Abstract
Background:
Adjuvant fluoropyrimidine-based chemotherapy substantially reduces recurrence and mortality after resection of stage 3 colon cancer. While standard doses of 5-fluorouracil and capecitabine are safe for most patients, the risk of severe toxicity is increased for the approximately 6% of patients with dihydropyimidine dehydrogenase (DPD) deficiency caused by pathogenic DPYD gene variants. Pre-treatment screening for pathogenic DPYD gene variants reduces severe toxicity but has not been widely adopted in the U.S.
Methods:
We conducted a cost-effectiveness analysis of DPYD genotyping prior to fluoropyrimidine-based adjuvant chemotherapy for stage 3 colon cancer, covering the c.1129–5923C>G (HapB3), c.1679T>G (*13), c.1905+1G>A (*2A), and c.2846A>T gene variants. We used a Markov model with a 5-year horizon, taking a U.S. healthcare perspective. Simulated patients with pathogenic DPYD gene variants received reduced-dose fluoropyrimidine chemotherapy. The primary outcome was the incremental cost-effectiveness ratio (ICER) for DPYD genotyping.
Results:
Compared with no screening for DPD deficiency, DPYD genotyping increased per-patient costs by $78 and improved survival by 0.0038 quality-adjusted life years (QALYs), leading to an ICER of $20,506/QALY. In one-way sensitivity analyses, The ICER exceeded $50,000 per QALY when the cost of the DPYD genotyping assay was greater than $286. In probabilistic sensitivity analysis using a willingness-to-pay threshold of $50,000/QALY DPYD genotyping was preferred to no screening in 96.2% of iterations.
Conclusions:
Among patients receiving adjuvant chemotherapy for stage 3 colon cancer, screening for DPD deficiency with DPYD genotyping is a cost-effective strategy for preventing infrequent but severe and sometimes fatal toxicities of fluoropyrimidine chemotherapy.
Introduction
Adjuvant chemotherapy reduces risk for cancer recurrence and improves survival in patients with stage 3 (node-positive) colon cancer.1 5-fluoruracil and its oral prodrug, capecitabine, are the essential components of adjuvant chemotherapy for colon cancer, usually given in conjunction with oxaliplatin. 5-fluorouracil and capecitabine, both classified as fluoropyrimidine chemotherapy agents, are safe for most patients at standard treatment doses. However, their therapeutic index is narrow. Toxicities linked to fluoropyrimidine chemotherapy range from mild to life-threatening, and may include diarrhea, mucositis, enteritis, neutropenia, thrombocytopenia, and palmar-plantar erythrodysesthesia.2 Fatal fluoropyrimidine toxicity is rare, occurring in less than 1% of treated patients.2–4 However, severe and sometimes fatal toxicity is greatly increased in patients with the syndrome of dihydropyrimidine dehydrogenase (DPD) deficiency,5–7 with risk of early treatment-related death among these patients estimated in the range of 2.3–10%.8, 9
DPD is the rate limiting enzyme in the metabolic clearance of 5-FU. DPD deficiency affects an estimated 3–8% of patient treated with fluoropyrimidine chemotherapy,10–12 often leading to severe toxicity. The most well-described causes of DPD deficiency are genetic variants of the DPYD gene, which encodes the DPD enzyme. Patients who are carriers of consensus pathogenic variants of the DPYD gene can be readily identified through genotyping assays, using peripheral blood. In recent years, multiple prospective studies have shown that screening for pathogenic DPYD gene variants prior to treatment with fluoropyrimidine chemotherapy, coupled with reduction of chemotherapy doses in patients with identified variants, leads to substantial reductions in severe treatment-related toxicity.8, 13, 14 On the basis of these findings, the European Medicines Agency (EMA) issued a recommendation in April of 2020 that all patients should be tested for DPD deficiency prior to starting a fluoropyrimidine-containing chemotherapy regimen.
Despite the growing evidence in favor of DPYD genotyping as a tool to prevent severe chemotherapy toxicity, there has been little uptake of this approach in the United States to date. The U.S. Food and Drug Administration (FDA) has yet to issue any updated recommendations regarding screening for DPD deficiency, and guidelines from the National Comprehensive Cancer Network [NCCN]) do not endorse or recommend any form of screening for DPD deficiency prior to fluoropyrimidine chemotherapy. Opponents of screening for DPD deficiency have argued that the benefits of this practice are too small to justify the costs, while proponents contend that screening is supported by the mounting evidence that this practice prevents severe toxicity and infrequent but avoidable deaths.15 To better weigh the costs and benefits of DPYD genotyping as a screening test for DPD deficiency, we conducted a model-based cost-effectiveness analysis from the U.S. healthcare perspective. Our model estimates the cost-effectiveness of DPYD genotyping prior to adjuvant chemotherapy for stage 3 colon cancer, compared with no screening for DPD deficiency.
