Abstract
Objectives
Evidence on the association between fasting blood glucose and mortality in non-diabetic patients who had a stroke is limited. We aimed to investigate the association of baseline fasting plasma glucose (FPG) with 1 year all-cause mortality in non-diabetic patients with acute cerebral infarction (ACI).
Design
A multicentre prospective cohort study.
Setting
Four grade A tertiary hospitals in the Xi'an district of China.
Participants
A total of 1496 non-diabetic patients within 7 days of ACI were included.
Main outcome measures
The outcome was 1 year all-cause mortality. Baseline FPG was analysed as a continuous variable and was divided into four quartiles (group Q1–group Q4). We used multivariable Cox regression analyses, curve fitting and Kaplan–Meier (K-M) analyses to explore the association of baseline FPG with 1 year all-cause mortality in non-diabetic patients with ACI.
Results
After controlling for confounders, multivariable Cox regression analyses indicated a 17% increase in 1 year all-cause mortality for every 1 mmol/L of baseline FPG increase (HR=1.17, 95% CI 1.02 to 1.35, p=0.030). Patients from the Q4 group had 2.08 times increased hazard of 1 year all-cause mortality compared with the Q1 group (HR=2.08, 95% CI 1.13 to 3.82, p=0.019), while the survival rate of patients in group Q4 was decreased compared with that in other groups (p<0.001). The curve fitting revealed a positive but non-linear association of baseline FPG with 1-year all-cause mortality in non-diabetic patients with ACI.
Conclusion
In non-diabetic patients with ACI, elevated baseline FPG is an independent risk factor for 1-year all-cause mortality, and the two are positively and non-linearly associated. These results suggest that high FPG should be seen as a concern in non-diabetic patients with ACI.
Keywords: Stroke, General endocrinology, Adult neurology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This was a multicentre, prospective study conducted only with non-diabetic patients with acute cerebral infarction.
The analysis method is comprehensive and effective in controlling relevant confounding factors.
The study had a large sample size and few were lost to follow-up.
The data included were all from grade A tertiary hospitals and may not represent data from smaller hospitals.
Introduction
Stroke is the second most prevalent cause of death worldwide. In the ranking of causes of death and disability, stroke ranks third in the world and first in China.1–3 Acute cerebral infarction (ACI) accounts for over 70% of all strokes.4 Therefore, it is necessary to identify and manage risk factors for mortality from stroke as early as possible.
Previous studies have demonstrated that people with diabetes have an increased risk of mortality from stroke.5 6 In China, a third of acute ischaemic stroke cases are thought to be due to diabetes mellitus or possible diabetes, with most patients having no known history of diabetes.5 However, the association of blood glucose with mortality in patients without diabetes who had a stroke has rarely been investigated. Many patients with acute stroke may present with hyperglycaemia, even without prediagnosed diabetes.7 In non-diabetic patients who had an acute stroke, elevated fasting blood glucose level is generally considered stress hyperglycaemia, defined as the spontaneous resolution of hyperglycaemia after the dissipation of acute illness.8 Previous studies have shown that stress hyperglycaemia with myocardial infarction and primary intracerebral haemorrhage is associated with higher mortality rates in non-diabetic patients.9 10 High blood glucose is an independent sign of hospitalised death rate in patients without diabetes.11 These findings suggest that differences in baseline blood glucose levels affect the association of blood glucose with mortality in hospitalised patients. Hence, it is of clinical value to study the association between baseline fasting plasma glucose (FPG) and mortality in non-diabetic patients with ACI.
However, the association of FPG with mortality in non-diabetic patients with ACI has rarely been explored. The results may also vary depending on country and region owing to different eating habits, lifestyles and economic levels. Therefore, we analysed the baseline FPG and relevant clinical information of non-diabetic patients with ACI in the Xi'an district of China, to explore the association of baseline FPG with 1 year all-cause mortality after the onset of ACI.
Methods
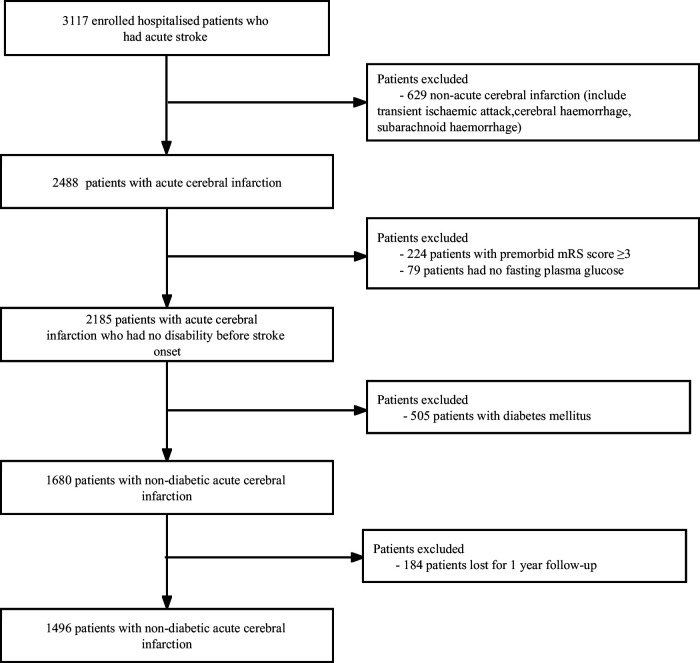
Study population
From January to December 2015, 3117 patients with acute stroke were continuously treated in four grade A tertiary hospitals in Xi'an. Excluding 629 patients with non-ACI, 224 patients with modified Rankin Scale scores ≥3 before onset, 79 patients without FPG, 505 patients with diabetes, and 184 patients lost to follow-up; 1496 non-diabetic patients with ACI were finally included. The detailed screening process of the study population is shown in figure 1. In this study, the inclusion criteria were as follows: patients with a clinical diagnosis of non-diabetic ACI, whose imaging diagnosis met the diagnostic criteria of the guideline from the American Heart Association/American Stroke Association and was verified by brain CT or cranial MRI;12 aged 18–97 years; onset time ≤7 days. All participants signed informed consent forms. Exclusion criteria were as follows: patients with non-ACI (including cerebral haemorrhage, subarachnoid haemorrhage and transient ischaemic attack); patients with diabetes mellitus (including diagnosis of diabetes at admission and discharge); and presence of non-cerebrovascular diseases associated with neurological declines, such as brain trauma and subdural haemorrhage.
