Abstract
We present a case of a woman who presented with a photosensitive skin rash and blisters on her extremities which did not improve with steroids. These were associated with polyarthralgia and a deranged liver function test on her admission. Further workup revealed that the patient has an undiagnosed porphyria cutanea tarda (PCT) and hereditary haemochromatosis. The patient later underwent regular venesections which improved her condition. This case report not only illustrates the challenge in diagnosing PCT but also aims to highlight the association between PCT and hereditary haemochromatosis.
Keywords: Dermatology, Liver disease, Haematology (incl blood transfusion)
Background
Hereditary haemochromatosis (HH) is a systemic iron-overload syndrome caused by genetic mutations affecting iron regulation, absorption and metabolism.1 The most common form of HH is due to homozygous mutations in the haemochromatosis (HFE) gene, which encodes HH protein. Secondary factors, such as alcohol use or excessive blood transfusions, can contribute to developing or exacerbating the pathogenesis of the disease.2 The signs and symptoms can vary widely due to the systemic nature of the disease. Thus, early disease recognition can be challenging for clinicians but is necessary in preventing organ dysfunction and damage. We present a patient with fragile and blistered skin diagnosed as porphyria cutanea tarda (PCT) which was the initial sign of underlying haemochromatosis. This case highlights the need to understand the pathogenesis of PCT and its associated roles towards iron overload.
Case presentation
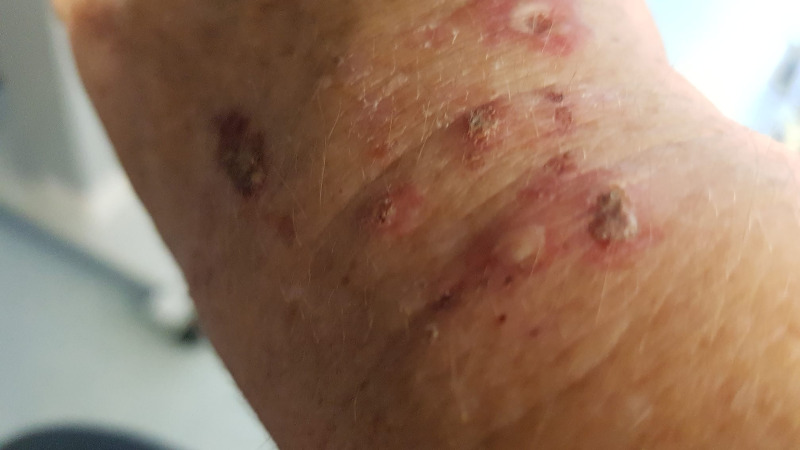
A woman in her 40s presented with a 2-month history of malaise, photosensitive skin rash on hands and feet (figures 1–3) and polyarthralgia affects the major joints of the knees, ankles and shoulders. She had not recently received any medications, including over-the-counter and antibiotics. At the time, the differential diagnosis included contact dermatitis, polymorphic light eruption and photosensitivity dermatitis. She was discharged from the hospital with a presumptive diagnosis of contact dermatitis caused by the usage of latex gloves at work, and a 2-week course of topical and oral steroids was prescribed.
Figure 1.
Photosensitive rash on dorsum of left hand.
Figure 2.
Photosensitive rash with excoriation marks on dorsum of right hand.
Figure 3.
Photosensitivity rash with excoriation marks on the dorsum of right hand.
Despite 2 weeks of daily steroids, her skin rash worsened over time. A new bullous rash formed on the dorsal aspect of the hands (figure 4). She was suspected of having vesiculobullous disease at the time, such as bullous pemphigoid and pemphigus vulgaris. A skin biopsy was performed due to the higher probability of vesiculobullous diseases. The skin biopsy revealed a sharply delineated region of ulceration indicative of excoriations, but no signs of a vesiculobullous condition. She was later scheduled for lupus testing due to the photosensitivity rash and arthralgia symptoms. Her antinuclear antibody and double-stranded DNA testing eventually turned out to be negative. On further enquiry, the patient clarified that consuming alcoholic drinks worsen her skin rash.
Figure 4.
Bullous on the dorsum of the patient’s left hand.
Investigations
She had a set of basic admission investigations (table 1). In view of the photosensitive nature of her skin rash, she was arranged to have an extensive porphyria test and the results are shown in table 2.
Table 1.
