Abstract
The C18-carboxypropylbetaine (CB-18) procedure for processing respiratory specimens for the detection of mycobacteria was shown to provide significant increases in sensitivity by smear and culture. However, the procedure also produced increased contamination, a loss in liquid culture sensitivity, and a reduction in smear specificity. Because of these observations, the toxicity of CB-18 and the nature of the contamination were characterized. Preincubation in 1 mM CB-18 impacted viability in a time-dependent fashion, but the magnitude of the loss was species and isolate dependent. Mycobacterium tuberculosis isolates were the most susceptible, losing 20 to 30% of the CFU within 30 min and 30 to 60% after 3 h, whereas Mycobacterium avium and Mycobacterium fortuitum isolates were unaffected by CB-18. In liquid culture, when the concentration of CB-18 exceeded 5 μg/ml, there was an impact on growth characteristics for the most susceptible M. tuberculosis isolate. In contrast, M. fortuitum isolates were able to grow in 100 μg of CB-18 per ml. In liquid culture, the deleterious effects of CB-18 were enhanced in the presence of antibiotics, whereas growth on solid media was not similarly affected. Supplementation of the resuspension buffer with 0.15% lecithin alleviated toxicity. Initial attempts to modify the CB-18 procedure to control contamination incorporated acids or alkalis; however, losses in culture sensitivity occurred. Studies to identify these contaminants led to the development of a sediment resuspension buffer that contained lytic enzymes to combat contamination and lecithin to alleviate toxicity. This formulation included lysozyme, zymolyase, and Cytophaga and Trichoderma extracts and was seen to reduce contamination to acceptable levels (<5%).
In industrialized countries, the “gold standard” for evaluating the presence of mycobacteria in respiratory specimens is culture. As with most diagnostic techniques, this method has both advantages and disadvantages. For example, while culture is extremely sensitive, it is also susceptible to contamination problems and absolutely requires that the acid-fast bacilli (AFB) survive specimen processing. The consequence of contamination would be a false-positive result, and a false-negative result would be the outcome of compromising mycobacterial viability during processing. Specimen processing is an essential prerequisite for culture because of the presence of other saprophytic and infectious organisms. Most processing agents impact viability to the extent that specimen processing becomes a tradeoff between killing as many contaminants as possible and trying not to kill all the mycobacteria (10, 12, 30, 32, 33). Yajko et al. (32) have shown that the efficacy of killing Mycobacterium tuberculosis with NaOH-based processing can exceed 90%.
In addition to the viability issue, all contemporary methods for processing specimens impact diagnostic sensitivity because of the buoyant nature of the mycobacteria. The loss of bacilli sustained through inefficient collection by centrifugation has been well documented (5–8, 15–20). A novel method for processing human respiratory specimens was recently introduced that increased smear sensitivity by 58% (P < 0.01) and aggregate culture sensitivity by approximately 43% (P < 0.01), relative to specimens processed by a method combining NALC and NaOH (NALC/NaOH) (24). In this study, statistically significant reductions in the time to a positive result of over 12 days in liquid culture (P < 0.01) and over 8 days on solid media (P < 0.025) were observed for tuberculous mycobacteria. This method was based on the use of the zwitterionic detergent N,N-dimethyl-N-(n-octadecyl)-N-(3-carboxypropyl) ammonium inner salt (Chemical Abstract Service no. 78195-27-4), also known as C18-carboxypropylbetaine (CB-18). The CB-18 procedure is the only method to date to compensate for the innate buoyancy of the mycobacteria in order to minimize the loss of mycobacteria (23) and maintain mycobacterial viability (22, 24) during specimen processing. While the less harsh processing conditions provided by the CB-18 method resulted in gains in culture sensitivity (24), the contamination rate in liquid culture for NALC/NaOH-processed respiratory specimens was 7.5%, compared to 20.8% for CB-18-processed specimens. There was also an unexplained reduction in liquid culture sensitivity of 33% with the CB-18 method, as well as a reduction in smear specificity of either 3.1% (comparison between processing methods) or 8.8% (comparison within the CB-18 method) relative to culture.
In order to optimize the gains in diagnostic sensitivity provided by this method, important parameters of CB-18 processing with respect to CB-18 toxicity, mycobacterial viability, and contamination were investigated. The contamination studies discussed took place before, during, and after the CB-18 pilot study (24). The toxicity and mycobacterial viability studies took place following the CB-18 pilot study in an effort to rationalize the observed loss in liquid culture sensitivity and smear specificity. Analysis of CB-18 toxicity led to the finding that lecithin, which has been shown to facilitate culture of mycobacteria (2, 9, 14, 29, 31), can also alleviate the deleterious effects of CB-18. This analysis, combined with an understanding of the nature of the contaminants breaking through the liquid culture system and the fact that CB-18 processing does not yield the same pH restrictions as contemporary methods, has led to the development of a simple sediment resuspension buffer that contains lecithin to alleviate toxicity and a series of lytic enzymes (lysozyme, zymolyase, Cytophaga extract, and Trichoderma extract [LZCT]) to reduce contamination to acceptable levels.
MATERIALS AND METHODS
Specimens.
Most specimens for this study were collected from either the general microbiology laboratory or the AFB laboratory at Quest Diagnostics—Baltimore, Baltimore, Md. (Quest). Other collection sites included the AFB laboratories of the D.C. Department of Human Services Bureau of Laboratories, Washington, D.C.; Johns Hopkins Medical Institutes, Baltimore, Md.; the Maryland Department of Health & Mental Hygiene, Baltimore, Md.; Quest Diagnostics—Teterboro, Teterboro, N.J.; and the University of Maryland at Baltimore, Baltimore, Md. (22, 24). Most AFB laboratory specimens were split such that equal portions of a given specimen could be processed by two different methods for comparative purposes (22, 24). Collection of respiratory specimens from the general microbiology and AFB laboratories at Quest for the contamination studies was performed as follows. On a daily basis, laboratory personnel collected discarded respiratory specimens which had been submitted for routine analysis. Typically, 2- to 3-ml portions of each specimen were removed for processing.
Purified isolates.
Several purified strains of bacteria, yeast, and fungi were used in these studies. The following strains were obtained from the American Type Culture Collection (Rockville, Md.): M. tuberculosis (ATCC 27294), Mycobacterium avium (ATCC 25291), Mycobacterium kansaii (ATCC 12478), Mycobacterium fortuitum (ATCC 6841), Mycobacterium xenopi (ATCC 19250), Mycobacterium gordonae (ATCC 14470), and Proteus mirabilis (ATCC 12453). The 571/573-S1 isolate previously described (24) was also used in this study. All M. tuberculosis isolates were confirmed by using molecular probes (Accuprobe [Gen-Probe, San Diego, Calif.) and biochemical assays (7). Other laboratory strains were derived from clinical specimens. These included Candida albicans, an Aspergillus species, a soil saprophyte, Staphylococcus epidermis, Staphylococcus aureus, Micrococcus luteus, a Bacillus species, Klebsiella pneumoniae, and Escherichia coli. All of these organisms were purified, identified, and maintained according to accepted procedures (13).
Liquid culture.
All liquid cultures employed the BACTEC 12B/460TB system (Becton-Dickinson, Cockeysville, Md.). All BACTEC 12B liquid cultures were supplemented with either reconstitution fluid (12B/RF), PANTA (12B/PANTA), or PANTA containing ceftazidime at a final concentration of 8 μg/ml (12B/PANTA/caz). Ceftazidime was made and added to liquid culture as previously described (24).
12B bottles inoculated with sediments from clinical specimens were monitored every other day for 2 weeks and then weekly for the next 6 weeks before being discarded. 12B bottles were considered positive when the growth index (GI) was ≥15. All positive samples were subjected to acid-fast staining with auramine-rhodamine (fluorochromes) according to the instructions of the manufacturer (DIFCO Laboratories, Inc., Detroit, Mich.) to assess the presence of AFB. Positive samples were also used to inoculate blood agar plates (Becton Dickinson) to assess the presence of contamination. Where indicated, contaminants were purified and identified as described below.