Figure 1.
Flow chart of study population screening. mRS, Modified Rankin Scale.
Data collection
The data were acquired from a multicentre observational cohort study of the Xi'an Stroke Registry Study.13 Relevant clinical data of the patients were collected, including baseline data (age, sex, medical insurance and education level), lifestyle (smoking and alcohol consumption), medical history (atrial fibrillation, hypertension and prior stroke), admission National Institutes of Health Stroke Scale (NIHSS) Score, admission heart rate, admission systolic blood pressure, admission diastolic blood pressure and concomitant pneumonia. Laboratory-related tests (triglyceride, total serum cholesterol, high-density lipoprotein, low-density lipoprotein, FPG, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, serum creatinine, blood urea nitrogen, blood uric acid and blood leucocyte count) were performed on the second day after an overnight fast on the first day of admission. All-cause mortality outcome events were collected at 3-month and 1-year follow-ups after enrolment. Smoking was defined as smoking at least one cigarette daily before stroke onset for 6 months or more; smoking cessation was defined as having previously met the definition of smoking but not smoking for consecutive 6 months before the stroke. Alcohol consumption was defined as having at least one drink per week (a standard drink equaled 120 mL of wine, 360 mL of beer or 45 mL of liquor). Pneumonia was defined as emerging pneumonia within 7 days of onset in a patient who had a stroke with non-mechanical ventilation. Pneumonia was diagnosed based on a typical chest X-ray; clinical symptoms such as a cough, purulent sputum, fever; and laboratory tests such as leucocyte count.14 Other relevant definitions were similar to those of the Chinese Intracranial Atherosclerosis Study.15
Grouping and data comparison
The baseline FPG was divided into four low to high groups: Q1 group, <4.52 mmol/L; Q2 group, 4.52–4.98 mmol/L; Q3 group, 4.99–5.55 mmol/L; and Q4 group, >5.55 mmol/L.
Statistical methods
Free Statistics software (V.1.7) and the statistical software package R (V.3.3.2) were used for data analysis. Measured data that conform to normal distribution are denoted as mean±SD, and analysis of variance was used for comparison among groups. Non-normally distributed variables are presented as median (IQR). The Kruskal–Wallis rank-sum test was adopted for multiple group comparisons. Multivariable Cox proportional hazard models were used to analyze the association of baseline FPG with 1-year mortality, and adjustment of confounding factors was determined according to whether the variable had >10% effect on the outcome effect value HR or the clinical significance of the variable’s impact on the outcome.16 17 Curve fitting was used to analyse the association of baseline FPG with 1-year mortality; the Kaplan–Meier method was used to analyse the survival rate between baseline FPG groups and 1-year mortality, and the log-rank test was used to assess the difference in 1-year survival rate among FPG quartiles. A two-tailed value of p<0.05 was considered statistically significant.
Results
Baseline characteristics
After the 1-year follow-up, 1496 patients remained in the study while 184 were lost to follow-up. The clinical features of the two groups were compared, and we found statistically significant differences in medical insurance type and education level but not in other features (online supplemental table 1). Moreover, the results showed that the predominant patient characteristic variables of the two groups were comparable. The analysed patients were well representative of the enrolled population.
bmjopen-2022-069716supp001.pdf (53.6KB, pdf)
Among the 1496 patients included in the study, 956 were men and 540 women; their mean age was 64.0 years. The mean baseline FPG level was 5.2±1.0 mmol/L. The baseline demographic, clinical and biochemical characteristics of the baseline FPG level quartile (Q1–Q4) groups were compared (table 1). There were significant differences in sex distribution, alcohol consumption, NIHSS Score on admission, body mass index, heart rate, total cholesterol, triglyceride, low-density lipoprotein, FPG, alanine aminotransferase, aspartate aminotransferase and leucocyte count among different baseline FPG levels (p<0.05). The 1-year mortality differed significantly among the groups (p<0.001). Baseline FPG levels among quartiles showed no statistically significant differences in age; medical insurance type; education level; smoking; prior stroke; hypertension; atrial fibrillation; pneumonia; systolic blood pressure at admission; diastolic blood pressure at admission; and levels of high-density lipoprotein, alkaline phosphatase, serum creatinine, blood urea nitrogen or uric acid.
Table 1.
