Abstract
Purpose: To present 2 cases of premature newborns with hyperbilirubinemia and retinopathy of prematurity (ROP) who could not be examined properly to assess for disease progression because of vitreous opacification in the setting of an icteric vitreous and frail health status. Methods: The cases and their findings were analyzed. Results: Given the sickness of the neonates and examination difficulty, intravitreal bevacizumab was administered in both eyes to prevent disease progression. During subsequent examinations, the patients remained stable until discharge from the neonatal intensive care unit and were followed in the outpatient clinic without complication. Conclusions: The ROP and vitreous opacification in our cases were thought to be caused by hyperbilirubinemia. Because of vitreous opacification, these patients could not be properly examined for ROP. Treatment with an intravitreal antivascular endothelial growth factor injection might be considered to delay disease development until the newborn is healthier and able to be examined.
Keywords: ROP screening, hyperbilirubinemia, icteric vitreous, premature newborns
Introduction
Retinopathy of prematurity (ROP) is a proliferative retinal vascular disease affecting the retina of premature infants. 1 Characterized by retinal ischemia, aberrant angiogenesis, fibrovascular proliferation, and progressive vitreoretinal traction, ROP accounts for 14% of childhood blindness in the United States and greater than 20% in developing nations. 1
Multiple risk factors for the development of ROP have been identified. Of these, low birth weight and gestational age carry a considerable risk. 2 Early identification and intervention are critical in preventing permanent vision loss. To decide whether a patient will undergo treatment, and if so when, it is crucial to assess the retina in detail with binocular indirect ophthalmoscopy or multimodal imaging, such as fluorescein angiography (FA) or fundus photography. 3
A poor view of the fundus is of major concern in ROP screening because the quality of the examination can be compromised by various factors. 3 In examinations performed in the neonatal intensive care unit (NICU), we observed that patients with hyperbilirubinemia undergoing ROP screening often demonstrated icteric vitreous. In addition, weekly fundus examination might not be feasible because of the preterm infant`s small size for gestational age and his or her fragile health conditions. In cases of undiagnosed advanced ROP or where treatment is delayed, consequences can include neovascularization, retinal detachment, and irreversible vision loss.1,3
Here, we present 2 cases of preterm newborns with ROP and vitreous opacification thought to be caused by hyperbilirubinemia.
Case Reports
Case 1
A male neonate with a gestational age of 23 weeks and birth weight of 500 g presented to Jackson Memorial Hospital for premature rupture of membranes. The neonate had respiratory distress syndrome, necrotizing enterocolitis, anemia of prematurity, and hyperbilirubinemia. He had received oxygen therapy and was admitted to the NICU for 54 days.
At birth, the patient’ total and direct bilirubin levels were 5.7 mg/dL and 4.7 mg/dL, respectively, and he did not receive treatment for hyperbilirubinemia. At 31 weeks postconceptual age (PCA), he was evaluated for the first time by a pediatric retina specialist. The ophthalmic examination showed an icteric eye and icteric vitreous, making the details of the retina vasculature difficult to access. At that time, his total and direct bilirubin levels were 5.2 mg/dL and 4.3 mg/dL, respectively.
At 32 weeks, a fundus examination was still difficult to interpret because of the increased icteric vitreous; however, the patient presented with stage 2 zone 1/2 (12 clock hours) ROP without plus disease in both eyes (Figure 1, A and B). His total and direct bilirubin levels were 13.3 mg/dL and 11.7 mg/dL, respectively. Given the sickness of the neonate and the difficulty in the examination, it was decided to administer intravitreal bevacizumab in both eyes to prevent disease progression.
Figure 1.