For all in vitro experiments with liquid culture, four replicate 12B bottles were inoculated (volumes indicated below) and checked every other day for growth. When the GI became positive, samples were checked daily until the maximum GI was achieved (GI = 999). Samples that did not become positive were checked every other day for 3 weeks and then for an additional 1 to 3 weeks before being discarded. Several samples became positive but did not enter the exponential phase of growth for the 4-week incubation. In those cases, samples were checked weekly after the third week; however, readings were done on 2 consecutive days to evaluate daily growth. The GI recorded on the second reading was used to plot the growth curves, which were plotted as the average of the group and analyzed with a two-tailed, heteroscediastic Student’s t test. All positive cultures from in vitro samples were subjected to acid-fast smear analysis by the Kinyoun method (7) to confirm the presence of AFB. Random isolates from each experiment were confirmed by Accuprobe.
Culture on solid media.
All processed clinical specimens, controls, or experiments analyzing growth characteristics on solid media used 7H11-selective plates (Becton Dickinson). Plates were placed in sealed plastic bags and incubated in 5 to 10% CO2. Plates derived from clinical specimens were checked weekly for growth for 8 weeks, and plates from in vitro experiments were checked after 3 weeks. Colony counts for in vitro experiments were reported as the average of the group ± the standard deviation. All positive cultures were subjected to acid-fast smear analysis to confirm the presence of AFB.
Identification of contaminants.
All contaminants were identified by morphology and/or Gram stain (Becton Dickinson) and then differentiated as either catalase positive or negative by standard techniques (13) or as oxidase positive or negative according to the manufacturer’s instructions (Becton Dickinson). Bacterial contaminants were then identified to the species level, and antibiotic sensitivities were determined with MicroScan panels (Dade International, West Sacramento, Calif.) according to the manufacturer’s instructions.
NALC/NaOH processing.
NALC/NaOH-processed specimens were treated with an equal volume of 3 to 4% NaOH containing 0.5% NALC–1.45% citrate for 20 min at room temperature, diluted with sterile filtered water, and then subjected to centrifugation at 3,800 × g for 20 min at 4°C. Following centrifugation, specimens were decanted and sediments were resuspended in 1 ml of sterile, filtered water and inoculated into 12B/PANTA or MGIT tubes (Becton Dickinson) and analyzed as described above or as described previously (24).
CB-18 stock.
CB-18 (Ecochem Research, Inc., Chaska, Minn.) was made as a 100-fold concentrate (100 mM) in 1:1 isopropanol-water. Buffered CB-18 was prepared and mixed with specimens as described below. Unused portions of diluted CB-18 were discarded.
CB-18 processing of clinical specimens.
The CB-18 protocol for processing clinical specimens was described previously (24). Briefly, samples were first treated with an equal volume of 0.5% NALC–1.45% citrate for 10 min at room temperature and then brought to a final volume of 40 ml with 4 ml of a 10-fold concentrate of buffered CB-18 and sterile, filtered water. The final concentration of added components was 50 mM Tris-HCl (pH 8.0), 0.1 mM NaCl, 1.0 mM CB-18, and 5 mM NALC. Specimens were then shaken (140 rpm) for 90 min at 37°C prior to centrifugation at 3,800 × g for 20 min at 30°C. Specimens were decanted, and sediments were resuspended in 0.5 ml of sterile, filtered water.
Acid and alkali modifications.
Initial attempts to control CB-18 contamination used either acid or alkali treatments before or after CB-18 addition. Pretreatment with NaOH (NaOH/CB-18) was accomplished by first mixing the specimen with an equal volume of 1% NaOH containing 1.45% sodium citrate and 0.5% NALC and then incubating it for 20 min at room temperature. The specimen was then neutralized with an amount of HCl equimolar to the amount of NaOH originally added to the specimen. Following neutralization, specimens were brought to a final volume of 40 ml with 4 ml of a 10-fold concentrate of buffered CB-18 and sterile, filtered water and processed as described above. All sediments were analyzed on 12B/PANTA.
Pretreatment with oxalic acid (OxAc/CB-18) was accomplished by first mixing the specimen with an equal volume of 5% oxalic acid (7) and then incubating it for 15 min at room temperature. The specimen was then neutralized with an amount of NaOH equimolar to the amount of oxalic acid originally added to the specimen. Following neutralization, specimens were brought to a final volume of 40 ml with 4 ml of a 10-fold concentrate of buffered CB-18 and sterile, filtered water and processed as described above. All sediments were analyzed on 12B/PANTA.
Treatments with acid following CB-18 processing (CB-18/Acid) were performed as previously described (24). Briefly, contaminated cultures (4.5 ml) were brought to a final volume of 10 ml with 1 ml of a 10-fold concentrate of buffered CB-18 and sterile, filtered water and incubated with shaking (140 rpm) for 90 min at 37°C. Following incubation, 500 μl of 10 N sulfuric acid was added to the specimen, which was then incubated for an additional 15 min at room temperature. The specimen was then neutralized with 1 ml of 5 N NaOH. Specimens were then brought to a final volume of 40 ml with 50 mM Tris-HCl (pH 8.0) and subjected to centrifugation and analysis on 12B/PANTA/caz.
CB-18/Tris-citrate buffer.
A Tris-citrate buffer was developed for use in conjunction with the lytic enzymes and was composed of 50 mM Tris-HCl, 12.5 mM citrate (pH 7.6), 1.5 mM NaCl, and 5 mM NALC. Tris-citrate buffer was prepared as a 20-fold stock by using 1 M Tris-HCl (pH 9.10) (at 25°C) and then adding citric acid monohydrate (Sigma, St. Louis, Mo.) to 0.25 M to adjust the pH to 7.6 and then adding NaCl to 30 mM (all concentrations final). Preparation of the CB-18/Tris-citrate buffer for use was accomplished by fully diluting the Tris-citrate stock (20:1) and CB-18 stock (100:1) immediately prior to use and then mixing with NALC to 5 mM. Prepared samples were mixed with this buffer as described below.
Lecithin.
A 100-fold concentrate of type X-E dried egg yolk lecithin (Sigma; catalog P-5394) was made by dissolving lecithin in 95% ethanol to 7.5% (wt/vol). Immediately prior to use, lecithin was diluted 50:1 in Tris-citrate buffer (with or without lytic enzymes) and then added to the sediments as described below.
CB-18/LZCT processing.
For CB-18/LZCT processing, specimens were processed with CB-18 as described above, with the exception that the processing solution was changed to the CB-18/Tris-citrate buffer described above, and sediments were resuspended in the LZCT/lecithin buffer described below (CB-18/LZCT).
A 10-fold stock of the LZCT lytic enzymes was made by diluting the 20-fold Tris-citrate stock in half (2:1) and adding lysozyme (Sigma) to 10 mg/ml, zymolyase (also known as lyticase [Sigma]) to 5,000 U/ml, Cytophaga extract (Sigma), to 1 mg/ml, and Trichoderma extract (Sigma) to 10 mg/ml. This stock was brought to the final volume with sterile, filtered water, gently mixed until all solid material was dissolved, and then quickly divided into 0.5-, 1-, and 2-ml portions and immediately stored at −20°C.
The LZCT sediment resuspension buffer containing lecithin (LZCT/lecithin) was prepared for use as a twofold concentrate. Immediately prior to use, the 10-fold LZCT enzyme stock was thawed and diluted 5:1 with water. Lecithin (above) was added by diluting the 100-fold lecithin stock 50:1 (to 0.15%) and then adding NALC to 5 mM. To the decanted specimens that had been processed with CB-18/Tris-citrate was added 1 ml of this twofold LZCT/lecithin mixture. The resuspended sediments were incubated at 37°C for 20 min. Portions (400 μl) of decontaminated sediments were removed directly to 12B/PANTA/caz. The fully diluted LZCT/lecithin formulation consisted of lysozyme at 1 mg/ml, zymolyase at 500 U/ml, Cytophaga extract at 0.1 mg/ml, and Trichoderma extract at 1 mg/ml in a combination of 50 mM Tris-HCl, 12.5 mM citrate (pH 7.6), 1.5 mM NaCl, 5 mM NALC, and 0.075% lecithin (all concentrations final).
Preparation of mycobacterial isolates.