Baseline data and clinical characteristics by baseline FPG quartiles in non-diabetic patients with ACI
| Variables | Overall n=1496 | Baseline FPG quartiles | P value | |||
| Q1 (n=369) |
Q2 (n=377) |
Q3 (n=372) |
Q4 (n=378) |
|||
| Age (years) | 64.0 (55.0,74.0) | 65.0 (54.0,74.0) | 65.0 (56.0,75.0) | 63.5 (55.0,73.0) | 65.0 (56.0,75.0) | 0.266 |
| Sex, n (%) | 0.026 | |||||
| Male | 956 (63.9) | 260 (70.5) | 233 (61.8) | 232 (62.4) | 231 (61.1) | |
| Female | 540 (36.1) | 109 (29.5) | 144 (38.2) | 140 (37.6) | 147 (38.9) | |
| Medical insurance type, n (%) | 0.259 | |||||
| Urban employees’ medical insurance | 663 (44.3) | 148 (40.1) | 164 (43.5) | 177 (47.6) | 174 (46) | |
| New type rural cooperative medical system | 637 (42.6) | 175 (47.4) | 168 (44.6) | 146 (39.2) | 148 (39.2) | |
| Commercial insurance | 4 (0.3) | 2 (0.5) | 1 (0.3) | 0 (0) | 1 (0.3) | |
| Out-of-pocket medical | 192 (12.8) | 44 (11.9) | 44 (11.7) | 49 (13.2) | 55 (14.6) | |
| Education level, n (%) | 0.737 | |||||
| Elementary or below | 736 (49.2) | 193 (52.3) | 190 (50.4) | 178 (47.8) | 175 (46.3) | |
| Middle school | 286 (19.1) | 69 (18.7) | 69 (18.3) | 74 (19.9) | 74 (19.6) | |
| High school or above | 474 (31.7) | 107 (29) | 118 (31.3) | 120 (32.3) | 129 (34.1) | |
| Smoking, n (%) | 0.539 | |||||
| Never smoking | 814 (54.4) | 186 (50.4) | 203 (53.8) | 208 (55.9) | 217 (57.4) | |
| Smoking cessation | 299 (20.0) | 83 (22.5) | 76 (20.2) | 67 (18) | 73 (19.3) | |
| Current smoking | 383 (25.6) | 100 (27.1) | 98 (26) | 97 (26.1) | 88 (23.3) | |
| Alcohol consumption, n (%) | 379 (25.3) | 100 (27.1) | 78 (20.7) | 109 (29.3) | 92 (24.3) | 0.042 |
| Prior stroke, n (%) | 395 (26.4) | 87 (23.6) | 91 (24.1) | 109 (29.3) | 108 (28.6) | 0.169 |
| Hypertension, n (%) | 1013 (67.7) | 241 (65.3) | 249 (66). | 254 (68.3) | 269 (71.2) | 0.312 |
| Atrial fibrillation, n (%) | 107 (7.2) | 21 (5.7) | 28 (7.4) | 21 (5.6) | 37 (9.8) | 0.091 |
| Admission NIHSS Score, (IQR) | 4.0 (2.0, 6.0) | 3.0 (2.0, 6.0) | 3.0 (2.0, 5.0) | 4.0 (2.0, 5.0) | 4.0 (2.0, 7.0) | < 0.001 |
| Pneumonia, n (%) | 0.134 | |||||
| No | 1428 (95.5) | 354 (95.9) | 365 (96.8) | 356 (95.7) | 353 (93.4) | |
| Yes | 68 (4.5) | 15 (4.1) | 12 (3.2) | 16 (4.3) | 25 (6.6) | |
| BMI (kg/m2) | 23.6±3.1 | 23.1±3.0 | 23.4±3.1 | 23.7±3.0 | 24.2±3.1 | < 0.001 |
| SBP on admission (mm Hg) | 145.0±21.6 | 143.7±21.5 | 145.5±21.5 | 145.7±21.6 | 145.3±21.9 | 0.579 |
| DBP on admission (mm Hg) | 85.8±12.6 | 85.9±13.0 | 86.3±12.5 | 86.0±12.3 | 85.1±12.5 | 0.639 |
| Heart rate (beats/min) | 74.4±10.2 | 73.2±9.5 | 74.3±10.6 | 74.6±10.0 | 75.7±10.5 | 0.01 |
| Total cholesterol (mmol/L) | 4.3±1.0 | 4.2±1.0 | 4.2±0.9 | 4.4±1.0 | 4.5±1.2 | < 0.001 |
| Triglycerides (mmol/L) | 1.6±1.4 | 1.5±1.0 | 1.5±1.0 | 1.6±1.3 | 1.8±2.0 | 0.002 |
| HDL cholesterol (mmol/L) | 1.1±0.3 | 1.1±0.3 | 1.1±0.3 | 1.1±0.3 | 1.2±0.3 | 0.855 |
| LDL cholesterol (mmol/L) | 2.6±0.8 | 2.4±0.8 | 2.5±0.7 | 2.6±0.8 | 2.7±0.9 | < 0.001 |
| FPG (mmol/L) | 5.2±1.0 | 4.2±0.3 | 4.7±0.1 | 5.2±0.2 | 6.5±1.1 | < 0.001 |
| Alanine aminotransferase (U/L) | 19.0 (14.0, 28.0) | 17.9 (13.0, 26.0) | 18.0 (13.0, 25.0) | 20.0 (14.0, 29.0) | 21.0 (15.0, 31.6) | < 0.001 |
| Aspartate aminotransferase (U/L) | 22.0 (18.0, 28.0) | 21.0 (17.0, 26.6) | 21.0 (17.0, 26.0) | 22.0 (18.0, 29.0) | 23.0 (18.1, 31.2) | < 0.001 |
| Alkaline phosphatase (U/L) | 79.3±36.3 | 76.7±26.3 | 78.2±26.4 | 82.3±51.9 | 80.1±34.2 | 0.191 |
| Serum creatinine (µmol/L) | 74.6±28.6 | 75.4±24.4 | 74.8±35.5 | 74.0±29.1 | 74.1±24.0 | 0.91 |
| Blood urea nitrogen (mmol/L) | 5.1±1.9 | 5.1±2.1 | 5.0±1.6 | 5.0±2.0 | 5.1±1.9 | 0.886 |
| Uric acid (µmol/L) | 291.4±96.8 | 294.6±89.4 | 293.3±96.2 | 288.2±95.6 | 289.6±105.5 | 0.791 |
| Leucocyte count (×109 /L) | 6.8±2.5 | 6.5±2.1 | 6.4±1.9 | 6.8±2.7 | 7.6±2.8 | < 0.001 |
| 1 year mortality, n (%) | 102 (6.8) | 18 (4.9) | 21 (5.6) | 20 (5.4) | 43 (11.4) | < 0.001 |
ACI, acute cerebral infarction; BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NIHSS, National Institutes of Health Stroke Scale; SBP, systolic blood pressure.