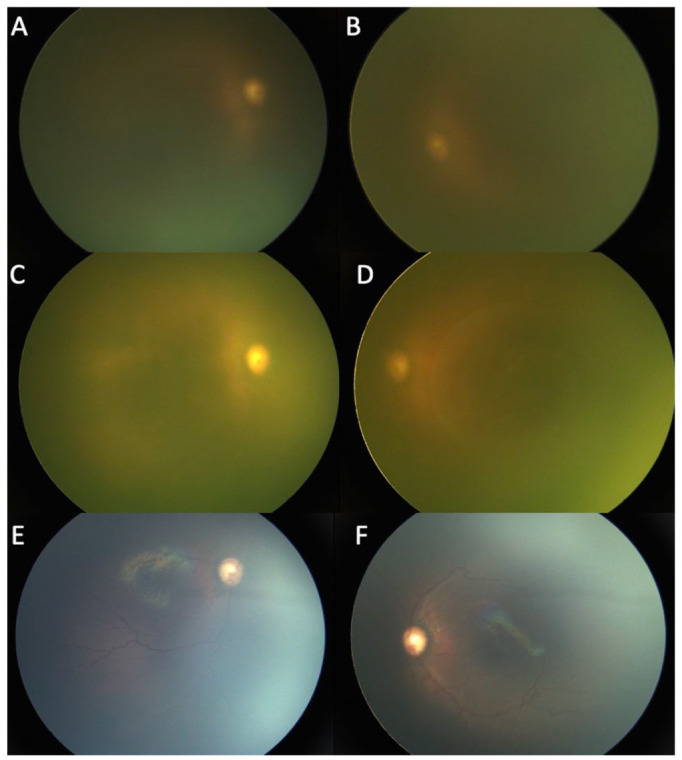
(A and B) Bilateral fundus photographs in Case 1 at 32 weeks postconceptional age (PCA) show a deteriorating view of the fundus. The total and direct bilirubin levels were 13.3 mg/dL and 11.7 mg/dL, respectively. Fundus photographs of the right eye (C) and left eye (D) in Case 1 at 33 weeks PCA show complete icteric vitreous obstructing fundus visualization. The total and direct bilirubin levels were 17.3 mg/dL and 15.2 mg/dL, respectively. Fundus photographs of the right eye (E) and left eye (F) in Case 1 at 35 weeks PCA show no ROP, no plus disease, and vessels reaching zone 2. The total and direct bilirubin levels were 4.5 mg/dL and 2.5 mg/dL, respectively.
At 33 weeks, the patient’s retinal details were not clear because of a very icteric vitreous (Figure 1, C and D). His total and direct bilirubin levels were 17.3 mg/dL and 15.2 mg/dL, respectively. At that moment, FA was attempted without benefit in the assessment of the retinal vasculature (Figure 2).
Figure 2.
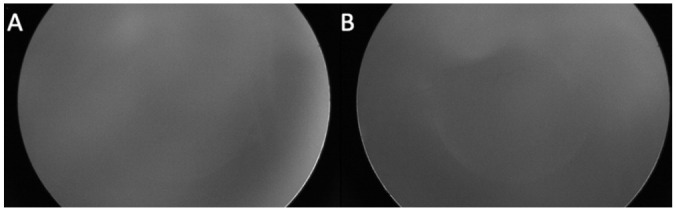
Fluorescein angiography of the right eye (A) and left eye (B) in Case 1 at 33 weeks postconceptional age shows complete icteric vitreous obstructing visualization.
At 35 weeks, the vitreous was less icteric, the ROP had regressed, and the vessels were reaching zone 2 (Figure 1, E and F). His total and direct bilirubin levels were 4.5 mg/dL and 2.5 mg/dL, respectively.
During subsequent weekly examinations, the patient remained stable until he was discharged from the NICU and followed in the outpatient clinic without complication. His total and direct bilirubin levels went back to normal at 0.5 mg/dL and 0.4 mg/dL, respectively.
Case 2
A female neonate with a gestational age of 23 weeks and birth weight of 500 g was born at Jackson Memorial Hospital as a result of the mother’s cervical incompetence. The neonate had respiratory distress syndrome, necrotizing enterocolitis, intraventricular hemorrhage, progressive hydrocephalus, and hyperbilirubinemia. She had received oxygen therapy and was admitted to the NICU for 189 days.
At birth, her total and direct bilirubin levels were 9.7 mg/dL and 7.4 mg/dL, respectively. She was treated for hyperbilirubinemia with phototherapy.
At 33.5 weeks PCA, the patient was evaluated for the first time by a pediatric retina specialist. An ophthalmic examination showed an icteric eye and icteric vitreous, making the details of the retinal vasculature difficult to assess. At that time her total and direct bilirubin levels were 5.2 mg/dL and 4.3 mg/dL, respectively.
At 34.3 weeks, a fundus examination was still difficult and the patient presented with stage 1 in zone 2 (6 clock hours) ROP without plus disease in both eyes. Her total and direct bilirubin levels were 6.7 mg/dL and 5.4 mg/dL, respectively.
At 35.3 weeks, the ROP progressed to stage 2 zone 2 (12 clock hours) in both eyes and the total and direct bilirubin levels were 14.7 mg/dL and 12.8 mg/dL, respectively. Given the sickness of the neonate and the difficulty in the examination, it was decided to administer intravitreal bevacizumab in both eyes to prevent disease progression. In the subsequent week, the patient’s retinal details were not evident given the icteric vitreous (Figure 3, A and B). FA was attempted but not informative (Figure 4). Her total and direct bilirubin levels were 20.9 mg/dL and 17.9 mg/dL, respectively.
Figure 3.
