Demicheli V, Jefferson T, Ferroni E, et al. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2018;2020. 10.1002/14651858.CD001269.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMillan M, Porritt K, Kralik D, et al. Influenza vaccination during pregnancy: a systematic review of fetal death, spontaneous abortion, and congenital malformation safety outcomes. Vaccine 2015;33:2108–17. 10.1016/j.vaccine.2015.02.068 [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2011. [Google Scholar]
- 22.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Omer SB, Clark DR, Madhi SA, et al. Efficacy, duration of protection, birth outcomes, and infant growth associated with influenza vaccination in pregnancy: a pooled analysis of three randomised controlled trials. Lancet Respir Med 2020;8:597–608. 10.1016/S2213-2600(19)30479-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regan AK, Munoz FM. Efficacy and safety of influenza vaccination during pregnancy: realizing the potential of maternal influenza immunization. Expert Rev Vaccines 2021;20:649–60. 10.1080/14760584.2021.1915138 [DOI] [PubMed] [Google Scholar]
- 25.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. 10.1136/bmj.328.7454.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bischoff AL, Følsgaard NV, Carson CG, et al. Altered response to A(H1N1)Pnd09 vaccination in pregnant women: a single blinded randomized controlled trial. PLoS ONE 2013;8:e56700. 10.1371/journal.pone.0056700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henkle E, Steinhoff MC, Omer SB, et al. The effect of exclusive breast-feeding on respiratory illness in young infants in a maternal immunization trial in Bangladesh. Pediatr Infect Dis J 2013;32:431–5. 10.1097/INF.0b013e318281e34f [DOI] [PubMed] [Google Scholar]
- 28.Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018;67:334–40. 10.1093/cid/ciy090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kostinov MP, Cherdantsev AP, Akhmatova NK, et al. Immunogenicity and safety of subunit influenza vaccines in pregnant women. ERJ Open Res 2018;4. 10.1183/23120541.00060-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kozuki N, Katz J, Englund JA, et al. Impact of maternal vaccination timing and influenza virus circulation on birth outcomes in rural Nepal. Int J Gynaecol Obstet 2018;140:65–72. 10.1002/ijgo.12341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munoz FM, Jackson LA, Swamy GK, et al. Safety and immunogenicity of seasonal trivalent inactivated influenza vaccines in pregnant women. Vaccine 2018;36:8054–61. 10.1016/j.vaccine.2018.10.088 [DOI] [PubMed] [Google Scholar]
- 32.Murray AF, Englund JA, Kuypers J, et al. Infant pneumococcal carriage during influenza, RSV, and hMPV respiratory illness within a maternal influenza immunization trial. J Infect Dis 2019;220:956–60. 10.1093/infdis/jiz212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nunes MC, Cutland CL, Jones S, et al. Efficacy of maternal influenza vaccination against all-cause lower respiratory tract infection hospitalizations in young infants: results from a randomized controlled trial. Clin Infect Dis 2017;65:1066–71. 10.1093/cid/cix497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nunes MC, Cutland CL, Jones S, et al. Duration of infant protection against influenza illness conferred by maternal immunization: secondary analysis of a randomized clinical trial. JAMA Pediatr 2016;170:840–7. 10.1001/jamapediatrics.2016.0921 [DOI] [PubMed] [Google Scholar]