Multivariable Cox regression analyses of the association of baseline FPG with all-cause mortality in non-diabetic patients with ACI
On considering FPG as a continuous variable, multivariable Cox regression analyses after adjustment for confounders showed a 27% increase in 3-month mortality (HR=1.27, 95% CI 1.02 to 1.58, p=0.030) and a 17% increase in 1-year mortality (HR=1.17, 95% CI 1.02 to 1.35, p=0.030) for every 1 mmol/L of increase in baseline FPG (table 2). After dividing baseline FPG into four quartile groups, group Q1 was taken as the reference. After adjustment for confounder, multivariable Cox regression analyses demonstrated nearly significant increase in the 3-month all-cause mortality in the Q4 group compared with the Q1 group (HR=2.43, 95% CI 0.98 to 6.04, p=0.056). Patients from the Q4 group had 2.08 times increased risk of 1-year all-cause mortality compared with the Q1 group (HR=2.08, 95% CI 1.13 to 3.82, p=0.019). There was no statistically significant difference in the risk of 3-month mortality and 1-year mortality between groups Q2 and Q3, respectively, compared with that in group Q1. In univariate and multivariable Cox regression analyses, the trend of death from the Q1 to Q4 groups had a significant difference at 3 months (p=0.006 and p=0.012 for trend tests) and 1 year (p=0.001 and p=0.005 for trend tests), as shown by trend tests (table 2).
Table 2.
Multivariable Cox regression analysis of the association between baseline FPG and all-cause mortality in non-diabetic patients with ACI
| Outcomes | N | Events, N (%) |
Unadjusted | Adjusted | ||
| HR (95% CI) |
P value | HR (95% CI) |
P value | |||
| All-cause mortality at 3 months | ||||||
| Baseline FPG (every increase of 1 mmol/L) | 1546 | 50 (3.2) | 1.35 (1.13 to 1.62) | 0.001 | 1.27 (1.02 to 1.58) | 0.03 |
| Q1 (<4.52) | 377 | 8 (2.1) | Reference | Reference | ||
| Q2 (4.52–4.98) | 385 | 8 (2.1) | 0.98 (0.37 to 2.6) | 0.963 | 0.94 (0.33 to 2.66) | 0.911 |
| Q3 (4.99–5.55) | 385 | 13 (3.4) | 1.63 (0.68 to 3.93) | 0.278 | 2.15 (0.84 to 5.52) | 0.111 |
| Q4 (>5.55) | 399 | 21 (5.3) | 2.61 (1.16 to 5.89) | 0.021 | 2.43 (0.98 to 6.04) | 0.056 |
| P for trend | 0.006 | 0.012 | ||||
| All-cause mortality at 12 months | ||||||
| Baseline FPG (every increase of 1 mmol/L) | 1496 | 102 (6.8) | 1.32 (1.16 to 1.50) | <0.001 | 1.17 (1.02 to 1.35) | 0.03 |
| Q1 (<4.52) | 369 | 18 (4.9) | Reference | Reference | ||
| Q2 (4.52–4.98) | 377 | 21 (5.6) | 1.14 (0.61 to 2.14) | 0.684 | 1.04 (0.53 to 2.03) | 0.913 |
| Q3 (4.99–5.55) | 372 | 20 (5.4) | 1.11 (0.59 to 2.1) | 0.745 | 1.37 (0.7 to 2.68) | 0.36 |
| Q4 (>5.55) | 378 | 43 (11.4) | 2.42 (1.39 to 4.19) | 0.002 | 2.08 (1.13 to 3.82) | 0.019 |
| P for trend | 0.001 | 0.005 | ||||
Adjusted variables included age, sex, smoking, alcohol consumption, NIHSS Score at admission, pneumonia, prior stroke, hypertension, atrial fibrillation, alkaline phosphatase, serum creatinine and body mass index;.
ACI, acute cerebral infarction; FPG, fasting plasma glucose; NIHSS, National Institutes of Health Stroke Scale.