All in vitro experiments examining CB-18 toxicity utilized bacilli that had been cultivated on 7H11-selective slants for approximately 2 to 3 weeks. A 0.5 MacFarland standard equivalent of a given isolate was prepared for use as follows: Cells were scraped off the slant, dispersed in 5 ml of sterile water, subjected to sonication in a water bath at 35 kHz (Gen-Probe, San Diego, Calif.) for 5 min, and then centrifuged at 1,000 × g for 5 min. The top 3 ml of the supernatant was collected, and the optical density was checked with a MicroScan Turbidity Meter (Baxter Diagnostics, Deerfield, Ill.) and adjusted with water as necessary to a 0.5 MacFarland standard equivalent. The suspension was diluted in Tris-citrate buffer for use as described below. All in vitro experiments with liquid culture used four replicate 12B bottles to analyze each sample. The inoculum was typically between 500 and 1,000 CFU. Quantitative culture was performed on a dilution (20,000:1) of each MacFarland stock in all experiments to determine bacterial load. Dilutions were made in Tris-citrate buffer, and four replicates were inoculated onto 7H11-selective plates.
CB-18 titration assay.
The CB-18 titration assay was designed to investigate the consequences of having CB-18 as a liquid culture component under clinically relevant circumstances. Growth of each isolate in increasing concentrations of CB-18 was examined by preparing a series of concentrated stocks of CB-18 (45-fold) in isopropanol-water (1:50) and then diluting 100 μl of each directly into 12B bottles. The final concentrations of CB-18 tested for M. tuberculosis, M. avium, and M. kansasii isolates were 5, 10, 15, 20, 25, 30, and 35 μg/ml. The final concentrations of CB-18 tested for M. fortuitum isolates were 5, 10, 20, 30, 40, 50, and 100 μg/ml. A 1:50 solution of isopropanol-water was used as the positive control in all experiments. The MacFarland stock of each isolate tested was first diluted 1,000:1 in Tris-citrate buffer, and then 300-μl portions were used to inoculate 12B/RF or 12B/PANTA/caz bottles containing the predefined amount of CB-18. Four replicates of each CB-18 concentration were inoculated with the diluted stock.
Lecithin neutralization assay.
The neutralization capacity of lecithin in monitoring CB-18 processing was evaluated with the 571/573-S1 isolate. In these experiments, the MacFarland stock was first diluted 50:1 in Tris-citrate buffer. Two 500:1 dilution stocks were then prepared by further diluting 10:1 into either Tris-citrate buffer or CB-18/Tris-citrate to simulate processed sediments. From each of these simulated sediment stocks, two 4-ml aliquots were placed in 50-ml conical tubes to generate one series of two tubes with buffer only and one series of two tubes with buffered CB-18. To one tube in each series was added 2 ml of Tris-citrate buffer (resuspension fluid without lecithin), and to the other tube in each series was added 2 ml of Tris-citrate buffer containing 0.15% lecithin. Each of the four tubes (1:2 buffer-sediment, 1:2 lecithin-sediment, 1:2 buffer–CB-18 sediment, and 1:2 lecithin–CB-18 sediment) was then inoculated (400 μl each, in quadruplicate) into 12B/RF and 12B/PANTA/caz.
Tuberculocidal assay.
The tuberculocidal characteristics of CB-18 processing were evaluated by initially diluting respective MacFarland stocks 200:1 in Tris-citrate buffer containing 1 mM CB-18 to a final volume of 20 ml. At 0, 30, 60, 90, 120, and 180 min, a 1-ml aliquot was removed and serially diluted 10:1 twice to achieve a 100:1 dilution. The first dilution was in Tris-citrate buffer, while the second dilution employed buffered lecithin (0.075% final). Aliquots of 200 μl each were then analyzed on 7H11-selective plates (five replicates for each time point). The number of bacilli surviving was calculated as a percent of the zero time point (± standard deviation).
Recovery on solid media.
The effect of CB-18 relative to recovery on solid media was accomplished with the 571/573-S1 isolate. In this assay, the MacFarland stock was first serially diluted to 1,000:1 in a final volume of 10 ml, and then 1-ml aliquots of this stock were transferred to two different series of 50-ml conical tubes. Each series was composed of three 50-ml tubes. The first tube in each series contained CB-18 at 255 μg/ml, the second tube in each series contained CB-18 at 191 μg/ml, and the third tube in each series contained CB-18 at 77 μg/ml (all concentrations final). The second series also contained lecithin at 0.05, 0.075, and 0.12%, respectively. The positive control contained 1 ml of the diluted MacFarland stock in an equivalent volume of Tris-citrate buffer. After addition of the diluted MacFarland stock, each of these seven tubes was then directly plated (200-μl aliquots) to 7H11-selective plates (five replicates each) and analyzed. Recovery was expressed as a percentage of the control.
RESULTS
Attempts to control contamination.
Prior to the CB-18 pilot study (24), the CB-18 processing method was used to examine 489 specimens derived from five AFB laboratories (22). An increase in culture sensitivity of 30% and a threefold increase in contamination were reported (Table 1). Early attempts to identify an acceptable procedure to control contamination involved combining the CB-18 procedure with contemporary acid-alkali processing methods. An alkaline pretreatment procedure (NaOH/CB-18) was developed and used to process 531 respiratory specimens from five AFB laboratories. Even though the NaOH/CB-18 method used 0.5% NaOH (final concentration), which was half the recommended concentration (7), the culture sensitivity among NaOH/CB-18-processed specimens was decreased by approximately 38% relative to NALC/NaOH-processed specimens (Table 1). Subsequently, an acid pretreatment protocol (OxAc/CB-18) was developed and evaluated using 224 specimens derived from five AFB laboratories. This method was also found to be unacceptable because it reduced the culture sensitivity by almost 62% (Table 1). The CB-18 pilot study described elsewhere (24) reverted to the unmodified procedure and used ceftazidime-fortified PANTA in an attempt to control contamination. Culture sensitivity increased by over 43% with the core CB-18 processing procedure, but the contamination rate again increased by almost threefold (Table 1). The CB-18/Acid protocol previously described (24) was developed as a redigestion procedure for contaminated liquid cultures in the CB-18 pilot study. The CB-18/Acid protocol provided acceptable results on 119 contaminated cultures, but the culture sensitivity was again unacceptable (Table 1). Treatments with alkali following CB-18 incubation were untenable due to the fact that betaines (e.g., CB-18) precipitate at high pH (25).
TABLE 1.
Culture sensitivity and contamination rate comparisons on split specimens processed by NALC/NaOH (4) and different versions of the CB-18 procedure
| Processing method or study (n) | No. of specimens AFB positive | Result by decontamination method
|
|||
|---|---|---|---|---|---|
| NALC/NaOH
|
CB-18
|
||||
| Contamination rate (%) | Culture sensitivity (%) | Contamination rate (%) | Culture sensitivity (%) | ||
| CB-18 preliminary study (489) | 37 | 5.0 | 62.2 | 16.1 | 81.1 |
| NaOH/CB-18 study (531) | 67 | 6.4 | 91.0 | 10.9 | 56.7 |
| OxAc/CB-18 study (224) | 27 | 5.9 | 96.3 | 6.7 | 37.0 |
| CB-18 pilot study (573) | 106 | 7.5 | 61.3 | 20.8 | 87.7 |
| CB-18/Acid redigestion (119)a | 18 | 16.0 | 27.7 | 6.9 | 22.2 |
The 119 specimens described in this study represent the subset of contaminated liquid cultures subjected to CB-18/Acid redigestion in the CB-18 pilot study (24). There were 18 AFB-positive specimens within this group. The corresponding 7H11-selective slants (from the original CB-18-processed specimens) identified 12 of these 18 (66.7%), and NALC/NaOH-processed specimens identified 5 of these 18 (27.7%), whereas CB-18/Acid-redigested specimens identified only 4 of these 18 (22.2%). The contamination rate among NALC/NaOH-processed specimens represents the contamination rate within this subset of 119 specimens, and the contamination rate among the CB-18/Acid-processed specimens represents subsequent contamination among liquid cultures following redigestion.
Contaminant spectrum study.
Fortification of the PANTA antibiotic supplement with ceftazidime in the CB-18 pilot study was based on the results of the contaminant spectrum study. This study analyzed the spectrum of non-AFB organisms breaking through the 12B/PANTA culture system following CB-18 processing. All specimens in the contaminant spectrum study were obtained from the general microbiology laboratory at Quest. The majority of the contaminants were gram-negative rods (GNR), and 62% of these GNR were members of the family Enterobacteriaceae (Table 2), suggesting that controlling contamination required an antibiotic supplement directed against GNR. Ceftazidime has excellent antipseudomonal properies and is also active against many other GNR (11). Based on the MicroScan sensitivity panels, 40 of the 48 GNR were sensitive to ceftazidime at 8 μg/ml (data not shown). Preliminary studies examined the result of culturing mycobacteria in cephalosporin-fortified 12B/PANTA. These in vitro studies used several mycobacterial isolates (M. tuberculosis ATCC 27294, M. avium ATCC 25291, M. kansasii ATCC 12478, M. fortuitum ATCC 6841, M. xenopi ATCC 19250, and M. gordonae ATCC 14470) and suggested that the 12B/PANTA/caz culture system would not significantly impact the viability of the mycobacteria under the conditions tested (data not shown).