- 35.Simões EAF, Nunes MC, Carosone-Link P, et al. Trivalent influenza vaccination randomized control trial of pregnant women and adverse fetal outcomes. Vaccine 2019;37:5397–403. 10.1016/j.vaccine.2019.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinhoff MC, Katz J, Englund JA, et al. Year-round influenza immunisation during pregnancy in Nepal: a phase 4, randomised, placebo-controlled trial. Lancet Infect Dis 2017;17:981–9. 10.1016/S1473-3099(17)30252-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinhoff MC, Omer SB, Roy E, et al. Neonatal outcomes after influenza immunization during pregnancy: a randomized controlled trial. CMAJ 2012;184:645–53. 10.1503/cmaj.110754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tapia MD, Sow SO, Tamboura B, et al. Maternal immunisation with Trivalent inactivated influenza vaccine for prevention of influenza in infants in Mali: a prospective, active-controlled, observer-blind, randomised phase 4 trial. Lancet Infect Dis 2016;16:1026–35. 10.1016/S1473-3099(16)30054-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vesikari T, Virta M, Heinonen S, et al. Immunogenicity and safety of a quadrivalent inactivated influenza vaccine in pregnant women: a randomized, observer-blind trial. Hum Vaccin Immunother 2020;16:623–9. 10.1080/21645515.2019.1667202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008;359:1555–64. 10.1056/NEJMoa0708630 [DOI] [PubMed] [Google Scholar]
- 41.Cowling BJ, Perera RAPM, Fang VJ, et al. Maternal antibodies against influenza in cord blood and protection against laboratory-confirmed influenza in infants. Clin Infect Dis 2020;71:1741–8. 10.1093/cid/ciz1058 [DOI] [PubMed] [Google Scholar]
- 42.Eick AA, Uyeki TM, Klimov A, et al. Maternal influenza vaccination and effect on influenza virus infection in young infants. Arch Pediatr Adolesc Med 2011;165:104–11. 10.1001/archpediatrics.2010.192 [DOI] [PubMed] [Google Scholar]
- 43.France EK, Smith-Ray R, McClure D, et al. Impact of maternal influenza vaccination during pregnancy on the incidence of acute respiratory illness visits among infants. Arch Pediatr Adolesc Med 2006;160:1277–83. 10.1001/archpedi.160.12.1277 [DOI] [PubMed] [Google Scholar]
- 44.Hulka JF. Effectiveness of polyvalent influenza vaccine in pregnancy. Report of a controlled study during an outbreak of Asian influenza. Obstet Gynecol 1964;23:830–7. [PubMed] [Google Scholar]
- 45.Regan AK, de Klerk N, Moore HC, et al. Effect of maternal influenza vaccination on hospitalization for respiratory infections in newborns: a retrospective cohort study. Pediatr Infect Dis J 2016;35:1097–103. 10.1097/INF.0000000000001258 [DOI] [PubMed] [Google Scholar]
- 46.Regan AK, Klerk N de, Moore HC, et al. Effectiveness of seasonal Trivalent influenza vaccination against hospital-attended acute respiratory infections in pregnant women: a retrospective cohort study. Vaccine 2016;34:3649–56. 10.1016/j.vaccine.2016.05.032 [DOI] [PubMed] [Google Scholar]
- 47.Sugimura T, Nagai T, Kobayashi H, et al. Effectiveness of maternal influenza immunization in young infants in Japan: maternal influenza immunization. Pediatr Int 2016;58:709–13. 10.1111/ped.12888 [DOI] [PubMed] [Google Scholar]
- 48.Yamada T, Abe K, Baba Y, et al. Vaccination during the 2013–2014 influenza season in pregnant Japanese women. Eur J Clin Microbiol Infect Dis 2015;34:543–8. 10.1007/s10096-014-2259-8 [DOI] [PubMed] [Google Scholar]
- 49.Benowitz I, Esposito DB, Gracey KD, et al. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin Infect Dis 2010;51:1355–61. 10.1086/657309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mølgaard-Nielsen D, Fischer TK, Krause TG, et al. Effectiveness of maternal immunization with Trivalent Inactivated influenza vaccine in pregnant women and their infants. J Intern Med 2019;286:469–80. 10.1111/joim.12947 [DOI] [PubMed] [Google Scholar]
- 51.Poehling KA, Szilagyi PG, Staat MA, et al. Impact of maternal immunization on influenza hospitalizations in infants. Am J Obstet Gynecol 2011;204:S141–8. 10.1016/j.ajog.2011.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sukumaran L, McCarthy NL, Kharbanda EO, et al. Infant hospitalizations and mortality after maternal vaccination. Pediatrics 2018;141:e20173310. 10.1542/peds.2017-3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson MG, Kwong JC, Regan AK, et al. Influenza vaccine effectiveness in preventing influenza-associated hospitalizations during pregnancy: a multi-country retrospective test negative design study, 2010–2016. Clin Infect Dis 2019;68:1444–53. 10.1093/cid/ciy737 [DOI] [PubMed] [Google Scholar]