Kaplan–Meier curve analyses and fitted curve analysis of the association of baseline FPG with 1-year mortality
Kaplan–Meier curve analysis revealed that the 1-year survival rate in Q4 was significantly lower than that in other groups (95.1% in Q1, 94.4% in Q2, 94.6% in Q3 and 88.6% in Q4, p<0.001) (figure 2). After controlling for potential confounders, fitted curve analysis revealed that baseline FPG had a positive but non-linear association with 1-year mortality in non-diabetic patients with ACI. The 1-year mortality significantly increased with the baseline FPG (figure 3). Consistent with the results of Cox regression analyses, especially in the fourth group, the risk of 1-year mortality increased significantly.
Figure 2.
Kaplan–Meier curve analyses are used to analyse the association of baseline FPG with 1-year mortality in non-diabetic patients with acute cerebral infarction (ACI). Q1 group, <4.52 mmol/L; Q2 group, 4.52–4.98 mmol/L; Q3 group, 4.99–5.55 mmol/L; and Q4 group, >5.55 mmol/L. FPG, fasting plasma glucose.
Figure 3.
The fitted curve shows a positive but non-linear association of baseline FPG with 1-year all-cause mortality in non-diabetic patients with acute cerebral infarction (ACI). The solid line represents the adjusted HR, the dashed line represents the 95% CI bands. FPG, fasting plasma glucose.
Subgroup analyses
A forest map analysis was applied to assess whether the association of baseline FPG with 1-year mortality was consistent across the subgroups (figure 4). Subgroup analysis revealed that the risk of 1-year mortality increased significantly with the increase in FPG in the subgroups of pneumonia subjects (HR=1.73, 95% CI 1.1 to 2.72), hypertensive subjects (HR=1.25, 95% CI 1.02 to 1.53) and the first onset of stroke subjects (HR=1.2, 95% CI 1.02 to 1.41).
Figure 4.
Subgroup analyses of the association of baseline fasting plasma glucose (FPG) with 1-year mortality in non-diabetic patients with acute cerebral infarction (ACI).
Discussion
In this multicentre, observational and registry-based study, we found that in non-diabetic patients with ACI, 1-year all-cause mortality significantly increased as baseline FPG increased. The baseline FPG had a positive but non-linear association with 1-year mortality. The stratification analyses revealed that in patients with pneumonia, hypertension or first stroke, with the increase in FPG, the risk of 1-year mortality was significantly increased, demonstrating the importance of enhancing FPG testing in these populations.
Previous studies have shown that elevated baseline FPG levels lead to poor stroke outcomes.18–20 However, Tziomalos et al have shown no direct relationship between stress hyperglycaemia and the prognosis of acute ischaemic stroke.21 Thus, the association between baseline FPG and stroke prognosis is controversial, particularly in non-diabetic patients. Previous research has concentrated on the association of baseline FPG with short-term mortality in non-diabetic patients who had a stroke,7 22 but an association with 1-year mortality has rarely been explored. Additionally, in these previous studies, patients with and without diabetes were studied together. It is rare to focus solely on non-diabetic patients. To provide evidence to support clinical treatment, our study focused on the association between baseline FPG and 1-year mortality in non-diabetic patients with ACI based on the Xi'an Stroke Registry Study. The advantage of our study design is that only non-diabetic patients at discharge were analysed, minimising the effect of diabetes on outcomes.
A systematic overview of epidemiological surveys and studies in 52 countries mentioned elevated FPG as a critical hazard for mortality in patients without diabetes who had a stroke.23 A study by Capes et al has shown that admission glucose levels >6.1–7.0 mmol/L in patients without diabetes after ischaemic stroke were related to an increased risk of hospitalisation or 30-day mortality (relative risk=3.28).7 Both their study and ours suggest that higher fasting blood glucose is associated with an increased mortality risk in non-diabetic patients with ACI. Stress hyperglycaemia was measured by FBG/HbA1c ratio in a study by Cai et al.24 Their study showed that patients in the highest quartile of the FBG/HbA1c ratio had an increased risk of all-cause death at 3 months (OR: 5.16) and at 12 months (OR: 2.59) after stroke. In contrast, our results showed that the 3-month all-cause mortality in Q4 (>5.55 mmol/L) was significantly increased compared with Q1 (<4.52 mmol/L). Compared with those in Q1, patients with baseline FPG in Q4 had 2.08 times increased risk of 1-year mortality (HR=2.08). They looked at all patients who had a stroke, we focused on ACI patients. In addition, they did not exclude patients with diabetes when they analysed the association between stress hyperglycaemia and all-cause death. Li et al found that the association between glucose/HbA1c ratio and 1-year mortality was significant in the patients without diabetes through a retrospective study of the China National Stroke Registry II.25 This conclusion is consistent with our findings. Their study represented national average data, while we studied regional data, so our data have some significance in regional prevention and control. The different results obtained in these studies may be related to differences in follow-up time, study design, racial and geographical differences. Although it is unknown how hyperglycaemia was treated in the acute phase of stroke, and there are no data on the use of hypoglycaemic agents,8 26 all of the above studies have shown that elevated baseline FPG is associated with a high risk of death in non-diabetic patients with ACI. This finding suggests that clinicians should watch the high baseline FPG in non-diabetic patients with ACI since it may be associated with an even greater risk of 1-year mortality.