Methods
We conducted a model-based cost-effectiveness analysis, using methods consistent with recommendations of the ISPOR CHEERS Task Force.16 We constructed a Markov model to estimate quality adjusted life years (QALYs) and treatment-related costs in patients receiving fluoropyrimidine-based adjuvant chemotherapy for stage 3 colon cancer. We modeled strategies of chemotherapy treatment with or without pretreatment DPYD genotyping to screen for DPD deficiency; the model structure is shown in Figure 1. In the screening arm of the model, all patients undergo DPYD genotyping, and patients who screen positive for a pathogenic DPYD gene variant receive reduced-dose fluoropyrimidine chemotherapy. In the no screening arm of the model, all patients receive standard-dose fluoropyrimidine-based chemotherapy, without prospective DPYD genotyping. All model cycles are six months long. Patients receive adjuvant chemotherapy in the first model cycle; outcomes modeled in the first cycle include grade 3–4 chemotherapy-related toxicity, toxicity-related hospitalization, treatment-related death (grade 5 toxicity), and non-treatment mortality. The main outcome modeled in subsequent six-month cycles is death from any cause. Cancer recurrence is assumed to be equivalent in the screening and no screening arms and is not explicitly modeled, with one exception. In the subgroup of patients with a “false positive” DPYD variant result the model allows for an increased risk of death related to reduced effectiveness of adjuvant treatment (due to use of reduced-dose fluoropyrimidine chemotherapy in patients with wild-type DPYD genotype).
Figure 1.
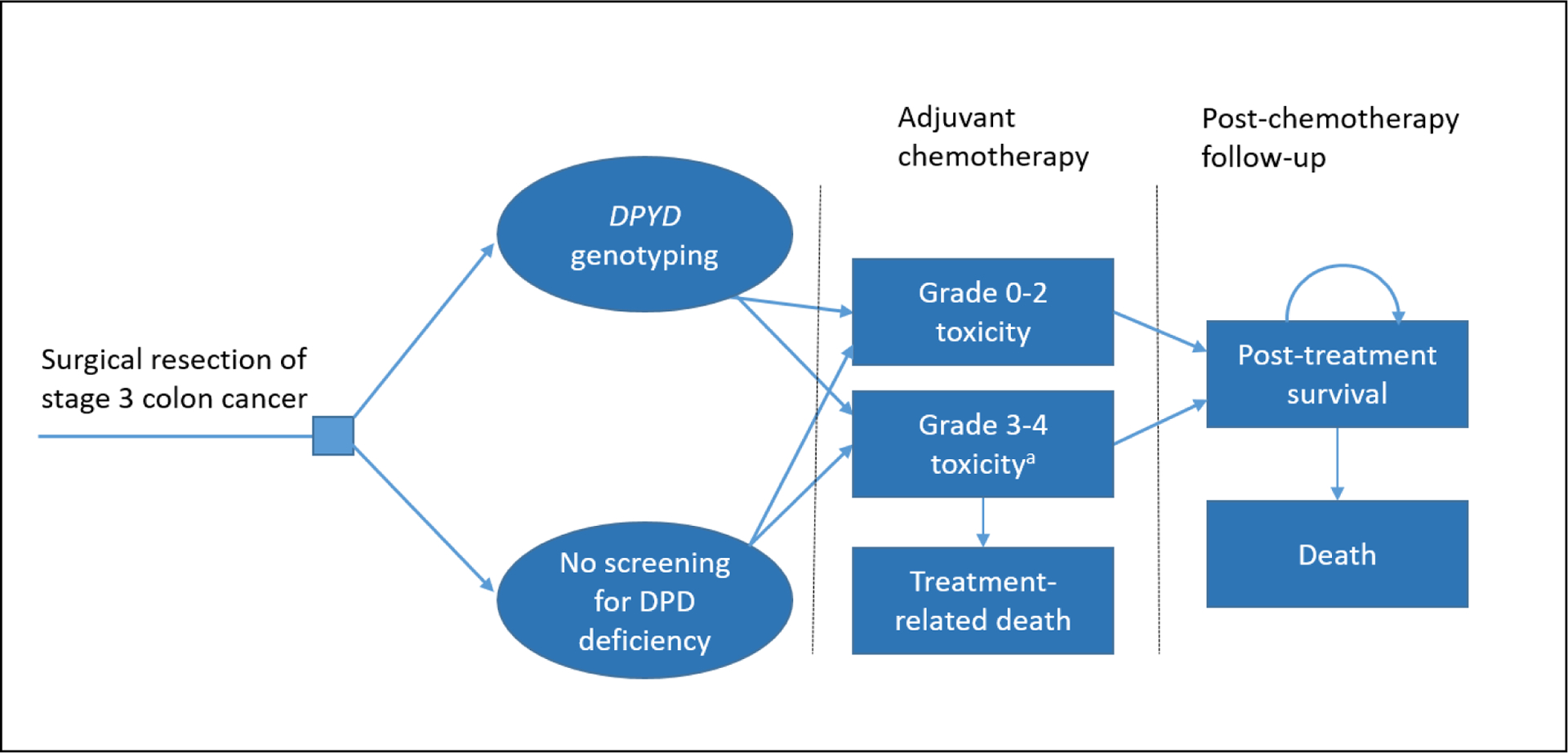
Structure of Markov model simulating adjuvant chemotherapy for stage 3 colon cancer
aA subset of patients with grade 3–4 toxicity experience toxicity-related hospitalizations.
