Abstract
Purpose: To describe the surgical technique and long-term outcomes of a modified split-thickness corneal patch grafting for conjunctival erosions that can be seen in patients with the Port Delivery System (PDS) implant. Methods: By way of retrospective review of medical records, this interventional case series identified 2 cases in which modified split-thickness corneal patch grafting was used to repair conjunctival erosion in patients with the PDS implant. Results: The surgical approach involved creating a small opening in the corneal graft over the center of the PDS implant to improve visibility and allow for easier access during subsequent refill-exchange procedures. At the last follow-up of 6.9 years and 5.6 years, there was no recurrence of conjunctival erosions in either patient. The PDS implants remained well covered with the split-thickness corneal graft and had undergone multiple implant refills without complication or difficulty. Conclusions: Modified split-thickness corneal patch grafting with central graft aperture offers another option for long-term successful management of conjunctival erosions in patients with a PDS, especially those who have failed prior repair, by allowing sufficient visibility and access for subsequent refill-exchange procedures.
Keywords: conjunctival erosion, corneal patch graft, neovascular age-related macular degeneration, port delivery system, ranibizumab
Introduction
The Port Delivery System (PDS) with ranibizumab (Genentech) is a novel drug-delivery system approved by the US Food and Drug Administration (FDA) for treatment of neovascular age-related macular degeneration (nAMD) in patients who have responded to at least 2 prior antivascular endothelial growth factor (anti-VEGF) injections.1,2 The PDS aims to reduce the high treatment burden of nAMD through the continuous release of anti-VEGF. In the pivotal phase 3 Archway trial, the PDS with ranibizumab demonstrated noninferiority and equivalent changes in best-corrected visual acuity (BCVA) from baseline compared with monthly intravitreal injections with ranibizumab, leading to its FDA approval. 1
In October 2022, Genentech initiated a voluntary recall of the Susvimo ocular implant and insertion tool assembly, including pulling the Susvimo (ranibizumab injection) drug vial and initial fill needle carton from commercial distribution in the United States. For patients who already had the implant but had not experienced septum dislodgement, after discussing with their provider and giving informed consent, refill-exchange procedures of ranibizumab could continue, providing visual and anatomical benefits.
The most concerning ocular adverse event in the Archway trial was endophthalmitis, which was observed in 1.6% of patients with a PDS in the study. Most eyes with endophthalmitis (3 of 4) in the Archway clinical trial had a history of preceding or concurrent conjunctival erosion or retraction. 1 This association highlighted the critical importance of meticulously handling conjunctiva and Tenon capsule during surgery as well as promptly managing conjunctival erosions and retraction in the postoperative course, which have been adopted in subsequent trials using the PDS. Best practices for management of PDS-associated conjunctival complications have been discussed previously, including reconstruction with split-thickness corneal graft as used in the Archway clinical trial.3,4 We describe a modified surgical technique in which a central aperture is added to a split-thickness corneal patch graft to repair conjunctival erosions in patients with a PDS and the long-term clinical outcomes associated with this technique.
Methods
Conjunctival revision with modified split-thickness corneal grafting procedure was performed in a surgical setting under local anesthesia with a peribulbar block. Following placement of a traction suture through the superotemporal cornea and inferonasal rotation of the globe for adequate visualization, blunt Westcott scissors were used to create a large peritomy in the quadrant with the PDS implant. Conjunctiva and Tenon capsule were meticulously dissected off of and adjacent to the implant. Extensive undermining was performed to ensure sufficient laxity of the tissue for optimal closure. Non-toothed forceps were used for conjunctival handling. Following cautery of the adjacent sclera, the 9.0 × 4.5-mm, half-moon-shaped, split-thickness graft (VisionGraft) was placed over the PDS implant.
As a modification to the partial-thickness corneal grafting technique, the portion of the graft that would be overlying the septum was marked. After removing the graft from the implant flange to avoid damage to the surgical tray, a No. 15 blade was used to create a small aperture about 1 mm (approximately the size of the PDS septum) in the center of the corneal graft to allow easier future access to the PDS septum. The half-moon-shaped, split-thickness corneal graft was then placed over the PDS implant and centered over the PDS implant flange. The corneal graft was sutured into place over the PDS implant using nonabsorbable, nonbraided sutures (9-0 or 10-0 nylon; Ethicon), which were buried. Conjunctival peritomy was closed using a 7-0 or 8-0 Vicryl (Ethicon) suture with scleral anchoring bites of at least 1-mm length at the apexes of the peritomy, ensuring that the PDS implant and corneal graft were fully covered with no traction on the conjunctival closure and that the peritomy was well opposed to the limbus with some overlap.