- 54.Thompson MG, Li D-K, Shifflett P, et al. Effectiveness of seasonal Trivalent influenza vaccine for preventing influenza virus illness among pregnant women: a population-based case-control study during the 2010–2011 and 2011–2012 influenza seasons. Clin Infect Dis 2014;58:449–57. 10.1093/cid/cit750 [DOI] [PubMed] [Google Scholar]
- 55.Adedinsewo DA, Noory L, Bednarczyk RA, et al. Impact of maternal characteristics on the effect of maternal influenza vaccination on fetal outcomes. Vaccine 2013;31:5827–33. 10.1016/j.vaccine.2013.09.071 [DOI] [PubMed] [Google Scholar]
- 56.Ludvigsson JF, Ström P, Lundholm C, et al. Maternal vaccination against H1N1 influenza and offspring mortality: population based cohort study and sibling design. BMJ 2015;351:h5585. 10.1136/bmj.h5585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahrens KA, Louik C, Kerr S, et al. Seasonal influenza vaccination during pregnancy and the risks of preterm delivery and small for gestational age birth: safety of influenza vaccination during pregnancy. Paediatr Perinat Epidemiol 2014;28:498–509. 10.1111/ppe.12152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arriola CS, Vasconez N, Thompson MG, et al. Association of influenza vaccination during pregnancy with birth outcomes in Nicaragua. Vaccine 2017;35:3056–63. 10.1016/j.vaccine.2017.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baum U, Leino T, Gissler M, et al. Perinatal survival and health after maternal influenza A(H1N1)Pdm09 vaccination: a cohort study of pregnancies stratified by trimester of vaccination. Vaccine 2015;33:4850–7. 10.1016/j.vaccine.2015.07.061 [DOI] [PubMed] [Google Scholar]
- 60.Beau AB, Hurault-Delarue C, Vidal S, et al. Pandemic A/H1N1 influenza vaccination during pregnancy: a comparative study using the EFEMERIS database. Vaccine 2014;32:1254–8. 10.1016/j.vaccine.2014.01.021 [DOI] [PubMed] [Google Scholar]
- 61.Black SB, Shinefield HR, France EK, et al. Effectiveness of influenza vaccine during pregnancy in preventing hospitalizations and outpatient visits for respiratory illness in pregnant women and their infants. Am J Perinatol 2004;21:333–9. 10.1055/s-2004-831888 [DOI] [PubMed] [Google Scholar]
- 62.Cantu J, Biggio J, Jauk V, et al. Selective uptake of influenza vaccine and pregnancy outcomes. J Matern Fetal Neonatal Med 2013;26:1207–11. 10.3109/14767058.2013.775419 [DOI] [PubMed] [Google Scholar]
- 63.Chambers CD, Johnson D, Xu R, et al. Risks and safety of pandemic H1N1 influenza vaccine in pregnancy: birth defects, spontaneous abortion, preterm delivery, and small for gestational age infants. Vaccine 2013;31:5026–32. 10.1016/j.vaccine.2013.08.097 [DOI] [PubMed] [Google Scholar]
- 64.Chambers CD, Johnson DL, Xu R, et al. Safety of the 2010-11, 2011-12, 2012-13, and 2013-14 seasonal influenza vaccines in pregnancy: birth defects, spontaneous abortion, preterm delivery, and small for gestational age infants, a study from the cohort arm of VAMPSS. Vaccine 2016;34:4443–9. 10.1016/j.vaccine.2016.06.054 [DOI] [PubMed] [Google Scholar]
- 65.Cleary BJ, Rice Ú, Eogan M, et al. A/H1N1 influenza vaccination in pregnancy: uptake and pregnancy outcomes – a historical cohort study. Eur J Obstet Gynecol Reprod Biol 2014;178:163–8. 10.1016/j.ejogrb.2014.04.015 [DOI] [PubMed] [Google Scholar]
- 66.Conlin AMS, Bukowinski AT, Sevick CJ, et al. Safety of the pandemic H1N1 influenza vaccine among pregnant U.S. military women and their newborns. Obstet Gynecol 2013;121:511–8. 10.1097/AOG.0b013e318280d64e [DOI] [PubMed] [Google Scholar]
- 67.Deinard AS, Ogburn P. A/NJ/8/76 influenza vaccination program: effects on maternal health and pregnancy outcome. Am J Obstet Gynecol 1981;140:240–5. 10.1016/0002-9378(81)90267-2 [DOI] [PubMed] [Google Scholar]