Source of model estimates
A summary of model estimates and their sources is shown in Table 1. We based our estimate for the prevalence of pathogenic DPYD gene variants on the prevalence of variants reported in NCCTG N0147, a U.S.-based cooperative group study which identified subjects with the c.1129–5923C>G (HapB3), c.1679T>G (*13), c.1905+1G>A (*2A), and c.2846A>T gene variants.5, 17 PCR-based tests represent the gold standard for genotype testing, and the analytic sensitivity and specificity of PCR-based genotyping tests is generally reported as ≥99%.18 In the base case analysis we modeled the sensitivity and specificity of PCR-based genotyping for DPYD gene variants as 99%.
Table 1.
Model parameters and sources
| Parameter name | Parameter estimate | Range for sensitivity analysis | Source |
|---|---|---|---|
| Probability of carrying a deleterious DPYD gene variant | 0.063 | 0.05, 0.08 | Lee et al., J Natl Cancer Inst, 20145, Lee et al., Pharmacogenet Genomics 201617 |
| Sensitivity of DPYD genotyping | 0.99 | - | Vendor website18 |
| Specificity of DPYD genotyping | 0.99 | 0.985, 0.999 | Vendor website |
| Probability of grade 3–4 toxicity | |||
| - DPYD variant, standard-dose chemotherapy | 0.73 | 0.65, 0.90 | Deenen et al., J Clin Oncol 20168 |
| - DPYD variant, reduced-dose chemotherapy | 0.39 | 0.34, 0.44 | Henricks et al., Lancet Oncol, 201813 |
| - DPYD wild-type, standard-dose chemotherapy | 0.23 | 0.18, 0.28 | Henricks et al., Lancet Oncol, 2018 |
| - DPYD wild-type, reduced dose chemotherapy | 0.23 | 0.18, 0.28 | Henricks et al., Lancet Oncol, 2018a |
| Probability of hospitalization | |||
| - DPYD variant, standard-dose chemotherapy | 0.297 | 0.25, 0.40 | Toffoli et al., Clin Pharm Ther, 201919 |
| - DPYD variant, reduced-dose chemotherapy | 0.188 | 0.15, 0.20 | Henricks et al., Lancet Oncol, 2018 |
| - DPYD wild-type, standard-dose chemotherapy | 0.144 | 0.12, 0.16 | Henricks et al., Lancet Oncol, 2018 |
| - DPYD wild-type, reduced dose chemotherapy | 0.144 | 0.12, 0.16 | Henricks et al., Lancet Oncol, 2018a |
| Health utility values | |||
| Baseline, prior to chemotherapy | 0.61 | 0.5, 0.9 | Best et al., Qual Life Res 201023 |
| −6 to 24 months after chemotherapy | 0.82 | 0.7, 1 | Ramsey et al., Cancer 200024 |
| -Year 3 after chemotherapy | 0.95 | 0.7, 1 | Ramsey et al., Cancer 2000 |
| - Years 4 to 5 after chemotherapy | 0.79 | 0.7, 1 | Ramsey et al., Cancer 2000 |
| - Utility during toxicity-related hospitalization (applied for 1 week) | 0.20 | 0, 0.48 | Author estimate |
| - Utility during grade 3–4 toxicity (applied for 3 months during chemotherapy treatment) | 0.48 | 0.40, 0.55 | Best et al., Qual Life Res 2010 |
| Probability of early treatment-related death | |||
| - DPYD variant, standard-dose chemotherapy | 0.023 | 0.013, 0.039 | Sharma et al, Oncologist 202120 |
| - DPYD variant, reduced-dose chemotherapy | 0.002 | 0.001, 0.005 | Derived from Sharma et al, Oncologist 2021 |
| - DPYD wild-type, standard-dose chemotherapy | 0.001 | 0.001, 0.002 | Sharma et al, Oncologist 2021 |
| - DPYD wild-type, reduced dose chemotherapy | 0.001 | 0.001, 0.002 | Sharma et al, Oncologist 2021a |
| Mortality after completion of adjuvant treatment period | |||
| - Until year 5 | Supplementary data table | Derived from SEER data (author analysis) | |
| - Until year 5, patients with “false positive” DPYD genotyping and reduced-dose chemotherapy | Hazard ratio (HR) of 1.32b | HR 1.00, 1.50 | Derived from22IMPACT investigators, Lancet ’95. |
| Costsc | |||
| - DPYD genotyping | $174.42 | $0, $300 | 25Clinical Laboratory Fee Schedule, CMS.gov |
| - Hospitalization for chemotherapy toxicity | $15,524.04 | $0, $30,000 | 26Roeland et al. J Clin Oncol ’18 (suppl, 112) |
| - Cancer recurrence after reduced-dose chemotherapy (DPYD wild-type) | $97,064.91 | $0, $120,000 | 27Mariotto et al. J Natl Cancer Inst ’11. |
Toxicities and adverse events extrapolated from observations in patients receiving standard-dose 5-FU chemotherapy.