Laboratory results during admission
| Labs (unit) | Normal range | Value |
| Plasma | ||
| Haemoglobin | 120–160 | 125 |
| Mean cell volume | 78–98 FL | 99.5↑ |
| White cell count | 4–11×109 /L | 7.6 |
| Platelets | 140–400×109 /L | 264 |
| C reactive protein | 0–5 mg/L | 6 ↑ |
| Amylase | 25–125 IU/L | 45 |
| Erythrocytes sedimentation rate | 0–12 first hour | 10 |
| Antinuclear antibody | – | Negative |
| Anti-CCP antibodies | <7 U/mL | 2.3 |
| dSDNA | <10 U/mL | 0.6 |
| Myeloperoxidase antibodies | <3.5 IU/mL | <0.2 |
| Proteinase 3 antibodies | <2 IU/mL | <0.2 |
| HIV antibodies | – | Negative |
| SARS-CoV-2 RNA | – | Not detected |
dSDNA, double-stranded DNA.
Table 2.
Laboratory results for porphyria screening
| Labs (unit) | Normal range | Value |
| Laboratory results for porphyria screening | ||
| Plasma | ||
| Porphobilinogen (PBG) | – | NA (Sample haemolysed, thus not being analysed) |
| Erythrocyte porphyrin | – | NA (Sample haemolysed, thus not being analysed) |
| PBG: creatinine ratio | – | NA (Sample haemolysed, thus not being analysed) |
| Urine | ||
| Porphyrins (nmol/L) | 25–114 | 1737 ↑ |
| Porphobilinogen | – | Negative |
| Porphyrin to creatinine ratio (nmol/mmol) | 0–30 | 226 ↑ |
| Total urine porphyrins:creatinine ratio (nmol/mmol) | 0–40 | 578 ↑ |
| Fractionated urine porphyrin analysis | – | Uroporphyrin (62% of total hepatacarboxylic porphyrin 31%) ↑ |
| Faecal | ||
| Total faecal porphyrin (nmol/g) | 10–200 | 352 ↑ |
| Fractionated faecal porphyrin analysis | – | Isocopropophyrin ↑ Heptacarboxylic Porphyrin ↑ |
| Laboratory results of her admission bloods | ||
| Plasma | ||
| Haemoglobin | 120–160 | 125 |
| Mean cell volume | 78–98 FL | 99.5↑ |
| White cell count | 4–11×109 /L | 7.6 |
| Platelets | 140–400×109 /L | 264 |
She continued to struggle with joint pain and fatigability. In view of her deranged liver function test (LFTs) on admission (table 3), she underwent a series of liver-related investigations. Liver screen, including hepatitis A, B and C, was unremarkable. Ultrasound and CT of liver subsequently showed fatty liver. She also underwent a haemochromatosis genetic screen which later revealed that she had type 1 haemochromatosis secondary to homozygous C282Y gene mutation.
Table 3.
Laboratory results of liver screen on admission
| Labs (unit) | Normal range | Value |
| Plasma | ||
| Alanine transaminase | 0–33 IU/L | 67 IU/L ↑ |
| Aspartate transferase | 0–32 IU/L | 27 IU/L |
| Alkaline phosphatase | 30–130 IU/L | 68 IU/L |
| Gamma-glutamyl transferase | 0–40 IU/L | 76 IU/L ↑ |
| Albumin | 35–50 g/L | 43 g/L |
| Bilirubin | 0–21 µmol/L | 8 µmol/L |
| Ferritin | 13–150 µg/L | 728 mg/L ↑ |
| Iron | 5.8–34.5 | 36.2 ↑ |
| Total iron binding capacity | 47–89 µg/L | 41.4 ↓ |
| Iron saturation | 15–50% | 87.4 ↑ |
| Alpha 1-antitrypsin | 0.9–2 g/L | 1.28 g/L |
| Alpha fetoprotein | 0–5.8 KU /L | 2.3 KU /L |
| Smooth muscle antibody | – | Negative |
| Liver kidney microsomal antibody | – | Negative |
| Mitochondrial antibody | – | Negative |
| Hepatitis A IgM antibody | – | Negative |
| Hepatitis B surface antigen | – | Negative |
| Hepatitis B core antibody | – | Negative |
| Hepatitis C antibody | – | Negative |
Treatment
The series of investigations confirmed the diagnosis of PCT on the background of type 1 haemochromatosis. She was tested for COVID-19, which had previously been identified as a potential trigger of porphyria in recent studies, and the results were negative. Her photosensivity rash is most likely caused by PCT. Homozygous C282Y gene mutation has indicated that she is at risk of developing iron overloading and clinical haemochromatosis. She subsequently received regular venesections on a monthly basis under the advice of a porphyria specialist with an aim to achieve serum ferritin level <25 mg/L and transferrin level <17% to prevent the relapse of PCT. She was advised to reduce alcohol intake as this might worsen her porphyria skin presentation, and to use sunblock daily to prevent worsening of her skin rashes.