TABLE 2.
Spectrum of contaminants isolated from CB-18-processed respiratory specimens
| Identification | No. (%) of specimens with resulta
|
||||
|---|---|---|---|---|---|
| Contamination spectrum study; Quest microbiology laboratory | CB-18 pilot study
|
LZCT studies
|
|||
| All AFB laboratories | Quest AFB laboratory | Quest microbiology laboratory | Quest AFB laboratory | ||
| Gram negative | |||||
| Pseudomonas sp. | 11b | 31c | 9d | 13d | 1d |
| Providencia stuartii | 13 | 7 | |||
| Proteus mirabilis | 7 | 1 | |||
| Alcaligenes sp. | 3 | 2 | 4 | 1 | |
| Morganella morganii | 3 | 1 | 2 | ||
| Serratia marcescens | 2 | 5 | 3 | ||
| Klebsiella sp. | 3 | 2 | |||
| Xanthomonas maltophilia | 1 | 5 | |||
| Acinetobacter sp. | 1 | 3 | 1 | ||
| Enterobacter sp. | 1 | 2 | |||
| Escherichia coli | 1 | 1 | |||
| Citrobacter freundii | 1 | ||||
| Flavobacterium breve | 1 | ||||
| Cedecae davisae | 1 | ||||
| Nonfermenter | 1 | ||||
| Total | 48 (84.2) | 55 (38.2) | 9 (25.7) | 30 (73.2) | 2 (100) |
| Gram positive | |||||
| Staphylococcus | 2 | 16 | 3 | 8 | |
| Cocci | 3 | 1 | 2 | ||
| Rods | 3 | 10 | 2 | ||
| Total | 5 (8.8) | 29 (20.1) | 6 (17.1) | 10 (24.4) | |
| Yeast | 3 (5.3) | 50 (34.7)e | 17 (48.6) | 1 (2.4) | |
| Fungal | 1 (1.8) | 9 (6.2) | 3 (8.6) | ||
| Unknown | 1 (0.7)f | ||||
| Total contaminants isolated | 57 | 144 | 35 | 41 | 2 |
| Total specimens contaminated | 40 (14.4g) | 119 (20.8g) | 31 (18.1g) | 36 (18.6g) | 2 (4.3g) |
| Total specimens processed | 277 | 573 | 171 | 194 | 46 |
The contamination spectrum study used CB-18/12B/PANTA, the CB-18 pilot study used CB-18/12B/PANTA/caz, and the LZCT studies used CB-18/LZCT/12B/PANTA/caz.
Of these 11 pseudomonads, 10 were P. aeruginosa.
Of these 31 pseudomonads, 24 were P. aeruginosa.
All of these pseudomonads were P. aeruginosa.
Candida albicans was by far the most common yeast isolate (≈90%).
One isolate was lost before identification and was judged to be bacterial in nature.
Contamination rate (percentage).
CB-18 pilot study.
The CB-18 pilot study (24) examined 573 split respiratory specimens from five different AFB laboratories. As in the contaminant spectrum study, GNR were the most common isolates; however, in contrast to earlier results, the percentage of yeast, fungi, and gram-positive contaminants was markedly increased (Table 2). Yeast and fungi accounted for 41% of the contaminating isolates and were present in approximately 50% of the contaminated specimens. The increase in mycologic contaminants (i.e., yeast and fungi) in the CB-18 pilot study was originally thought to be an anomaly due to the inclusion of different clinical sites or a delay in processing between methods (24). However, examination of the 171 specimens from Quest’s laboratory revealed that the change in contaminant spectrum was not an anomaly (Table 2), because while the contamination rate from Quest was still high, approximately 57% of the contaminants were mycologic.
CB-18/LZCT evaluation studies.
Because mycologic contaminants were a significant source of contamination in AFB specimens, and because CB-18 does not impose pH restrictions as would acid- or alkali-based decontamination methods, a procedure was developed using a mixture of lysozyme, zymolyase, Cytophaga extract, and Trichoderma extract as a means to alleviate contamination. The buffer was modified to be more compatible with the LZCT enzyme formulation (Tris-citrate), but not impact the efficacy of the CB-18 procedure (data not shown). Microscopic and cultural examination of purified laboratory strains of fungi, Candida, Staphylococcus species, M. luteus, Bacillus species, K. pneumoniae, E. coli, and P. mirabilis (ATCC 12453), processed by the CB-18/LZCT procedure confirmed that this combination had significant efficacy against all mycologic contaminants, some efficacy against certain gram-positive bacteria, minimal efficacy against most gram-negative bacteria, and no efficacy against mycobacteria (data not shown). Mycobacterial isolates used in these studies included M. tuberculosis (ATCC 27294), M. avium (ATCC 25291), M. kansaii (ATCC 12478), M. fortuitum (ATCC 6841), M. xenopi (ATCC 19250), and M. gordonae (ATCC 14470). The addition of 0.15% lecithin to the lytic enzyme formula did not compromise its ability to affect vulnerable organisms (data not shown).
The CB-18/LZCT procedure was then used on 194 specimens from the general microbiology laboratory at Quest (Table 2), and 46 specimens from the AFB laboratory at Quest (Table 2). The contamination rate among CB-18-processed specimens from the general microbiology laboratory was not significantly impacted, whereas the contamination rate among specimens from the AFB laboratory was reduced to acceptable levels (<5%).
CB-18 titrations.
The loss in liquid culture sensitivity and smear specificity in the CB-18 pilot study (24) suggested that CB-18 might be toxic. The concentration of CB-18 in liquid cultures following inoculation depends on the quantity of the processing fluid remaining after decanting, as well as the volume used to resuspend the sediment. The concentration of CB-18 during specimen processing (24) was 1 mM (383 μg/ml). In an extreme case (e.g., a thick, heavy sputum), resuspension would provide little if any dilution, resulting in a CB-18 concentration of approximately 35 to 40 μg/ml following inoculation. Conversely, in a thin bronchial wash, resuspension would provide a significant dilution resulting in a concentration of CB-18 of about 5 μg/ml following inoculation.
The titration assay was initially designed to examine CB-18 concentrations of 5 to 35 μg/ml in either 12B/RF or 12B/PANTA/caz. A total of 11 M. tuberculosis isolates (10 clinical isolates and the ATCC type strain), 4 M. avium complex isolates (3 clinical isolates, and the ATCC type strain), 5 M. fortuitum isolates (4 clinical isolates, and the ATCC type strain), and the M. kansasii ATCC type strain were examined in the titration assay described or an abbreviated version of this assay. Two M. tuberculosis isolates were selected because they represented the extremes in susceptibility to the experimental parameters. Eight of the M. tuberculosis clinical isolates were grouped with the ATCC 27294 type strain based on their growth characteristics in CB-18, while the other three clinical isolates reacted similarly to the 571/573-S1 isolates (two of these three [571-S1 and 573-S1] were from the same patient [24]). The M. avium complex isolates tested in this assay reacted similarly to the M. avium type strain, while the rapidly growing mycobacteria exhibited characteristics similar to the M. fortuitum type strain.