Increased hazard of death is expressed as compared to SEER-derived survival data described in the row above.
Costs expressed in 2020 US dollars.
Estimates for the risk of treatment-related toxicity and hospitalization came from Henricks et al13 (NCT02324452; a large, prospective study of dose-reduced chemotherapy for patients with pathogenic DPYD mutations, with a comparison group of patients with wild-type DPYD genotyping), Deenen et al8 (which described grade ≥3 toxicities in a historical cohort of patients with the DPYD *2A variant who received standard-dose fluoropyrimidine chemotherapy), and Toffoli et al19 (which reported on hospitalizations in a historical cohort of patients with DPYD *2A, *13, c.2846A>T, or HapB3 variants who received standard-dose chemotherapy.) The risk of treatment-related death, conditional on DPYD genotype and standard vs reduced-dose chemotherapy, was taken from the systematic review by Sharma et al.20 There is scant evidence to estimate the risk of treatment-related death in patients with pathogenic DPYD variants receiving reduced-dose chemotherapy; however, two prospective studies suggest that this risk is similar to the risk of treatment-related death in patients with wild-type DPYD genotype receiving standard-dose chemotherapy,8, 13 and our analysis uses an estimate of 0.2% (two times the risk of a patient with wild-type DPYD genotype receiving standard-dose chemotherapy.)
Estimates for mortality in the five years after completing adjuvant chemotherapy were based on our analysis of data from the U.S. Surveillance, Epidemiology, and End Results (SEER) program for patients with stage 3 colorectal cancer (see Table 2).21 For patients receiving reduced-dose chemotherapy after a “false positive” DPYD genotype test, we modeled a 32% increase in the hazard of death. This estimate is the inverse of the 24% reduction in the hazard of death associated with receipt of fluoropyrimidine chemotherapy in the IMPACT meta-analysis,22 reflecting a conservative assumption that the fluoropyrimidine component of adjuvant chemotherapy is ineffective in patients with a normal (wild-type) DPYD genotype who receive reduced-dose therapy.
Table 2.
Six month interval probability of death following adjuvant chemotherapy for stage 3 colon cancer
| Model stage | Time from treatment | Probability of death |
|---|---|---|
| 1 | 0 to <6 months | 0.017 |
| 2 | 6 to <12 months | 0.034 |
| 3 | 12 to <18 months | 0.041 |
| 4 | 18 to <24 months | 0.042 |
| 5 | 24 to <30 months | 0.044 |
| 6 | 30 to <36 months | 0.041 |
| 7 | 36 to <42 months | 0.04 |
| 8 | 42 to <48 months | 0.035 |
| 9 | 48 to <54 months | 0.039 |
| 10 | 54 to <60 months | 0.038 |
Probability of death in six month intervals derived from the authors’ analysis of SEER data for patients with stage 3 colon cancer receiving adjuvant chemotherapy.
Utility values for specific health states were derived from published studies of patients with colorectal cancer.23, 24 The model incorporated monetary costs for DPYD genotyping, derived from the Clinical Laboratory Fee Schedule of the Centers for Medicare and Medicaid Services.25 The estimated cost of hospitalization for chemotherapy toxicity was derived from a U.S. study of inpatient hospitalization costs in patients with chemotherapy-induced nausea and vomiting.26 For patients with “false positive” DPYD genotyping results, we modeled additional costs associated with cancer recurrence and death.27 We assumed that other costs would accrue equally across both arms of the model. All costs were adjusted to 2020 U.S. dollars, using the Personal Health Care price index and the Personal Consumption Expenditure health component price index.28 Future costs were discounted at 3% per year.
Sensitivity analysis
We performed one-way sensitivity analyses of key model estimates including prevalence of patients with DPYD gene variants, sensitivity and specificity of DPYD genotyping, health utility values before, during and after adjuvant chemotherapy, cost of DPYD genotyping, cost of hospitalization related to chemotherapy toxicity, and increased mortality risk and cost in patients with “false positive” DPYD genotyping. The range of parameter values tested in the sensitivity analyses is shown in Table 1. We also conducted probabilistic sensitivity analysis to demonstrate parameter uncertainty by sampling each parameter’s value from a distribution. We used a uniform distribution for each parameter, constraining minimum and maximum values to correspond with the parameter’s upper and lower bounds.
Results
Compared with no screening for DPD deficiency, DPYD genotyping was associated with an incremental cost of $78 and an incremental effectiveness of 0.0038 QALYs. The ICER for DPYD genotyping was $20,506/QALY. In one-way sensitivity analyses, the cost-effectiveness of DPYD genotyping was sensitive to the input parameters for test cost and the cost of hospitalization for chemotherapy-related toxicity. The ICER exceeded $50,000 per QALY when the cost of the DPYD genotyping assay was greater than $286. The strategy of DPYD genotyping became dominant to the “no screening” strategy (with lower cost and greater quality-adjusted survival) when the cost of DPYD genotyping was less than $96 or when the cost of hospitalization related to chemotherapy toxicity was greater than $27,778.