When controlling for relevant confounders, multivariable Cox regression analyses demonstrated a 17% increase in 1-year mortality for every 1 mmol/L of baseline FPG increase (table 2). The fitted curve shows a positive but non-linear association of baseline FPG with 1-year mortality (figure 3). Using Cox regression models with restricted cubic splines, Zhu et al found a non-linear relationship between the level of glucose-to-Hemoglobin A1c (HbA1c) ratio and the risk of 12-month all-cause mortality.27 In addition, a previous study by our team suggested that FPG in non-diabetic patients with ACI had a J-shaped curve relationship with 1-year stroke recurrence.28 These results indicate that FPG has different effects on 1-year mortality and recurrence of stroke, highlighting that elevated baseline FPG should be regarded as a concern in non-diabetic patients with ACI.
Subgroup analyses showed no significant difference among different subgroups in patients with pneumonia, hypertension or first stroke; however, the 1-year mortality increased significantly with the increase in baseline FPG. Zhao et al showed a significant increase in 60-day mortality in patients with stroke-associated pneumonia, a common complication.29 Moreover, Heikinheimo et al showed that in young patients with ischaemic stroke, pneumonia was associated with an increased hazard of long-term mortality.30 The results of these studies are consistent with our findings. Many previous studies have demonstrated that hypertension is associated with the risk of death from stroke and is a predictor of long-term stroke mortality.31 As such, controlling hypertension reduces the risk of death from acute stroke.32 In this study, in patients with a first stroke, the hazard of 1-year mortality increased with the increase in baseline FPG. Previous studies have been concerned about the relationship between blood glucose and stroke mortality, while few subgroup analyses have examined whether the stroke occurred for the first time. Therefore, more relevant research is needed to verify this conclusion.
Although the underlying mechanisms of the association of baseline FPG with stroke mortality are not fully understood, several potential mechanisms warrant further exploration. First, elevated baseline FPG level in non-diabetic patients with ACI is often thought to be a stress response caused by extremely complicated interactions of counter-regulatory hormones such as cortisol, catecholamines, glucagon and cytokines. ACI may affect cytokine production and hormone disorders, leading to inflammation. Indeed, neurohormonal disorders may cause patients to experience more severe damage than those who do not develop stress hyperglycaemia.7 8 33 Second, intracellular acidosis, which leads to mitochondrial dysfunction, occurs in ischaemic brain tissue.34 Hyperglycaemia significantly worsens intracortical acidosis in the ischaemic penumbra region, aggravating mitochondrial function damage.7 35 36 Third, studies have shown that endothelial dysfunction in vivo is associated with abnormal glucose homoeostasis.37 38 Compared with chronic sustained hyperglycaemia, glucose fluctuations have a more specific trigger on oxidative stress and a more significant detrimental effect on endothelial function, which are vital factors that lead to vascular events.37–39 Additionally, previous studies have shown that aspartate aminotransferase and alanine aminotransferase positively correlate with mortality after ischaemic stroke.40 Moreover, stroke-specific mortality increases with the elevation of low-density lipoprotein.41 In our study, among the clinical characteristics by baseline FPG quartiles in non-diabetic patients with ACI (table 1), aspartate aminotransferase, alanine aminotransferase and low-density lipoprotein were also elevated in the elevated FPG population. Although the median of ALT and AST is indeed within the normal range, the levels of some ALT and AST in the Q4 group of FPG were significantly higher than the normal range. Thus, the mechanism by which FPG may affect stroke mortality through aspartate aminotransferase, alanine aminotransferase and low-density lipoprotein can offer new insights into the association of FPG with stroke mortality.
Our study has some limitations that need to be improved. First, data on patients with a final diagnosis of diabetes after 1-year follow-up were unavailable for this study; therefore, the effect of diabetes on outcomes cannot be completely ruled out. Second, we did not record the occurrence of blood glucose fluctuations and hypoglycaemia, both of which impact vascular function and physical condition, leading to a poor prognosis. Third, as data on patients who received intravenous thrombolysis and/or underwent mechanical thrombectomy were unavailable, we could not analyse these two groups. Fourth, there was selection bias in the enrolled patients. All hospitals selected for this study were grade A tertiary hospitals; they might not be representative of the current situation of patients with ACI in other smaller community hospitals. Therefore, further large-scale studies will be conducted in different regions and populations to validate our findings.
Conclusions
Our study demonstrated that elevated baseline FPG is an independent risk factor for 1-year all-cause mortality in non-diabetic patients with ACI in the Xi'an region. Baseline FPG had a positive but non-linear association with 1-year mortality, and higher baseline FPG significantly increased 1-year mortality. Our findings may help clinicians to assess the 1-year all-cause mortality in non-diabetic patients with ACI in the acute phase.
Supplementary Material
Acknowledgments
The authors thank all the imaging and laboratory technicians, medical staff and nurses from the participating hospitals.
Footnotes
DZ and ZL contributed equally.
Contributors: DZ, ZL and PL are joint first authors. DZ, ZL and PL designed the study. HZ, WG, QL, CH, JW, PL, QC, MZ and YH collected the data. YW, XL and FW were involved in data cleaning, mortality follow-up and verification. DZ and ZL analysed the data. DZ, ZL and PL drafted the manuscript. SW contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content and approved the final version of the manuscript. SW had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and approved the final manuscript. SW is the guarantor of the work.