- 68.Dodds L, MacDonald N, Scott J, et al. The association between influenza vaccine in pregnancy and adverse neonatal outcomes. J Obstet Gynaecol Can 2012;34:714–20. 10.1016/S1701-2163(16)35336-1 [DOI] [PubMed] [Google Scholar]
- 69.Fabiani M, Bella A, Rota MC, et al. A/H1N1 pandemic influenza vaccination: a retrospective evaluation of adverse maternal, fetal and neonatal outcomes in a cohort of pregnant women in Italy. Vaccine 2015;33:2240–7. 10.1016/j.vaccine.2015.03.041 [DOI] [PubMed] [Google Scholar]
- 70.Fell DB, Sprague AE, Liu N, et al. H1N1 influenza vaccination during pregnancy and fetal and neonatal outcomes. Am J Public Health 2012;102:e33–40. 10.2105/AJPH.2011.300606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Getahun D, Fassett MJ, Peltier MR, et al. Association between seasonal influenza vaccination with pre- and postnatal outcomes. Vaccine 2019;37:1785–91. 10.1016/j.vaccine.2019.02.019 [DOI] [PubMed] [Google Scholar]
- 72.Håberg SE, Trogstad L, Gunnes N, et al. Risk of fetal death after pandemic influenza virus infection or vaccination. N Engl J Med 2013;368:333–40. 10.1056/NEJMoa1207210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heikkinen T, Young J, van Beek E, et al. Safety of MF59-Adjuvanted A/H1N1 influenza vaccine in pregnancy: a comparative cohort study. Am J Obstet Gynecol 2012;207:177. 10.1016/j.ajog.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 74.Källén B, Olausson PO. Vaccination against H1N1 influenza with Pandemrix® during pregnancy and delivery outcome: a Swedish register study: H1N1 influenza vaccination during pregnancy. BJOG 2012;119:1583–90. 10.1111/j.1471-0528.2012.03470.x [DOI] [PubMed] [Google Scholar]
- 75.Kharbanda EO, Vazquez-Benitez G, Romitti PA, et al. First trimester influenza vaccination and risks for major structural birth defects in offspring. J Pediatr 2017;187:234–9. 10.1016/j.jpeds.2017.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Launay O, Krivine A, Charlier C, et al. Low rate of pandemic A/H1N1 2009 influenza infection and lack of severe complication of vaccination in pregnant women: a prospective cohort study. PLoS ONE 2012;7:e52303. 10.1371/journal.pone.0052303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Legge A, Dodds L, MacDonald NE, et al. Rates and determinants of seasonal influenza vaccination in pregnancy and association with neonatal outcomes. CMAJ 2014;186:E157–64. 10.1503/cmaj.130499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin T-H, Lin S-Y, Lin C-H, et al. AdimFlu-S® influenza A (H1N1) vaccine during pregnancy: the Taiwanese pharmacovigilance survey. Vaccine 2012;30:2671–5. 10.1016/j.vaccine.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 79.Ludvigsson JF, Ström P, Lundholm C, et al. Risk for congenital malformation with H1N1 influenza vaccine: a cohort study with sibling analysis. Ann Intern Med 2016;165:848–55. 10.7326/M16-0139 [DOI] [PubMed] [Google Scholar]
- 80.Ludvigsson JF, Zugna D, Cnattingius S, et al. Influenza H1N1 vaccination and adverse pregnancy outcome. Eur J Epidemiol 2013;28:579–88. 10.1007/s10654-013-9813-z [DOI] [PubMed] [Google Scholar]
- 81.Ma F, Zhang L, Jiang R, et al. Prospective cohort study of the safety of an influenza A(H1N1) vaccine in pregnant Chinese women. Clin Vaccine Immunol 2014;21:1282–7. 10.1128/CVI.00375-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mackenzie IS, MacDonald TM, Shakir S, et al. Influenza H1N1 (swine flu) vaccination: a safety surveillance feasibility study using self-reporting of serious adverse events and pregnancy outcomes: Scottish safety swine flu study. Br J Clin Pharmacol 2012;73:801–11. 10.1111/j.1365-2125.2011.04142.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McHugh L, Andrews RM, Ware RS. Birth outcomes for Australian mother-infant pairs who received an influenza vaccine during pregnancy 2012-2014: the Flumum study. Vaccine 2017;35:4492–3. 10.1016/j.vaccine.2017.06.008 [DOI] [PubMed] [Google Scholar]