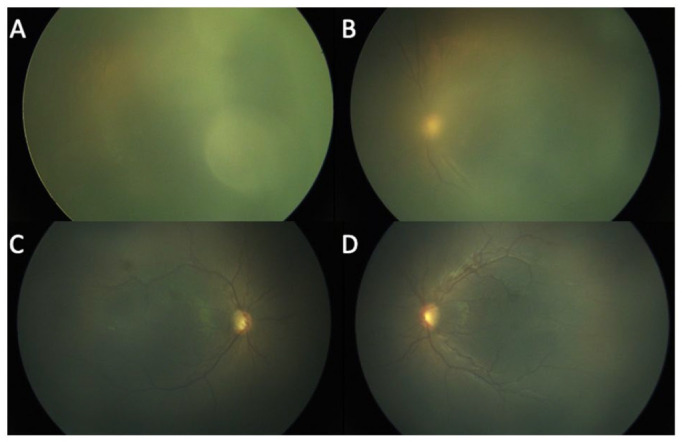
Fundus photographs of the right eye (A) and left eye (B) in Case 2 at 36.3 weeks postconceptional age (PCA) show the patient’s retina could not be well examined because of the icteric vitreous. The total and direct bilirubin levels were at 20.9 mg/dL and 17.9 mg/dL, respectively. Fundus photographs of the right eye (C) and left eye (D) in Case 2 at 37.3 weeks PCA show that a vitreous was less icteric, the ROP had regressed, and the vessels were reaching zone 2. The total and direct bilirubin levels were at 4.5 mg/dL and 2.5 mg/dL, respectively.
Figure 4.
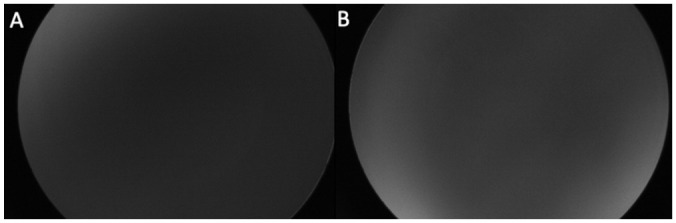
(A and B) On bilateral fluorescein angiography photographs, the patient’s retina could not be well examined because of the icteric vitreous.
At 37.3 weeks, the vitreous was less icteric, the ROP had regressed, and the vessels were reaching zone 2. At that time, the total and direct bilirubin levels were 5.1 mg/dL and 3.4 mg/dL, respectively.
During subsequent weekly examinations, the patient remained stable until she was discharged from the NICU and followed in the outpatient clinic without complication. Her total and direct bilirubin levels were normal at 0.5 mg/dL and 0.4 mg/dL, respectively.
Conclusions
We present 2 cases of premature newborns with hyperbilirubinemia and ROP who could not be examined properly to assess the progression of ROP because of vitreous opacification in the setting of an icteric vitreous and a very frail and tenuous health status. We hypothesized that high bilirubin obstructs the visibility the retinal vasculature by making the vitreous gel icteric. The age of the child, the presence of a tunica vasculosa, and the at times hazy cornea do not allow for a detailed retinal examination at a critical time.
The inability to perform detailed examinations of the fundus in a timely fashion during ROP screening can lead to uncertainty regarding the diagnosis and treatment of these patients 4 and might lead to irreversible changes that cause visual loss. Thus, in such patients, if a diagnosis of ROP is made, providers may consider treatment with an intravitreal injection of an antivascular endothelial growth factor (anti-VEGF) agent to avoid disease progression. The treatment would also allow the neonate to improve without the need for weekly examinations if the neonate is not physically able to tolerate the examinations. Special consideration should be paid to the status and trends of the patient’s bilirubin levels because these likely correspond to the degree of icteric vitreous.
Hyperbilirubinemia is common in newborns. 5 Elevated bilirubin in preterm and full-term babies result from different mechanisms, including decreased bilirubin uptake by hepatocytes, excess bilirubin load in hepatocytes, and impaired bilirubin conjugation.6,7 Although hyperbilirubinemia is common in term newborns (60%), it is more prevalent (80%) and severe in preterm infants because of the underdeveloped liver.6,7 In full-term infants, unconjugated bilirubin levels less than 12 mg/dL and conjugated bilirubin levels greater than 15 mg/dL require treatment. In preterm infants, there is no safe threshold for hyperbilirubinemia and treatment is based on gestational age and clinical factors. 8 For these patients, the treatment is usually phototherapy and, less common, exchange transfusions. 9
Given the difficulty of examination in the setting of hyperbilirubinemia in extreme premature, medically complex newborns, we imagined that performing FA could clarify the disease. This was attempted without benefit in the assessment of the retinal vasculature. Also, in infants whose health is already frail, the length of the angiography is at times intolerable. Furthermore, because of the wavelength of this examination, hyperbilirubinemia with an icteric vitreous may not only obstruct indirect fundus ophthalmoscopy but also angiographic details. This occurs because bilirubin has a bichromophoric nature, causing it to both absorb and emit light. 10 It absorbs light primarily at a wavelength of 450 to 460 nm and fluoresces at a wavelength of 510 to 570 nm.10,11 Meanwhile, FA uses a water-soluble orange dye that absorbs blue light at a wavelength of 465 to 490 nm and emits green light at a wavelength of 520 to 530 nm, which overlaps with that of bilirubin.12,13 Therefore, the fluorescence of bilirubin can also affect the quality of fundus photograph and FA studies. Monitoring ROP progression in these patients can be challenging.