Outcome and follow-up
She is currently under the care of the haematologist and receiving regular venesections in the haematology day unit in the local district hospital. She has recently changed her job to become a community care worker which involves less driving and therefore less sun exposure. She has also reduced her alcohol intake significantly to the UK recommended alcohol intake (<14 units per week) and this has helped in preventing flare up for porphyria skin presentations.
Her skin rash has improved significantly after two sessions of venesection within 6 months (figures 5–6). At 6 months follow-up, her serum ferritin has improved to 75 mg/L with a normal ALT level of 21 IU/L (table 4). She will be reviewed by a local gastroenterologist for follow-up for her haemochromatosis diagnosis.
Figure 5.
Remaining photosensitivity rash on left dorsum of the hand 6 months postvenesection.
Figure 6.
Remaining photosensitivity rash on right dorsum of the hand 6 months postvenesection.
Table 4.
Laboratory results of liver screen 6 months later
| Labs (unit) | Normal range | Value |
| Plasma | ||
| Ferritin | 13–150 µg/L | 75 |
| Iron | 5.8–34.5 | 13.2 |
| Total iron binding capacity | 47–89 µg/L | 42.5 ↓ |
| Iron saturation | 15%–50% | 31.3 |
| Alanine transaminase | 0–33 IU/L | 24 |
Discussion
HH is the most common inherited hepatic disease as well as the most common autosomal recessive disorder.3 PCT could be triggered by alcohol, excessive vitamin C intake, oral contraceptives, hepatitis C infection and iron overload coupled with underlying genetic susceptibility.4 During the COVID-19 pandemic, COVID-19 has been identified as a potential trigger of porphyria. Bardak et al described a patient with known acute intermittent porphyria (AIP) having an acute AIP attack provoked by COVID-19 infection.5 Stadlbauer et al reported similar occurrences in 2021, in which acute porphyria flare up occurred in patients with a history of AIP.6 However, there is currently insufficient evidence to link COVID-19 infection with PCT. Our patient was tested negative for COVID-19 and her PCT is most likely caused by iron overload from undiagnosed haemochromatosis.
Despite a proven relationship between PCT and haemochromatosis in the medical literature, diagnosing PCT can be difficult due to its diverse aetiologies and may result in a missed diagnosis.4 The pathogenesis relating the two conditions necessitates the understanding of haem synthesis in addition to the effects of iron metabolism on the pathway.
PCT is caused by a mutation of the uroporphyrinogen decarboxylase (UPD) gene on chromosome 1p34 leading to a deficiency of UPD, which is responsible for converting uroporphyrinogen III into coproporphyrinogen III (figure 7).7 8 Iron can affect the haem synthesis pathway through several mechanisms. Through formation of reactive oxidative species, iron overload affects the quantity and activity of UPD.9 Moreover, iron induces uroporphyrin production by inducing aminolevulinate synthase.3 As a result, uroporphyrinogen accumulates in different tissues giving rise to a varied clinical presentation. The accumulation of uroporphyrinogen in skin with superimposed environmental risk factors can lead to the classic blistering appearance of PCT.
Figure 7.
Haem synthesis biochemical illustration (NB Figure is created by this case report’s authors).
Other cases have further discussed the pathogenetic association between haemochromatosis and PCT. Bovenschen and Vissers described a case in 2009 that also emphasised this relationship.10 According to the case records, a young Caucasian woman entered with significant fragility and blistering on the dorsal side of both hands. A porphyria screen confirmed PCT, and an investigation revealed multiple active blisters, erosions, erythematous macules, dyspigmentation, atrophic scarring and milia on the sun-exposed dorsum of the hands. This patient also had increased ALT, ferritin and iron levels, increasing the potential of primary haemochromatosis. She underwent haemochromatosis genetic testing, which found a homozygous C282Y missense mutation in the HFE gene.
In 2022, Larrondo and Gosch projected a similar scenario.11 According to the case report, a middle-aged woman presented with blisters and photosensitive skin erosions over the dorsum of her hands, forearms, cheeks and forehead. Topical corticosteroids and antibiotics had no effect on the patient. Porphyria screening tests were carried out based on clinical suspicion and skin lesion presentation results, which ultimately supported the PCT diagnosis. The increased ferritin and LFTs have shown haemochromatosis. This patient was determined to have the C282Y mutation in the HFE gene after genetic testing.