- 84.McHugh L, Binks MJ, Gao Y, et al. Influenza vaccination in pregnancy among a group of remote dwelling aboriginal and Torres Strait Islander mothers in the Northern territory: the 1+1 healthy start to life study. Commun Dis Intell 2019;43. 10.33321/cdi.2019.43.33 [DOI] [PubMed] [Google Scholar]
- 85.McHugh L, Marshall HS, Perrett KP, et al. The safety of influenza and pertussis vaccination in pregnancy in a cohort of Australian mother-infant pairs, 2012–2015: the Flumum study. Clin Infect Dis 2019;68:402–8. 10.1093/cid/ciy517 [DOI] [PubMed] [Google Scholar]
- 86.McMorrow ML, Rossi L, Meiring S, et al. A retrospective observational cohort study of the effect of antenatal influenza vaccination on birth outcomes in Cape town, South Africa, 2015-2016. Influenza Other Respir Viruses 2021;15:446–56. 10.1111/irv.12836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mohammed H, Roberts CT, Grzeskowiak LE, et al. Safety and protective effects of maternal influenza vaccination on pregnancy and birth outcomes: a prospective cohort study. EClinicalMedicine 2020;26:100522. 10.1016/j.eclinm.2020.100522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Munoz FM, Greisinger AJ, Wehmanen OA, et al. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol 2005;192:1098–106. 10.1016/j.ajog.2004.12.019 [DOI] [PubMed] [Google Scholar]
- 89.Nordin JD, Kharbanda EO, Benitez GV, et al. Maternal safety of Trivalent Inactivated influenza vaccine in pregnant women. Obstet Gynecol 2013;121:519–25. 10.1097/AOG.0b013e3182831b83 [DOI] [PubMed] [Google Scholar]
- 90.Nordin JD, Kharbanda EO, Vazquez Benitez G, et al. Maternal influenza vaccine and risks for preterm or small for gestational age birth. J Pediatr 2014;164:1051–7. 10.1016/j.jpeds.2014.01.037 [DOI] [PubMed] [Google Scholar]
- 91.Ohfuji S, Deguchi M, Tachibana D, et al. Safety of influenza vaccination on adverse birth outcomes among pregnant women: a prospective cohort study in Japan. Int J Infect Dis 2020;93:68–76. 10.1016/j.ijid.2020.01.033 [DOI] [PubMed] [Google Scholar]
- 92.Ohfuji S, Deguchi M, Tachibana D, et al. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018;217:878–86. 10.1093/infdis/jix629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Olsen SJ, Mirza SA, Vonglokham P, et al. The effect of influenza vaccination on birth outcomes in a cohort of pregnant women in Lao PDR, 2014–2015. Clin Infect Dis 2016;63:487–94. 10.1093/cid/ciw290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Omer SB, Goodman D, Steinhoff MC, et al. Maternal influenza immunization and reduced likelihood of prematurity and small for gestational age births: a retrospective cohort study. PLoS Med 2011;8:e1000441. 10.1371/journal.pmed.1000441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pasternak B, Svanström H, Mølgaard-Nielsen D, et al. Vaccination against pandemic A/H1N1 2009 influenza in pregnancy and risk of fetal death: cohort study in Denmark. BMJ 2012;344:e2794. 10.1136/bmj.e2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pasternak B, Svanström H, Mølgaard-Nielsen D, et al. Risk of adverse fetal outcomes following administration of a pandemic influenza A(H1N1) vaccine during pregnancy. JAMA 2012;308:165–74. 10.1001/jama.2012.6131 [DOI] [PubMed] [Google Scholar]
- 97.Regan AK, Moore HC, de Klerk N, et al. Seasonal Trivalent influenza vaccination during pregnancy and the incidence of stillbirth: population-based retrospective cohort study. Clin Infect Dis 2016;62:1221–7. 10.1093/cid/ciw082 [DOI] [PubMed] [Google Scholar]