In conclusion, hyperbilirubinemia may produce an icteric vitreous in which the yellowish vitreous obstructs fundus examination as well as FA. Thus, screening and monitoring ROP in such patients can be especially arduous. In our patients, it is possible that spontaneous regression of ROP would have occurred; however, after weighing the benefits and drawbacks, we chose to provide intravitreal anti-VEGF treatment to stop the condition from progressing. If beneficial, a physician may consider anti-VEGF injections after individually assessing patients whose frail health status will not tolerate weekly examination. Of course, a detailed discussion with the caretakers of the infants is of utmost necessity.
Footnotes
Ethical Approval: This case report was conducted in accordance with the Declaration of Helsinki. The collection and evaluation of all protected patient health information was performed in a US Health Insurance Portability and Accountability Act–compliant manner.
Statement of Informed Consent: Informed consent was obtained prior to performing the procedure, including permission for publication of all photographs and images included herein.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors alone are responsible for the content and writing of this article. Dr. Berrocal has financial interests in Aerie Pharmaceuticals, ProQR therapeutics, Oculus surgical, Alcon, Allergan, Bayer, DORC, Phoenix Clinical, Vizunex Medical Systems, Zeiss, Novartis. Drs. Cruz, Dr. Al-khersan, and Miss Lopez-Cañizares have indicated they do not have any conflict of interest relevant to this article to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH Center Core under Grant P30EY014801; Research to Prevent Blindness-Unrestricted under Grant (GR004596); and US Department of Defense under Grant W81XWH-13-1-0048. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
ORCID iDs: Ashley López-Cañizares  https://orcid.org/0000-0002-5902-6286
https://orcid.org/0000-0002-5902-6286
Audina Maria Berrocal  https://orcid.org/0000-0002-2446-2184
https://orcid.org/0000-0002-2446-2184
References
- 1. Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007;10:133-140. [DOI] [PubMed] [Google Scholar]
- 2. Alajbegovic-Halimic J, Zvizdic D, Alimanovic-Halilovic E, Dodik I, Duvnjak S. Risk factors for retinopathy of prematurity in premature born children. Med Arch. 2015;69(6):409-413. doi: 10.5455/medarh.2015.69.409-413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Valikodath N, Cole E, Chiang MF, Campbell JP, Chan R. Imaging in retinopathy of prematurity. Asia Pac J Ophthalmol (Phila). 2019;8(2):178-186. doi: 10.22608/APO.201963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gubernick JA, Rosenberg HK, Ilaslan H, Kessler A. US approach to jaundice in infants and children. Radiographics. 2000;20(1): 173-195. [DOI] [PubMed] [Google Scholar]
- 5. Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84:77-82. [DOI] [PubMed] [Google Scholar]
- 6. Boyer JL. Bile formation and secretion. Compr Physiol. 2013;3(3): 1035-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joseph A, Samant H. Jaundice. Updated May 8, 2022. In: StatPearls [Internet]. StatPearls Publishing; 2022. Jan-. [Google Scholar]
- 8. Ennever JF. Blue light, green light, white light, lighter: treatment of neonatal jaundice. Clin Perinatol. 1990;17(2):467-481. [PubMed] [Google Scholar]
- 9. Tan KL. The pattern of bilirubin response of phototherapy for neonatal hyperbilirubinemia. Pediatr Res. 1982;16(8):670-674. [DOI] [PubMed] [Google Scholar]
- 10. Lepore D, Ji MH, Ying GS, et al. Early angiographic signs of retinopathy of prematurity requiring treatment. Eye (Lond). 2021; 35(11):3094-3101. doi: 10.1038/s41433-020-01305-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu R, Teich W, Frenzel F, et al. Optical characterization of sodium fluorescein In Vitro and Ex Vivo. Front Oncol. 2021;11:654300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scanlon PH. Update on screening for sight-threatening diabetic retinopathy. Ophthalmic Res. 2019;62(4):218-224. doi: 10.1159/000499539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Christopoulos TK, Diamandis EP. Fluorescence immunoassay. In: Diamandis EP, Christopoulos TK, eds. Immunoassay. Academic Press; 1996:309-335. doi: 10.1016/B978-012214730-2/50015-7 [DOI] [Google Scholar]