Mogl et al reported underlying haemochromatosis and hepatocellular carcinoma (HCC) in a PCT patient in 2007.12 The patient was initially tested for HCC after having elevated levels of alpha-fetoprotein and aspartate transferase. For the previous 25 years, he had vitiligous skin lesions on sun-exposed areas. He was eventually diagnosed with PCT after a battery of porphyria tests due to doctors’ considerable clinical concern on the skin look. His MR cholangiography after left hemihepatectomy indicated evidence of iron overload, prompting genetic testing for haemochromatosis, which found a homozygous C282Y mutation.
Individuals in the aforementioned case reports had blisters and weak skin, as well as high ferritin and iron levels, prompting clinicians to perform additional tests for porphyria and haemochromatosis. Given how linked the two pathologies are, more robust guidelines should be implemented with more emphasis towards searching for and identifying the underlying cause.
The diagnosis of haemochromatosis could be missed and leading to inappropriate management and/or a prolonged disease course with potential complications (figure 8), as patients may present only with PCT symptoms. Blood and faecal porphyrins are needed to confirm the diagnosis and type of PCT. However, a high index of suspicion for an underlying pathology is paramount to the timely diagnosis and treatment of haemochromatosis. Therefore, clinicians should consider screening patients for suspected haemochromatosis using iron and ferritin studies. Genetic studies may confirm the presence of the disease on revealing HFE mutations. Management consists of treating the underlying cause, removal of exacerbating factors, photoprotection and recurrent phlebotomies. Our patient responded well to phlebotomies and was given regular follow-up appointments for further monitoring. She was instructed to avoid triggers such as sunlight, and her skin lesions improved significantly and resolved after several venesections.
Figure 8.

Possible cycle of misdiagnosing patients with underlying haemochromatosis who initially present with PCT (NB Figure is created by this case report’s authors). PCT, porphyria cutanea tarda.
Patient’s perspective.
While I was working a year ago, I noticed a rash appearing on both of my hands and feet. It was a very warm summer and the heat seemed to make it worse and irritable. I scratch my hands out of irritation but this made it worse. I remember returning home that evening very upset and distressed due to the pain in my hands and feet over the scratched sites. I then applied some cream on my hands and feet to try and soothe it and wore gloves to stop me scratching.
I went to the hospital the following day and was reviewed by acute medical doctors who were unsure what the skin rash and the underlying cause of it. I was kept in a medical ward overnight for observation. I was then discharged from the hospital on the following day after being reviewed by a medical consultant, and advised to return to acute medical clinics for regular reviews. I was reviewed in clinics several times over the next few weeks and was told that the skin rash might be due to an allergic reaction towards the latex gloves which I usually wear at work. Therefore, I was given some steroid cream to apply on the rash for 2 weeks.
Unfortunately, there was no improvement in my skin rash. I began to have blisters over my hands. They took a biopsy from my right hand where one of the blisters broke open. The skin biopsy was a very painful and unpleasant experience. The biopsy site was then stitched and dressed with bandages. I was sent home after this and still not knowing what was causing the blisters and rash.
I returned back to the hospital on the following days to have the hand dressing changed, and repeat blood tests. I was later found out to have extremely high iron and ferritin levels as my body was producing too much iron. I was unsure what had caused the abnormal blood test and skin rash and blisters until being reviewed in an acute medical clinic by an acute medical registrar. He explained to me about the possible cause of the abnormal blood test and skin presentation and the following investigation plan.
After numerous investigations which consisted of blood, urine and stool tests, ultrasound and CT scans, they have eventually reached the diagnosis of a rare blood condition known as porphyria, along with a rare liver condition, called haemochromatosis. I then had genetic testing for haemochromatosis and this has come back as positive.
My porphyria diagnosis was a rare condition and it was very difficult to be managed in the local district hospital. The acute medical registrar subsequently referred me to a porphyria specialist who is in Cardiff. My case was discussed with the porphyria specialist, and I was advised to avoid sunlight as this would worsen my skin rash. I was also being told to have regular venesections for my porphyria and haemochromatosis via the local haematologist. I began to read up on online articles on websites regarding porphyria and haemochromatosis, and this has helped in understanding my condition better.