- 98.Richards JL, Hansen C, Bredfeldt C, et al. Neonatal outcomes after antenatal influenza immunization during the 2009 H1N1 influenza pandemic: impact on preterm birth, birth weight, and small for gestational age birth. Clin Infect Dis 2013;56:1216–22. 10.1093/cid/cit045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rubinstein F, Micone P, Bonotti A, et al. Influenza A/H1N1 MF59 adjuvanted vaccine in pregnant women and adverse perinatal outcomes: multicentre study. BMJ 2013;346:f393. 10.1136/bmj.f393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sammon CJ, Snowball J, McGrogan A, et al. Evaluating the hazard of foetal death following H1N1 influenza vaccination; a population based cohort study in the UK GPRD. PLoS One 2012;7:e51734. 10.1371/journal.pone.0051734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shakib JH, Korgenski K, Presson AP, et al. Influenza in infants born to women vaccinated during pregnancy. Pediatrics 2016;137:e20152360. 10.1542/peds.2015-2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sheffield JS, Greer LG, Rogers VL, et al. Effect of influenza vaccination in the first trimester of pregnancy. Obstet Gynecol 2012;120:532–7. 10.1097/AOG.0b013e318263a278 [DOI] [PubMed] [Google Scholar]
- 103.Singh M, Tanvir T, Nagoji D, et al. Influenza vaccine: a viable option to protect pregnant women and infants from seasonal flu: a retrospective hospital‐based study in India. Int J Clin Pract 2019;73:e13361. 10.1111/ijcp.13361 [DOI] [PubMed] [Google Scholar]
- 104.Speake HA, Pereira G, Regan AK. Risk of adverse maternal and foetal outcomes associated with inactivated influenza vaccination in first trimester of pregnancy. Paediatr Perinat Epidemiol 2021;35:196–205. 10.1111/ppe.12715 [DOI] [PubMed] [Google Scholar]
- 105.Trotta F, Da Cas R, Spila Alegiani S, et al. Evaluation of safety of A/H1N1 pandemic vaccination during pregnancy: cohort study. BMJ 2014;348:g3361. 10.1136/bmj.g3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van der Maas N, Dijs-Elsinga J, Kemmeren J, et al. Safety of vaccination against influenza A (H1N1) during pregnancy in the Netherlands: results on pregnancy outcomes and infant’s health: cross-sectional linkage study. BJOG: Int J Obstet Gy 2016;123:709–17. 10.1111/1471-0528.13329 [DOI] [PubMed] [Google Scholar]
- 107.Yamada T, Yamada T, Morikawa M, et al. Pandemic (H1N1) 2009 in pregnant Japanese women in Hokkaido: pandemic (H1N1) 2009 in Japan. J Obstet Gynaecol Res 2012;38:130–6. 10.1111/j.1447-0756.2011.01644.x [DOI] [PubMed] [Google Scholar]
- 108.Zerbo O, Modaressi S, Chan B, et al. No association between influenza vaccination during pregnancy and adverse birth outcomes. Vaccine 2017;35:3186–90. 10.1016/j.vaccine.2017.04.074 [DOI] [PubMed] [Google Scholar]
- 109.Coenders A, Koopmans NK, Broekhuijsen K, et al. Adjuvanted vaccines in pregnancy: no evidence for effect of the adjuvanted H1N1/09 vaccination on occurrence of preeclampsia or intra-uterine growth restriction. Eur J Obstet Gynecol Reprod Biol 2015;187:14–9. 10.1016/j.ejogrb.2015.01.011 [DOI] [PubMed] [Google Scholar]
- 110.Donahue JG, Kieke BA, King JP, et al. Association of spontaneous abortion with receipt of Inactivated influenza vaccine containing H1N1Pdm09 in 2010-11 and 2011-12. Vaccine 2017;35:5314–22. 10.1016/j.vaccine.2017.06.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Donahue JG, Kieke BA, King JP, et al. Inactivated influenza vaccine and spontaneous abortion in the vaccine safety datalink in 2012-13, 2013-14, and 2014-15. Vaccine 2019;37:6673–81. 10.1016/j.vaccine.2019.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huang W-T, Tang F-W, Yang S-E, et al. Safety of inactivated monovalent pandemic (H1N1) 2009 vaccination during pregnancy: a population-based study in Taiwan. Vaccine 2014;32:6463–8. 10.1016/j.vaccine.2014.09.054 [DOI] [PubMed] [Google Scholar]
- 113.Irving SA, Kieke BA, Donahue JG, et al. Trivalent inactivated influenza vaccine and spontaneous abortion. Obstet Gynecol 2013;121:159–65. 10.1097/aog.0b013e318279f56f [DOI] [PubMed] [Google Scholar]
- 114.Louik C, Ahrens K, Kerr S, et al. Risks and safety of pandemic H1N1 influenza vaccine in pregnancy: exposure prevalence, preterm delivery, and specific birth defects. Vaccine 2013;31:5033–40. 10.1016/j.vaccine.2013.08.096 [DOI] [PubMed] [Google Scholar]