Results
Case 1
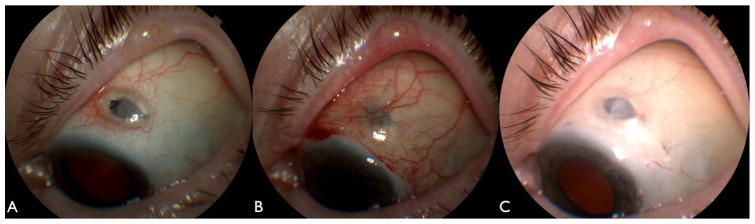
An 80-year-old woman with a history of nAMD underwent uncomplicated implantation of the PDS in the left eye as part of a clinical trial. On presentation, BCVA was 20/32 in the left eye. Two months following PDS implantation, she developed a conjunctival erosion at the nasal edge of the implant (Figure 1A). She initially underwent surgical repair with a rotational conjunctival flap, in which great care was taken to mobilize conjunctiva and Tenon capsule to avoid subsequent traction on the closure (Figure 1B). Three months following that repair, the patient developed a second conjunctival erosion overlying the nasal edge of the PDS implant (Figure 1C). She underwent repair with a modified split-thickness corneal patch graft as presently described (Figure 1D).
Figure 1.
An external photograph taken 2 months following insertion of the Port Delivery System demonstrates a conjunctival erosion overlying the nasal implant flange (A). An external photographs taken 3 weeks following repair with a rotational conjunctival flap demonstrates a well-covered implant (B). However, at 3 months, a recurrent conjunctival erosion developed over the nasal flange (C). Four months following repair of the conjunctival erosion with a modified split-thickness corneal patch graft, the implant was well covered (D).
Postoperatively, the patient had no additional PDS-related complications and required no subsequent interventions. At her last follow-up visit 6.9 years following the repair, BCVA remained 20/32 in the left eye. The patient had undergone 10 refill-exchange procedures of the PDS every 24 weeks without complication. The patient’s PDS implant remained well covered with the corneal patch graft and intact overlying conjunctiva.
Case 2
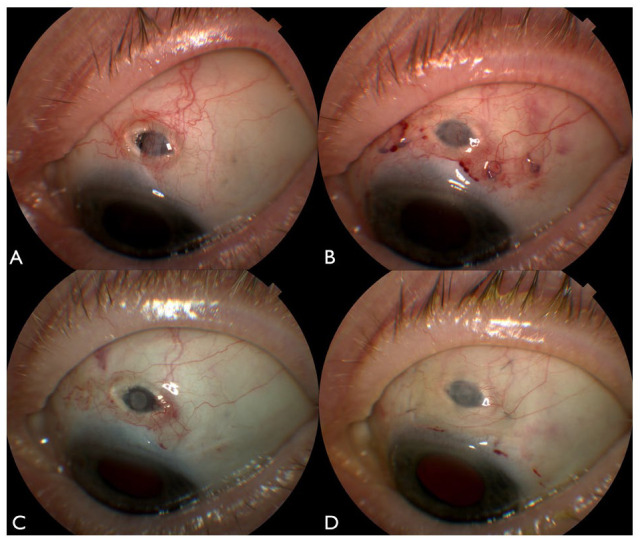
A 79-year-old woman with a history of nAMD underwent implantation of the PDS in the left eye within a clinical trial. On presentation her BCVA was 20/32. Three months following implantation, she developed a small conjunctival erosion at the nasal edge of the PDS. Following initial conservative management, the conjunctival erosion enlarged (Figure 2A), and the patient underwent primary repair with conjunctival revision and modified split-thickness corneal patch grafting to treat the conjunctival erosion using the approach we have described. Postoperatively the patient had no additional complications related to the PDS and required no further surgical procedures (Figure 2, B and C). At the last follow-up 5.6 years after the repair of the conjunctival erosion, her BCVA was 20/50 and the patient had successfully undergone 11 refill-exchange procedures every 24 weeks without complications. The PDS remained well covered with the corneal patch graft and intact overlying conjunctiva.
Figure 2.
An external photograph 3 months following insertion of the Port Delivery System demonstrates a conjunctival erosion overlying the nasal implant flange (A). External photographs 1 week (B) and 1 year (C) following repair of the conjunctival erosion with a modified split-thickness corneal patch graft show a well-covered implant.
Conclusions
In the Phase 3 Archway trial, the rate of PDS-associated conjunctival erosions, defined as a full-thickness breakdown of the conjunctiva in the area of the device flange, was 2.4%. 1 The rate of conjunctival retraction, defined as the recession of the conjunctival peritomy, was 2.0%. Among the 11 cases with at least one of these conjunctival complications in the trial, surgical intervention was needed in 9 patients, and 3 cases of endophthalmitis occurred in 5 patients with conjunctival retraction. 1 The rate of endophthalmitis in the Archway trial and its association with conjunctival complications highlighted the need for meticulous conjunctival opening, dissection, and closure-anchoring sutures, including a partial-thickness scleral pass to ensure that conjunctiva is well opposed to the limbus covering the PDS implant without tension. 3
The rate of PDS-related conjunctival erosions in the Ladder and Archway Trials, 2.8% at a mean of 22 months and 2.4% at 40 weeks respectively, is similar to those reported in the glaucoma literature.1,2 The Tube Versus Trabeculectomy study reported a conjunctival erosion rate following glaucoma drainage device implantation of 5% at 5 years. Levinson et al 5 performed a large retrospective review of 702 glaucoma drainage devices and found a conjunctival erosion rate of 5.8% over a mean follow-up of 34 months, with 16.3% of patients with conjunctival erosions developing endopthalmitis.1,2,6 Reported risk factors for the exposure of glaucoma drainage devices have included female sex and White race. 7
While the glaucoma drainage device literature is helpful in evaluting observed rates of conjunctival erosion and for considering surgical repair techniques, there are notable differences that affect its applicability to the treatment of patients with PDS. Glaucoma drainage devices differ significantly in their profile and structure from PDS implants, such as requiring patch-graft coverage when the glaucoma drainage device is initially implanted. In addition, the PDS implant has the unique need of a clear visualization of the PDS septum and an ability to access it during subsequent refill-exchange procedures.