I have been experiencing widespread body ache during this period of time, in which the haematologist and my doctor (General Practitioner) couldn’t understand the underlying cause of it since the type of porphyria that I am having would only affect the skin. I was told by the acute medical registrar that my joints ache could be due to haemochromatosis. Besides that, I have also experienced insomnia in which I have to take sleeping tablets to help in my sleep. It was a difficult period of time for me as I also suffered from anxiety and depression.
The skin rash and blisters over my hands and feet started to heal and left some scars after having several sessions of venesections in the haematology department over the following months. I continue to cover my skin with sunblock and clothing from sunlight to prevent flare up of the skin rash and blisters. I am glad that I have not had another flare up of the skin blisters over the past 8 months. I continue to have regular follow-up by a haematologist in the hospital for my condition. Finding out about the diagnosis of porphyria along with haemochromatosis have been a long and challenging journey. I hope that I will be able to share my experience with healthcare professionals to raise awareness of porphyria, and remind them of the possibility of such a diagnosis, should they come across a similar skin presentation in the future.
Learning points.
Patients presenting with photosensitive skin rash should have a prompt search for the underlying cause as opposed to exclusively symptomatic treatment.
There is an association between porphyria cutanea tarda and hereditary haemochromatosis; a high index of suspicion is paramount to the timely diagnosis and treatment.
A multidisciplinary team approach, involving dermatology, haematology and gastroenterology, is essential to achieving timely patient care.
Footnotes
Contributors: JWG, CKO and KMA contributed equally in the case report. JWG and CKO contributed in writing the abstract, case presentation, tables, conclusion sections as well as manuscript reviewing. JWG, CKO and KMA contributed in writing most of the discussion and learning points sections.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Brissot P, Pietrangelo A, Adams PC, et al. Haemochromatosis. Nat Rev Dis Primers 2018;4:18016. 10.1038/nrdp.2018.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bulaj ZJ, Phillips JD, Ajioka RS, et al. Hemochromatosis genes and other factors contributing to the pathogenesis of porphyria CUTANEA TARDA. Blood 2000;95:1565–71. 10.1182/blood.v95.5.1565.005k42_1565_1571 [DOI] [PubMed] [Google Scholar]
- 3.Bonkovsky HL, Lambrecht RW. Hemochromatosis, iron overload, and porphyria cutanea tarda. Hemochromatosis 2000:453–67. 10.1017/CBO9780511666476 [DOI] [Google Scholar]
- 4.Frank J, Poblete-Gutiérrez P, Weiskirchen R, et al. Hemochromatosis gene sequence deviations in German patients with porphyria cutanea tarda. Physiol Res 2006;55 Suppl 2:S75–83. 10.33549/physiolres.930000.55.S2.75 [DOI] [PubMed] [Google Scholar]
- 5.Bardak AE, Alada MO, Senkal N, et al. Acute intermittent porphyria attack triggered by COVID-19 infection. Cureus 2023;15:e37412. 10.7759/cureus.37412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stadlbauer V, Jud P, Hackl G, et al. Impact of COVID-19 on rare diseases: report of three cases of acute hepatic porphyria affected by the pandemic. Zeitschrift Für Gastroenterologie 2021. 10.1055/s-0041-1734279 [DOI] [Google Scholar]
- 7.Frank J, Christiano AM. The genetic bases of the porphyrias. Skin Pharmacol Physiol 1998;11:297–309. 10.1159/000029853 [DOI] [PubMed] [Google Scholar]
- 8.Moore MR. The biochemistry of Heme synthesis in porphyria and in the porphyrinurias. Clin Dermatol 1998;16:203–23. 10.1016/s0738-081x(97)00201-0 [DOI] [PubMed] [Google Scholar]
- 9.Sampietro M, Fiorelli G, Fargion S. Iron overload in porphyria cutanea tarda. Haematologica 1999;84:248–53. [PubMed] [Google Scholar]
- 10.Bovenschen HJ, Vissers WHPM. Primary hemochromatosis presented by porphyria CUTANEA TARDA: a case report. Cases J 2009;2:7246. 10.4076/1757-1626-2-7246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larrondo J, Gosch M. Porphyria cutanea tarda due to primary hemochromatosis. Am J Med 2020;133:e681–2. 10.1016/j.amjmed.2020.03.053 [DOI] [PubMed] [Google Scholar]
- 12.Mogl M-T, Pascher A, Presser S-J, et al. An unhappy triad: hemochromatosis, porphyria cutanea tarda and hepatocellular carcinoma-A case report. World J Gastroenterol 2007;13:1998–2001. 10.3748/wjg.v13.i13.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]