In the probabilistic sensitivity analysis, we conducted a Monte Carlo simulation of 100,000 iterations. At the willingness-to-pay threshold of $50,000 per QALY DPYD genotyping was preferred to no screening in 96.2% of iterations, and DPYD genotyping dominated no screening (with lower cost and higher QALYs) in 47.5% of iterations. Figure 2 depicts a plot of incremental costs and QALYs for each of the 100,000 Monte Carlo iterations.
Figure 2.
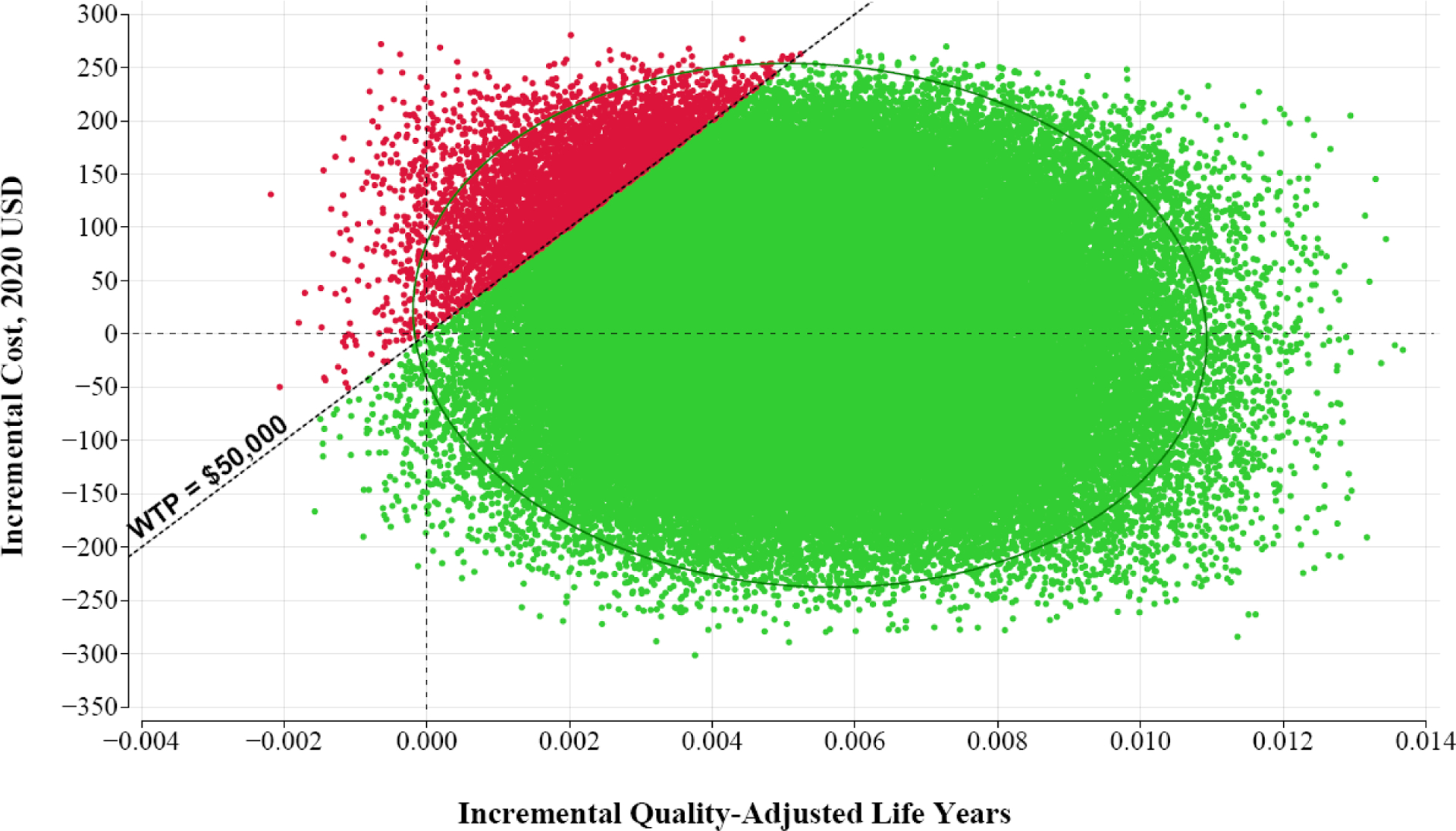
Monte Carlo plot of 100,000 model iterations from probabilistic sensitivity analysis
Each plotted point represents an iteration of the probabilistic sensitivity analysis. The diagonal line indicates a willingness-to-pay (WTP) threshold of $50,000 and the ellipse represents the 95% confidence interval. DPYD genotyping is preferred to the no screening strategy for green points below the WTP threshold.