Funding: The study was supported by the Scientific Research Project of the Xi’an Health Commission (Grant nos. 2020ms03, 2020yb05, 2021yb33 and 2022qn11), the Science and Technology Plan Project of Xi’an city (Grant No.22YXYJ0061 and 22YXYJ0074), the Science and Technology Program of Shaanxi Province (Grant nos. 2021SF-333, 2022SF-381 and 2022SF-507), the Project of Shaanxi Administration of Traditional Chinese Medicine (Grant no. 2022-SLRH-LJ-013), and the Scientific Research Project of the Shaanxi Health Commission (Grant no. 2022C005).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the Ethics Committee of Xi’an No.1 Hospital (Approval No. 2014(5)), Registration number: ChiCTR-EOC-17012190. Participants gave informed consent to participate in the study before taking part.
References
- 1.Collaborators GBDS . Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol 2021;20:795–820. 10.1016/S1474-4422(21)00252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma Q, Li R, Wang L, et al. Temporal trend and attributable risk factors of stroke burden in China, 1990-2019: an analysis for the global burden of disease study 2019. Lancet Public Health 2021;6:e897–906. 10.1016/S2468-2667(21)00228-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the global burden of disease study 2017. The Lancet 2019;394:1145–58. 10.1016/S0140-6736(19)30427-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W, Jiang B, Sun H, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation 2017;135:759–71. 10.1161/CIRCULATIONAHA.116.025250 [DOI] [PubMed] [Google Scholar]
- 5.Hu G, Gu H, Jiang Y, et al. Prevalence and in-hospital outcomes of diabetes among acute ischemic stroke patients in China: results from the Chinese stroke center alliance. J Neurol 2022;269:4772–82. 10.1007/s00415-022-11112-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacIntosh BJ, Cohen E, Colby-Milley J, et al. Diabetes mellitus is associated with poor in-hospital and long-term outcomes in young and Midlife stroke survivors. J Am Heart Assoc 2021;10:e019991. 10.1161/JAHA.120.019991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 2001;32:2426–32. 10.1161/hs1001.096194 [DOI] [PubMed] [Google Scholar]
- 8.Dungan KM, Braithwaite SS, Preiser JC. Stress Hyperglycaemia. Lancet 2009;373:1798–807. 10.1016/S0140-6736(09)60553-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capes SE, Hunt D, Malmberg K, et al. Stress Hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. The Lancet 2000;355:773–8. 10.1016/S0140-6736(99)08415-9 [DOI] [PubMed] [Google Scholar]
- 10.Fogelholm R, Murros K, Rissanen A, et al. Admission blood glucose and short term survival in primary intracerebral haemorrhage: a population based study. J Neurol Neurosurg Psychiatry 2005;76:349–53. 10.1136/jnnp.2003.034819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Umpierrez GE, Isaacs SD, Bazargan N, et al. Hyperglycemia: an independent marker of in-hospital mortality in patients with Undiagnosed diabetes. J Clin Endocrinol Metab 2002;87:978–82. 10.1210/jcem.87.3.8341 [DOI] [PubMed] [Google Scholar]
- 12.Kleindorfer DO, Towfighi A, Chaturvedi S, et al. Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline from the American heart Association/American stroke Association. Stroke 2021;52:e364–467. 10.1161/STR.0000000000000375 [DOI] [PubMed] [Google Scholar]
- 13.Zou F, Tian Y, Wu W, et al. Design and implementation of management system for stroke data mining based on non-structured electronic medical record. China Digital Medicine 2015;10:41–4. [Google Scholar]
- 14.Smith CJ, Kishore AK, Vail A, et al. Diagnosis of stroke-associated pneumonia: recommendations from the pneumonia in stroke consensus group. Stroke 2015;46:2335–40. 10.1161/STROKEAHA.115.009617 [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Zhao X, Liu L, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and Occlusions in China: the Chinese intracranial Atherosclerosis (CICAS) study. Stroke 2014;45:663–9. 10.1161/STROKEAHA.113.003508 [DOI] [PubMed] [Google Scholar]
- 16.Kurth T, Walker AM, Glynn RJ, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of Nonuniform effect. Am J Epidemiol 2006;163:262–70. 10.1093/aje/kwj047 [DOI] [PubMed] [Google Scholar]
- 17.Jaddoe VWV, de Jonge LL, Hofman A, et al. First trimester fetal growth restriction and cardiovascular risk factors in school age children: population based cohort study. BMJ 2014;348:g14. 10.1136/bmj.g14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimoyama T, Kimura K, Uemura J, et al. Elevated glucose level adversely affects infarct volume growth and neurological deterioration in non-diabetic stroke patients, but not diabetic stroke patients. Eur J Neurol 2014;21:402–10. 10.1111/ene.12280 [DOI] [PubMed] [Google Scholar]
- 19.Bruno A, Levine SR, Frankel MR, et al. Admission glucose level and clinical outcomes in the NINDS RT-PA stroke trial. Neurology 2002;59:669–74. 10.1212/WNL.59.5.669 [DOI] [PubMed] [Google Scholar]
- 20.Desilles J-P, Meseguer E, Labreuche J, et al. Diabetes mellitus, admission glucose, and outcomes after stroke Thrombolysis: a Registry and systematic review. Stroke 2013;44:1915–23. 10.1161/STROKEAHA.111.000813 [DOI] [PubMed] [Google Scholar]