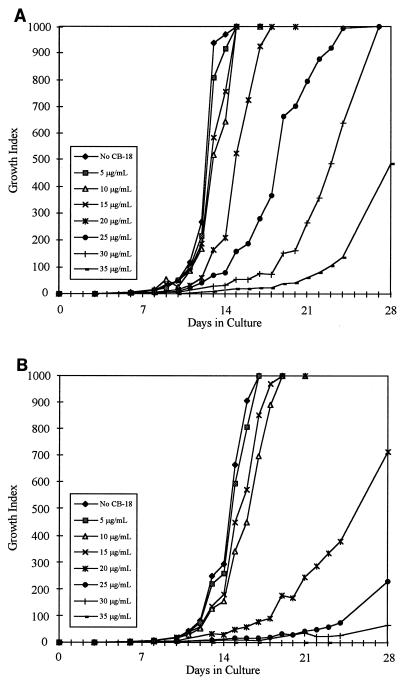
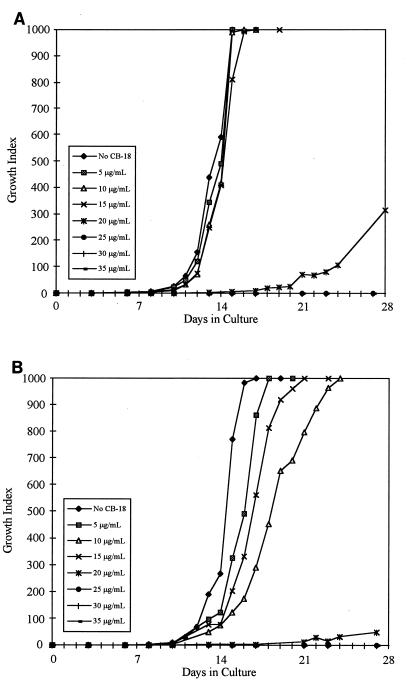
Statistical analysis of the growth curves of the M. tuberculosis isolates ATCC 27294 (Fig. 1A and B) and 571/573-S1 (Fig. 2A and B) showed significant differences between these two isolates with respect to the CB-18 concentrations at which the time to a positive result and the time to maximum GI were affected (Table 3). These two isolates also differed with respect to the concentration of CB-18 that completely suppressed growth. Alternatively, the synergy between CB-18 and the antibiotics affected the time to maximum GI similarly for both M. tuberculosis isolates. The 571/573-S1 isolate was the most sensitive isolate tested, especially to changes in the time to maximum GI between 15 and 20 μg of CB-18 per ml (data not shown).
FIG. 1.
Growth curves of M. tuberculosis ATCC 27294 in reconstitution fluid (A) or PANTA/caz (B) at various concentrations of CB-18.
FIG. 2.
Growth curves of M. tuberculosis 571/573-S1 in reconstitution fluid (A) or PANTA/caz (B) at various concentrations of CB-18.
TABLE 3.
Culture results for statistically significant concentrations of CB-18 (P < 0.05)
| Critical parameter for isolate | Time [days] to positive result [GI ≥ 15] (CB-18 concn [μg/ml])
|
Time [days] to max GI [999] (CB-18 concn [μg/ml])
|
CB-18 concn [μg/ml] with no growth
|
|||
|---|---|---|---|---|---|---|
| 12B/RF | 12B/PANTA/caz | 12B/RF | 12B/PANTA/caz | 12B/RF | 12B/PANTA/caz | |
| M. tuberculosis ATCC 27294 | ||||||
| Control | 8.8 ± 0.5a | 10.2 ± 0.5 | 13.5 ± 1.0 | 16.8 ± 0.5 | ||
| Time at significant concn of CB-18 | >35 | 35 | ||||
| First | 10.5 ± 0.6b (20)c | 12.5 ± 1.0 (20) | 17.5 ± 0.6 (20) | 18.8 ± 0.5 (10) | ||
| Second | 12.5 ± 1.0d (30)e | 15.0 ± 1.6 (25) | 21.8 ± 2.5 (25) | >28 (20) | ||
| M. tuberculosis 571/573-S1 | ||||||
| Control | 9.2 ± 0.5 | 10.8 ± 1.0 | 15.0 ± 0.0 | 16.0 ± 0.8 | ||
| Time at significant concn of CB-18 | 25 | 25 | ||||
| First | 11.0 ± 0.8 (10) | 12.5 ± 1.0 (10) | >28 (20) | 17.8 ± 0.5 (5) | ||
| Second | 18.2 ± 1.0 (20) | >28 (20)f | >28 (25) | >28 (20) | ||
| M. avium ATCC 25291 | ||||||
| Control | 6.0 ± 0.0 | 8.8 ± 1.0 | 10.2 ± 0.5 | 16.8 ± 2.1 | ||
| Time at significant concn of CB-18 | >35 | >35 | ||||
| First | 7.5 ± 0.6 (30) | 11.8 ± 1.0 (15) | 11.2 ± 0.5 (15) | 21.0 ± 2.4 (15) | ||
| Second | Not tested (>35) | 16.0 ± 2.7 (20) | 13.8 ± 1.0 (20) | >28 (25) | ||
| M. kansasii ATCC 12478 | ||||||
| Control | 3.0 ± 0.0 | 4.5 ± 0.6 | 7.0 ± 0.0 | 8.8 ± 0.5 | ||
| Time at significant concn of CB-18 | 30 | >35 | ||||
| First | 4.5 ± 0.6 (20) | 5.8 ± 0.3 (25) | >28 (25)g | 24.0 ± 2.0 (20) | ||
| Second | 6.2 ± 0.5 (25) | Not tested (>35) | >28 (30) | >28 (25) | ||
| M. fortuitum ATCC 6841 | ||||||
| Control | 1.0 ± 0.0 | 1.0 ± 0.0 | 2.0 ± 0.0 | 3.2 ± 0.5 | ||
| Time at significant concn of CB-18 | >100 | >100 | ||||
| First | 2.5 ± 0.6 (30) | 6.8 ± 1.7 (30) | 4.8 ± 1.7 (30) | 5.0 ± 0.8 (10) | ||
| Second | 7.2 ± 0.5 (100) | 10.0 ± 1.4 (40) | 8.8 ± 1.5 (40) | 10.5 ± 1.7 (30) | ||
This is the average time (± standard deviation) to a given result in the absence of CB-18.
This time represents the average time (± standard deviation) to a given result at the lowest CB-18 concentration at which the first statistically significant change occurred.
This is the CB-18 concentration at which the first statistically significant change occurred for a given result relative to the control result.
This time represents the average time (± standard deviation) to a given result at the second statistically significant CB-18 concentration.
This concentration represents the next, or second, statistically significant change that occurred relative to the CB-18 concentration at which the first statistically significant change occurred.
Although the graph of this result appears to be positive, only one of the four replicates became positive during the 4-week incubation.
The time to maximum GI result at 20 μg/ml (17.8 ± 8.8 days) was not significantly different (P = 0.081) from the control result because of the wide replicate-to-replicate variation in this series of bottles.
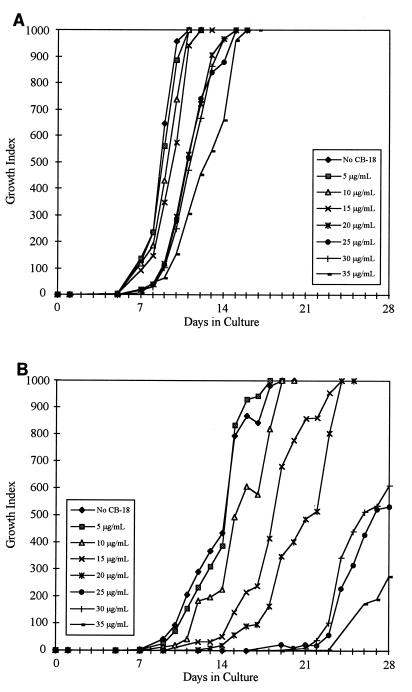
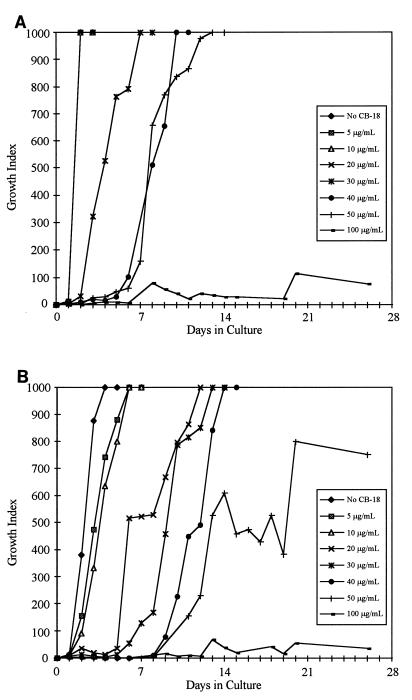
Slow-growing, nontuberculous mycobacteria tested in this assay exhibited interesting characteristics (Table 3). The M. avium type strain, ATCC 25291, was less susceptible to the deleterious effects of CB-18 (Fig. 3A), but the time to a positive result was affected to a greater degree when CB-18 was combined with PANTA/caz (Fig. 3B). The M. kansasii type strain, ATCC 12478, was affected by CB-18 in a manner similar to that of M. tuberculosis (data not shown), but the combination of CB-18/PANTA/caz was not synergistic. For example, the concentration of CB-18 required to produce a statistically significant change in the time to a positive result was increased in the presence of PANTA/caz (Table 3). Growth characteristics of the rapidly growing mycobacteria, exemplified by the M. fortuitum type strain, ATCC 6841 (Fig. 4A and B), were markedly different from those of the slow-growing mycobacteria. The titration assay was adjusted to examine increased CB-18 concentrations for the rapid growers. Growth in CB-18 at levels relevant to the CB-18 pilot study insignificantly affected the growth of the M. fortuitum isolates; however, the synergy between CB-18 and the antibiotics affected the time to maximum GI more than for the M. tuberculosis isolates (Table 3).