Management of conjunctival erosion in a patient with PDS implant and flange exposure as was seen in our patients consists of topical antibiotics and prompt surgical intervention to minimize the risk of infection. A rotational conjunctival flap can be performed to cover the implant, taking care to thoroughly undermine the conjunctiva and Tenon capsule to avoid tension on the closure. 4 A rotational conjunctival flap with extensive undermining was performed in our first patient, but a recurrent conjunctival erosion developed at the edge of the flange.
A conjunctival revision with a modified split-thickness corneal patch graft as described in this report may be effective in cases of recurrent erosion and can be considered for repair of primary conjunctival erosion in a patient with PDS, such as in our second case. The addition of a small aperture in the center of the partial-thickness corneal graft overlying the septum of PDS implant can allow for easier visualization and may not increase resistance during future refill-exchange procedures, minimizing potential obstacles that could be encountered with an intact graft. In both patients reported here, multiple implant refill-exchange procedures were performed without complications over 5 to 6 years following placement of the split-thickness corneal patch graft. Notably, partial-thickness corneal graft repairs performed by other sugeons in the Archway clincial trial did not involve creating an opening in the graft as described here. 4
The PDS with ranibizumab is an innovative drug-delivery system aimed at decreasing the treatment burden of nAMD. As with many innovative systems, refinement of device and technique are often needed to optimize outcomes. For the PDS this was first demonstrated in the Phase 2 Ladder trial, where optimization of the surgical procedure to include laser ablation of the pars plana choroidal vasculature decreased the rate of vitreous hemorrhage from 50% to 4.5%. Similarly, the rate of conjunctival complications may be reduced by meticulous conjunctival and Tenon capsule handling throughout the surgical insertion as well as the prompt recognition and surgical management of conjunctiva-related complications. In our 2 patients, prompt conjunctival revision with a modified split-thickness corneal patch grafting technique was succesful at repairing the conjunctiva, ensuring continued long-term stability in the coverage of the PDS implant and ongoing ability to continue the treatment with uncomplicated refill-exchange procedures every 24 weeks. In conclusion, the modified split-thickness corneal patch graft can be considered for management of conjunctival erosions in patients with PDS.
Footnotes
Ethical Approval: The study complied with the guidelines for human studies and animal welfare regulations.
Statement of Informed Consent: The subjects gave informed consent for the publication of cases and images.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Miller received research support from Genentech, Regeneron, Novartis, Iveric Bio; Shareholder Vortex Surgical, Employee Alcon. Dr. Shildkrot and Dr. Menezes are employed by Genentech. Dr. Ebert has no disclosures.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Yevgeniy (Eugene) Shildkrot  https://orcid.org/0000-0003-0536-8528
https://orcid.org/0000-0003-0536-8528
References
- 1. Holekamp NM, Campochiaro PA, Chang MA, et al. Archway randomized phase 3 trial of the port delivery system with ranibizumab for neovascular age-related macular degeneration. Ophthalmology. 2022;129(3):295-307. [DOI] [PubMed] [Google Scholar]
- 2. Campochiaro PA, Marcus DM, Awh CC, et al. The port delivery system with ranibizumab for neovascular age-related macular degeneration: results from the randomized phase 2 ladder clinical trial. Ophthalmology. 2019;126(8):1141-1154. [DOI] [PubMed] [Google Scholar]
- 3. Graff JM, Sheth VS, Chang RT, Menezes AR, Barteselli G, Malhotra VK. Conjunctiva and Tenon’s capsule handling in the Port Delivery System with ranibizumab implant insertion procedure: surgical pearls. Ophthalmic Surg Lasers Imaging Retina. 2022;53(5):266-273. [DOI] [PubMed] [Google Scholar]
- 4. Awh CC, Barteselli G, Makadia S, et al. Management of key ocular adverse events in patients implanted with the Port Delivery System with ranibizumab. Ophthalmol Retina. 2022;6(11):1028-1043. [DOI] [PubMed] [Google Scholar]
- 5. Levinson JD, Giangiacomo AL, Beck AD, et al. Glaucoma drainage devices: risk of exposure and infection. Am J Ophthalmol. 2015;160(3):516-521.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gedde SJ, Herndon LW, Brandt JD, et al. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804-814.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Muir KW, Lim A, Stinnett S, Kuo A, Tseng H, Walsh MM. Risk factors for exposure of glaucoma drainage devices: a retrospective observational study. BMJ Open. 2014;4(5):e004560. [DOI] [PMC free article] [PubMed] [Google Scholar]