Discussion
DPD deficiency is an uncommon condition that predisposes patients to severe, potentially fatal toxicity from treatment with fluoropyrimidine chemotherapies (5-fluorouracil or capecitabine).2 A large proportion of patients with DPD deficiency can be detected through genotyping for pathogenic variants of the DPYD gene, and two high-quality prospective clinical trials have demonstrated that DPYD genotyping, linked with chemotherapy dose reductions for variant carriers, leads to substantial reductions in severe chemotherapy toxicities.8, 13 In this analysis, we evaluated the cost-effectiveness of DPYD genotyping prior to adjuvant chemotherapy for stage 3 colon cancer from the perspective of the U.S. healthcare system. In the base-case analysis, we found that DPYD genotyping improved quality-adjusted survival, with an ICER of $20,506 per QALY. Probabilistic sensitivity analysis showed that the ICER was less than $50,000/QALY in 96% of the model simulations—with 47.5% of showing that DPYD genotyping dominated the no screening strategy. We conclude that DPYD genotyping is highly likely to be cost-effective from a U.S. healthcare perspective, whether that is measured in reference to the widely-cited threshold of $100,000/QALY, or in reference to the cost-effectiveness of colon cancer treatments that are widely used in the U.S.29–31
A number of prior studies have evaluated the cost and outcomes of DPYD genotyping from European and Canadian perspectives. Henricks and colleagues performed a cost analysis32 of DPYD genotyping from a Dutch perspective, using data from their pivotal prospective trial (NCT02324452).13 Their analysis did not formally assess the effectiveness of DPYD genotyping (did not estimate incremental QALYs), but concluded that DPYD genotyping was likely cost-saving due to reduced costs of toxicity management in the context of the trial’s patient population.32 Murphy and colleagues retrospectively estimated savings from DPYD genotyping in a cohort of 134 patients from a single center in Ireland.33 They found that the cost of DPYD genotyping for these 134 patients would have been considerably less than the cost of toxicity-related hospitalizations incurred in 5 patients with retrospectively identified pathogenic DPYD gene variants. In 2021 Ontario Health (the Canadian provincial health authority) conducted a Health Technology Assessment of DPYD genotyping; this assessment focused on short-term costs and benefits of DPYD genotyping occurring within a six-month time horizon.34 The assessment found that DPYD genotyping dominated no screening for DPD deficiency, with incremental savings of $145 and a gain of 0.0011 QALYs. Additional studies have concluded that management costs for chemotherapy-related toxicity are higher in patients who are carriers of pathogenic DPYD gene variants.19, 35
Our study adds to prior research by using formal cost-effectiveness methods and incorporating evidence-based parameter estimates relevant to the U.S. healthcare setting. Our model uses conservative assumptions regarding the clinical utility of DPYD genotyping for improving quality-adjusted survival. For example, our model incorporates the hypothetical risk that DPYD genotyping could produce false-positive results in 1% of patients (specificity = 99%), leading to unwarranted chemotherapy dose-reductions and potential loss of therapeutic benefit from adjuvant chemotherapy. Even with this conservative approach, we found that the clinical benefits of DPYD genotyping outweighed harms, leading to an incremental improvement in QALYs.
The cost of DPYD genotyping is an important parameter in our model. We modeled the cost of genotyping at $174—the allowable amount listed in the Medicare Clinical Laboratory Fee Schedule. Alternatively, sensitivity analysis showed that DPYD genotyping dominates the no screening approach when the test cost is less than $96. These cost estimates assume that DPYD genotyping has not been previously completed and must be ordered a la carte prior to chemotherapy treatment. As the cost of germline genomic testing continues to decrease (with some vendors offering whole genome sequencing at less than $1000), it is increasingly feasible to imagine that many patients will have panel testing for actionable germline genetic variants as part of their initial oncologic evaluation.36 In this case the marginal cost of assessing a patient’s DPYD genotype could fall essentially to zero.
One notable aspect of our results is that the average benefit of DPYD genotyping is small, as reflected by the incremental survival benefit of 0.0038 QALYs. However, this small average benefit masks large effects in the tails of the probability distribution. Most patients in our model (>96.8%) experience neither benefit nor harm from DPYD genotyping, while all of the benefits of testing occur in the 6.3% of patients with detectable, pathogenic DPYD gene variants. A small proportion of patients is spared from treatment-related death (1 in 764 patients), and a larger proportion avoid grade 3–4 toxicity (1 in 48 patients).