FIG. 3.
Growth curves of M. avium ATCC 25291 in reconstitution fluid (A) or PANTA/caz (B) at various concentrations of CB-18.
FIG. 4.
Growth curves of M. fortuitum ATCC 6841 in reconstitution fluid (A) or PANTA/caz (B) at various concentrations of CB-18.
Experiments with the ATCC 27294 and 571/573-S1 M. tuberculosis isolates and various inocula with respect to the CB-18 concentration showed that above ∼105 CFU, the effects of CB-18 could be overcome (data not shown). The greatest variation between isolates within a given group was in the degree of induced susceptibility caused by CB-18.
Lecithin neutralization buffer.
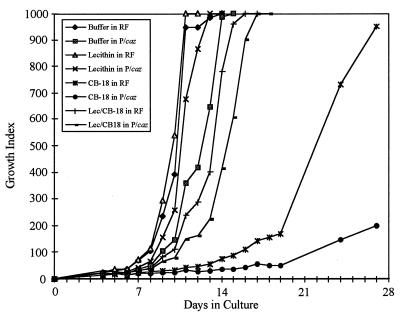
Based on reports that lecithin can neutralize quaternary ammonium compounds (14, 29) and support growth in liquid media (2, 9, 31) a lecithin buffer was formulated for use as a sediment resuspension solution. In order to create the worst possible situation that might be encountered in a clinical laboratory, the most susceptible isolate, 571/573-S1, was added to 2 parts of processing solution (mock supernatant) at 383 μg/ml and mixed with 1 part of resuspension buffer containing 0.15% lecithin. Following inoculation, this produced a CB-18 concentration of approximately 20 μg/ml and a lecithin concentration of approximately 0.05%. Analysis of this condition against the appropriate controls (Fig. 5) showed that there were no statistically significant differences in the time to a positive result between 12B/RF (3.8 ± 0.5 days), 12B/PANTA/caz (5.2 ± 1.5 days), lecithin/RF (4.0 ± 0.8 days), lecithin/PANTA/caz (5.2 ± 1.0 days), CB-18/RF (6.5 ± 2.9 days), CB-18/PANTA/caz (5.2 ± 2.5 days), CB-18/lecithin/RF (5.0 ± 1.2 days), and CB-18/lecithin/PANTA/caz (3.8 ± 0.5 days). However, this was due to larger-than-normal variations in a few replicates in this experiment. The difference in the time to reach maximum GI between 12B/RF (12.2 ± 1.5 days) and CB-18/RF (26.0 ± 2.0 days) was both large and statistically significant (P < 0.0001), whereas the difference between 12B/RF and CB-18/lecithin/RF (15.2 ± 0.5 days), while statistically significant (P = 0.022), was much less. The differences in the time to reach maximum GI among 12B/PANTA/caz (14.2 ± 0.5 days), lecithin/PANTA/caz (13.0 ± 0.0 days), CB-18/PANTA/caz (>28 days), and CB-18/lecithin/PANTA/caz (16.8 ± 0.5 days) were all statistically significant (P < 0.01). Lecithin appeared to restore growth characteristics of the 571/573-S1 isolate to some degree. Based on the work of Woodley and Smithwick (31) and others (2, 9), the expectation that the lecithin resuspension buffer would not have a significant impact on the sensitivity of the BACTEC 12B/460TB assay, even if used directly, was confirmed (data not shown).
FIG. 5.
Growth curves of 571/573-S1 resuspended in and planted in buffer/RF, buffer/PANTA/caz (P/caz), lecithin/RF, lecithin/PANTA/caz, CB-18/RF, CB-18/PANTA/caz, lecithin (Lec)/CB-18/RF, and lecithin/CB-18/PANTA/caz.
Tuberculocidal action of CB-18.
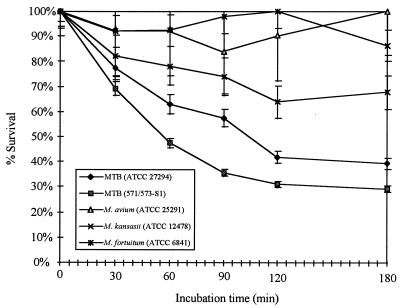
These experiments were designed to examine the time-dependent tuberculocidal action of CB-18 at the concentration used (1 mM or 383 μg/ml) to process specimens in the clinical study (24). Losses in CFU with respect to time were statistically significant (P < 0.01) for the M. kansasii isolate and both M. tuberculosis isolates, but changes in CFU with respect to time for the M. avium and M. fortuitum isolates were not statistically significant (P > 0.1). As was seen in the titration assays, the isolates most sensitive to these experimental conditions were the two M. tuberculosis isolates, with the 571/573-S1 isolate the most susceptible to CB-18 exposure (Fig. 6). Among susceptible isolates, the greatest percentage of bacilli was lost in the first 30 to 60 min. Thereafter, the slope gradually declined and approached zero at approximately 3 h. There appeared to be a refractory population of bacilli that was isolate and species independent.
FIG. 6.
Time-dependent kill curves of M. tuberculosis (MTB) ATCC 27294, M. tuberculosis 571/573-S1, M. avium ATCC 25291, M. kansasii ATCC 12478, and M. fortuitum ATCC 6841 following incubation in 1 mM CB-18.
CB-18 and solid media.
The action of CB-18 on M. tuberculosis viability on solid media was evaluated with the 571/573-S1 isolate because of its particular susceptibility to CB-18. In these experiments, there was a minimal incubation period in order to avoid complications associated with the tuberculocidal action of CB-18, and yet also duplicate CB-18 concentrations that might be encountered in a clinical laboratory. For example, if 250 μl, 1 ml, or 2 ml of the supernatant (at a concentration of 383 μg/ml) remained in the specimen following decanting and standard operating procedure called for 1 ml of resuspension fluid to be added to all specimens, then the concentrations of CB-18 in the inoculum would be 77, 191, and 255 μg/ml, respectively. When lecithin was added to the assay, the concentration of lecithin in the resuspension buffer was assumed to be 0.15%, which would result in lecithin concentrations of 0.12, 0.075, and 0.05%, respectively, in the inoculum. At the highest concentration of CB-18 tested, in the absence of lecithin, only two-thirds of the input bacilli were recovered (Table 4). This was the only statistically significant loss in viable CFU relative to the control (P < 0.01). Even at 191 μg/ml, CB-18 in the absence of lecithin losses could not be considered significant (P = 0.066). At 255 μg of CB-18 per ml in the presence of lecithin, the loss in CFU was not statistically significant (P = 0.22). As in the liquid culture results, lecithin alleviated the deleterious effects of CB-18. It was also observed that at 255 μg/ml, in the absence of lecithin, the colonies appeared mucoid as well as smaller, rounder, and more petite than normal. At the lower CB-18 concentrations, or in the presence of lecithin, colonies appeared more normal.
TABLE 4.
Percent survival of 571/573-S1 on 7H11-selective plates at different CB-18 concentrations in the presence or absence of lecithin
| Concn (μg/ml) of CB-18 in inoculation solution | % Survival (±SD) after inoculation onto 7H11 ina:
|
|
|---|---|---|
| CB-18/buffer | CB-18/lecithin | |
| 255 | 67.7 ± 9.1 | 92.7 ± 4.1 |
| 191 | 87.9 ± 4.1 | 100 ± 7.6 |
| 77 | 95.9 ± 2.8 | 100 ± 9.3 |
Bacilli were diluted to 2,385 ± 260 CFU/ml in inoculation solution.