- 21.Tziomalos K, Dimitriou P, Bouziana SD, et al. Stress hyperglycemia and acute ischemic stroke in-hospital outcome. Metabolism 2017;67:99–105. 10.1016/j.metabol.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 22.Marulaiah SK, Reddy MP, Basavegowda M, et al. Admission hyperglycemia an independent Predictor of outcome in acute ischemic stroke: A longitudinal study from a tertiary care hospital in South India. Niger J Clin Pract 2017;20:573–80. 10.4103/1119-3077.206368 [DOI] [PubMed] [Google Scholar]
- 23.Danaei G, Lawes CMM, Vander Hoorn S, et al. Global and regional mortality from ischaemic heart disease and stroke attributable to higher-than-optimum blood glucose concentration: comparative risk assessment. Lancet 2006;368:1651–9. 10.1016/S0140-6736(06)69700-6 [DOI] [PubMed] [Google Scholar]
- 24.Cai Z-M, Zhang M-M, Feng R-Q, et al. Fasting blood glucose-to-Glycated hemoglobin ratio and all-cause mortality among Chinese in-hospital patients with acute stroke: a 12-month follow-up study. BMC Geriatr 2022;22:508. 10.1186/s12877-022-03203-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Quan K, Wang Y, et al. Effect of stress hyperglycemia on neurological deficit and mortality in the acute ischemic stroke people with and without diabetes. Front Neurol 2020;11:576895. 10.3389/fneur.2020.576895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrari F, Moretti A, Villa RF. Hyperglycemia in acute ischemic stroke: Physiopathological and therapeutic complexity. Neural Regen Res 2022;17:292–9. 10.4103/1673-5374.317959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu B, Pan Y, Jing J, et al. Stress hyperglycemia and outcome of non-diabetic patients after acute ischemic stroke. Front Neurol 2019;10:1003. 10.3389/fneur.2019.01003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Z, Lin X, Lin W, et al. A J-shaped curve relationship between baseline fasting blood glucose and 1-year stroke recurrence in non-diabetic patients with acute cerebral infarction in Xi'An. Front Neurol 2021;12:698793. 10.3389/fneur.2021.698793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao J, Li L, Zhen N, et al. Microbiology and outcomes of institutionalized patients with stroke-associated pneumonia: an observational cohort study. Front Microbiol 2021;12:720051. 10.3389/fmicb.2021.720051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heikinheimo T, Broman J, Haapaniemi E, et al. Preceding and Poststroke infections in young adults with first-ever ischemic stroke: effect on short-term and long-term outcomes. Stroke 2013;44:3331–7. 10.1161/STROKEAHA.113.002108 [DOI] [PubMed] [Google Scholar]
- 31.Abdo R, Abboud H, Salameh P, et al. Mortality and predictors of death Poststroke: data from a multicenter prospective cohort of Lebanese stroke patients. J Stroke Cerebrovasc Dis 2019;28:859–68. 10.1016/j.jstrokecerebrovasdis.2018.11.033 [DOI] [PubMed] [Google Scholar]
- 32.Alhazzani AA, Mahfouz AA, Abolyazid AY, et al. In hospital stroke mortality: rates and determinants in southwestern Saudi Arabia. Int J Environ Res Public Health 2018;15:927. 10.3390/ijerph15050927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts GW, Quinn SJ, Valentine N, et al. Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J Clin Endocrinol Metab 2015;100:4490–7. 10.1210/jc.2015-2660 [DOI] [PubMed] [Google Scholar]
- 34.Beppu K, Sasaki T, Tanaka KF, et al. Optogenetic countering of glial acidosis suppresses glial glutamate release and ischemic brain damage. Neuron 2014;81:314–20. 10.1016/j.neuron.2013.11.011 [DOI] [PubMed] [Google Scholar]
- 35.Rinkel LA, Nguyen TTM, Guglielmi V, et al. High admission glucose is associated with poor outcome after Endovascular treatment for ischemic stroke. Stroke 2020;51:3215–23. 10.1161/STROKEAHA.120.029944 [DOI] [PubMed] [Google Scholar]
- 36.Anderson RE, Tan WK, Martin HS, et al. Effects of glucose and Pao2 modulation on cortical intracellular acidosis, NADH redox state, and infarction in the ischemic Penumbra. Stroke 1999;30:160–70. 10.1161/01.str.30.1.160 [DOI] [PubMed] [Google Scholar]
- 37.Vehkavaara S, Seppälä-Lindroos A, Westerbacka J, et al. In vivo endothelial dysfunction characterizes patients with impaired fasting glucose. Diabetes Care 1999;22:2055–60. 10.2337/diacare.22.12.2055 [DOI] [PubMed] [Google Scholar]
- 38.Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006;295:1681–7. 10.1001/jama.295.14.1681 [DOI] [PubMed] [Google Scholar]
- 39.Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008;57:1349–54. 10.2337/db08-0063 [DOI] [PubMed] [Google Scholar]
- 40.Choi KM, Han K, Park S, et al. Implication of liver enzymes on incident cardiovascular diseases and mortality: A nationwide population-based cohort study. Sci Rep 2018;8:3764. 10.1038/s41598-018-19700-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rong S, Li B, Chen L, et al. Association of low-density lipoprotein cholesterol levels with more than 20-year risk of cardiovascular and all-cause mortality in the general population. J Am Heart Assoc 2022;11:e023690. 10.1161/JAHA.121.023690 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-069716supp001.pdf (53.6KB, pdf)
Data Availability Statement
Data are available upon reasonable request.