Our analysis has limitations. Literature-based estimates are unavailable for some of the parameters in our model, including estimates for the precise sensitivity and specificity of DPYD genotyping and the effectiveness of reduced-dose adjuvant chemotherapy in patients with or without DPYD gene variants. As described above, we used conservative estimates for these model parameters so that our analysis would have a tendency to underestimate the cost-effectiveness of DPYD genotyping. We only modeled one strategy of screening for DPD deficiency, with DPYD genotyping. While DPYD genotyping is the only clinical test for DPD deficiency that is widely available in the U.S., alternative tests are used in Europe and other countries, including the plasma uracil concentration and a “multi-parametric” approach.12, 14, 37 We did not model the clinical utility of DPYD genotyping in patients from distinct ancestral populations, and it is likely that DPYD genotyping is less sensitive for DPD deficiency in non-white patients, who are less likely to carry the canonical DPYD gene variants described in early studies of white patients of European ancestry.38
In summary, we found that DPYD genotyping improves quality-adjusted survival and is highly likely to be cost-effective among patients receiving adjuvant chemotherapy for stage 3 colon cancer. Our analysis uses parameter estimates that are relevant to the U.S. health care setting, and we conclude that U.S. health authorities should include DPYD genotyping in clinical care guidelines for patients who will receive fluoropyrimidine chemotherapy, consistent with the EMA’s recent recommendation in favor of universal screening for DPD deficiency.37 Further study is warranted to evaluate how the clinical utility, cost-effectiveness, and equity of DPYD genotyping compare with other modalities of screening for DPD deficiency.
Acknowledgments
Research reported in this manuscript was supported by The Dartmouth-Hitchcock Cancer Research Fellows Program and by the National Cancer Institute Cancer Center Support Grant 5P30CA023108 to the Dartmouth-Hitchcock Norris Cotton Cancer Center, as well as by The Dartmouth Clinical and Translational Science Institute, under award number UL1TR001086 from the National Center for Advancing Translational Sciences of the National Institutes of Health. This research was presented in abstract form as part of the 2021 American Society of Clinical Oncology Gastrointestinal Cancers Symposium (January 15, 2021 [virtual meeting]).
References
- 1.Meyers BM, Cosby R, Quereshy F, Jonker D. Adjuvant Chemotherapy for Stage II and III Colon Cancer Following Complete Resection: A Cancer Care Ontario Systematic Review. Clin Oncol (R Coll Radiol). 2017;29:459–465. [DOI] [PubMed] [Google Scholar]
- 2.Innocenti F, Mills SC, Sanoff H, Ciccolini J, Lenz HJ, Milano G. All You Need to Know About DPYD Genetic Testing for Patients Treated With Fluorouracil and Capecitabine: A Practitioner-Friendly Guide. JCO Oncol Pract. 2020;16:793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. [DOI] [PubMed] [Google Scholar]
- 4.Grothey A, Sobrero AF, Shields AF, et al. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N Engl J Med. 2018;378:1177–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee AM, Shi Q, Pavey E, et al. DPYD variants as predictors of 5-fluorouracil toxicity in adjuvant colon cancer treatment (NCCTG N0147). J Natl Cancer Inst. 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terrazzino S, Cargnin S, Del Re M, Danesi R, Canonico PL, Genazzani AA. DPYD IVS14+1G>A and 2846A>T genotyping for the prediction of severe fluoropyrimidine-related toxicity: a meta-analysis. Pharmacogenomics. 2013;14:1255–1272. [DOI] [PubMed] [Google Scholar]
- 7.Rosmarin D, Palles C, Church D, et al. Genetic markers of toxicity from capecitabine and other fluorouracil-based regimens: investigation in the QUASAR2 study, systematic review, and meta-analysis. J Clin Oncol. 2014;32:1031–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deenen MJ, Meulendijks D, Cats A, et al. Upfront Genotyping of DPYD*2A to Individualize Fluoropyrimidine Therapy: A Safety and Cost Analysis. J Clin Oncol. 2016;34:227–234. [DOI] [PubMed] [Google Scholar]
- 9.Rai K, Batukbhai BDO, Brooks GA. Risk of treatment-related death in carriers of pathogenic DPYD polymorphisms treated with fluoropyrimidine chemotherapy: A systematic review and patient-level analysis. J Clin Oncol. 2019;37:e15132–e15132. [Google Scholar]
- 10.van Kuilenburg AB. Dihydropyrimidine dehydrogenase and the efficacy and toxicity of 5-fluorouracil. Eur J Cancer. 2004;40:939–950. [DOI] [PubMed] [Google Scholar]
- 11.Mattison LK, Fourie J, Desmond RA, Modak A, Saif MW, Diasio RB. Increased prevalence of dihydropyrimidine dehydrogenase deficiency in African-Americans compared with Caucasians. Clin Cancer Res. 2006;12:5491–5495. [DOI] [PubMed] [Google Scholar]
- 12.Meulendijks D, Henricks LM, Jacobs BAW, et al. Pretreatment serum uracil concentration as a predictor of severe and fatal fluoropyrimidine-associated toxicity. Br J Cancer. 2017;116:1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henricks LM, Lunenburg C, de Man FM, et al. DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol. 2018;19:1459–1467. [DOI] [PubMed] [Google Scholar]
- 14.Boisdron-Celle M, Capitain O, Faroux R, et al. Prevention of 5-fluorouracil-induced early severe toxicity by pre-therapeutic dihydropyrimidine dehydrogenase deficiency screening: Assessment of a multiparametric approach. Semin Oncol. 2017;44:13–23. [DOI] [PubMed] [Google Scholar]
- 15.Lunenburg C, Henricks LM, Guchelaar HJ, et al. Prospective DPYD genotyping to reduce the risk of fluoropyrimidine-induced severe toxicity: Ready for prime time. Eur J Cancer. 2016;54:40–48. [DOI] [PubMed] [Google Scholar]
- 16.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)--explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16:231–250. [DOI] [PubMed] [Google Scholar]
- 17.Lee AM, Shi Q, Alberts SR, et al. Association between DPYD c.1129–5923 C>G/hapB3 and severe toxicity to 5-fluorouracil-based chemotherapy in stage III colon cancer patients: NCCTG N0147 (Alliance). Pharmacogenet Genomics. 2016;26:133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ARUP Laboratories. Dihydropyrimidine Dehydrogenase (DPYD), 3 Variants. Vol 2021.