DISCUSSION
As observed in the CB-18 pilot study, the method used for processing specimens significantly impacted all diagnostic parameters (24). When CB-18 was used to process human respiratory specimens, there were increases in both smear and aggregate culture sensitivity relative to NALC/NaOH-processed specimens, as well as statistically significant reductions in the time to positive results by liquid and solid culture for M. tuberculosis. Unfortunately, there were also a reduction in smear specificity, a loss in liquid culture sensitivity, and an increase in the contamination rate. The focus of these experiments was to understand and overcome these problems.
The presence of saprophytic and infectious microorganisms in respiratory specimens necessitates a decontamination step. Specimen processing amounts to balancing the killing of mycobacteria with that of the contaminants. This is also true with the CB-18 processing method. While the loss of mycobacteria due to exposure to CB-18 was not as severe as the 90% loss described by Yajko et al. (32), the bactericidal action of CB-18 depended on the time of exposure, as well as the mycobacterial species, and even the isolate. The bactericidal and bacteriostatic activity of the n-alkyl betaines has been known for some time. Voss (28) reported on the activity of C16-sulfopropylbetaine, and Tsubone et al. (25–27) studied the action of phosphobetaines, particularly C16-phosphoethylbetaine (PB-16). PB-16 proved to be very active against gram-positive microorganisms, ineffective against gram-negative bacteria, and variable with respect to mycologic organisms (26). Regardless, while CB-18 processing could be expected to provide some decontamination capacity, the contamination rates were unacceptably high, and CB-18 appeared to be toxic to some degree.
Prior to the CB-18 pilot study, in an effort to control contamination, the core CB-18 protocol was modified to resemble contemporary acid-alkali processing procedures. While these attempts succeeded in controlling contamination, they did so at the expense of culture sensitivity. Ceftazidime was added to the culture system for the pilot study (24) to reduce gram-negative contamination while avoiding caustic chemicals. It was difficult to ascertain the impact of ceftazidime on contamination. The redigestion procedure used in the CB-18 pilot study (24) was the CB-18/Acid method. The poor sensitivity observed among redigested specimens possibly resulted because CB-18, used under acidic conditions, may have functioned as a quaternary ammonium salt (25).
A comparison of the results between the CB-18 pilot study and the contaminant spectrum study suggested that there was an innate difference in the potential contaminant that would break through PANTA/caz in CB-18-processed specimens, and the difference depended on whether the specimen was obtained from the general microbiology laboratory or the AFB laboratory. This suggested that an impact on the contamination rate could be affected by eliminating mycologic contaminants. While discontinuing contemporary decontamination methods increased the magnitude of the contamination problem, it eliminated the pH restriction and created a new opportunity to solve this problem with lytic enzymes. The LZCT formulation contained lysozyme, a muraminidase; zymolyase, a combination of endoglucanase and protease activities; Cytophaga extract, a combination of protease and amidase activities; and Trichoderma extract, a combination of cellulase, pectinase, and protease activities (1). This combination successfully minimized mycologic contamination in CB-18-processed respiratory specimens. While gram-negative contaminants still pose a significant problem, there are extracts such as the Micromonospora extract of Suzuki et al. (21) that degrade the capsules of gram-negative bacteria. Unfortunately, this extract is not commercially available.
The lytic enzyme solution appears to reduce contamination in several ways. First, these enzymes may simply lyse vulnerable organisms. Second, if the LZCT enzymes do not lyse the contaminants, they may destabilize their structural integrity so that the antibiotics function more effectively. Third, the inoculum may be so reduced by the process of lysing or destabilizing structural integrity that it allows the antibiotics to function more efficiently on organisms that are not vulnerable to the enzymes. For example, Eng et al. (4) have shown that the MIC of ceftazidime for Pseudomonas aeruginosa increases with increasing inoculum. If the LZCT procedure reduced the inoculum to the degree that the antibiotics, particularly ceftazidime, overcame the inoculum effect, then the gram-negative contamination might be affected by LZCT decontamination as well. Finally, the enzymes may have been active when the sediment was inoculated into the 12B bottle so that it affected the integrity of these contaminants during incubation. Several specimens exhibited initial rapid growth, which then subsided within 24 h. LZCT-treated samples were not examined until the GI rose above 100. It should be noted that the contaminant spectrum is particularly dependent on specimen type; the LZCT formulation used here is likely not effective on all specimen types.
The reduction in liquid culture sensitivity and loss in smear specificity in the pilot study (24) also suggested that CB-18 was somewhat toxic. An in vitro analysis of CB-18 toxicity suggested that the critical diagnostic factor was not so much that the bacilli had been processed in CB-18 at a high concentration (383 μg/ml), but that the cells had been prepared and inoculated in such a manner that the concentration of CB-18 during culture was above a critical level. In other words, while CB-18 had tuberculocidal activity and exposure to CB-18 reduced the number of viable organisms, this action appeared to be secondary to the deleterious effects caused by the presence of CB-18 during growth in liquid media. The CB-18 level in liquid culture must exceed 5 μg/ml to begin to affect the growth characteristics of the most susceptible mycobacteria. The time to maximum GI was more sensitive to increasing concentrations of CB-18 than the time to a positive result. This was consistent with the times to positivity reported in the clinical study (24), and the reduction in these times was probably associated with increased recovery. While the critical amount of CB-18 in liquid culture is species and isolate dependent, CB-18 must be at >20 μg/ml to substantively affect viability.
Altering the chemical structure of CB-18 (23) or reducing the concentration of CB-18 below 20 μg/ml would likely ensure growth of all mycobacteria, although some would grow more than others. The concentration of CB-18 during culture can be minimized either by reducing the inoculum, by diluting the remaining supernatant, or by washing the sediment. Reducing inoculum size or increasing the resuspension volume would diminish culture sensitivity on smear-negative specimens by enhancing sampling error. Washing the sediment would not affect culture sensitivity, but would add an unwelcome step. Alternatively, lecithin can be used as a resuspension buffer component. Lecithin may neutralize CB-18 in a manner similar to its interaction with quaternary ammonium compounds (14, 29), stabilize the cell walls of mycobacteria (3), or support mycobacterial growth (2, 9, 31).
The susceptibility to CB-18 and synergy between CB-18 and PANTA/caz in these experiments was surprising and may explain the unusual results observed in the clinical study (24); specifically, the loss in both liquid culture sensitivity and smear specificity. Of the 41 smear-positive (including smear ±) and culture-negative specimens, only 32% could be confirmed. The 571/573-S1 isolates were investigated further, because both specimens from this patient were smear positive and yet negative in 12B/PANTA/caz (24). Unconfirmed smear-positive and culture-negative specimens may have been either false positives or false negatives. Based on the concentration-dependent susceptibility to CB-18, the synergistic effects between increasing CB-18 concentrations and the PANTA/caz antibiotic supplement, and the historical specificity of acid-fast staining, the majority of these specimens were probably false negative by culture. This suggests that further increases in sensitivity are possible with CB-18 processing. In light of the differential effects of CB-18 on slowly growing versus rapidly growing mycobacteria, it is interesting to note that the 14 smear-positive and liquid culture-negative specimens identified by solid media only included 5 M. tuberculosis, 4 M. avium complex, and 2 M. fortuitum isolates and 1 M. chelonae, 1 M. szulgai, and 1 M. kansasii isolate (24).
The results on solid media were consistent with the results of the pilot study. No further refinements are required to employ this diagnostic mode. The agar in solid media may neutralize CB-18 in some manner, or capillary action may remove CB-18 from the microenvironment of the bacilli during growth, both of which affect the reliability of solid media. While we did not test other media such as Lowenstein-Jensen (L-J) Gruft or L-J Mycobactosel, the 7H11-selective media was preferred because it is effective against mycologic contamination and contains the broadest combination of antibiotics. Regardless, the addition of lecithin to the resuspension buffer should ensure the reliability of culture on solid media.
Culture techniques in mycobacteriology will be required for the foreseeable future because of the need for susceptibility testing. While the CB-18 method provided improved diagnostic sensitivity, the original report indicated potential complications related to contamination and liquid culture sensitivity, as well as smear specificity (24). The experimental data presented explain the loss in liquid culture sensitivity and indicate that the loss in smear specificity also might result from toxicity and the induced susceptibility caused by the presence of CB-18 in antibiotic-containing media. When used in combination with lecithin and the appropriate lytic enzymes, the CB-18 method can be employed without excessive contamination or reductions in either smear specificity or liquid culture sensitivity.