- 115.Louik C, Kerr S, Van Bennekom CM, et al. Safety of the 2011-12, 2012-13, and 2013-14 seasonal influenza vaccines in pregnancy: preterm delivery and specific malformations, a study from the case-control arm of VAMPSS. Vaccine 2016;34:4450–9. 10.1016/j.vaccine.2016.06.078 [DOI] [PubMed] [Google Scholar]
- 116.Panagiotakopoulos L, McCarthy NL, Tepper NK, et al. Evaluating the association of stillbirths after maternal vaccination in the vaccine safety datalink. Obstet Gynecol 2020;136:1086–94. 10.1097/AOG.0000000000004166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yadav K, Lewis RJ. Immortal time bias in observational studies. JAMA 2021;325:686–7. 10.1001/jama.2020.9151 [DOI] [PubMed] [Google Scholar]
- 118.Fell DB, Dimitris MC, Hutcheon JA, et al. Guidance for design and analysis of observational studies of fetal and newborn outcomes following COVID-19 vaccination during pregnancy. Vaccine 2021;39:1882–6. 10.1016/j.vaccine.2021.02.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nunes MC, Cutland CL, Moultrie A, et al. Immunogenicity and safety of different dosing schedules of Trivalent Inactivated influenza vaccine in pregnant women with HIV: a randomised controlled trial. Lancet HIV 2020;7:e91–103. 10.1016/S2352-3018(19)30322-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Regan AK, Tracey L, Blyth CC, et al. A prospective cohort study comparing the reactogenicity of Trivalent influenza vaccine in pregnant and non-pregnant women. BMC Pregnancy Childbirth 2015;15:61. 10.1186/s12884-015-0495-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Regan AK, Tracey LE, Blyth CC, et al. A prospective cohort study assessing the reactogenicity of pertussis and influenza vaccines administered during pregnancy. Vaccine 2016;34:2299–304. 10.1016/j.vaccine.2016.03.084 [DOI] [PubMed] [Google Scholar]
- 122.Glover C, Crawford N, Leeb A, et al. Active SMS-based surveillance of adverse events following Immunisation with influenza and pertussis-containing vaccines in Australian pregnant women using Ausvaxsafety. Vaccine 2020;38:4892–900. 10.1016/j.vaccine.2020.04.056 [DOI] [PubMed] [Google Scholar]
- 123.Regan AK, Blyth CC, Effler PV. Using SMS technology to verify the safety of seasonal Trivalent influenza vaccine for pregnant women in real time. Med J Aust 2013;199:744–6. 10.5694/mja13.10712 [DOI] [PubMed] [Google Scholar]
- 124.Bonhoeffer J, Kochhar S, Hirschfeld S, et al. Global alignment of immunization safety assessment in pregnancy – the GAIA project. Vaccine 2016;34:5993–7. 10.1016/j.vaccine.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 125.McGowan J, Sampson M, Salzwedel DM, et al. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 2016;75:40–6. 10.1016/j.jclinepi.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 126.Canadian Agency of Drugs and Technologies in Health (CADTH) . Grey matters: a practical tool for searching health-related grey literature. 2019. Available: https://www.cadth.ca/resources/finding-evidence/grey-matters [Accessed 04 Dec 2020].
- 127.The World Bank Group . The world by income and region 2022. 2022. Available: https://datatopics.worldbank.org/world-development-indicators/the-world-by-income-and-region.html [Accessed 05 Apr 2022].
- 128.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. 2000. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Accessed 07 Dec 2020].
- 129.Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 2020;368:l6890. 10.1136/bmj.l6890 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-066182supp001.pdf (10.2MB, pdf)
bmjopen-2022-066182supp002.xlsx (126.4KB, xlsx)
bmjopen-2022-066182supp003.xlsx (583.8KB, xlsx)
bmjopen-2022-066182supp004.xlsx (144.5KB, xlsx)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information. All relevant data are provided within the manuscript and its supporting files. Our supplemental files include additional detail regarding the methods and findings of our review, and we also have provided spreadsheets containing all of our gathered data.