- 19.Toffoli G, Innocenti F, Polesel J, et al. The Genotype for DPYD Risk Variants in Patients With Colorectal Cancer and the Related Toxicity Management Costs in Clinical Practice. Clin Pharmacol Ther. 2019;105:994–1002. [DOI] [PubMed] [Google Scholar]
- 20.Sharma BB, Rai K, Blunt H, Zhao W, Tosteson TD, Brooks GA. Pathogenic DPYD Variants and Treatment-Related Mortality in Patients Receiving Fluoropyrimidine Chemotherapy: A Systematic Review and Meta-Analysis. Oncologist. 2021;26:1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER 18 Regs Custom Data (with additional treatment fields), Nov 2018 Sub (2000–2016) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission.: National Cancer Institute; 2019. [Google Scholar]
- 22.Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet. 1995;345:939–944. [PubMed] [Google Scholar]
- 23.Best JH, Garrison LP, Hollingworth W, Ramsey SD, Veenstra DL. Preference values associated with stage III colon cancer and adjuvant chemotherapy. Qual Life Res. 2010;19:391–400. [DOI] [PubMed] [Google Scholar]
- 24.Ramsey SD, Andersen MR, Etzioni R, et al. Quality of life in survivors of colorectal carcinoma. Cancer. 2000;88:1294–1303. [PubMed] [Google Scholar]
- 25.CMS.gov: Clinical Laboratory Fee Schedule Files. Vol 2021.
- 26.Roeland E, Nipp RD, Ruddy KJ, et al. Inpatient hospitalization costs associated with nausea and vomiting among patients with cancer. J Clin Oncol. 2018:112–112. [Google Scholar]
- 27.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunn A, Grosse SD, Zuvekas SH. Adjusting Health Expenditures for Inflation: A Review of Measures for Health Services Research in the United States. Health Serv Res. 2018;53:175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shankaran V, Ortendahl JD, Purdum AG, et al. Cost-Effectiveness of Cetuximab as First-line Treatment for Metastatic Colorectal Cancer in the United States. Am J Clin Oncol. 2018;41:65–72. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein DA, Chen Q, Ayer T, et al. Bevacizumab for Metastatic Colorectal Cancer: A Global Cost-Effectiveness Analysis. Oncologist. 2017;22:694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherman SK, Lange JJ, Dahdaleh FS, et al. Cost-effectiveness of Maintenance Capecitabine and Bevacizumab for Metastatic Colorectal Cancer. JAMA Oncol. 2019;5:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henricks LM, Lunenburg C, de Man FM, et al. A cost analysis of upfront DPYD genotype-guided dose individualisation in fluoropyrimidine-based anticancer therapy. Eur J Cancer. 2019;107:60–67. [DOI] [PubMed] [Google Scholar]
- 33.Murphy C, Byrne S, Ahmed G, et al. Cost Implications of Reactive Versus Prospective Testing for Dihydropyrimidine Dehydrogenase Deficiency in Patients With Colorectal Cancer: A Single-Institution Experience. Dose Response. 2018;16:1559325818803042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DPYD Genotyping in Patients Who Have Planned Cancer Treatment With Fluoropyrimidines: A Health Technology Assessment. Ont Health Technol Assess Ser. 2021;21:1–186. [PMC free article] [PubMed] [Google Scholar]
- 35.Cortejoso L, García-González X, García MI, García-Alfonso P, Sanjurjo M, López-Fernández LA. Cost-effectiveness of screening for DPYD polymorphisms to prevent neutropenia in cancer patients treated with fluoropyrimidines. Pharmacogenomics. 2016;17:979–984. [DOI] [PubMed] [Google Scholar]
- 36.Hicks JK, Howard R, Reisman P, et al. Integrating Somatic and Germline Next-Generation Sequencing Into Routine Clinical Oncology Practice. JCO Precis Oncol. 2021:884–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.European Medicines Agency. 5-Fluorouracil (i.v.), capecitabine and tegafur containing products: Pre-treatment testing to identify DPD-deficient patients at increased risk of severe toxicity. Vol 2021.
- 38.da Rocha JEB, Lombard Z, Ramsay M. Potential Impact of DPYD Variation on Fluoropyrimidine Drug Response in sub-Saharan African Populations. Front Genet. 2021;12:626954. [DOI] [PMC free article] [PubMed] [Google Scholar]