REFERENCES
- 1.Andrews B A, Asenjo J A. Enzymatic lysis and disruption of microbial cells. Trends Biotechnol. 1987;5:273–277. [Google Scholar]
- 2.Cutler R R, Wilson P, Clarke F V. Evaluation of a modified BACTEC method to study the activity of disinfectants against Mycobacterium tuberculosis. Tubercle Lung Dis. 1987;76:254–260. doi: 10.1016/s0962-8479(05)80014-0. [DOI] [PubMed] [Google Scholar]
- 3.Durand E, Welby M, Laneelle G, Tocanne J-F. Phase behavior of cord factor and related bacterial glycolipids toxins. Eur J Biochem. 1979;93:103–112. doi: 10.1111/j.1432-1033.1979.tb12799.x. [DOI] [PubMed] [Google Scholar]
- 4.Eng R H K, Smith S M, Cherubin C. Inoculum effect of new β-lactam antibiotics on Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984;26:42–47. doi: 10.1128/aac.26.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gebre N, Karlsson U, Jonsson G, Macaden R, Wolde A, Assefa A, Miorner H. Improved microscopical diagnosis of pulmonary tuberculosis in developing countries. Trans R Soc Trop Med Hyg. 1995;89:191–193. doi: 10.1016/0035-9203(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 6.Hanks J H, Clark H F, Feldman H. Concentration of tubercle bacilli from sputum by chemical flocculation methods. J Lab Clin Med. 1938;23:736–746. [Google Scholar]
- 7.Kent P T, Kubica G P. Public health mycobacteriology. A guide for the level III laboratory. Atlanta, Ga: Centers for Disease Control; 1985. [Google Scholar]
- 8.Klein G C, Maltz M, Cummings M M, Fish C H. Efficacy of centrifugation as a method of concentrating tubercle bacilli. Am J Clin Pathol. 1952;22:581–585. doi: 10.1093/ajcp/22.6_ts.581. [DOI] [PubMed] [Google Scholar]
- 9.Kononov Y, Ta K D, Heifets L. Effect of egg yolk on growth of Mycobacterium tuberculosis in 7H12 liquid medium. J Clin Microbiol. 1988;26:1395–1397. doi: 10.1128/jcm.26.7.1395-1397.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krasnow I, Wayne L G. Sputum digestion. I. The mortality rate of tubercle bacilli in various digestion systems. Am J Clin Pathol. 1966;45:352–355. [PubMed] [Google Scholar]
- 11.Kucers A, Bennett N M. The use of antibiotics. A comprehensive review with clinical emphasis. 4th ed. Philadelphia, Pa: J. B. Lippincott Co.; 1988. pp. 525–542. [Google Scholar]
- 12.Mitchison D A, Allen B W, Carrol L, Dickinson J M, Aber V R. A selective oleic acid albumin agar medium for tubercle bacilli. J Med Microbiol. 1972;5:165–175. doi: 10.1099/00222615-5-2-165. [DOI] [PubMed] [Google Scholar]
- 13.Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1996. [Google Scholar]
- 14.Patterson R A. A new method for the isolation of M. tuberculosis. Am J Public Health. 1956;46:1429–1430. doi: 10.2105/ajph.46.11.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratnam S, March S B. Effect of relative centrifugal force and centrifugation time on sedimentation of mycobacteria in clinical specimens. J Clin Microbiol. 1986;23:582–585. doi: 10.1128/jcm.23.3.582-585.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rickman T W, Moyer N P. Increased sensitivity of acid-fast smears. J Clin Microbiol. 1980;11:618–620. doi: 10.1128/jcm.11.6.618-620.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts G D, Koneman E W, Kim Y K. Mycobacterium. In: Balows A, Hausler W J Jr, Herrmann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C: American Society for Microbiology; 1991. pp. 304–339. [Google Scholar]
- 18.Robinson L, Stovall W D. Factors influencing the demonstration of tubercle bacilli by concentration methods. J Lab Clin Med. 1941;27:84–91. [Google Scholar]
- 19.Silverstolpe L. Förbättrad metod för påvisande av tuberkelbakterier. Nord Med. 1948;40/48:2220–2222. [Google Scholar]
- 20.Sommers H M, Good R C. Mycobacterium. In: Lennette E H, Balows A, Hausler W J Jr, Shadomy H J, editors. Manual of clinical microbiology. 4th ed. Washington, D.C: American Society for Microbiology; 1985. pp. 216–248. [Google Scholar]
- 21.Suzuki K, Uyeda M, Shibata M. Serratia marcescens-lytic enzyme produced by Micromonospora sp. strain no. 152. Agric Biol Chem. 1985;49:1719–1726. [Google Scholar]
- 22.Thornton C G, Llorin O J, Wolfe D M, Romagnoli M, Hooper N, Turner J, Lim R G, Merz W G, Libonati J P, Joseph J M, Schwalbe R S, Moody M, Passen S. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. A novel method for processing mycobacteria using C18-carboxypropylbetaine, abstr. U-50; p. 109. [Google Scholar]
- 23.Thornton, C. G. August 1997. Methods for processing mycobacteria. U.S. patent 5,658,749.
- 24.Thornton C G, MacLellan K M, Brink T L, Jr, Lockwood D E, Romagnoli M, Turner J, Merz W G, Schwalbe R S, Moody M, Lue Y, Passen S. A novel method for processing respiratory specimens for the detection of mycobacteria by using C18-carboxypropylbetaine: blinded study. J Clin Microbiol. 1997;36:1996–2003. doi: 10.1128/jcm.36.7.1996-2003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsubone K, Uchida N. Synthesis of 2-(N-2-hydroxyalkyl-N,N-dimethylammonio)ethyl hydrogen phosphates and their physiochemical and antimicrobial properties. J Am Oil Chem Soc. 1990;67:394–399. [Google Scholar]
- 26.Tsubone K, Uchida N, Ito Y. Relationship between structure and antimicrobial activity of 2-(N,N,N-trialkylammonio)alkyl hydrogen phosphates. J Pharm Sci. 1991;80:441–444. doi: 10.1002/jps.2600800509. [DOI] [PubMed] [Google Scholar]
- 27.Tsubone K. Correlation between antimicrobial activity and chelating ability of 2-(N,N,N-trialkylammonio)alkyl hydrogen phosphates. J Pharm Sci. 1991;80:1051–1054. doi: 10.1002/jps.2600801110. [DOI] [PubMed] [Google Scholar]
- 28.Voss J G. Effects of organic cations on the gram-negative cell wall and their bactericidal activity with ethylenediamine-tetra-acetate and surface active agents. J Gen Microbiol. 1967;48:391–400. doi: 10.1099/00221287-48-3-391. [DOI] [PubMed] [Google Scholar]
- 29.Wayne L G. Some observations on the use of benzalkonium chloride (Zephiran) in tuberculosis bacteriology. Am Rev Respir Dis. 1959;80:912–913. [Google Scholar]
- 30.Wayne L G, Krasnow I, Kidd G. Finding the “hidden positive” in tuberculosis eradication programs. Am Rev Respir Dis. 1962;86:537–541. doi: 10.1164/arrd.1962.86.4.537. [DOI] [PubMed] [Google Scholar]
- 31.Woodley C L, Smithwick R W. Radiometric method for pyrazinamide susceptibility testing of Mycobacterium tuberculosis in egg-yolk-enriched BACTEC 12A medium. Antimicrob Agents Chemother. 1988;32:125–127. doi: 10.1128/aac.32.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yajko D M, Wagner C, Tevere V J, Kocagöz T, Hadley W K, Chambers H F. Quantitative culture of Mycobacterium tuberculosis from clinical sputum specimens and dilution endpoint of its detection by the Amplicor PCR assay. J Clin Microbiol. 1995;33:1944–1947. doi: 10.1128/jcm.33.7.1944-1947.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yegian D, Budd V. Toxic effect of sodium hydroxide on tubercle bacilli. Am J Clin Pathol. 1952;22:456–460. doi: 10.1093/ajcp/22.5.456. [DOI] [PubMed] [Google Scholar]